- Clinical Laboratory, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, The Fifth School of Clinical Medicine of Zhejiang Chinese Medical University, Huzhou, Zhejiang, China
Background: It is unclear whether the systemic inflammation response index (SIRI) can predict the prognosis of patients with hepatocellular carcinoma (HCC). Consequently, the present study focused on systematically identifying the relationship between SIRI and the prognosis of patients with HCC through a meta-analysis.
Methods: Systematic and comprehensive studies were retrieved from PubMed, Web of Science, Embase, and the Cochrane Library from their inception to August 10, 2023. The role of SIRI in predicting overall survival (OS) and progression-free survival (PFS) in HCC was determined using pooled hazard ratios (HRs) and 95% confidence intervals (CIs). Odds ratios (ORs) and 95% CIs were pooled to analyze the correlations between SIRI and the clinicopathological features of HCC.
Results: Ten articles involving 2,439 patients were included. An elevated SIRI was significantly associated with dismal OS (HR=1.75, 95% CI=1.52–2.01, p<0.001) and inferior PFS (HR=1.66, 95% CI=1.34–2.05, p<0.001) in patients with HCC. Additionally, according to the combined results, the increased SIRI was significantly related to multiple tumor numbers (OR=1.42, 95% CI=1.09–1.85, p=0.009) and maximum tumor diameter >5 cm (OR=3.06, 95% CI=1.76–5.30, p<0.001). However, the SIRI did not show any significant relationship with sex, alpha-fetoprotein content, Child-Pugh class, or hepatitis B virus infection.
Conclusion: According to our results, elevated SIRI significantly predicted OS and PFS in patients with HCC. Moreover, the SIRI was significantly associated with tumor aggressiveness.
Systematic review registration: https://inplasy.com/inplasy-2023-9-0003/, identifier INPLASY202390003.
Introduction
Primary liver cancer ranks sixth among cancers in terms of morbidity and is the third most common cause of cancer-associated mortality worldwide (1). As estimated by GLOBCAN, 905,677 new liver cancer cases and 830,180 liver cancer-associated deaths were reported globally in 2020 (1). Hepatocellular carcinoma (HCC), the most frequent subtype of liver cancer, affects approximately 75% of patients worldwide (2). Approximately 72% of the HCC cases are reported in Asia (over 50% in China), and 10%, 7.8%, 5.1%, 4.6%, and 0.5% in Europe, Africa, North America, Latin America, and Oceania, respectively (3). In general, surgery, local thermal ablation, liver transplantation (LT), transcatheter arterial chemoembolization (TACE), and systemic therapy are the main treatments for HCC and have shown efficacy in reducing the mortality rates of HCC (4). Despite this, the long-term survival rates of patients remain unsatisfactory, and recurrence rates are high (5). Among patients with localized or metastatic HCC, the 5-year overall survival (OS) rate is < 10% (6). In addition, up to 70% of patients experience recurrence after undergoing treatment with a curative intent (6). Therefore, the identification of effective prognostic biomarkers is pivotal for risk stratification and adjunctive treatment development in patients with HCC.
Accumulating evidence suggests that immune responses and inflammation influence tumor progression and metastasis (7). Many inflammatory blood-based indices, such as the neutrophil-to-lymphocyte ratio (NLR) (8), platelet-to-lymphocyte ratio (PLR), albumin-to-globulin ratio (AGR) (9), lymphocyte-to-monocyte ratio (LMR) (10), and C-reactive protein-to-albumin ratio (CAR) (11), are significant prognostic markers of different cancer types. The systemic inflammation response index (SIRI) is a novel hematologic parameter that was first proposed in 2016 (12) and is determined using the following formula: SIRI = (neutrophil × monocyte)/lymphocyte count. SIRI has been widely suggested to exhibit a significant and powerful value in predicting solid tumors such as bladder cancer (13), non-small cell lung cancer (NSCLC) (14), gastric cancer (15), breast cancer (16), and ovarian cancer (17). The impact of the SIRI on predicting HCC prognosis has also been explored; however, no consistent findings have been found (18–27). In certain studies, elevated SIRI was found to be a significant prognostic marker of HCC (23–25), while other studies have shown no obvious relationship between SIRI and HCC survival (21, 27). This meta-analysis aimed to accurately identify the prognostic effects of SIRI in patients with HCC. Additionally, the relationship between SIRI and clinicopathological features of HCC was explored.
Materials and methods
Study guideline
The present meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (28). Our meta-analysis protocol was registered in INPLASY (registration number: INPLASY202390003) and can be found at https://inplasy.com/inplasy-2023-9-0003/.
Ethics statement
Data in this study were extracted from publications, and, as a result, ethical approval or patient consent was waived.
Literature search
The PubMed, Web of Science, Embase, and Cochrane Library databases were comprehensively searched from their inception to August 10, 2023, using the following search terms: (systemic inflammation response index or systemic inflammatory response index) and (hepatocellular carcinoma, hepatocellular cancer, HCC, or liver cancer). A detailed search strategy for each database is provided in Supplementary Data Sheet 1. The language used in this study was English. To identify additional eligible articles, we manually searched the reference lists of each retrieved article.
Inclusion and exclusion criteria
Articles satisfying the following criteria were recruited: (1) HCC was diagnosed based on pathology or histology; (2) studies investigating the relationship between SIRI and prognosis of patients with HCC; (3) those with available or calculable hazard ratios (HRs) and 95% confidence intervals (CIs); (4) those mentioning threshold SIRI; (5) those reporting survival outcomes such as OS, disease-free survival (DFS), progression-free survival (PFS), or cancer-specific survival (CSS); and (6) English language articles. The following studies were excluded: (1) meeting abstracts, reviews, comments, letters, and case reports; (2) animal studies; and (3) those including overlapping patients.
Data extraction and quality assessment
The studies were reviewed and data were independently extracted from qualified studies by two reviewers (SZ and ZT). Any dispensaries were resolved through negotiation until a consensus was reached. The following information was collected: first author, publication year, country, study design, sample size, age, sex, study center, study period, Child-Pugh class, treatment, threshold, threshold selection method, survival outcomes, survival analysis type, follow-up, HRs, and 95%CIs for survival outcomes. If eligible studies underwent propensity score matching (PSM) analysis, the data for the entire population were extracted and analyzed to avoid selection bias. OS and PFS were defined as the primary and secondary survival outcomes, respectively. Two researchers (SZ and ZT) evaluated the literature quality using the Newcastle-Ottawa Scale (NOS) (29) and crosschecked our results. The NOS assesses literature quality from three perspectives: selection, comparability, and outcome measurement. The NOS score ranges from 0 to 9, with studies scoring ≥ 6 points considered to be of high quality.
Statistical analysis
The significance of the SIRI in predicting the OS and PFS of patients with HCC was evaluated based on the combined HRs and 95% CI. Interstudy heterogeneity was evaluated using the Higgins I2 statistic and Cochran’s Q test. The random-effects model was applied when I2 was >50% or P was <0.1; otherwise, the fixed-effects model was used. Diverse factor-stratified subgroup analyses were conducted to identify sources of heterogeneity. The relationships between SIRI and the clinicopathological characteristics of HCC were analyzed using combined odds ratios (ORs) and 95% CIs. A sensitivity analysis was used to evaluate the consistency of the findings. Meta-regression was conducted to evaluate the effect of clinicopathological factors on the overall results. Begg’s test, funnel plots, and Egger’s test were used to examine possible publication bias. Stata software (version 12.0; Stata Corp., College Station, TX, USA) was used for graph generation and statistical analyses. Statistical significance was set at P<0.05.
