- 1Division of Infectious Diseases, Department of Internal Medicine, State Key Laboratory of Complex Severe and Rare Disease, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Center for Tuberculosis Research, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Clinical Epidemiology Unit, Peking Union Medical College, International Clinical Epidemiology Network, Beijing, China
- 4Department of Rheumatology and Clinical Immunology, Clinical Immunology Center, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objective: The aim of this study was to investigate the clinical traits and consequences of systemic lupus erythematosus (SLE) complicated by active cytomegalovirus (CMV) infection.
Methods: This retrospective review involved the examination of medical records for patients diagnosed with SLE who had an active CMV infection at the time of their discharge from Peking Union Medical College Hospital between June 2016 and December 2022. The consistency between plasma CMV deoxyribonucleic acid (DNA) viral load and pp65 antigenemia was analyzed using the chi-square test. Related factors for CMV disease in SLE complicated by active CMV infection patients were analyzed by univariate analysis and multivariable stepwise logistic regression. Cox hazards regression analysis was used to determine predictors for all-cause mortality and CMV recurrence within 3 months.
Results: A total of 206 patients were enrolled in this study. Of the 123 patients who were detected with both plasma CMV DNA viral load and pp65 antigenemia within an interval not exceeding 72 h, the consistency between plasma CMV DNA viral load and pp65 antigenemia was not good (Kappa = −0.304, p < 0.001). Plasma CMV DNA viral load ≥ 1,600 copies/mL [odds ratio (OR) 4.411, 95% CI 1.871–10.402, p = 0.001], current glucocorticoids dose (equivalent to prednisolone) ≥60 mg/d (OR 2.155, 95% CI 1.071–4.334, p = 0.031), and elevated alanine transaminase (OR 3.409, 95% CI 1.563–7.435, p = 0.002) were significant clinical clues indicating CMV disease in SLE. Multivariable Cox hazards regression analysis showed that CMV organ involvement [hazard ratio (HR) 47.222, 95% CI 5.621–396.689, p < 0.001], SLE multi-system involvement (HR 1.794, 95% CI 1.029–3.128, p = 0.039), and elevated hypersensitive C-reactive protein (hsCRP) (HR 5.767, 95% CI 1.190–27.943, p = 0.030) were independent risk factors for 3-month all-cause mortality. CMV organ involvement (HR 3.404, 95% CI 1.074–10.793, p = 0.037) was an independent risk factor for CMV recurrence within 3 months.
Conclusion: In SLE patients, plasma CMV DNA viral load seemed to have a higher value in the diagnosis of CMV disease; patients with CMV organ involvement, SLE multi-system involvement, and elevated hsCRP might have a higher risk of 3-month all-cause mortality; and patients with CMV organ involvement might have a higher risk of CMV recurrence within 3 months.
Introduction
Cytomegalovirus (CMV), a double-stranded deoxyribonucleic acid (DNA) virus, is known to establish a state of lifelong latency following infection (1, 2). In China, seroprevalence of adult CMV has been reported to be as high as 90% (3). Under specific circumstances, CMV can be reactivated, leading to recurrent infections. Typically, CMV infection is either symptomless or manifests as mononucleosis-like symptoms, including fever, sore throat, and fatigue. Nevertheless, CMV harbors the potential to inflict damage upon diverse organs, including the lungs, liver, gastrointestinal tract, central nervous system, and retina, consequently amplifying the mortality risk for immunocompromised individuals (4–6). The most common methods of CMV infection diagnosis are plasma CMV DNA viral load and pp65 antigenemia test. The reliability of CMV infection diagnostic test raises questions due to the variability introduced by factors such as sample stability and leukopenia.
The prevalence of systemic lupus erythematosus (SLE) in China ranges from approximately 30/100,000 to 70/100,000 (7). Patients who received glucocorticoids (GCs) (equivalent PSL dose ≥20 mg/d, plus treatment course ≥14 days, or total equivalent PSL dose >700 mg), biological agents, or immunosuppressants are considered in an immunosuppressive state (8). Infection is the leading cause of death in SLE patients; SLE patients who received immunosuppressive treatment are at increased risk of infection, and the identification and prevention of infections, such as active CMV infection, should be strengthened (9). When SLE patients develop fever or other clinical symptoms suspected of active CMV infection, CMV infection should also be considered and actively tested. Previous studies have reported that the prevalence of CMV antigenemia was 35.1% in whole patients with autoimmune diseases, up to 58.6% in patients with SLE, and 11.4% in patients with non-SLE autoimmune diseases (10). In another single-center study, CMV DNA was detected in 17.0% (142/834) of patients who received corticosteroid therapy for RD (11). The coexistence of CMV infection and SLE requires many complex considerations. Active CMV infection can mimic clinical manifestations of SLE (12, 13) and treatment options for SLE patients may be limited because of serious infection. On the other hand, CMV plays a potential role in triggering SLE and SLE disease activity (14, 15). It is thus important to study CMV infection in SLE patients.
Although some scholars have conducted active exploration in the clinical significance of CMV infection in human immunodeficiency virus (HIV)-infected patients and transplant recipients, there is still a lack of understanding of active CMV infection in SLE patients. This study endeavors to summarize the characteristics and outcomes of SLE patients complicated by active CMV infection, investigate the association and diagnostic efficacy of plasma CMV DNA viral load and pp65 antigenemia, and explore risk factors for all-cause mortality and CMV recurrence within 3 months. This work is valuable for the management of CMV infection in SLE population.
Materials and methods
Study design and patients
This study follows a retrospective cohort design, in which we included SLE patients who were hospitalized and had active CMV infection at Peking Union Medical College Hospital between June 2016 and December 2022. Inclusion criteria were as follows: (1) age 16 years or older; (2) meeting the 2009 American College of Rheumatology (ACR) SLE classification criteria (16); and (3) satisfying the diagnostic criteria for active CMV infection. Exclusion criteria were as follows: (1) cases with an overlap syndrome (where SLE overlaps with other rheumatic diseases); and (2) transplantation or other non-SLE immunocompromising conditions.
We collected data on patient demographics; disease progression; the SLE disease activity index (SLEDAI)-2000 (17); medication usage including GCs, immunosuppressants, and biological agents; plasma CMV DNA viral load; and pp65 antigenemia, as well as laboratory findings such as routine blood tests. All laboratory analyses were performed by the Laboratory Department of Peking Union Medical College Hospital. This study received approval from the institutional ethics committee at Peking Union Medical College Hospital (No. I-23PJ1192).
