
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 03 March 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.993860
This article is part of the Research TopicInsights in Inflammation: 2022View all 13 articles
 Zhiyong Long1*†
Zhiyong Long1*† Ying Deng2†
Ying Deng2† Qi He2†
Qi He2† Kailin Yang3
Kailin Yang3 Liuting Zeng4
Liuting Zeng4 Wensa Hao3
Wensa Hao3 Yuxuan Deng5
Yuxuan Deng5 Jiapeng Fan6
Jiapeng Fan6 Hua Chen3
Hua Chen3Objective: To explore the efficacy and safety of Iguratimod (IGU) intervention in the treatment of Ankylosing Spondylitis (AS).
Methods: We used computer to search literature databases, collected randomized controlled trials (RCTs) related to IGU treatment of AS, and searched the relevant literature in each database until Sep. 2022. Two researchers independently carried out literature screening, data extraction, and evaluation and analysis of the risk of bias in the included studies, and then used Rev Man5.3 software for meta-analysis. The protocol is CRD42020220798.
Results: A total of 10 RCTs involves in 622 patients were collected. The statistical analysis showed that IGU can decrease the BASDAI score (SMD -1.62 [-2.20, -1.05], P<0.00001. Quality of evidence: low), the BASFI score (WMD -1.30 [-1.48, -1.12], P<0.00001. Quality of evidence: low) and the VAS (WMD -2.01 [-2.83, -1.19], P<0.00001. Quality of evidence: very low). Meanwhile, the addition of IGU into the conventional therapy would not increase the adverse events (RR 0.65 [0.43, 0.98], P=0.04. Quality of evidence: moderate).
Conclusion: IGU may be an effective and safe intervention for AS.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?, identifier CRD42020220798.
Ankylosing Spondylitis (AS) is a chronic inflammatory autoimmune disease, which mainly involves axial joint involvement, which may be accompanied by extra-articular manifestations. In severe cases, spinal deformity and joint stiffness may occur. One of the current features of AS is the high prevalence rate (0.86% in Western European white population) and the low incidence rate (1, 2). Patients can live with the disease for many years, and fusion of the spine or peripheral joints can occur in the late stage, causing the patient to lose motor function and living ability, and bring a heavy economic burden to the family and society (3, 4). Inflammation and pathological new bone formation are the two most important pathological features of AS. The early stage of AS is mainly manifested by inflammation and the bone erosion and destruction caused by it, and the late stage causes ectopic new bone formation (4, 5). As the initiating factor, inflammation runs through the entire process of disease development. There are many studies on it at present. The research on pathological new bone formation and the development of corresponding therapeutic drugs are still in the initial stage (5).
The therapeutic drugs for AS currently used clinically mainly include non-steroidal anti-inflammatory drugs (NSAIDs) and biological agents (TNF-α blockers). Although these drugs have achieved good anti-inflammatory effects, they have certain limitations and side effects, and there is no clear evidence for the role of AS new bone formation (6, 7). NSAIDs, as the first-line drugs recommended by AS treatment guidelines, have good anti-inflammatory and analgesic effects, but they need to be taken for a long time and have side effects such as cardiovascular, gastrointestinal and renal toxicity (8, 9). Similarly, TNF-α blockers are not effective for some patients, they are also very expensive, and there are reports that they may increase the risk of cancer (10). Therefore, new drugs for the treatment of AS are urgently needed clinically.
Iguratimod (IGU) is a new type of small molecule anti-rheumatic drug, which has the effects of non-steroidal anti-inflammatory drugs (NSAID) and disease mitigating anti-rheumatic drugs (DMARD). At present, it has been widely used clinically in China and Japan for the treatment of rheumatoid arthritis (RA) (11). IGU not only inhibits related inflammation-related signaling pathways and the expression of inflammatory factors (NF-κB and IL-17 inflammatory signaling pathways) (12), but also inhibits osteoclast differentiation (RANKL signaling pathway), promote osteoblast function (BMP/Dlx5/Osterix signaling pathway), and reduce cartilage destruction (MMPs family related factors) (13, 14), so as to play a bone protection role. At present, clinical randomized controlled trial (RCT)s showed the efficacy of IGU on AS (15–24), but there is no relevant research to systematic review and meta-analyze these RCTs to provide new evidence. Therefore, this research will evaluate the effectiveness and safety of IGU intervention in AS through systematic reviews and meta-analysis for the first time, in order to provide new evidence for clinical use.
This systematic review and meta-analysis were conducted strictly in accordance with the protocol [CRD42020220798 (see Supplementary Material)].
(1) Participants: Patients diagnosed with AS. All patients are at least 18 years old, and there are no restrictions on gender, race, and region. (2) Intervention methods: The intervention of the experimental group is IGU, used alone or in combination with the control group’s drugs. The intervention of the control group was conventional therapy. (3) Outcomes: Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), VAS and adverse events; secondary outcomes are ESR, CRP, TNF-α, back pain score, SOD, CTX-I, β⁃CTX, OPG. (4) Study design: Randomized controlled trial without any limitations.