Results
Study selection process
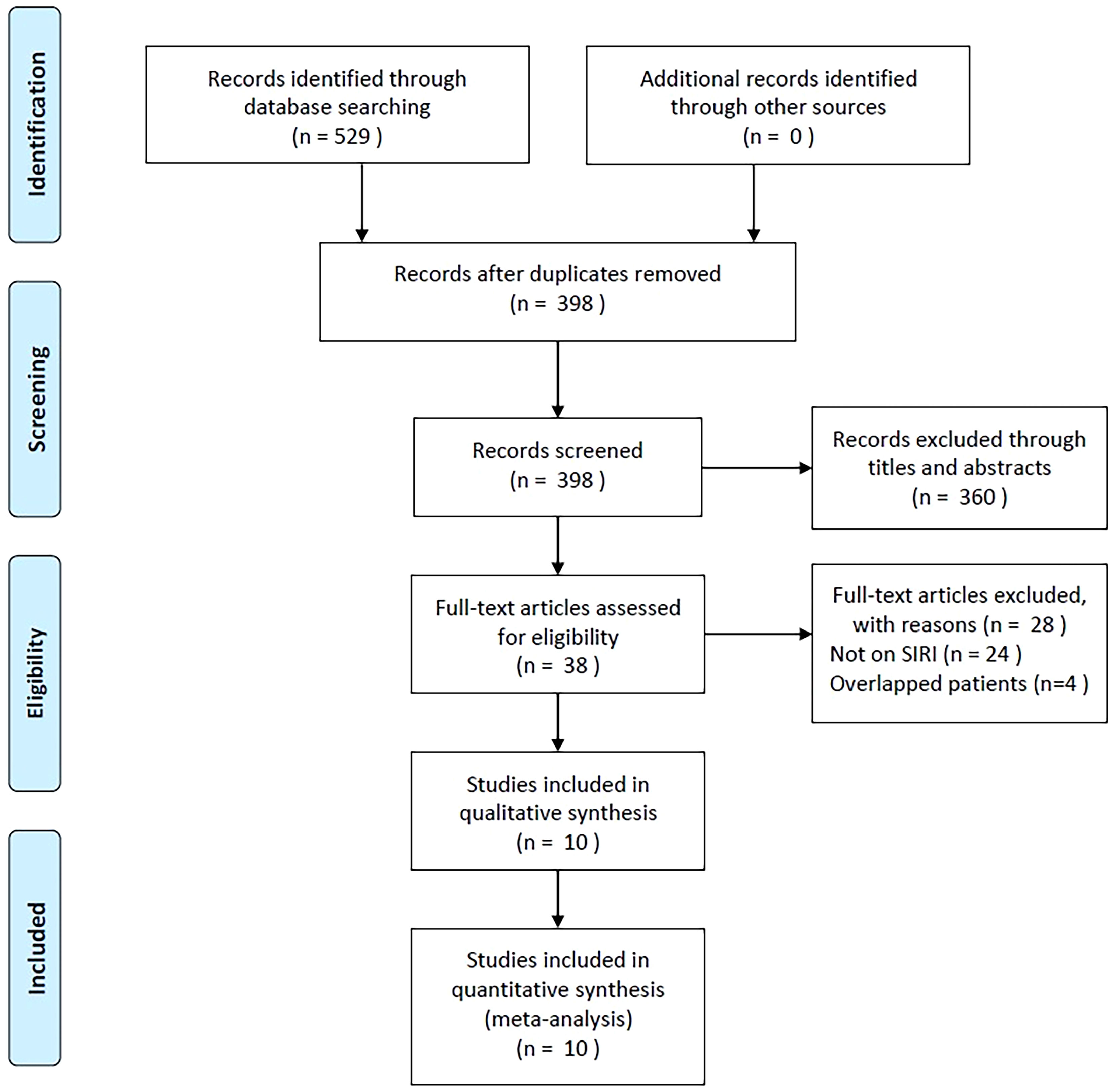
As shown in Figure 1, 529 studies were identified through primary literature retrieval, and 398 records were retained following the removal of duplicates. Subsequently, 360 articles were discarded by title and abstract screening owing to their irrelevance. Then, the full texts of 38 articles were examined, and another 28 were excluded because they did not focus on SIRI (n=24) or recruited overlapping patients (n=4). Finally, the present meta-analysis recruited 10 articles involving 2,439 patients (18–27) (Figure 1; Table 1).
Included study features
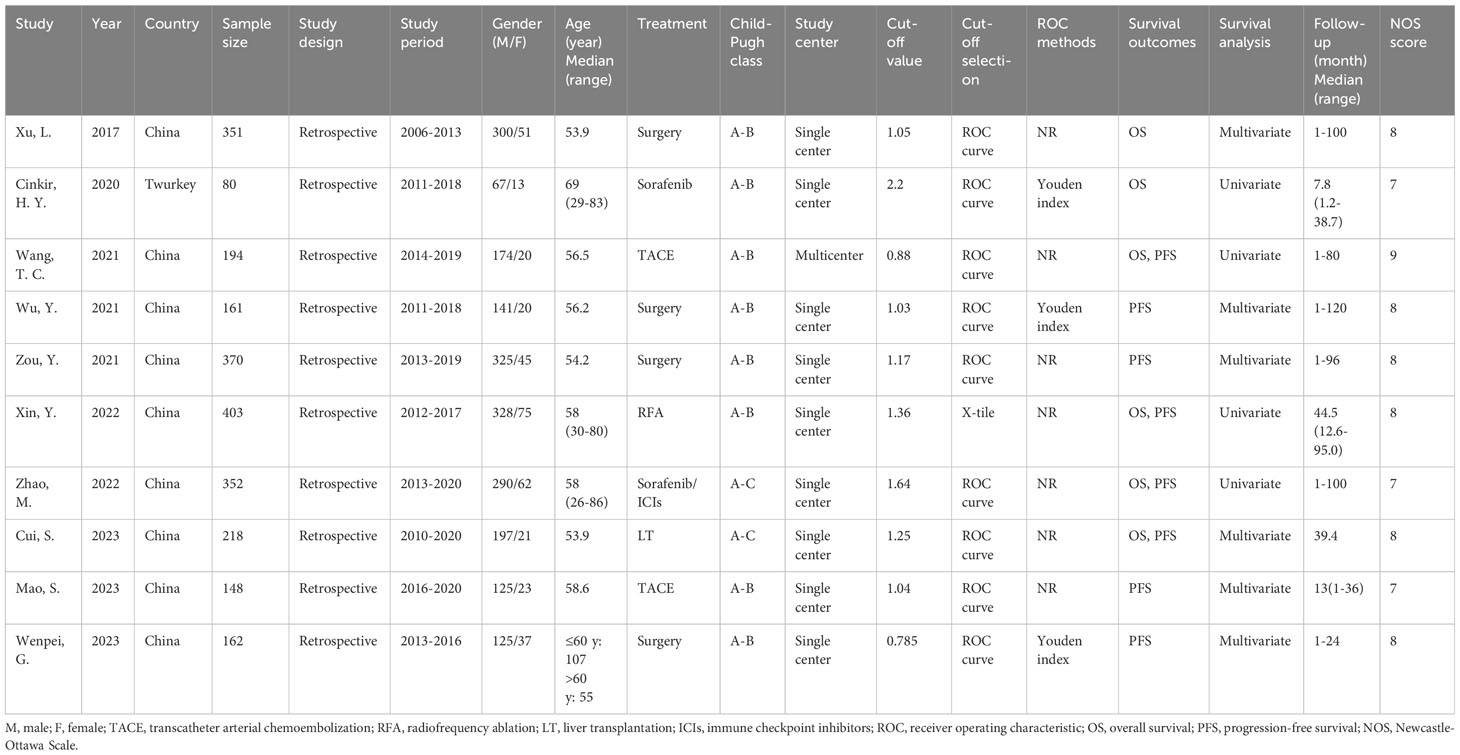
Table 1 presents the baseline characteristics of the selected articles. The publication years of these articles ranged between 2017 and 2023 and all had a retrospective design. Nine studies were conducted in China (18, 20–27) while one was conducted in Turkey (19). The sample size across the selected articles ranged from 80 to 403 (median, 206). We therefore selected 200 for subgroup analysis of sample size. Four studies treated patients with HCC with surgery (18, 21, 22, 27), two studies used TACE