Laboratory testing
Plasma CMV DNA viral load: The CMV DNA diagnostic blood kit (Sansure Biotech, China) with a limit of detection of 500 copies/ml was performed to extract DNA extraction of the plasma sample. A real-time fluorescent quantitative polymerase chain reaction (PCR) detection of CMV DNA was performed on a LightCycler 480 Detection System (Roche, US) using Therma-Base Taqman technologies.
CMV pp65 antigenemia: The CMV Brite assay (IQ products BV, Groningen, the Netherlands) was performed as instructed. EDTA anti-coagulated whole blood samples (for patients with neutropenia, at least 5–6 ml of blood was drawn) were processed and approximately 1.5×105/0.1 mL cells were immunofluorescence stained with anti-CMV pp65 antibodies (C10/C11 and IgG1) after fixation. Then, they were re-stained with FITC-labeled rabbit anti-mouse IgG conjugate. The slides were read using a fluorescence microscope (Olympus BX51, Tokyo, Japan). Polylobate perinuclear yellow-green fluorescent staining of leukocytes was used to determine positive CMV antigenemia. One or more CMV antigen-positive cells present per duplicate stain was considered positive.
Definition
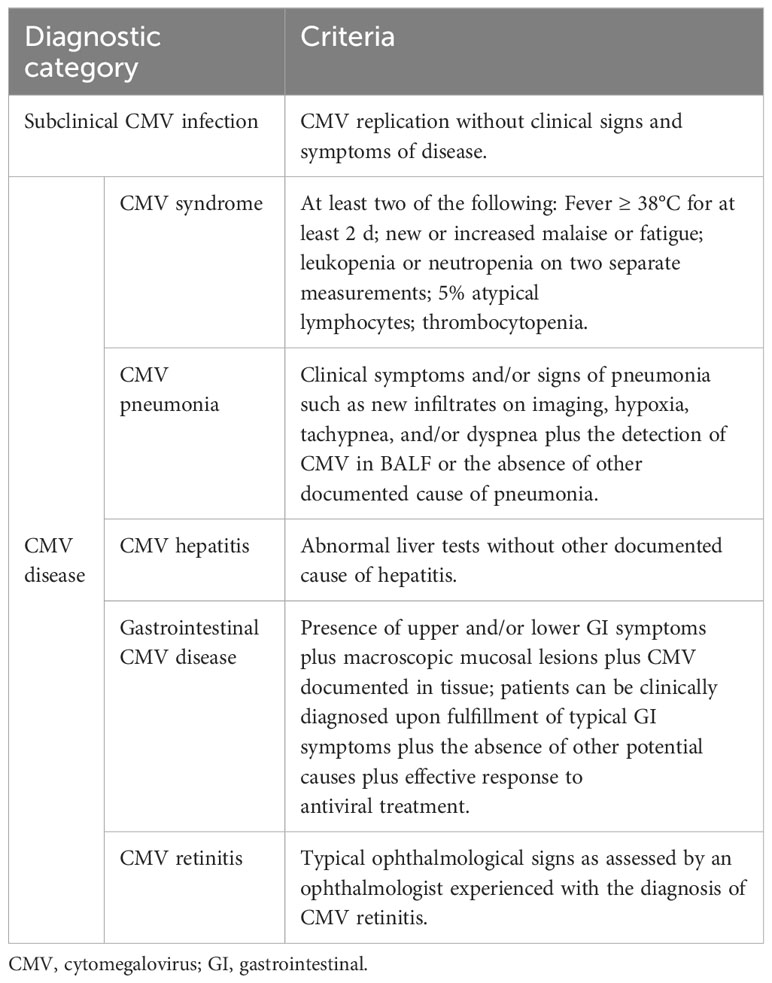
Active CMV infection: presence of CMV replication in tissue, blood, or other bodily fluids regardless of symptomatology. CMV replication is detected by nucleic acid testing, antigen testing, H&E stain, and/or immunohistochemistry. Active infections were classified as subclinical CMV infection or CMV disease based on the presence or absence of symptoms related to CMV.
Subclinical CMV infection: CMV replication without clinical signs and symptoms of disease.
CMV disease: CMV infection that is accompanied by clinical signs and symptoms, including CMV syndrome (fever, malaise, atypical lymphocytosis, leukopenia or neutropenia, and thrombocytopenia) and CMV organ involvement (e.g., pneumonia, hepatitis, gastrointestinal disease, and retinitis) (Table 1). The diagnosis of CMV infection/disease was independently established by two infectious disease specialists, with any disagreements resolved through consultation with a third senior physician.
CMV recurrence: plasma CMV DNA viral load or pp65 antigenemia turned positive again within a temporal window spanning from 7 days to 3 months regardless of symptomatology.
Statistical analysis
Normally distributed variables are typically represented as means with a standard deviation (SD), while non-normally distributed variables are often indicated by the median and interquartile range (IQR). Categorical variables are usually expressed as percentages (%). To compare continuous variables that follow a normal distribution, the group t-test was employed, while for continuous variables that do not conform to normal distribution, the Wilcoxon test was used. When comparing categorical data between groups, the chi-square test or Fisher’s exact test was utilized. We used the chi-square test to assess the consistency between plasma CMV DNA viral load and pp65 antigenemia. Univariate and multivariable stepwise logistic regression analyses were employed to investigate factors related to CMV disease in SLE patients complicated by active CMV infection. Predictors of all-cause mortality and CMV recurrence within 3 months were identified through Cox hazards regression analysis. Statistical significance was determined for p-values < 0.05. Data analysis was carried out using Statistical Package for Social Sciences (SPSS) software version 26 and R programming software.
