- 1Department of Cardiothoracic Surgery, Zhongshan City People’s Hospital, Zhongshan, China
- 2Department of Pulmonary Oncology, Zhongshan City People’s Hospital, Zhongshan, China
- 3Department of Radiotherapy, Zhongshan City People’s Hospital, Zhongshan, China
Background: We compared the real-world efficacy and safety of neoadjuvant chemoimmunotherapy to chemotherapy alone in patients with stage III non-small-cell lung cancer (NSCLC).
Participants and methods: A total of 59 consecutive patients were finally selected and divided into two groups: the neoadjuvant chemotherapy group (n = 33) and the neoadjuvant chemoimmunotherapy group (n = 26). The primary endpoint was disease-free survival (DFS). The secondary endpoints were pathological response, clinical response, and adverse events. All patients were followed up to collect perioperative pathology and clinical data.
Results: The objective response rate (ORR), pathological complete response (pCR), and major pathological response (MPR) were significantly higher in the neoadjuvant chemoimmunotherapy group than in the neoadjuvant chemotherapy group (73.1% vs. 45.5%, 34.6% vs. 3.0%, and 65.3% vs. 15.1%, respectively; P < 0.05). There was no statistically significant difference in disease-free survival between the neoadjuvant chemoimmunotherapy and neoadjuvant chemotherapy groups (P = 0.129). Patients in the neoadjuvant chemoimmunotherapy group had a higher rate of tumor regression than those in neoadjuvant chemotherapy group (37.0% [25 patients] vs. 29.0% [33 patients], P = 0.018). However, no discernible correlation between MPR achievement and the degree of tumor shrinkage was observed in either group (P > 0.05). The cumulative MPR rates were 42.3, 50, and 65.3% for 2, 3, and ≥ 4 cycles, respectively, in the neoadjuvant chemoimmunotherapy group and 9.1, 12.1, and 15.1% for ≤ 2, 3, and ≥ 4 cycles, respectively, in the neoadjuvant chemotherapy group. Moreover, No statistical difference was observed between the two groups regarding postoperative complications, resection range, operation time, surgical method, and extent of resection (P > 0.05). Although the incidence of grades III–IV adverse events was higher in the neoadjuvant chemotherapy group than in the neoadjuvant chemoimmunotherapy group (33.3% vs. 4.6%, P = 0.042), there was no significant difference in the incidence of adverse events between the two groups (64.6% vs. 83.6%, P = 0.072).
Conclusion: In stage III NSCLC, neoadjuvant chemoimmunotherapy achieved higher pathological and clinical remission rates than chemotherapy alone, with compromising safety, making it an attractive choice for neoadjuvant therapy.
Introduction
Currently, the treatment of stage III non-small-cell lung cancer (NSCLC) remains a significant challenge. Existing literature suggests that the 5-year survival rate following surgery in patients with stage III NSCLC is only 13–36%, whereas that for surgery combined with adjuvant radiotherapy and for chemotherapy alone is merely 20 and 45%, respectively (1, 2). The National Comprehensive Cancer Network guidelines of the United States have suggested the use of nivolumab monoclonal antibody along with platinum-based doublet chemotherapy as a neoadjuvant therapy regimen for NSCLC (3). However, studies specifically focusing on the use of neoadjuvant chemoimmunotherapy for stage III NSCLC are lacking. To the best of our knowledge, there are also currently no studies discussing the derived patterns of major pathological responses (MPRs) following neoadjuvant chemoimmunotherapy. In this real-world study, we examined the clinical and pathological data of patients with stage III NSCLC who underwent pre-operative neoadjuvant therapy. We aimed to compare the safety and efficacy of neoadjuvant chemoimmunotherapy with those of neoadjuvant chemotherapy, paying special attention to the pattern of MPR occurrence, which could serve as a beneficial reference for selecting the most appropriate neoadjuvant therapy for stage III NSCLC.
Patients and methods
This real-world study included patients diagnosed with stage III NSCLC between October 2017 and October 2023, who were divided into two groups: the neoadjuvant chemotherapy group and the neoadjuvant chemoimmunotherapy group. This study was conducted in accordance with the Declaration of Helsinki. All patients provided informed consent.
The inclusion criteria were as follows: 1) stage III diagnosis of NSCLC via imaging and cytological examination, with all tumors identified as primary lung cancer; 2) preoperative assessment by multiple senior surgeons, deeming the lesion resectable, despite a large tumor volume involving the carina, bronchus, or pulmonary vessels, with hilar or mediastinal lymph node metastases; 3) Karnofsky performance status score ≥ 80, indicating the capacity to tolerate neoadjuvant therapy; 4) normal liver and kidney functions; 5) anticipated survival time of > 3 months; 6) sufficient pulmonary function for the expected pneumonectomy; and 7) negative for EGFR and ALK gene mutations.
The exclusion criteria were as follows: 1) distant metastasis or surgical contraindications; 2) dysfunction of the liver, kidney, or other organs; 3) autoimmune diseases (e.g., diseases due to human immunodeficiency virus) or long-term use of immunosuppressive drugs; and 4) intolerance to immunotherapy or chemotherapy.
Patients treated with a combination of programmed cell death-1 monoclonal antibody and platinum-based doublet chemotherapy were included in the immunochemotherapy group, whereas those treated only with platinum-based doublet chemotherapy were included in the chemotherapy group. The immunotherapy drugs included pembrolizumab, tislelizumab, sintilimab, camrelizumab, and nivolumab. Chemotherapy was based on the following first-line regimen for advanced NSCLC.
Four patients underwent treatment with gemcitabine 1000 mg/m2 on days 1 and 8 and platinum-based therapy, whereas two received etoposide 100 mg/m2 on days 1–3 and platinum-based therapy. A 21-day cycle was followed for both immunotherapy and chemotherapy, in which the tumor efficacy was assessed every two cycles. Regular examinations via hematology and imaging were performed during the treatment period.
Chest tomography or positron emission tomography-computed tomography reassessment was performed every two cycles, and the efficacy of the treatment was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (4). Pathological complete response (pCR) was defined as neoadjuvant therapy-induced tumor regression with no visible residual tumor on pathological examination. A major pathological response (MPR) was defined as neoadjuvant therapy-induced tumor regression with ≤ 10% residual tumor on pathological examination (MPR includes pCR) (5, 6). Non-MPR was defined as a major pathological response not being reached.
The time to surgery after neoadjuvant therapy was taken as the duration from treatment completion to surgical intervention. Any adverse events (AEs) occurring from the initiation of the medication under study to 1 month after treatment completion, regardless of any causal relationship with the trial drugs, were considered AEs. AEs were evaluated using the Common Toxicity Criteria Document for Adverse Events, version 5.0 of the US National Cancer Institute.
Patients were monitored every 3 months until October 2023. The primary endpoint was disease-free survival (DFS), which was defined as the period from surgery to the discovery of disease recurrence or death. The secondary endpoints were pathological response, clinical response, and AEs.
Statistical analysis
All statistical analyses were performed using Statistical Product and Service Solutions, version 25.0. Count data are presented as numbers and percentages. Intergroup comparisons were conducted using the χ2 test. Independent sample t-tests were used for measurement information. The effect size was estimated according to the relative risk and corresponding 95% confidence intervals, survival analysis comparisons using the Kaplan–Meier survival curve analysis, and correlation analysis using point-biserial correlation. The significance levels for all tests were established at α = 0.05.