We searched the ClinicalTrials, the China National Knowledge Infrastructure Databases (CNKI), Web of Science, Pubmed, The Chinese Science and Technology Periodical Database (VIP), EMBASE, Wan Fang Database, CiNii Research, J-STAGE, National Diet Library Digital Collections (NDLDC), Chinese Biomedical Database (CBM), Medline Complete, Cochrane Library. The retrieval time is up to Sep. 2022. The search strategy was shown in Table S1. All included studies were screened by two researchers according to the search criteria. If there is a disagreement between the two, the two researchers will discuss and resolve with the other researchers.
In order to collect the sample size, baseline conditions, treatment plan, treatment time, outcomes and other information included in the RCTs, a table was made to facilitate the extraction of relevant data and retrieval records. Data extraction was carried out independently by two researchers, and differences were resolved through discussions with other researchers. RCT quality assessment is carried out according to the risk of bias tool included in the Cochrane Handbook or Systematic Reviews of Interventions Version (25). The following aspects are evaluated for each study: random sequence generation and allocation hiding (selection bias), blinding (performance bias and detection bias), incomplete outcome data (detection bias), selective reporting (reporting bias) and other bias. The results of the analysis are divided into: “yes” (low risk of bias), “no” (high risk of bias), and “unclear” (unknown risk of bias).
Review Manager 5.3 software was used for statistical analysis (26). For continuous variables such as BASDAI, BASFI, VAS, ESR, CRP, the mean difference (MD) was used to describe the effect size, and the confidence interval (CI) is 95%. For dichotomous variables such as adverse event indicators, relative risk (RR) was used to describe the impact, and the CI is set to 95%. The χ2 test was used to analyze the heterogeneity between the results. In the case of low heterogeneity (P>0.1, I2<50%), a fixed effects model analysis was performed. If there is heterogeneity between the studies, a random effects model was used. The publication bias was detected by STATA 15 with Egger method (continuous variable) and Harbord methods (dichotomous variable) for primary outcomes. P>0.1 is considered to have no publication bias.
The total records identified through database searching and other sources were 53. Forty (40) were excluded based on the title and abstract and 13 for more detailed evaluation. Three (3) of 13 records were excluded because they were not RCTs (27–29) (Figure 1). All patients in those RCTs come from China and involves in 622 participants. The age range of patients is 20-50 years old, and the course of treatment is at least 12 weeks and the maximum is 24 weeks. The details of study characteristics are presented in Table 1.
The summary and graph of risk of bias ware shown in Figures 2, 3.
The random sequences of all RCTs are generated by random number table method, so we evaluate them as low risk of bias. Meanwhile, only Yang et al. (21) describe an acceptable method of allocation concealment, while other RCTs did not describe an acceptable method of allocation concealment. Therefore, Yang et al. (21) were rated as having a low risk of bias, while others were rated as having an unclear risk of bias.
Zeng et al. (20) and Yang et al. (21) stated in the RCT that the blind method was used, but did not describe the specific implementation process of the blind method, so we thought its risk of bias is unclear. Other studies did not specify whether to use blinding, and their main outcome are subjective evaluation indicators (such as BASDAI, BASFI, VAS), which are easily affected by non-blinding, so we believe that their risk of bias is high.
All RCTs do not have incomplete outcome data and selective reporting, so we evaluate them as low risk.
Other sources of bias were not observed in 8 RCTs; therefore, the risks of other bias of the RCTs were low.
Eight RCTs (15, 17–23) utilized BASDAI to assess the improvement of AS, which include 247 patients in IGU group and 225 patients in control group. The heterogeneity test showed that P<0.00001, I2 = 86%, which suggest that the heterogeneity is high, and the random effects model was used for analysis. The meta-analysis results show that compared with the control group, the BASDAI in the IGU group was lower (SMD -1.62 [-2.20, -1.05], P<0.00001; random effect model) (Figure 4).
Five RCTs (15, 18, 19, 21, 24) utilized BASFI to assess the improvement of AS, including 146 patients in IGU group and 122 patients in control group. The heterogeneity test showed that P=0.27, I2 = 23%, which suggest that the heterogeneity is low, and the fixed effects model was used for analysis. The meta-analysis results show that compared with the control group, the BASFI in the IGU group was lower (WMD -1.30 [-1.48, -1.12], P<0.00001; fixed effect model) (Figure 5).
Four RCTs (15, 16, 18, 19, 22) utilized VAS to assess the improvement of AS, including 137 patients in IGU group and 135 patients in control group. The heterogeneity test showed that P<0.00001, I2 = 95%, which suggest that the heterogeneity is high, and the random effects model was used for analysis. The meta-analysis results show that compared with the control group, the VAS in the IGU group was lower (WMD -2.01 [-2.83, -1.19], P<0.00001; random effect model) (Figure 6).
