- 1Department of Haematology-Oncology, National University Cancer Institute, Singapore (NCIS), National University Hospital, Singapore, Singapore
- 2Yong Loo Lin School of Medicine and Cancer Science Institute (CSI), National University of Singapore (NUS), Singapore, Singapore
- 3Yong Loo Lin School of Medicine, National University Centre for Cancer Research (N2CR) and Cancer Science Institute (CSI), National University of Singapore, Singapore, Singapore
Ovarian cancer (OC) is an aggressive malignancy characterized by a complex immunosuppressive tumor microenvironment (TME). Immune checkpoint inhibitors have emerged as a breakthrough in cancer therapy by reactivating the antitumor immune response suppressed by tumor cells. However, in the case of OC, these inhibitors have failed to demonstrate significant improvements in patient outcomes, and existing biomarkers have not yet identified promising subgroups. Consequently, there remains a pressing need to understand the interplay between OC tumor cells and their surrounding microenvironment to develop effective immunotherapeutic approaches. This review aims to provide an overview of the OC TME and explore its potential as a therapeutic strategy. Tumor-infiltrating lymphocytes (TILs) are major actors in OC TME. Evidence has been accumulating regarding the spontaneous TILS response against OC antigens. Activated T-helpers secrete a wide range of inflammatory cytokines with a supportive action on cytotoxic T-cells. Simultaneously, mature B-cells are recruited and play a significant antitumor role through opsonization of target antigens and T-cell recruitment. Macrophages also form an important subset of innate immunity (M1-macrophages) while participating in the immune-stimulation context. Finally, OC has shown to engage a significant natural-killer-cells immune response, exerting direct cytotoxicity without prior sensitization. Despite this initial cytotoxicity, OC cells develop various strategies to induce an immune-tolerant state. To this end, multiple immunosuppressive molecules are secreted to impair cytotoxic cells, recruit regulatory cells, alter antigen presentation, and effectively evade immune response. Consequently, OC TME is predominantly infiltrated by immunosuppressive cells such as FOXP3+ regulatory T-cells, M2-polarized macrophages and myeloid-derived suppressor cells. Despite this strong immunosuppressive state, PD-1/PD-L1 inhibitors have failed to improve outcomes. Beyond PD-1/PD-L1, OC expresses multiple other immune checkpoints that contribute to immune evasion, and each representing potential immune targets. Novel immunotherapies are attempting to overcome the immunosuppressive state and induce specific immune responses using antibodies adoptive cell therapy or vaccines. Overall, the OC TME presents both opportunities and obstacles. Immunotherapeutic approaches continue to show promise, and next-generation inhibitors offer exciting opportunities. However, tailoring therapies to individual immune characteristics will be critical for the success of these treatments.
1 Introduction
Ovarian cancer (OC) is a leading cause of women mortality by cancer, with more than 200,000 death annually (1). Significant progress was made this last decade regarding the molecular characterization of OC, particularly the discovery of recurrent alterations in the homologous recombination (HR) pathway, such as BRCA1 and 2 mutations, among others. OC has served as a model demonstrating that inhibitors of the poly-ADP ribose polymerase (PARPi) are synthetically lethal in case of deficient HR (HRD) leading to a tremendous improvement in progression-free and overall survival (2–6). Approximately 40% of newly diagnosed OC cases are estimated to be, with half of them having BRCA mutations, making them potential beneficiaries of maintenance PARPi after a positive response to platinum-based chemotherapy (7, 8). However, despite these advancements, most HRD patients will eventually recur and effective strategies in the relapse setting are still lacking. Furthermore, for the majority of OC patients not classified as HRD, and those with no objective response to platinum, no targeted therapy has demonstrated a survival benefit highlighting the need for new effective treatment strategies.
The majority of OC cases are diagnosed at advanced stages (stage III/IV) and typically present with pelvic masses, peritoneal nodules and, less frequently, distant metastases (lymph nodes, lung and liver) (9). Classically, OC will spread and disseminates via malignant ascites composed of free-floating tumors cells or spheroids alongside with a complex ecosystem (10). The tumor cells, together with their surrounding non-malignant cells, extracellular matrix and various signaling molecules, create a unique tumor microenvironment (TME) that promotes cancer progression.
Over the past twenty years, targeting TME has become a key therapeutic strategy in solid tumors (11). The first successes involved Programmed-death 1(PD-1)/Programmed-Death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways which are crucial mediators of cancer cells evasion from antitumoral T-cell-mediated cytotoxicity (12). Consequently, immune-checkpoint inhibitors (ICIs) targeting PD-1/PD-L1, and CTLA-4 were the first to be developed, demonstrating unprecedented benefits in selected patients and reshaping the therapeutic landscape for numerous cancers (13). Naturally, these ICIs were also tested on OC patients but ICIs as monotherapy or combined with chemotherapy have not been associated with any statistical significant survival benefits in phase III trials (14–17). It is suspected that the strongly immunosuppressive context and the number of actors involved in the OC TME are responsible for these disappointing results. Current data suggests that targeting the OC TME looks like an elusive goal, but it is also likely that focusing on T-cell activity and PD-1/PD-L1 pathway is too narrow in the context of the OC TME, and only an extensive characterization and more comprehensive understanding of the complex interaction between OC tumor cells and its microenvironment might change this paradigm.
2 Tumor-infiltrating lymphocytes in OC microenvironment
2.1 T-cells
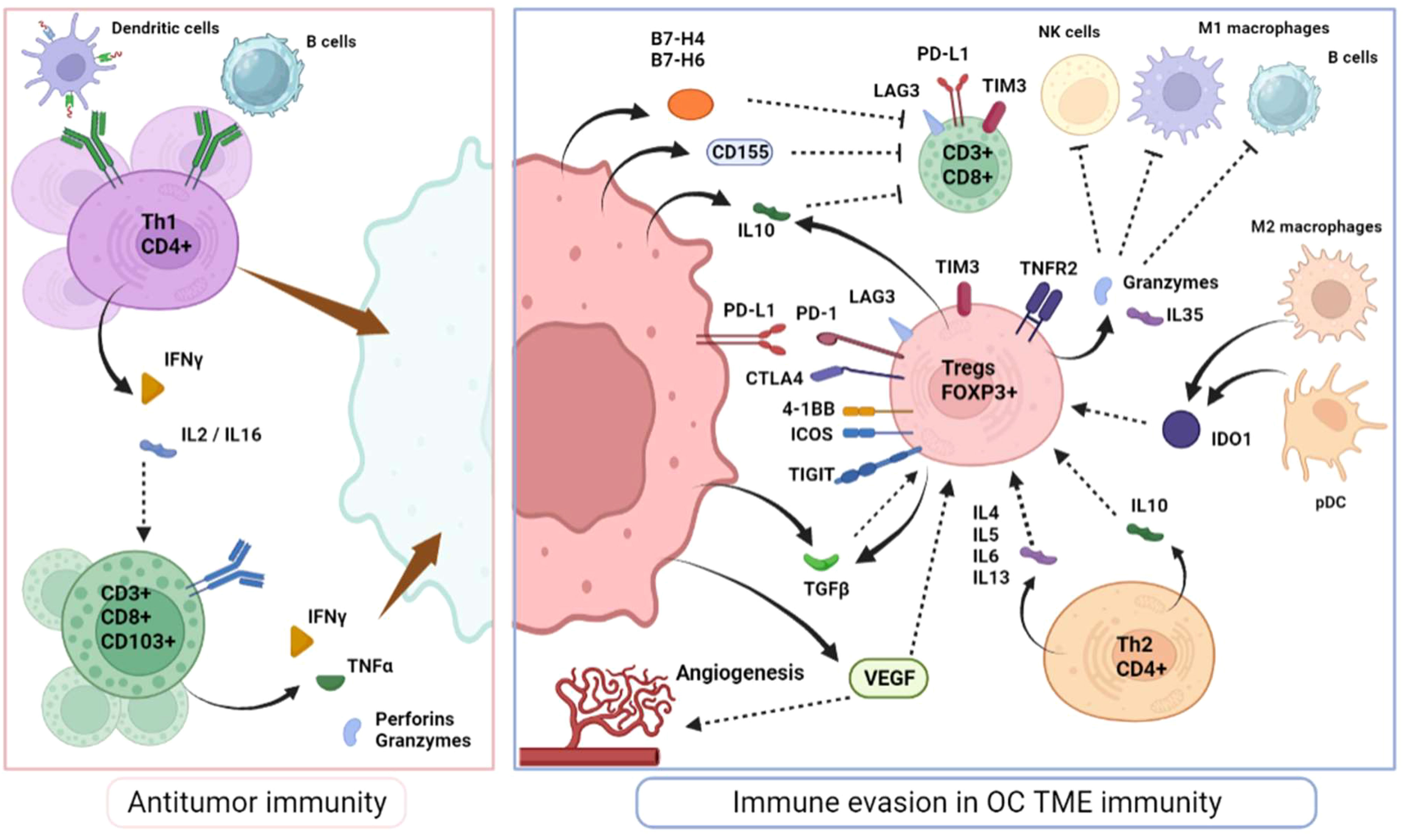
Intraepithelial cytotoxic T-cells (usually defined as CD8+) recognize cancer-specific antigens carried by presenting cells and generate local inflammation via cytokine release, particularly interferon-gamma (IFNγ) and tumor necrosis factor alpha (TNFα) and recruitment of secondary immune actors that lead to the elimination of tumor cells (Figure 1). Therefore, they are a critical determinant of antitumor adaptative immunity. In OC, similar to many other solid tumors, a high infiltration of cytotoxic TILs appears to be a positive prognosis biomarker (18, 19). Importantly, this prognostic impact is particularly associated with TILs’ within the tumor epithelium rather than in stromal areas (20). However, both the abundance of CD8+ T-cell in primary tumors or metastatic localizations retain prognostic value (20). Among cytotoxic T-cells, it is well-established that several subpopulations coexist, exerting a broad range of inflammatory effects and supportive functions. CD44 and CD69 are typically associated with the initial activation of CD8+ T-cells. Subsequently, effector T-cells tend to express at their surface, the CD103 marker, a component of the alpha E/beta 7 integrin that binds to E-cadherin on epithelial cells, the killer cell lectin-like receptor G1 (KLRG1) and IL-2 receptor subunit-α (CD25) while downregulating L-selectin (CD62L), IL-7 receptor subunit-α (CD127) and CD27 (21, 22). CD103+ TILS have been linked to improved survival rates in OC patients (23, 24). Additionally, alternative effector CD8+ T-cells subsets (Tc2, Tc9 and Tc17 cells) display complementary inflammatory effects and mediate CD4+ T-helpers through specific interleukins secretion, particularly IL-4, IL-9 and IL-17, although their identification in vivo remains challenging (25–27).
CD4+ T-helpers (Th) are activated by antigen-presenting cells and have dualistic effects. On one hand, they provide cytokine support (Interleukine-2, IL-16 and IFNγ) to effectors cells like CD8+ T-cells and macrophages and trigger the recruitment of dendritic cells, consequently enhancing the duration of cytotoxic response (Th1 cells). Thus, most reports have observed a survival benefit associated with the number of CD4+ TILs in OC TME (28, 29). On the other hand, the Th2 profile drives the immunosuppressive context by the secretion of IL-4, 5, 6 and 10 and may explain why the prognostic value of CD4+ cells is inconsistent (30).
Based on the TCGA dataset and then confirmed on independent cohorts, HRD tumors (associated with a BRCA1/2 mutation or not) exhibit a higher neoantigen load compared to HR proficient tumors (31). In addition, BRCA1 mutations have been associated with a modest increase in TILs infiltration (32). However, this was not the case for BRCA2 mutations (33).
Regulatory T-cells (Tregs) expressing FOXP3 transcription factors, are central actors in immune-regulation to avoid self-tissue toxicity (autoimmunity) but have a detrimental effect on the antitumor immune response (34). They can arise from naïve CD4+ exposed locally to transforming growth factor beta (TGFβ) and express elevated levels of CD25, or develop in the thymus and be recruited in the tumor TME (natural Tregs) (35). Tegs isolated from OC are highly activated as they tend to express multiple immunomodulatory molecules including PD-1, 4-1BB, TIGIT and ICOS and secrete important immunosuppressive factors such as IL-10, TGFβ and various granzymes (granzyme B, and perforins) that directly target other immune cells (36, 37). Indoleamine 2,3-dioxygenase (IDO1), vascular endothelial growth factor (VEGF), and B7-H4 are highly expressed in OC TME and have significant negative impact on effector T-cell proliferation while favoring Tregs proliferation and contributing to OC immune evasion (Figure 1) (38, 39). Moreover, a Treg subpopulation expressing the TNF receptor 1 (TNFR2), particularly immunosuppressive, is highly present in malignant ascites and could represent another factor driving OC dissemination (40).
Overall, increased Tregs infiltration (usually defined as CD4+/FOXP3+ phenotype) is correlated with immune tolerance and poor prognosis, including in OC (41–44). Consistently, a higher CD8/FOXP3 ratio appears to be correlated with improved outcomes (45, 46). Similarly, based on gene expression profiles, large retrospective studies have confirmed the large variability in TILs composition and the prognostic implications of specific immune infiltrates (47, 48). However, CD8+ T cells and Tregs were not found to be related with patients’ prognosis highlighting the probable coexistence of different functional subtypes not yet identified by this type of analyses (48).