Results
Baseline characteristics of the patients
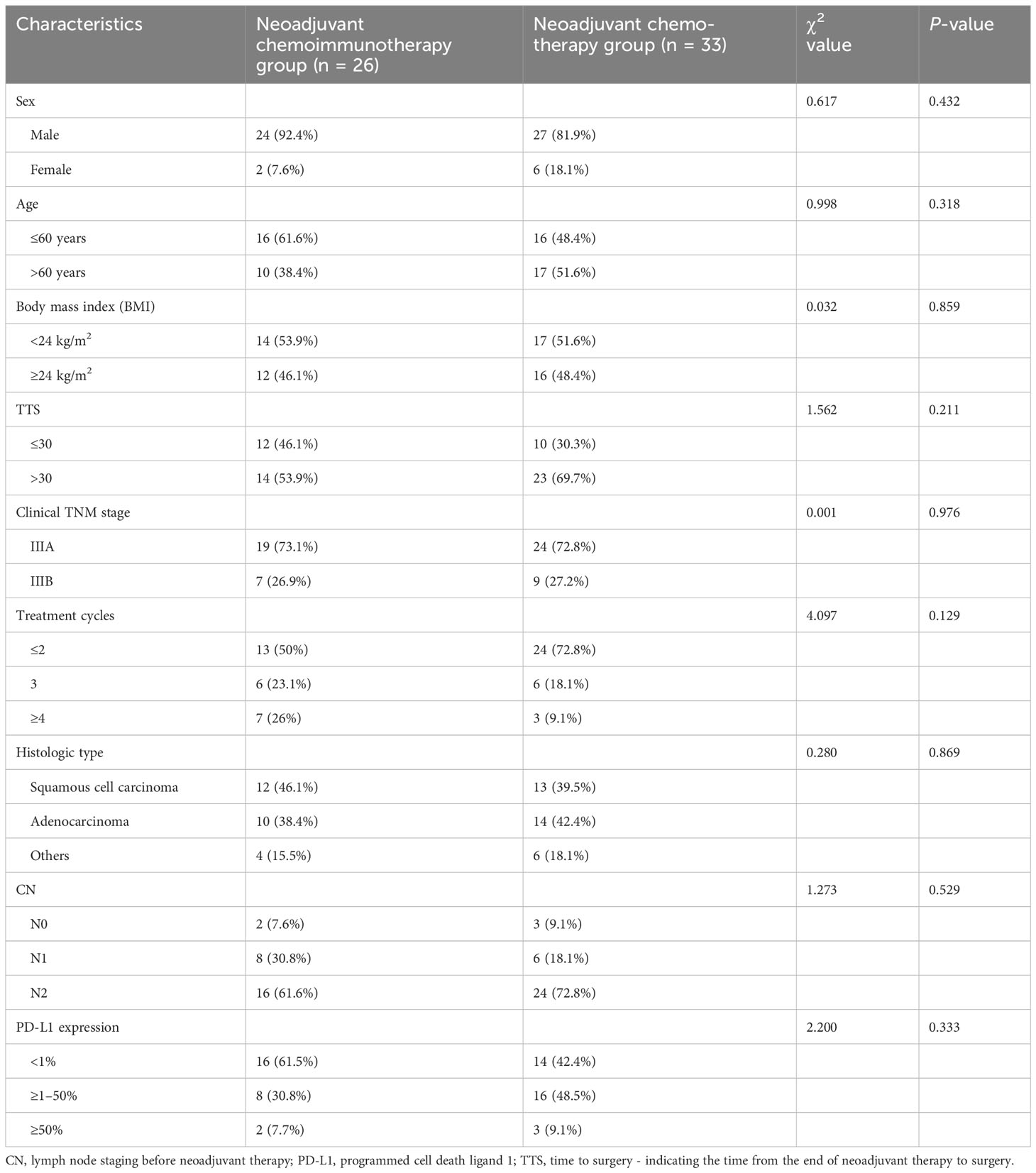
The neoadjuvant therapy groups comprised 59 patients who fulfilled the defined inclusion criteria. Figure 1 shows the treatment processes followed in the two groups, and Table 1 shows the clinical data of the enrolled patients. General clinical data comparison revealed no statistically significant difference between the two groups (P > 0.05).
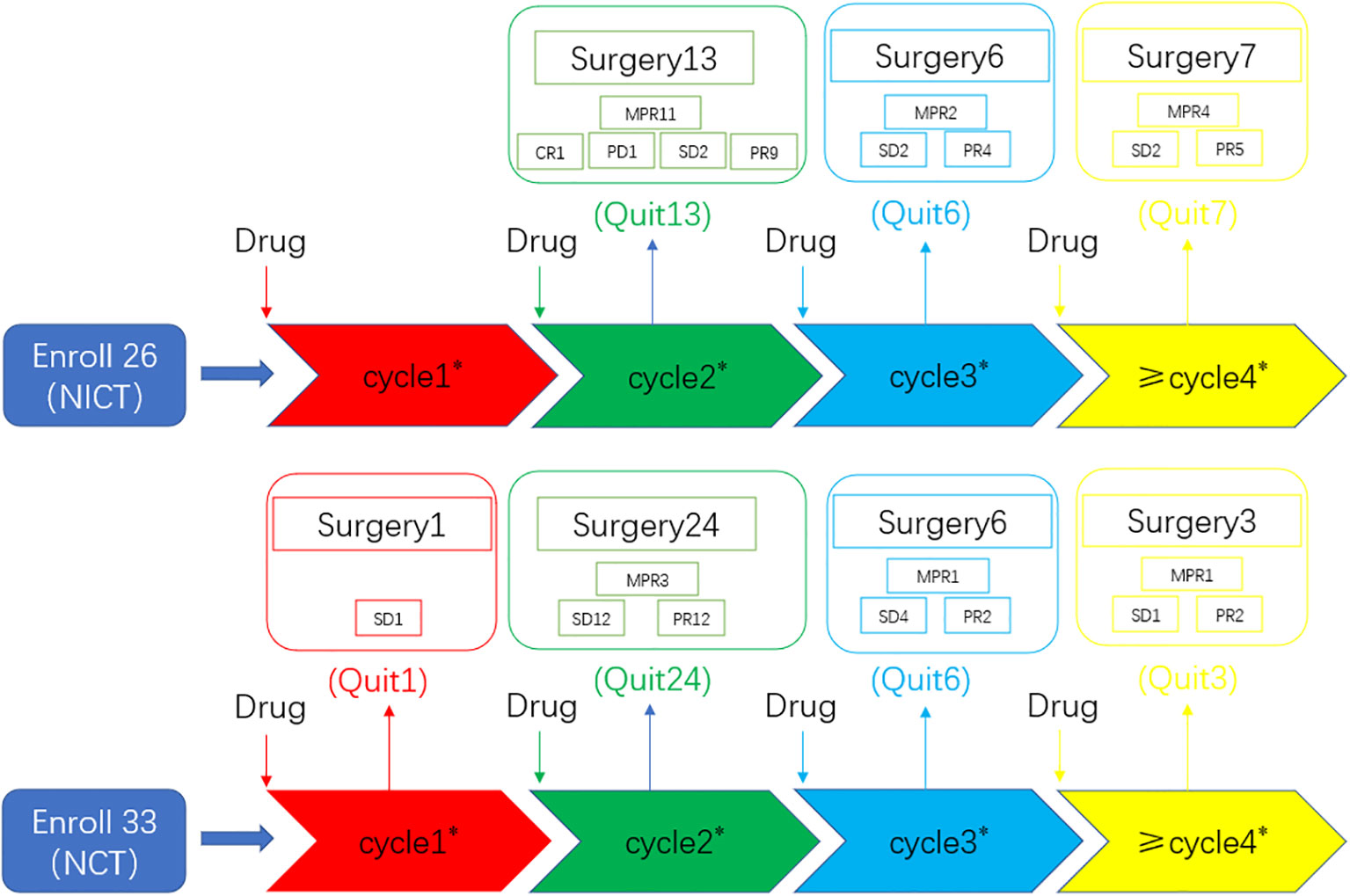
Figure 1 Overview of the treatment processes. A total of 59 consecutive patients were ultimately selected and divided into two groups: the neoadjuvant chemotherapy group (n = 33) and the neoadjuvant chemoimmunotherapy group (n = 26). All patients underwent randomization and received a neoadjuvant treatment followed by surgery. CR, complete response; NCT, neoadjuvant chemotherapy group; NICT, neoadjuvant chemoimmunotherapy group; MPR, major pathological response; PD, progressive disease; PR, partial response; SD, stable disease. Numeric values represent the number of patients. Number * represents the number of neoadjuvant therapy cycles. “Quit” signifies patients who underwent surgery after completing neoadjuvant therapy.