Six RCTs (15–17, 19, 21, 22) utilized ESR to assess the improvement of AS, which involves in 209 patients in IGU group and 185 patients in control group. The heterogeneity test showed that P<0.00001, I2 = 90%, which suggest that the heterogeneity is high, and the random effects model was used for analysis. The meta-analysis results show that compared with the control group, the ESR in the IGU group was lower (WMD -10.01 [-14.72, -5.29], P<0.0001; random effect model) (Figure 7).
Seven RCTs (16, 17, 19–22, 24) utilized CRP to assess the improvement of AS, which involves in 251 patients in IGU group and 226 patients in control group. The heterogeneity test showed that P<0.00001, I2 = 99%, which suggest that the heterogeneity is high, and the random effects model was used for analysis. The meta-analysis results show that compared with the control group, the CRP in the IGU group was lower (WMD -10.11 [-14.55, -5.66], P<0.00001; random effect model) (Figure 8).
Four RCTs (18, 21, 23, 24) utilized TNF-α to assess the improvement of AS, which involves in 129 patients in IGU group and 128 patients in control group. The heterogeneity test showed that P<0.00001, I2 = 94%, which suggest that the heterogeneity is high, and the random effects model was used for analysis. The meta-analysis results show that compared with the control group, the TNF-α in the IGU group was lower (WMD -6.21 [-7.96, -4.47], P<0.00001; random effect model) (Figure 9).
Two RCTs (16, 24) utilized SOD to assess the improvement of AS, which involves in 76 patients in IGU group and 73 patients in control group. The heterogeneity test showed that P<0.00001, I2 = 95%, which suggest that the heterogeneity is high, and the random effects model was used for analysis. The meta-analysis results show that there was no significant difference in SOD between the experimental group and the control group (WMD 3.97 [-42.07, 50.01], P=0.87; random effect model) (Figure 10).
Two RCTs (16, 24) utilized CTX-I to assess the improvement of AS, which involves in 76 patients in IGU group and 73 patients in control group. The heterogeneity test showed that P<0.0001, I2 = 94%, which suggest that the heterogeneity is high, and the random effects model was used for analysis. The meta-analysis results show that there was no significant difference in CTX-I between the experimental group and the control group (WMD -0.29 [-0.60, 0.01], P=0.06; random effect model) (Figure 11).
Only Qiu et al. (16) reported back pain score, and they found that IGU can improve back pain score (P<0.05). Only Pang et al. (18) reported β⁃CTX and OPG levels, and they found that IGU can reduce β⁃CTX level and increase OPG level (P<0.05).
Nine RCTs (15–22, 24) (284 patients in experimental group and 258 patients in control group) reported adverse events. The heterogeneity test P=0.37, I2 = 8%, indicating that the included studies are heterogeneous, and the fix effects model is used for analysis. The results of meta-analysis showed that incidence of adverse events in IGU group was lower (RR 0.65 [0.43, 0.98], P=0.04; fix effect model) (Figure 12).
The publication bias of the primary outcomes was detected by STATA 15.0. (1) BASDAI: The publication bias detection suggests that the possibility of publication bias was small (P=0.302) (Figure 13A). (2) BASFI: The publication bias detection suggests that the possibility of publication bias was small (P=0.420) (Figure 13B). (3) VAS: The publication bias detection suggests that the possibility of publication bias was small (P=0.531) (Figure 13C). (4) Adverse events: The publication bias detection suggests that the possibility of publication bias was small (P=0.844) (Figure 13D).
The subgroup analysis was performed according to the duration (Table 2). The results of subgroup analysis showed that BASDAI, VAS, CRP, and TNF-α improved after 12 weeks of IGU treatment, and also improved after 24 weeks of treatment. However, for ESR, the addition of IGU treatment improved ESR at 12 weeks, while 24 weeks after the intervention showed no significant difference in ESR compared with the control group. For adverse events, the results showed that the 12-week intervention did not lead to an increase in the occurrence of adverse events, and the adverse events of long-term use (24 weeks) may be lower than that of the control group.
This research included 10 RCTs with 622 participants. In addition to ClinicalTrials.gov, we also searched the Chinese Clinical Trial Registry and found that currently ongoing randomized controlled studies are: ChiCTR1800019227 and ChiCTR2000029112. The meta-analysis results showed that IGU can decrease the BASDAI score, BASFI score and VAS. IGU can also reduce inflammation levels (decreasing ESR, CRP and TNF-α). Most of the results are highly heterogeneous, especially VAS, ESR, CRP and TNF-α. It may be because both BASDAI and VAS are subjective measurement indicators, and the subjective feelings of patients with different RCTs are not uniform. ESR, CRP and TNF-α are individual biochemical indicators, and patients in different RCTs are different due to different conditions. All studies reported adverse reactions and no patient deaths were reported. Compared with the control group, the adverse events of the IGU group was lower. This shows that the addition of IGU will not cause additional adverse events to patients, and the occurrence of adverse events may be lower in IGU treatment over 24 weeks.