Results
Demographic and clinical characteristics
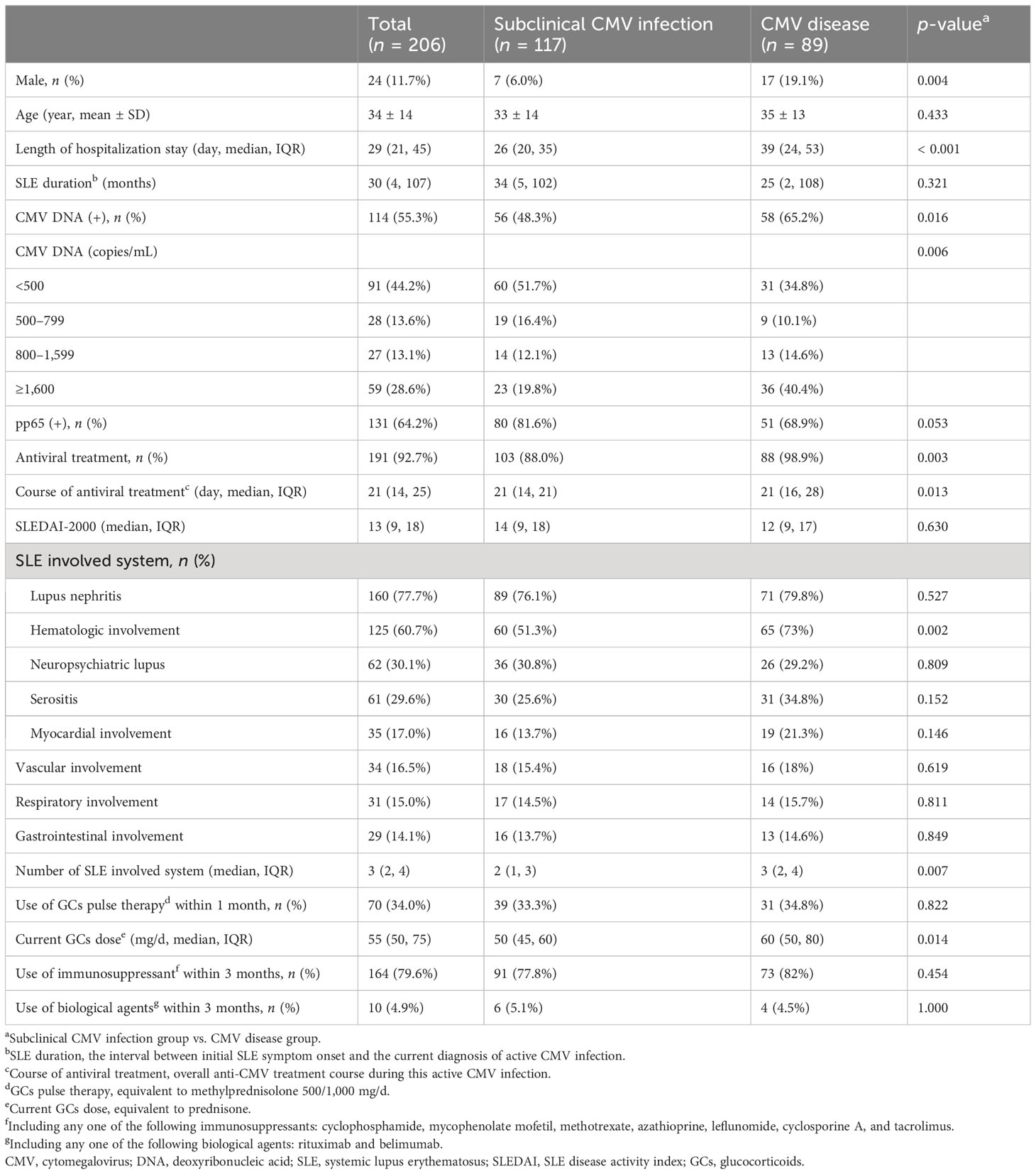
A total of 206 patients with SLE were enrolled in the study. In the overall study population, the average age of participants was 34 ± 14 years; 88.3% were women. In terms of SLE system involvement, lupus nephritis was the most common (160/206, 77.7%), followed by hematologic involvement (125/206, 60.7%), neuropsychiatric lupus (62/206, 30.1%), and serositis (61/206, 29.6%). Of the 206 patients enrolled, 204 patients (99.0%) were treated with GCs within 1 month, 70 patients (34.0%) received GCs pulse therapy (equivalent to methylprednisolone 500/1,000 mg/d), 164 patients (79.6%) were treated with immunosuppressants within 3 months [including cyclophosphamide (CTX), methotrexate (MTX), mycophenolate mofetil (MMF), azathioprine (AZA), cyclosporine A (CsA), leflunomide (LEF), and tacrolimus (FK506)], and 10 patients (4.9%) were treated with biological agents within 3 months (including rituximab and belimumab). Among the patients enrolled, 191 (92.7%) received antiviral therapy, and 15 (7.3%) have not been treated with antiviral therapy.
Of the 206 patients enrolled, 114 (55.3%) were positive for plasma CMV DNA viral load and 131 (64.2%) were positive for pp65 antigen. The 206 patients were grouped into the subclinical CMV infection group (n = 117) and the CMV disease group (n = 89) according to the definition above. The latter included CMV syndrome (56/89, 62.9%), CMV pneumonia (17/89, 19.1%), CMV hepatitis (13/89, 14.6%), CMV gastroenteritis (2/89, 2.2%), and retinitis (1/89, 1.1%). As shown in Table 2, compared with the subclinical CMV infection group, the proportion of male (p = 0.004), positive CMV DNA (p = 0.016), plasma CMV DNA viral load ≥ 1,600 copies/mL (p < 0.001), and received anti-CMV therapy (p = 0.003) were higher in the CMV disease group, and their hospital stays (p < 0.001) and course of antiviral treatment (p = 0.013) tended to be longer. The number of SLE involved system (p = 0.007) and current GCs dose [equivalent to prednisolone (PSL)] (p = 0.014) were significantly higher than in the subclinical CMV infection group. No significant differences were observed between the two groups in terms of SLEDAI score, GCs pulse therapy within 1 month, and immunosuppressive or biological agent use within 3 months. Antivirals were started pre-emptively in 103 (88.0%) patients without clinical symptoms of active CMV infection.
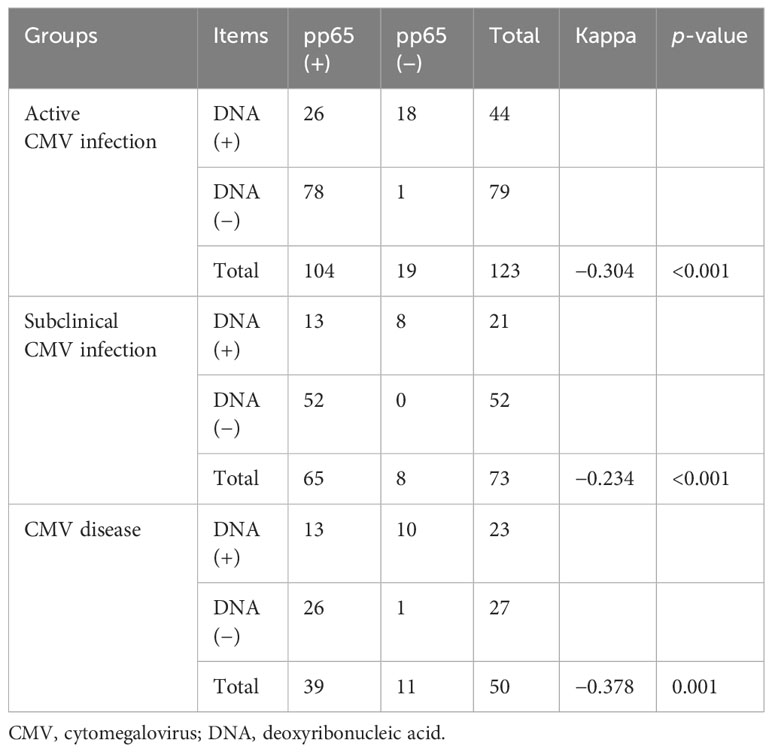
Consistency analysis of plasma CMV DNA viral load and pp65 antigenemia
Of the 123 SLE patients who were detected with both plasma CMV DNA viral load and pp65 antigen within an interval not exceeding 72 h and no treatment adjustments within the interval, 44 (35.8%) were positive for CMV DNA viral load, 104 (84.6%) were positive for pp65 antigen, 26 (21.1%) were positive for both CMV DNA viral load and pp65 antigen, and 1 was negative for both CMV DNA viral load and pp65 antigen (proven by the detection of CMV DNA in the bronchoalveolar lavage, compatible clinical and radiographic findings and effective antiviral response). There was a lack of consistency between plasma CMV DNA viral load and pp65 antigenemia (Table 3).
