- 1Surgical Oncology Group, Frazer Institute, The University of Queensland, Brisbane, QLD, Australia
- 2Department of Surgery, Princess Alexandra Hospital, Brisbane, QLD, Australia
The poor treatment response of oesophageal adenocarcinoma (OAC) leads to low survival rates. Its increasing incidence makes finding more effective treatment a priority. Recent treatment improvements can be attributed to the inclusion of the tumour microenvironment (TME) and immune infiltrates in treatment decisions. OAC TME is largely immunosuppressed and reflects treatment resistance as patients with inflamed TME have better outcomes. Priming the tumour with the appropriate neoadjuvant chemoradiotherapy treatment could lead to higher immune infiltrations and higher expression of immune checkpoints, such as PD-1/PDL-1, CTLA4 or emerging new targets: LAG-3, TIM-3, TIGIT or ICOS. Multiple trials support the addition of immune checkpoint inhibitors to the current standard of care. However, results vary, supporting the need for better response biomarkers based on TME composition. This review explores what is known about OAC TME, the clinical significance of the various cell populations infiltrating it and the emerging therapeutical combination with a focus on immune checkpoints inhibitors.
1 Introduction
Oesophageal cancer is the sixth most lethal cancer worldwide (1). Although most cases are oesophageal squamous cell carcinoma (OSCC), its incidence is decreasing while the incidence of oesophageal adenocarcinoma (OAC) is rapidly rising worldwide (2). OAC cases outnumber OSCC in developed countries, which can be attributed to epidemiological risk factors. Known risks factors include increasing age, male sex, obesity, gastro-oesophageal reflux disease (GORD), smoking, and diets low in fibre (3). Over time GORD can lead to Barrett’s oesophagus (BO), a precancerous lesion with intestinal and gastric metaplasia composed of columnar and goblets cells replacing the normal squamous epithelia in the oesophagus. Symptoms appear at late stages, leading to late-diagnosis and poor survival. OAC 5-year survival is amongst the lowest (4). Perioperative chemotherapy or neoadjuvant chemo(radio)therapy (NAC) with surgery remains the standard of care for curative intent disease. However, with the advent of immunotherapy therapeutic options have expanded to include immune checkpoint inhibitors (ICI) in both the curative and palliative settings (5, 6). Significant treatment improvement has been made in other cancer types, but ICI response in OAC is moderate. Only a minority of patients shows a complete or partial response when treated with immunotherapy (7). Treatment strategies across cancers are moving towards personalised medicine. Hence, it is crucial to stratify patients and identify biomarkers to predict treatment efficacy and minimize toxicity.
Immunotherapy efficiency relies on the patient’s immune system, and more specifically on the immune cells surrounding and infiltrating the tumour. This immune infiltration, together with other cell populations, composes the tumour micro-environment (TME). Due to its potential predictive and prognostic value, the TME is becoming a major research focus to better tailor therapeutic strategies.
This review will explore the current knowledge concerning the OAC TME and focus on the clinical importance of considering the TME to guide therapeutic approaches.
2 OAC TME
Cancer is a genetic disease underpinned by DNA mutations that allow unregulated cell growth. These DNA mutations can result in the production of abnormal proteins, known as tumour antigens, which are eliminated by the host immune system in a process known as immunosurveillance. Tumours may escape immunosurveillance through several active mechanisms that results in the recruitment and modulation of diverse cell populations within the TME including immune and stromal cells. Therefore, characterising the TME in OAC is crucial to enabling informed patient selection to predict treatment response to standard therapies and ICIs and ultimately improve patient outcomes (8).
2.1 TME composition and clinical significance
Tumours evolve to form a complex ecosystem composed of cancerous cells infiltrated by diverse immune cells, mainly T cells, B cells and macrophages, and a rich stroma, mainly constituted of cancer-associated fibroblasts (CAFs), endothelial cells forming blood vessels and extracellular matrix components (9, 10). Depending on the proportion, location, and phenotype of the different cell populations, the TME switches between being pro- or anti-inflammatory (11). The TME communicates with tumour cells through cytokines, chemokines, and growth factors, leading to a dynamic tumour evolution following the environmental cues.
Chronic exposure of the oesophagus to gastric acid reflux favours a chronically inflamed environment. Recent studies showed a progressive shift in the immune populations present in the oesophagus during the evolution from BO to OAC (12, 13). Immune populations progressively invade BO microenvironment, however a diminution of the immune infiltrate is noted between a highly dysplastic oesophagus and OAC. The profiles of released cytokines and chemokines also support a more inflamed environment in OAC while immune-stimulating cytokines only show slight increase during the evolution from BO to OAC. Some evidence support the important role of CAFs in the development of BO and progression to OAC (14, 15). These immune changes contribute to the establishment of a pro-tumorigenic environment promoting OAC development (16).