Current research shows that IGU, as a new type of anti-rheumatic drug, has good anti-inflammatory and immunosuppressive effects, and may be a potential drug for the treatment of AS in the future. The main clinical features of AS include inflammatory back pain caused by myositis and inflammation of other parts of the axial skeleton, peripheral arthritis, enteritis and anterior uveitis (30). In addition to inflammation, AS is also characterized by new bone formation in sacroiliac joints (SIJ) and the spine (31). Theories about the pathogenesis of AS include misfolding during the assembly of human leukocyte antigen (HLA)-B27, which leads to endoplasmic reticulum stress and unfolded protein response (UPR) (32). The activation of UPR gene leads to the release of TNF-α and IL-17, which is very important in the development of AS (33). The COX-2/PGE2 pathway is also important in the pathogenesis of AS (34). In addition, current evidence shows that MIF can promote inflammation and bone formation in AS (35). MIF also interacts with IL-17 and TNF-α pathways by up-regulating the expression and secretion of IL-17 and induces the production of TNF-α (35).
IGU plays an important role in suppressing immunity, inflammation, and maintaining bone balance. (1) In terms of inhibiting inflammatory factors and osteoclast intracellular signaling pathways: Bao et al. found in collagen-induced arthritis mice (CIA) that IGU can inhibit IL-17 expression while reducing TNF-α, IL-1β and IL-6 levels (36). Xu et al. confirmed that IGU can block the IL-17 pathway by targeting Act1, and IL-17 is an important cytokine involved in bone destruction in RA patients (37). The NF-κB pathway is an important intracellular conduction pathway in the process of osteoclast activation. Kohno et al. found that IGU can interfere with the translocation of NF-κB p65 into the nucleus and inhibit the activity of NF-κB (38). (2) In terms of inhibiting bone resorption: RANKL is an important signal to initiate osteoclast activation. Zhang et al. confirmed in vitro experiments that in mouse RAW264.7 cells, IGU can inhibit the number of osteoclasts induced by RANKL and reduce bone resorption pits (39). Guo et al. also found in bone marrow monocytes that IGU strongly inhibited RANKL-mediated osteoclastogenesis and bone resorption in a dose-dependent manner (40). IGU can also inhibit RANKL-induced osteoclast development and bone resorption in the PPARγ/c-Fos signaling pathway, and can also reduce the expression of downstream osteoclast marker genes (41). In addition, IGU not only inhibited the production of RANKL, but also significantly decreased the ratio of RANKL/OPG in serum and IL-1β-induced RA-FLSs (42). IGU inhibits the generation, differentiation, migration and bone resorption of osteoclasts induced by RANKL, and reduces the expression of nuclear activated T cell factor (NFAT) c1 and downstream osteoclast marker genes (43). These effects collectively show the effect of IGU attenuating bone erosion. Gan et al. found that IGU significantly inhibited RANKL-induced osteoclast differentiation, migration and bone resorption in RAW264.7 cells in a dose-dependent manner; the mechanism was related to the activation of MAPK and NF-κB pathways (44). It shows that IGU has a direct inhibitory effect on the formation and function of osteoclasts. In addition to osteoclasts, MMPs produced by FLSs also play an important role in cartilage destruction in spondylitis (45). Du et al. treated FLS with different doses of IGU in vitro and then stimulated them with TNF-α, IL-1β or IL-17A. MMP-3 was significantly inhibited by 5 μg/ml IGU, but MMP-1 was significantly inhibited at 50 μg/ml. Clinical trials found that after 24 weeks of IGU (25mg, 22 times a day) treatment, the levels of MMP-1 and MMP-3 were significantly reduced (46). All these suggest that IGU prevents MMP-1 and MMP-3 from protecting cartilage (43). (3) In terms of promoting bone formation: Kohji Kuriyama et al. found that IGU can promote the differentiation of mouse bone marrow stromal cells ST2 and embryonic osteoblast precursor cells MC3T3-E1 into osteoblasts in vitro, and can promote BMP-2 so as to induce bone formation in vivo (47). In addition, Osterix is a core transcription factor that regulates bone formation and plays a key role in the differentiation of osteoblasts (48), while IGU can increase the expression of Osterix and osteocalcin (41). Song et al. also found that IGU can increase the expression of Dlx5 and Osterix and regulate the p38 pathway to promote osteoblast differentiation and maturation in mesenchymal stem cells (49). (4) In the aspect of regulating immunity: IGU can regulate immune balance by regulating T cells and related cytokine levels. Studies have shown that IGU can significantly reduce the number of Th1, Th17, follicular helper T (Tfh) cells and related transcription factors and cytokine levels, increase the number of regulatory T cells (Treg) and related transcription factors and cytokine levels (50–52). IGU also reduced the apoptosis of peripheral blood mononuclear cells, the content of IFN-γ in CD3 + T cells and the level of IL-8 in peripheral blood (53). In addition, in regulating B cells, IGU can also inhibit PKC pathway and its downstream target EGR1, thereby inhibiting B cell terminal differentiation into mature plasma cells to reduce the production of autoantibodies (54). In summary, IGU can be controlled by multiple targets, and it can inhibit cartilage and bone destruction in the pathological process of AS, and has the basis of bone protection (see Figure 14).
