- 1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, China
- 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 3State Key Laboratory of Southwestern Chinese Medicine Resources, Hospital of Chengdu University of Traditional Chinese Medicine, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Zanthoxylum bungeanum Maxim., commonly known as Chinese prickly ash, is a well-known spice and traditional Chinese medicine ingredient with a rich history of use in treating inflammatory conditions. This review provides a comprehensive overview of the botanical classification, traditional applications, and anti-inflammatory effects of Z. bungeanum, with a specific focus on its polyphenolic components. These polyphenols have exhibited considerable promise, as evidenced by preclinical studies in animal models, suggesting their therapeutic potential in human inflammatory diseases such as ulcerative colitis, arthritis, asthma, chronic obstructive pulmonary disease, cardiovascular disease, and neurodegenerative conditions. This positions them as a promising class of natural compounds with the potential to enhance human well-being. However, further research is necessary to fully elucidate their mechanisms of action and develop safe and effective therapeutic applications.
1 Introduction
Chinese prickly ash, also known as Hua Jiao in Mandarin, belongs to the genus Zanthoxylum in the Rutaceae family (1). Widely cultivated in Asia, including China, Japan, India, and Korea (2), the genus comprises approximately 250 species, with 41 found in China (Table 1) (3). Chinese prickly ash, or Hua Jiao, is a popular spice and traditional Chinese medicine ingredient specifically derived from Zanthoxylum bungeanum Maxim. and Zanthoxylum schinifolium, according to the Pharmacopoeia of the People’s Republic of China (4). This review, we will focus on Zanthoxylum bungeanum Maxim. (Z. bungeanum).
Zanthoxylum bungeanum Maxim., commonly known as Honghuajiao, is a deciduous shrub with a height range of 3-7 meters, bearing small, crimson fruits measuring 4-5 mm in diameter. The flowering period spans from April to May, while fruit ripening occurring between August and October. Z. bungeanum holds significant importance in both traditional Chinese medicine and cuisine. The earliest record of its use in China can be traced back to the “Book of Songs,” a compilation of folk poetry from the Western Zhou period, underscoring a history of over two thousand years of utilization (5). The dried fruit follicles of Z. bungeanum are integral to Chinese cuisine, often incorporated for their distinctive flavor and numbing taste (1). Additionally, leaves at various stages of maturity serve as ingredients and seasonings in Chinese culinary practices (6).
In traditional Chinese medicine, Z. bungeanum is esteemed for its properties in warming the spleen and stomach, alleviating pain, and demonstrating anthelmintic and antipruritic effects (4). It is also recognized for promoting the flow of Qi and dispelling coldness (5). Decoctions of Z. bungeanum find primary application in treating conditions such as stomachaches accompanied by sensations of coldness and dampness, vomiting, intestinal disorders, diarrhea, ascarid infections, schistosomiasis, and rheumatic joint inflammations (5, 7). Externally, the plant is used to address issues like bruises, eczema, and snakebites (2).
Z. bungeanum also features prominently in Indian and Nepalese folk medicine. Its decoction serves as an aromatic tonic for fevers, and as a carminative and stomachic remedy for dyspepsia, cholera, and toothaches (7).
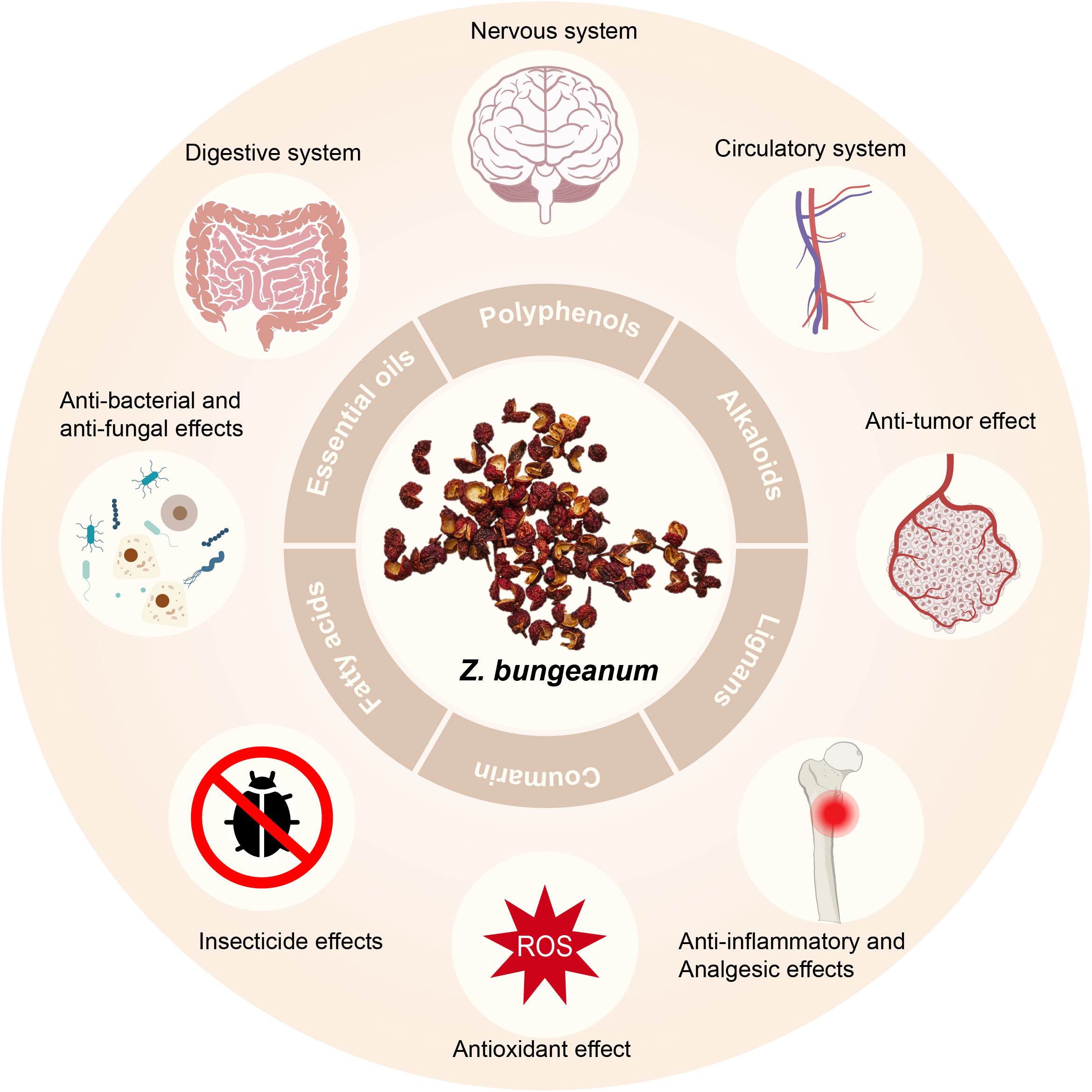
Current research endeavors have demonstrated the pharmacological effects of Z. bungeanum on the gastrointestinal, neurological, and cardiovascular systems. Additionally, it exhibits anti-inflammatory and analgesic properties, along with displaying antioxidant, anti-tumor, antibacterial, antifungal, and insecticidal effects (2) (Figure 1).
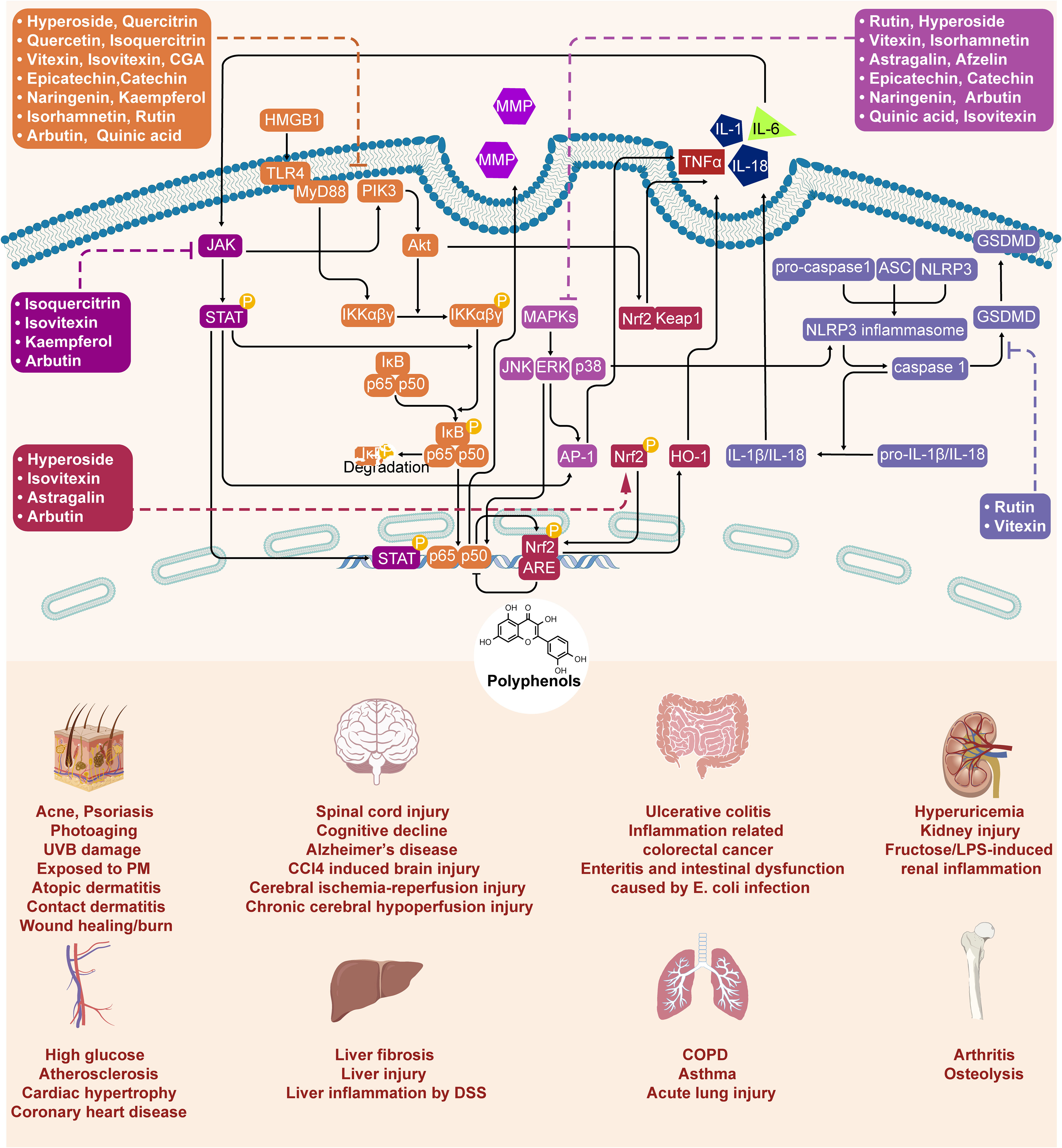
Inflammation constitutes an adaptive response of the immune system to deleterious stimuli, encompassing pathogens, cellular injury, and toxic agents. Its principal role is protective, expelling these detrimental agents from the body and instigating the recovery process. However, unbridled inflammation can also be deleterious, culminating in conditions such as atherosclerosis, type 2 diabetes, and rheumatoid arthritis (8). Empirical evidence corroborates the noteworthy anti-inflammatory attributes of polyphenols. They possess the capacity to ameliorate inflammation in various diseases induced by inflammation, such as inflammatory bowel disease and acute pancreatitis (9). The molecular mechanisms underlying the anti-inflammatory activities of polyphenols involve scavenging free radicals, modulating the activity of inflammatory cells, inhibiting enzymes linked to pro-inflammatory attributes like COX2, iNOS, and LOX, suppressing NF-κB and AP-1, and impeding the activation of MAPK, protein kinase C, and Nrf2 (10).