treatment (20, 26), and one each used sorafenib (19), radiofrequency ablation (RFA) (23), sorafenib/immune checkpoint inhibitors (ICIs) (24), and LT (25). Nine studies were single-center studies (18, 19, 21–27) and one was a multicenter study (20). The threshold SIRI was 0.785–2.2 (median, 1.11). We, therefore, used 1.1 for subgroup analysis of cut-off value in the following analyses. Nine articles used the receiver operating characteristic (ROC) curve to determine the best threshold (18–22, 24–27) and one study applied the X-tile software (23). Six studies reported the significance of SIRI in the OS prediction of HCC (18–20, 23–25) and eight studies reported the association between SIRI and PFS (20–27). Six studies reported HRs and 95%CIs using multivariate analysis (18, 21, 22, 25–27) and four studies used univariate analysis (19, 20, 23, 24). The NOS scores of all eligible studies were 7–9, suggesting a high quality (Table 1).
SIRI and OS
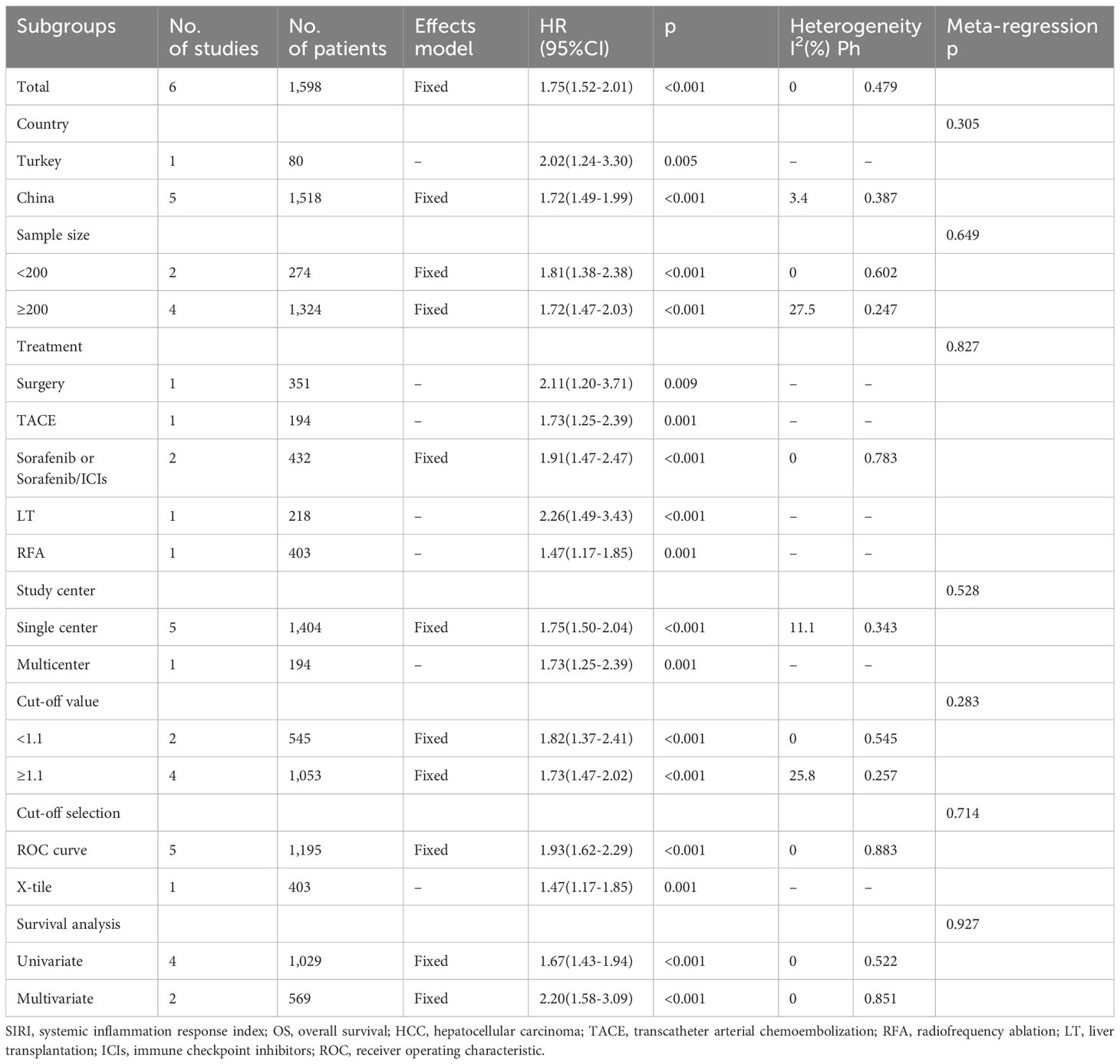
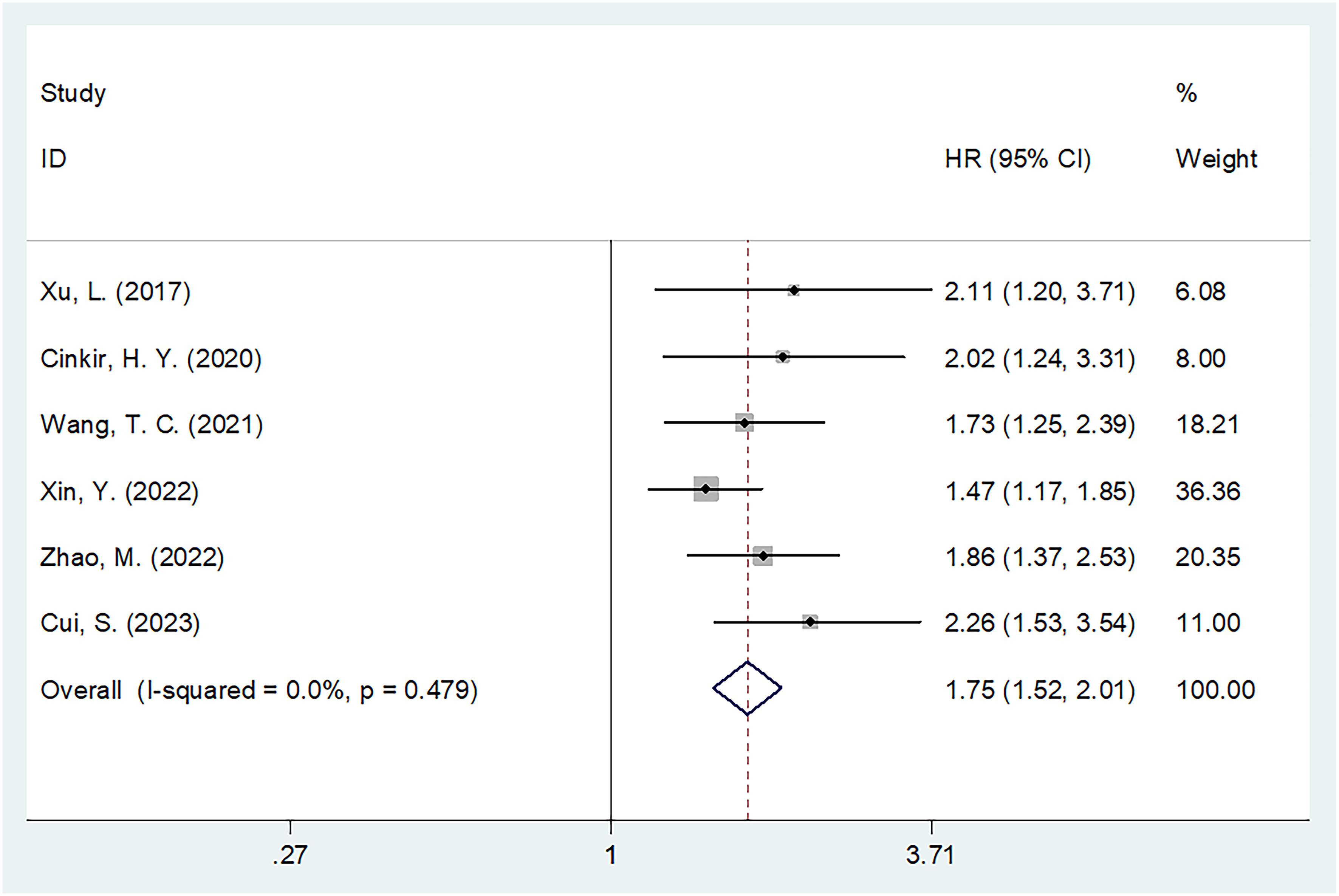
