- 1The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Department of Neurology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
Background: Pain is a common symptom in multiple sclerosis (MS), especially neuropathic pain, which has a significant impact on patients’ mental and physical health and quality of life. However, risk factors that related to neuropathic pain, still remain unclear.
Objective: The study aimed to explore the risk factors of neuropathic pain among MS patients.
Materials and methods: This retrospective study examined the consecutive patients diagnosed with MS in the Department of Neurology of Guangdong Provincial Hospital of Chinese Medicine between August 2011 and October 2022. Neuropathic pain was defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system”. Demographic and clinical features were obtained from the electronic system of the hospital.
Results: Our cohort revealed that the prevalence of patients with neuropathic pain in MS was 34.1%. The results indicated that the longer the spinal lesions, the greater the neuropathic pain risks (2-4: OR, 13.3(2.1-82), >5: OR, 15.2(2.7-86.8), p for tread: 0.037). Meanwhile, multivariate regression analysis showed that cervical and thoracic lesions (OR 4.276, 95% CI 1.366-13.382, P = 0.013), upper thoracic lesions (T1-T6) (OR 3.047, 95% CI 1.018-9.124, P = 0.046) were positively correlated with neuropathic pain, while basal ganglia lesions (OR 0.188, 95% CI 0.044-0.809, P = 0.025) were negatively correlated with neuropathic pain among MS patients.
Conclusion: Extended spinal lesions (≥3 spinal lesions), cervical and thoracic lesions, upper thoracic lesions were independent risk factors of neuropathic pain among MS patients. Furthermore, our study found that the longer the spinal lesions, the greater the neuropathic pain risks.
1 Introduction
Multiple sclerosis (MS) is an autoimmune, inflammatory disorder of the central nervous system characterized by multiple demyelination scattered in the brain and spinal cord with a chronic course and various clinical symptoms. Pain is one of the most troubling and refractory clinical symptoms in MS(1–3), especially the neuropathic pain(4, 5). The prevalence of patients with neuropathic pain in MS varies between 21% and 58%(6–8), which interferes the quality of life domains(3, 5). There are several definitions of neuropathic pain, such as International Association for the Study of Pain (IASP) criteria(9), Networks-American Pain Society Pain Taxonomy (AAPT) criteria(10), and some validated questionnaires(11). The IASP definition of neuropathic pain was that “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system”, which was used in our study. Meanwhile, a study had divided the neuropathic pain into three types: ongoing extremity pain, trigeminal neuralgia and Lhermitte’s phenomenon(12). So far, both the underlying mechanism and effective treatment of neuropathic pain associated with MS are still unclear(13). According to previous studies, neuropathic pain may arise from lesions within the somatosensory nervous system(7, 14) which was found to be closely related to the pathophysiology of MS(4). Although recent research suggested female, higher disability and longer course were more likely to suffer neuropathic pain (15), several clinical studies suggested that clinical factors (such as disability and disease duration) and demographic features (age) were associated with neuropathic pain in patients with MS(16, 17). Since the risk factors of neuropathic pain are still unclear, our study aims to investigate the risk and protective factors of neuropathic pain among MS patients.
2 Methods
2.1 Patients
We retrospectively screened the consecutive patients diagnosed with MS in the Department of Neurology of Guangdong Provincial Hospital of Chinese Medicine between August 2011 and October 2022. All patients met the 2017 revised McDonald criteria. Patients with incomplete clinical data were excluded. Neuropathic pain (NP) was defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” and was assessed by a neurologist according to the IASP criteria (9). It means that “If patient’s pain is described within the area affected by an MS lesion in the brain or spinal cord, and associated with sensory changes in the same neuroanatomically plausible distribution, it is considered that the person has neuropathic pain”(10). Headache (with the exception of trigeminal neuralgia) is not considered as neuropathic pain in our study. Patients were classified into NP Group and non-NP Group based on the presence of neuropathic pain. Then, we analyzed the risk factors of MS patients. Demographic and clinical features such as sex, age of the first episode, Extended disability status Scale (EDSS) of the first onset, symptoms of the first episode including neuropathic pain and pain location, localization of lesions of the whole course of the disease on Magnetic Resonance Imaging (MRI) were collected from the hospital system database. In our study, two associate professors respectively interpreted the imaging data to determine their locations of lesions and the length of involved spinal segments. If the two neurologists had different views, a third senior neurologist made the final judgment.
The study was approved by the Institutional Review Boards (Ethical Committee of Guangdong Provincial Hospital of Chinese Medicine ID ZE2022-165-01). Written informed consents were not required.