Comparison of the efficacy of neoadjuvant therapies
The objective response rate (ORR) of the neoadjuvant chemoimmunotherapy group was significantly higher than that of the neoadjuvant chemotherapy group (P = 0.033). Similarly, the pCR and MPR rates were significantly higher in the neoadjuvant chemoimmunotherapy group than in the neoadjuvant chemotherapy group (P = 0.004, P = 0.004). However, no statistical difference was observed in terms of complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD), and disease control rate (DCR) between the two groups (P > 0.05). The postoperative occurrence of positive lymph node metastasis, pleural invasion, vascular invasion, and nerve invasion revealed no statistical difference between the groups (P > 0.05). These details are presented in Table 2. A major pathological response occurred in 19 of the 26 patients in the neoadjuvant chemoimmunotherapy group and in 5 of the 33 patients in the neoadjuvant chemotherapy group (relative risk [RR]: 4.315; 95% confidence interval [CI]: 1.836 to 10.142) (Figure 2A). A pCR occurred in 9 of 26 patients in the neoadjuvant chemoimmunotherapy group and in 1 of 33 patients in the neoadjuvant chemotherapy group (RR: 11.423; 95% CI: 1.544 to 84.493) (Figure 2B). The MPR group included three cases of postoperative recurrence, whereas the non-MPR group included 15 cases of postoperative recurrence. A statistically significant difference was observed in DFS postoperatively between the MPR and non-MPR groups (P = 0.048; Figure 3A). The neoadjuvant chemoimmunotherapy group included four cases of postoperative recurrence, whereas the neoadjuvant chemotherapy group recorded 14 cases. No statistical difference was observed in DFS between these groups postoperatively (P = 0.129; Figure 3B).
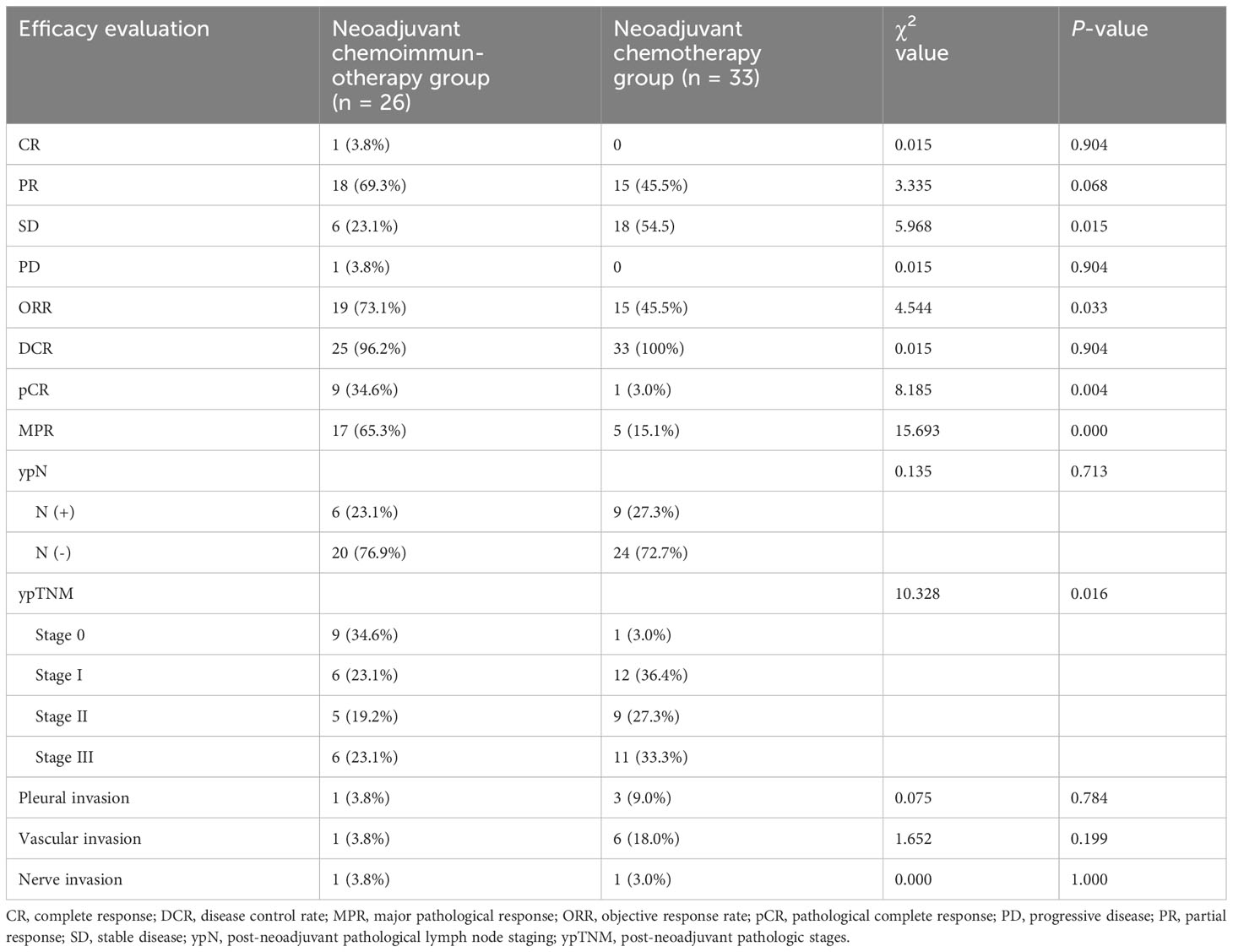
Table 2 Comparison of the efficacy after neoadjuvant therapy in 59 patients with non-small-cell lung cancer.
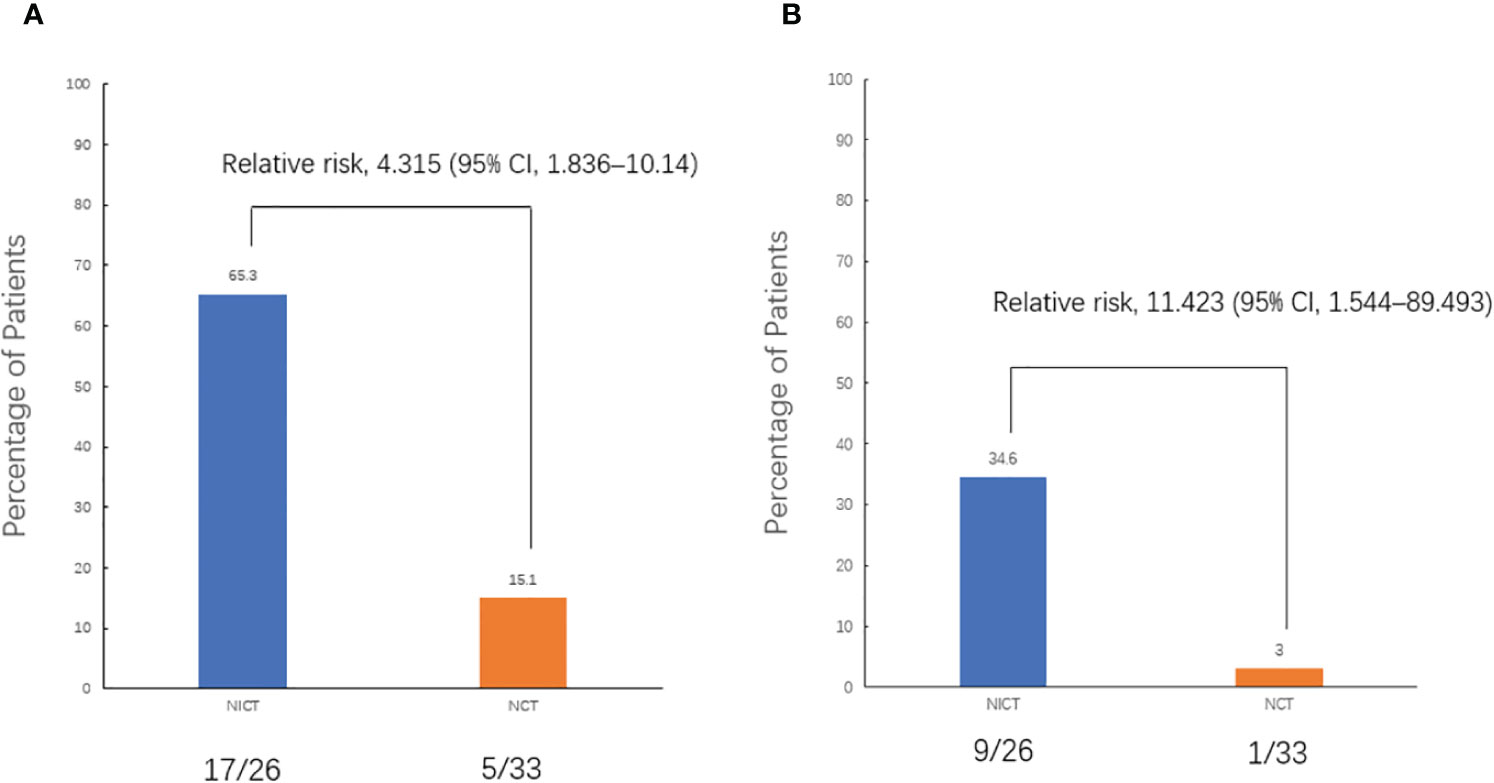
Figure 2 Pathological response. (A) Comparison of the major pathological response (MPR) rates between the two groups. (B) Comparison of the pathological complete response (pCR) rates between the two groups. All patients underwent randomization and received a neoadjuvant treatment. The assessment of pathological response was valid for all patients who underwent surgery (59 patients). pCR was defined as 0% residual tumor on pathological examination, and major pathological response (MPR) was defined as neoadjuvant therapy-induced tumor regression with ≤ 10% residual tumor on pathological examination. CI, confidence interval; NCT, neoadjuvant chemotherapy group; NICT, neoadjuvant chemoimmunotherapy group.