Six studies involving 1,598 patients (18–20, 23–25) provided data on the significance of SIRI in predicting OS in HCC. There was no obvious heterogeneity (I2 = 0, P=0.479); therefore, we adopted a fixed-effects model. As shown in Table 2 and Figure 2, the HR was 1.75 (95% CI=1.52–2.01, p<0.001), suggesting a relationship between elevated SIRI and dismal OS in patients with HCC. Subgroup analysis revealed that the predictive role of SIRI in OS remained consistent across various factors including country, treatment, sample size, study center, threshold, threshold selection method, or survival analysis type (Table 2).
SIRI and PFS
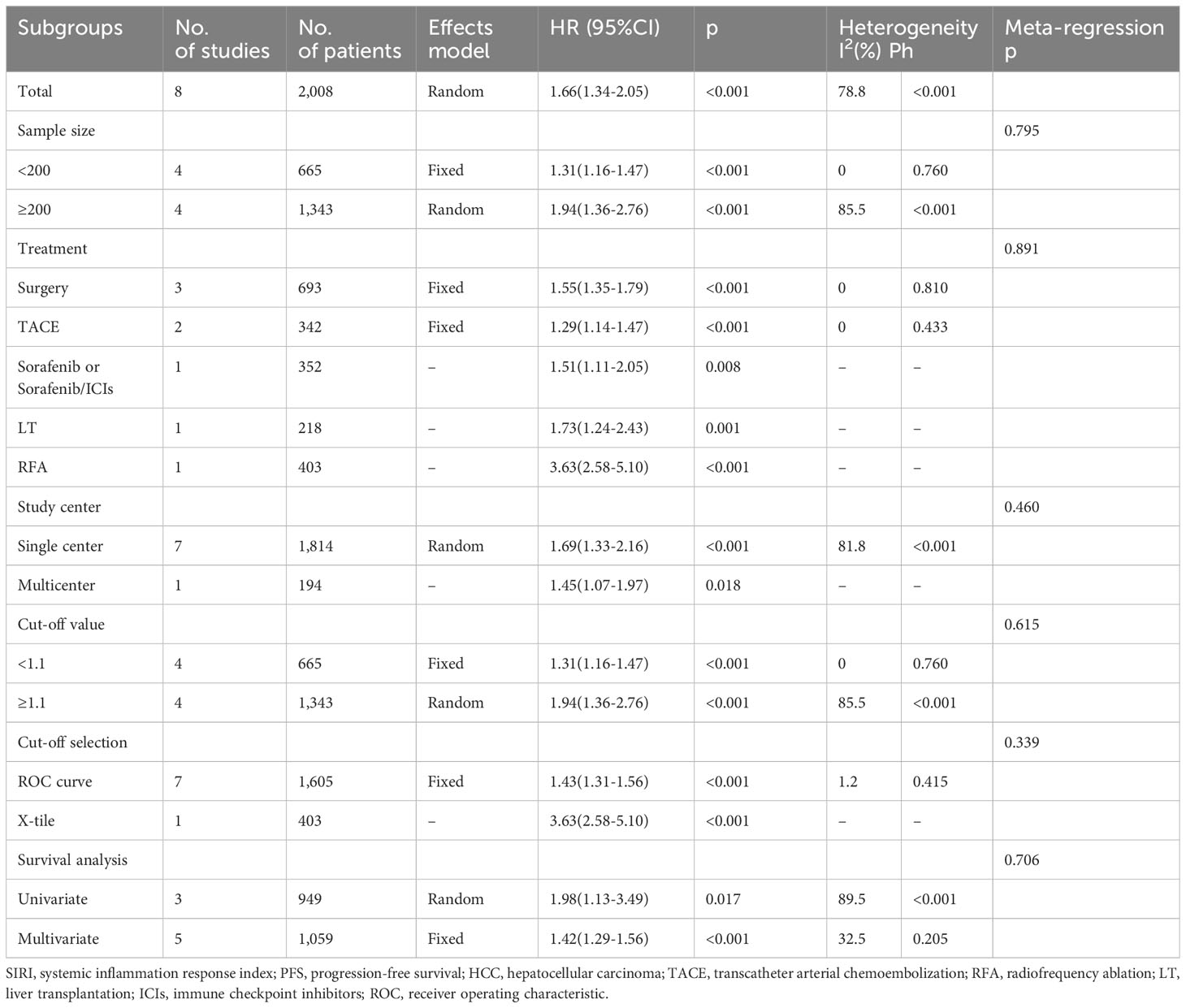
Altogether, eight articles comprising 2,008 patients (20–27) reported a correlation between SIRI and PFS in patients with HCC. Because of significant heterogeneity, we employed the random-effects model (I2 = 78.8%, P<0.001; Table 3 and Figure 3). Based on the pooled results, an increased SIRI significantly predicted inferior PFS in HCC (HR=1.66, 95% CI=1.34–2.05, p<0.001; Figure 3; Table 3). Subgroup analysis indicated that elevated SIRI still significantly predicted PFS in HCC, which was unaffected by country, treatment, sample size, study center, threshold, threshold selection method, or survival analysis type (Table 3).