Several studies describing the TME in OAC associated clinical outcomes to the presence or absence of specific cell populations. It is widely accepted that a higher level of total immune cells, or total tumour-infiltrating lymphocytes, is linked to longer overall survival (OS) independently from the chosen therapeutic approach (17–22). Derks et al. reported OAC had a lower T cell density compared to gastric tumours (23). It is important to distinguish between phenotypes of the infiltrating T cell subpopulation. Higher immunoregulatory T cell infiltrations have been found in non-responders to NAC, whereas complete responders showed higher tissue-resident or circulating memory T cells (24, 25). Stein et al. found contradictory results with a positive correlation between high regulatory T cell infiltration and OS (26). Effector T cells can also become ineffective or differentiate to an immunoregulatory phenotypes with the overexpression of specific surface proteins, known as immune checkpoints. These include programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte-associated protein 4 (CTLA-4), lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin and mucin domain 3 (TIM-3) or inducible T-cell costimulator (ICOS) (Figure 1). Several studies linked the high expression of immune checkpoints with treatment response or OS (22, 27). The location of the immune infiltrate is also a key parameter. Patients with a higher T cell infiltration in the tumour core had a better response to NAC and a significant survival benefit (19, 25, 26). However, this benefit was not observed in patients with high T cell infiltration in the tumour invasive margin or in the tumour periphery. Other studies reported CD8+ T cells located in the stroma were also a prognostic marker associated with better OS (21, 22). This emphasises the importance to precisely characterise T cell infiltration with subpopulation phenotyping while assessing the spatial distribution of these cells.
Other immune populations play a role in cancer progression and treatment, but fewer studies described them in OAC. Haddad et al. reported an enrichment of macrophages with an anti-inflammatory phenotype in the TME (21). High anti-inflammatory macrophage infiltration has been linked to NAC non-responders and poor survival in small OAC cohorts (24, 28). Derks et al. reported that contrary to CD8+ T cells being included from the tumour core, macrophages seemed to be able to infiltrate it quite homogeneously (23). Mylod et al. reported low levels of infiltrating NK cells compared to circulating NK cells (29).
Emerging evidence suggests that stromal cells play an important role. Courrech Staal et al. validated a stroma score in OAC biopsies, a higher score was associated with better survival (30). Huai et al. found that OAC stromal cell infiltration was correlated with tumour stage (31). Activated CAFs correlated with poor OS and favoured OAC growth and invasion through paracrine communication (32). Sharpe et al. reported CAFs drive resistance to chemotherapy and found that blocking CAFs phosphodiesterase type 5 would prevent normal fibroblasts to differentiate into CAFs (33). Manousopoulou et al. found many differentially expressed genes between normal fibroblasts and CAFs in OAC tumours, supporting the pro-oncogenic phenotype shift happening during stroma remodelling (34). Moreover, the stroma is the environment favouring angiogenesis, a crucial phenomenon for cancer oxygenation, growth and later invasion to distant sites (10). Endothelial cells appeared to be an indicator of good pathological response to chemotherapy (35).
Lymph node and distant site metastases worsen the prognosis. Lower levels of immune cells, specifically CD8+ T cells, have been associated with higher lymph node invasion (17, 18, 21, 36). Macrophages tend to have an immunoregulatory phenotype in nodal-spread OAC (28). Dos Santos Cunha et al. also observed mast cells and NK cells in metastasised OAC were favourable prognostic factors. These findings support the hypothesis that immune surveillance failure participates in cancer invasion.
2.2 Immune classification of the TME and clinical significance
First initiated by Galon et al., the Immunoscore was described for colorectal cancer and was validated as a reliable biomarker of immunotherapy response (37, 38). The use of the Immunoscore has been extended beyond colorectal cancer and appears to be a strong prognostic factor for other solid cancer types, including OAC (39–41). The initial Immunoscore relied on the density of CD8+ effectors and CD3+CD45RO+ memory T cells presence in the tumour core and invasive margin. The Immunoscore was established as a prognostic factor for OSCC (42). However, when applied to OAC, Conroy et al. noticed a higher expression of CD8+ and CD45RO+ T cells in the stromal compared to the tumoural regions and failed to demonstrate an association with survival (16). Patient classification in different immune groups can be tailored for a specific cancer type by considering additional immune populations associated with treatment response and survival. It appears that OAC immune stratification requires further markers.
Recent studies focused on defining OAC immune groups. Naeini et al. identified four immune clusters based on proportions of immune cells in the TME (8). The immune hot group was enriched in immune cell infiltrate and associated with the best OS, while the immune suppressed group was enriched in macrophages but depleted of lymphocytes and associated with the worst OS. The immune moderate group with moderate levels of lymphocytes and depleted of other immune cells and the immune cold group lacking all immune cells showed moderate OS. More studies found several immune clusters in OAC patients and support that inflamed TME correlated with better outcomes (41, 43).
Establishing an OAC-specific immune score chart combining several immune features of the TME would be highly informative to predict treatment response and adjust the regimen administered to patients (44).