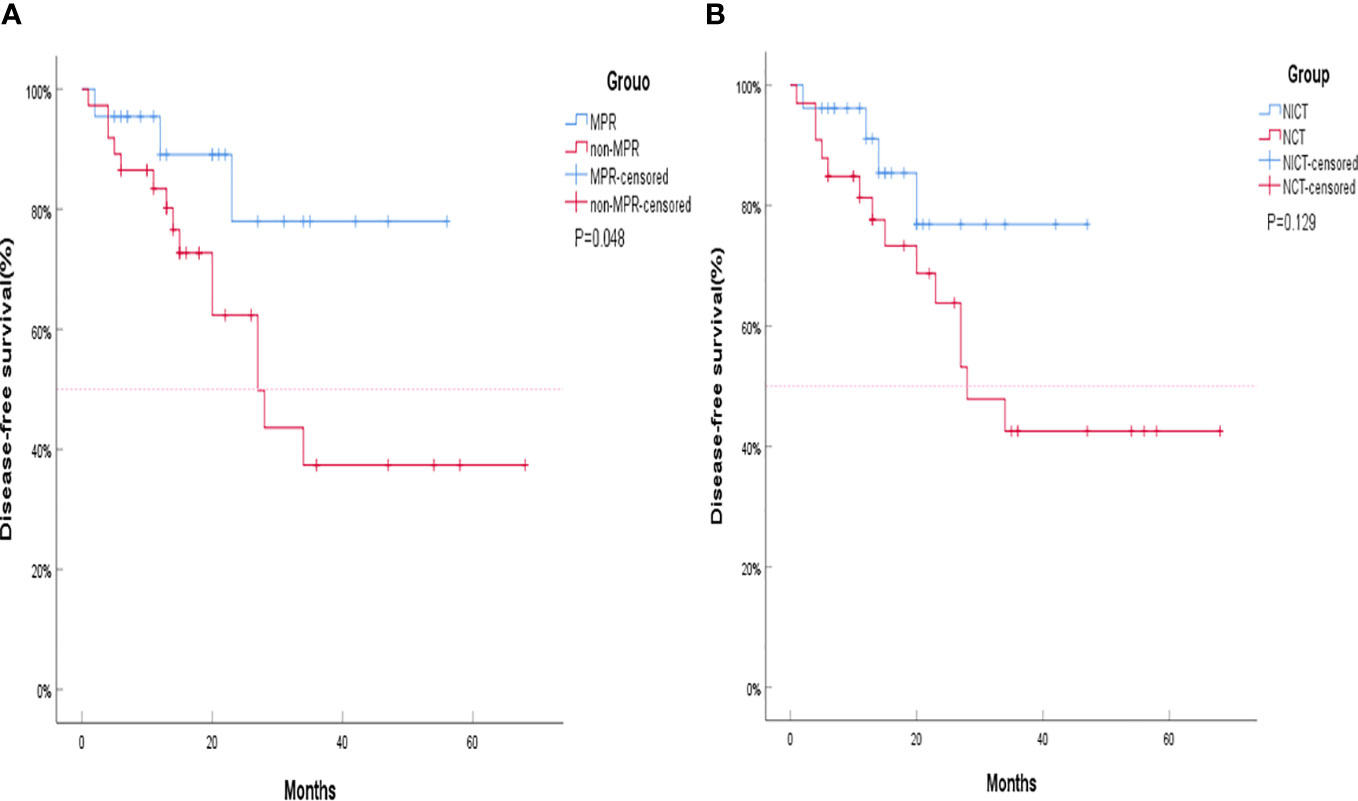
Figure 3 Kaplan–Meier curves for survival stratified by disease-free survival (DFS). (A) DFS stratified by treatment plan. (B) DFS stratified by major pathological response (MPR). The MPR group and non-MPR group are represented by Kaplan–Meier curves for survival stratified by DFS. The neoadjuvant chemotherapy group (NCT) and neoadjuvant chemoimmunotherapy (NICT) group are represented by Kaplan–Meier curves for survival stratified by DFS. Although the DFS of the NICT group and the NCT group did not show statistical differences, the separation of the two curves was already clear, and the DFS of the NICT group showed a trend of benefit. The shorter curve of the NICT group could be attributed to its later development compared to NCT, resulting in insufficient enrollment and follow-up time, which is also the reason that significant differences were not achieved. NCT, neoadjuvant chemotherapy group; NICT, neoadjuvant chemoimmunotherapy group; MPR, major pathological response.
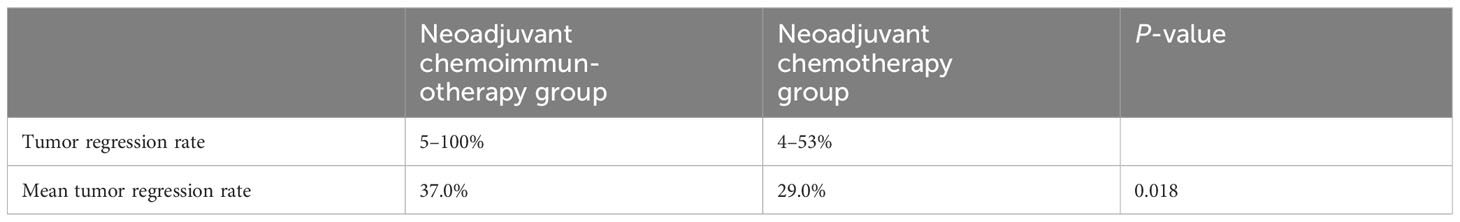
All patients in the neoadjuvant chemotherapy group experienced tumor regression (tumor regression rate: 4–53%; Figure 4A). The neoadjuvant chemoimmunotherapy group included 25 cases of tumor regression (tumor regression rate: 5–100%; Figure 4B). The tumor regression rate was higher in the neoadjuvant chemoimmunity group than in the neoadjuvant chemotherapy group (37.0% vs. 29.0%, P = 0.018; Table 3). The achievement of MPR in both groups showed no correlation with the degree of tumor shrinkage (P > 0.05; Table 4). The cumulative MPR rates were 42.3%, 50%, and 65.3%, while the cumulative adverse event rates were 76.9%, 84.2%, and 84.6%, for 2, 3, and ≥ 4 cycles, respectively, in the neoadjuvant chemoimmunotherapy group (Figure 5A). In the neoadjuvant chemotherapy group, these rates were 9.1%, 12.1%, and 15.1% (MPR rate), and 66.6%, 63.6%, and 63.6% (cumulative adverse event rate) for ≤ 2, 3, and ≥ 4 cycles, respectively (Figure 5B). The cumulative MPR rate of patients in the neoadjuvant chemoimmunotherapy group continuously increased with the number of treatment courses.