Black plain arrows depict a secretion. Dotted stem arrows represent a inhibitory or a stimulatory signal. Tapered arrows represent a cytolysis effect.
2.2 B-cells
B-cells are classically known for their role in the antibody response against foreign antigens. As such, tumor-infiltrating B-cells stimulate antitumor immunity through opsonization of target antigens, complement activation and T-cell recruitment (49). However, recent studies have shown that they can also modulate immunity via the secretion of cytokine or other immunomodulatory molecules and through direct cell-cell interactions (50). Multiple B-cells subsets have been identified so far, including memory B-cells, antibody secreting B-cells (plasma cells), germinal center B-cells, regulatory B-cells (Bregs), etc. each with distinct surface markers. All of these subsets have been identified in OC, along with evidence of autoantibody response against OC antigens, such as P53 and NY-ESO-1 highlighting the importance of these immune cells in OC (51, 52).
Bregs are important immunosuppressive cells in the OC TME; they produce IL-10 and TGFβ, express several immune checkpoints at their surface (PD1, PDL1, OX40 etc) to impair CD4+ and CD8+ T cell proliferation and can promote tumor growth and progression via IL-35 signaling (53–55).
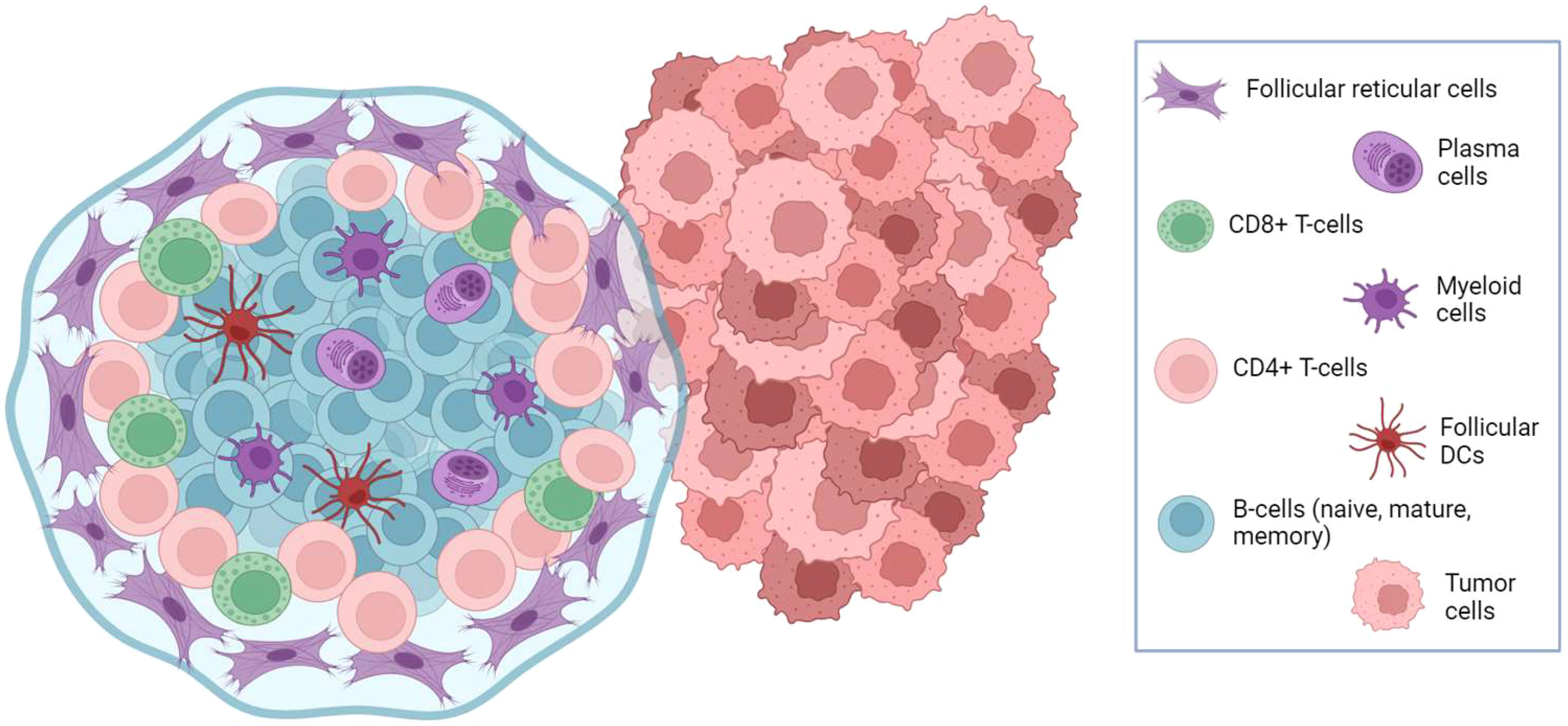
Mature B-cells (CD20+) and antibody-secreting B-cells (CD19+) are reported to be associated with favorable outcomes in OC (56–58). Furthermore, tumor infiltration by plasma cells can participate in the recruitment of CD8+ and CD4+ T-cell and follicular dendritic cells in organized lymphoid aggregates inside tumor stroma (Figure 2), resembling lymph nodes architecture and called tertiary lymphoid structures (TLS). TLS are documented in around 30% of OC but tend to be associated with improved overall prognosis (59). Similarly, the coexistence of a high density of CD20+ B-cells with high levels of CD8+ and CD4+ T-cell is associated with favorable outcomes (57).
Conversely, some studies have suggested that high B-cell infiltration is associated with poor prognosis (60, 61). These discrepancies suggest that both the assessment of the absolute abundance of B-cells and the evaluation of the relative infiltration by different B-cells subtypes are necessary when addressing their clinical relevance. In addition, while the understanding of the importance of B cells in the antitumor immune response is rapidly growing, there is a clear need for better markers to precisely characterize B-cell infiltration.
2.3 NK cells
NK lymphocytes are a part of innate immunity and they exert direct cell toxicity using perforin and granzymes. They also produce various chemokines and inflammatory factors (IFNγ, TNFα, CCL5, XCL1, XCL2) against tumor cells without prior sensitization, mostly in an antigen-independent manner (62). NK activity is tightly regulated by the balance between inhibitory and stimulatory signals. They are classically activated by CD16, NKp30 and NKG2D, while inhibited by MHC proteins present at the surface of healthy cells (63). Additionally, they can influence B-cell and T-cell shaping as well as the selection of immature dendritic cells (DC) depending on the antigenic signal (64–66). Interestingly, the OC TME has shown to contain multiple NK inhibitory signals such as macrophage migration inhibitory factor (MIF) responsible for inhibiting NKG2D signal and B7-H6 ligand, which contributes to the downregulation of NKp30 signaling (67, 68).
In OC, NK cells often co-infiltrate tumor stroma along with CD8+ T-cells and are observed in high numbers in malignant ascites (24). Consequently, higher NK infiltration (CD56+) appears as a positive predictive biomarker (69). Conversely, some authors have observed the opposite effect when considering different markers for NK cell detection. For instance, higher CD16+ cells infiltration appears to be associated with poorer prognosis (61). Taken together, NK cells seem to be crucial components of the OC microenvironment. However, the lack of robust biomarkers to assess their relative abundance and potential impairment is a major limitation to existing data.
3 Myeloid precursors, dendritic cells and macrophages
3.1 Tumor-associated macrophages
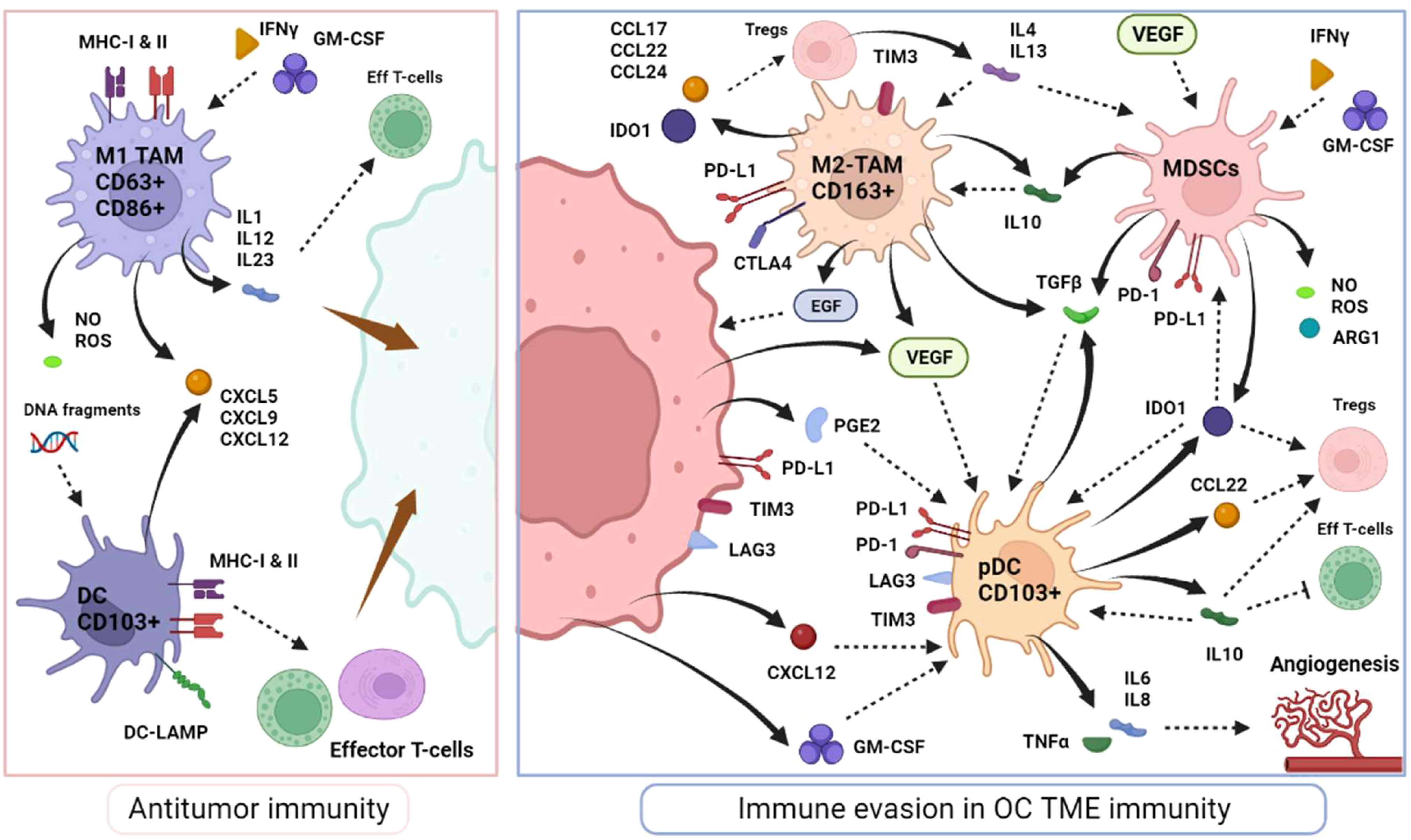
Macrophages are a key component of innate immune response in humans and the most abundant immune cell within the TME (70). Macrophages residing in the TME are called TAMs and can originate from circulating monocytes recruited and matured locally, or from the differentiation of tissue-resident macrophages (71). They are programmed to participate in antitumoral immunity as M1-polarized macrophages, characterized by their phagocytic functions, defined as CD63+CD86+, and stimulated by IFNγ and granulocyte/macrophage colony-stimulation factor (GM-CSF). Their inflammatory activity involves various inflammatory molecules such as IL-1, IL-12 and IL-23, TNFα, and chemokine ligands (CXCL5, CXCL9 and CXCL12) as well as tumor-specific antigen presentation via major histocompatibility complex molecules (MHC class 1 and 2) (72, 73). In the context of OC, high M1-TAMs infiltration (along with a high M1/M2 ratio) constitute a good prognostic biomarker (74–76)
On the other hand, it has been noted that M1-macrophages display strong plasticity and can transform into immune-suppressive protumoral immune cells as M2-polarized macrophages, under stimulation by prostaglandin E2 (PGE2), IL-4, IL-10 and IL-13 (77, 78). M2-macrophages express high levels of scavenger receptor class B (CD163) and secrete a variety of immunosuppressive molecules, such as IL-10, IL-13, TGFβ, CCL17, CCL22 and CCL24, which participate in monocytes differentiation to M2-macrophages (79, 80). They effectively modulate effector T-cell proliferation [through the expression of PD-L1 and CTLA4 receptors and arginase-1 (ARG1) activity] and enhance Tregs recruitment (mediated by CCL22) (81, 82). Aside from immunosuppression, M2 TAMs are involved in a wide range of actions including angiogenesis (VEGF secretion), TME remodeling and extracellular matrix degradation, adipocytes interaction and chemoresistance, all of which promote tumor progression and dissemination (83–88). In OC, M2-like TAMs play a role in the formation of spheroids during peritoneal dissemination via the secretion of epidermal growth (EGF) and VEGF and the absence of M2-like TAMs (as well as EGFR-blockage) seems to prevent metastatic dissemination in mouse models (Figure 3) (89, 90). Across various solid tumors, including ovarian cancer, a higher M2/M1-like ratio have shown to be correlated with advanced stage, high histologic grade, lymphovascular invasion and poorer outcomes (91, 92).