Study sample size was based on our previous study, which found that extended spinal lesions was more prevalent among patients with neuropathic pain in NMOSD (OR 4.41, 95% CI 1.54-12.62)(18). We assumed the occurrence rate of NP among MS patients with extended spinal lesions was 65% and OR was 2. Then, a sample size of 80 patients with an alpha of 0.05 and a beta of 0.10 was required to reach the power over 90%.
2.2 Statistical analyses
Continuous variables were presented as means, standard deviations (SD) or medians and ranges, while categorical factors were presented as number with percentage. The categorical variables were analyzed with a chi-square test. Using an independent two-sample Student’s t-test and a nonparametric test for continuous variables with normal distribution and data that were not normally distributed, respectively. To assess the independent risk factors of neuropathic pain among MS patients, the association between demographic and clinical variables and neuropathic pain was tested using univariate and multivariate linear regression models. P values < 0.05 were considered statistically significant.
3 Result
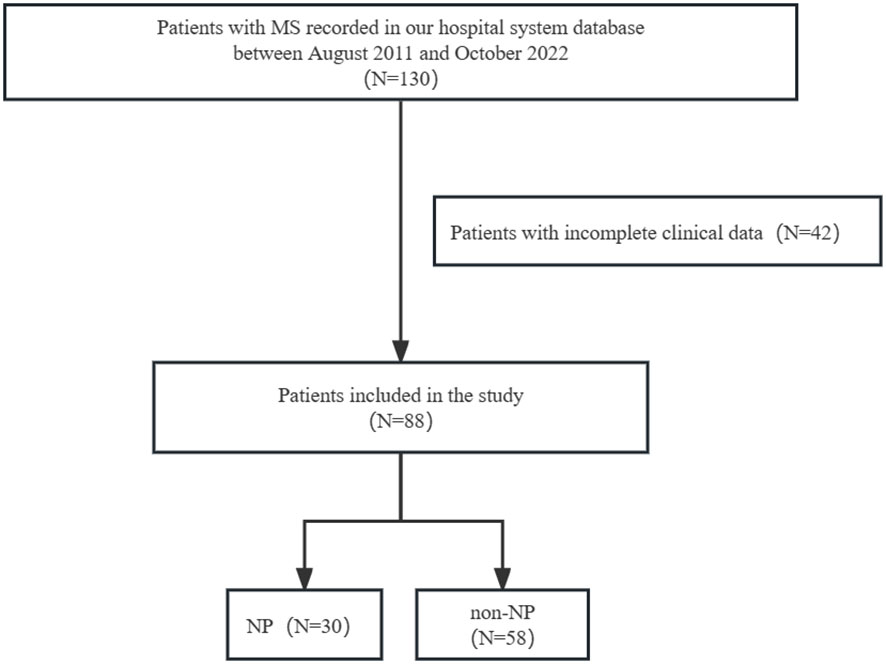
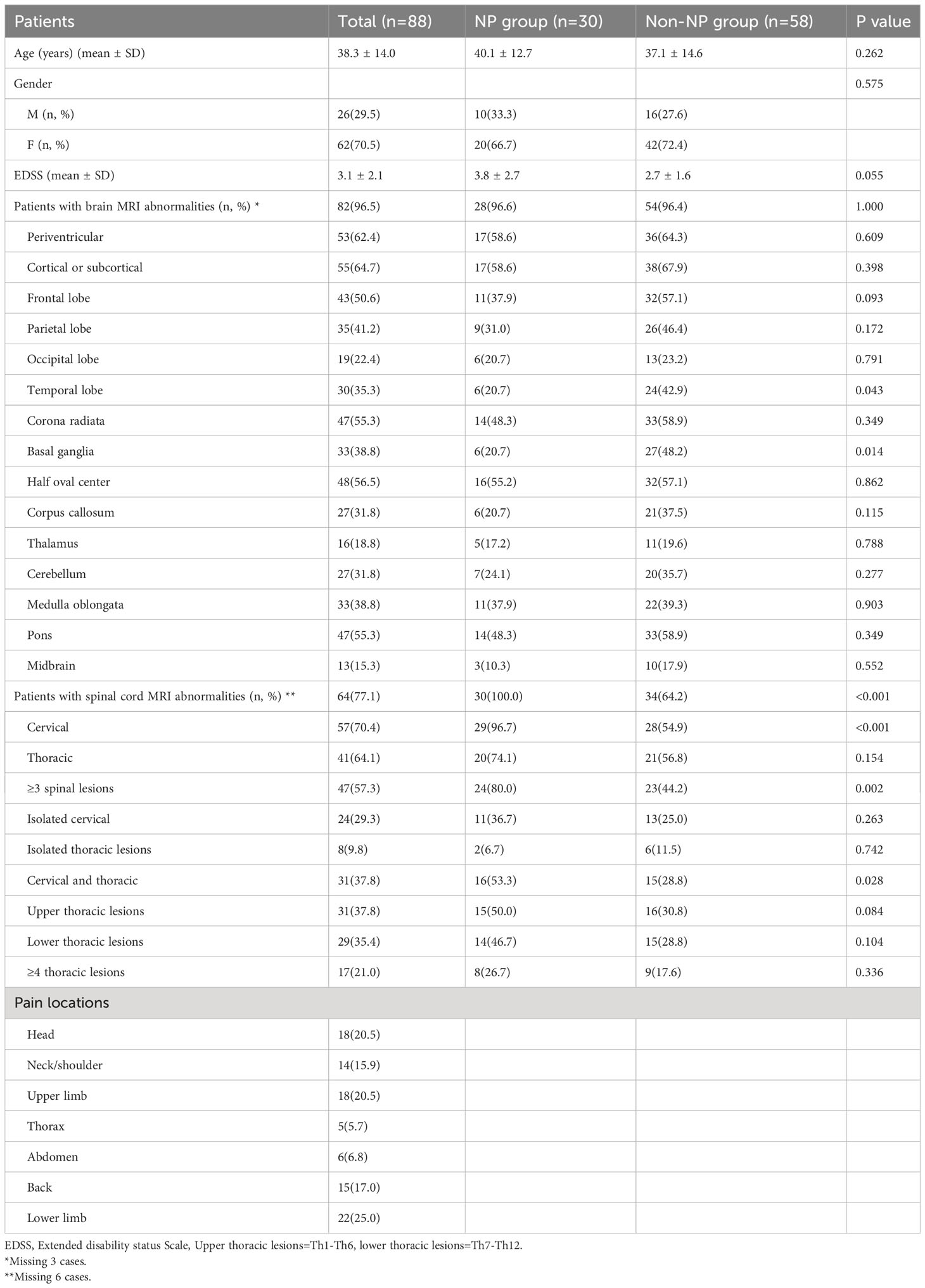
Totally, 130 patients with MS were screened and 88 patients were enrolled in our study. Patients with incomplete clinical data were excluded (N=42) (Figure 1). Out of 88 patients, 62(70.5%) were female, with an average age of 38.3(SD 14.0) years at the first attack and an EDSS score of 3.1(SD 2.1) at the first onset. The most reported pain