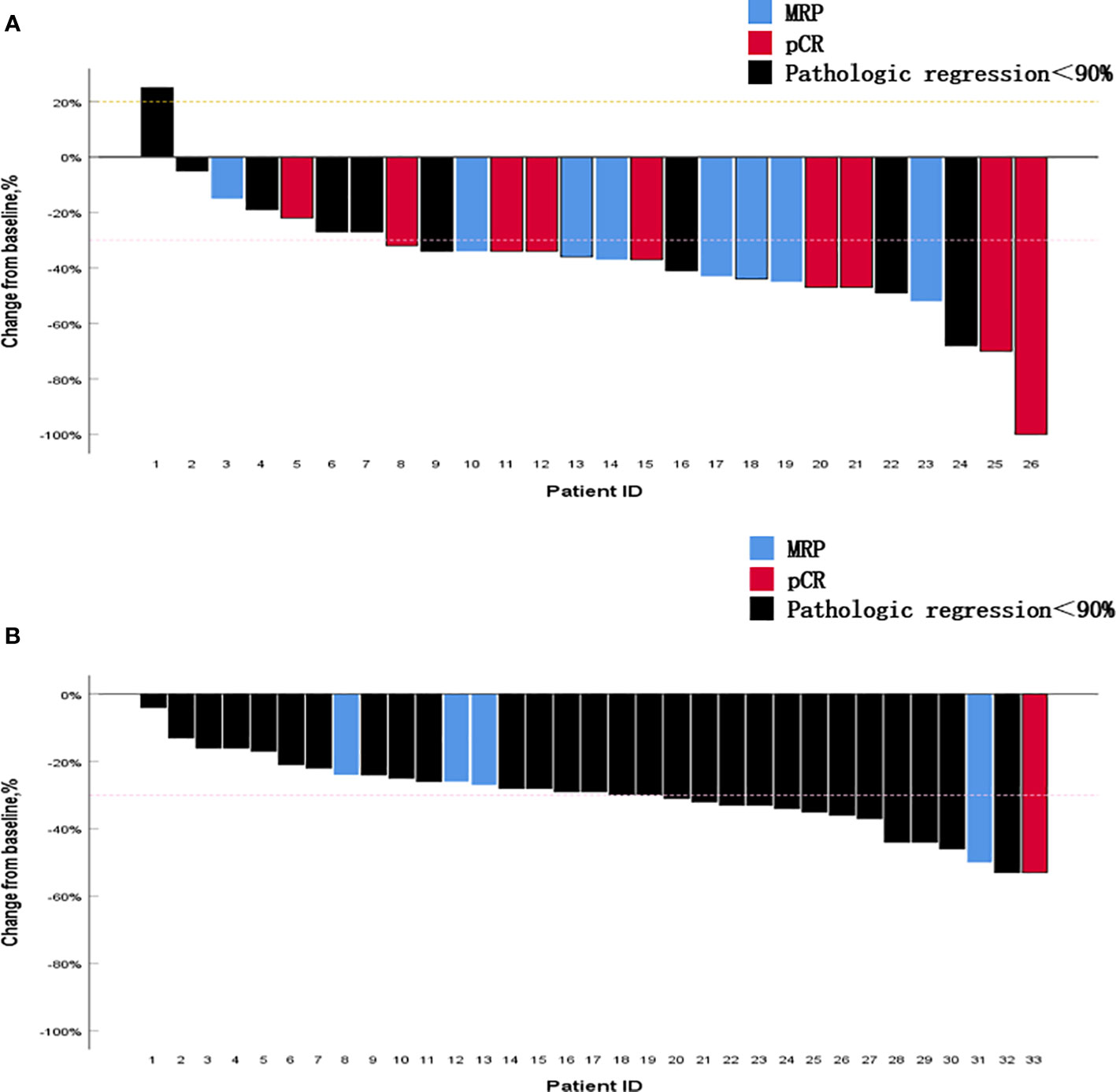
Figure 4 Percentage change in the diameter of the maximum target lesion from baseline. (A) Neoadjuvant chemoimmunotherapy group and (B) neoadjuvant chemotherapy group. The figure shows the change in the maximum tumor diameter after neoadjuvant therapy in all patients. It is noteworthy that most tumors that obtained pathological complete response (pCR) or major pathological response (MPR) did not exhibit corresponding complete regression in their imaging findings. MPR, major pathological response; pCR, pathological complete response.
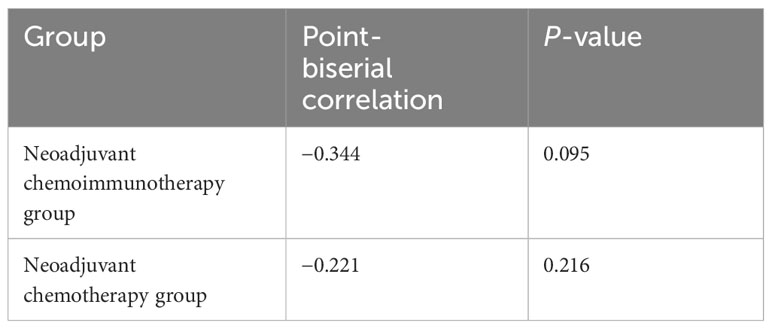
Table 4 Correlation analysis between the degree of tumor shrinkage and the major pathological response.
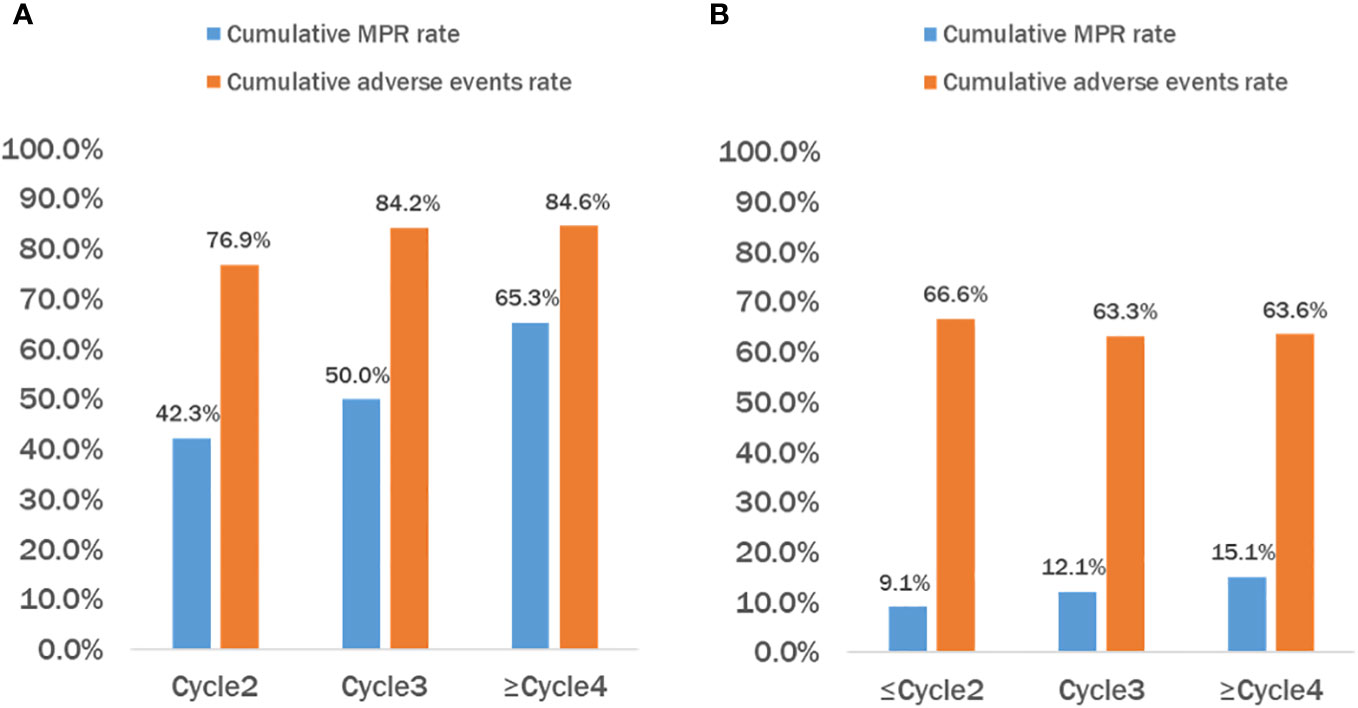
Figure 5 Cumulative MPR rate and cumulative adverse events rate of patients in the chemoimmunotherapy and chemotherapy groups. (A) Neoadjuvant chemoimmunotherapy (NICT) group and (B) neoadjuvant chemotherapy (NCT) group. It can be observed that as the treatment cycle increases, the cumulative major pathological response (MPR) rate in the NICT group also continues to rise, but the incidence of cumulative adverse events reaches a plateau after 3–4 courses of treatment. While the cumulative MPR rate in the NCT group also increased, it was significantly lower than that in the NICT group, and the cumulative adverse events continued to increase accordingly. NCT, neoadjuvant chemotherapy group; NICT, neoadjuvant chemoimmunotherapy group; MPR, major pathological response.