M2-like TAMs are the most represented immune cell in OC TME and play a pivotal role in OC carcinogenesis. Altogether, the interplay between OC and macrophages, and the subsequent balance between M1-inflammed macrophages versus M2-immunosuppressive cells, is likely critically important in the natural history of ovarian cancer.
3.2 Dendritic cells
Dendritic cells mainly home into lymph nodes or TLS and act as the most potent antigen-presenting cells. After capturing antigens, DCs mature into active DCs to present these foreign antigens on major histocompatibility complex, MHC-I and MHC-II, stimulating effector T-cells while providing inflammatory cytokines support (favoring Th1 immunity and cytotoxic T-cells proliferation) (93, 94). The abundance of mature DC (CD103+) appears to be correlated with the density of their secreted ligands such as CXCL9, CXCL10 and lysosomal-associated membrane protein 3 (DC-LAMP) and is associated with improved outcomes in OC (95–97). In OC, the abundance of DC-LAMP+ cells appears to be robustly associated with effective antitumor T-cell activity and better outcomes (96).
Nonetheless, in OC TME, tumor cells have shown to subvert normal physiological activity of DCs into an immature or an immunosuppressive phenotype, inhibiting T-cell activity instead of enhancing it. A subset of DC called plasmacytoid DCs (pDCs) is generally associated with inflammatory impairment. The immunosuppressive microenvironment, comprising CXL12, TGFβ, IL-10, VEGF, and IDO1, secreted by tumor cells (particularly in malignant ascites), Tregs and other immunosuppressive cells, drives the proliferation of immature and pDCS (98–100). In OC, immature DCs can secrete IL-6, IL-10, IDO1, ICOS-L and TGFβ among others, express PD-L1 and B7-H4 and thus, effectively disrupt the normal maturation of myeloid precursors, actively suppress T-cell responses, expand potent Tregs and consequently significantly weaken antitumor immunity (Figure 3) (101–105). In addition, pDCs are responsible for stimulating angiogenesis by the production of IL-8 and TNFα (98). Overall, most studies have revealed that the presence of pDCs correlates with low TILs infiltration, neoangiogenesis and, herein, is associated with poor prognosis (106, 107).
Therefore, the OC TME appears to profoundly alter DCs functions from potent inflammatory actors into central immunosuppressive mediators, fostering effective immune evasion and carcinogenic progression. The pivotal immunosuppressive role of DCs in the TME has led to considerable efforts in the development of DC-based vaccines intended to restore antigen-presenting capacity and improve antitumor immunity (108).
3.3 Myeloid-derived suppressor cells
Myeloid derived suppressor cells (MDSCs) are immature progenitors to myeloid cells such as dendritic cells and macrophages and they are stimulated by tumor cells to promote tumor growth and immunosuppression, particularly via inhibition of T-cells (109). The MDSCs are major producers of IL-10 in the TME, as well as a vast range of inflammatory mediators such as ARG1, nitric oxide (NO), reactive-oxygen species (ROS), TGFβ and IDO1, reinforcing their pivotal role in immune tolerance (110–112). In addition multiple factors highly expressed in the OC TME, have been shown to enhance MDSCs recruitment, such as cyclooxygenase-2 (COX2), prostaglandins, GM-CSF, and VEGF (Figure 3) (113–115). Other factors in the TME also tend to activate MDSCs, including IFNγ, IL-4 and TGFβ (111, 116, 117).
Active MDSCs inhibit effector CD4+ and CD8+ T-cells and attenuate NK-mediated cytotoxicity while also interfering with their migration capacity (118). Interestingly, OC favors their recruitment via inflammatory mediators like PGE2 which has been shown to induce the expression of PD-L1, CXCR4 and its ligand CXCL12 promoting angiogenesis, MDSCs accumulation and Tregs recruitment (119–121). Consistently, the OC TME contains high levels of tumor-infiltrating MDSCs, and an elevated number of MDSCs is associated with advanced stages, high-grade tumors, and poor prognosis (120, 122–124).
Nonetheless, the lack of specific markers has hampered their precise characterization and the understanding of their impact on immunosuppression
Black plain arrows depict a secretion. Dotted stem arrows represent a inhibitory or a stimulatory signal. Tapered arrows represent a cytolysis effect.
4 Compositional heterogeneity within the OC TME
The understanding of the composition and cross-talk between TME components remain critical in decoding the complex workings of the TME. Conventional bulk transcriptome analysis has made substantial contributions to our comprehension of the TME components. Bioinformatic deconvolution methods, such as CIBERSORTx and xCell, enable the inference of multiple cell types from bulk RNA-seq data, thus unveiling the heterogeneity of the TME. These methods employ predetermined gene signatures that represent each cell type, providing a general estimate of cell type abundance based on bulk data (125, 126). Immune deconvolution of TCGA data defined three immune subtypes: immune-activated, immune-suppressed and immune-desert, characterized by differing abundances of CD8+ T cells, M1 and M2 macrophages, and exhibiting distinct survival trends (127). Notably, neutrophil infiltration was associated with poor survival outcomes in only two out of the four molecular subtypes identified in HGSOC (128), underscoring the necessity to understand the tumor context and its surrounding components in order to elucidate the various immune-related influences on survival.
The emergence of single-cell RNA sequencing (scRNA-seq) technology has empowered us to explore the transcriptomic diversity of tumors and microenvironment components with unprecedented resolution (129). By incorporating unique molecular identifiers (UMIs) in droplet-based protocols, scRNA-seq allows the simultaneous analysis of thousands of individual cells from a single biopsy. This facilitates the detection of small cell populations that may hold prognostic significance. Several scRNA-seq studies have characterized components of OC TME, revealing extensive subpopulations of tumor, stromal and immune cells. For instance, scRNA-seq profiling of 12 HGSOC biopsies obtained from various sites (ovarian, peritoneal and omentum) revealed the existence of 32 stromal subclusters. Among these, distinct functional phenotypes of mesothelial cells, endothelial cells and fibroblasts were identified, with several showing correlation with poor survival. In another study focused on the landscape of infiltrating T cells in seven cases of HGSOC, 22 subclusters of T cells were detected, comprising 7 clusters of CD4+ and 15 clusters of CD8+ cells, each characterized by their unique signature genes (130). This discovery underscores the inherent functional differences within the T cell compartment.
Significantly, distinct TME niches can coexist within the same patient. Profiling OC at different tumor sites has revealed compositionally diverse TMEs within individual patients. In a study involving multi-site biopsies from 42 HGSOC patients. Comparative analysis revealed an enrichment of dysfunctional T cell in adnexal sites compared to non-adnexal sites (131). Similarly, the myeloid and DCs compartment exhibited significant compositional variations between solid tumor foci and ascites, both within and among patients. These findings illustrate the existence of site-specific immunophenotypes, that may impact the response to current treatment modalities. Differences in immune context across various tumor sites may thus contribute to the limited efficacy of current immunotherapy regimens. Approaches potentially effective for ovarian tumors may not necessarily apply as effectively to omental localizations and vice versa.
5 Spatial influences in TME
In addition to compositional differences in the TME, the classification of ovarian tumors into “immune-hot”, “immune-desert” or “immune-excluded” categories can be stratified based on the histological localization of CD8+ T cells. “Immune-hot” tumors are characterized by the presence and high density of CD8+ T cells within the tumor bed. “Immune-desert” tumors are lack CD8+ T cells in both the tumor bed and the tumor edges. Lastly, “Immune-excluded” tumors are defined by the retention of CD8+ T cells at the tumor edges without entering the tumor islets. Similarly, considering stromal CAFs known to regulate tumor immunity, the spatial positioning of CAFs also holds significance in mediating immunosuppression in cancer. In lung cancer, the histological presentation of alpha-smooth muscle actin (αSMA) CAFs lining tumor aggregates instead of being spread across the stroma is associated with decreased T cell infiltration into tumor nests, indicating a more “immune-excluded” phenotype. These observation underscore the importance of cell localization in mediating different states of the TME (132). Indeed, mounting evidence across various cancer types suggests that the spatial positioning of the cellular components within the TME, in relation to tumor cells, immune compartments, and vasculature, plays a crucial role in regulating anti- or pro-tumoral responses and understanding intricate intercellular networks (133–135).
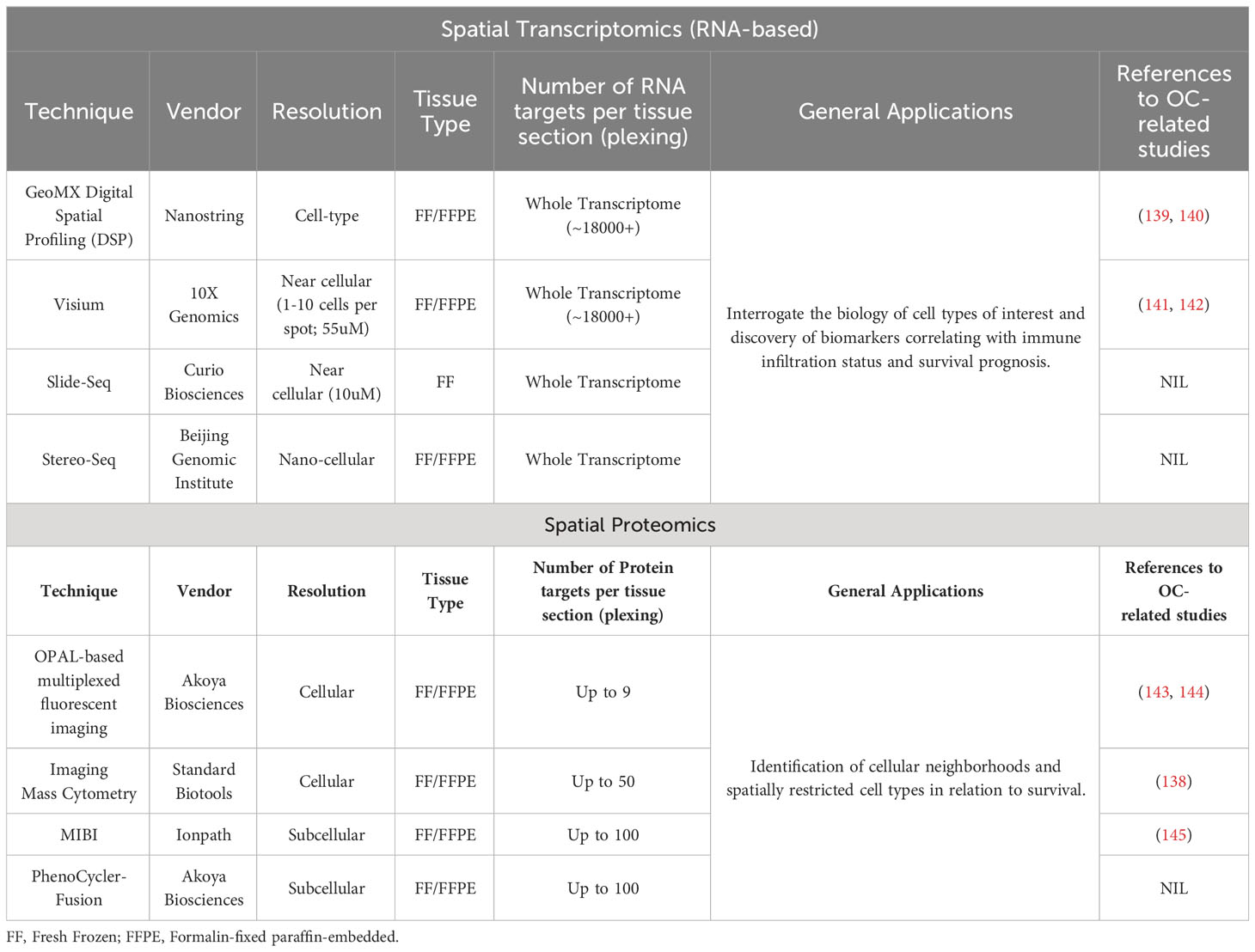
Our current understanding of the OC TME has been primarily shaped by traditional methods of immune deconvolution, such as immunohistochemistry (IHC), and hematoxylin and eosin (H&E) tissue staining. However, these low-content methods have limitations in their ability to comprehensively unravel the spatial architecture of the TME, which hinders the discovery of novel distributions of cell populations. Advanced spatial techniques include transcriptomic-based approaches such as Nanostring GeoMx (136) and 10X Visium, and protein-based methods, including OPAL-based multiplexed fluorescent imaging (137) and imaging mass cytometry (138). A key advantage of these techniques allows for the detection of multiple genes or proteins of interest within the spatial architecture, which were restricted in conventional IHC or in-situ hybridization transcriptomic methods. Table 1 highlights some of these spatial platforms in research.