3 Treatment evolution in OAC and modulation of the TME
3.1 First generation of treatment for OAC and its impact on the TME
The TME is dynamic and evolves with tumour progression or via administration of cytotoxic therapies. Few studies have investigated the effect of cytotoxic therapies on the OAC TME. Understanding how both chemotherapy and radiotherapy regimens effect the different TME profiles of OAC will allow better treatment selection based on the patient’s TME profile. Previous studies have found no difference in infiltrating T cell subpopulations between patients who underwent different NAC treatments or surgery alone (18, 21, 43). However, Soeratram et al. found an increase of CD8+ T cell density in inflamed post-NAC tumours (22). Furthermore, two recent studies found a higher T cell infiltrate after therapy where there was a poor pathological response. Croft et al. performed a single cell analysis comparing treatment-naive and NAC-treated OAC samples (35). The most notable proportion changes in the TME profiles were a reduction of the NK and T cell populations, and an increase of B cells, endothelial cells and fibroblast populations. However, poor pathological responders kept a higher proportion of NK and T cells compared to good responders. This result was supported by Koemans et al. showing that pathological non-responders had a higher CD8+ T cell infiltration which was associated with worse OS, whilst no association between T cell infiltration and survival could be found for good and moderate responders (45).
Fewer studies investigated other components of OAC TME following NAC. Cao et al. noticed the correlation between macrophage pro- and anti-inflammatory phenotype ratio and survival is diminished after therapy (28). NAC appears to also greatly impact CAFs based on the high number of differentially expressed genes identified by Croft et al. between pre- and post-treatment samples (35).
NAC also appears to influence immune checkpoint expression. Conflicting results support the complexity of OAC response to treatment. Several studies found immune checkpoints, such as PD-1, CTLA-4, TIGIT, TIM-3, LAG-3 or ICOS, to be significantly upregulated in infiltrating T cells following NAC (46, 47). An OAC cell line study validated ex-vivo also suggested increased expression of several immune checkpoint expression following radiotherapy (48). Soeratram et al. found increased stromal PD-L1+ T cells after NAC, suggesting that PD-1/PD-L1 blockade may be the recommended therapeutic strategy following chemoradiotherapy (22). In contrast, Galvin et al. demonstrated a significant reduction of intra-tumoural PD-1 expression following NAC (49).
These findings on OAC TME modulation combined with previous evidence in other cancer types, support the potential role of chemo(radio)therapy in switching cold tumours to hot through triggering an immune response from dying tumour cells or new neoantigen generation (46, 50). This could prime tumours to be more responsive to immunotherapy.
3.2 Immunotherapy landscape in OAC
ICI is now an established therapy aimed at preventing the inhibition of the anti-tumour immune response (44). The earliest trials investigating ICIs in OAC focused on treating advanced/metastatic oesophageal or gastro-oesophageal cancers with anti-PD-1, or anti-PD-L1 monoclonal antibodies (6, 51, 52) and have been summarised in a previous review (7). Results varied, but overall, in the advanced/palliative setting ICI appears to improve patient survival compared to chemotherapy alone. CheckMate-577 is the largest adjuvant phase III study to date and investigated adjuvant nivolumab in patients with residual disease post CRT and surgery (6). Although disease-free survival rates were improved in OAC patients, pathological response was poor. Furthermore, various trials have incorporated PD-1 blockade in different lines of therapy and have found that patients expressing high levels of PD-1 have improved outcomes (NCT04802876) (53). These results suggest that an immune inflamed TME in OAC is necessary to derive benefit from immunotherapy. Thus, more trials incorporated PD-1 blockade in therapeutic protocols, even in the neoadjuvant or adjuvant setting (Keynote-585 (NCT03221426), NCT02918162) (54, 55). In the neoadjuvant setting results are conflicting.
Several trials investigated the feasibility of combining chemotherapy with anti-PD-L1 antibodies. Gemstone-303 (NCT03802591) noted a modest but significant improvement in OS and PFS when patients underwent PD-L1 blockade combined with chemotherapy (56). MATTERHORN (NCT04592913) reports more patients with a pathological complete response when anti-PD-L1 antibodies were added to chemotherapy compared to chemotherapy alone (57). DANTE (NCT03421288) showed beneficial effects of the addition of PD-L1 blockade to chemotherapy on pathological regression, especially for patients with higher PD-L1 expression (58). Finally, the addition of anti-PD-L1 antibodies did not increase the number of responders in the PERFECT trial (NCT03087864) (59). In the PERFECT trial, a biological sub-study was performed and suggested TME features could be used as response biomarkers (59). Good pathological responders had low expression of genes linked to ICI resistance and were found to have higher OS and PFS after PD-L1 blockade compared to chemotherapy-alone in good responders. Focusing on the non-responders, two subgroups were identified, with either high infiltration of CD8+ T cells presenting an exhausted phenotypes or with very low CD8+ T cell levels. These results were found in a small patient cohort (n=40) but showed the role of the TME in ICI response. Arbore et al. also reported a subset of CD8+ T cells were correlated with a better response rate to PD-1 blockade, confirming the potential of TME features as predictors of ICI response (24).