The relationship of SIRI with clinicopathological characteristics of HCC
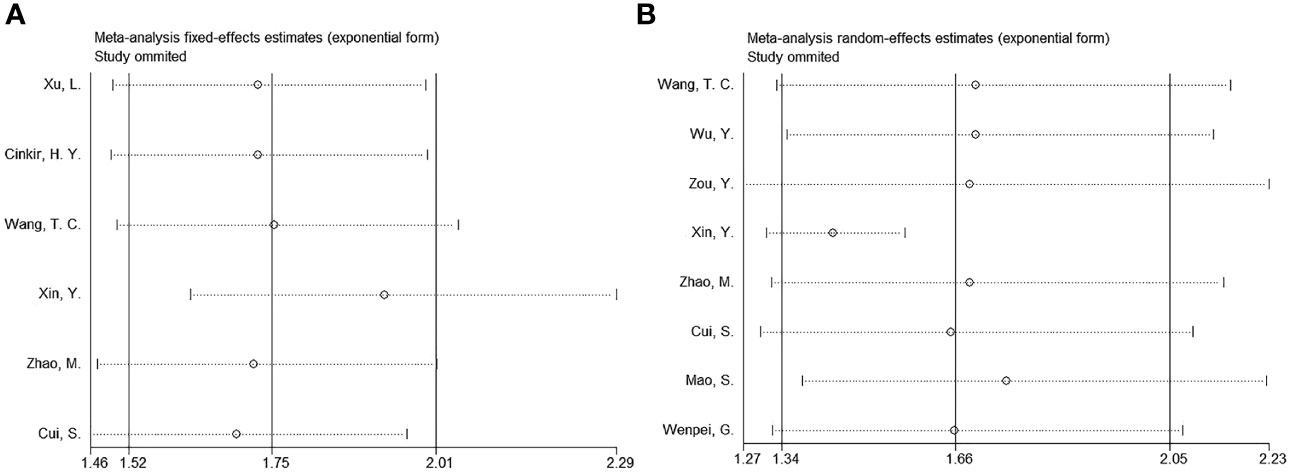
Four studies involving 1,111 patients investigated the relationship between the SIRI and the clinicopathological characteristics of HCC (20, 23, 24, 27). According to Figure 4 and Table 4, our combined results suggested a significant relationship between increased SIRI and multiple tumor numbers (OR=1.42, 95% CI=1.09–1.85, p=0.009) and maximum tumor diameter >5 cm (OR=3.06, 95% CI=1.76–5.30, p<0.001). Nonetheless, SIRI was not significantly related to sex (OR=1.10, 95% CI=0.88–1.51, p=0.559), Child-Pugh class (OR=1.46, 95% CI=0.52–4.11, p=0.476), alpha-fetoprotein (AFP) level (OR=1.02, 95% CI=0.80–1.30, p=0.880), or hepatitis B virus infection (OR=1.12, 95% CI=0.78–1.61, p=0.550) (Figure 4; Table 4).
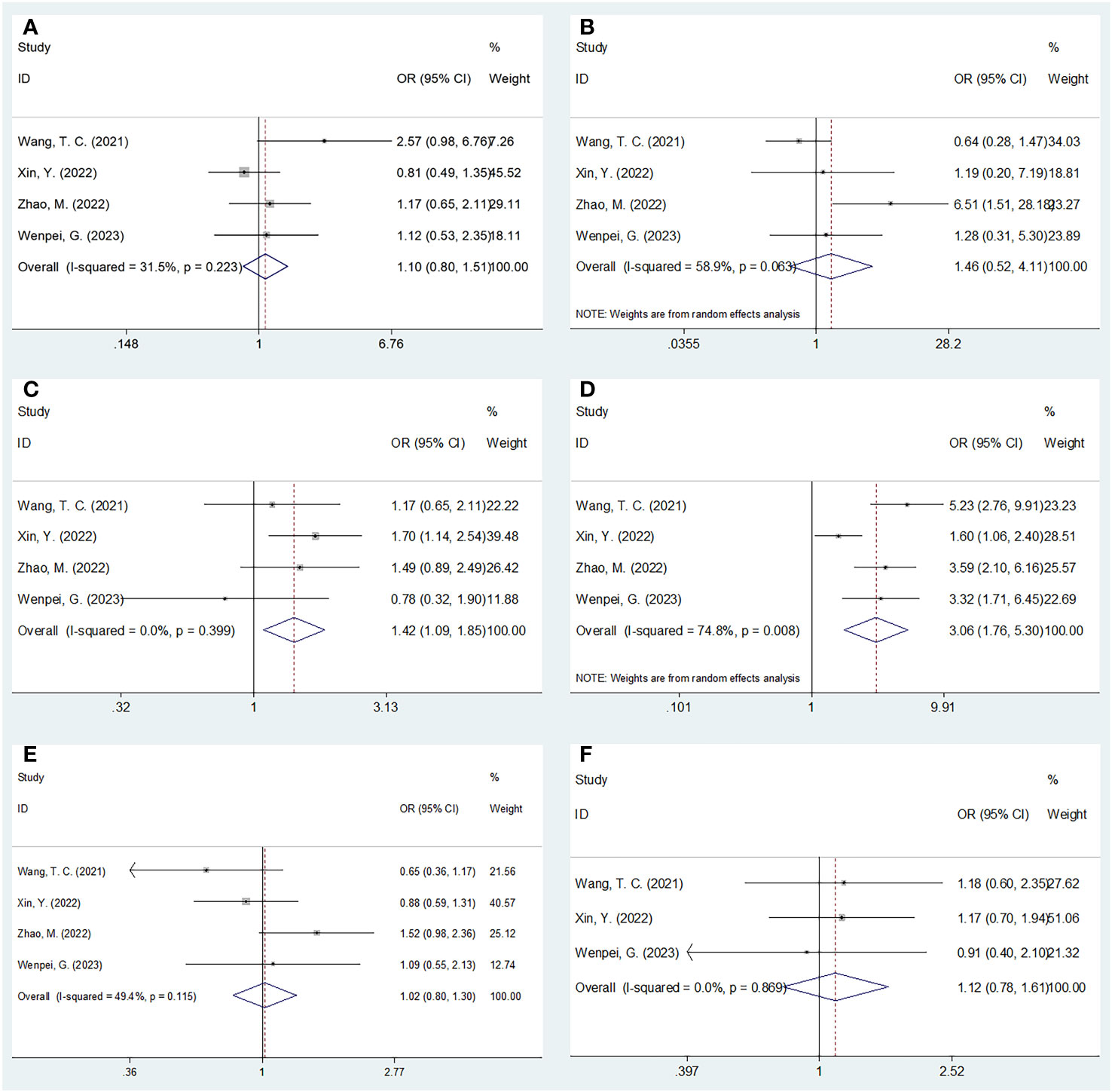
Figure 4 Forest plots of the associations between SIRI and clinicopathological factors in HCC. (A) Gender (male vs female); (B) Child-Pugh class (B-C vs A); (C) Tumor number (multiple vs solitary); (D) Maximum tumor diameter (>5 cm vs ≤5 cm); (E) AFP (ng/ml) (≥400 vs <400); and (F) HBV (+) (yes vs no).