was observed in lower limb (25.0%), head (20.5%), and upper limb (20.5%) among the total population. Nine patients only had headaches were not included in NP group. Spinal cord lesions were detected in 64 (77.1%) MS patients on MRI. The most common type of spinal lesion was cervical lesions (70.4%) followed by thoracic lesions (64.1%). 47(57.3%) patients had extended spinal lesions (≥3 spinal lesions). 31(37.8%) patients had upper thoracic lesions (T1-T6). 31(37.8%) patients had cervical and thoracic lesions. 29(35.4%) patients had lower thoracic lesions (T7-T12). 24(29.3%) patients had isolated cervical lesions. 17(21.0%) patients had extended thoracic lesions (≥4 thoracic lesions). 8(9.8%) patients had isolated thoracic lesions (Table 1).
The total prevalence of neuropathic pain in our study was 34.1% (30/88). The prevalence of ongoing extremity pain, trigeminal neuralgia and Lhermitte’s phenomenon was separately 21.6%, 3.4% and 4.5%. No significant difference was observed between NP and non-NP group in terms of gender (P = 0.575), age of onset (P = 0.262) and EDSS (P = 0.055). A higher number of cases with extended spinal cord lesions (80.0% vs. 44.2%, P = 0.002), cervical and thoracic lesions (53.3% vs. 28.8%, P = 0.028), were observed in NP group. No significant difference was observed between the groups in relation to upper and lower thoracic lesions (p > 0.05). In addition, non-NP group had higher basal ganglia lesions (20.7% vs. 48.2%; P = 0.014) and temporal lobe lesions (20.7% vs. 42.9%; P = 0.043) compared to NP group (Table 1).