Concerning other major immune checkpoint of interest, CTLA-4, a phase-II trial (NCT01585987) completed in 2015 compared the efficacy of ipilimumab to the best standard of care for advanced gastro-oesophageal junction cancers. However, the results did not show benefit in OS or PFS for patients treated with ipilimumab (60). Since, the administration of anti-CTLA-4 antibodies has been investigated in combination with anti-PD-1 or anti-PD-L1 therapies. Checkmate-649 (NCT02872116) did not demonstrate better survival with nivolumab/ipilimumab combination (61). However, Checkmate-648 used the same treatment combination on OSCC patients and found improved OS compared to chemotherapy alone (62). A combination of durvalumab and tremelimumab treatment showed encouraging results in a small cohort of patients (n=114) by a phase I/II trial (NCT02340975) (63). However, targeting CTLA-4 does not appear to be the most beneficial approach for OAC patients.
A recent trial (NCT05187338) is looking at a triplex checkpoint inhibitor combination therapy with ipilimumab, pembrolizumab and durvalumab for a range of solid tumours. It will surely be interesting to compare how patients with oesophageal cancer respond to this combination therapy and the range of adverse effects.
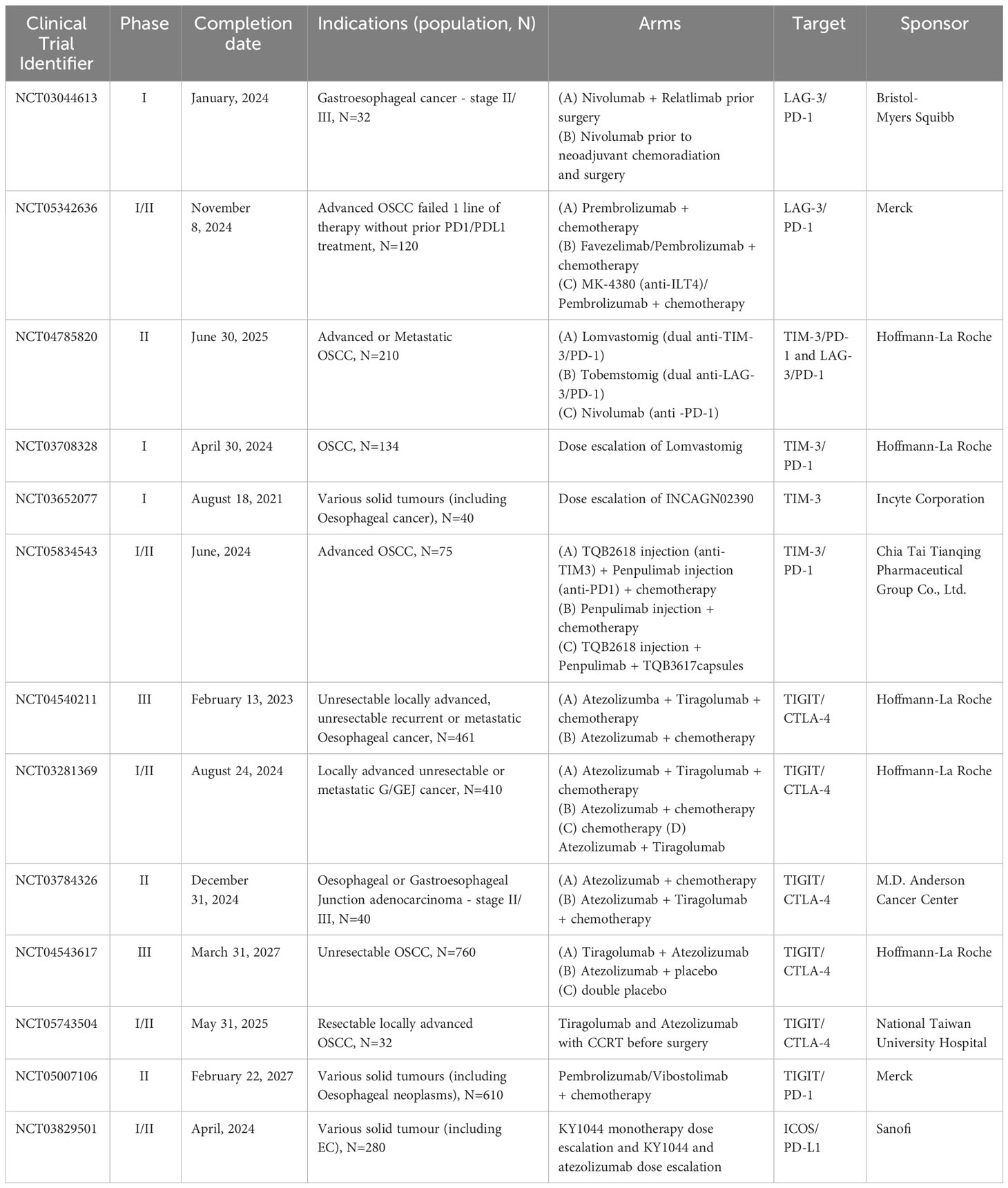
The PERFECT study showed high number of CD8+ T cells with a high expression of exhaustion markers, such as PD-1, TIM-3 or LAG-3 in non-responders (59). This suggests that patients responding moderately or not at all to the current immunotherapy options may benefit from a new generation of immunotherapies, targeting different immune checkpoints, such as TIM-3, LAG-3, TIGIT or ICOS (16, 44, 64). Trials focussing on dual blockade combinations (LAG-3/PD-1, TIM-3/PD-1 or TIGIT/CTLA-4) are still at early stages of completion and feasibility, tolerability and safety exploration (Table 1). These mainly phase I or II trials represent the first step to a better understanding of TME response to these immune checkpoints in oesophageal cancers.