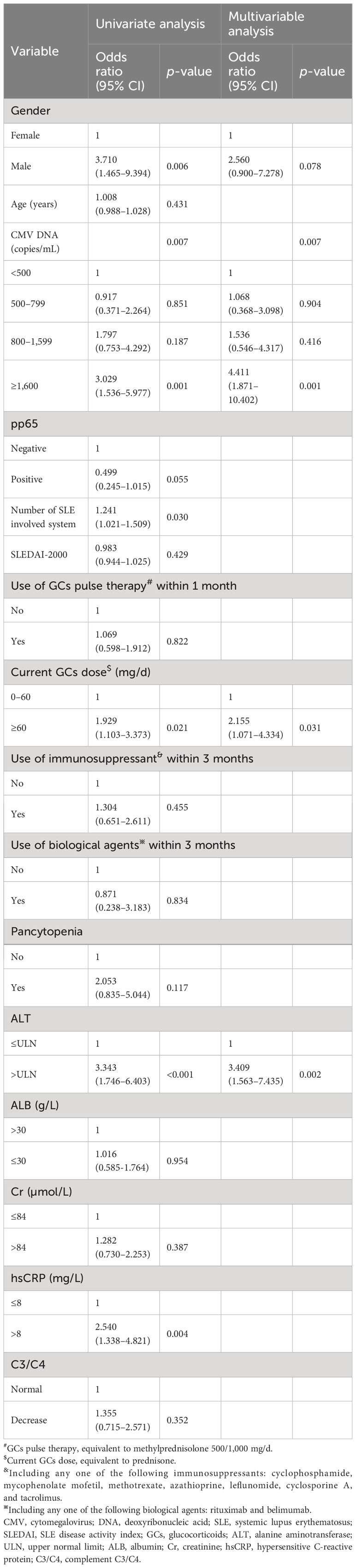
Related factors for CMV disease in SLE complicated by active CMV infection patients
Related factors for CMV disease were analyzed in 206 SLE patients. The univariate analysis findings can be seen in Table 4. Subsequently, variables such as gender, CMV DNA viral load, pp65 antigenemia, number of SLE involved system, current GCs dose ≥ 60 mg/d, alanine transaminase (ALT), and hypersensitive C-reactive protein (hsCRP) were included in a multivariable logistic regression model. The finding indicated that plasma CMV DNA viral load ≥ 1,600 copies/mL (OR 4.411, 95% CI 1.871–10.402, p = 0.001), current glucocorticoids dose (equivalent to PSL) ≥ 60 mg/d (OR 2.155, 95% CI 1.071–4.334, p = 0.031), and elevated ALT (OR 3.409, 95% CI 1.563–7.435, p = 0.002) were independent related factors for CMV disease.
Risk factors for 3-month all-cause mortality in SLE complicated by active CMV infection patients
Ten patients died within 3 months; all patients who died had CMV disease. The median time to death was 21.5 days (IQR 7.75–53). All patients who died were female, and the average age was 39 ± 10 years. All patients who died were treated with GCs within 1 month, of which four patients (4/10, 40.0%) received GCs pulse therapy. Nine patients (9/10, 90.0%) were treated with immunosuppressants within 3 months (including CTX in six patients, MMF in seven patients, CsA in one patient, and FK506 in one patient). Non-patients were treated with biological agents within 3 months.
Risk factors for 3-month all-cause mortality were analyzed in the 206 SLE complicated by active CMV infection patients. Differences in survival by subgroup were tested with Cox regression, after testing that proportional hazard assumptions were satisfied. We conducted a univariate Cox regression analysis involving gender, age, SLE duration, SLEDAI-2000, plasma CMV DNA viral load, the use and dosage of medications, and laboratory examinations. The results are shown in Table 5. CMV organ involvement, plasma CMV DNA viral load, number of SLE involved system, lymphopenia, moderate to severe anemia, and hsCRP were included in a multivariable Cox regression model. The finding indicated that CMV organ involvement [hazard ratio (HR) 47.222, 95% CI 5.621–396.689, p < 0.001], number of SLE involved system (HR 1.794, 95% CI 1.029–3.128, p = 0.039), and elevated hsCRP (HR 5.767, 95% CI 1.190–27.943, p = 0.030) were independent risk factors for 3-month all-cause mortality. Survival curves of 3-month all-cause mortality in patients with or without CMV organ involvement are shown in Figure 1.
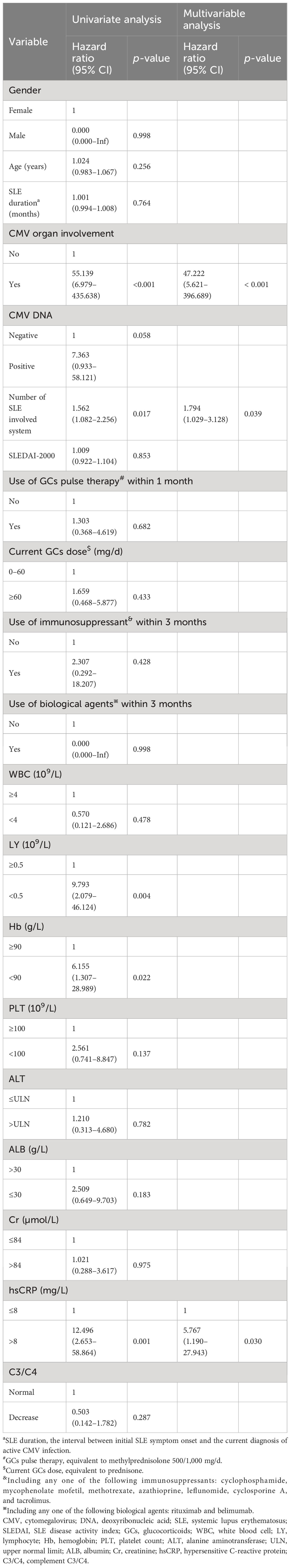
Table 5 Risk factors for 3-month all-cause mortality in SLE complicated by active CMV infection patients.