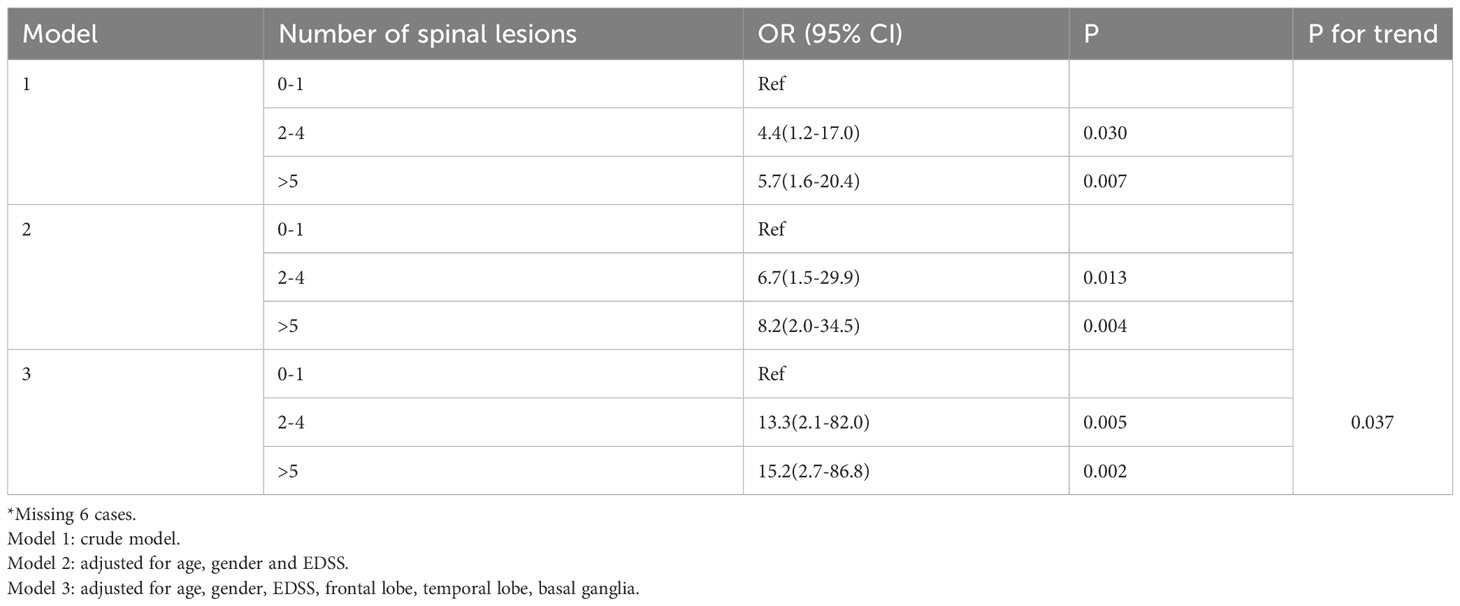
The multivariate logistic regression model found that extended spinal lesions (≥3 spinal lesions) (OR 11.878, 95% CI 2.930-48.157, P = 0.001), cervical and thoracic lesions (OR 4.276, 95% CI 1.366-13.382, P = 0.013) and upper thoracic lesions (T1-T6) (OR 3.047, 95% CI 1.018-9.124, P = 0.046) were identified as risk factors for neuropathic pain among MS patients. By contrast, those with basal ganglia lesions (OR 0.188, 95% CI 0.044-0.809, P = 0.025) had lower risk. Furthermore, we categorized the patients according to the tertiles of the spinal lesions (0-1, 2-4, >5) and the results indicated that the longer the spinal lesions, the greater the neuropathic pain risks (2-4: OR, 13.3(2.1-82), >5: OR, 15.2(2.7-86.8), p for tread: 0.037). Meanwhile, age might be associated with neuropathic pain (OR 1.046, 95% CI 1.000-1.094, P=0.051), too (Table 2, 3).
4 Discussion
Our study identified a significant association between the spinal cord lesions and neuropathic pain among MS patients.
4.1 The criteria and prevalence of neuropathic pain of MS
Neuropathic pain is a common and serious symptom of MS that greatly affects the patients’ quality of life. According to previous studies, the diagnosis of neuropathic pain was mainly defined through the following methods: IASP criteria (15) AAPT criteria (19), questionnaire evaluation (20), and clinical symptoms (21). Douleur Neuropathique 4 Questions (DN4), McGill Pain Questionnaire (MPQ) and Pain Disability Questionnaire (PDQ), are the most commonly used for the diagnosis of neuropathic pain(11). In Heitmann’s study, neuropathic pain was defined as PDQ ≧̸19 and the prevalence of neuropathic pain was 4.2% (20). Solaro’s study used DN4 scale combining with the criteria of the IASP to diagnose the neuropathic pain and the incidence of neuropathic pain is 13.8% (15). Therefore, there were different criteria of neuropathic pain and the prevalence of neuropathic pain varied in different studies.
In our study, we also found the prevalence of the headache was 20.5%. According to the literature, headache is common in the population with MS. The prevalence of headache in MS patients was 2-56% (22, 23). However, the mechanism and influence of headache on MS are still unclear (24). More research is needed for exploration in the future.