Immune checkpoints play a major role in cancer immune evasion. Blocking the signals is one approach to promoting T-cell activity. Another approach is to inject checkpoint-deficient polyclonal T cells to replenish the effector population capable of targeting and killing tumour cells. Rapa Therapeutics is currently conducting a phase-I/II trial in several solid tumours, including oesophageal tumours, adding RAPA-201 cells to the standard of care chemotherapy protocols (NCT05144698). However, new targets are required as some ICI seem to decrease immune checkpoint expression on cells, which may contribute to developing ICI resistance (46). Trials attempt to assess the feasibility, safety and benefits from CLDN18.2 blockade (NCT03653507) (65), or RTK inhibitors added to PD-1 blockade (NCT04662710) (66).
4 Discussion and future perspectives
Despite tremendous results in other cancers, the mechanisms of immunotherapy response in OAC remain to be better understood in order to improve survival. There is growing evidence that the TME plays a critical role in treatment response and patient survival, particularly the infiltrating immune populations or stromal cells. OAC tumours are highly heterogeneous and surrounded by a largely immunosuppressive TME. However, individual study findings are conflicting due to the variability of the different study designs (sample collection, processing, used markers) or patient cohorts (demographics, stages). Efforts need to be directed towards better defining cell populations with multiple markers in large patient cohorts. Recent spatial technologies will provide additional details concerning the location and organisation of the immune infiltrate. Incorporating these insights with deep learning algorithms will lead to a better and more refined patient stratification. Grouping patients based on their tumour immune profiles appears to be a promising approach for advising the appropriate treatment or priming regimen for the tumour (8, 43). To date, patients with an immune enriched TME have better outcomes supporting the crucial role of the immune infiltrate. The second wave of blockade targeting LAG-3, TIM-3, TIGIT or ICOS will contribute to explore the anti-tumour immune response further.
Author contributions
CB: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. JL: Writing – review & editing. SB: Writing – review & editing. AB: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. JL is supported by the UQ Philip Walker Surgery Research Scholarship. SB received funding through the 2021 Priority-driven Collaborative Cancer Research Scheme and funded by Cure Cancer with the support of Cancer Australia (2010313). SB and AB received further funding from the Metro South Health Research Support Scheme (RSS_2022_039).
Acknowledgments
The figure was created with BioRender.com. The authors thank Dr. Vanessa Bonazzi for her insightful comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BO, Barrett’s oesophagus; CAF, cancer-associated fibroblasts; GORD, gastro-oesophageal reflux disease; ICI, Immune Checkpoint Inhibitor; NAC, neoadjuvant chemo(radio)therapy; OAC, oesophageal adenocarcinoma; OS, overall survival; OSCC, Oesophageal Squamous Cell Carcinoma; TME, tumour microenvironment.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol (2021) 18(6):432–43. doi: 10.1038/s41575-021-00419-3
3. Wong MCS, Hamilton W, Whiteman DC, Jiang JY, Qiao Y, Fung FDH, et al. Global Incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci Rep (2018) 8(1):4522. doi: 10.1038/s41598-018-19819-8
4. Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology (2022) 163(3):649–58 e2. doi: 10.1053/j.gastro.2022.05.054
5. Valkema MJ, Mostert B, Lagarde SM, Wijnhoven BPL, van Lanschot JJB. The effectivity of targeted therapy and immunotherapy in patients with advanced metastatic and non-metastatic cancer of the esophagus and esophago-gastric junction. Updates Surg (2023) 75(2):313–23. doi: 10.1007/s13304-022-01327-0
6. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
7. Lonie JM, Barbour AP, Dolcetti R. Understanding the immuno-biology of oesophageal adenocarcinoma: Towards improved therapeutic approaches. Cancer Treat Rev (2021) 98:102219. doi: 10.1016/j.ctrv.2021.102219
8. Naeini MM, Newell F, Aoude LG, Bonazzi VF, Patel K, Lampe G, et al. Multi-omic features of oesophageal adenocarcinoma in patients treated with preoperative neoadjuvant therapy. Nat Commun (2023) 14(1):3155. doi: 10.1038/s41467-023-38891-x