These techniques are conducted on fresh frozen or FFPE tissues, preserving the tissue architecture and cellular morphology. This allows for information to be obtained from intact archival tissue in the original physiological context. The simultaneous detection of multiple genes or proteins in a single-tissue section enables the in-depth exploration of the biology of relevant cell types and their phenotypes within the TME, reaching unprecedented levels of detail (146). For example, application of imaging mass cytometry (IMC) on HGSOC tissues, allowed for the detection of 21 proteins of interest simultaneously in a single tissue section enabling profound levels of cell phenotyping, and capable of distinguishing functional differences within the same cell type, such as the detection of M1- from M2-polarized macrophages (138). The greater number of targets detected generally unveils the TME composition beyond the capabilities of traditional IHC modalities which detects only one single marker in a tissue sample. In addition, the preservation of spatial information facilitates the identification of unique spatial patterns of immune cells within the TME. It is established that cells closely clustered in proximity are likely to engage in stronger and more frequent interactions, potentially leading to functional remodeling of the TME and influencing treatment responses. These spatial patterns enable the identification of specific cellular neighborhoods and cell-cell crosstalk with distinct anti-tumor characteristics. For instance, multiplexed imaging analysis of HGSOC tissues revealed a prognostically relevant proliferative Ki67+ tumor cell population associated with enhanced spatial tumor-immune interactions involving cytotoxic and helper T cells in BRCA-mutated tumors, correlating with improved survival. Interestingly, the prognostic role of CD8+ T cells was conveyed only by their spatial arrangement near Ki67+ tumor cells rather than their relative abundance (147). In the TOPACIO trial (148) where OC patients received a PARP inhibitor (niraparib) combined with an anti-PD-1 antibody (pembrolizumab), spatial analysis of TME cellular neighborhoods revealed spatial clustering of exhausted CD8+ T cells with PDL1+ macrophages as a central factor in driving response towards PD1/PDL1-based immunotherapy (149). In a study examining CAFs in short-term versus long-term OC survivors, the APOE(CAFs) - LRP5(tumor) interaction pattern between CAFs and tumor cells at the tumor-stroma interface was associated with short-term survival. This specific cell-cell interaction may play a crucial role in modulating the malignant phenotype of OC, potentially serving as a spatially resolved prognostic biomarker for patient survival.
Overall, spatial features within the TME, including the spatial interactions of immune cell subpopulations, have the potential to be linked to treatment response and hold promise for more effective immunotherapeutic strategies and patient stratification in OC.
6 Non-cellular immune evasion actors
6.1 Immune-checkpoints
PD-L1 is a coregulatory molecule secreted on the surface of multiple immune cells and cancer cells. When it binds to its receptor PD-1, mainly present on lymphocytes, it generates an inhibitory signal (150). Importantly, PD-L1 expression on TILS can be induced by various factors secreted by TILS themselves or NK cells, such as IFNγ (151, 152). OC infiltrating DCs and MDSCs can also express both PD-1 and PD-L1 on their surface, promoting T-cell exhaustion (105, 121). However, there are inconsistent results regarding the expression of PD-L1 in OC, as each report uses different scoring system, different positivity thresholds, and different antibodies. It is generally accepted that around 30% of ovarian cancer cells show significant (≥5%) PD-L1 expression, whereas a higher rate of cases present concomitant PD-L1 on TILS (153–155). As for infiltrating lymphocytes, no significant association between PD-L1 expression and BRCA1/2 mutations has been observed (154, 156). Paradoxically, high PD-L1 expression is generally associated with improved outcomes, in particular in case of high number of CD8+ TILS (153, 157–159). Nonetheless, these results may simply suggest that PD-L1 expression behaves as a surrogate marker of CD8+ TILS infiltration.
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is another transmembrane receptor, belonging to the CD28 family, expressed on the T-cell surface that binds to CD80/CD86 (B7 ligand) to transmit an inhibitory signal. Upon T-cell activation, CTLA-4 is released to the cell surface and regulates effector T-cell and Tregs proliferation, B-cell response, and indirectly IL-2 production (160, 161). Physiologically, CTLA-4 exerts an inhibitory action on both effector and regulatory immune cells to avoid uncontrolled inflammation. Consequently, CTLA-4 inhibitors have been developed to promote T-cell expansion and increase antitumor response. In the context of OC, significant heterogeneity exists between CTLA-4 expression, suggesting different levels of B7-ligand pathway inhibition (162). Herein, it is anticipated that only the subset of patients with high CTLA-4 expression will benefit from CTLA-4 specific inhibition.
Likewise, lymphocyte activating 3 (LAG3) is also an coinhibitory checkpoint expressed on various mature immune cells, including activated CD4+ and CD8+ T-cells, FOXP3+ Tregs, NK cells and DCs (163–166). The activated LAG3 signal (induced by MHC class-II molecules) acts synergistically with PD-L1 signaling in disrupting CD4+ and CD8+ functions, inducing their apoptosis while favoring Tregs proliferation (167). Its expression has been found to be associated with increased PD-L1 expression and a higher number of CD8+ T-cells in OC TME (154, 168). Around 10% of TILS infiltrating the OC TME express LAG3 without any significant association with tumor mutational profile or patient outcomes (156, 169).
T cell immunoglobulin and mucin domain containing protein 3 (TIM3), also known as hepatitis A virus cellular receptor 2, is an immune checkpoint receptor present on the surface of dysfunctional CD4+ and CD8+ T-cells, Tregs, NK-cells, M2-macrophages and DCs (170–172). On T-cells, it binds to galectin-9 and carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1) carrying both an inhibitory signal inducing effector T-cell exhaustion on one hand, and a stimulatory signal enhancing Tregs immunosuppressive state on the other hand (173). At the same time, TIM3 is constitutively expressed on DCs and macrophages and appears to favor their immunosuppressive functions within the TME, although this mechanism is still unclear (174, 175). Like LAG3 and PD-L1, the expression of TIM3 is significantly correlated with TILS in OC (176). Importantly, TIM3 expression on OC TILS is very frequent, and most TIM3+ cells coexpress one or multiple other coregulators simultaneously (156, 177).
Platinum remains the cornerstone in the treatment of OC and is associated with compelling activity in 1st line, with response rates of more than 70% (178). Consequently, platinum chemotherapy has been increasingly used in the neoadjuvant chemotherapy (NACT) setting to decrease tumor burden and help achieve completeness in surgery (179). This window-of-opportunity between diagnostic biopsies and interval debulking surgery has allowed the comparative assessment of OC immunogenicity before and after chemotherapy exposure. Interestingly, multiple teams have demonstrated immunogenic cell death with a positive impact of NACT on CD4+, CD8+ and NK-cells, suggesting an antitumor immune response induced by the chemotherapy (46, 180–182). Conversely, some reports have also observed a significant increase in PD-L1, CTLA4, and LAG3 expression under NACT which probably tempers the immunogenicity (156, 182–184). Altogether, these observations suggest that NACT primes an immunocompetent TME in which ICIs could be more efficient.
6.2 Cytokines, VEGF and interleukins
IDO1 is an immune regulatory factor induced by the inflammation cascade, including signaling by interferons, IL-10 and TGFβ). It is often secreted by cancer cells, DCs and macrophages, and rarely by TILS, and its expression generates an immune-permissive TME (185). IDO1 plays a crucial role in activating Tregs, allowing a stable and IDO1-independent immunosuppressive TME (186). Additionally, IDO1 appears to upregulate PD-L1 and CTLA-4 expression, indirectly affecting T-cell activation. Furthermore, IDO1 also contributes to the recruitment of MDSCs and enhances their suppressor functions (187, 188). In OC, IDO1 is frequently expressed, and higher expression is associated with chemoresistance and poor prognosis (189).
VEGF is a family of proteins regulated by hypoxia-induced genes (HIF) and EGF, and its role in OC angiogenesis, progression and intraperitoneal dissemination is well-established (190, 191). VEGF has also been shown to induce the evasion of innate and adoptive immune response by inhibiting the maturation of DCs and promoting Tregs activity. In the context of OC, a large amount of VEGF is secreted in the TME, particularly in malignant ascites, in contrast to normal ovarian tissue and non-malignant ascites (192, 193). Recently, it has been observed that VEGF expression can be induced by multiples factors present in OC TME, such as PGE2, TGFβ, and TNFα, reinforcing its close relationship with the immunosuppressive context (194, 195). Elevated levels of VEGF are associated with poor prognosis in OC patients (99, 196, 197). Based on these considerations, bevacizumab, a VEGF inhibitor, has been intensively evaluated in OC patients and has demonstrated higher response rates and improved outcomes. It is now FDA-approved and routinely prescribed (198, 199). Interestingly, bevacizumab has been shown to significantly increase T-cells and B-cells infiltration, enhance DC maturation and block Tregs proliferation in colorectal and breast models (200–202). Altogether, these reports suggest that using VEGF-targeting agents may give positive effects on the OC TME and enhance antitumor immune responses.
As previously mentioned, the cytokine context within the OC TME, plays a crucial role in creating an immunosuppressive state that allows tumor progression and immune evasion. IL-10 produced by various immune cells and OC cells themselves, is a soluble factor which acts as a potent negative regulator of antitumor inflammation. Its precise mechanisms on effector immune cells are not fully understood, but it likely involves the induction of other inhibitory factors like PGE2, the active blockage of DC maturation, the induction of PD-1 expression and the deletion of antigen presentation by ovarian tumor cells (203, 204). Interestingly, PD-1 blockade can lead to a high release of IL-10 in OC TME, suggesting a compensatory mechanism to escape inflammatory response (204). Similarly, IL-6 produced mainly by macrophages and cancer cells, plays a critical role in promoting angiogenesis, ascites development, and T-cell exhaustion (205, 206). Overall, both IL-10 and IL-6 are independent predictors of poorer outcomes in OC (206–209).
TGFβ is another cytokine that is highly expressed in OC TME and has a dual role, acting as both a tumor suppressor and a promotor of tumor progression and immunosuppression. Cancer cells that escape the immunosuppressive effects of TGFβ can overexpress it to promote invasiveness, neoangiogenesis and immune evasion (210, 211). TGFβ directly affects the activity of T-cells, NK-cells and DCs and can regulate the release of inflammatory cytokines (212).
The OC TME is deeply influenced by numerous other cytokines as well, with each having a different impact on progression and immune activity. Understanding the roles of these cytokines and their interactions in the TME is crucial for identifying potential targets to overcome immunoresistance and improve therapeutic outcomes.
Within the intricate landscape of the OC TME, non-immune elements have emerged as integral contributors to immune evasion, offering insights into potential therapeutic strategies. Cancer-associated fibroblasts (CAFs), activated by TGF-β signaling and governed by aberrant signaling pathways, have been identified as key orchestrators of immunosuppression. They promote extracellular matrix remodeling, notably collagen engineering, that physically hinder immune cell infiltration (213). Furthermore, CAFs engage metabolic reprogramming, fostering a nutrient-depleted TME unfavorable for effector T cell function (144). Endothelial cells within the tumor vasculature also play a pivotal role, participating in immune evasion by regulating immune cell trafficking and immune checkpoint molecule expression (214). Notably, autotaxin, an enzyme produced by various cell types in the TME, generates lysophosphatidic acid (LPA), which can enhance tumor growth and impair immune cell function through LPA receptor signaling (215). Collectively, these non-immune elements underscore once again the complexity of immune evasion mechanisms while representing potential targets for therapeutic interventions (216, 217)
7 TME particularities of rare epithelial OC subtypes
7.1 Clear cell ovarian cancer
Clear-cell ovarian cancer (OCCC) represents the second most frequent subtype of epithelial ovarian cancer, comprising approximately 15% of cases (218). Unlike high-grade serous OC, OCCC is often associated with endometriosis, and is typically diagnosed in younger women. It is also characterized by a tendency to present as localized diseases (219, 220). Molecularly, OCCC displays distinct profiles compared to other subtypes, with high rates of ARID1A loss-of-function and PIK3CA activating mutations, while P53 loss-of-function mutations are observed in only 20% of cases (221, 222). These molecular characteristics suggest the presence of a strong immunosuppressive microenvironment. The PI3K/AKT/mTOR pathway is reported to promote immune evasion by increasing resistance to cytotoxic T-cell induced apoptosis, reinforcing Tregs functionality and escaping death receptor signals via PD-L1 and LAG3 expression (223–226). Furthermore, endometriosis and ARID1A mutations also contribute independently to the immunosuppressive environment. they have been shown to reduce T-cell infiltration and enrich the TME with PD-L1 expression and various other immunosuppressive cytokines, including IL2, IL6, IL10, TGFβ and TNFα (227–231). Interestingly, recent studies reported that coexistent PIK3CA/ARID1A mutations in OCCC are responsible for high levels of IL-6 and this mechanism may promote cancer growth and as such, could represent a potential therapeutic target in OCCC (232). Overall, OCCC appears to be the least infiltrated OC subtype by CD8+ TILS and, contrary to the others subtypes, it has been suggested to have a negative impact on survival (44, 231, 233).
7.2 Mucinous ovarian cancer
Mucinous OC (MOC) is known to have a poor response to platinum-based chemotherapy and advanced diseases are associated with a dismal prognosis (234). Molecularly, MOC often carries KRAS mutations, which are commonly associated with P53 mutations and ERBB2 amplifications, resembling the molecular background of colorectal carcinomas (235). One interesting aspect of MOC is that around 20% of cases exhibit mismatch repair deficiency, leading to a very high mutational load, increased neoantigens levels and potential sensitivity to immune checkpoint inhibitors (236). However, the immune context of MOC is poorly understood and limited data are available. In this regard, a recent study by Meagher et al, investigated the TME of MOC in 23 cases and reported high heterogeneity across these tumors. They observed that advanced stages had a higher number of M2-like macrophages and a higher density of FOXP3 Tregs in the tumor stroma was associated to poorer prognosis. Overall, MOC were not found to have an immune competent TME as most of the cases were classified as “cold” tumors based on T-cell infiltration and PD-L1 expression, which may explain its unfavorable prognosis and suggests resistance to immunotherapy even though there is no published clinical data supporting this assumption (237).