4.2 Extended spinal lesions were independent risk factors of neuropathic pain
Consistent with previous studies, 34.1% of patients experienced neuropathic pain in our study. Meanwhile, we found that extended spinal lesions (≥3 spinal lesions), cervical and thoracic lesions and upper thoracic lesions (T1-T6) independently contributed to the risk of neuropathic pain among patients with MS. The spinal cord plays a crucial role in the pathogenesis of pain, especially in neuromyelitis optica spectrum disorder (NMOSD) and MS. As is well known, neuropathic pain of MS is caused by demyelinating lesions in pain perception areas (brain and spinal cord). The spinothalamic tract is the main sensory ascending pathway, consisting of the anterior spinothalamic tract that transmits tactile sensation and the lateral spinothalamic tract that transmits pain and warmth sensation. Previous study had indicated that spinothalamic dysfunctions was the mechanism of MS patients with neuropathic pain (12, 25). Therefore, damage to the spinothalamic tract may be a possible mechanism for the occurrence of neuropathic pain in patients with spinal cord lesions. Another suspected mechanism might be related to the destruction of the autonomic intermediomedial nucleus located in the upper/middle segment of the thoracic spinal cord. These neurons project bilaterally to the superficial dorsal horn of the lumbosacral region, which may contribute to the occurrence of pain (26). In addition, from anatomical and pathophysiological perspective, lesions in the thalamus and parietal cortex are likely related to neuropathic pain and these association were verified by some studies (27, 28). However, in our study, we didn’t find the association of parietal lobe lesions as well as the thalamic lesions with the neuropathic pain which should be investigated by further studies.
Although spinal cord lesions were of vital importance in the diagnosis and monitoring of disease recurrence and progression of MS, few studies have investigated the relation between pain and the length of involved spinal segments in patients with MS. The association between spinal cord and neuropathic pain is still controversial. Research had showed that MS patients with upper/mid-thoracic spinal cord demyelinating lesions were more likely to experience neuropathic pain (OR 155.0, 95% CI 17.0–1414.0, P < 0.001) (26). Similarly, some studies also showed that thoracic cord lesions, especially the upper thoracic lesions (T1-T6) were closely related to pain among NMOSD patients, and indicated more severe pain (29–31). Likewise, our previous study has shown that extended thoracic lesions (≥4 thoracic lesions) were strong predictors of neuropathic pain in NMOSD patients (18). On the basis of this study, our further exploration found cervical and thoracic lesions and upper thoracic lesions (T1-T6) are positively associated with neuropathic pain.
Furthermore, we found that the longer the spinal lesions, the greater the neuropathic pain risks (2-4: OR 13.3(2.1-82), >5, OR 15.2(2.7-86.8), p for tread: 0.037). This is the first report to demonstrate the linear correlation between the length of spinal cord lesions and neuropathic pain in MS. Our study found association between neuropathic pain and spinal cord lesions levels in MS, similar in some respects to NMOSD. These results suggested that NMOSD and MS may develop with common mechanisms in patients with neuropathic pain. Because the exploration and research on the pain mechanism and risk factors of MS were scare, more prospective cohort studies were needed.
4.3 Basal ganglia lesions might not be risk factors of neuropathic pain
In our study, basal ganglia lesions (OR 0.188, 95% CI 0.044-0.809, P = 0.025) were negatively correlated with neuropathic pain in MS patients. The basal ganglia was considered as a brain region of nociceptive sensorimotor integration (32). Painful diseases of the nervous system such as Parkinson (33), migraine (34) and Huntington’s disease (35), have been proposed to be related to the basal ganglia. In Parkinson’s disease, deep brain stimulation (DBS) may alleviate pain symptoms, with the subthalamic nucleus (STN) located in the basal ganglia circuit as the most commonly used target for treatment. Research has shown that in Parkinson’s disease, abnormal and pathological enhancement of STN activity was observed, and pain sensitivity and central sensitization in Parkinson’s disease mice could be improved by inhibiting overactive STN neurons. The underlying mechanism may relate to a pathway which is composed of STN, the substantia nigra pars reticulata and the lateral parabrachial nucleus (36). The destructed inhibitory inputs of the pathways might lead to inadequate response to pain, which may explain the negative correlation between basal ganglia lesions and neuropathic pain in our cohort. However, other studies of MS didn’t find such relationship between basal ganglia lesions and neuropathic pain. Further studied were needed to explore the possible association.