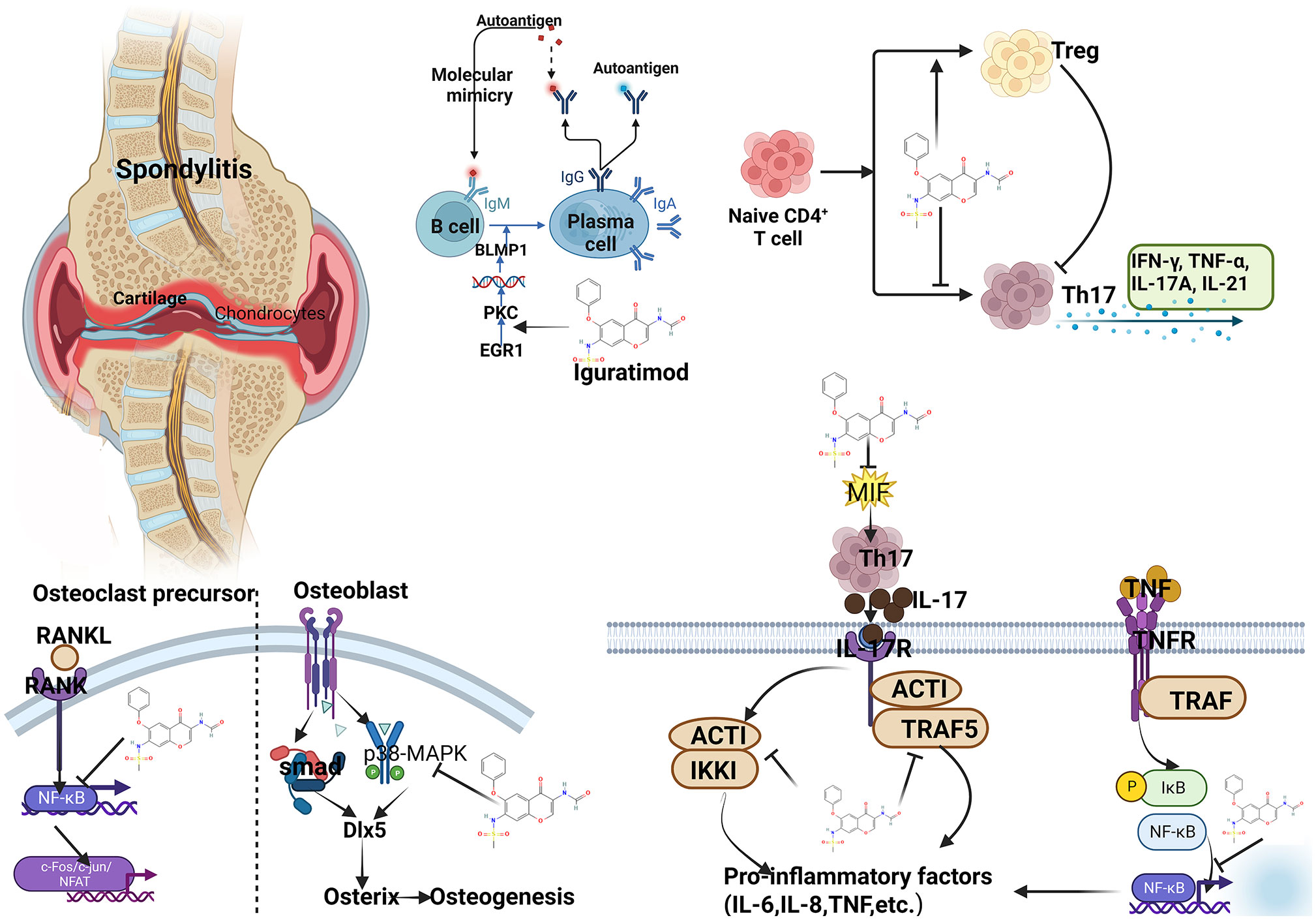
Figure 14 Summary of mechanism of IGU treating AS (PKC, protein kinase C; EGR1, early growth response 1; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IL, interleukin; RANKL, NF-κB receptor activating factor ligand; MIF, Macrophage migration inhibitory factor; TRAF, tumor necrosis factor receptor-associated factor).
To promote the conclusion, the GRADE tool was utilized to rate the quality of the evidence (55). According to the GRADE handbook (56), the evidence was judged to be moderate to very low (Table 3).