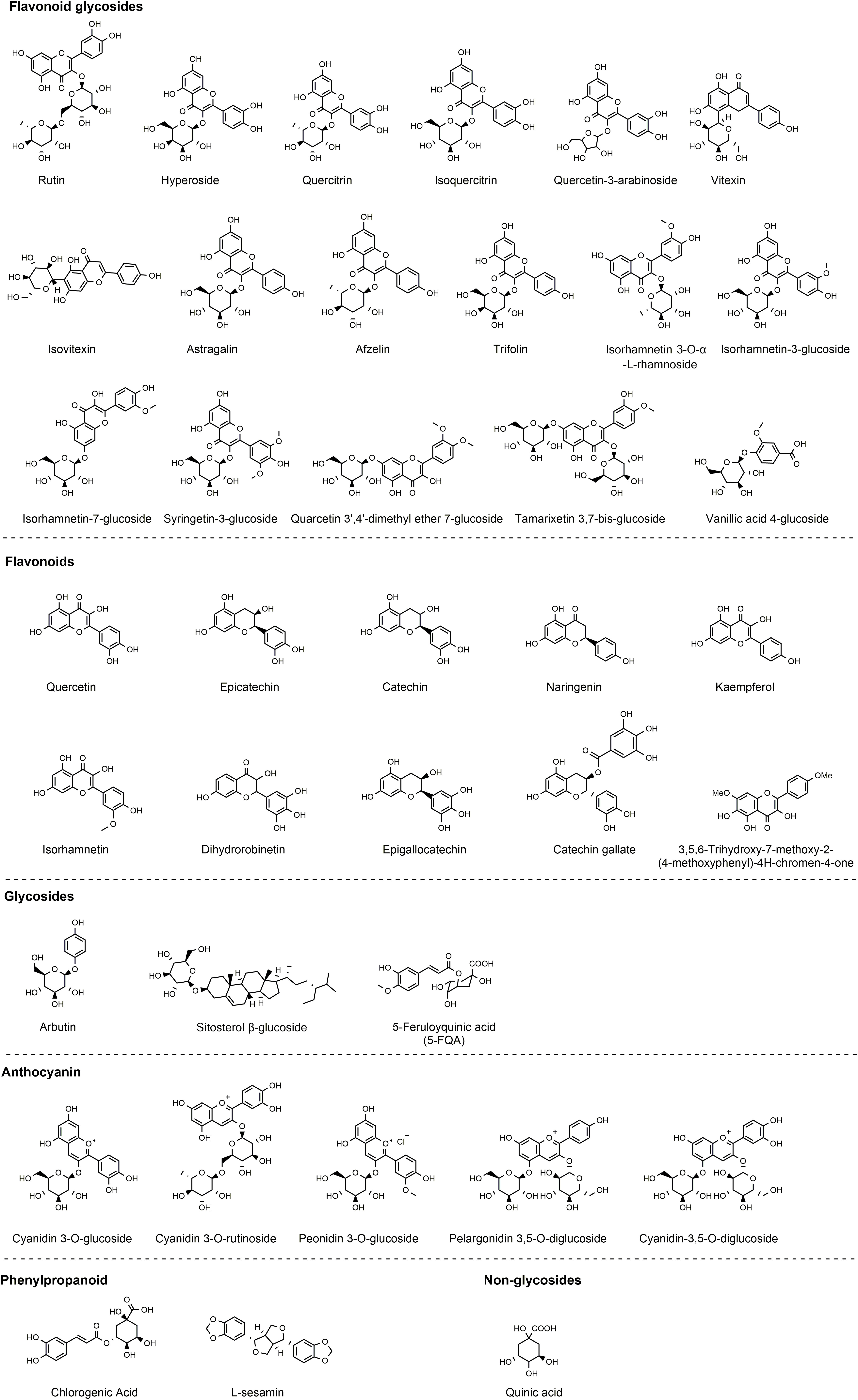
Currently, more than 140 constituents have been identified in Zanthoxylum bungeanum, encompassing polyphenols, alkaloids, lignans, coumarin, fatty acids, essential oils, and others (2, 11, 12). Among these, more than 40 polyphenols have been ascertained in Z. bungeanum, categorized into various types based on their chemical structures, including flavonoid glycosides, flavonoids, glycosides, phenylpropanoid, anthocyanin and non-glycosides. These polyphenolic compounds have exhibited promising anti-inflammatory effects on disorders affecting diverse organs and systems, comprising ulcerative colitis, arthritis, pain, asthma, UVB-induced skin damage, and cognitive function of the brain ulcerative colitis (13), arthritis (14), pain (15), asthma (16), UVB skin damage (17), and cognitive function of the brain (18). Polyphenols derived from Z. bungeanum proficiently inhibit inflammatory cytokines and modulate NF-κB, p38-MAPK, TLR4, Erk1/2, JNK, and Nrf2/HO-1 pathways to exert their anti-inflammatory effects.
In this review, we summarize the polyphenolic compounds present in Zanthoxylum bungeanum (Z. bungeanum) and the therapeutic effects of Z. bungeanum on inflammation, with a particular emphasis on the polyphenols. Recent research suggests that Z. bungeanum polyphenols have the potential to significantly contribute to the management and prevention of inflammatory conditions. Further in-depth research is needed to promote their health benefits.
2 Composition and structure of polyphenols in Z. bungeanum
Both the leaves and seeds of Z. bungeanum contain polyphenolic compounds, predominantly comprising flavonoid glycosides. Research conducted by three independent groups (19–21) provides substantial evidence of the polyphenol richness in the leaves, characterized by potent antioxidant properties. Noteworthy constituents include 5-feruloyquinic acid, vanillic acid-4-glucoside, quercetin-3-arabinoside, chlorogenic acid, epicatechin, quinic acid, syringetin-3-glucoside, quercetin, isorhamnetin-3-glucoside, trifolin, afzelin, hyperoside, isovitexin, quercitrin, trifolin, rutin, isorhamnetin 3-O-α-L-rhamnoside, astragalin, and isoquercitrin (19–21). In the outer coverings of Z. bungeanum fruits, Xiong et al. have identified tamarixetin 3,7-bis-glucoside, quarcetin 3’,4’-dimethyl ether 7-glucoside, 3,5,6-trihydroxy-7,4’-dimethoxyflavone, hyperoside, sitosterol β-glucoside, quercetin, quercitrin, isorhamnetin 7-glucoside, rutin, arbutin, and L-sesamin (22). Additionally, the research conducted by Jia’s group has revealed the presence of epigallocatechin, dihydrorobinetin, naringenin, catechin, kaempferol, catechin gallate, and isorhamnetin are identified by Jia’s group (23). Recently, with the advancement of technology such as the application of high-throughput sequencing techniques, a series of polyphenolic compounds with lower concentrations in Z. bungeanum have been identified. The identification of polyphenols in Z. bungeanum has expanded from approximately 40 types to over 150 types (24), thanks to these technological developments. Due to words limit, our review specifically revisits polyphenols with higher concentrations in Z. bungeanum, focusing on those extensively studied for their anti-inflammatory activities (Figure 2).
Investigation of the structure-activity relationships of Z. bungeanum polyphenols reveals a correlation between elevated antioxidant efficacy and the presence of a hydroxyl (-OH) group at both the 4’ position on the B ring and the 7 position on the A ring. Moreover, adjacent -OH groups on the B and/or A rings significantly enhanced antioxidant capabilities. Additionally, the diverse structures of these polyphenols suggest that they may display different antioxidant capacities in solution or oil-in-water emulsion reactions (20). Z. bungeanum polyphenols have demonstrated effective radical scavenging activities in DPPH, ABTS (21), FRAP, lipid peroxidation inhibition assays (20), and superoxide anion (19). Furthermore, polyphenols have been reported to protect Escherichia coli under peroxide stress (20) and concurrently reduce reactive oxygen species (ROS) levels in HT-29 cells without inducing any cell toxicity (19). Moreover, polyphenols have a cell-protective impact, mitigating oxidative damage in PC12 cells caused by H2O2 (21).
3 Inflammatory diseases and polyphenols in Z. bungeanum
A combination of polyphenols found in Zanthoxylum bungeanum has demonstrated anti-inflammatory effectiveness in both in vivo and in vitro experiments. The ethyl acetate fraction of Z. bungeanum has been identified as the primary active component in enhancing cognitive function in aging mice with D-galactose-induced cognitive decline. This fraction contains several polyphenols, such as hyperoside, chlorogenic acid, quercetin-3β-d-glucoside, rutin, and epicatechin. It aids in reducing neuroinflammation, inhibiting the NLRP3/caspase-1 pathway, GSDMD, and downstream pyroptosis, both in the mouse model and in BV-2 cells subjected to LPS and ATP treatment, leading to overall cognitive improvements (25).
The treatment with Z. bungeanum pericarp extract (ZBE), predominantly composed of rutin, isoquercitrin, and quercitrin, has demonstrated effectiveness in protecting mice with dextran sulfate sodium (DSS)-induced ulcerative colitis (UC). It has been observed to mitigate body weight loss, prevent colonic shortening, reduce disease activity index scores, and inhibit myeloperoxidase activity. ZBE is found to inhibit caspase-1, ASC, NLRP3, TLR4, subsequent MAPK and NF-κB pathways, and the production of TNFα, IL-12, and IL-1β, both in vitro in the LPS-triggered J774.1 cell model and in vivo. Concurrently, activation of PPARγ is detected (13).
In the subsequent section, we will individually discuss the research pertaining to the anti-inflammatory effects of each polyphenolic component found in Z. bungeanum. We will categorize the 40 polyphenolic constituents of Z. bungeanum into various groups based on their chemical compositions: flavonoids, flavonoid glycosides, glycosides, phenylpropanoid, anthocyanin, and nonglycosides (Figure 2). Please note that we do not aim to provide an exhaustive or comprehensive list of all anti-inflammatory studies for each component here. Instead, we have selected those with high citation counts or the most recent research to provide an overview of the association between inflammation and polyphenols in Z. bungeanum.
3.1 Flavonoid glycosides
3.1.1 Rutin
Rutin is a flavonoid with well-established anti-inflammatory properties (26) Administered at doses of 50-100 mg/kg, rutin exhibits protective effects against hepatotoxicity induced by cyclophosphamide (CP), a potent anticancer agent, in rats. This protection is associated with decreased levels of pro-inflammatory cytokines and signaling molecules, including IL-6, TNFα, iNOS, COX2, p38-MAPK, and NF-κB. Histopathological analysis reveals substantial structural damage to the liver caused by CP, effectively reversed through prior administration of rutin (27). Rutin has also demonstrated the preservation of the vascular barrier integrity in human umbilical vein endothelial cells stimulated by LPS and in an acetic acid-induced mouse mode (28). It effectively reduced hyperpermeability induced by LPS, TNFα, and HMGB1, and suppressed both TNFα production and NF-κB activation triggered by LPS (28). Beyond its anti-inflammatory and vascular protective effects, rutin has demonstrated neuroprotective and anti-colitic properties. In a rat model of spinal cord injury, rutin administration significantly attenuated histological alterations and reduced tissue damage. This was associated with decreased levels of oxidative stress markers, pro-inflammatory cytokines, and caspase-1 (29). In a mouse model of DSS-induced colitis, rutin significantly improved several key indicators of disease severity, including the disease activity score, colon length, and the integrity of goblet cells and colon epithelium. Rutin also reduced the expression of a range of oxidative-inflammatory markers, including IgE, IgM, iNOS, HO-1, and ICAM-1, and restored the balance among effector cells, regulatory cells, and B cells. The study revealed a substantial increase in the activation of the PI3K/Akt/GSK3β/MAPKs/NF-κB and p38/MK2 pathways during DSS-induced colitis in the animal subjects, a condition that rutin treatment effectively mitigated. In silico studies supported the specificity of rutin’s interaction with these pathways (30).
In terms of pharmacokinetics, orally administered rutin is absorbed in the small intestine, transferred to the liver via the bloodstream, and eliminated through bile and the kidneys (31). Major metabolites include sulfates and glucuronides of quercetin (32). In rats, Zhang et al. reported elimination rate half-life, area under the curve, and plasma clearance values of 3.345 minutes, 5750 μg min/ml, and 5.891 mL/min/kg, respectively (33). Intravenous rutin accumulates in the liver, with a significant portion then transferred to the small intestine, and is also detected in the lung post-injection (31). Interactions between rutin and drugs were studied as well. Rutin reduces the anticoagulant effect of racemic warfarin by 31% when co-administered orally. This outcome was ascribed to a noteworthy 77% rise in the unbound formation clearance of both oxidative and reductive metabolites, coupled with an elevation in the unbound renal clearance of the more potent S-enantiomer of warfarin (34). Rutin also significantly decreases the oral Cmax and AUC of cyclosporine by 63.2% and 57.2%, respectively, through the activation of Pgp transporter and CYP3A enzyme (35).
3.1.2 Hyperoside
Hyperoside is another flavonoid known for its anti-inflammatory properties. In mouse peritoneal macrophages subjected to LPS stimulation, hyperoside inhibited TNFα, IL-6, and NO production by 32.3%, 41.3%, and 30%, respectively. Moreover, hyperoside reduced NF-κB activation and IκB-α degradation (36). This compound also exhibits anti-neuroinflammation effect in vitro and in vivo (37, 38). In the LPS-induced HT22 murine neuronal cell line, hyperoside enhances cell survival and mitigates inflammation, oxidative stress, and apoptosis. This effect is achieved by amplifying SIRT1, triggering the activation of both Wnt/β-catenin and sonic hedgehog pathways (38). In rats, 50 mg/kg hyperoside protected against cerebral ischemia-reperfusion injury by mitigating oxidative stress, inflammation, and cell death. Rats treated with hyperoside exhibited significantly enhanced neurological function and a substantial reduction in the ratio of cerebral infarction volume (37).
Hyperoside also attenuate several vascular inflammatory responses initiated by elevated glucose levels in human umbilical vein endothelial cells and mice. These responses include vascular permeability, monocyte attachment, CAMs expression, ROS formation, and NF-κB activation (39). Furthermore, hyperoside’s anti-arthritic properties have also been verified both in vitro and in vivo. It can suppress inflammation and prevent cartilage breakdown by influencing the PI3K/AKT/NF-κB and MAPK signaling pathways, as well as the interplay between the Nrf2/HO-1 and NF-κB signaling pathways (40). Hyperoside also inhibited OVA-induced airway hyperresponsiveness in mice through activation of Nrf2/HO-1 (41). In a rat model of antiphospholipid syndrome (APS), hyperoside at a dose of 40 mg/kg led to increased fetal weight, reduction of fetal resorption rates, and reduced pregnancy loss by modulating the mTOR/S6K and TLR4/MyD88/NF-kB signaling pathways (42).
3.1.3 Quercitrin
Quercitrin demonstrates the ability to attenuate carbon tetrachloride (CCl4) induced brain injury by suppressing ROS, MDA, TNFα, and IL-6 (43). Furthermore, it exhibits protective effects against skin damage induced by UVB damage. This protection is achieved through the reduction of ROS, NF-κB activation, and DNA damage triggered by UVB exposure. Quercitrin also restores the diminished expression of catalase and the GSH/GSSG ratio due to UVB exposure (17). In a study involving mice with Alzheimer’s disease, quercitrin inhibits the activation and proliferation of microglia, decreases the accumulation of amyloid-β plaques, and improves cognitive impairment by inhibiting inflammation. Specifically, this compound inhibits the level of IL-1α, IL-17A, IL-6, and G-CSF in peripheral blood, as well as IL-1α, IL-4, IL-6, Eotaxin, CXCL-1, MIP-1α, MIP-1β and G-CSF in the brain, thereby alleviating systemic inflammation in the 5XFAD mice (44).