Sensitivity analysis and meta-regression
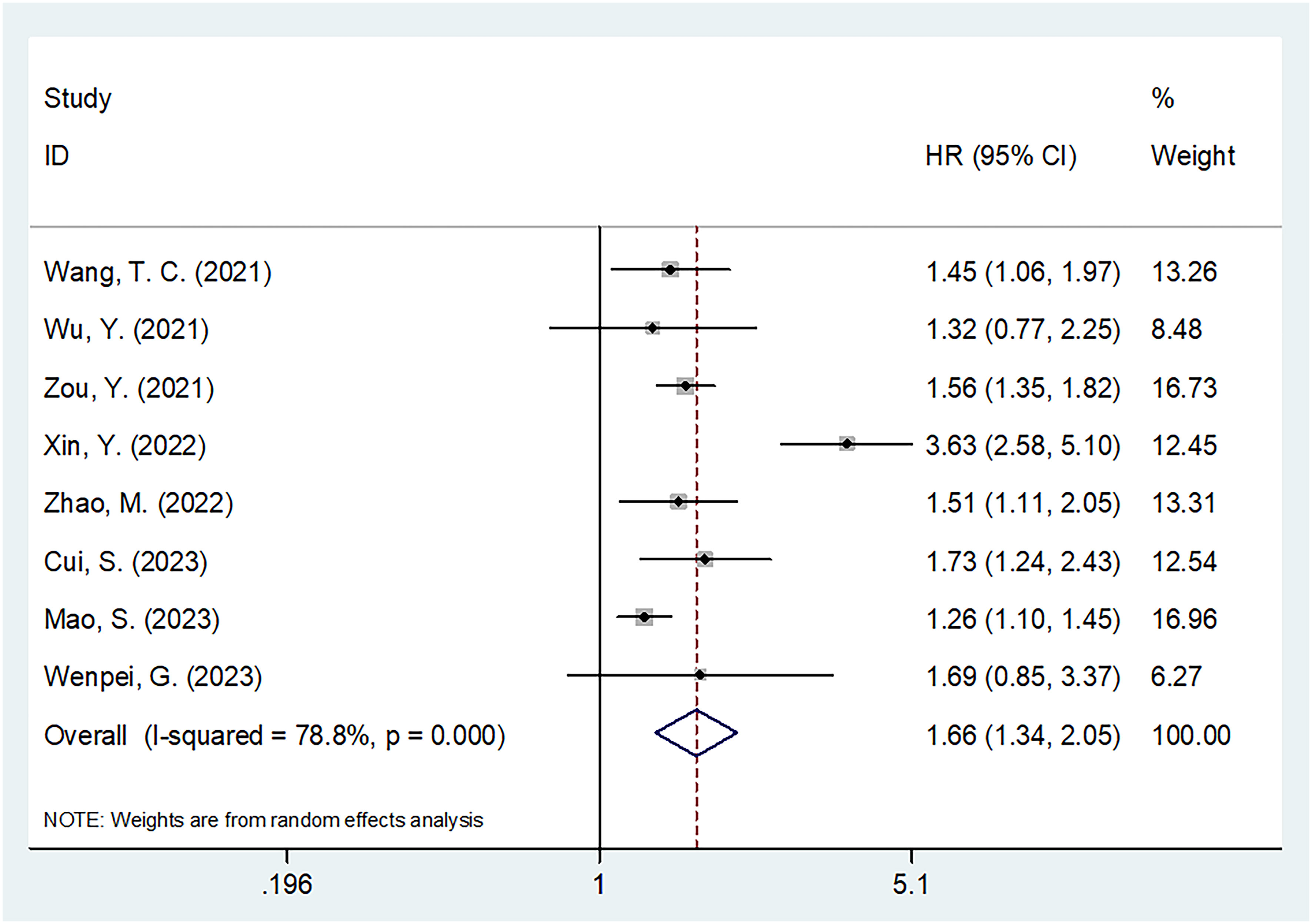
By removing studies study-by-study, sensitivity analysis revealed that none of the studies had an effect on OS or PFS, suggesting that all results remained consistent (Figure 5). Meta-regression showed that none of the factors significantly influenced the overall results of OS and PFS (Tables 2, 3).
Publication bias
Begg’s test, funnel plots, and Egger’s test were used to assess potential publication bias. The funnel plot did not exhibit any significant asymmetry in the OS or PFS (Figure 6). Moreover, the results indicated no significant publication bias for OS (Begg’s p=0.133; Egger’s p=0.358) or PFS (Begg’s p=0.536; Egger’s p=0.302).

Figure 6 Publication test by Begg’s test and Egger’s test. (A) Begg’s test for OS, p=0.133; (B) Egger’s test for OS, p=0.358; (C) Begg’s test for PFS, p=0.536; and (D) Egger’s test for PFS, p=0.302.
Discussion
The prognostic significance of SIRI in patients with HCC remains inconsistent. This meta-analysis collected data from 10 articles involving 2,439 patients. According to our results, elevated SIRI levels were markedly associated with shortened OS and inferior PFS in patients with HCC. Additionally, a high SIRI was significantly correlated with multiple tumor numbers and tumor size >5 cm in HCC. Publication bias tests and subgroup analyses were conducted to verify the reliability of the findings. Collectively, SIRI serves as a promising and cost-effective factor for predicting the short- and long-term prognoses of patients with HCC. To the best of our knowledge, this is the first meta-analysis to explore the potential of SIRI in predicting the prognosis of patients with HCC.
SIRI was calculated using the following formula: SIRI= neutrophil count × monocyte count/lymphocyte count. Therefore, a high SIRI could be the result of increased neutrophils, increased monocytes, and/or decreased lymphocytes. Currently, the accurate predictive mechanism of SIRI in HCC prognosis remains largely unclear and can be interpreted as follows: First, neutrophils recruited to a tumor site can produce inflammatory factors, such as interleukin-1 (IL-1) and IL-6, promoting the growth and metastasis of tumor cells (7). Activated neutrophils can suppress T cell proliferation and cytotoxicity by binding PD-L1 on the neutrophil surface to PD-1 on the T cell surface (30). This may promote tumor immune evasion and malignant growth, ultimately resulting in a shorter lifespan in cancer patients (31). Second, monocytes may differentiate into tumor-associated macrophages (TAMs) (32). TAMs secrete several inflammatory factors that affect the tumor microenvironment, thereby promoting tumor occurrence, metastasis, and relapse (33). Many studies have demonstrated the presence of TAM infiltration in the HCC matrix, accelerating tumor angiogenesis, growth, metastasis, and immunosuppression (34, 35). Third, lymphocytes play a critical role in cellular anti-tumor responses. Tumor-infiltrating lymphocytes (TILs) have a pivotal impact on the anticancer immune microenvironment and are involved in multiple stages of tumor progression (36). The presence of lymphocyte infiltration in tumor tissues is associated with improved therapeutic outcomes. However, when the number of lymphocytes in the tumor microenvironment decreases, anti-tumor ability decreases, resulting in immune tolerance and tumor escape (37). Taken together, a high SIRI may significantly predict the prognosis of patients with HCC.