4.4 Gender and age may not associate with neuropathic pain
The correlation between gender, age and neuropathic pain in patients with MS was inconsistent in previous studies. Recently, a meta-analysis surveyed 6671 MS patients and found that prevalence of neuropathic pain was higher for females (74.17%, 95% CI: 65.34; 81.38) than male patients (28.93%, 95% CI: 22.28; 36.63) (11). Meanwhile, the possible mechanisms that contribute to this phenomenon might be the influence of sex hormones, genetic and anatomical differences (37). However, another study found neuropathic pain in MS patients is not correlated with gender (females vs males = 27.5% vs 36.4%, p = 0.715) (38). As to the association between age and neuropathic pain, some suggested that they had no association (39, 40). Conversely, another previous study reported that the presence of neuropathic pain is associated with age [OR 1.03, 95% CI 1.01–1.04, P = 0.002] in MSF (15). In our study, we suggested that neuropathic pain is neither associated with gender nor with age. However, we found a trend towards increasing neuropathic pain alone with age (OR 1.046, 95% CI 1.000-1.094, P = 0.051). Larger studies were need to confirm the association of neuropathic pain and demographic features.
4.5 Limitation
There were some limitations of the present study. Firstly, the evaluation of the scales was not obtained while the retrospective data collection. Therefore, we cannot determine whether intracranial and spinal cord lesions were associated with the severity and pain-related accompanying symptoms of neuropathic pain. Secondly, results from single-center study are less responsive to broader population, larger cohort studies were need to explore in more detail.
5 Conclusions
Our study indicated that for MS patients, the longer the spinal lesions, the greater the neuropathic pain risks. Meanwhile, extended spinal lesions (≥3 spinal lesions), cervical and thoracic lesions, upper thoracic lesions (T1-T6) were independent risk factors of neuropathic pain. Early attention to spinal cord lesions may be beneficial for pain management in MS patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of Guangdong Provincial Hospital of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because data were collected retrospectively and analyzed anonymously, individual patient consents were not required.
Author contributions
HO: Conceptualization, Data curation, Formal analysis, Investigation, Software, Visualization, Writing – original draft. XL: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. HX: Resources, Validation, Writing – review & editing. YiZ: Data curation, Investigation, Writing – review & editing. ZZ: Resources, Validation, Writing – review & editing. GC: Data curation, Writing – review & editing. ZL: Data curation, Writing – review & editing. HC: Data curation, Writing – review & editing. JZ: Data curation, Writing – review & editing. HM: Data curation, Writing – review & editing. CZ: Data curation, Writing – review & editing. LQ: Data curation, Writing – review & editing. YuZ: Funding acquisition, Project administration, Supervision, Writing – review & editing. MZ: Methodology, Project administration, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the “State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ46 and SZ2022KF22)”, “Guangzhou Science and Technology Project (202002020034 and 202201020506)”, “Guangdong Province Key Laboratory of Chinese Medicine Emergency Special Project (2019KT1340)”, “Guangdong Provincial Bureau of Traditional Chinese Medicine research project (20225030)”, “National Administration of Traditional Chinese Medicine (0102016401)”, “NATCM’s Project of High-level Construction of Key TCM Disciplines (zyyzdxk-2023154)”. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Clifford DB, Trotter JL. Pain in multiple sclerosis. Arch Neurol (1984) 41(12):1270–2. doi: 10.1001/archneur.1984.04050230052017
2. Kalia LV, O'Connor PW. Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Multiple Sclerosis (Houndmills Basingstoke England) (2005) 11(3):322–7. doi: 10.1191/1352458505ms1168oa
3. Solaro C, Trabucco E, Messmer Uccelli M. Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep (2013) 13(1):320. doi: 10.1007/s11910-012-0320-5
4. Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA, Chandran S, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain (2013) 154(5):632–42. doi: 10.1016/j.pain.2012.12.002
5. Drulovic J, Basic-Kes V, Grgic S, Vojinovic S, Dincic E, Toncev G, et al. The prevalence of pain in adults with multiple sclerosis: A multicenter cross-sectional survey. Pain Med (Malden Mass.) (2015) 16(8):1597–602. doi: 10.1111/pme.12731
6. O'Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain (2008) 137(1):96-111. doi: 10.1016/j.pain.2007.08.024
7. Nurmikko TJ, Gupta S, Maclver K. Multiple sclerosis-related central pain disorders. Curr Pain Headache Rep (2010) 14(3):189–95. doi: 10.1007/s11916-010-0108-8
8. Kahraman T, Özdoğar AT, Ertekin Ö, Özakbaş S. "Frequency, type, distribution of pain and related factors in persons with multiple sclerosis. Multiple Sclerosis Related Disord (2019) 28:221–5. doi: 10.1016/j.msard.2019.01.002
9. Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology (2008) 70(18):1630–5. doi: 10.1212/01.wnl.0000282763.29778.59
10. Widerström-Noga E, Loeser JD, Jensen TS, Finnerup NB. AAPT diagnostic criteria for central neuropathic pain. J Pain (2017) 18(12):1417–26. doi: 10.1016/j.jpain.2017.06.003
11. Rodrigues P, da Silva B, Trevisan G. A systematic review and meta-analysis of neuropathic pain in multiple sclerosis: Prevalence, clinical types, sex dimorphism, and increased depression and anxiety symptoms. Neurosci Biobehav Rev (2023) 154:105401. doi: 10.1016/j.neubiorev.2023.105401
12. Truini A, Barbanti P, Pozzilli C, Cruccu G. A mechanism-based classification of pain in multiple sclerosis. J Neurol (2013) 260(2):351–67. doi: 10.1007/s00415-012-6579-2
13. Iannitti T, Kerr BJ, Taylor BK. Mechanisms and pharmacology of neuropathic pain in multiple sclerosis. Curr Topics In Behav Neurosci (2014) 20:75–97. doi: 10.1007/7854_2014_288
14. Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain (2019) 160(1):53–9. doi: 10.1097/j.pain.0000000000001365
15. Solaro C, Cella M, Signori A, Martinelli V, Radaelli M, Centonze D, et al. Identifying neuropathic pain in patients with multiple sclerosis: a cross-sectional multicenter study using highly specific criteria. J Neurol (2018) 265(4):828–35. doi: 10.1007/s00415-018-8758-2
16. Stenager E, Knudsen L, Jensen K. Acute and chronic pain syndromes in multiple sclerosis. A 5-year follow-up study. Ital J Neurological Sci (1995) 16(9):629–32. doi: 10.1007/BF02230913
17. Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain (2007) 127(1-2):35–41. doi: 10.1016/j.pain.2006.07.015
18. Li X, Xu H, Zheng Z, Ouyang H, Chen G, Lou Z, et al. The risk factors of neuropathic pain in neuromyelitis optica spectrum disorder: a retrospective case-cohort study. BMC Neurol (2022) 22(1):304. doi: 10.1186/s12883-022-02841-9
19. Rivel M, Achiron A, Dolev M, Stern Y, Zeilig G, Defrin R. Unique features of central neuropathic pain in multiple sclerosis: Results of a cluster analysis. Eur J Pain (London England) (2022) 26(5):1107–22. doi: 10.1002/ejp.1934
20. Heitmann H, Biberacher V, Tiemann L, Buck D, Loleit V, Selter RC, et al. Prevalence of neuropathic pain in early multiple sclerosis. Multiple Sclerosis (Houndmills Basingstoke England) (2016) 22(9):1224–30. doi: 10.1177/1352458515613643
21. Krupina NA, Churyukanov MV, Kukushkin ML, Yakhno NN. Central neuropathic pain and profiles of quantitative electroencephalography in multiple sclerosis patients. Front In Neurol (2019) 10:1380. doi: 10.3389/fneur.2019.01380
22. Mirmosayyeb O, Barzegar M, Nehzat N, Shaygannejad V, Sahraian MA, Ghajarzadeh M. The prevalence of migraine in multiple sclerosis (MS): A systematic review and meta-analysis. J Clin Neurosci Off J Neurosurgical Soc Australasia (2020) 79:33–8. doi: 10.1016/j.jocn.2020.06.021
23. Wang L, Zhang J, Deng Z-R, Zu M-D, Wang Y. The epidemiology of primary headaches in patients with multiple sclerosis. Brain Behav (2021) 11(1):e01830. doi: 10.1002/brb3.1830
24. Sahai-Srivastava S, Wang SL, Ugurlu C, Amezcua L. Headaches in multiple sclerosis: Cross-sectional study of a multiethnic population. Clin Neurol Neurosurg (2016) 143:71–5. doi: 10.1016/j.clineuro.2016.01.017
25. Svendsen KB, Jensen TS, Hansen HJ, Bach FW. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain (2005) 114(3):473–81. doi: 10.1016/j.pain.2005.01.015