Quercetin and quercitrin, common flavonoids in vegetables, are frequently compared (45). Theoretical calculations clarify that the oxygen atom located on the B rings could serve as the primary site for alterations in electron cloud density, providing insights into how quercetin and quercitrin exert their anti-inflammatory and ROS scavenging effects (46). In LPS-stimulated RAW264.7 cells, both compounds markedly decrease NO and ROS production, as well as the expression of TNFα, IL-1β, and IL-6 (46). However, Comalada et al. reported that unlike quercitrin, quercetin can reduce the expression of cytokines and iNOS by inhibiting the NF-κB pathway in vitro in bone marrow-derived macrophages, without affecting c-Jun N-terminal kinase activity. The group revealed that quercitrin’s in vivo impact in a rat colitis model induced by DSS may be attributed to the liberation of quercetin, which occurs following the breakdown of glycosides by intestinal microbiota. In other words, quercitrin releases quercetin to exert its anti-inflammatory influence, achieved by inhibiting the NF-κB pathway (45).
3.1.4 Isoquercitrin
Isoquercitrin has undergone tested in an LPS-stimulated RAW264.7 cell model, revealing its ability to decrease NO production, downregulate the expression of PGE2, COX2, iNOS, and NF-κB p65 protein, and reduce the mRNA levels of IL-1, IL-6, PTGES2, and MCP-1 (47). Moreover, at a dosage of 20 mg/kg, isoquercitrin has demonstrated the capacity to protect denervated muscle from atrophy. This protective effect is achieved by reducing the levels of IL-1β, TNFα, and IL-6 and inactivating the JAK/STAT3 signaling pathway in the target muscle (48).
3.1.5 Vitexin
Vitexin exhibits anti-inflammatory properties in the OVA-induced mouse allergic asthma model at doses of ranging from 0.2 to 5 mg/kg. Specifically, vitexin mitigates the migration of eosinophils, neutrophils, and mononuclear cells prompted by OVA within bronchoalveolar lavage fluid (BALF). Examination of lung tissue reveals that vitexin effectively suppresses the invasion of leukocytes, mucus production, and development of pulmonary edema. It also moderates the escalation of Th2 cytokines in BALF and reduces the concentration of IgE in the plasma (49). Vitexin has also demonstrated anti-inflammatory effects in chronic cerebral hypoperfusion injury in a rat model of persistent bilateral common carotid artery occlusion and in HT22 mouse hippocampal neuronal cells exposed to oxygen and glucose deprivation followed by reoxygenation injury. The findings confirm vitexin’s ability to modulate Epac and NLRP3. Additionally, in the rat model, vitexin has shown the potential in diminishing the severity of ongoing pathological harm in the cortex and hippocampus and preventing further decline in cognitive function (18). Moreover, vitexin inhibits inflammatory pain in various mouse models of inflammation-related pain, including acetic acid-induced writhing, pain-like behavior prompted by phenyl-p-benzoquinone, capsaicin, complete Freund’s adjuvant (CFA), and both phases of the formalin test. It also alleviates mechanical and thermal hyperalgesia triggered by capsaicin, carrageenan, and chronic CF. TRPV1 is considered the key target (50). Additionally, vitexin alleviates liver inflammation in a DSS-induced colitis model by inhibiting the TLR4/NF-κB signaling pathway activation. Administration of vitexin results in lower ALT and TC levels in the livers of mice suffering from liver injury. It also reduces the release of IL-6, TNFα, and IL-1β induced by DSS (51). Furthermore, vitexin inhibits the movement of neutrophils toward areas of inflammation by suppressing the p38, ERK1/2, and JNK pathways (52).
3.1.6 Isovitexin
Isovitexin effectively alleviates contact dermatitis in mice triggered by ginkgolic acids, leading to a significant reduction in ear swelling, splenomegaly, and inflammatory cell infiltration. Subsequent investigations have revealed that isovitexin can impede the MAPK and STAT signaling pathways, along with the phosphorylation of SHP2 (53). In the mouse models of kidney injury induced by cyclophosphamide (CP) (54), liver injury triggered by LPS/d-galactosamine (55), and acute lung injury induced by LPS (56), isovitexin demonstrates its therapeutic effects via inhibiting NF-κB activation and inducing Nrf2 and HO-1 expression. In the kidney injury model, isovitexin mitigates CP-induced increases in serum BUN and creatinine, and curbs TNFα, IL-1β, and IL-6 (54). Isovitexin substantially diminishes liver injury, evidenced by reduced histopathological changes and lower AST and ALT levels. It also reduces TNFα levels, MPO activity, and MDA content (55). Pretreatment with isovitexin significantly alleviates acute lung injury, as demonstrated by reduced histopathological changes, diminished granulocyte infiltration, and subdued endothelial activation. Additionally, it lowers VCAM-1 and ICAM-1 expression, reduces MPO and MDA levels, and enhances GSH and SOD (56).
3.1.7 Astragalin
Astragalin notably alleviates inflammatory reactions and bone damage in both DBA/1J mice with collagen-induced arthritis and human fibroblast-like synoviocytes. It reduces joint swelling, arthritis index, and bone erosion, while also inhibiting the production of IL-1β, TNFα, IL-6, and IL-8. Moreover, a decrease in MMP-1, MMP-3, and MMP-13 levels has also been observed in chondrocytes, synovial cells, and TNFα-induced MH7A cells. Additionally, astragalin inhibits p38, JNK phosphorylation, and c-Jun/AP-1 activation (57). Furthermore, through the ROS and MAPK signaling pathway, the process of osteoclastogenesis in inflammatory osteolysis is alleviated by astragalin (58). In an OVA-challenged mouse model, astragalin at doses of 10-20 mg/kg impedes mast cell recruitment, preventing airway thickening and alveolar emphysema (59).
3.1.8 Afzelin
Afzelin performs anti-inflammatory effect in two in vitro experiments (60, 61). In human keratinocytes exposed to particulate matter (PM), a widespread airborne contaminant, afzelin mitigates inflammation and ROS production. It also inhibits p38 kinase, as well as the transcription factors c-Fos and c-Jun (61). The inhibitory effect of afzelin on the p38 kinase pathway contributes to its protective effect of human keratinocytes and epidermal equivalent models exposed to UVB, resulting in a reduction of IL-6, TNFα, and PGE2 release induced by UVB (60).
3.2 Flavones
3.2.1 Quercetin
Quercetin stands out as one of the extensively researched polyphenols in Z. bungeanum. showcasing therapeutic potential in addressing inflammatory conditions, particularly arthritis (62, 63). In a study involving women with rheumatoid arthritis, a daily supplement of 500mg quercetin over 8 weeks resulted in significant improvements in the clinical symptoms, disease activity, hs-TNFα levels, and health assessment questionnaire outcomes (62). For rabbits with surgically-induced osteoarthritis (OA), a 4-week gavage treatment of 25 mg/kg quercetin demonstrated increased SOD and TIMP-1 expressions, reduced MMP-13 expression, and mitigation of OA degeneration, comparable to the effects observed in the celecoxib-treated group (63). Quercetin’s impact extends to inflammation-based pain models, as intraperitoneal and oral administrations significantly suppressed pain induced by phenyl-p-benzoquinone and acetic acid. It also mitigated the second phase of pain intensity escalation caused by formalin and carrageenin. This compound further demonstrated its efficacy in curtailing hypernociception stimulated by TNFα and CXCL1, along with reducing carrageenin-induced IL-1β production (15). Moreover, in RAW264.7 cells stimulated with LPS, quercetin significantly reduced the production of NO, inducible NO synthase, and IL-6. It also hindered the relocation of NF-κB to the cell nucleus and suppressed the activation of Erk1/2 and JNK. In DNCB-induced atopic dermatitis mouse model, quercetin exhibited anti-inflammatory effects, as evidenced by improvements in ear thickness, serum IgE levels, and histological analysis (64).
Regarding the pharmacokinetic aspects of quercetin, initial metabolism occurs in the small intestine through processes like glucuronidation and O-methylation. The subsequent breakdown and processing take place in the liver after reaching it through the hepatic portal vein. Notably, gut bacteria, especially clostridium orbiscindens, play a role in the breakdown process in the large intestine. Key metabolites found in human plasma include quercetin-3-glucuronide, quercetin-3-sulfate, and isorhamnetin-3-glucosidic acid. Quercetin distribution involves various organs (lungs, kidneys, heart, and liver), with the lungs exhibiting the highest concentrations. Conjugates are predominantly present in the blood and are excreted in urine (65).
Pharmacokinetic and pharmacodynamic interactions between quercetin and drugs have been unveiled in studies. Competitive binding to serum albumin influence on cytochrome P450, glycoproteins, and other factors modify drug profiles, affecting treatment outcomes for infectious diseases, cardiovascular diseases, diabetes, and cancer (65). For example, quercetin competes with erlotinib for binding to bovine serum albumin, potentially contributing to increased adverse events associated with erlotinib use (66). Additionally, combined treatment with quercetin and methotrexate significantly reduces inflammatory mediators in collagen-induced arthritis mice, suggesting quercetin’s potential as an adjuvant to enhance anti-rheumatic monotherapy (67).
3.2.2 Epicatechin
Epicatechin exhibits dose-dependent reduction in TNFα-induced increase of JNK, p38, and ERK1/2 phosphorylation, nuclear AP-1-DNA interaction, activation of the NF-κB signaling pathway, nuclear NF-κB-DNA binding, p65 nuclear translocation, and PPARγ expression in 3T3-L1 adipocytes (68). A dosage of 20 mg/kg epicatechin proves effective in mitigating inflammation in the renal cortex of fructose-fed rats (69), while a higher dose of 80 mg/kg demonstrates efficacy in alleviating LPS-induced renal inflammation in rats (70). In both studies, downregulation of TNFα, iNOS and IL-6 are observed (69, 70). Furthermore, a dosage of 15 mg/kg epicatechin exhibits anti-inflammatory properties in mice experiencing LPS-induced acute lung injury, achieved by directly impeding the function of the p38-MAPK signaling pathway (71). Epicatechin also shows significant effects in mitigating atherosclerosis, specifically reducing severe lesions by 27% in ApoE*3-Leiden mice, without affecting plasma lipids. Additionally, it successfully countered diet-induced increases in inflammatory markers such as serum amyloid A and human C-reactive protein (72).
Concerning the pharmacokinetic parameters, orally administered epicatechin is initially absorbed in the duodenum, with the majority (70%) being absorbed in the lower intestine after catabolism by the gut microbiome. Over 80% of ingested epicatechin is absorbed, and the gut microbiome plays a crucial role in its metabolism, yielding more than 20 identifiable metabolites. These metabolites are then mainly excreted through urine (73).
3.2.3 Catechin
Catechin mitigates coronary heart disease in rats induced by pituitrin injection and a high-fat diet by inhibiting, lipoprotein-associated phospholipase A2, C-reactive protein, TNFα, and IL-6. Simultaneously, catechin treatment also demonstrates the inhibition of NF-κB and upregulation of FXR, p-STAT3, and p-Akt expression levels (74). High fructose consumption over a six-week period in rats induces a series of metabolic problems, including insulin resistance, dyslipidemia, obesity, reduced plasma adiponectin, and inflammation of adipose tissue. Supplementing their diet with 20 mg/kg/day of catechin effectively enhances all these parameters. In the TNFα induced 3T3-L1 adipocyte model, catechin inhibits inflammation by suppressing MAPKs, JNK and p38 activation, and preventing PPAR-γ reduction (75). At a dose of 75-300 mg/kg, catechin alleviates allergic symptoms such as sneezing and nose rubbing in mice suffering from OVA-induced allergic rhinitis. It reduces the levels of ovalbumin-specific IgE, IL-5, IL-13, restoring the balance between Th2 and Th1 cells. The potential mechanism of action involves the inhibition of TSLP expression in epithelial cells through the modulation of the NF-κB/TSLP pathway by catechin (76).
3.2.4 Naringenin
Naringenin significantly inhibits paw swelling and pathological changes in the joint tissue in the SD rat model of complete Freund’s adjuvant-induced arthritis. Additionally, IL-1β, TNFα, and IL-6 in serum are notably suppressed (14). Naringenin demonstrates neuroprotective effects by ameliorating neuroinflammation through the inhibition of p38-MAPK and STAT-1. In neuroglial cells induced by LPS/IFN-γ, this compound reduces the production of TNFα and NO, along with the expression of iNOS, thereby preventing neuron death induced by inflammation (77). Furthermore, naringenin inhibits pain behavior in mice triggered by various inflammatory stimuli, including acute pain caused by the use of acetic acid, PBQ, formalin, capsaicin, and CFA, as well as the provocation of mechanical hyperalgesia through subplantar injection of capsaicin, CFA, carrageenan, or PGE2. The mechanism of naringenin involves the activation of NF-κB and the inhibition of IL-1β, IL-33, TNFα, and oxidative stress. Additionally, naringenin activates the analgesic NO-cyclic GMP-PKG-ATP sensitive K+ channel pathway (78). Naringenin also exhibits anti-inflammatory effects in respiratory inflammation. In a murine COPD model, characterized by 90 days of cigarette smoke exposure-induced initiation, 20-80mg/kg of naringenin significantly improves pulmonary function, reduced inflammatory cells, and inhibits IL-8, TNFα, and MMP-9 in mouse BALF and serum. Suppression of the NF-κB pathway is also observed in mice treated with naringenin (79).