Notably, the degree of liver fibrosis may significantly affect SIRI. As these data were rarely evaluated in the included papers, they were not analyzed. However, the development of HCC in cirrhotic and non-cirrhotic livers differs significantly. Future studies are needed to investigate the prognostic value of SIRI for HCC in both cirrhotic and non-cirrhotic liver groups. Evaluating liver function is essential for determining the prognosis of HCC although the Child-Pugh score alone is not sufficient to do so. A major prognostic factor for HCC is portal hypertension, which may affect the SIRI; however, this has rarely been examined in the included studies. None of the included studies provided data on portal hypertension. Therefore, we expect future studies to evaluate the prognostic value of the SIRI under different circumstances of portal hypertension. Sarcopenia is another important prognostic factor in patients with solid tumors (38–42). Current evidence shows that sarcopenia is independently associated with a poor prognosis in various cancers (38–42). Previous studies have also indicated that many patients with HCC have sarcopenia (43–45). The SIRI is associated with tumor characteristics but may also be influenced by general health status, particularly sarcopenia. However, the included studies did not present data on sarcopenia. Therefore, we expect that the correlation between SIRI and sarcopenia in HCC can be investigated in future studies.
Recently, meta-analyses have been conducted to determine whether SIRI can be used to predict the prognosis of solid tumors. A meta-analysis conducted by Wang et al. found that the SIRI independently predicted the prognosis and survival status of nasopharyngeal carcinoma based on 3,187 patients (46). Another meta-analysis of 10,754 cases by Zhou et al. showed that a high SIRI was related to shorter OS and DFS/recurrence-free survival/PFS in various cancers (47). In addition, in a meta-analysis of 14 studies, Wei et al. demonstrated that the SIRI is a useful factor for predicting dismal prognostic outcomes during malignancy treatment (48). The present study identified an obvious relationship between SIRI and survival in HCC, which is in accordance with findings in other cancer types.
This study has certain limitations. First, the articles included were from Asian countries, particularly China. Although only articles in the English language were included, the applicability of our results should be noted. Second, the SIRI cut-off values differed, possibly inducing heterogeneity in the present study. Third, the sample size was relatively small. Therefore, large-scale prospective trials using standard SIRI cut-off values should be conducted for further validation.
Conclusions
In conclusion, the present study showed that elevated SIRI levels significantly predicted OS and PFS in patients with HCC. Moreover, the SIRI was significantly associated with tumor aggressiveness. The SIRI could be used as a promising prognostic index for HCC in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SZ: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft. ZT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1291840/full#supplementary-material
SUPPLEMENTARY DATA SHEET 1 | The detailed search strategies of each database in this meta-analysis.
Abbreviations
SIRI, systemic inflammation response index; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; OR, odds ratio; AFP, alpha-fetoprotein; HBV, hepatitis B virus; LT, liver transplantation; TACE, transcatheter arterial chemoembolization; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; CAR, C-reactive protein-to-albumin ratio; AGR, albumin-to-globulin ratio; LMR, lymphocyte-to-monocyte ratio; NSCLC, non-small cell lung cancer; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; DFS, disease-free survival; CSS, cancer-specific survival; NOS, Newcastle-Ottawa Scale; OR,odds ratio; RFA, radiofrequency ablation; ICIs, immune checkpoint inhibitors; TAMs, tumor-associated macrophages; TILs, tumor-infiltrating lymphocytes.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology (2021) 73 Suppl 1:4–13. doi: 10.1002/hep.31288
3. Torimura T, Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int (2022) 42:2042–54. doi: 10.1111/liv.15130
4. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
5. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
6. Sperandio RC, Pestana RC, Miyamura BV, Kaseb AO. Hepatocellular carcinoma immunotherapy. Annu Rev Med (2022) 73:267–78. doi: 10.1146/annurev-med-042220-021121
7. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
8. Gao Y, Zhang Z, Li Y, Chen S, Lu J, Wu L, et al. Pretreatment neutrophil-to-lymphocyte ratio as a prognostic biomarker in unresectable or metastatic esophageal cancer patients with anti-PD-1 therapy. Front Oncol (2022) 12:834564. doi: 10.3389/fonc.2022.834564
9. Taguchi S, Kawai T, Nakagawa T, Nakamura Y, Kamei J, Obinata D, et al. Prognostic significance of the albumin-to-globulin ratio for advanced urothelial carcinoma treated with pembrolizumab: a multicenter retrospective study. Sci Rep (2021) 11:15623. doi: 10.1038/s41598-021-95061-z
10. Taha HF, Kamel LM, Embaby A, Abdelaziz LA. Prognostic significance of lymphocyte-to-monocyte ratio in patients with classical Hodgkin lymphoma before and after receiving first-line chemotherapy. Contemp Oncol (Poznan Poland) (2022) 26:69–77. doi: 10.5114/wo.2022.115459
11. Alkurt EG, Durak D, Turhan VB, Sahiner IT. Effect of C-reactive protein-to-albumin ratio on prognosis in gastric cancer patients. Cureus (2022) 14:e23972. doi: 10.7759/cureus.23972
12. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer (2016) 122:2158–67. doi: 10.1002/cncr.30057
13. Yilmaz H, Cinar NB, Avci IE, Telli E, Uslubas AK, Teke K, et al. The systemic inflammation response index: An independent predictive factor for survival outcomes of bladder cancer stronger than other inflammatory markers. Urol Oncol (2023) 41:256. doi: 10.1016/j.urolonc.2022.11.011
14. Zuo R, Zhu F, Zhang C, Ma J, Chen J, Yue P, et al. The response prediction and prognostic values of systemic inflammation response index in patients with advanced lung adenocarcinoma. Thorac Cancer (2023) 14:1500–11. doi: 10.1111/1759-7714.14893
15. Yazici H, Yegen SC. Is systemic inflammatory response index (SIRI) a reliable tool for prognosis of gastric cancer patients without neoadjuvant therapy? Cureus (2023) 15:e36597. doi: 10.7759/cureus.36597
16. Zhu M, Chen L, Kong X, Wang X, Fang Y, Li X, et al. The systemic inflammation response index as an independent predictor of survival in breast cancer patients: A retrospective study. Front Mol Biosci (2022) 9:856064. doi: 10.3389/fmolb.2022.856064
17. Huang H, Wu K, Chen L, Lin X. Study on the application of systemic inflammation response index and platelet-lymphocyte ratio in ovarian Malignant tumors. Int J Gen Med (2021) 14:10015–22. doi: 10.2147/ijgm.S346610