26. Okuda DT, Melmed K, Matsuwaki T, Blomqvist A, Craig ADB. Central neuropathic pain in MS is due to distinct thoracic spinal cord lesions. Ann Clin Trans Neurol (2014) 1(8):554–61. doi: 10.1002/acn3.85
27. Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis–prevalence and clinical characteristics. Eur J Pain (London England) (2005) 9(5):531–42. doi: 10.1016/j.ejpain.2004.11.005
28. Poncet-Megemont L, Dallel R, Chassain C, Perrey A, Mathais S, Clavelou P, et al. Whole-body reversible neuropathic pain associated with right parieto-temporal operculum single inflammatory lesion in a patient with multiple sclerosis: A case report. Eur J Pain (London England) (2019) 23(10):1763–6. doi: 10.1002/ejp.1464
29. Tackley G, Vecchio D, Hamid S, Jurynczyk M, Kong Y, Gore R, et al. Chronic neuropathic pain severity is determined by lesion level in aquaporin 4-antibody-positive myelitis. J Neurology Neurosurgery Psychiatry (2017) 88(2):165–9. doi: 10.1136/jnnp-2016-314991
30. Asseyer S, Kuchling J, Gaetano L, Komnenić D, Siebert N, Chien C, et al. Ventral posterior nucleus volume is associated with neuropathic pain intensity in neuromyelitis optica spectrum disorders. Multiple Sclerosis Related Disord (2020) 46:102579. doi: 10.1016/j.msard.2020.102579
31. Ayzenberg I, Richter D, Henke E, Asseyer S, Paul F, Trebst C, et al. Pain, depression, and quality of life in neuromyelitis optica spectrum disorder: A cross-sectional study of 166 AQP4 antibody-seropositive patients. Neurology(R) Neuroimmunology Neuroinflamm (2021) 8(3):e985. doi: 10.1212/NXI.0000000000000985
32. Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain (1995) 60(1):3-38. doi: 10.1016/0304-3959(94)00172-B
33. Blanchet PJ, Brefel-Courbon C. Chronic pain and pain processing in Parkinson's disease. Prog In Neuropsychopharmacol Biol Psychiatry (2018) 87(Pt B):200–6. doi: 10.1016/j.pnpbp.2017.10.010
34. Huang Y, Zhang Y, Hodges S, Li H, Yan Z, Liu X, et al. The modulation effects of repeated transcutaneous auricular vagus nerve stimulation on the functional connectivity of key brainstem regions along the vagus nerve pathway in migraine patients. Front In Mol Neurosci (2023) 16:1160006. doi: 10.3389/fnmol.2023.1160006
35. Li J, Wang Y, Yang R, Ma W, Yan J, Li Y, et al. Pain in Huntington's disease and its potential mechanisms. Front In Aging Neurosci (2023) 15:1190563. doi: 10.3389/fnagi.2023.1190563
36. Jia T, Wang Y-D, Chen J, Zhang X, Cao J-L, Xiao C, et al. A nigro-subthalamo-parabrachial pathway modulates pain-like behaviors. Nat Commun (2022) 13(1):7756. doi: 10.1038/s41467-022-35474-0
37. Presto P, Mazzitelli M, Junell R, Griffin Z, Neugebauer V. Sex differences in pain along the neuraxis. Neuropharmacology (2022) 210:109030. doi: 10.1016/j.neuropharm.2022.109030
38. Kasap Z, Uğurlu H. Pain in patients with multiple sclerosis. Turkish J Phys Med Rehabil (2023) 69(1):31–9. doi: 10.5606/tftrd.2022.10524
39. Truini A, Galeotti F, La Cesa S, Di Rezze S, Biasiotta A, Di Stefano G, et al. Mechanisms of pain in multiple sclerosis: a combined clinical and neurophysiological study. Pain (2012) 153(10):2048–54. doi: 10.1016/j.pain.2012.05.024
Keywords: spinal cord lesions, basal ganglia, multiple sclerosis, neuropathic pain, MRI
Citation: Ouyang H, Li X, Xu H, Zhan Y, Zheng Z, Chen G, Lou Z, Chen H, Zhang J, Mao H, Zhang C, Qin L, Zhao Y and Zhao M (2024) Risk factors of neuropathic pain in multiple sclerosis: a retrospective case-cohort study. Front. Immunol. 15:1309583. doi: 10.3389/fimmu.2024.1309583
Received: 08 October 2023; Accepted: 12 January 2024;
Published: 29 January 2024.
Edited by:
Marta Altieri, Sapienza University of Rome, ItalyReviewed by:
Samar Farouk Ahmed, Minia University, EgyptGianfranco De Stefano, Sapienza University of Rome, Italy
Bonaventura Casanova, La Fe Hospital, Spain
Copyright © 2024 Ouyang, Li, Xu, Zhan, Zheng, Chen, Lou, Chen, Zhang, Mao, Zhang, Qin, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhao, Mzc2NDc5ODA4QHFxLmNvbQ==; Yuanqi Zhao, dGNtMjAwOEAxMjYuY29t
 Huiying Ouyang
Huiying Ouyang Xiaojun Li1
Xiaojun Li1 Yibo Zhan
Yibo Zhan Zequan Zheng
Zequan Zheng Zhenzhen Lou
Zhenzhen Lou Jiahui Zhang
Jiahui Zhang Yuanqi Zhao
Yuanqi Zhao Min Zhao
Min Zhao