Comparison of the perioperative indicators of neoadjuvant therapies
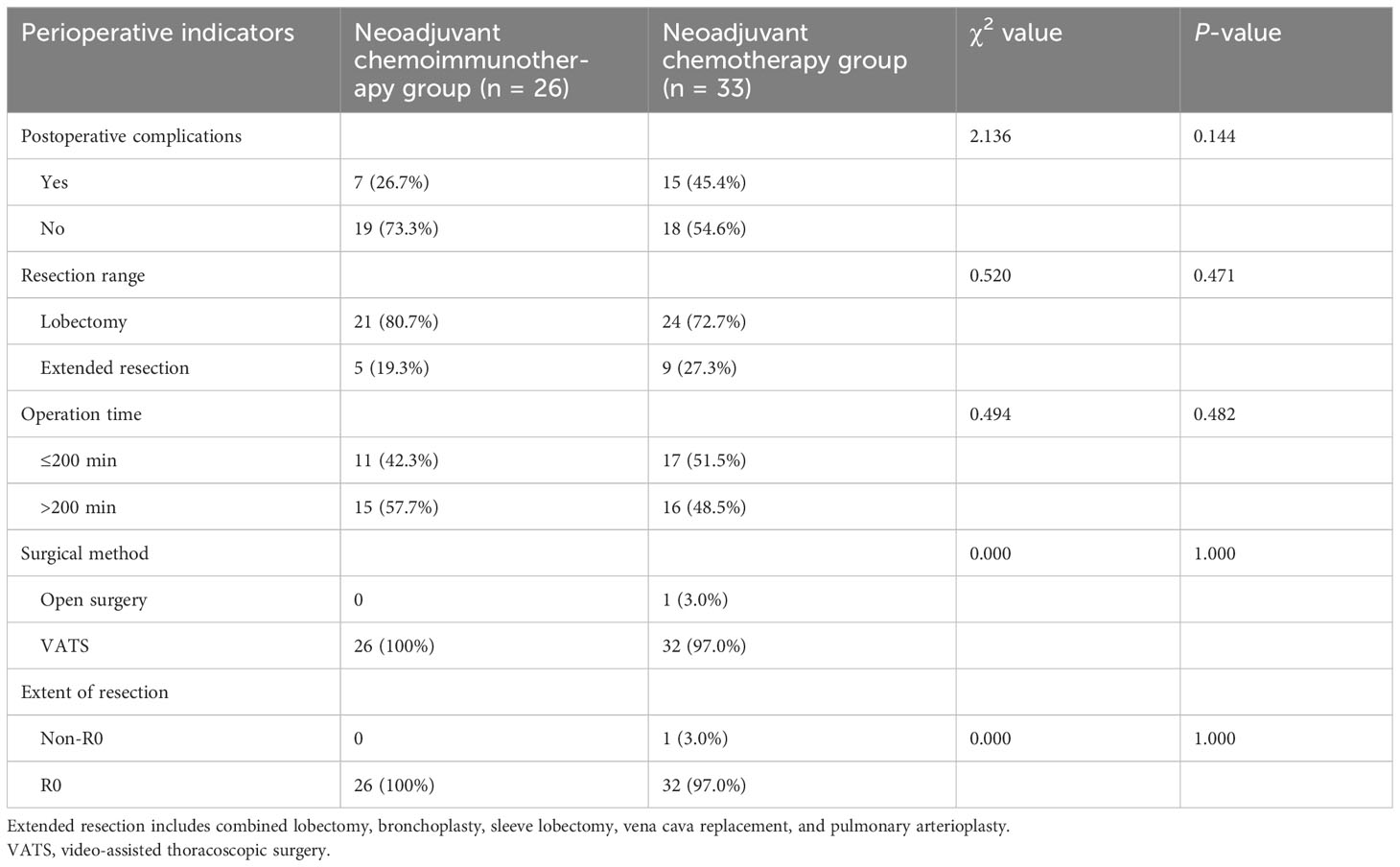
All 59 patients underwent surgical treatment. Analysis of postoperative complications, resection range, operation time, surgical method, and extent of resection exhibited no statistical difference between the two groups (P > 0.05; Table 5).
Comparison of the AEs of neoadjuvant therapies
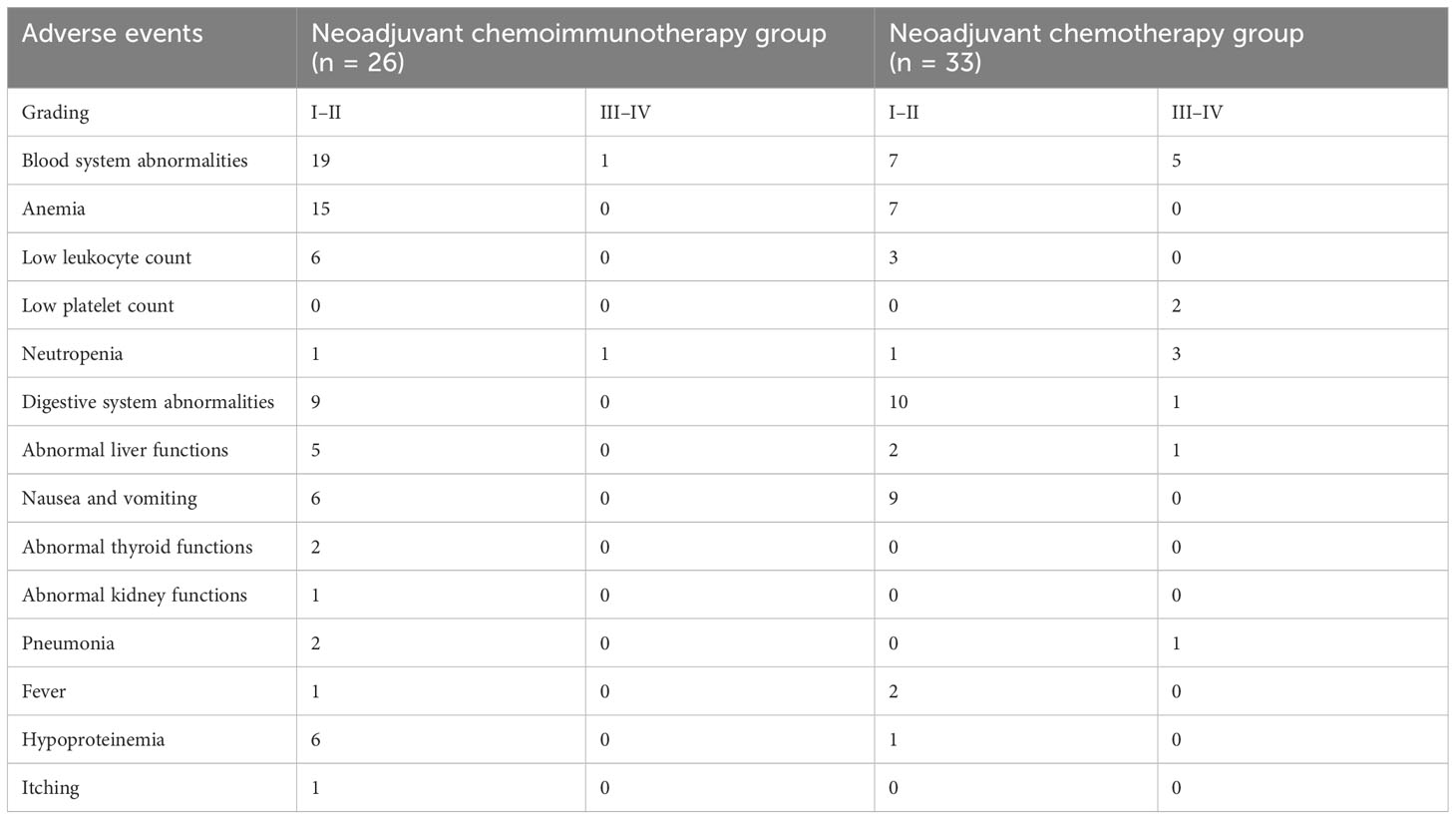
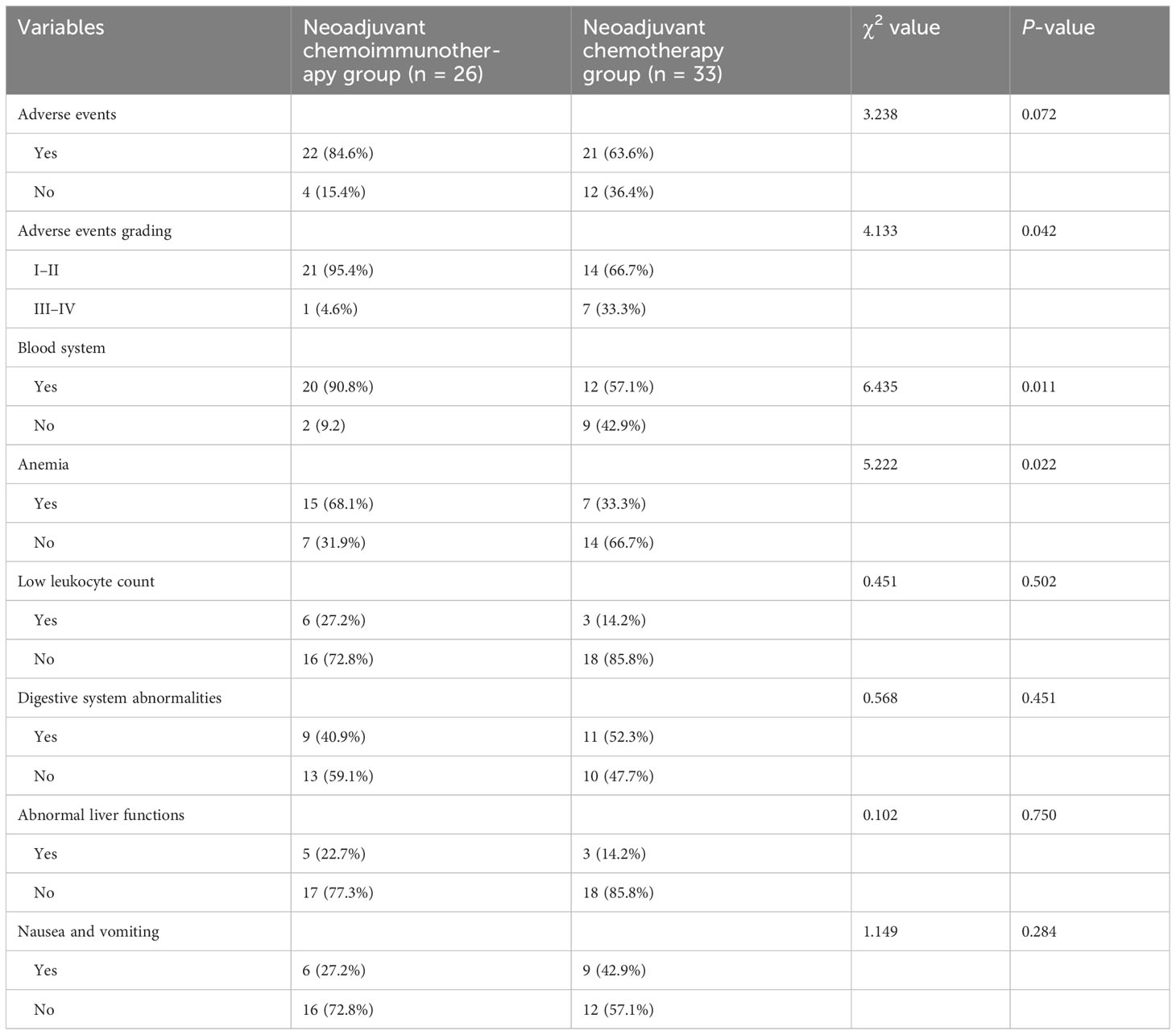
Table 6 presents the AEs of all patients in the study. The occurrence of grades III–V AEs was significantly higher in the neoadjuvant chemotherapy group than in the neoadjuvant chemoimmunotherapy group (P = 0.042). Blood system-related AEs were the most common in both groups (neoadjuvant chemoimmunotherapy group: 95.4%; neoadjuvant chemotherapy group: 57.1%). However, the incidence of AEs, such as low leukocyte count, liver function abnormalities, and nausea and vomiting, did not differ significantly between the two groups (P = 0.072; Table 7).
Discussion
Several studies (7, 8) have shown that chemotherapy not only induces immunogenic cell death (ICD) in tumor cells, leading to an anti-tumor immune response, but also improves the tumor microenvironment by removing some of the immunosuppressive cells and increasing the number of immune cells with anti-tumor effects, thus enhancing the anti-tumor immune response from the surface. Therefore, the combination of neoadjuvant immunotherapy with chemotherapy may be effective in treating cancer. The results of several clinical studies of neoadjuvant chemoimmunotherapy versus neoadjuvant chemotherapy in stage I–III NSCLC have shown that the pCR and MPR rates of the neoadjuvant chemoimmunotherapy group were significantly higher than those of the neoadjuvant chemotherapy alone group (9–11); however, most of these studies enrolled patients with a wider range of clinical stages, including stages I–III. The present study enrolled a better homogeneity of patients with stage III NSCLC, and used a variety of chemoimmunotherapy combination regimens, which is closer to the real-world situation, and also achieved better efficacy. Our findings are consistent with those of a prospective trial of neoadjuvant nivolumab in combination with platinum-based two-agent chemotherapy versus chemotherapy alone in stage III lung cancer (NCT03838159) (12). The 2014 (University of Texas Anderson Lung Cancer Study Group) Oncology Expert Consensus (13) concluded that the MPR is associated with long-term prognosis of patients with lung cancer. In our study, 59 patients were divided into MPR and non-MPR groups, and the DFS of patients in the MPR