18. Xu L, Yu S, Zhuang L, Wang P, Shen Y, Lin J, et al. Systemic inflammation response index (SIRI) predicts prognosis in hepatocellular carcinoma patients. Oncotarget (2017) 8:34954–60. doi: 10.18632/oncotarget.16865
19. Cinkir HY, Dogan I. Comparison of inflammatory indexes in patients treated with sorafenib in advanced hepatocellular carcinoma: A single-center observational study. Erciyes Med J (2020) 42:201–6. doi: 10.14744/etd.2020.82246
20. Wang TC, An TZ, Li JX, Pang PF. Systemic inflammation response index is a prognostic risk factor in patients with hepatocellular carcinoma undergoing TACE. Risk Manag Healthc Policy (2021) 14:2589–600. doi: 10.2147/rmhp.S316740
21. Wu Y, Tu C, Shao C. Inflammatory indexes in preoperative blood routine to predict early recurrence of hepatocellular carcinoma after curative hepatectomy. BMC Surg (2021) 21:178. doi: 10.1186/s12893-021-01180-9
22. Zou Y, Chen Z, Lou Q, Han H, Zhang Y, Chen Z, et al. A novel blood index-based model to predict hepatitis B virus-associated hepatocellular carcinoma recurrence after curative hepatectomy: guidance on adjuvant transcatheter arterial chemoembolization choice. Front Oncol (2021) 11:755235. doi: 10.3389/fonc.2021.755235
23. Xin Y, Zhang X, Li Y, Yang Y, Chen Y, Wang Y, et al. A systemic inflammation response index (SIRI)-based nomogram for predicting the recurrence of early stage hepatocellular carcinoma after radiofrequency ablation. Cardiovasc interventional Radiol (2022) 45:43–53. doi: 10.1007/s00270-021-02965-4
24. Zhao M, Duan X, Mi L, Shi J, Li N, Yin X, et al. Prognosis of hepatocellular carcinoma and its association with immune cells using systemic inflammatory response index. Future Oncol (London England) (2022) 18:2269–88. doi: 10.2217/fon-2021-1087
25. Cui S, Cao S, Chen Q, He Q, Lang R. Preoperative systemic inflammatory response index predicts the prognosis of patients with hepatocellular carcinoma after liver transplantation. Front Immunol (2023) 14:1118053. doi: 10.3389/fimmu.2023.1118053
26. Mao S, Shan Y, Yu X, Huang J, Fang J, Wang M, et al. A new prognostic model predicting hepatocellular carcinoma early recurrence in patients with microvascular invasion who received postoperative adjuvant transcatheter arterial chemoembolization. Eur J Surg Oncol (2023) 49:129–36. doi: 10.1016/j.ejso.2022.08.013
27. Wenpei G, Yuan L, Liangbo L, Jingjun M, Bo W, Zhiqiang N, et al. Predictive value of preoperative inflammatory indexes for postoperative early recurrence of hepatitis B-related hepatocellular carcinoma. Front Oncol (2023) 13:1142168. doi: 10.3389/fonc.2023.1142168
28. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
30. Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut (2017) 66:1900–11. doi: 10.1136/gutjnl-2016-313075
31. He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res CR (2015) 34:141. doi: 10.1186/s13046-015-0256-0
32. Cavassani KA, Meza RJ, Habiel DM, Chen JF, Montes A, Tripathi M, et al. Circulating monocytes from prostate cancer patients promote invasion and motility of epithelial cells. Cancer Med (2018) 7:4639–49. doi: 10.1002/cam4.1695
33. Mano Y, Aishima S, Fujita N, Tanaka Y, Kubo Y, Motomura T, et al. Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology J Immunopathol Mol Cell Biol (2013) 80:146–54. doi: 10.1159/000346196
34. Yan C, Yang Q, Gong Z. Tumor-associated neutrophils and macrophages promote gender disparity in hepatocellular carcinoma in zebrafish. Cancer Res (2017) 77:1395–407. doi: 10.1158/0008-5472.Can-16-2200
35. Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, et al. Aging and cancer: The role of macrophages and neutrophils. Ageing Res Rev (2017) 36:105–16. doi: 10.1016/j.arr.2017.03.008
36. Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol (Northwood London England) (2012) 29:1817–26. doi: 10.1007/s12032-011-0006-x
37. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer (2011) 105:93–103. doi: 10.1038/bjc.2011.189
38. Lin WL, Nguyen TH, Lin CY, Wu LM, Huang WT, Guo HR. Association between sarcopenia and survival in patients with gynecologic cancer: A systematic review and meta-analysis. Front Oncol (2022) 12:1037796. doi: 10.3389/fonc.2022.1037796
39. Gan H, Lan J, Bei H, Xu G. The impact of sarcopenia on prognosis of patients with pancreatic cancer: A systematic review and meta-analysis. Scott Med J (2023) 68:133–48. doi: 10.1177/00369330231187655
40. He J, Luo W, Huang Y, Song L, Mei Y. Sarcopenia as a prognostic indicator in colorectal cancer: an updated meta-analysis. Front Oncol (2023) 13:1247341. doi: 10.3389/fonc.2023.1247341
41. Park A, Orlandini MF, Szor DJ, Junior UR, Tustumi F. The impact of sarcopenia on esophagectomy for cancer: a systematic review and meta-analysis. BMC Surg (2023) 23:240. doi: 10.1186/s12893-023-02149-6
42. Xiong J, Chen K, Huang W, Huang M, Cao F, Wang Y, et al. Prevalence and effect on survival of pre-treatment sarcopenia in patients with hematological Malignancies: a meta-analysis. Front Oncol (2023) 13:1249353. doi: 10.3389/fonc.2023.1249353
43. Li X, Huang X, Lei L, Tong S. Impact of sarcopenia and sarcopenic obesity on survival in patients with primary liver cancer: a systematic review and meta-analysis. Front Nutr (2023) 10:1233973. doi: 10.3389/fnut.2023.1233973
44. Liu J, Luo H, Huang L, Wang J. Prevalence of sarcopenia among patients with hepatocellular carcinoma: A systematic review and meta−analysis. Oncol Lett (2023) 26:283. doi: 10.3892/ol.2023.13869
45. Prokopidis K, Affronti M, Testa GD, Ungar A, Cereda E, Smith L, et al. Sarcopenia increases mortality risk in liver transplantation: a systematic review and meta-analysis. Panminerva Med (2023). doi: 10.23736/s0031-0808.23.04863-2
46. Wang L, Qin X, Zhang Y, Xue S, Song X. The prognostic predictive value of systemic immune index and systemic inflammatory response index in nasopharyngeal carcinoma: A systematic review and meta-analysis. Front Oncol (2023) 13:1006233. doi: 10.3389/fonc.2023.1006233
47. Zhou Q, Su S, You W, Wang T, Ren T, Zhu L. Systemic inflammation response index as a prognostic marker in cancer patients: A systematic review and meta-analysis of 38 cohorts. Dose-response Publ Int Hormesis Soc (2021) 19:15593258211064744. doi: 10.1177/15593258211064744
Keywords: SIRI, meta-analysis, hepatocellular carcinoma, prognosis, evidence-based medicine
Citation: Zhang S and Tang Z (2024) Prognostic and clinicopathological significance of systemic inflammation response index in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Front. Immunol. 15:1291840. doi: 10.3389/fimmu.2024.1291840
Received: 10 September 2023; Accepted: 05 February 2024;
Published: 26 February 2024.
Edited by:
Paulo Rodrigues-Santos, University of Coimbra, PortugalReviewed by:
Xiaobin Gu, First Affiliated Hospital of Zhengzhou University, ChinaMichal Michalak, Poznan University of Medical Sciences, Poland
Copyright © 2024 Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhining Tang, Tangzhining0203@163.com
 Sunhuan Zhang
Sunhuan Zhang Zhining Tang
Zhining Tang