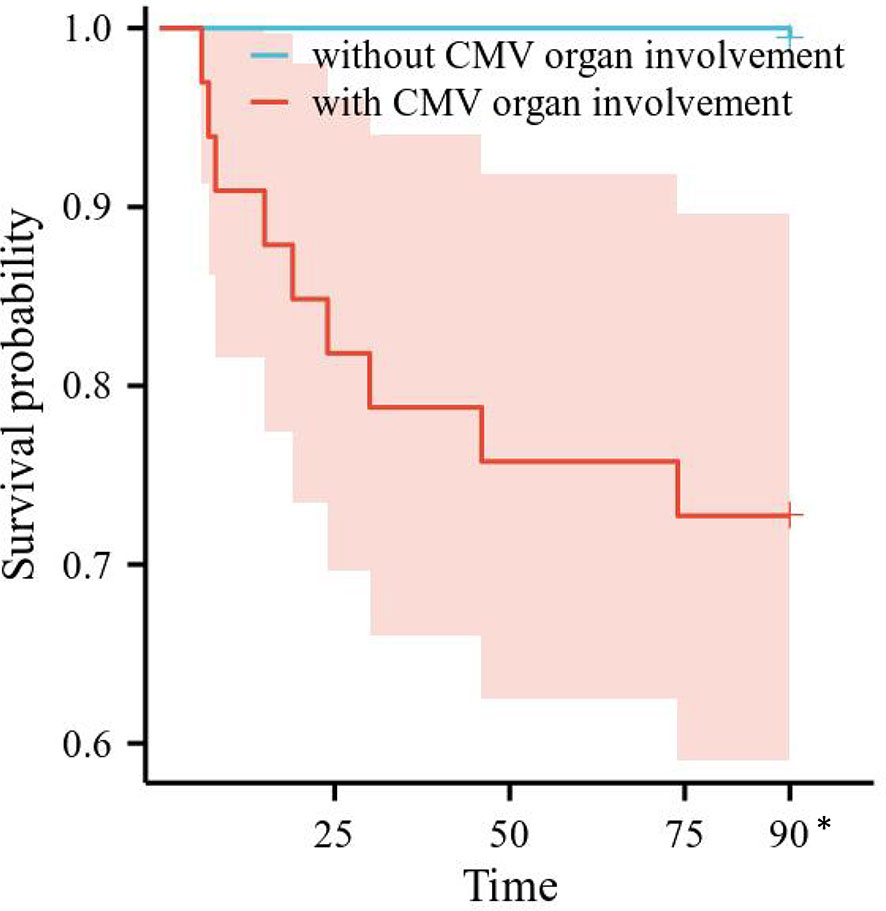
Figure 1 Survival curves of all-cause mortality in patients with or without CMV organ involvement. CMV, cytomegalovirus. *All the patients censored at this time point.
Risk factors for CMV recurrence within 3 months in SLE complicated by active CMV infection patients
A total of 12 patients experienced CMV recurrence within 3 months. The median time to recurrence was 53 days (IQR 15.5–67.5). All recurrent patients were female, and the average age was 37 ± 16 years. All recurrent patients were treated with GCs within 1 month before CMV reactivation, of which one patient (8.3%) received GCs pulse therapy. All recurrent patients were treated with immunosuppressants within 3 months (including CTX in seven patients, MMF in seven patients, CsA in three patients, and FK506 in two patients). None of the recurrent patients were treated with biological agents.
Risk factors for CMV recurrence within 3 months were analyzed in the 206 SLE complicated by active CMV infection patients. Differences in CMV recurrence by subgroup were tested with Cox regression, after testing that proportional hazard assumptions were satisfied. We conducted univariate Cox regression analysis on gender, age, SLE duration, SLEDAI-2000, plasma CMV DNA viral load, the use and dosage of medications, and laboratory examinations. The results are shown in Table 6. CMV organ involvement, plasma CMV DNA viral load, and C3/C4 were incorporated into a multivariable Cox regression model. The outcomes revealed that CMV organ involvement (HR 3.404, 95% CI 1.074–10.793, p = 0.037) independently posed a risk for CMV recurrence within 3 months. Survival curves of CMV recurrence in patients with or without CMV organ involvement are shown in Figure 2.
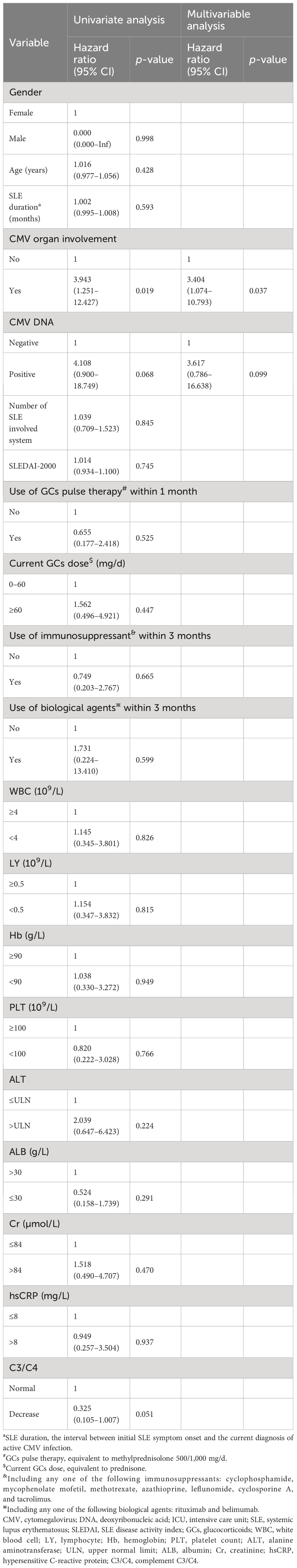
Table 6 Risk factors for CMV recurrence within 3 months in SLE complicated by active CMV infection patients.