The strengths of this review is that this we firstly conducted a systematic review and meta-analysis about IGU on AS. This study not only found that adding IGU to conventional therapy can improve AS, but also showed that it does not increase adverse reactions. However, the limitations is that most of the RCTs included this time did not use blinding, and did not hide the allocation of interventions, leading to a high risk of bias in the results. The number of RCTs included in this study is small, and the number of participants involved is not more than 1,000, which may affect the accuracy of the results. Moreover, most of the patients included in the study included this time are Chinese, which may affect the applicability of the results. Therefore, high-quality RCTs involving more countries and regions are needed in the future to revise or verify the results of this meta-analysis.
Through the systematic evaluation and meta-analysis of this study, it can be clarified that IGU as a new multi-targeted DMARD may have multiple benefits in the treatment of AS.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
LZ, YiD, KY, HC are responsible for the study concept and design. LZ, YiD, QH, ZL, KY, WH, YuD, JF, HC are responsible for the data collection, data analysis and interpretation. LZ and KY drafted the paper. HC supervised the study. All authors contributed to the article and approved the submitted version.
Author JF was employed by company ZCCC Jinzhu Transportation Construction Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.993860/full#supplementary-material
2. Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatol (Oxford) (2014) 53(4):650–7. doi: 10.1093/rheumatology/ket387
3. Smith JA. Update on ankylosing spondylitis: Current concepts in pathogenesis. Curr Allergy Asthma Rep (2015) 15(1):489. doi: 10.1007/s11882-014-0489-6
4. Zhang X, Aubin JE, Inman RD. Molecular and cellular biology of new bone formation: Insights into the ankylosis of ankylosing spondylitis. Curr Opin Rheumatol (2003) 15(4):387–93. doi: 10.1097/00002281-200307000-00004
5. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet (20172017) 390(10089):73–84. doi: 10.1016/S0140-6736(16)31591-4
6. Garcia-Montoya L, Gul H, Emery P. Recent advances in ankylosing spondylitis: Understanding the disease and management. F1000Res (2018) 7:F1000 Faculty Rev-1512. doi: 10.12688/f1000research.14956.1
7. Raychaudhuri SP, Deodhar A. The classification and diagnostic criteria of ankylosing spondylitis. J Autoimmun (2014) 48-49:128–33. doi: 10.1016/j.jaut.2014.01.015
8. Marsico F, Paolillo S, Filardi PP. NSAIDs and cardiovascular risk. J Cardiovasc Med (Hagerstown) (20172017) 18 Suppl 1. Special Issue on The State of the Art for the Practicing Cardiologist: The 2016 Conoscere E Curare Il Cuore (CCC) Proceedings from the CLI Foundation:e40-e43. doi: 10.2459/JCM.0000000000000443
9. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci (2013) 16(5):821–47. doi: 10.18433/j3vw2f
10. Lopetuso LR, Mocci G, Marzo M, D'Aversa F, Rapaccini GL, Guidi L, et al. Harmful effects and potential benefits of anti-tumor necrosis factor (TNF)-α on the liver. Int J Mol Sci (2018) 19(8):2199. doi: 10.3390/ijms19082199
11. Shrestha S, Zhao J, Yang C, Zhang J. Relative efficacy and safety of iguratimod monotherapy for the treatment of patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol (2020) 39(7):2139–50. doi: 10.1007/s10067-020-04986-9
12. Lina C, Conghua L, Nan Z. PingCombined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol (2011) 31(4):96–605. doi: 10.1007/s10875-011-9542-6
13. Wu YX, Sun Y, Ye YP, Zhang P, Guo JC, Huang JM, et al. Iguratimod prevents ovariectomyinduced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferatoractivated receptorgamma.Mol. Med Rep.16 (2017) 6):8200–8. doi: 10.3892/mmr.2017.7648
14. Zhao L, Mu B, Zhou R, Cheng Y. Huang.Iguratimod ameliorates bleomycin-induced alveolar inflammation and pulmonary fibrosis in mice by suppressing expression of matrix metalloproteinase-9.Int. J Rheumatol Dis (2019) 22(4):686–94. doi: 10.1111/1756-185X.13463
15. Qiu YY, Tang Y, Rui JB, Li J. Observation on the clinical effect of iguratimod in the treatment of refractory ankylosing spondylitis. J Jiangsu Univ (Medical Edition) (2016) 26(03):235–9. (in Chinese).
16. Yuan FF, Chen YH, Lin JX, Luo J. Efficacy of iguratimod combined with methotrexate in the treatment of refractory ankylosing spondylitis and its effect on serum SOD and CTX-I levels in patients. J Pharm Epidemiol (2020) 29(03):163–165+205. (in Chinese).
17. Pang LX, Zheng ZH, Li ZQ, Li Y, Wu ZB. Efficacy of iguratimod combined with etanercept in the treatment of ankylosing spondylitis. J Trop Med (2020) 20(04):118–21. (in Chinese).
18. Lin YP, Liu H, Gao JT. Preliminary observation on the treatment of ankylosing spondylitis with iguratimod. J Clin Rational Use (2019) 012(014):9–13. (in Chinese).
19. Xu BJ, Mo SQ, Xue XQ, Wu Y. Study on the efficacy and safety of iguratimod in the treatment of ankylosing spondylitis. New Med (2019) 50(12):915–8. (in Chinese).