Delving into the pharmacokinetic characteristics, orally administrated naringenin exhibits limited absorption in the human gastrointestinal tract, yielding a modest 15% oral bioavailability. The absorption process encompasses both passive diffusion and active transport mechanisms. Once absorbed, naringenin swiftly distribute to vital organs such as the liver, cerebrum, kidney, spleen, and heart, suggesting potential neuroprotection within the central nervous system. Remarkably, naringenin demonstrates high permeability across blood-brain barrier models. The enterohepatic recycling of naringenin plays a crucial role, contributing to hepatic conjugate excretion in bile and participating in the enteric excretion of phase II conjugation. Post-absorption, Naringenin undergoes a significant metabolic process involving glucuronidation, resulting in the detection of 98% of naringenin−o−β−d−glucuronide in plasma. Before absorption in the caecum, naringenin undergoes hydrolysis by beta–glucosidase in the small intestine. Further metabolism by intestinal bacterial microflora produces p−hydroxybenzoic acid, p−hydroxyphenylpropionic acid, and p−coumaric acid, which manifest in plasma and urine. Ultimately, flavonoid excretion primarily occurs through two pathways: the biliary and urinary pathways (80).
3.2.5 Kaempferol
In OVA challenged asthmatic mouse models, oral intake of kaempferol mitigated the increase in eosinophil major basic protein and eotaxin-1 expression, achieve through the transactivation inhibition of NF-κB. Consequently, this reduction leads to decreased accumulation of eosinophils in the airways and lung tissue (16). Furthermore, kaempferol demonstrates the ability to control vascular inflammation in an atherosclerosis rabbit model with a high-cholesterol diet for ten weeks. Following treatment with kaempferol, decreased levels of IL-1β, TNFα, and MDA, an increase in serum SOD activity, and a reduction in the gene and protein expression of aortic E-selectin, ICAM-1, VCAM-1, and MCP-1 are observed (81). In a rat model simulating cerebral ischemia/reperfusion by occluding the middle cerebral artery for 60 minutes and then reperfusion, kaempferol is administered at doses of 25-100 mg/kg. The treatment significantly reduces the volume of cerebral infarction following cerebral ischemia-reperfusion, alleviated inflammation, and prevented the breakdown of the blood-brain barrier, thereby improving the neurological outcome on the 7th day after cerebral ischemia reperfusion. Additionally, reduced nuclear translocation and phosphorylation of the transcription factor NF-κB p65 are observed (82). What’s more, kaempferol exerts a protective effect on osteoarthritis chondrocytes by regulating the XIST/miR-130a/STAT3 axis, thereby inhibiting inflammation and extracellular matrix degradation (83).
Limited absorption and minimal oral bioavailability are observed with kaempferol. Its lipophilic nature allows for passive absorption, diffusion facilitation, and active transport. Metabolism in the liver results in the formation of glucuronic acid and sulfate conjugates, while intestinal enzymes in the small intestine contribute to its processing. Aglycogens, produced through the metabolism of kaempferol by colonic microbiota, are further transformed into 4-hydroxyphenylacetic acid, 4-methylphenol, and phloroglucinol. These metabolites undergo absorption into the systemic circulation, distribution to tissues, and eventual excretion in feces or urine (84).
Notably, the administration of a 12 mg/kg kaempferol dose demonstrated a substantial improvement in oral etoposide bioavailability in rats, showing a 64% enhancement compared to lower doses of 47% and 15%. At the highest dose, 12 mg/kg kaempferol exhibited a 26% increase in intravenous etoposide bioavailability. This intriguing finding suggests potential hepatic CYP3A4 inhibition and implicates kaempferol in reducing the unpredictable oral bioavailability of etoposide (85).
3.2.6 Isorhamnetin
Isorhamnetin possesses the ability to inhibit inflammation and provide renal protection. In a rat model of type 2 diabetes induced by a high-fat diet and streptozotocin, isorhamnetin significantly improved the renal function. The study reported that Isorhamnetin inhibited NF-κB signaling activity, resulting in reductions in IL-1β, IL-6, TNFα, TGF-β1, and ICAM-1 levels, as well as the mitigation of oxidative stress in diabetic rats and glomerular mesangial cells (86). Research conducted by Dou’s team demonstrated that isorhamnetin exerts beneficial effects on TNBS- and DSS-induced mouse inflammatory bowel disease (IBD) by upregulating xenobiotic metabolism mediated by PXR and concomitantly downregulating NF-κB signaling. Isorhamnetin inhibited the expression of IL-6 and TNFα, as well as the mRNA levels of ICAM-1, iNOS, TNFα, COX2, IL-6, IL-2, through the aforementioned pathways (87). Isorhamnetin has been found to inhibit neuroinflammation. In BV2 microglial cells stimulated with LPS, isorhamnetin significantly inhibits NO and PEG2, as well as IL-1β, TNFα, iNOS and COX2. Research on its anti-inflammatory mechanism indicates that isorhamnetin controls neuroinflammation by inhibiting the TLR4/MyD88/NF-kB pathway (88). Moreover, isorhamnetin exhibits efficacy in asthma. In TNFα-induced human bronchial epithelial cell line BEAS-2B, isorhamnetin at concentrations of 20-40 μM can reduce cellular proliferation and notably suppress the expression of CXCL10, IL-1β, IL-6, and IL-8. Furthermore, treatment with isorhamnetin downregulates the phosphorylation of the NF-κB and MAPK pathways in this model (89).
In the context of collagen-induced arthritis, isorhamnetin at doses ranging from 10 to 20 mg/kg significantly alleviate arthritis, improving arthritis score, joint damage score, and inflammation score. Isorhamnetin can also regulate the production of cytokines such as IL-1β, TNFα, IL-6, IL-10, IL-17A, IL-17F, and IL-35, while mitigating oxidative stress (90).
3.3 Glycosides
Arbutin significantly enhances kidney function in rats experiencing LPS-induced acute kidney damage. It reduces inflammation and cell death by modulating the PI3K/Akt/Nrf2 pathway after LPS exposure both in vivo and in vitro. Moreover, the Akt inhibitor GDC effectively inhibits this arbutin-induced improvement in vitro (91). Additionally, arbutin protects mice from isoproterenol (ISO)-induced cardiac hypertrophy. Pre-treatment with arbutin notably inhibits the TLR4/NF-κB pathway, resulting in decreased IL-6 and TNFα (92). In a DSS-induced mouse colitis model, arbutin significantly mitigates symptoms such as elevated disease activity index, loss of body weight, and increased colon weight-to-length ratio. This anti-inflammatory impact is contingent upon the control of JAK2 and the suppression of IL-1β, TNFα, and IL-6. Arbutin also suppresses inflammatory responses in epithelial (IEC6) and immune (RAW264.7) cells triggered by LPS. However, these benefits, both in vitro and in vivo, can be negated by the JAK2 inhibitor AG490 (93). In addressing metabolic issues, arbutin is found to suppress high-glucose-induced inflammation in adult human retinal pigment epithelial cells via upregulation of SIRT1, which provides a novel therapeutic target for diabetic retinopathy management (94). In arbutin-treated LPS-triggered BV2 murine microglial cells, inhibition of NO production, and reduced expression of COX2 and iNOS are observed. Arbutin significantly diminishes the expression of IL-1β, IL-6, MCP-1, and TNFα. Additionally, it impedes the nuclear transcriptional and translocation activity of NF-κB (95).
Jin’s group developed arbutin-loaded gelatine methacryloyl-Liposome microspheres (GM-Lipo@ARB), offering extended arbutin release and notable cartilage targeting. The microspheres decrease inflammation in IL-1β-stimulated arthritic chondrocytes and maintain cartilage matrix equilibrium through NF-κB inhibition and Nrf2 pathway activation. Application of the GM-Lipo@ARB lessens inflammation and oxidative stress in articular cartilage, effectively decelerating osteoarthritis progression in a mouse model (96).
3.4 Phenylpropanoid
The anti-inflammatory effects of chlorogenic acid (CGA) have been investigated in LPS-stimulated RAW 264.7 macrophages and BV2 microglial cells. CGA inhibits the production of NO, IL-1β, IL-6, TNFα, CXCL1, COX2, and iNOS. A possible mechanism of action involves the reduction of ninjurin1 level and nuclear translocation of NF-κB (97). CGA also downregulates the TLR4/MyD88/NF-κB signaling pathway (98, 99). Through this pathway, CGA can potently inhibit CCl4-induced liver fibrosis in rats (98), and alleviate renal inflammation in a mouse model of hyperuricemia induced by hypoxanthine and potassium oxonate (99). Animal experiments have confirmed that the systemic administration of CGA can help alleviate both inflammatory and neuropathic pain (100).
The hydrophilic nature of CGA essentially impedes its passage through the lipophilic membrane barrier, resulting in low absorption. Absorption likely occurs in the stomach rather than the small intestine. Caffeic acid is detected in plasma and urine 1.5 hours after a CGA-supplemented meal, along with derivatives like ferulic acid and isoferulic acid. These derivatives result from CGA hydrolysis in the small intestinal mucosa. CGAs’ absorption and metabolism are relatively low, constituting about one-third of total intake in the upper gastrointestinal tract. The remaining two-thirds reach the colon, where intense microbial metabolism occurs. Microflora-derived esterase hydrolyzes CGA, producing microbial metabolites, comprising 57.4% of the total CGA consumed, emphasizing the crucial role of gut microbiota in CGA metabolism and biological properties (101).
3.5 Anthocyanin
Anthocyanins, a member of the polyphenolic family in Z. bungeanum, contribute to the crimson coloration of its fruit peel. (102). In total, five types of anthocyanins with clear chemical structure have been identified in Z. bungeanum (24, 102–104).
The anti-inflammatory efficacy of cyanidin 3-O-glucoside (C3G) has been demonstrated across various in vivo and in vitro models. C3G exhibits the ability to safeguard mice from chronic skin damage induced by UVB exposure, leading to notable improvements in UVB-induced epidermal hyperplasia, collagen fiber preservation, ROS levels, and the expression of COX-2 and IL-6 (105). Furthermore, C3G demonstrates protective effects in rats against cecal ligation and puncture (CLP)-induced acute lung injury (ALI), enhancing their survival rate. C3G treatment results in reduced serum levels of TNF-α, IL-1β, and IL-6, along with the inhibition of COX-2 protein expression and PGE2 production in the lung, potentially through the suppression of the NF-κB signaling pathway (106). C3G also exerts anti-neuroinflammatory effects. In LPS-stimulated BV2 microglia, C3G effectively suppresses microglial activation and the levels of neurotoxic mediators and pro-inflammatory cytokines. Moreover, there is observed suppression of the NF-κB and p38 MAPK signaling pathways (107). Additionally, in TNBS-challenged mice, C3G significantly ameliorates clinical symptoms and mitigates histological damage, possibly by protecting the intestinal barrier and suppressing inflammatory cytokine secretion (108).
Cyanidin 3-O-rutinoside, peonidin 3-O-glucoside, pelargonidin 3,5-O-diglucoside, cyanidin-3,5-O-diglucoside have limited study in inflammatory disorders. Only a few in vitro studies were found (109, 110).
3.6 Non-glycosides
Research on the anti-inflammatory effects of quinic acid is limited. However, one study shown that quinic acid mitigates vascular inflammation in TNFα-stimulated vascular smooth muscle cells by reducing MAPK phosphorylation and inhibiting NF-κB activation (111).
Limited study has been conducted on the anti-inflammatory effects of catechin gallate, epigallocatechin, dihydrorobinetin, quercetin-3-arabinoside, quarcetin 3’,4’-dimethyl ether 7-glucoside, isorhamnetin-3-glucoside, isorhamnetin 7-glucoside, isorhamnetin 3-O-α-L-rhamnoside, tamarixetin 3,7-bis-glucoside, 3,5,6-trihydroxy-7,4’-dimethoxy flavone, sitosterol β-glucoside, trifolin, vanillic acid-4-glucoside, syringetin-3-glucoside, L-sesamin, and 5-feruloyquinic acid.
4 Direct target of Z. bungeanum polyphenols
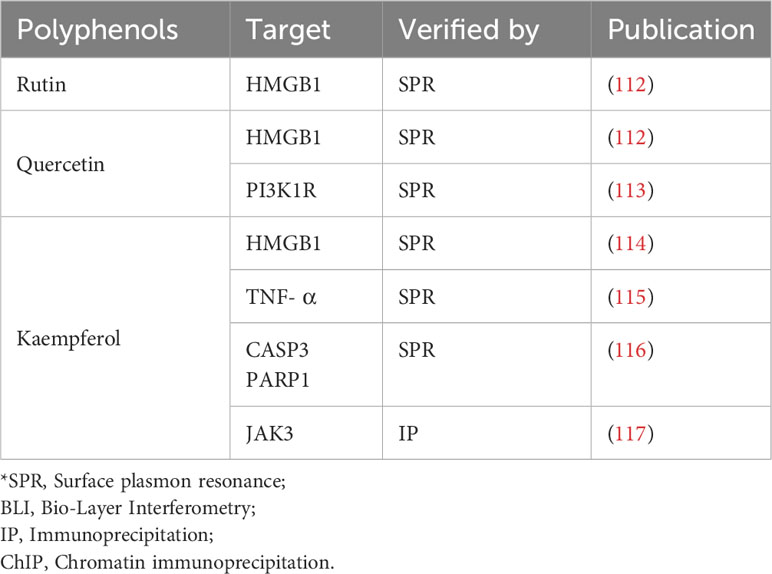
In the preceding sections, we primarily delineated the anti-inflammatory pharmacological activities of Z. bungeanum polyphenols, highlighting their modulation of inflammation through signaling pathways, including NF-κB, MAPK, Nrf2/keap1, and the NLRP3 inflammasome (Figure 3). However, to date, limited research has been conducted on the direct targeting of proteins or genes associated with inflammation by Z. bungeanum polyphenols. In this section, we consolidate and summarize the pertinent studies investigating the direct interactions of Z. bungeanum polyphenols with inflammatory-related proteins or genes (Table 2).