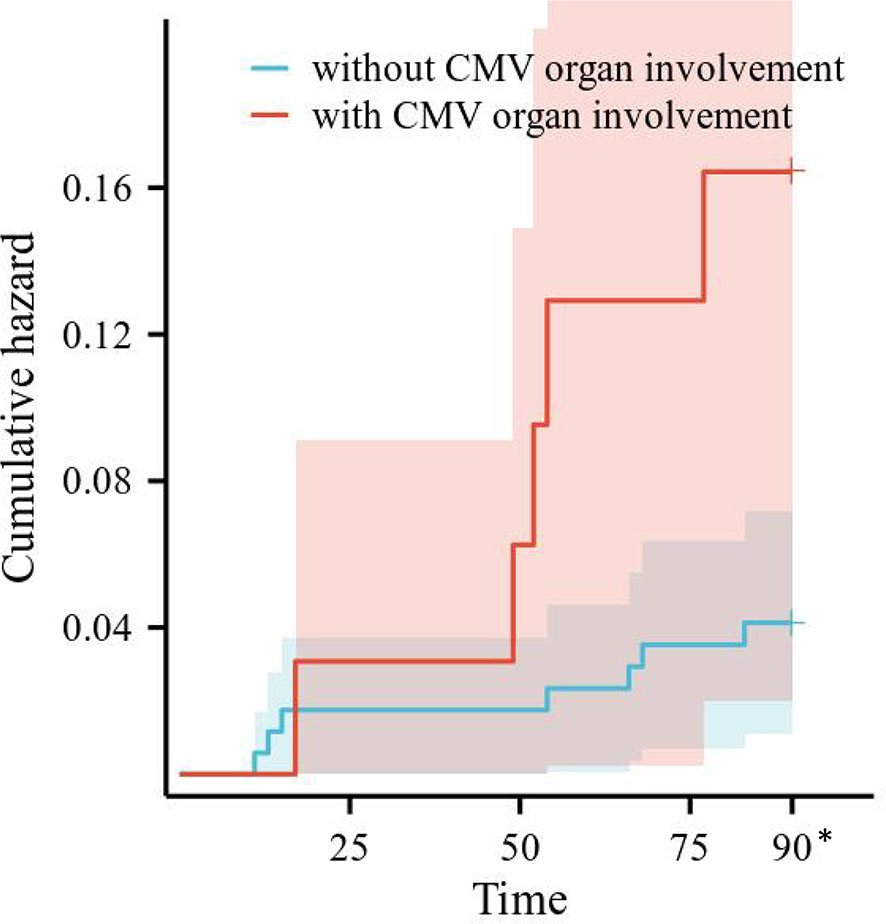
Figure 2 Survival curves of CMV recurrence in patients with or without CMV organ involvement. CMV, cytomegalovirus. *All the patients censored at this time point.
Discussion
In the present study, we conducted an exploration of the clinical features and prognosis in SLE patients complicated by active CMV infection. This will provide information on management strategies of CMV infection in the SLE population.
Few studies have explored the association and diagnostic value between plasma CMV DNA viral load and pp65 antigenemia in the SLE population. Inconsistent with previous findings in the transplant population (18, 19), a study compared the CMV DNA viral load and pp65 antigenemia test in the SLE patients for monitoring the development of CMV disease, and they found that pp65 antigenemia had a higher sensitivity (87%) but poor specificity (7.6%), CMV DNA viral load had a moderate sensitivity (66.1%) and specificity (55.3%), and there was no consistency between plasma CMV DNA viral load and pp65 antigenemia (Kappa = −0.176, p = 0.055) (19). In addition, a systematic review revealed that a high CMV DNA viral load was linked to the development of CMV disease in individuals with SLE (20). In this study, there was a lack of consistency between plasma CMV DNA viral load and pp65 antigenemia in the SLE population (Kappa < 0, p < 0.05), and plasma CMV DNA viral load seemed to have a higher value in diagnosing CMV disease. There may be cross-reactive antigens related to CMV pp65 in SLE patients (21, 22), thereby reducing the diagnostic value of pp65 antigenemia in CMV disease.
Several studies linked treatment drugs to CMV disease. Xue Y et al. reported that the CMV pneumonia group received higher doses of PSL [median (range) 32 (4–100) mg vs. 20 (1–50) mg/d, respectively, p < 0.010] and more frequently immunosuppressants (79% vs. 58%%p < 0.010) than the subclinical CMV infection group in the RD population (9). Another study of 38 SLE patients observed that the CMV disease group received a higher PSL dosage compared to the non-CMV disease group [mean (SD) 25.9 (17.1) mg/d vs. 9.0 (4.1) mg/d, respectively, p = 0.006], and the use of AZA 1 month prior to admission was more common in the CMV disease group (35% vs. 5.6%, p = 0.045) (23). The results of this study were partially similar to those of previous studies, which indicate that higher doses of GCs were associated with CMV disease (OR = 2.155, p = 0.031), while there were no significant differences in the GCs pulse therapy and immunosuppressant usage. The discrepancy may be due to different definition of CMV infection, types and activity of RD.
We proposed a certain level of positive association between CMV disease and SLE activity. Xue Y et al. found that patients with CMV pneumonia had a longer SLE duration compared to patients without CMV disease [median (range) 8 (0.03–360) months vs. 3 (0.25–156) months, respectively, p < 0.05] (9). Results of a clinical study in autoimmune disease patients (mostly SLE) complicated by active CMV infection showed that the deceased subgroup had a significantly higher SLEDAI-2000 than the alive subgroup (p = 0.072) (23). In the present study, number of SLE involved system was higher in the CMV disease group than in the subclinical CMV infection group, while there were no significant differences in the SLEDAI-2000 and SLE disease duration. In addition, SLE multi-system involvement was associated with 3-month all-cause mortality (HR = 1.794, p = 0.039). Although they are most widely used for the assessment of SLE activity, number of involved system and SLEDAI-2000 have some limitations, e.g., insufficient attention to the rare but important symptoms of SLE. The relationship between CMV disease and SLE activity should be confirmed in the future.
One study of 56 SLE patients with CMV diseases revealed a significant difference in the percentage of patients who had CMV end-organ diseases between the mortality and survival groups (83.33% vs. 25%, HR = 15.000, p = 0.001) (24). In a single-center-based nested case–control study, 113 patients who underwent haploidentical HSCT (2.92%) experienced CMV disease, and the overall mortality was higher in patients with CMV pneumonia, disseminated CMV disease, and CMV encephalitis (61.7%, 57.1%, and 40.0%, respectively) (25). In our study, CMV organ involvement accounted for 37.1% (33/89) of SLE complicated by CMV disease, with case fatality rates within 3 months of up to 27.3% (9/33); CMV organ involvement (HR = 47.222, p < 0.001) and elevated hsCRP, a well-known clinical biomarker for inflammation (HR = 5.767, p = 0.030), were independent risk factors for 3-month all-cause mortality in SLE patients complicated by active CMV infection.