20. Zeng HQ, Kong WH, Zhuang P, Dong HJ, Yin ZH, Xinpeng C, et al. Observation on the efficacy of iguratimod in the treatment of ankylosing spondylitis. Hainan Med (2016) 27(01):118–20. (in Chinese).
21. Li Y, Li K, Zhao Z, Wang Y, Jin J, Guo J, et al. Randomised, double-blind, placebo-controlled study of iguratimod in the treatment of active spondyloarthritis. Front Med (Lausanne) (2021) 8:678864. doi: 10.3389/fmed.2021.678864
22. Bai YJ, Wang XY, Yao YJ. Observation on the clinical effect of iguratimod in the treatment of axial spondyloarthritis. Chin Med Innovation (2021) 18(2):44–7. doi: 10.3969/j.issn.1674-4985.2021.02.011. (in Chinese).
23. Li X, Pan T, Chen MP. Observation of the curative effect of iguratimod in the treatment of ankylosing spondylitis. J Med Theor Prac (2021) 34(17):3009–11.
24. Zhang W. Efficacy of iguratimod combined with celecoxib in the treatment of ankylosing spondylitis. J Med Inf (2022) 35(15):114–6. (in Chinese).
25. Deeks JJ, Higgins JP, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane handbook or systematic reviews of interventions version 6.1.0. UK: The Cochrane Collaboration (2020) 2020.
26. Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analyzing data and undertaking meta-analyses. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. UK: The Cochrane Collaboration (2020).
27. Huang BJ, Ma JX. Observation on the short-term curative effect of iguratimod in the treatment of ankylosing spondylitis. Chin Community Physician (2018) 34(13):92–3.
28. Luo Y, Zheng N, Wu R. Is iguratimod effective in refractory axial spondyloarthritis? Scand J Rheumatol (2018) 47(6):518–20. doi: 10.1080/03009742.2017.1390150
29. Xu YW, Tao YL, Zhang H, Dai SM. Observation on the clinical efficacy of iguratimod in the treatment of axial spondyloarthritis. Shanghai Med J (2021) 44(06):421–4. doi: 10.19842/j.cnki.issn.0253-9934.2021.06.013
30. Braun J, Sieper J. Ankylosing spondylitis. Lancet (2007) 369(9570):1379–90. doi: 10.1016/S0140-6736(07)60635-7
31. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet (2017) 390(10089):73–84. doi: 10.1016/S0140-6736(16)31591-4
32. Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N. Pathogenesis of ankylosing spondylitis - recent advances and future directions. Nat Rev Rheumatol (2017) 13(6):359–67. doi: 10.1038/nrrheum.2017.56
33. Pedersen SJ, Maksymowych WP. The pathogenesis of ankylosing spondylitis: An update. Curr Rheumatol Rep (2019) .21(10):58. doi: 10.1007/s11926-019-0856-3
34. Schett G, Lories RJ, D’Agostino MA, Elewaut D, Kirkham B, Soriano ER, et al. Enthesitis: From pathophysiology to treatment. Nat Rev Rheumatol (2017) 13(12):731–41. doi: 10.1038/nrrheum.2017.188
35. Ranganathan V, Ciccia F, Zeng F, Sari I, Guggino G, Muralitharan J, et al. Macrophage migration inhibitory factor induces inflammation and predicts spinal progression in ankylosing spondylitis. Arthritis Rheum (2017) 69(9):1796–806. doi: 10.1002/art.40175
36. Du F, Lü LJ, Fu Q, Dai M, Teng JL, Fan W, et al. T-614, a novel immunomodulator, attenuates joint inflammation and articular damage in collagen-induced arthritis. Arthritis Res Ther (2008) 10(6):R136. doi: 10.1186/ar2554
37. Luo Q, Sun Y, Liu W, Qian C, Jin B, Tao F, et al. A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J Immunol (2013) 191(10):4969–78. doi: 10.4049/jimmunol.1300832
38. Kohno M, Aikawa Y, Tsubouchi Y, Hashiramoto A, Yamada R, Kawahito Y, et al. Inhibitory effect of T-614 on tumor necrosis factor-alpha induced cytokine production and nuclear factor-kappaB activation in cultured human synovial cells. J Rheumatol (2001) 28(12):2591–6.