5 Clinical trials of Z. bungeanum polyphenols
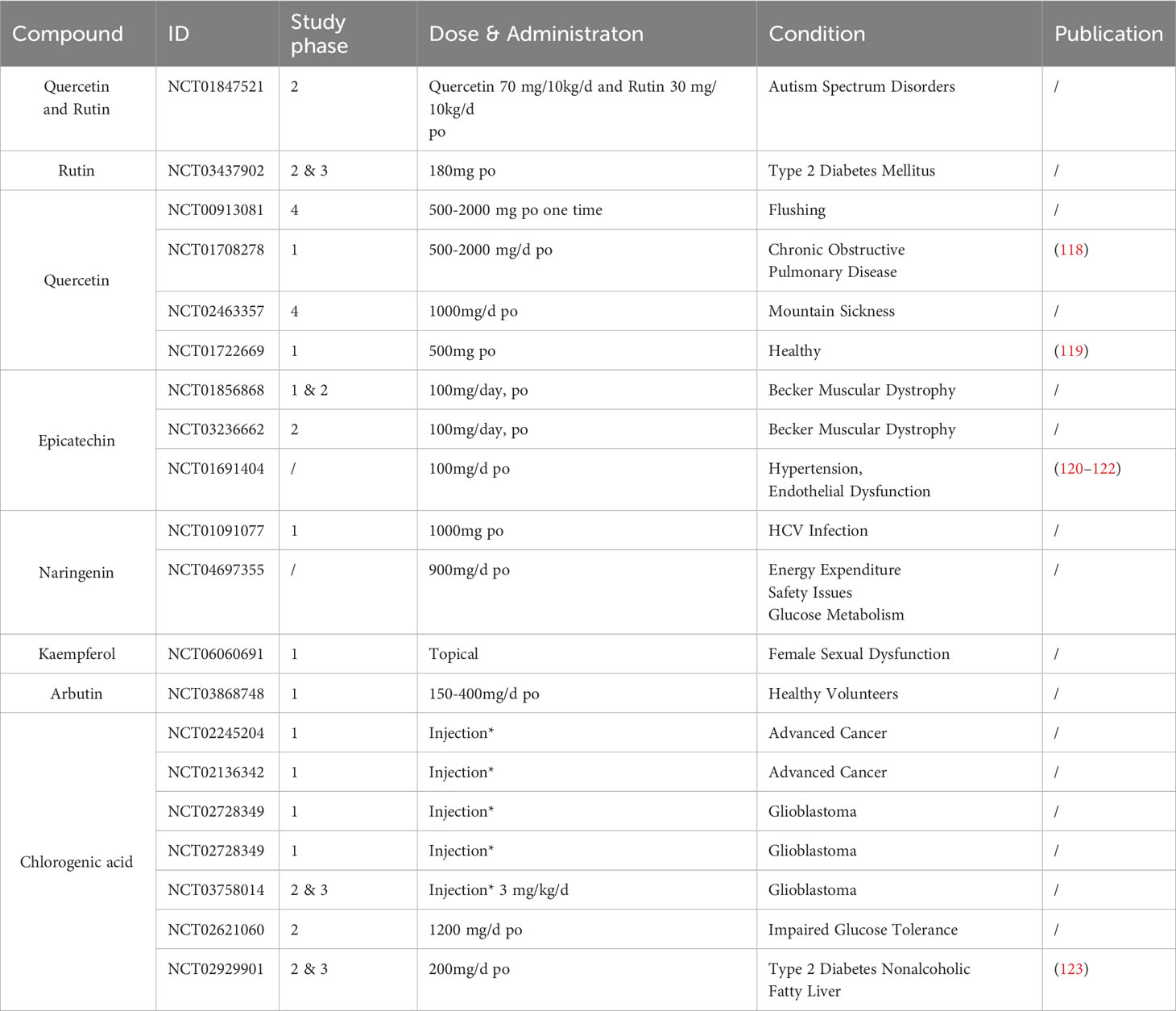
Currently, several clinical trials have utilized Z. bungeanum polyphenols; however, their application in inflammatory conditions remains limited. Table 3 below summarizes completed clinical studies on Z. bungeanum polyphenols to date. Notably, no severe adverse reactions associated with these polyphenols have been reported across these clinical investigations, providing a certain degree of evidence supporting their safety profile.
6 Inflammatory diseases and other compositions in Z. bungeanum
6.1 Alkaloids
Alkaloids, such as hydroxy-alpha-sanshool (HAS), constitute the characteristic compounds in Z. bungeanum, contributing to the notable sensation of numbness in the mouth (124). In a rat model of type 2 diabetes mellitus (T2DM), Zanthoxylum alkylamides (ZA), a mixed extract containing hydroxyl-γ-sanshool, hydroxyl-β-sanshool, and hydroxyl-α-sanshool, demonstrated the ability to control inflammation and address protein metabolism disorders, consequently ameliorating T2DM. The PI3K/Akt/forkhead box O signaling pathway and the TNFα/NF-κB pathway are implicated in this process (125).
Among the alkaloids in Z. bungeanum, HAS has been extensively studied for its anti-inflammatory effects. HAS exhibits a neuroprotective effect on H2O2-stimulated PC12 cells without inducing cytotoxicity in normal PC12 cells. The suppression of apoptosis is achieved by regulating the PI3K/Akt signaling pathway(126). Oral administration of HAS markedly improves spontaneous locomotion, cognitive function, and histopathological injuries in a mouse model of Alzheimer’s disease induced by D-galactose and AlCl3. The therapeutic effect of HAS involves the mitigation of oxidative stress damage and the activation of the Nrf2/HO-1 signaling pathway (127). As one of the main active ingredients in the herbal medicine TU-100, HAS enhances the production of antimicrobial defense molecules (ADM) by intestinal epithelial cells. TU-100, administered orally, prevents weight loss and colon ulceration in both TNBS-induced type-1 model colitis and OXN-induced type-2 model colitis. This suggests that HAS possesses anti-inflammatory properties and could potentially serve as a beneficial treatment agent for UC through the promotion of ADM production (128).
Zanthoxylin, another major alkaloid of Z. bungeanum, exhibits anti-inflammatory and pain-relieving effects in a variety of animal models. In mice, zanthoxylin alleviates pain in both general and formaldehyde-induced pain models. Its mechanism of action involves binding to the α7nAChR receptor and activating the JAK2/STAT3 signaling pathway, thereby inhibiting inflammation and reducing the production of pro-inflammatory cytokines such as IL-6 and TNFα (129).
6.2 Fatty acid
Research on the anti-inflammatory properties of fatty acids in Z. bungeanum predominantly focuses on Z. bungeanum seed oil (ZBSO). The primary components of ZBSO include eicosoic acid, linolenic acid, linoleic acid, oleic acid, palmitic acid, arachidonic acid, stearic acid, eicosenoic acid, and docosahexenoic acid (130). In LPS-triggered lung epithelial cells, ZBSO effectively inhibits the production of pro-inflammatory cytokines and chemokines, including IL-6, IL-10, TNFα, PGE2, MMP2, MMP9, MCP1, and COX2. This inhibition is achieved by blocking the TLR4/MyD88/NF-κB signaling pathway. Additionally, ZBSO inhibits the nuclear translocation of NF-κB/p65 (131).
Zanthoxylum bungeanum Maxim seed (ZBMS), rich in oleic acid, linoleic acid, and α-linolenic acid, exhibits potential for treating asthma and stress-related disorders (132). ZBMS protects mice from histamine/acetylcholine-induced asthma, reduces citric acid-induced cough in guinea pigs, and increases swimming endurance and survival time in mice, indicating a positive anti-stress effect. In an OVA-induced airway inflammation mouse model, ZBMS treatment improved lung peak inspiratory airflow in a dose-dependent manner (132).
Another group examined ZBSO in an OVA-induced asthmatic mouse model, demonstrating its efficacy in alleviating airway inflammation, attenuates lung tissue injury and airway remodeling, and inhibits leukocytes and eosinophils infiltration into the airway. ZBSO also reduces IL-5 and IL-4 in the bronchial airway, attenuates the induction of ICAM-1 and TNFα mRNA and protein expression levels, and alleviates ERK, JNK phosphorylation, c-fos and c-JUN induction in the lung tissue (133). ZBSO exhibits effective anti-inflammatory properties in the wound healing process. In SD rat models with deep second-degree burns, topical ZBSO application resulted in decreased levels of TNFα, IL-6, and IL-1β in serum, elevated IκBα, and reduced p-IκBα and p-NF-κB p65 expression (134). In copper comb-induced rat burn model, ZBSO can reduce the level of thiobarbituric acid reactant, IL-6, TNFα, increase GSH level and promote wound recovery (135). ZBSO also inhibits inflammation in bone-destroying diseases. In RAW264.7 cells stimulated with NF-κB ligand (RANKL), ZBSO decreases NF-κB, TNFα, NFATc1, and TRAP, leading to the inhibition of osteoclastogenesis. Among the fatty acids in ZBSO, alpha-linolenic acid (ALA) exhibits the strongest effect. In ovariectomized osteoporotic rats, preventive and therapeutic interventions with ALA resulted in decreased levels of IL-1β, IL-6, TAK1, TRAP, NFATc1, and TNFα (136).
6.3 Z. bungeanum essential oil
Z. bungeanum essential oil (ZBEO) is the primary source of the distinctive flavor of Sichuan pepper, with terpenoids being a major component of ZBEO (2). ZBEO has demonstrated anti-inflammatory effects in various skin disease models. In a guinea pig model of psoriasis, ZBEO treatment significantly improved Baker scores and reduced inflammatory cell infiltration (137). In a mouse model of ultraviolet-induced skin photoaging, topical application of ZBEO improved photoaging damage, reduced skin thickening, and attenuated inflammatory cell infiltration. ZBEO also inhibits the levels of MMP9, MMP1, and MMP3 in skin tissue, enhance the activity of CAT, SOD, and GSH-Px/GPX, and reduced the production of the lipid peroxidation byproduct MDA. Furthermore, ZBEO effectively suppress the expression of TNFα, IL-6, IL-1β, and IL-1α (138). In a HaCaT cell inflammatory model induced by Propionibacterium acnes (P. acnes), pretreatment with ZBEO reduced the levels of TNFα, IL-1β, IL-8, and IL-6, as well as the mRNA levels of TLR2, IL-8, IL-6, and NF-κB (139).
ZBEO also shows therapeutic effects in gastrointestinal disorders due to its anti-inflammatory properties. ZBEO has demonstrated protective effects against DSS-induced colitis in mice. ZBEO doses of 20-80 mg/kg reduced myeloperoxidase activity, colonic pathological damage, colon length shortening, disease activity index, and DSS-induced weight loss (140, 141). Administration of ZBEO significantly reduced IL-1β, IL-12 (140), TNFα, VCAM-1, TLR8, and IL-11 (141) mRNA levels. ZBEO is reported to inhibit inflammation in colitis in mice by regulating the PPARγ and NF-κB pathways, and suppressing NLRP3 activation (140). Next-generation sequencing (NGS) verifies that ZBEO increases VCAM-1 and CYP, and suppresses CXCL and S100A8 to attenuate UC symptoms (141). In vitro studies also demonstrate that ZBEO can reverse the imbalanced expression of IL-1β, IL-6, IL-10, and TNFα in LPS-induced NCM460 colon epithelial cells (141).
ZBEO inhibited enteritis and intestinal dysfunction caused by E. coli infection in mice. Histopathological observations indicated that ZBEO significantly improved the impairment of intestinal tissue structure, which could be associated with its inhibitory effect on the gene expression of inflammatory cytokines such as IL-8, TNFα, TLR4, and TLR2 (142). Atomized inhalation of ZBEO protects mouse from inflammation related colorectal cancer by reducing inflammation and cancer transformation. Furthermore, a decrease in AChE activity, an increase in ChAT activity, an increase in α7nAChR expression, and a decrease in IL-6 mRNA levels are observed in ZBEO treated group (143).
6.4 Other extractions
Z. bungeanum-cake-separated moxibustion (ZBCS-moxi) is a traditional Chinese therapy that has been employed for centuries to treat rheumatoid arthritis. A recent study assessed the anti-inflammatory effects of ZBCS-moxi in a rat model of rheumatoid arthritis. The study found that rats treated with ZBCS-moxi for three weeks exhibited a significant reduction in paw volume, pannus formation, synovial hyperplasia of synovial membranes, and levels of TNFα and IL-1β in serum (144).
These findings suggest that Zanthoxylin and ZBCS-moxi may have therapeutic potential for the treatment of inflammation and pain. However, more research is needed to confirm these findings through clinical trials.
7 Discussion
Zanthoxylum bungeanum Maxim., or Chinese prickly ash, holds a rich history spanning over two millennia in traditional Chinese medicine (5). This herb has been extensively used orally and topically to address various ailments, including gastrointestinal discomfort, arthritis, and bruises (5, 7). Its significance extends beyond China, finding a place in traditional medical practices in countries such as India and Nepal (7). Additionally, the unique flavor and numbing taste of the dried fruit follicles of Z. bungeanum have made it a significant ingredient in Chinese cuisine (1). Over time, research on and applications of Z. bungeanum have expanded significantly. Z. bungeanum exhibits diverse pharmacological activities such as anti-inflammatory, analgesic, antibacterial, and anti-tumor properties, showcasing therapeutic effects on multiple organ systems, including the gastrointestinal tract, cardiovascular system, and nervous system (2). The plant contains over 140 compounds, including polyphenols, alkaloids, lignans, coumarin, fatty acids, and essential oils (2, 11, 12). Beyond its polyphenolic content, constituents like hydroxy-alpha-sanshool, a mixed extract of fatty acids, and essential oil extraction from Z. bungeanum, have proven anti-inflammatory efficacious in various systems, such as the nervous system (127) and digestive system (140, 141). As research progresses, the application of Z. bungeanum in both medical and daily contexts continues to broaden, promising potential benefits to human health.
Polyphenols from Z. bungeanum emerge as a promising class of natural compounds with potential health benefits, particularly in preventing and treating inflammatory diseases. Numerous studies have highlighted their anti-inflammatory and antioxidant properties through a variety of mechanisms, including:
● Inhibiting pro-inflammatory cytokine production, such as IL-1β, TNFα, and IL-6.