7.3 Low-grade ovarian cancer
Low-grade OC (LGOC) is a relatively rare epithelial OC subtype, accounting for less than 10% of. It typically occurs in younger women and is often diagnosed at an advanced stage. Compared to high-grade serous OC, LGOC is generally associated with more favorable outcomes (238). Their molecular background is characterized by a low mutational burden and recurrent mutations in the RAS/RAF pathway (239). However, little is known regarding about the immune infiltrate in LGOC. Studies have reported that LGOC tend to have fewer TILS and a lower number of CD68+ TAMs compared to high-grade tumors (240, 241). In addition, the TAMs associated with LGOC appear to be less polarized into M2-like macrophages (lower CD163/CD68 ratio) compared to HGOC (241). Taken together, given the low immune infiltration in LGOC, it is thought that these tumors may not be ideal candidates for immunotherapies.
8 Therapeutic implications
8.1 Targeting immune-checkpoint inhibitors
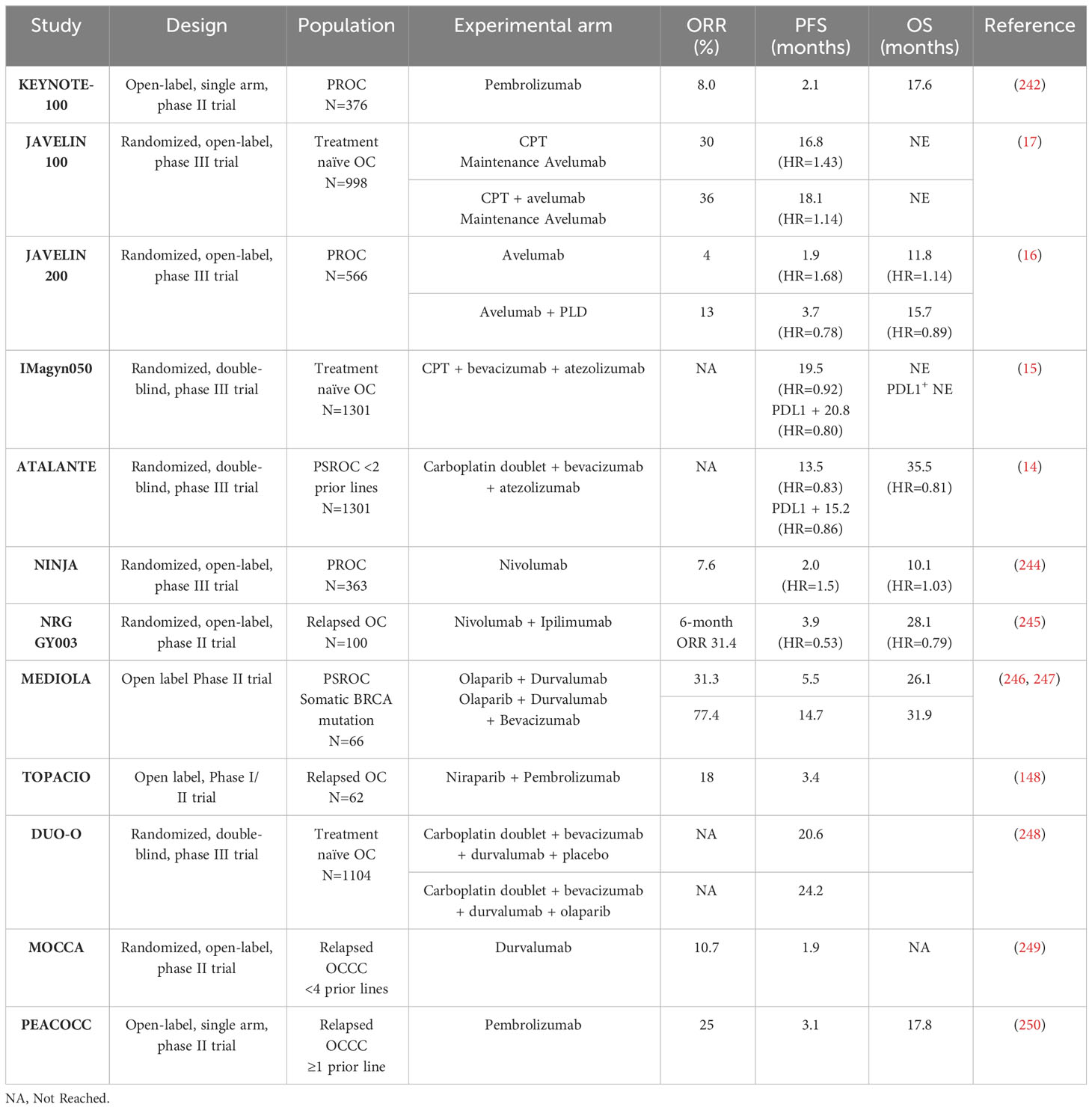
The principle behind targeting immune checkpoints is to block the natural inhibitory pathway that cancer cells use to suppress the activity of T cells, thereby allowing the immune system to maintain an effective immune response. The first class of ICI approved were PD-1/PD-L1 inhibitors, which block the interaction between PD-1 receptor on T-cells and the PD-L1 ligand, enabling T cells to remain active and attack cancer cells (150). In OC, PD-1/PD-L1 inhibitors monotherapy has shown limited activity, with an objective response rate (ORR) of approximately 10% and disease stabilization in around 30% of cases (16, 242, 243). To improve activity, considering the strong rationale of immunogenic cell death induced by cytotoxic agents and the close relationship between angiogenesis and TME, investigators have explored combinations strategies with chemotherapy and bevacizumab. However, large phase 3 trials have failed to demonstrated any benefit of PD-1/PD-L1 inhibitor combinations (Table 2) (14–17).
Despite the challenges in identifying reliable biomarkers for predicting response to ICI, some studies have shown that patients with higher PD-L1 expression (based on CPS score) may benefit more from adding PD-1/PD-L1 inhibitors to the standard treatment regimen. In the IMAGYN050 trial, patients with ≥5% expression of PD-L1 on the immune cells, or patients with ≥1% positive tumor cells, seemed to benefit from adding atezolizumab (PD-L1 inhibitor) to chemotherapy with bevacizumab (HR=0.64 [0.43-0.96] and HR=0.41 [0.19-0.90]) (15). However, these corresponded to only 20% and 6% of the overall population. Conversely, in the JAVELIN 200 and ATALANTE trials, PD-L1 positive patients did not benefit from adding ICI nor did the CD8+/PD-L1+ positive subgroup (14, 16). Interestingly, in some trials, patients with OCCC appeared to benefit more from ICI therapy. Consequently, dedicated trials have tested ICI in this population and reported encouraging responses with 11-25% ORR and up to 38.5% when associated with bevacizumab (249–251). Disappointingly, the only randomized trial comparing single-agent chemotherapy to durvalumab (PD-1 inhibitor) in OCCC patients, did not show improved outcomes in the ICI arm, highlighting the need for better biomarkers (249).
The complexity of the OC TME, including the multiplicity of immunomodulators, is most likely the reason for these contradictory results. To overcome these challenges, next-generation trials are exploring new targets, including other immunoregulatory checkpoints more suitable for the OC TME. Combinations of a PD-1 inhibitors with CTLA-4 inhibitors (nivolumab+ipilimumab) have demonstrated increased activity compared to monotherapy with a 31.4% ORR, versus 12.2% with nivolumab alone, at the cost of frequent severe adverse events (245). Likewise, various large ongoing trials are evaluating the benefits of adding a CTLA-4 inhibitor to a PD-1/PD-L1 inhibitor plus chemotherapy. Furthermore, clinical trials with ICI targeting novel checkpoints like TIM3 and LAG3, are being tested in OC, and the results are eagerly awaited (NCT02608268, NCT03099109, NCT04611126, NCT03538028, NCT03365791).
PROC: platinum-resistant OC; PSROC: platinum-sensitive recurrent OC; CPT: Carboplatin+Paclitaxel; PLD: Pegylated liposomal doxorubicine; OCCC: clear-cell ovarian cancerAs previously mentioned, HRD tumors tend to present higher levels of TILS and neoantigens load, suggesting a potential combination strategy of ICI with PARP inhibitors (31, 32). Additionally, PARP inhibitors have shown to induce immunogenic cell death through the activation of the cytosolic DNA sensor cyclic GMP-AMP synthetase (cGAS) and stimulator of interferon genes (STING) pathway (252), further enhancing the potential synergy between PARP inhibition and immunotherapy. The MEDIOLA trial, which tested the combination of olaparib and durvalumab, reported provocative results in the BRCA-mutated population (whilst naïve of PARP inhibitor) with an impressive 72% ORR (247). However, in the TOPACIO trial, a similar association combining pembrolizumab and niraparib, showed a lower ORR of 18% and 65% disease control rate in an heterogenous OC population. Interestingly, this association appeared particularly effective in the platinum-refractory and non-HRD populations (148). Finally, the DUO-O trial, which investigated the addition of durvalumab to olaparib and bevacizumab reported a significant improvement in outcomes in the BRCAwt/HRD population as expected with a PARPi in this context. However, similar to the TOPACIO trial, the BRCAwt/HRD-negative population also appeared to benefit from the combination even though PARPi alone have not shown effectiveness in this scenario (248). Altogether, these results suggest that combining PARP inhibitors with ICI may be a promising approach, especially in populations that are not usually candidate for PARP inhibitors. Nonetheless, the trial was not specifically designed to explore the benefits of the combination versus PARPi alone, and further dedicated trials are necessary to confirm these findings and identify the specific subset of patients that would derive the greatest benefit from this combination.
Overall, a multitude of ICI strategies are currently being explored in OC, and many of these trials consider an all-comer population. Translational studies to identify biomarkers that can predict response to ICI will be crucial in optimizing the use of these strategies in the future.
8.2 Monoclonal antibodies, cancer vaccines, and T-cell engagers
Cancer vaccines hold great promise in harnessing the patient’s own immune system to target tumor-associated antigens and induce specific immune responses against cancer cells. Several approaches have been tested in OC, showing encouraging activity in early trials. One strategy involves using cancer-testis/cancer-germline antigens such as NY-ESO-1, which are proteins aberrantly expressed in OC, and have demonstrated consistent CD4+ and CD8+ T-cell responses in OC patients leading to prolonged responses (253, 254). Another promising strategy involves DC vaccines, which capitalizes on the critical role of DCs in stimulating both adaptative and innate immunity. Schematically, DCs are collected from the patient’s blood, matured in vitro and then loaded with the specific antigen of interest. The matured, antigen loaded DCs are then injected back into the patient, stimulating a targeted immune response against the tumor. For example, vaccination with autologous DCs targeting Mucin 1 (MUC1), a glycoprotein highly expressed in ovarian carcinomas, resulted in a strong MUC1-specific T cell response and prolonged survival in patients with advanced OC (255). Similarly, a DC vaccine targeting folate receptor alpha (FRα) demonstrated a strong cytotoxic T-cell response (composed of IL-17 producing T cells) and prolonged remission in patients with heavily pretreated OC (256).
Additionally, monoclonal antibodies have been developed to mimic tumor antigens in order to induce a self-tumor antigen-specific response (anti-idiotypic antibody). Abagovomab was one such antibody imitating the tumor-associated antigen CA125. However. despite a sustained immune response and encouraging antitumor efficacy in early-phase trials, the drug failed to demonstrate a survival benefit in OC patients in later trials (257). The next-generation antibody oregovomab was designed to generate an immune complex with tumoral CA125, which is then processed by DCs and subsequently responsible for downstream CA125-specific antitumor immune response (258). Unfortunately, after an initial efficacy signal in a subset of OC patients with favorable prognostic characteristics, the phase III clinical trial did not show any statistical survival difference between oregovomab maintenance and placebo (259). Nonetheless, the FLORA-5 study (NCT04498117) is testing oregovomab in combination with chemotherapy in newly diagnosed OC and might demonstrate promising results.
Despite the promising results, and even if vaccine therapies are steadily moving forward, there are still multiple unresolved challenges. Manufacturing personalized vaccines for each patient can be time-consuming and costly, limiting their accessibility (260). In addition, as above-mentioned, DCs are highly plastic and can acquire an immunosuppressive phenotype over time, potentially limiting their long-term efficacy. Ongoing research is focused on developing more efficient and cost-effective methods of vaccine production and enhancing the stability and potency of dendritic cell vaccines. As research in this field continues to advance, cancer vaccines hold the potential to become an integral part of OC treatment, complementing other therapeutic modalities and improving patient outcome.