group was significantly higher than that in the non-MPR group, suggesting that an MPR indicates a good prognosis. Results from clinical studies have indicated that there is generally a higher MPR rate in neoadjuvant chemoimmunotherapy groups compared to neoadjuvant chemotherapy groups. Additionally, the DFS of neoadjuvant chemoimmunotherapy groups has been shown to be significantly better than that of neoadjuvant chemotherapy groups (9, 10, 14, 15). However, in the present study, we found that the DFS of the neoadjuvant chemoimmunotherapy group was not significantly higher than that of the neoadjuvant chemotherapy group (P = 0.129). This may be attributed to the short follow-up time in this study, as well as the fact that the neoadjuvant chemoimmunotherapy group had not yet reached the median follow-up time, which led to immature DFS data; however, the results indicated that the 2-year DFS rate of the neoadjuvant chemoimmunotherapy group was 76.9% higher than that of the neoadjuvant chemotherapy group, at 63.8%, suggesting that patients may potentially benefit from neoadjuvant chemoimmunotherapy. Therefore, it can be assumed that the new modality of neoadjuvant chemoimmunotherapy has better disease outcomes compared to neoadjuvant chemotherapy alone in stage III NSCLC, and may show better efficacy in terms of pathologic remission and thus a better prognosis.
The main purpose of neoadjuvant therapy is to shrink the tumor, reduce the stage, and improve the resectability of surgery. In this study, we found that the tumor regression rate of the neoadjuvant chemoimmunotherapy group was significantly higher than that of the neoadjuvant chemotherapy group, suggesting that neoadjuvant chemoimmunotherapy can more effectively achieve tumor shrinkage, which is conducive to radical surgical resection. The CheckMate-816 (11) results also suggested that the neoadjuvant chemoimmunotherapy group had a higher rate of R0 resection, a higher percentage of minimally invasive surgeries, and a lower proportion of total lung resections. By analyzing the correlation between patients’ tumor regression rate and MPR, it was found that there was no significant correlation between the two, which is similar to the findings of a phase 2 clinical trial (NCT04304248) (16). This suggests that although the tumor regression is not significant on imaging, the actual residual tumor activity may have been significantly reduced. Therefore, for patients undergoing neoadjuvant treatment with chemotherapy plus immunotherapy, aggressive surgery should be performed if the tumor is considered potentially resectable and more accurate evaluation methods should be adopted.