● Suppressing the NF-κB and MAPK signaling pathway, central to inflammation.
● Activating the Nrf2/HO-1 signaling pathway, protecting cells from oxidative damage.
● Modulating the immune response, promoting regulatory T cells and suppressing inflammatory T cells.
While much of the current research on polyphenols of Z. bungeanum has been conducted in vitro or in animal models, promising preclinical data suggest therapeutic potential for a range of inflammatory diseases in humans, including ulcerative colitis, arthritis, asthma, chronic obstructive pulmonary disease, cardiovascular disease, and neurodegenerative diseases. In addition to their anti-inflammatory effects, polyphenols of Z. bungeanum have demonstrated other beneficial properties, such as anti-cancer, anti-diabetic, anti-bacterial, and neuroprotective effects.
Several clinical trials have tested Z. bungeanum polyphenols in non- inflammatory diseases, indicating the safety of these compound. Future research should prioritize human clinical trials to validate the clinical efficacy of polyphenols of Z. bungeanum on inflammatory diseases. Additionally, researchers should investigate:
● Optimal dosages and long-term safety of polyphenols of Z. bungeanum.
● Synergistic or antagonistic interactions of polyphenols of Z. bungeanum with other bioactive substances.
● Effects of polyphenols of Z. bungeanum on specific biomarkers of inflammation and disease activity.
● Mechanisms by which polyphenols of Z. bungeanum exert their beneficial effects.
Ultimately, research outcomes may contribute to the development of novel therapeutic interventions and dietary recommendations that harness the power of polyphenols of Z. bungeanum to improve human health and well-being. For example, polyphenols of Z. bungeanum could be used to develop:
● New drugs or dietary supplements for the prevention and treatment of inflammatory diseases.
● Functional foods or fortified beverages that promote overall health and well-being.
● Personalized nutrition plans that take into account individual genetic and environmental risk factors.
Overall, the polyphenols of Z. bungeanum are a promising class of natural compounds with the potential to play a significant role in human health and well-being. Further research is needed to fully elucidate their mechanisms of action and develop safe and effective therapies for human use.
Author contributions
GH: Funding acquisition, Investigation, Writing – review & editing. JQ: Conceptualization, Data curation, Writing – original draft. ZP: Validation, Visualization, Writing – original draft. XW: Resources, Validation, Visualization, Writing – original draft. NZ: Visualization, Writing – review & editing. XJ: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82273559 and 82073473), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21036), the Clinical Research Innovation Project, West China Hospital, Sichuan University (2019HXCX10) and Sichuan University-Zigong Special Fund for University-Local Science and Technology Cooperation (2021CDZG-21).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, et al. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci (2008) 11(7):772–9. doi: 10.1038/nn.2143
2. Zhang M, Wang J, Zhu L, Li T, Jiang W, Zhou J, et al. Zanthoxylum bungeanum maxim. (Rutaceae): A systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int J Mol Sci (2017) 18(10):2172. doi: 10.3390/ijms18102172
3. Institute of Botany, Chinese Academy of Sciences. Flora of China. China: Institute of Botany Chinese Academy of Sciences (2007-2019).
4. Commission CP. Pharmacopoeia of the People’s Republic of China. China: China Medical Science Press (2020).
5. Institute of Botany, Chinese Academy of Sciences. Flora Reipublicae Popularis Sinicae. China: Institute of Botany Chinese Academy of Sciences (2005-2019).
6. Zhengyi D, Bingyin S, Kegong K, Yugong D. Analysis of the main nutritional labeling in the tender bud of Zanthoxylum bungeanum. J Northwest Forest Univ (2005) 01):179–180 + 185.
7. Wagner H, Bauer R, Melchart D, Xiao P-G, Staudinger A. Pericarpium Zanthoxyli Huajiao. In: Wagner H, Bauer R, Melchart D, Xiao P-G, Staudinger A, editors. Chromatographic Fingerprint Analysis of Herbal Medicines: Thin-layer and High Performance Liquid Chromatography of Chinese Drugs. Vienna: Springer Vienna (2011). p. 191–202.
8. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget (2018) 9(6):7204–18. doi: 10.18632/oncotarget.23208
9. Shapiro H, Singer P, Halpern Z, Bruck R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut (2007) 56(3):426–35. doi: 10.1136/gut.2006.094599
10. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev (2016) 2016:7432797. doi: 10.1155/2016/7432797
11. Wang K, Meng X-H, Chai T, Wang C-B, Sang C-Y, Wang W-F, et al. Chemical constituents from the fruits of Zanthoxylum bungeanum and their chemotaxonomic significance. Biochem System Ecol (2021) 99:104356. doi: 10.1016/j.bse.2021.104356
12. Bao Y, Yang L, Fu Q, Fu Y, Tian Q, Wang C, et al. The current situation of Zanthoxylum bungeanum industry and the research and application prospect. A review. Fitoterapia (2023) 164:105380. doi: 10.1016/j.fitote.2022.105380
13. Zhang Z, Liu J, Shen P, Cao Y, Lu X, Gao X, et al. Zanthoxylum bungeanum pericarp extract prevents dextran sulfate sodium-induced experimental colitis in mice via the regulation of TLR4 and TLR4-related signaling pathways. Int Immunopharmacol (2016) 41:127–35. doi: 10.1016/j.intimp.2016.10.021
14. Zhu L, Wang J, Wei T, Gao J, He H, Chang X, et al. Effects of naringenin on inflammation in complete freund’s adjuvant-induced arthritis by regulating bax/bcl-2 balance. Inflammation (2015) 38(1):245–51. doi: 10.1007/s10753-014-0027-7
15. Valério DA, Georgetti SR, Magro DA, Casagrande R, Cunha TM, Vicentini FTMC, et al. Quercetin reduces inflammatory pain: inhibition of oxidative stress and cytokine production. J Natural Prod (2009) 72(11):1975–9. doi: 10.1021/np900259y
16. Gong J-H, Shin D, Han S-Y, Kim J-L, Kang Y-H. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J Nutr (2011) 142(1):47–56. doi: 10.3945/jn.111.150748
17. Yin Y, Li W, Son Y-O, Sun L, Lu J, Kim D, et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol Appl Pharmacol (2013) 269(2):89–99. doi: 10.1016/j.taap.2013.03.015
18. Zhang Q, Fan Z, Xue W, Sun F, Zhu H, Huang D, et al. Vitexin regulates Epac and NLRP3 and ameliorates chronic cerebral hypoperfusion injury. Can J Physiol Pharmacol (2021) 99(10):1079–87. doi: 10.1139/cjpp-2021-0034%M33915055
19. Yang L-C, Li R, Tan J, Jiang Z-T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. Grown in hebei, China, and their radical scavenging activities. J Agric Food Chem (2013) 61(8):1772–8. doi: 10.1021/jf3042825
20. Zhang Y, Wang D, Yang L, Zhou D, Zhang J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PloS One (2014) 9(8):e105725. doi: 10.1371/journal.pone.0105725
21. Zhong K, Li X-J, Gou A-N, Huang Y-N, Bu Q, Gao H. Antioxidant and cytoprotective activities of flavonoid glycosides-rich extract from the leaves of zanthoxylum bungeanum. J Food Nutr Res (2014) 2(7):349–56. doi: 10.12691/jfnr-2-7-4
22. Xiong Q, Shi D, Mizuno M. Flavonol glucosides in pericarps of Zanthoxylum bungeanum. Phytochemistry (1995) 39(3):723–5. doi: 10.1016/0031-9422(94)00965-V
23. Jia W, Wang X. Zanthoxylum bungeanum as a natural pickling spice alleviates health risks in animal-derived foods via up-regulating glutathione S-transferase, down-regulating cytochrome P450 1A. Food Chem (2023) 411:135535. doi: 10.1016/j.foodchem.2023.135535
24. Han N, Sun L, Zhang J, Yuan W, Wang C, Zhao A, et al. Transcriptomics integrated with metabolomics to characterize key pigment compounds and genes related to anthocyanin biosynthesis in Zanthoxylum bungeanum peel. Physiol Plant (2023) 175(5):e14031. doi: 10.1111/ppl.14031
25. Zhao M, Dai Y, Li P, Wang J, Ma T, Xu S. Inhibition of NLRP3 inflammasome activation and pyroptosis with the ethyl acetate fraction of Bungeanum ameliorated cognitive dysfunction in aged mice. Food Funct (2021) 12(21):10443–58. doi: 10.1039/D1FO00876E
26. Muvhulawa N, Dludla PV, Ziqubu K, Mthembu SXH, Mthiyane F, Nkambule BB, et al. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol Res (2022) 178:106163. doi: 10.1016/j.phrs.2022.106163
27. Nafees S, Rashid S, Ali N, Hasan SK, Sultana S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chemico-Biol Interact (2015) 231:98–107. doi: 10.1016/j.cbi.2015.02.021
28. Lee W, Ku S-K, Bae J-S. Barrier protective effects of rutin in LPS-induced inflammation in vitro and in vivo. Food Chem Toxicol (2012) 50(9):3048–55. doi: 10.1016/j.fct.2012.06.013
29. Wu J, Maoqiang L, Fan H, Zhenyu B, Qifang H, Xuepeng W, et al. Rutin attenuates neuroinflammation in spinal cord injury rats. J Surg Res (2016) 203(2):331–7. doi: 10.1016/j.jss.2016.02.041
30. Sharma A, Tirpude NV, Kumari M, Padwad Y. Rutin prevents inflammation-associated colon damage via inhibiting the p38/MAPKAPK2 and PI3K/Akt/GSK3β/NF-κB signalling axes and enhancing splenic Tregs in DSS-induced murine chronic colitis. Food Funct (2021) 12(18):8492–506. doi: 10.1039/D1FO01557E
31. Choi MH, Rho JK, Kang JA, Shim HE, Nam YR, Yoon S, et al. Efficient radiolabeling of rutin with 125I and biodistribution study of radiolabeled rutin. J Radioanal Nucl Chem (2016) 308(2):477–83. doi: 10.1007/s10967-015-4415-8
32. Yang C-Y, Hsiu S-L, Wen K-C, Lin S-P, Tsai S-Y, Hou Y-C, et al. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J Food Drug Anal (2005) 13(3):244–50. doi: 10.38212/2224-6614.2517
33. Zhang P, Gou Y-Q, Gao X, Bai R-B, Chen W-X, Sun B-L, et al. The pharmacokinetic study of rutin in rat plasma based on an electrochemically reduced graphene oxide modified sensor. J Pharm Anal (2016) 6(2):80–6. doi: 10.1016/j.jpha.2015.12.003
34. Chan APE, Hegde A, Chen X. Effect of rutin on warfarin anticoagulation and pharmacokinetics of warfarin enantiomers in rats. J Pharm Pharmacol (2010) 61(4):451–8. doi: 10.1211/jpp.61.04.0006
35. Yu CP, Wu PP, Hou YC, Lin SP, Tsai SY, Chen CT, et al. Quercetin and rutin reduced the bioavailability of cyclosporine from Neoral, an immunosuppressant, through activating P-glycoprotein and CYP 3A4. J Agric Food Chem (2011) 59(9):4644–8. doi: 10.1021/jf104786t
36. Kim S-J, Um J-Y, Hong S-H, Lee J-Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am J Chin Med (2011) 39(01):171–81. doi: 10.1142/s0192415x11008737
37. He J, Li H, Li G, Yang L. Hyperoside protects against cerebral ischemia-reperfusion injury by alleviating oxidative stress, inflammation and apoptosis in rats. Biotechnol Biotechnol Equip (2019) 33(1):798–806. doi: 10.1080/13102818.2019.1620633
38. Huang J, Zhou L, Chen J, Chen T, Lei B, Zheng N, et al. Hyperoside attenuate inflammation in HT22 cells via upregulating SIRT1 to activities wnt/β-catenin and sonic hedgehog pathways. Neural Plastic (2021) 2021:8706400. doi: 10.1155/2021/8706400