9. Davern M, Donlon NE, Power R, Hayes C, King R, Dunne MR, et al. The tumour immune microenvironment in oesophageal cancer. Br J Cancer. (2021) 125(4):479–94. doi: 10.1038/s41416-021-01331-y
10. Fu T, Dai LJ, Wu SY, Xiao Y, Ma D, Jiang YZ, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol (2021) 14(1):98. doi: 10.1186/s13045-021-01103-4
11. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
12. Sundaram S, Kim EN, Jones GM, Sivagnanam S, Tripathi M, Miremadi A, et al. Deciphering the immune complexity in esophageal adenocarcinoma and pre-cancerous lesions with sequential multiplex immunohistochemistry and sparse subspace clustering approach. Front Immunol (2022) 13:874255. doi: 10.3389/fimmu.2022.874255
13. Lagisetty KH, McEwen DP, Nancarrow DJ, Schiebel JG, Ferrer-Torres D, Ray D, et al. Immune determinants of Barrett's progression to esophageal adenocarcinoma. JCI Insight (2021) 6(1):e143888. doi: 10.1172/jci.insight.143888
14. Wang J, Zhang G, Wang J, Wang L, Huang X, Cheng Y. The role of cancer-associated fibroblasts in esophageal cancer. J Transl Med (2016) 14:30. doi: 10.1186/s12967-016-0788-x
15. Fujiya T, Asanuma K, Koike T, Okata T, Saito M, Asano N, et al. Nitric oxide could promote development of Barrett's esophagus by S-nitrosylation-induced inhibition of Rho-ROCK signaling in esophageal fibroblasts. Am J Physiol Gastrointest Liver Physiol (2022) 322(1):G107–G16. doi: 10.1152/ajpgi.00124.2021
16. Conroy MJ, Kennedy SA, Doyle SL, Hayes B, Kavanagh M, van der Stok EP, et al. A study of the immune infiltrate and patient outcomes in esophageal cancer. Carcinogenesis. (2021) 42(3):395–404. doi: 10.1093/carcin/bgaa101
17. Dos Santos Cunha AC, Simon AG, Zander T, Buettner R, Bruns CJ, Schroeder W, et al. Dissecting the inflammatory tumor microenvironment of esophageal adenocarcinoma: mast cells and natural killer cells are favorable prognostic factors and associated with less extensive disease. J Cancer Res Clin Oncol (2023) 149(10):6917–29. doi: 10.1007/s00432-023-04650-0
18. Noble F, Mellows T, McCormick Matthews LH, Bateman AC, Harris S, Underwood TJ, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother. (2016) 65(6):651–62. doi: 10.1007/s00262-016-1826-5
19. Schoemmel M, Loeser H, Kraemer M, Wagener-Ryczek S, Hillmer A, Bruns C, et al. Distribution of tumor-infiltrating-T-lymphocytes and possible tumor-escape mechanisms avoiding immune cell attack in locally advanced adenocarcinomas of the esophagus. Clin Transl Oncol (2021) 23(8):1601–10. doi: 10.1007/s12094-021-02556-2
20. Gao Y, Guo W, Geng X, Zhang Y, Zhang G, Qiu B, et al. Prognostic value of tumor-infiltrating lymphocytes in esophageal cancer: an updated meta-analysis of 30 studies with 5,122 patients. Ann Transl Med (2020) 8(13):822. doi: 10.21037/atm-20-151
21. Haddad R, Zlotnik O, Goshen-Lago T, Levi M, Brook E, Brenner B, et al. Tumor lymphocyte infiltration is correlated with a favorable tumor regression grade after neoadjuvant treatment for esophageal adenocarcinoma. J Pers Med (2022) 12(4):627. doi: 10.3390/jpm12040627
22. Soeratram TT, Creemers A, Meijer SL, de Boer OJ, Vos W, Hooijer GK, et al. Tumor-immune landscape patterns before and after chemoradiation in resectable esophageal adenocarcinomas. J Pathol (2022) 256(3):282–96. doi: 10.1002/path.5832
23. Derks S, de Klerk LK, Xu X, Fleitas T, Liu KX, Liu Y, et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann Oncol (2020) 31(8):1011–20. doi: 10.1016/j.annonc.2020.04.011
24. Arbore G, Albarello L, Bucci G, Punta M, Cossu A, Fanti L, et al. Preexisting immunity drives the response to neoadjuvant chemotherapy in esophageal adenocarcinoma. Cancer Res (2023) 83(17):2873–88. doi: 10.1158/0008-5472.CAN-23-0356
25. Goedegebuure RSA, Harrasser M, de Klerk LK, van Schooten TS, van Grieken NCT, Eken M, et al. Pre-treatment tumor-infiltrating T cells influence response to neoadjuvant chemoradiotherapy in esophageal adenocarcinoma. Oncoimmunology (2021) 10(1):1954807. doi: 10.1080/2162402X.2021.1954807
26. Stein AV, Dislich B, Blank A, Guldener L, Kroll D, Seiler CA, et al. High intratumoural but not peritumoural inflammatory host response is associated with better prognosis in primary resected oesophageal adenocarcinomas. Pathology (2017) 49(1):30–7. doi: 10.1016/j.pathol.2016.10.005
27. Humphries MP, Craig SG, Kacprzyk R, Fisher NC, Bingham V, McQuaid S, et al. The adaptive immune and immune checkpoint landscape of neoadjuvant treated esophageal adenocarcinoma using digital pathology quantitation. BMC Cancer (2020) 20(1):500. doi: 10.1186/s12885-020-06987-y