Despite effective antiviral treatment, a proportion of patients may experience relapse. Risk factors for CMV recurrence are incompletely characterized; most studies have been conducted in transplant recipients. An investigation indicated that 19.4% (33/170) of solid organ transplant recipients encountered a relapse of CMV within 6 months, and low absolute lymphocyte count was an independent predictor for the recurrence of CMV disease (HR 1.11, p = 0.009) (26). Some scholars have proposed that high CMV DNA load at diagnosis was associated with risk of recurrence (27, 28). To our knowledge, our research is the first study to analyze risk factors for CMV recurrence in SLE patients. We found that the CMV recurrence rate within 3 months was only 5.8% in SLE patients, much lower than that in transplant recipients (19.4%–73%) (26, 29, 30). Notably, CMV organ involvement was an independent risk factor for CMV recurrence within 3 months (HR = 3.404, p = 0.037). The varying outcomes could be attributed to disparities in the study population, antiviral therapy, the time frame retrospectively examined, and so forth.
Threshold of plasma CMV DNA viral load or pp65 antigenemia for preemptive therapy may be different in different risk populations. Although low threshold is likely to be more clinically meaningful in patients with a higher infection risk, choosing a very low threshold may lead to unnecessary treatment. A systematic review revealed that the timing of preemptive therapy was not uniform across the studies (31), and several international guidelines recommend that medical institutions define thresholds for triggering therapy based on risk categories and center data (20, 32). In our study, of the 15 patients who did receive anti-CMV therapy, the median plasma viral load was 500 copies/mL, the median WBC count of peripheral blood with positive CMV pp65 antigen was 3, and none of the patients died or developed target organ invasion within 3 months. In a retrospective cohort analysis of non-immunocompromised patients with CMV reactivation, the use of ganciclovir did not show any significant connection with long-term outcomes; therefore, antiviral treatment in such cases may not be deemed necessary unless there are concerns of organ involvement (33). Studies on rheumatic disease patients complicated by active CMV infection also found that pp65 turned negative spontaneously in some patients (17, 34). Thus, antiviral therapy may not be necessary for some patients with subclinical CMV infection or even CMV syndrome. The relatively limited sample size of the untreated group emphasizes the need for more expansive studies in the future.
This study inevitably has some limitations. First, the enrolled patients were hospitalized at Peking Union Medical College Hospital; they seemed to be more seriously ill, and some patients were excluded because of grossly incomplete medical records; thus, there is a potential for selection bias in this study. Second, some factors such as viral replication kinetics and lymphocyte subsets cannot be further analyzed due to missing data. Third, because of the limited number of observed outcome events, the estimated incidences have broad confidence intervals, which necessitate further research for validation. Lastly, this study cannot establish a causal relationship, emphasizing the need for prospective investigations in the future.
Conclusion
In summary, our study explored the clinical characteristics and outcomes of SLE complicated by active CMV infection. We found that peripheral blood CMV DNA viral load seemed to have a higher value in the diagnosis of CMV disease; patients with CMV organ involvement, SLE multi-system involvement, and elevated hsCRP might have a higher risk of 3-month all-cause mortality; and patients with CMV organ involvement might have a higher risk of CMV recurrence within 3 months. These findings contribute to CMV management targeting SLE patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Peking Union Medical College Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study did not involve contact with participants or any intervention.
Author contributions
YC: Writing – original draft. LFZ: Data curation, Methodology, Writing – review & editing. YCL: Writing – review & editing. YL: Writing – review & editing. LDZ: Writing – review & editing. BZ: Writing – review & editing. GR: Writing – review & editing. XS: Funding acquisition, Writing – review & editing. XL: Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-043).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stern L, Withers B, Avdic S, Gottlieb D, Abendroth A, Blyth E, et al. Human cytomegalovirus latency and reactivation in allogeneic hematopoietic stem cell transplant recipients. Front Microbiol. (2019) 10:1186. doi: 10.3389/fmicb.2019.01186
2. Freer G, Quaranta P, Pistello M. Evaluation of T cell immunity against human cytomegalovirus: impact on patient management and risk assessment of vertical transmission. J Immunol Res. (2016) 2016:9384813. doi: 10.1155/2016/9384813
3. Fang FQ, Fan QS, Yang ZJ, Peng YB, Zhang L, Mao KZ, et al. Incidence of cytomegalovirus infection in Shanghai, China. Clin Vaccine Immunol. (2009) 16:1700–3. doi: 10.1128/CVI.00385-08
4. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. (2010) 50:1439–47. doi: 10.1086/652438
5. Siegal DS, Hamid N, Cunha BA. Cytomegalovirus colitis mimicking ischemic colitis in an immunocompetent host. Heart Lung. (2005) 34:291–4. doi: 10.1016/j.hrtlng.2004.08.009
6. Bernard S, Germi R, Lupo J, Laverrière MH, Masse V, Morand P, et al. Symptomatic cytomegalovirus gastrointestinal infection with positive quantitative real-time PCR findings in apparently immunocompetent patients: a case series. Clin Microbiol Infect. (2015) 21:1121.e1–7. doi: 10.1016/j.cmi.2015.05.016
7. Tian XP, Li MT, Zeng XF. The challenges and future development of the management of systemic lupus erythematosus in China: a concise annual report of 2020. Chin J Internal Med. (2022) 61:611–6. doi: 10.2478/rir-2022-0006
8. Ramirez JA, Musher DM, Evans SE, Dela Cruz C, Crothers KA, Hage CA, et al. Treatment of community-acquired pneumonia in immunocompromised adults: A consensus statement regarding initial strategies. Chest. (2020) 158:1896–911. doi: 10.1016/j.chest.2020.05.598
9. Li M, Zhao Y, Zhang Z, Huang C, Liu Y, Gu J, et al. Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus. Rheumatol Immunol Res. (2020) 1:5–23. doi: 10.2478/rir-2020-0009
10. Cui J, Yan W, Xie H, Xu S, Wang Q, Zhang W, et al. Cytomegalovirus antigenemia in patients with autoimmune and non-autoimmune diseases in Beijing: A 10-year single hospital experience. PloS One. (2019) 14:e0221793. doi: 10.1371/journal.pone.0221793
11. Xue Y, Jiang L, Wan WG, Chen YM, Zhang J, Zhang ZC. Cytomegalovirus pneumonia in patients with rheumatic diseases after immunosuppressive therapy: a single center study in China. Chin Med J (Engl). (2016) 129:267–73. doi: 10.4103/0366-6999.174490
12. Vasquez V, Barzaga RA, Cunha BA. Cytomegalovirus-induced flare of systemic lupus erythematosus. Heart Lung. (1992) 21:407–8.
13. Cooray M, Manolakos JJ, Wright DS, Haider S, Patel A. Parvovirus infection mimicking systemic lupus erythematosus. CMAJ. (2013) 185:1342–4. doi: 10.1503/cmaj.121565
14. Sekigawa I, Nawata M, Seta N, Yamada M, Iida N, Hashimoto H. Cytomegalovirus infection in patients with systemic lupus erythematosus. Clin Exp Rheumatol. (2002) 20:559–64.