39. Ku S-K, Kwak S, Kwon OJ, Bae J-S. Hyperoside inhibits high-glucose-induced vascular inflammation in vitro and in vivo. Inflammation (2014) 37(5):1389–400. doi: 10.1007/s10753-014-9863-8
40. Sun K, Luo J, Jing X, Xiang W, Guo J, Yao X, et al. Hyperoside ameliorates the progression of osteoarthritis: An in vitro and in vivo study. Phytomedicine (2021) 80:153387. doi: 10.1016/j.phymed.2020.153387
41. Ye P, Yang X-l, Chen X, Shi C. Hyperoside attenuates OVA-induced allergic airway inflammation by activating Nrf2. Int Immunopharmacol (2017) 44:168–73. doi: 10.1016/j.intimp.2017.01.003
42. Wei A, Song Y, Ni T, Xiao H, Wan Y, Ren X, et al. Hyperoside attenuates pregnancy loss through activating autophagy and suppressing inflammation in a rat model. Life Sci (2020) 254:117735. doi: 10.1016/j.lfs.2020.117735
43. Ma J-Q, Luo R-Z, Jiang H-X, Liu C-M. Quercitrin offers protection against brain injury in mice by inhibiting oxidative stress and inflammation. Food Funct (2016) 7(1):549–56. doi: 10.1039/C5FO00913H
44. Wang L, Sun J, Miao Z, Jiang X, Zheng Y, Yang G. Quercitrin improved cognitive impairment through inhibiting inflammation induced by microglia in Alzheimer’s disease mice. NeuroReport (2022) 33(8):327. doi: 10.1097/WNR.0000000000001783
45. Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur J Immunol (2005) 35(2):584–92. doi: 10.1002/eji.200425778
46. Tang J, Diao P, Shu X, Li L, Xiong L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264.7 cells: in vitro assessment and a theoretical model. BioMed Res Int (2019) 2019:7039802. doi: 10.1155/2019/7039802
47. Lee E-H, Park H-J, Jung H-Y, Kang I-K, Kim B-O, Cho Y-J. Isoquercitrin isolated from newly bred Green ball apple peel in lipopolysaccharide-stimulated macrophage regulates NF-κB inflammatory pathways and cytokines. 3 Biotech (2022) 12(4):100. doi: 10.1007/s13205-022-03118-1
48. Shen Y, Zhang Q, Huang Z, Zhu J, Qiu J, Ma W, et al. Isoquercitrin delays denervated soleus muscle atrophy by inhibiting oxidative stress and inflammation. Front Physiol (2020) 11:988. doi: 10.3389/fphys.2020.00988
49. Venturini CL, Macho A, Arunachalam K, de Almeida DAT, Rosa SIG, Pavan E, et al. Vitexin inhibits inflammation in murine ovalbumin-induced allergic asthma. Biomed Pharmacother (2018) 97:143–51. doi: 10.1016/j.biopha.2017.10.073
50. Borghi SM, Carvalho TT, Staurengo-Ferrari L, Hohmann MSN, Pinge-Filho P, Casagrande R, et al. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J Natural Prod (2013) 76(6):1141–9. doi: 10.1021/np400222v
51. Duan S, Du X, Chen S, Liang J, Huang S, Hou S, et al. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed Pharmacother (2020) 121:109683. doi: 10.1016/j.biopha.2019.109683
52. Rosa SIG, Rios-Santos F, Balogun SO, Martins D. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine (2016) 23(1):9–17. doi: 10.1016/j.phymed.2015.11.003
53. Zhang Y, Qi Z, Wang W, Wang L, Cao F, Zhao L, et al. Isovitexin inhibits ginkgolic acids-induced inflammation through downregulating SHP2 activation. Front Pharmacol (2021) 12:630320. doi: 10.3389/fphar.2021.630320
54. Liu S, Zhang X, Wang J. Isovitexin protects against cisplatin-induced kidney injury in mice through inhibiting inflammatory and oxidative responses. Int Immunopharmacol (2020) 83:106437. doi: 10.1016/j.intimp.2020.106437
55. Hu J-j, Wang H, Pan C-w, Lin M-x. Isovitexin alleviates liver injury induced by lipopolysaccharide/d-galactosamine by activating Nrf2 and inhibiting NF-κB activation. Microbial Pathogen (2018) 119:86–92. doi: 10.1016/j.micpath.2018.03.053
56. Lv H, Yu Z, Zheng Y, Wang L, Qin X, Cheng G, et al. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/nrf2 pathways. Int J Biol Sci (2016) 12(1):72–86. doi: 10.7150/ijbs.13188
57. Jia Q, Wang T, Wang X, Xu H, Liu Y, Wang Y, et al. Astragalin suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis and in human fibroblast-like synoviocytes. Front Pharmacol (2019) 10:94. doi: 10.3389/fphar.2019.00094
58. Xing F, Geng L, Guan H, Liu D, Li Y, Zeng L, et al. Astragalin mitigates inflammatory osteolysis by negatively modulating osteoclastogenesis via ROS and MAPK signaling pathway. Int Immunopharmacol (2022) 112:109278. doi: 10.1016/j.intimp.2022.109278
59. Kim Y-H, Choi Y-J, Kang M-K, Park S-H, Antika LD, Lee E-J, et al. Astragalin inhibits allergic inflammation and airway thickening in ovalbumin-challenged mice. J Agric Food Chem (2017) 65(4):836–45. doi: 10.1021/acs.jafc.6b05160
60. Shin SW, Jung E, Kim S, Kim J-H, Kim E-G, Lee J, et al. Antagonizing effects and mechanisms of afzelin against UVB-induced cell damage. PloS One (2013) 8(4):e61971. doi: 10.1371/journal.pone.0061971
61. Kim JH, Kim M, Kim JM, Lee MK, Seo SJ, Park KY. Afzelin suppresses proinflammatory responses in particulate matter-exposed human keratinocytes. Int J Mol Med (2019) 43(6):2516–22. doi: 10.3892/ijmm.2019.4162
62. Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J Am Coll Nutr (2017) 36(1):9–15. doi: 10.1080/07315724.2016.1140093
63. Wei B, Zhang Y, Tang L, Ji Y, Yan C, Zhang X. Protective effects of quercetin against inflammation and oxidative stress in a rabbit model of knee osteoarthritis. Drug Dev Res (2019) 80(3):360–7. doi: 10.1002/ddr.21510
64. Lee HN, Shin SA, Choo GS, Kim HJ, Park YS, Kim BS, et al. Anti−inflammatory effect of quercetin and galangin in LPS−stimulated RAW264.7 macrophages and DNCB−induced atopic dermatitis animal models. Int J Mol Med (2018) 41(2):888–98. doi: 10.3892/ijmm.2017.3296
65. Ding K, Jia H, Jiang W, Qin Y, Wang Y, Lei M. A double-edged sword: focusing on potential drug-to-drug interactions of quercetin. Rev Bras Farmacognosia (2023) 33(3):502–13. doi: 10.1007/s43450-022-00347-6
66. Wani TA, Bakheit AH, Zargar S, Alanazi ZS, Al-Majed AA. Influence of antioxidant flavonoids quercetin and rutin on the in-vitro binding of neratinib to human serum albumin. Spectrochim Acta A Mol Biomol Spectrosc (2021) 246:118977. doi: 10.1016/j.saa.2020.118977
67. Haleagrahara N, Hodgson K, Miranda-Hernandez S, Hughes S, Kulur AB, Ketheesan N. Flavonoid quercetin–methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology (2018) 26(5):1219–32. doi: 10.1007/s10787-018-0464-2
68. Vazquez-Prieto MA, Bettaieb A, Haj FG, Fraga CG, Oteiza PI. (–)-Epicatechin prevents TNFα-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Arch Biochem Biophys (2012) 527(2):113–8. doi: 10.1016/j.abb.2012.02.019
69. Prince PD, Lanzi CR, Toblli JE, Elesgaray R, Oteiza PI, Fraga CG, et al. Dietary (–)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radical Biol Med (2016) 90:35–46. doi: 10.1016/j.freeradbiomed.2015.11.009
70. Prince PD, Fischerman L, Toblli JE, Fraga CG, Galleano M. LPS-induced renal inflammation is prevented by (–)-epicatechin in rats. Redox Biol (2017) 11:342–9. doi: 10.1016/j.redox.2016.12.023
71. Xing J, Yu Z, Zhang X, Li W, Gao D, Wang J, et al. Epicatechin alleviates inflammation in lipopolysaccharide-induced acute lung injury in mice by inhibiting the p38 MAPK signaling pathway. Int Immunopharmacol (2019) 66:146–53. doi: 10.1016/j.intimp.2018.11.016
72. Morrison M, van der Heijden R, Heeringa P, Kaijzel E, Verschuren L, Blomhoff R, et al. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis (2014) 233(1):149–56. doi: 10.1016/j.atherosclerosis.2013.12.027
73. Ottaviani JI, Borges G, Momma TY, Spencer JPE, Keen CL, Crozier A, et al. The metabolome of [2-14C](–)-epicatechin in humans: implications for the assessment of efficacy, safety and mechanisms of action of polyphenolic bioactives. Sci Rep (2016) 6(1):29034. doi: 10.1038/srep29034
74. Tu S, Xiao F, Min X, Chen H, Fan X, Cao K. Catechin attenuates coronary heart disease in a rat model by inhibiting inflammation. Cardiovasc Toxicol (2018) 18(5):393–9. doi: 10.1007/s12012-018-9449-z
75. Vazquez Prieto MA, Bettaieb A, Rodriguez Lanzi C, Soto VC, Perdicaro DJ, Galmarini CR, et al. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Mol Nutr Food Res (2015) 59(4):622–33. doi: 10.1002/mnfr.201400631
76. Pan Z, Zhou Y, Luo X, Ruan Y, Zhou L, Wang Q, et al. Against NF-κB/thymic stromal lymphopoietin signaling pathway, catechin alleviates the inflammation in allergic rhinitis. Int Immunopharmacol (2018) 61:241–8. doi: 10.1016/j.intimp.2018.06.011
77. Vafeiadou K, Vauzour D, Lee HY, Rodriguez-Mateos A, Williams RJ, Spencer JPE. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch Biochem Biophys (2009) 484(1):100–9. doi: 10.1016/j.abb.2009.01.016
78. Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, et al. Naringenin reduces inflammatory pain in mice. Neuropharmacology (2016) 105:508–19. doi: 10.1016/j.neuropharm.2016.02.019
79. Liu J, Yao J, Zhang J. Naringenin attenuates inflammation in chronic obstructive pulmonary disease in cigarette smoke induced mouse model and involves suppression of NF-κB. J Microbiol Biotechnol (2018) 28. doi: 10.4014/jmb.1810.10061
80. Joshi R, Kulkarni YA, Wairkar S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci (2018) 215:43–56. doi: 10.1016/j.lfs.2018.10.066
81. Kong L, Luo C, Li X, Zhou Y, He H. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis (2013) 12(1):115. doi: 10.1186/1476-511X-12-115
82. Li W-H, Cheng X, Yang Y-L, Liu M, Zhang S-S, Wang Y-H, et al. Kaempferol attenuates neuroinflammation and blood brain barrier dysfunction to improve neurological deficits in cerebral ischemia/reperfusion rats. Brain Res (2019) 1722:146361. doi: 10.1016/j.brainres.2019.146361
83. Xiao Y, Liu L, Zheng Y, Liu W, Xu Y. Kaempferol attenuates the effects of XIST/miR-130a/STAT3 on inflammation and extracellular matrix degradation in osteoarthritis. Future Med Chem (2021) 13(17):1451–64. doi: 10.4155/fmc-2021-0127
84. Jin S, Zhang L, Wang L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. Biomed Pharmacother (2023) 165:115215. doi: 10.1016/j.biopha.2023.115215
85. Li C, Li X, Choi JS. Enhanced bioavailability of etoposide after oral or intravenous administration of etoposide with kaempferol in rats. Arch Pharm Res (2009) 32(1):133–8. doi: 10.1007/s12272-009-1127-z
86. Qiu S, Sun G, Zhang Y, Li X, Wang R. Involvement of the NF-κB signaling pathway in the renoprotective effects of isorhamnetin in a type 2 diabetic rat model. BioMed Rep (2016) 4(5):628–34. doi: 10.3892/br.2016.636
87. Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, et al. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem (2014) 25(9):923–33. doi: 10.1016/j.jnutbio.2014.04.006
88. Kim SY, Jin CY, Kim CH, Yoo YH, Choi SH, Kim GY, et al. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-κB, blocking the TLR4 pathway and reducing ROS generation. Int J Mol Med (2019) 43(2):682–92. doi: 10.3892/ijmm.2018.3993
89. Ren X, Han L, Li Y, Zhao H, Zhang Z, Zhuang Y, et al. Isorhamnetin attenuates TNF-α-induced inflammation, proliferation, and migration in human bronchial epithelial cells via MAPK and NF-κB pathways. Anatom Rec (2021) 304(4):901–13. doi: 10.1002/ar.24506
90. Wang X, Zhong W. Isorhamnetin attenuates collagen-induced arthritis via modulating cytokines and oxidative stress in mice. Int J Clin Exp Med (2015) 8(9):16536–42.