As previously mentioned, T-cell immune activation is mainly MHC restricted. Therefore, the impairment of antigen presentation by MHC molecules is an important immune evasion mechanism and a major limitation for T-cell activity (261, 262). T-cell engaging bispecific antibodies (bsAbs or BiTEs) are antibodies that have been designed to target both tumor-associated antigens and a T-cell molecule (most frequently CD3). This design directs polyclonal T-cells to the tumor resulting in tumor-specific cytotoxicity (263). As a result, bsAbs can crosslink cancer cells with T-cells independently of MHC action, limiting the potential for immune escape. Given the encouraging results in hematological malignancies, bsAbs are currently in clinical development for solid tumors. In OC, several tumors antigens are being tested including WT1 (264), MUC16 (265), claudin 6 (266) and folate receptor alpha (267). Notably, the toxicity profile of these therapies is of considerable concern due to the high rates of severe cytokine release syndromes (CRS) and immune effector cell-associated neurotoxicity (ICANS) (268, 269).
8.3 Adoptive cellular therapies
Adoptive cell therapy, including chimeric antigen receptor (CAR) T-cell therapy, is an innovative approach in cancer immunotherapy that involves the infusion of autologous immune cells that have been stimulated and expanded ex-vivo to target cancer cells. This approach aims to harness the patient’s preexisting antitumor response and enhance it, through the use of genetically modified immune cells.
The first trials, few decades ago, used TILS transfer in heavily pretreated diseases and showed encouraging activity and prolonged survival (270). More recently, the combination of ACT with PD-1/PD-L1 inhibitors has shown improved T-cell expansion and increased T-cell reactivity in OC patients, leading to promising antitumor activity even though it was tested only in a very limited number of patients (271).
Unlike autologous TILS infusion, CAR-T cells have been genetically engineered to target a specific tumor antigen, allowing an increased immune response with minimal off-tumor effect (272). Importantly, CAR-T cells are not MHC restricted and, as such, exert a highly efficient and specific tumor cytoxicity. In the context of OC, several potential antigens have been proposed as targets for CAR-T cell therapy due to their high expression levels and specificity. The most promising targets in OC are Mucin 16 (MUC16), mesothelin (MSLN), Folate receptor 1 (FOLR1) and tumor-associated glycoprotein 72 (TAG72) and have achieved promising responses both in vitro and in vivo (273–276). Clinical trials are actively recruiting patients with OC to test the safety and efficacy of these therapies (277). Finally, next-generation CAR cell therapies are also exploring the use of engineered NK cells, which are thought to limit systemic toxicity while enhancing the antitumor activity (278).
Although cellular immunotherapies come with important limitations including off-target toxicity and challenges in accessibility and manufacturing, they also hold great promise in OC treatment. Given the strong spontaneous antitumor response observed in vivo, they represent a potential breakthrough in the field of OC treatment.
9 Conclusions
In conclusion, despite preclinical data continue supporting the use of immunotherapy combinations, ICI is not yet firmly established in OC therapeutic landscape. This review aimed to summarize the potent immune evasion mechanisms deployed, the exceptional diversity of the immune cells and ligands recruited, presenting the OC TME both hindered by obstacles and a field of great opportunity. However, certain critical elements deeply intertwined into these pathways, such as extracellular matrix modeling, fibroblast recruitment, hypoxia, and metabolic reprogramming, as well as host factors, were intentionally overlooked in this work. These aspects gave been extensively covered by other works. For example, Yang et al., provided an extensive review of the immune and non-immune cellular components of the TME and how they can be targeted beyond immune-checkpoint blockade (279). Works by Kandalaft et al. and Colombo and colleagues, focused on the most promising strategies to target the TME considering the immunosuppressive context of OC and the potential future biomarkers (280, 281). Finally, Baci and colleagues reviewed the complex innate immune response in the OC TME and how it might either support or limit cancer progression and treatment sensitivity (282).
OC TME is a complex and dynamic landscape that plays a critical role in promoting tumor progression, invasion, dissemination, and treatment resistance. The immune cells and components within the TME possess both antitumor effects and immunosuppressive actions, creating a delicate balance that allows cancer cells to evade immune surveillance and continue to thrive. Understanding the interactions and polarization of these immune cells is essential for developing effective immunotherapeutic strategies. While there has been significant progress in understanding various features of the TME, such as extracellular matrix remodelling, cancer-associated fibroblasts, and metabolic reprogramming, there are still many unsolved enigmas and challenges to address. Cutting-edge techniques like single-cell sequencing and spatial molecular assays hold promise for unravelling the complexities of the TME and its interactions. In OC, it is likely to think that histological subtypes and molecular backgrounds be critical features to consider in the interpretation of results.
Immunotherapeutic approaches in ovarian cancer continue to show promise, and next-generation inhibitors, innovative combinations, vaccines, and cellular therapies offer exciting opportunities. However, finding the right target, tailoring therapies to individual patients’ immune characteristics, and carefully considering endpoints and potential toxicity will be critical for the success of these treatments.
Author contributions
FB-D: Data curation, Writing – original draft, Writing – review & editing. LCWX: Data curation, Writing – review & editing. DT: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. FB-D received support from the Nuovo Soldati Foundation (2021) and the National University of Singapore President’s Graduate Fellowship (2023). This research is supported by the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist Award (MOH-001006) and the Pangestu Family Foundation Gynaecological Cancer Research Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim B-G, Oaknin A, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial. J Clin Oncol (2023) 41:609–17. doi: 10.1200/JCO.22.01549
3. Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med (2018) 379:2495–505. doi: 10.1056/NEJMoa1810858
4. Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med (2019) 381:2416–28. doi: 10.1056/NEJMoa1911361
5. González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med (2019) 381:2391–402. doi: 10.1056/NEJMoa1910962
6. Monk BJ, Parkinson C, Lim MC, O’Malley DM, Oaknin A, Wilson MK, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol (2022) 40:3952–64. doi: 10.1200/JCO.22.01003
7. Elvin JA, He Y, Sun J, Odunsi K, Szender JB, Moore KN, et al. Comprehensive genomic profiling (CGP) with loss of heterozygosity (LOH) to identify therapeutically relevant subsets of ovarian cancer (OC). JCO (2017) 35:5512–2. doi: 10.1200/JCO.2017.35.15_suppl.5512
8. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature (2011) 474:609–15. doi: 10.1038/nature10166
9. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin (2018) 68:284–96. doi: 10.3322/caac.21456
10. Kim S, Kim B, Song YS. Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci (2016) 107:1173–8. doi: 10.1111/cas.12987
11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
12. Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol (2013) 13:227–42. doi: 10.1038/nri3405
13. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer (2018) 6:8. doi: 10.1186/s40425-018-0316-z
14. Kurtz JE, Pujade-Lauraine E, Oaknin A, Belin L, Tsibulak I, Cibula D, et al. LBA30 Phase III ATALANTE/ov29 trial: Atezolizumab (Atz) versus placebo with platinum-based chemotherapy (Cx) plus bevacizumab (bev) in patients (pts) with platinum-sensitive relapse (PSR) of epithelial ovarian cancer (OC). Ann Oncol (2022) 33:S1397. doi: 10.1016/j.annonc.2022.08.026
15. Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol (2021) 39:1842–55. doi: 10.1200/JCO.21.00306
16. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard I-L, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol (2021) 22:1034–46. doi: 10.1016/S1470-2045(21)00216-3
17. Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22:1275–89. doi: 10.1016/S1470-2045(21)00342-9
18. Hwang W-T, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol (2012) 124:192–8. doi: 10.1016/j.ygyno.2011.09.039
19. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med (2003) 348:203–13. doi: 10.1056/NEJMoa020177
20. Leffers N, Gooden MJM, de Jong RA, Hoogeboom B-N, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother (2009) 58:449–59. doi: 10.1007/s00262-008-0583-5
21. Mittrücker H-W, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch Immunol Ther Exp (2014) 62:449–58. doi: 10.1007/s00005-014-0293-y
22. Gorfu G, Rivera-Nieves J, Ley K. Role of β7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med (2009) 9(7):836–50. doi: 10.2174/156652409789105525
23. Bösmüller H-C, Wagner P, Peper JK, Schuster H, Pham DL, Greif K, et al. Combined immunoscore of CD103 and CD3 identifies long-term survivors in high-grade serous ovarian cancer. Int J Gynecologic Cancer (2016) 26(4):671–9. doi: 10.1097/IGC.0000000000000672
24. Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res (2014) 20:434–44. doi: 10.1158/1078-0432.CCR-13-1877
25. Visekruna A, Ritter J, Scholz T, Campos L, Guralnik A, Poncette L, et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur J Immunol (2013) 43:606–18. doi: 10.1002/eji.201242825
26. Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med (1994) 180:1715–28. doi: 10.1084/jem.180.5.1715
27. Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest (2013) 123:247–60. doi: 10.1172/JCI63681
28. Nesbeth YC, Martinez DG, Toraya S, Scarlett UK, Cubillos-Ruiz JR, Rutkowski MR, et al. CD4+ T cells elicit host immune responses to MHC class II-negative ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol (2010) 184:5654–62. doi: 10.4049/jimmunol.0903247
29. Pinto MP, Balmaceda C, Bravo ML, Kato S, Villarroel A, Owen GI, et al. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol Oncol (2018) 151:10–7. doi: 10.1016/j.ygyno.2018.07.025
30. Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature (2008) 453:1051–7. doi: 10.1038/nature07036
31. Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget (2016) 7:13587–98. doi: 10.18632/oncotarget.7277
32. Fujiwara M, McGuire VA, Felberg A, Sieh W, Whittemore AS, Longacre TA. Prediction of BRCA1 germline mutation status in women with ovarian cancer using morphology-based criteria: identification of a BRCA1 ovarian cancer phenotype. Am J Surg Pathol (2012) 36:1170–7. doi: 10.1097/PAS.0b013e31825d9b8d
33. Morse CB, Toukatly MN, Kilgore MR, Agnew KJ, Bernards SS, Norquist BM, et al. Tumor infiltrating lymphocytes and homologous recombination deficiency are independently associated with improved survival in ovarian carcinoma. Gynecol Oncol (2019) 153:217–22. doi: 10.1016/j.ygyno.2019.02.011
34. Miyara M, Sakaguchi S. Human FoxP3(+)CD4(+) regulatory T cells: their knowns and unknowns. Immunol Cell Biol (2011) 89:346–51. doi: 10.1038/icb.2010.137
35. Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol (2013) 4:152. doi: 10.3389/fimmu.2013.00152
36. Toker A, Nguyen LT, Stone SC, Yang SYC, Katz SR, Shaw PA, et al. Regulatory T cells in ovarian cancer are characterized by a highly activated phenotype distinct from that in melanoma. Clin Cancer Res (2018) 24:5685–96. doi: 10.1158/1078-0432.CCR-18-0554
37. Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity (2007) 27:635–46. doi: 10.1016/j.immuni.2007.08.014
38. Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res (2007) 67:8900–5. doi: 10.1158/0008-5472.CAN-07-1866
39. Qiao S, Hou Y, Rong Q, Han B, Liu P. Tregs are involved in VEGFA/ VASH1-related angiogenesis pathway in ovarian cancer. Transl Oncol (2023) 32:101665. doi: 10.1016/j.tranon.2023.101665
40. Govindaraj C, Scalzo-Inguanti K, Madondo M, Hallo J, Flanagan K, Quinn M, et al. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin Immunol (2013) 149:97–110. doi: 10.1016/j.clim.2013.07.003
41. Shang B, Liu Y, Jiang S, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep (2015) 5:15179. doi: 10.1038/srep15179
42. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med (2004) 10:942–9. doi: 10.1038/nm1093
43. Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res (2005) 11:8326–31. doi: 10.1158/1078-0432.CCR-05-1244
44. Ovarian Tumor Tissue Analysis (OTTA) Consortium, Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W, et al. Dose-response association of CD8+ Tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol (2017) 3:e173290. doi: 10.1001/jamaoncol.2017.3290
45. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA (2005) 102:18538–43. doi: 10.1073/pnas.0509182102
46. Leary A, Genestie C, Blanc-Durand F, Gouy S, Dunant A, Maulard A, et al. Neoadjuvant chemotherapy alters the balance of effector to suppressor immune cells in advanced ovarian cancer. Cancer Immunol Immunother (2021) 70:519–31. doi: 10.1007/s00262-020-02670-0
47. Zhou X, Bing Y, Yu Z, Zheng-Jiang L. Profiles of immune infiltration in ovarian cancer and their clinical significance: a gene expression-based study Eur J Gynaecol Oncol (2021) 42:346–52. doi: 10.31083/j.ejgo.2021.02.5347
48. Gao Y, Chen L, Cai G, Xiong X, Wu Y, Ma D, et al. Heterogeneity of immune microenvironment in ovarian cancer and its clinical significance: a retrospective study. Oncoimmunology (2020) 9:1760067. doi: 10.1080/2162402X.2020.1760067
49. Rodríguez-Pinto D. B cells as antigen presenting cells. Cell Immunol (2005) 238:67–75. doi: 10.1016/j.cellimm.2006.02.005
50. Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol (2017) 14:662–74. doi: 10.1038/cmi.2017.35
51. Gnjatic S, Ritter E, Büchler MW, Giese NA, Brors B, Frei C, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U.S.A. (2010) 107:5088–93. doi: 10.1073/pnas.0914213107
52. Stone B, Schummer M, Paley PJ, Thompson L, Stewart J, Ford M, et al. Serologic analysis of ovarian tumor antigens reveals a bias toward antigens encoded on 17q. Int J Cancer (2003) 104:73–84. doi: 10.1002/ijc.10900
53. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol (2002) 3:944–50. doi: 10.1038/ni833
54. Zhang Y, Morgan R, Podack ER, Rosenblatt J. B cell regulation of anti-tumor immune response. Immunol Res (2013) 57:115–24. doi: 10.1007/s12026-013-8472-1
55. Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, et al. IL35-producing B cells promote the development of pancreatic neoplasia. Cancer Discovery (2016) 6:247–55. doi: 10.1158/2159-8290.CD-15-0843
56. Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PloS One (2009) 4:e6412. doi: 10.1371/journal.pone.0006412
57. Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res (2012) 18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234
58. Lundgren S, Berntsson J, Nodin B, Micke P, Jirström K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J Ovarian Res (2016) 9:21. doi: 10.1186/s13048-016-0232-0
59. Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res (2016) 22:3005–15. doi: 10.1158/1078-0432.CCR-15-2762
60. Yang C, Lee H, Jove V, Deng J, Zhang W, Liu X, et al. Prognostic significance of B-cells and pSTAT3 in patients with ovarian cancer. PloS One (2013) 8:e54029. doi: 10.1371/journal.pone.0054029
61. Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol (2006) 125:451–8. doi: 10.1309/15B66DQMFYYM78CJ
62. Chiossone L, Dumas P-Y, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol (2018) 18:671–88. doi: 10.1038/s41577-018-0061-z
63. Rosenberg J, Huang J. CD8+ T cells and NK cells: parallel and complementary soldiers of immunotherapy. Curr Opin Chem Eng (2018) 19:9–20. doi: 10.1016/j.coche.2017.11.006
64. Ferlazzo G, Moretta L. Dendritic cell editing by natural killer cells. Crit Rev Oncog (2014) 19:67–75. doi: 10.1615/critrevoncog.2014010827
66. Krebs P, Barnes MJ, Lampe K, Whitley K, Bahjat KS, Beutler B, et al. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood (2009) 113:6593–602. doi: 10.1182/blood-2009-01-201467
67. Pesce S, Tabellini G, Cantoni C, Patrizi O, Coltrini D, Rampinelli F, et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology (2015) 4:e1001224. doi: 10.1080/2162402X.2014.1001224
68. Krockenberger M, Kranke P, Häusler S, Engel JB, Horn E, Nürnberger K, et al. Macrophage migration-inhibitory factor levels in serum of patients with ovarian cancer correlates with poor prognosis. Anticancer Res (2012) 32:5233–8. Available at: http://ar.iiarjournals.org/content/32/12/5233.abstract.