39. Gan K, Yang L, Xu L, Feng X, Zhang Q, Wang F, et al. Iguratimod (T-614) suppresses RANKL-induced osteoclast differentiation and migration in RAW264.7 cells via NF-κB and MAPK pathways. Int Immunopharmacol (2016) 35:294–300. doi: 10.1016/j.intimp.2016.03.038
40. Wu YX, Sun Y, Ye YP, Zhang P, Guo JC, Huang JM, et al. Iguratimod prevents ovariectomyinduced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferatoractivated receptorγ. Mol Med Rep (2017) 16(6):8200–8. doi: 10.3892/mmr.2017.7648
41. Wu YX, Sun Y, Ye YP, Zhang P, Guo JC, Huang JM, et al. Iguratimod prevents ovariectomy−induced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferator−activated receptor−γ. Mol Med Rep (2017) 16(6):8200–8. doi: 10.3892/mmr.2017.7648
42. Wang XT, Li P, Xu TS, Ding R, Zhang X. BiEffect of iguratimod and methotrexate on RANKL and OPG expression in serum and IL-1beta-induced fibroblast-like synoviocytes from patients with rheumatoid arthritis. Cell Mol Biol (Noisy-le-grand) (2016) 62(12):44–50. doi: 10.14715/cmb/2016.62.13.8
43. Ren YH, Dong W, Liu H, Liu L. Clinical observation of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. China Pharm (2017) 28(32):4530–2.
44. Gan K, Yang L, Xu L, Feng X, Zhang Q, Wang F, et al. Iguratimod (T-614) suppresses RANKL-induced osteoclast differentiation and migration in RAW264.7 cells via NF-kappaB and MAPK pathways. Int Immunopharmacol (2016) 35:294–300. doi: 10.1016/j.intimp.2016.03.038
45. Reveille JD. Biomarkers in axial spondyloarthritis and low back pain: A comprehensive review. Clin Rheumatol (2022) 41(3):617–34. doi: 10.1007/s10067-021-05968-1
46. Du F, Lu LJ, Teng JL, Shen N, Ye P, Bao. CD. T-614 alters the production of matrix metalloproteinases (MMP-1 andMMP-3) and inhibits the migratory expansion of rheumatoid synovial fibroblasts, in vitro. Int Immunopharmacol (2012) 13(1):54–60. doi: 10.1016/j.intimp.2012.03.003
47. Kuriyama K, Higuchi C, Tanaka K, Yoshikawa H, Itoh K. A novel anti-rheumatic drug, T-614, stimulates osteoblastic differentiation in vitro and bone morphogenetic protein-2-induced bone formation in vivo. Biochem Biophys Res Commun (2002) 299(5):903–9. doi: 10.1016/S0006-291X(02)02754-7
48. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing tranion factor osterix is required for osteoblast differentiation and bone formation. Cell (2002) 108(1):17–29. doi: 10.1016/S0092-8674(01)00622-5
49. Song J, Liu H, Zhu Q, Miao Y, Wang F, Yang F, et al. T-614 promotes osteoblastic cell differentiation by increasing Dlx5 expression and regulating the activation of p38 and NF-κB. BioMed Res Int (2018) 2018:4901591. doi: 10.1155/2018/4901591
50. Wang Q, Xiao Y, Zhang Y, Cai R, Wei J. Modulation of Th17 cells and treg cell imbalance in patients with rheumatoid arthritis by iguratimod. Chin J Med Equip (2018) 31(10):6–8. (In Chinese).
51. Jiang H, Gao H, Wang Q, Wang M, Wu B. Molecular mechanisms and clinical application of iguratimod: A review. BioMed Pharmacother (2020) 122:109704. doi: 10.1016/j.biopha.2019.109704
52. Zeng L, He Q, Yang K, Hao W, Yu G, Chen H. A systematic review and meta-analysis of 19 randomized controlled trials of iguratimod combined with other therapies for sjogren's syndrome. Front Immunol (2022) 13:924730. doi: 10.3389/fimmu.2022.924730
53. Liu D, Liu CF, Wang N, Min XY, Ma N, Lin Y, et al. The research of effects of Iguratimod(T-614) on the apoptosis of peripheral blood mononuclear cell and TH1 in rheumatoid arthritis. Value Health (2014) 17(7):A772. doi: 10.1016/j.jval.2014.08.321
54. Ye Y, Liu M, Tang L, Du F, Liu Y, Hao P, et al. Iguratimod represses b cell terminal differentiation linked with the inhibition of PKC/EGR1 axis. Arthritis Res Ther (2019) 21(1):92. doi: 10.1186/s13075-019-1874-2
55. GRADEpro GDT. GRADEpro guideline development tool [Software] Vol. 2015. Australia: McMaster University (2015). (developed by Evidence Prime, Inc.).
Keywords: Iguratimod, ankylosing spondylitis, systematic review, meta-analysis, randomized controlled trial
Citation: Long Z, Deng Y, He Q, Yang K, Zeng L, Hao W, Deng Y, Fan J and Chen H (2023) Efficacy and safety of Iguratimod in the treatment of Ankylosing Spondylitis: A systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 14:993860. doi: 10.3389/fimmu.2023.993860
Received: 14 July 2022; Accepted: 10 January 2023;
Published: 03 March 2023.
Edited by:
Rudolf Lucas, Augusta University, United StatesReviewed by:
Jun Deng, Shanghai Jiao Tong University, ChinaCopyright © 2023 Long, Deng, He, Yang, Zeng, Hao, Deng, Fan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Long, MjIxMjQ3MTQzOEBxcS5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.