91. Zhang B, Zeng M, Li B, Kan Y, Wang S, Cao B, et al. Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine (2021) 82:153466. doi: 10.1016/j.phymed.2021.153466
92. Nalban N, Sangaraju R, Alavala S, Mir SM, Jerald MK, Sistla R. Arbutin attenuates isoproterenol-induced cardiac hypertrophy by inhibiting TLR-4/NF-κB pathway in mice. Cardiovasc Toxicol (2020) 20(3):235–48. doi: 10.1007/s12012-019-09548-3
93. Wang L, Feng Y, Wang J, Luo T, Wang X, Wu M, et al. Arbutin ameliorates murine colitis by inhibiting JAK2 signaling pathway. Front Pharmacol (2021) 12:683818. doi: 10.3389/fphar.2021.683818
94. Ma C, Zhang D, Ma Q, Liu Y, Yang Y. Arbutin inhibits inflammation and apoptosis by enhancing autophagy via SIRT1. Adv Clin Exp Med (2021) 30(5):535–44. doi: 10.17219/acem/133493
95. Lee H-J, Kim K-W. Anti-inflammatory effects of arbutin in lipopolysaccharide-stimulated BV2 microglial cells. Inflammation Res (2012) 61(8):817–25. doi: 10.1007/s00011-012-0474-2
96. Jin J, Liu Y, Jiang C, Shen Y, Chu G, Liu C, et al. Arbutin-modified microspheres prevent osteoarthritis progression by mobilizing local anti-inflammatory and antioxidant responses. Mater Today Bio (2022) 16:100370. doi: 10.1016/j.mtbio.2022.100370
97. Hwang SJ, Kim Y-W, Park Y, Lee H-J, Kim K-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res (2014) 63(1):81–90. doi: 10.1007/s00011-013-0674-4
98. Shi H, Dong L, Jiang J, Zhao J, Zhao G, Dang X, et al. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology (2013) 303:107–14. doi: 10.1016/j.tox.2012.10.025
99. Zhou X, Zhang B, Zhao X, Lin Y, Wang J, Wang X, et al. Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct (2021) 12(12):5637–49. doi: 10.1039/D0FO03199B
100. Bagdas D, Gul Z, Meade JA, Cam B, Cinkilic N, Gurun MS. Pharmacologic overview of chlorogenic acid and its metabolites in chronic pain and inflammation. Curr Neuropharmacol (2020) 18(3):216–28. doi: 10.2174/1570159X17666191021111809
101. Li L, Su C, Chen X, Wang Q, Jiao W, Luo H, et al. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J Agric Food Chem (2020) 68(24):6464–84. doi: 10.1021/acs.jafc.0c01554
102. Chen X, Wei Z, Zhu L, Yuan X, Wei D, Peng W, et al. Efficient approach for the extraction and identification of red pigment from Zanthoxylum bungeanum Maxim and its antioxidant activity. Molecules (2018) 23(5):1109. doi: 10.3390/molecules23051109
103. Zheng T, Han J, Su K-x, Sun B-y, Liu S-m. Regulation mechanisms of flavonoids biosynthesis of Hancheng Dahongpao peels (Zanthoxylum bungeanum Maxim) at different development stages by integrated metabolomics and transcriptomics analysis. BMC Plant Biol (2022) 22(1):251. doi: 10.1186/s12870-022-03642-5
104. Zhang J, Yu L, Han N, Wang D. Characterization of phenolic chemotypes, anatomy, and histochemistry of Zanthoxylum bungeanum Maxim. Ind Crops Prod (2023) 193:116149. doi: 10.1016/j.indcrop.2022.116149
105. Peng Z, Hu X, Li X, Jiang X, Deng L, Hu Y, et al. Protective effects of cyanidin-3-O-glucoside on UVB-induced chronic skin photodamage in mice via alleviating oxidative damage and anti-inflammation. Food Front (2020) 1(3):213–23. doi: 10.1002/fft2.26
106. Yan X, Wu L, Li B, Meng X, Dai H, Zheng Y, et al. Cyanidin-3-O-glucoside attenuates acute lung injury in sepsis rats. J Surg Res (2015) 199(2):592–600. doi: 10.1016/j.jss.2015.06.013
107. Kaewmool C, Udomruk S, Phitak T, Pothacharoen P, Kongtawelert P. Cyanidin-3-O-glucoside protects PC12 cells against neuronal apoptosis mediated by LPS-stimulated BV2 microglial activation. Neurotoxic Res (2020) 37(1):111–25. doi: 10.1007/s12640-019-00102-1
108. Gan Y, Fu Y, Yang L, Chen J, Lei H, Liu Q. Cyanidin-3-O-glucoside and cyanidin protect against intestinal barrier damage and 2,4,6-trinitrobenzenesulfonic acid-induced colitis. J Med Food (2020) 23(1):90–9. doi: 10.1089/jmf.2019.4524
109. Hao RL, Shan SH, Yang DD, Zhang HM, Sun Y, Li ZY. Peonidin-3-O-glucoside from purple corncob ameliorates nonalcoholic fatty liver disease by regulating mitochondrial and lysosome functions to reduce oxidative stress and inflammation. Nutrients (2023) 15(2):372. doi: 10.3390/nu15020372
110. Lee HY, Kim JS. Cherry fruit anthocyanins cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside protect against blue light-induced cytotoxicity in HaCaT cells. Appl Biol Chem (2023) 66(1):3. doi: 10.1186/s13765-023-00767-5
111. Jang S-A, Park DW, Kwon JE, Song HS, Park B, Jeon H, et al. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed Pharmacother (2017) 96:563–71. doi: 10.1016/j.biopha.2017.10.021
112. Shen P, Peng Y, Zhou X, Jiang X, Raj R, Ge H, et al. A comprehensive spectral and in silico analysis on the interactions between quercetin, isoquercitrin, rutin and HMGB1. LWT (2022) 169:113983. doi: 10.1016/j.lwt.2022.113983
113. Gong P, Wang D, Cui D, Yang Q, Wang P, Yang W, et al. Anti-aging function and molecular mechanism of Radix Astragali and Radix Astragali preparata via network pharmacology and PI3K/Akt signaling pathway. Phytomedicine (2021) 84:153509. doi: 10.1016/j.phymed.2021.153509
114. Shen P, Sun Y, Jiang X, Zhou X, Nian B, Wang W, et al. Interaction of bioactive kaempferol with HMGB1: Investigation by multi-spectroscopic and molecular simulation methods. Spectrochimica Acta Part A: Mol Biomol Spectrosc (2023) 292:122360. doi: 10.1016/j.saa.2023.122360
115. Wang S, Shi X, Li J, Huang Q, Ji Q, Yao Y, et al. A small molecule selected from a DNA-encoded library of natural products that binds to TNF-α and attenuates inflammation in vivo. Adv Sci (2022) 9(21):2201258. doi: 10.1002/advs.202201258
116. Wang S, Liu Y, Wang Q, Xu X, Huang T, Dong P, et al. Utilizing Network Pharmacology and Molecular Docking Integrated Surface Plasmon Resonance Technology to Investigate the Potential Targets and Mechanisms of Tripterygium wilfordii against Pulmonary Artery Hypertension. Evid Based Complement Alternat Med (2022) 2022:9862733. doi: 10.1155/2022/9862733
117. Cortes JR, Perez-G M, Rivas MD, Zamorano J. Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK31. J Immunol (2007) 179(6):3881–7. doi: 10.4049/jimmunol.179.6.3881
118. Han MK, Barreto TA, Martinez FJ, Comstock AT, Sajjan US. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir Res (2020) 7(1):e000392. doi: 10.1136/bmjresp-2018-000392
119. Stopa JD, Neuberg D, Puligandla M, Furie B, Flaumenhaft R, Zwicker JI. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight (2017) 2(1):e89373. doi: 10.1172/jci.insight.89373
120. Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (Pre)Hypertensive adults: A randomized double-blind, placebo-controlled, crossover trial. J Nutr (2015) 145(7):1459–63. doi: 10.3945/jn.115.211888
121. Dower JI, Geleijnse JM, Gijsbers L, Zock PL, Kromhout D, Hollman PC. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am J Clin Nutr (2015) 101(5):914–21. doi: 10.3945/ajcn.114.098590
122. Van den Eynde MDG, Geleijnse JM, Scheijen J, Hanssen NMJ, Dower JI, Afman LA, et al. Quercetin, but not epicatechin, decreases plasma concentrations of methylglyoxal in adults in a randomized, double-blind, placebo-controlled, crossover trial with pure flavonoids. J Nutr (2018) 148(12):1911–6. doi: 10.1093/jn/nxy236
123. Mansour A, Mohajeri-Tehrani MR, Karimi S, Sanginabadi M, Poustchi H, Enayati S, et al. Short term effects of coffee components consumption on gut microbiota in patients with non-alcoholic fatty liver and diabetes: A pilot randomized placebo-controlled, clinical trial. Excli J (2020) 19:241–50. doi: 10.17179/excli2019-2021
124. Galopin CC, Furrer SM, Goeke A. Pungent and Tingling Compounds in Asian Cuisine. In: Challenges in Taste Chemistry and Biology. United States: American Chemical Society (2003). p. 139–52.
125. Wei X, Yang B, Chen X, Wen L, Kan J. Zanthoxylum alkylamides ameliorate protein metabolism in type 2 diabetes mellitus rats by regulating multiple signaling pathways. Food Funct (2021) 12(8):3740–53. doi: 10.1039/D0FO02695F
126. Li R-L, Zhang Q, Liu J, Sun J-y, He L-Y, Duan H-X, et al. Hydroxy-α-sanshool possesses protective potentials on H2O2-stimulated PC12 cells by suppression of oxidative stress-induced apoptosis through regulation of PI3K/akt signal pathway. Oxid Med Cell Longevity (2020) 2020:3481758. doi: 10.1155/2020/3481758
127. Liu Y, Meng X, Sun L, Pei K, Chen L, Zhang S, et al. Protective effects of hydroxy-α-sanshool from the pericarp of Zanthoxylum bungeanum Maxim. On D-galactose/AlCl3-induced Alzheimer’s disease-like mice via Nrf2/HO-1 signaling pathways. Eur J Pharmacol (2022) 914:174691. doi: 10.1016/j.ejphar.2021.174691
128. Kaneko A, Kono T, Miura N, Tsuchiya N, Yamamoto M. Preventive effect of TU-100 on a type-2 model of colitis in mice: possible involvement of enhancing adrenomedullin in intestinal epithelial cells. Gastroenterol Res Pract (2013) 2013:384057. doi: 10.1155/2013/384057
129. Lei L, Yang Y. Study on the ameliorating effect of zanthoxylin on pain and and the influence on inflammatory mediators. World Clin Drug (2023) 44(01):32–8. doi: 10.13683/j.wph.2023.01.006
130. Xia L, You J, Li G, Sun Z, Suo Y. Compositional and antioxidant activity analysis of Zanthoxylum bungeanum seed oil obtained by supercritical CO2 fluid extraction. J Am Oil Chemists’ Soc (2011) 88(1):23–32. doi: 10.1007/s11746-010-1644-4
131. Hou J, Wang J, Meng J, Zhang X, Niu Y, Gao J, et al. Zanthoxylum bungeanum seed oil attenuates LPS-induced BEAS-2B cell activation and inflammation by inhibiting the TLR4/myD88/NF-κB signaling pathway. Evidence-Based Complement Altern Med (2021) 2021:2073296. doi: 10.1155/2021/2073296
132. Tang W, Xie Q, Guan J, Jin S, Zhao Y. Phytochemical profiles and biological activity evaluation of Zanthoxylum bungeanum Maxim seed against asthma in murine models. J Ethnopharmacol (2014) 152(3):444–50. doi: 10.1016/j.jep.2014.01.013
133. Wang JQ, Li XW, Liu M, Wang SC, Cao ZF. Inhibitory effect of Zanthoxylum bungeanum seed oil on ovalbumin−induced lung inflammation in a murine model of asthma. Mol Med Rep (2016) 13(5):4289–302. doi: 10.3892/mmr.2016.5050
134. Li XQ, Kang R, Huo JC, Xie YH, Wang SW, Cao W. Wound-healing activity of Zanthoxylum bungeanum Maxim seed oil on experimentally burned rats. Pharmacogn Mag (2017) 13(51):363–71. doi: 10.4103/pm.pm_211_16
135. Moujun XTL, Zhongqiang HYG. Regulatory effect of Zanthoxylum bungeanum seed oil on wound healing and serum inflammatory factors in rats with burn injury. China J Clin Pharmacol (2020) 36(13):1821–4. doi: 10.13699/j.cnki.1001-6821.2020.13.013
136. Deng Y, Li W, Zhang Y, Li J, He F, Dong K, et al. α-linolenic acid inhibits RANKL-induced osteoclastogenesis in vitro and prevents inflammation in vivo. Foods (2023) 12(3):682. doi: 10.3390/foods12030682
137. Yang H, Xin PJ, Guo X, Zengfang H. The rapeutic effects of solution with essential oil Zanthoxylum bungeanum Maxim on psoriasis-like pathological changes of Guinea pig. China Trop Med (2012) 12(05):545–7. doi: 10.13604/j.cnki.46-1064/r.2012.05.045
138. Qinyue Z. Essential oil of zanthoxylum bungeanum maxim prevents ultraviolet irradiation-induced cutaneous photoaging in mice. [Master’s Thesis]. Southwest Jiaotong University (2020).
139. Chun Y, Yao W, Fang-ting H, Tian-li Z, Yang L, Xiao-fang P. Inhibitory effect of Zanthoxylum bungeanum essential oil on cell inflammation caused by P. acnes. Modern Prev Med (2019) 46(14):2617–21. doi: 10.1039/C6FO01739H
140. Zhang Z, Shen P, Liu J, Gu C, Lu X, Li Y, et al. In vivo study of the efficacy of the essential oil of Zanthoxylum bungeanum pericarp in dextran sulfate sodium-induced murine experimental colitis. J Agric Food Chem (2017) 65(16):3311–9. doi: 10.1021/acs.jafc.7b01323
141. Zhang H, Guo ZQ, Wang X, Xian J, Zou L, Zheng C, et al. Protective mechanisms of Zanthoxylum bungeanum essential oil on DSS-induced ulcerative colitis in mice based on a colonic mucosal transcriptomic approach. Food Funct (2022) 13(18):9324–39. doi: 10.1039/d1fo04323d
142. Hong L, Jing W, Qing W, Anxiang S, Mei X, Qin L, et al. Inhibitory effect of Zanthoxylum bungeanum essential oil (ZBEO) on Escherichia coli and intestinal dysfunction. Food Funct (2017) 8(4):1569–76. doi: 10.1039/C6FO01739H
143. Tingru W, Hao W, Jie Z, Chuan Z, Fengming Y. Effect of atomization inhalation of huajiao(Zanthoxylum bungeanum) essential oil on inflammatory and cancer transformation in CAC mice and its mechanism. Chin Arch OF TRADITION Chin Med (2022) 40(10):77–81 + 264-265. doi: 10.13193/j.issn.1673-7717.2022.10.017
Keywords: Zanthoxylum bungeanum Maxim., inflammation, polyphenols, inflammatory disease, NF-κB
Citation: Qi J, Pan Z, Wang X, Zhang N, He G and Jiang X (2024) Research advances of Zanthoxylum bungeanum Maxim. polyphenols in inflammatory diseases. Front. Immunol. 15:1305886. doi: 10.3389/fimmu.2024.1305886
Received: 02 October 2023; Accepted: 08 January 2024;
Published: 26 January 2024.
Edited by:
Danila Cianciosi, Università Politecnica delle Marche, ItalyReviewed by:
Md Tajmul, National Institute of Diabetes and Digestive and Kidney Diseases (NIH), United StatesLei Zhang, University of Waterloo, Canada
Copyright © 2024 Qi, Pan, Wang, Zhang, He and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian Jiang, amlhbmd4aWFuQHNjdS5lZHUuY24=; Gu He, aGVndUBzY3UuZWR1LmNu
 Jinxin Qi
Jinxin Qi Zhaoping Pan1,2
Zhaoping Pan1,2 Xiaoyun Wang
Xiaoyun Wang Gu He
Gu He Xian Jiang
Xian Jiang