69. Hoogstad-van Evert JS, Maas RJ, van der Meer J, Cany J, van der Steen S, Jansen JH, et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget (2018) 9:34810–20. doi: 10.18632/oncotarget.26199
70. Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med (2019) 8:4709–21. doi: 10.1002/cam4.2327
71. Hourani T, Holden JA, Li W, Lenzo JC, Hadjigol S, O’Brien-Simpson NM. Tumor associated macrophages: origin, recruitment, phenotypic diversity, and targeting. Front Oncol (2021) 11:788365. doi: 10.3389/fonc.2021.788365
72. Ramanathan S, Jagannathan N. Tumor associated macrophage: A review on the phenotypes, traits and functions. Iran J Cancer Prev (2014) 7:1–8.
73. Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett (2008) 267:204–15. doi: 10.1016/j.canlet.2008.03.028
74. Macciò A, Gramignano G, Cherchi MC, Tanca L, Melis L, Madeddu C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep (2020) 10:6096. doi: 10.1038/s41598-020-63276-1
75. Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res (2014) 7:19. doi: 10.1186/1757-2215-7-19
76. He Y, Zhang M, Wu X, Sun X, Xu T, He Q, et al. High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PloS One (2013) 8:e79769. doi: 10.1371/journal.pone.0079769
77. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol (2000) 164:6166–73. doi: 10.4049/jimmunol.164.12.6166
78. Van Dalen FJ, Van Stevendaal MHME, Fennemann FL, Verdoes M, Ilina O. Molecular repolarisation of tumour-associated macrophages. Molecules (2019) 24:9. doi: 10.3390/molecules24010009
79. Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol (2008) 18:349–55. doi: 10.1016/j.semcancer.2008.03.004
80. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
81. Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem (2007) 101:805–15. doi: 10.1002/jcb.21159
82. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity (2014) 41:49–61. doi: 10.1016/j.immuni.2014.06.010
83. Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res (2010) 70:5270–80. doi: 10.1158/0008-5472.CAN-10-0012
84. Feng Y, Ye Z, Song F, He Y, Liu J. The role of TAMs in tumor microenvironment and new research progress. Stem Cells Int (2022) 2022:5775696. doi: 10.1155/2022/5775696
85. El-Arabey AA, Denizli M, Kanlikilicer P, Bayraktar R, Ivan C, Rashed M, et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signalling (2020) 68:109539. doi: 10.1016/j.cellsig.2020.109539
86. Nowak M, Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells (2020) 9(5):1299. doi: 10.3390/cells9051299
87. Hao J, Yan F, Zhang Y, Triplett A, Zhang Y, Schultz DA, et al. Expression of adipocyte/macrophage fatty acid–binding protein in tumor-associated macrophages promotes breast cancer progression. Cancer Res (2018) 78:2343–55. doi: 10.1158/0008-5472.CAN-17-2465
88. Gharpure KM, Pradeep S, Sans M, Rupaimoole R, Ivan C, Wu SY, et al. FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat Commun (2018) 9:2923. doi: 10.1038/s41467-018-04987-y
89. Etzerodt A, Moulin M, Doktor TK, Delfini M, Mossadegh-Keller N, Bajenoff M, et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med (2020) 217:e20191869. doi: 10.1084/jem.20191869
90. Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest (2016) 126:4157–73. doi: 10.1172/JCI87252
91. Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol (2017) 147:181–7. doi: 10.1016/j.ygyno.2017.07.007
92. Zhang Q, Liu L, Gong C, Shi H, Zeng Y, Wang X, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS One (2012) 7:e50946. doi: 10.1371/journal.pone.0050946
93. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol (2020) 20:7–24. doi: 10.1038/s41577-019-0210-z
94. Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res (2005) 65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043
95. Bronger H, Singer J, Windmüller C, Reuning U, Zech D, Delbridge C, et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br J Cancer (2016) 115:553–63. doi: 10.1038/bjc.2016.172
96. Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer (2018) 6:139. doi: 10.1186/s40425-018-0446-3
97. Zhang Z, Huang J, Zhang C, Yang H, Qiu H, Li J, et al. Infiltration of dendritic cells and T lymphocytes predicts favorable outcome in epithelial ovarian cancer. Cancer Gene Ther (2015) 22:198–206. doi: 10.1038/cgt.2015.7
98. Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res (2004) 64:5535–8. doi: 10.1158/0008-5472.CAN-04-1272
99. Kassim SK, El-Salahy EM, Fayed ST, Helal SA, Helal T, Azzam EE, et al. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem (2004) 37:363–9. doi: 10.1016/j.clinbiochem.2004.01.014
100. Bouzin C, Brouet A, De Vriese J, DeWever J, Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy1. J Immunol (2007) 178:1505–11. doi: 10.4049/jimmunol.178.3.1505
101. Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med (2001) 7:1339–46. doi: 10.1038/nm1201-1339
102. Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, et al. Indoleamine 2,3-dioxygenase–expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood (2009) 114:555–63. doi: 10.1182/blood-2008-11-191197
103. Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med (2012) 209:495–506. doi: 10.1084/jem.20111413
104. Conrad C, Gregorio J, Wang Y-H, Ito T, Meller S, Hanabuchi S, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res (2012) 72:5240–9. doi: 10.1158/0008-5472.CAN-12-2271
105. Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol (2011) 186:6905–13. doi: 10.4049/jimmunol.1100274
106. Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res (2011) 71:5423–34. doi: 10.1158/0008-5472.CAN-11-0367
107. Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol (2013) 93:343–52. doi: 10.1189/jlb.0812397
108. Zhang X, He T, Li Y, Chen L, Liu H, Wu Y, et al. Dendritic cell vaccines in ovarian cancer. Front Immunol (2021) 11:613773. doi: 10.3389/fimmu.2020.613773
109. Zhang C, Wang S, Liu Y, Yang C. Epigenetics in myeloid derived suppressor cells: a sheathed sword towards cancer. Oncotarget (2016) 7:57452–63. doi: 10.18632/oncotarget.10767
110. Hart KM, Byrne KT, Molloy MJ, Usherwood EM, Berwin B. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front Immunol (2011) 2:29. doi: 10.3389/fimmu.2011.00029
111. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol (2009) 9:162–74. doi: 10.1038/nri2506
112. Okła K, Wertel I, Polak G, Surówka J, Wawruszak A, Kotarski J. Tumor-associated macrophages and myeloid-derived suppressor cells as immunosuppressive mechanism in ovarian cancer patients: progress and challenges. Int Rev Immunol (2016) 35:372–85. doi: 10.1080/08830185.2016.1206097
113. Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol (2007) 179:977–83. doi: 10.4049/jimmunol.179.2.977
114. Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res (2004) 64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757
115. Wong JL, Obermajer N, Odunsi K, Edwards RP, Kalinski P. Synergistic COX2 induction by IFNγ and TNFα Self-limits type-1 immunity in the human tumor microenvironment. Cancer Immunol Res (2016) 4:303–11. doi: 10.1158/2326-6066.CIR-15-0157
116. Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol (2003) 170:270–8. doi: 10.4049/jimmunol.170.1.270
117. Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell (2008) 13:23–35. doi: 10.1016/j.ccr.2007.12.004
118. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol (2012) 12:253–68. doi: 10.1038/nri3175
119. Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res (2011) 71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449
120. Komura N, Mabuchi S, Shimura K, Yokoi E, Kozasa K, Kuroda H, et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol Immunother (2020) 69:2477–99. doi: 10.1007/s00262-020-02628-2
121. Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med (2003) 9:562–7. doi: 10.1038/nm863
122. Okła K, Czerwonka A, Wawruszak A, Bobiński M, Bilska M, Tarkowski R, et al. Clinical relevance and immunosuppressive pattern of circulating and infiltrating subsets of myeloid-derived suppressor cells (MDSCs) in epithelial ovarian cancer. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.00691
123. Horikawa N, Abiko K, Matsumura N, Hamanishi J, Baba T, Yamaguchi K, et al. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res (2017) 23:587–99. doi: 10.1158/1078-0432.CCR-16-0387
124. Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun (2018) 9:1685. doi: 10.1038/s41467-018-03966-7
125. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods (2015) 12:453–7. doi: 10.1038/nmeth.3337
126. Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol (2017) 18:220. doi: 10.1186/s13059-017-1349-1
127. Shen X, Gu X, Ma R, Li X, Wang J. Identification of the immune signatures for ovarian cancer based on the tumor immune microenvironment genes. Front Cell Dev Biol (2022) 10:772701. doi: 10.3389/fcell.2022.772701
128. Liu R, Hu R, Zeng Y, Zhang W, Zhou H-H. Tumour immune cell infiltration and survival after platinum-based chemotherapy in high-grade serous ovarian cancer subtypes: A gene expression-based computational study. EBioMedicine (2020) 51:102602. doi: 10.1016/j.ebiom.2019.102602
129. Qian J, Olbrecht S, Boeckx B, Vos H, Laoui D, Etlioglu E, et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res (2020) 30:745–62. doi: 10.1038/s41422-020-0355-0
130. Olbrecht S, Busschaert P, Qian J, Vanderstichele A, Loverix L, Van Gorp T, et al. High-grade serous tubo-ovarian cancer refined with single-cell RNA sequencing: specific cell subtypes influence survival and determine molecular subtype classification. Genome Med (2021) 13:111. doi: 10.1186/s13073-021-00922-x
131. Vázquez-García I, Uhlitz F, Ceglia N, Lim JLP, Wu M, Mohibullah N, et al. Ovarian cancer mutational processes drive site-specific immune evasion. Nature (2022) 612:778–86. doi: 10.1038/s41586-022-05496-1
132. Grout JA, Sirven P, Leader AM, Maskey S, Hector E, Puisieux I, et al. Spatial positioning and matrix programs of cancer-associated fibroblasts promote T-cell exclusion in human lung tumors. Cancer Discovery (2022) 12:2606–25. doi: 10.1158/2159-8290.CD-21-1714
133. Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun (2017) 8:15095. doi: 10.1038/ncomms15095
134. Ji AL, Rubin AJ, Thrane K, Jiang S, Reynolds DL, Meyers RM, et al. Multimodal analysis of composition and spatial architecture in human squamous cell carcinoma. Cell (2020) 182:497–514.e22. doi: 10.1016/j.cell.2020.05.039
135. Gartrell-Corrado RD, Chen AX, Rizk EM, Marks DK, Bogardus MH, Hart TD, et al. Linking transcriptomic and imaging data defines features of a favorable tumor immune microenvironment and identifies a combination biomarker for primary melanoma. Cancer Res (2020) 80:1078–87. doi: 10.1158/0008-5472.CAN-19-2039
136. Merritt CR, Ong GT, Church SE, Barker K, Danaher P, Geiss G, et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat Biotechnol (2020) 38:586–99. doi: 10.1038/s41587-020-0472-9
137. Tan WCC, Nerurkar SN, Cai HY, Ng HHM, Wu D, Wee YTF, et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun (Lond) (2020) 40:135–53. doi: 10.1002/cac2.12023
138. Zhu Y, Ferri-Borgogno S, Sheng J, Yeung T-L, Burks JK, Cappello P, et al. SIO: A spatioimageomics pipeline to identify prognostic biomarkers associated with the ovarian tumor microenvironment. Cancers (Basel) (2021) 13:1777. doi: 10.3390/cancers13081777
139. Wang DY-T, Tan TZ, Tai Y-T, Ye J, Lin W-C, Wei L-H, et al. Case study: Digital spatial profiling of metastatic clear cell carcinoma reveals intra-tumor heterogeneity in epithelial-mesenchymal gradient. bioRxiv (2021) 2021.05.27.445912. doi: 10.1101/2021.05.27.445912
140. Wang Y, Huang P, Wang BG, Murdock T, Cope L, Hsu F-C, et al. Spatial transcriptomic analysis of ovarian cancer precursors reveals reactivation of IGFBP2 during pathogenesis. Cancer Res (2022) 82:4528–41. doi: 10.1158/0008-5472.CAN-22-1620