The duration of neoadjuvant immunotherapy is currently inconclusive, with most studies generally opting for 2–4 cycles. The current CheckMate-159 trial (17) gave two cycles of treatment with an MPR of 45%, and the NADIM study (18) gave three cycles of treatment with an MPR of 83%. The present study found that the cumulative MPR rate of patients in the neoadjuvant chemoimmunotherapy group increased with the number of treatment cycles. However, the cumulative adverse event rate of patients also increased in both groups. Due to the small sample size, our findings require further confirmation regarding whether: 1) extending the number of cycles improves the MPR rate and 2) the balance between the patient’s tolerance and the number of cycles of treatment needs exploration.
Several studies have found that surgical resection of the lungs after neoadjuvant immunotherapy is feasible and does not delay the timing to surgery. In addition, the overall perioperative outcome is relatively safe, similar to that of patients receiving neoadjuvant platinum-based chemotherapy (19, 20). Similarly, the findings of the present study suggested that neoadjuvant chemoimmunotherapy did not significantly increase surgery-related AEs and that surgery performed after neoadjuvant chemoimmunotherapy had a safety profile similar to neoadjuvant chemotherapy. Theoretically, the addition of immunotherapy inevitably increases drug-related AEs. In this study, the incidence of AEs was slightly higher in the neoadjuvant chemoimmunotherapy group, but grade I–II AEs were predominant, and the incidence of grade III and above AEs was much lower than that in the neoadjuvant chemotherapy group, which had less impact on the subsequent surgical treatment. According to a study, neoadjuvant chemoimmunotherapy not only has a synergistic anti-tumor effect but may also be caused by different antitumor mechanisms of the two drugs, thus not producing overlapping toxic reactions (21). Overall, compared to traditional neoadjuvant chemotherapy alone throughout the perioperative period, neoadjuvant chemoimmunotherapy is safer and limits the augmentation of adverse drug reactions. Therefore, neoadjuvant chemoimmunotherapy may be a safe treatment modality.
In conclusion, neoadjuvant chemoimmunotherapy not only has better efficacy than chemotherapy in stage III NSCLC but also has a reliable safety profile, which is an option for preoperative neoadjuvant treatment of stage III NSCLC. However, this was a small-sample retrospective clinical study with limited results; therefore, more large-sample, multicenter, prospective clinical studies are needed to verify the conclusions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Zhongshan City People’s Hospital (Zhongshan, China). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SHZ: Writing – original draft, Data curation, Formal Analysis, Investigation. YLiu: Data curation, Formal Analysis, Writing – original draft. KJL: Data curation, Investigation, Methodology, Writing – original draft. JKZ: Conceptualization, Supervision, Validation, Writing – review & editing. HLL: Investigation, Project administration, Supervision, Validation, Writing – review & editing. YMW: Project administration, Supervision, Validation, Writing – review & editing. HYY: Investigation, Supervision, Validation, Writing – review & editing. YLiang: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. JJZ: Conceptualization, Data curation, Project administration, Validation, Writing – review & editing. WZH: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Zhongshan Municipal Science and Technology Bureau (No. 2022B1140).
Acknowledgments
The authors wish to thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript; Zhongshan People’s Hospital for date preparation; and all participating investigators.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AEs, adverse events; BMI, body mass index; CI, confidence interval; CN, lymph node staging before neoadjuvant therapy; CR, complete remission; DCR, disease control rate; DFS, disease-free survival; MPR, major pathological response; NCT, neoadjuvant chemotherapy group; NICT, neoadjuvant immuno-chemotherapy group; NSCLC, non-small cell lung cancer; ORR, objective response rate; pCR, pathological complete response; PD, progressive disease; PD-L1, programmed cell death ligand 1; PR, partial remission; RR, relative risk; SD, stable disease; TTS, time to surgery - indicating the time from the end of neoadjuvant therapy to surgery; VATS, video-assisted thoracoscopic surgery; ypN, post-neoadjuvant pathological lymph node staging; ypTNM, post-neoadjuvant pathologic stages.
References
1. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
2. Shin S, Kim HK, Cho JH, Choi YS, Kim K, Kim J, et al. Adjuvant therapy in stage IIIA-N2 non-small cell lung cancer after neoadjuvant concurrent chemoradiotherapy followed by surgery. J Thorac Dis (2020) 12:2602–13. doi: 10.21037/jtd.2020.03.23
3. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines® Insights: non-small cell lung cancer, version 2.2023. J Natl Compr Canc Netw (2023) 21:340–50. doi: 10.6004/jnccn.2023.0020
4. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
5. Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol (2012) 7:825–32. doi: 10.1097/JTO.0b013e318247504a
6. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol (2018) 29:1853–60. doi: 10.1093/annonc/mdy218
7. Wu J, Waxman DJ. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett (2018) 419:210–21. doi: 10.1016/j.canlet.2018.01.050
8. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ (2014) 21(1):15–25. doi: 10.1038/cdd.2013.67
9. Zhang B, Xiao H, Pu X, Zhou C, Yang D, Li X, et al. A real-world comparison between neoadjuvant chemoimmunotherapy and chemotherapy alone for resectable non-small cell lung cancer. Cancer Med (2023) 12:274–86. doi: 10.1002/cam4.4889
10. Dai F, Wu X, Wang X, Li K, Wang Y, Shen C, et al. Neoadjuvant immunotherapy combined with chemotherapy significantly improved patients' overall survival when compared with neoadjuvant chemotherapy in non-small cell lung cancer: a cohort study. Front Oncol (2022) 12:1022123. doi: 10.3389/fonc.2022.1022123
11. Spicer J, Wang C, Tanaka F, Saylors GB, Chen K-N, Liberman M, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol (2021) 39:8503. doi: 10.1200/jco.2021.39.15_suppl.8503
12. Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med (2023) 389:504–13. doi: 10.1056/NEJMoa2215530
13. Hellmann MD, Chaft JE, William WN Jr., Rusch V, Pisters KMW, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol (2014) 15:e42–50. doi: 10.1016/S1470-2045(13)70334-6
14. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Abstract CT005: AEGEAN: A phase 3 trial of neoadjuvant durvalumab+ chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Cancer Res (2023) 83(8):CT005. doi: 10.1158/1538-7445.AM2023-CT005
15. Lu S, Wu L, Zhang W, Zhang P, Wang WX, Fang WT, et al. Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): Interim event-free survival (EFS) analysis of the phase III Neotorch study. J Clin Oncol (2023) 41(36):425126. doi: 10.1200/JCO.2023.41.16_suppl.8501
16. Zhao Z-R, Yang C-P, Chen S, Yu H, Lin Y-B, Lin Y-B, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology (2021) 10:1996000. doi: 10.1080/2162402X.2021.1996000
17. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med (2018) 378:1976–86. doi: 10.1056/NEJMoa1716078
18. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21:1413–22. doi: 10.1016/s1470-2045(20)30453-8
19. Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg (2019) 158:269–76. doi: 10.1016/j.jtcvs.2018.11.124
20. Yang C-FJ, McSherry F, Mayne NR, Wang X, Berry MF, Tong B, et al. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann Thorac Surg (2018) 105:924–9. doi: 10.1016/j.athoracsur.2017.09.030
Keywords: non-small-cell lung cancer, neoadjuvant therapy, chemotherapy, immunotherapy, efficacy, safety
Citation: Zhou S, Liu Y, Liu K, Zhang J, Liang H, Wu Y, Ye H, Liang Y, Zhang J and Huang W (2023) Comparison of neoadjuvant chemoimmunotherapy and chemotherapy alone for resectable stage III non-small cell lung cancer: a real-world cohort study. Front. Immunol. 14:1343504. doi: 10.3389/fimmu.2023.1343504
Received: 23 November 2023; Accepted: 11 December 2023;
Published: 22 December 2023.
Edited by:
Tiezheng Hou, University College London, United KingdomReviewed by:
Jiade J. Lu, Heyou International Health System, ChinaGuozhen Cui, Zunyi Medical University, China
Yaojun Zhang, Sun Yat-sen University Cancer Center (SYSUCC), China
Bin Zheng, Fujian Medical University Union Hospital, China
Copyright © 2023 Zhou, Liu, Liu, Zhang, Liang, Wu, Ye, Liang, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weizhao Huang, Z2FybWluZzk3QDEyNi5jb20=; Jingjing Zhang, amluZ2ppbmdmcmVpYnVyZ0AxMjYuY29t; Yi Liang, enNsaWFuZ3lpQHllYWgubmV0
†These authors have contributed equally to this work
 Sihao Zhou
Sihao Zhou Yi Liu1†
Yi Liu1† Weizhao Huang
Weizhao Huang