28. Cao W, Peters JH, Nieman D, Sharma M, Watson T, Yu J. Macrophage subtype predicts lymph node metastasis in oesophageal adenocarcinoma and promotes cancer cell invasion in vitro. Br J Cancer (2015) 113(5):738–46. doi: 10.1038/bjc.2015.292
29. Mylod E, Melo AM, Donlon NE, Davern M, Bhardwaj A, Reynolds JV, et al. Fractalkine elicits chemotactic, phenotypic, and functional effects on CX3CR1(+)CD27(-) NK cells in obesity-associated cancer. J Immunol (2021) 207(4):1200–10. doi: 10.4049/jimmunol.2000987
30. Courrech Staal EF, Smit VT, van Velthuysen ML, Spitzer-Naaykens JM, Wouters MW, Mesker WE, et al. Reproducibility and validation of tumour stroma ratio scoring on oesophageal adenocarcinoma biopsies. Eur J Cancer (2011) 47(3):375–82. doi: 10.1016/j.ejca.2010.09.043
31. Huai Q, Guo W, Han L, Kong D, Zhao L, Song P, et al. Identification of prognostic genes and tumor-infiltrating immune cells in the tumor microenvironment of esophageal squamous cell carcinoma and esophageal adenocarcinoma. Transl Cancer Res (2021) 10(4):1787–803. doi: 10.21037/tcr-20-3078
32. Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol (2015) 235(3):466–77. doi: 10.1002/path.4467
33. Sharpe BP, Hayden A, Manousopoulou A, Cowie A, Walker RC, Harrington J, et al. Phosphodiesterase type 5 inhibitors enhance chemotherapy in preclinical models of esophageal adenocarcinoma by targeting cancer-associated fibroblasts. Cell Rep Med (2022) 3(6):100541. doi: 10.1016/j.xcrm.2022.100541
34. Manousopoulou A, Hayden A, Mellone M, Garay-Baquero DJ, White CH, Noble F, et al. Quantitative proteomic profiling of primary cancer-associated fibroblasts in oesophageal adenocarcinoma. Br J Cancer (2018) 118(9):1200–7. doi: 10.1038/s41416-018-0042-9
35. Croft W, Evans RPT, Pearce H, Elshafie M, Griffiths EA, Moss P. The single cell transcriptional landscape of esophageal adenocarcinoma and its modulation by neoadjuvant chemotherapy. Mol Cancer (2022) 21(1):200. doi: 10.1186/s12943-022-01666-x
36. Kotsafti A, Fassan M, Cavallin F, Angerilli V, Saadeh L, Cagol M, et al. Tumor immune microenvironment in therapy-naive esophageal adenocarcinoma could predict the nodal status. Cancer Med (2023) 12(5):5526–35. doi: 10.1002/cam4.5386
37. Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet (2018) 391(10135):2128–39. doi: 10.1016/S0140-6736(18)30789-X
38. Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the 'Immunoscore' in the classification of Malignant tumours. J Pathol (2014) 232(2):199–209. doi: 10.1002/path.4287
39. Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med (2012) 10:205. doi: 10.1186/1479-5876-10-205
40. Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. (2020) 20(11):662–80. doi: 10.1038/s41568-020-0285-7
41. Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell (2021) 39(6):845–65.e7. doi: 10.1016/j.ccell.2021.04.014
42. Noma T, Makino T, Ohshima K, Sugimura K, Miyata H, Honma K, et al. Immunoscore signatures in surgical specimens and tumor-infiltrating lymphocytes in pretreatment biopsy predict treatment efficacy and survival in esophageal cancer. Ann Surg (2023) 277(3):e528–e37. doi: 10.1097/SLA.0000000000005104
43. Lonie JM, Brosda S, Bonazzi VF, Aoude LG, Patel K, Brown I, et al. The oesophageal adenocarcinoma tumour immune microenvironment dictates outcomes with different modalities of neoadjuvant therapy – results from the AGITG DOCTOR trial and the cancer evolution biobank. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1220129
44. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov (2019) 18(3):197–218. doi: 10.1038/s41573-018-0007-y
45. Koemans WJ, van Dieren JM, van den Berg JG, Meijer GA, Snaebjornsson P, Chalabi M, et al. High CD8(+) tumour-infiltrating lymphocyte density associates with unfavourable prognosis in oesophageal adenocarcinoma following poor response to neoadjuvant chemoradiotherapy. Histopathology (2021) 79(2):238–51. doi: 10.1111/his.14361
46. Davern M, Donlon NE, O’ Connell F, Sheppard AD, Hayes C, King R, et al. Cooperation between chemotherapy and immune checkpoint blockade to enhance anti-tumour T cell immunity in oesophageal adenocarcinoma. Transl Oncol (2022) 20:101406. doi: 10.1016/j.tranon.2022.101406
47. Kelly RJ, Zaidi AH, Smith MA, Omstead AN, Kosovec JE, Matsui D, et al. The dynamic and transient immune microenvironment in locally advanced esophageal adenocarcinoma post chemoradiation. Ann Surg (2018) 268(6):992–9. doi: 10.1097/SLA.0000000000002410