15. Rozenblyum EV, Allen UD, Silverman ED, Levy DM. Cytomegalovirus infection in childhood-onset systemic lupus erythematosus. Int J Clin Rheumtol. (2013) 8:137–146. doi: 10.2217/ijr.12.82
16. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. (2012) 64:2677–86. doi: 10.1002/art.34473
17. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29:288–91.
18. Cardeñoso L, Pinsky BA, Lautenschlager I, Aslam S, Cobb B, Vilchez RA, et al. CMV antigenemia and quantitative viral load assessments in hematopoietic stem cell transplant recipients. J Clin Virol. (2013) 56:108–12. doi: 10.1016/j.jcv.2012.10.001
19. Ma YY. Clinical analysis of systemic lupus erythematosus with CMV infection [doctor's thesis]. China (Beijing): Peking Union Medical College (2014).
20. Choo HMC, Cher WQ, Kwan YH, Fong WWS. Risk factors for cytomegalovirus disease in systemic lupus erythematosus (SLE): a systematic review. Adv Rheumatol. (2019) 59:12. doi: 10.1186/s42358-019-0055-y
21. HoHsieh A, Wang CM, Wu YJ, Chen A, Chang MI, Chen JY. B cell epitope of human cytomegalovirus phosphoprotein 65 (HCMV pp65) induced anti-dsDNA antibody in BALB/c mice. Arthritis Res Ther. (2017) 19:65. doi: 10.1186/s13075-017-1268-2
22. Hsieh AH, Kuo CF, Chou IJ, Tseng WY, Chen YF, Yu KH, et al. Human cytomegalovirus pp65 peptide- induced autoantibodies cross-reacts with TAF9 protein and induces lupus-like autoimmunity in BALB/c mice. Sci Rep. (2020) 10:9662. doi: 10.1038/s41598-020-66804-1
23. Tsai WP, Chen MH, Lee MH, Yu KH, Wu MW, Liou LB. Cytomegalovirus infection causes morbidity and mortality in patients with autoimmune diseases, particularly systemic lupus: in a Chinese population in Taiwan. Rheumatol Int. (2012) 32:2901–8. doi: 10.1007/s00296-011-2131-4
24. Hung M, Huang DF, Chen WS, Lai CC, Chen MH, Liao HT, et al. The clinical features and mortality risk factors of cytomegalovirus infection in patients with systemic lupus erythematosus. J Microbiol Immunol Infect. (2019) 52:114–21. doi: 10.1016/j.jmii.2018.12.002
25. Meng XY, Fu HX, Zhu XL, Wang JZ, Liu X, Yan CH, et al. Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation. Ann Hematol. (2020) 99:2659–70. doi: 10.1007/s00277-020-04201-4
26. Gardiner BJ, Nierenberg NE, Chow JK, Ruthazer R, Kent DM, Snydman DR. Absolute lymphocyte count: A predictor of recurrent cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis. (2018) 67:1395–402. doi: 10.1093/cid/ciy295
27. Sia IG, Wilson JA, Groettum CM, Espy MJ, Smith TF, Paya CV. Cytomegalovirus (CMV) DNA load predicts relapsing CMV infection after solid organ transplantation. J Infect Dis. (2000) 181:717–20. doi: 10.1086/315242
28. Helanterä I, Lautenschlager I, Koskinen P. The risk of cytomegalovirus recurrence after kidney transplantation. Transpl Int. (2011) 24:1170–8. doi: 10.1111/tri.2011.24.issue-12
29. Wagner-Drouet E, Teschner D, Wolschke C, Janson D, Schäfer-Eckart K, Gärtner J, et al. Standardized monitoring of cytomegalovirus-specific immunity can improve risk stratification of recurrent cytomegalovirus reactivation after hematopoietic stem cell transplantation. Haematologica. (2021) 106:363–74. doi: 10.3324/haematol.2019.229252
30. Camargo JF, Anderson AD, Rosa R, Kimble E, Komanduri KV, Morris MI. Use of maintenance therapy and incidence of recurrent Cytomegalovirus DNAemia among allogeneic hematopoietic cell transplant recipients. Transpl Infect Dis. (2019) 21:e13054. doi: 10.1111/tid.13054
31. Giménez E, Torres I, Albert E, Piñana JL, Hernández-Boluda JC, Solano C, et al. Cytomegalovirus (CMV) infection and risk of mortality in allogeneic hematopoietic stem cell transplantation (Allo-HSCT): A systematic review, meta-analysis, and meta-regression analysis. Am J Transplant. (2019) 19:2479–94. doi: 10.1111/ajt.15515
32. Hakki M, Aitken SL, Danziger-Isakov L, Michaels MG, Carpenter PA, Chemaly RF, et al. American society for transplantation and cellular therapy series: #3-prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. (2021) 27:707–19. doi: 10.1016/j.jtct.2021.05.001
33. Park GE, Ki HK, Ko JH. Impact of antiviral treatment on long-term prognosis in non-immunocompromised patients with CMV reactivation. BMC Infect Dis. (2021) 21:414. doi: 10.1186/s12879-021-06098-4
Keywords: systemic lupus erythematosus, cytomegalovirus, organ involvement, mortality, recurrence
Citation: Chen Y, Zhang L, Liu Y, Liu Y, Zhao L, Zhou B, Ruan G, Shi X and Liu X (2024) Clinical features and prognosis of systemic lupus erythematosus complicated by active cytomegalovirus infection: a retrospective cohort study. Front. Immunol. 15:1323923. doi: 10.3389/fimmu.2024.1323923
Received: 18 October 2023; Accepted: 09 February 2024;
Published: 28 February 2024.
Edited by:
Lazaros Ignatios Sakkas, University of Thessaly, GreeceReviewed by:
Fahd Adeeb, RCSI and UCD Malaysia Campus, MalaysiaGsrsnk Naidu, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2024 Chen, Zhang, Liu, Liu, Zhao, Zhou, Ruan, Shi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochun Shi, c2hpeGNoNzcyMkAxNjMuY29t; Xiaoqing Liu, bGl1eHFAcHVtY2guY24=
†These authors have contributed equally to this work and share first authorship
 Yan Chen
Yan Chen Lifan Zhang1,2,3†
Lifan Zhang1,2,3† Yuchen Liu
Yuchen Liu Lidan Zhao
Lidan Zhao Baotong Zhou
Baotong Zhou Xiaochun Shi
Xiaochun Shi Xiaoqing Liu
Xiaoqing Liu