48. Donlon NE, Davern M, O'Connell F, Sheppard A, Heeran A, Bhardwaj A, et al. Impact of radiotherapy on the immune landscape in oesophageal adenocarcinoma. World J Gastroenterol (2022) 28(21):2302–19. doi: 10.3748/wjg.v28.i21.2302
49. Galvin KC, Conroy MJ, Doyle SL, Dunne MR, Fahey R, Foley E, et al. Extratumoral PD-1 blockade does not perpetuate obesity-associated inflammation in esophageal adenocarcinoma. Cancer Lett (2018) 418:230–8. doi: 10.1016/j.canlet.2018.01.039
50. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med (2007) 13(9):1050–9. doi: 10.1038/nm1622
51. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
52. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (2021) 398(10302):759–71. doi: 10.1016/S0140-6736(21)01234-4
53. Prat A, Paz-Ares L, Juan M, Felip E, Garralda E, Gonzalez B, et al. SOLTI-1904 ACROPOLI TRIAL: efficacy of spartalizumab monotherapy across tumor-types expressing high levels of PD1 mRNA. Future Oncol (2022). doi: 10.2217/fon-2022-0660
54. Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol (2019) 15(9):943–52. doi: 10.2217/fon-2018-0581
55. Manji GA, Lee S, Del Portillo A, May M, Ana SS, Alouani E, et al. Chemotherapy and immune checkpoint blockade for gastric and gastroesophageal junction adenocarcinoma. JAMA Oncol (2023). doi: 10.1001/jamaoncol.2023.4423
56. Zhang X, Wang J, Wang G, Zhang Y, Fan Q, Chuangxin L, et al. LBA79 GEMSTONE-303: Prespecified progression-free survival (PFS) and overall survival (OS) final analyses of a phase III study of sugemalimab plus chemotherapy vs placebo plus chemotherapy in treatment-naïve advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. Ann Oncol (2023) 34:S1319. doi: 10.1016/j.annonc.2023.10.080
57. Janjigian YY, Al-Batran SE, Wainberg ZA, Van Cutsem E, Molena D, Muro K, et al. LBA73 Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. Ann Oncol (2023) 34:S1315–S6. doi: 10.1016/j.annonc.2023.10.074
58. Al-Batran S-E, Lorenzen S, Thuss-Patience PC, Homann N, Schenk M, Lindig U, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol (2022) 40(Suppl 16):4003. doi: 10.1200/JCO.2022.40.16_suppl.4003
59. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res (2021) 27(12):3351–9. doi: 10.1158/1078-0432.CCR-20-4443
60. Moehler MH, Cho JY, Kim YH, Kim JW, Bartolomeo MD, Ajani JA, et al. A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1L) chemotherapy in patients with unresectable, locally advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer. J Clin Oncol (2016) 34(15_suppl):4011–. doi: 10.1200/JCO.2016.34.15_suppl.4011
61. Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. (2022) 603(7903):942–8. doi: 10.1038/s41586-022-04508-4
62. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med (2022) 386(5):449–62. doi: 10.1056/NEJMoa2111380
63. Kelly RJ, Lee J, Bang Y-J, Almhanna K, Murphy MAB, Catenacci DVT, et al. Safety and efficacy of durvalumab in combination with tremelimumab, durvalumab monotherapy, and tremelimumab monotherapy in patients with advanced gastric cancer. J Clin Oncol (2018) 36(15_suppl):4031–. doi: 10.1200/JCO.2018.36.15_suppl.4031
64. Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol (2023) 16(1):101. doi: 10.1186/s13045-023-01499-1
65. Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med (2023) 29(8):2133–41. doi: 10.1038/s41591-023-02465-7
66. Yanez PE, Ben-Aharon I, Rojas C, Eyzaguirre DA, Hubert A, Araya H, et al. First-line lenvatinib plus pembrolizumab plus chemotherapy versus chemotherapy in advanced/metastatic gastroesophageal adenocarcinoma (LEAP-015): Safety run-in results. J Clin Oncol (2023) 41(Suppl 4):411. doi: 10.1200/JCO.2023.41.4_suppl.411
Keywords: oesophageal adenocarcinoma, tumour microenvironment, immune infiltrate, immunotherapy, treatment response, immune checkpoints
Citation: Belle CJ, Lonie JM, Brosda S and Barbour AP (2023) Tumour microenvironment influences response to treatment in oesophageal adenocarcinoma. Front. Immunol. 14:1330635. doi: 10.3389/fimmu.2023.1330635
Received: 31 October 2023; Accepted: 30 November 2023;
Published: 13 December 2023.
Edited by:
John Reynolds, St. James’s Hospital, IrelandReviewed by:
Dina Schneider, Lentigen Technology, United StatesCopyright © 2023 Belle, Lonie, Brosda and Barbour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clemence J. Belle, Yy5iZWxsZUB1cS5lZHUuYXU=
 Clemence J. Belle
Clemence J. Belle James M. Lonie
James M. Lonie Sandra Brosda
Sandra Brosda Andrew P. Barbour1,2
Andrew P. Barbour1,2