141. Ferri-Borgogno S, Zhu Y, Sheng J, Burks JK, Gomez JA, Wong KK, et al. Spatial transcriptomics depict ligand-receptor cross-talk heterogeneity at the tumor-stroma interface in long-term ovarian cancer survivors. Cancer Res (2023) 83:1503–16. doi: 10.1158/0008-5472.CAN-22-1821
142. Stur E, Corvigno S, Xu M, Chen K, Tan Y, Lee S, et al. Spatially resolved transcriptomics of high-grade serous ovarian carcinoma. iScience (2022) 25:103923. doi: 10.1016/j.isci.2022.103923
143. Hoppe MM, Jaynes P, Wardyn JD, Upadhyayula SS, Tan TZ, Lie S, et al. Quantitative imaging of RAD51 expression as a marker of platinum resistance in ovarian cancer. EMBO Mol Med (2021) 13:e13366. doi: 10.15252/emmm.202013366
144. Desbois M, Udyavar AR, Ryner L, Kozlowski C, Guan Y, Dürrbaum M, et al. Integrated digital pathology and transcriptome analysis identifies molecular mediators of T-cell exclusion in ovarian cancer. Nat Commun (2020) 11:5583. doi: 10.1038/s41467-020-19408-2
145. Ptacek J, Locke D, Finck R, Cvijic M-E, Li Z, Tarolli JG, et al. Multiplexed ion beam imaging (MIBI) for characterization of the tumor microenvironment across tumor types. Lab Invest (2020) 100:1111–23. doi: 10.1038/s41374-020-0417-4
146. Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science (2016) 353:78–82. doi: 10.1126/science.aaf2403
147. Launonen I-M, Lyytikäinen N, Casado J, Anttila EA, Szabó A, Haltia U-M, et al. Single-cell tumor-immune microenvironment of BRCA1/2 mutated high-grade serous ovarian cancer. Nat Commun (2022) 13:835. doi: 10.1038/s41467-022-28389-3
148. Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol (2019) 5:1141–9. doi: 10.1001/jamaoncol.2019.1048
149. Färkkilä A, Gulhan DC, Casado J, Jacobson CA, Nguyen H, Kochupurakkal B, et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun (2020) 11:1459. doi: 10.1038/s41467-020-15315-8
150. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med (2000) 192:1027–34. doi: 10.1084/jem.192.7.1027
151. Burger GA, Nesenberend DN, Lems CM, Hille SC, Beltman JB. Bidirectional crosstalk between epithelial-mesenchymal plasticity and IFNγ-induced PD-L1 expression promotes tumour progression. R Soc Open Sci (2022) 9:220186. doi: 10.1098/rsos.220186
152. Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol (2006) 45:520–8. doi: 10.1016/j.jhep.2006.05.007
153. Wang Q, Lou W, Di W, Wu X. Prognostic value of tumor PD-L1 expression combined with CD8+ tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int Immunopharmacol (2017) 52:7–14. doi: 10.1016/j.intimp.2017.08.017
154. Whitehair R, Peres LC, Mills AM. Expression of the immune checkpoints LAG-3 and PD-L1 in high-grade serous ovarian carcinoma: relationship to tumor-associated lymphocytes and germline BRCA status. Int J Gynecol Pathol (2020) 39:558–66. doi: 10.1097/PGP.0000000000000657
155. Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA (2007) 104:3360–5. doi: 10.1073/pnas.0611533104
156. Blanc-Durand F, Genestie C, Galende EY, Gouy S, Morice P, Pautier P, et al. Distribution of novel immune-checkpoint targets in ovarian cancer tumor microenvironment: A dynamic landscape. Gynecol Oncol (2021) 160:279–84. doi: 10.1016/j.ygyno.2020.09.045
157. Martin de la Fuente L, Westbom-Fremer S, Arildsen NS, Hartman L, Malander S, Kannisto P, et al. PD-1/PD-L1 expression and tumor-infiltrating lymphocytes are prognostically favorable in advanced high-grade serous ovarian carcinoma. Virchows Arch (2020) 477:83–91. doi: 10.1007/s00428-020-02751-6
158. Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol (2016) 141:293–302. doi: 10.1016/j.ygyno.2016.03.008
159. Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget (2016) 7:1486–99. doi: 10.18632/oncotarget.6429
160. Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity (2016) 44:955–72. doi: 10.1016/j.immuni.2016.05.002
161. Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med (1996) 183:2541–50. doi: 10.1084/jem.183.6.2541
162. Yang K, Zhao W, Lou G, Rong Z, Xu H, Wang W, et al. Immunophenotyping of ovarian cancer with clinical and immunological significance. An Front Immunol (2018) 9:757. doi: 10.3389/fimmu.2018.00757
163. He Y, Rivard CJ, Rozeboom L, Yu H, Ellison K, Kowalewski A, et al. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci (2016) 107:1193–7. doi: 10.1111/cas.12986
164. Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, et al. Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PloS One (2014) 9:e109080. doi: 10.1371/journal.pone.0109080
165. Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood (2003) 102:2130–7. doi: 10.1182/blood-2003-01-0273
166. Huang C-T, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity (2004) 21:503–13. doi: 10.1016/j.immuni.2004.08.010
167. Huang R-Y, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget (2015) 6:27359–77. doi: 10.18632/oncotarget.4751
168. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1–specific CD8 + T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci (2010) 107:7875–80. doi: 10.1073/pnas.1003345107
169. Kim H-S, Kim J-Y, Lee YJ, Kim SH, Lee J-Y, Nam EJ, et al. Expression of programmed cell death ligand 1 and immune checkpoint markers in residual tumors after neoadjuvant chemotherapy for advanced high-grade serous ovarian cancer. Gynecol Oncol (2018) 151:414–21. doi: 10.1016/j.ygyno.2018.08.023
170. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature (2002) 415:536–41. doi: 10.1038/415536a
171. Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, et al. Promotion of tissue inflammation by the immune receptor tim-3 expressed on innate immune cells. Science (2007) 318:1141–3. doi: 10.1126/science.1148536
172. Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol (2009) 39:2492–501. doi: 10.1002/eji.200939274
173. Sakuishi K, Ngiow SF, Sullivan JM, Teng MWL, Kuchroo VK, Smyth MJ, et al. TIM3+FOXP3+ regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. OncoImmunology (2013) 2:e23849. doi: 10.4161/onci.23849
174. de Mingo Pulido Á, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, et al. TIM-3 regulates CD103+ Dendritic cell function and response to chemotherapy in breast cancer. Cancer Cell (2018) 33:60–74.e6. doi: 10.1016/j.ccell.2017.11.019
175. Jiang X, Zhou T, Xiao Y, Yu J, Dou S, Chen G, et al. Tim-3 promotes tumor-promoting M2 macrophage polarization by binding to STAT1 and suppressing the STAT1-miR-155 signaling axis. OncoImmunology (2016) 5:e1211219. doi: 10.1080/2162402X.2016.1211219
176. Tu L, Guan R, Yang H, Zhou Y, Hong W, Ma L, et al. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int J Cancer (2020) 147:423–39. doi: 10.1002/ijc.32785
177. Sawada M, Goto K, Morimoto-Okazawa A, Haruna M, Yamamoto K, Yamamoto Y, et al. PD-1+ Tim3+ tumor-infiltrating CD8 T cells sustain the potential for IFN-γ production, but lose cytotoxic activity in ovarian cancer. Int Immunol (2020) 32:397–405. doi: 10.1093/intimm/dxaa010
178. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet (2015) 386:249–57. doi: 10.1016/S0140-6736(14)62223-6
179. Elies A, Rivière S, Pouget N, Becette V, Dubot C, Donnadieu A, et al. The role of neoadjuvant chemotherapy in ovarian cancer. Expert Rev Anticancer Ther (2018) 18:555–66. doi: 10.1080/14737140.2018.1458614
180. Stanske M, Wienert S, Castillo-Tong DC, Kreuzinger C, Vergote I, Lambrechts S, et al. Dynamics of the intratumoral immune response during progression of high-grade serous ovarian cancer. Neoplasia (2018) 20:280–8. doi: 10.1016/j.neo.2018.01.007
181. Pölcher M, Braun M, Friedrichs N, Rudlowski C, Bercht E, Fimmers R, et al. Foxp3(+) cell infiltration and granzyme B(+)/Foxp3(+) cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer Immunol Immunother (2010) 59:909–19. doi: 10.1007/s00262-010-0817-1
182. Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol (2017) 28:651–7. doi: 10.1093/annonc/mdw625
183. Böhm S, Montfort A, Pearce OMT, Topping J, Chakravarty P, Everitt GLA, et al. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin Cancer Res (2016) 22:3025–36. doi: 10.1158/1078-0432.CCR-15-2657
184. Bansal A, Srinivasan R, Rohilla M, Rai B, Rajwanshi A, Suri V, et al. Immunotyping in tubo-ovarian high-grade serous carcinoma by PD-L1 and CD8+ T-lymphocytes predicts disease-free survival. APMIS (2021) 129:254–64. doi: 10.1111/apm.13116
185. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol (2016) 37:193–207. doi: 10.1016/j.it.2016.01.002
186. Sharma MD, Baban B, Chandler P, Hou D-Y, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest (2007) 117:2570–82. doi: 10.1172/JCI31911
187. Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, et al. Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep (2015) 13:412–24. doi: 10.1016/j.celrep.2015.08.077
188. Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discovery (2012) 2:722–35. doi: 10.1158/2159-8290.CD-12-0014
189. Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res (2005) 11:6030–9. doi: 10.1158/1078-0432.CCR-04-2671
190. Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology (2005) 69 Suppl 3:4–10. doi: 10.1159/000088478
191. Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev (2012) 31:143–62. doi: 10.1007/s10555-011-9337-5
192. Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in Malignant ascites. Ann Surg Oncol (1999) 6:373–8. doi: 10.1007/s10434-999-0373-0
193. Santin AD, Hermonat PL, Ravaggi A, Cannon MJ, Pecorelli S, Parham GP. Secretion of vascular endothelial growth factor in ovarian cancer. Eur J Gynaecol Oncol (1999) 20:177–81.
194. Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res (2007) 67:585–92. doi: 10.1158/0008-5472.CAN-06-2941
195. Liao S, Liu J, Lin P, Shi T, Jain RK, Xu L. TGF-beta blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin Cancer Res (2011) 17:1415–24. doi: 10.1158/1078-0432.CCR-10-2429
196. Guo B-Q, Lu W-Q. The prognostic significance of high/positive expression of tissue VEGF in ovarian cancer. Oncotarget (2018) 9:30552–60. doi: 10.18632/oncotarget.25702
197. Hartenbach EM, Olson TA, Goswitz JJ, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Lett (1997) 121:169–75. doi: 10.1016/s0304-3835(97)00350-9
198. Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med (2014) 370:734–43. doi: 10.1056/NEJMoa1309748
199. Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol (2015) 16:928–36. doi: 10.1016/S1470-2045(15)00086-8
200. Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother (2008) 57:1115–24. doi: 10.1007/s00262-007-0441-x
201. Manzoni M, Rovati B, Ronzoni M, Loupakis F, Mariucci S, Ricci V, et al. Immunological effects of bevacizumab-based treatment in metastatic colorectal cancer. Oncology (2011) 79:187–96. doi: 10.1159/000320609
202. Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res (2013) 73:539–49. doi: 10.1158/0008-5472.CAN-12-2325
203. Brencicova E, Jagger AL, Evans HG, Georgouli M, Laios A, Attard Montalto S, et al. Interleukin-10 and prostaglandin E2 have complementary but distinct suppressive effects on Toll-like receptor-mediated dendritic cell activation in ovarian carcinoma. PloS One (2017) 12:e0175712. doi: 10.1371/journal.pone.0175712
204. Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J, et al. IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res (2017) 77:6667–78. doi: 10.1158/0008-5472.CAN-17-0740
205. Rabinovich A, Medina L, Piura B, Segal S, Huleihel M. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res (2007) 27:267–72.
206. Lane D, Matte I, Rancourt C, Piché A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer (2011) 11:210. doi: 10.1186/1471-2407-11-210
207. Mustea A, Könsgen D, Braicu EI, Pirvulescu C, Sun P, Sofroni D, et al. Expression of IL-10 in patients with ovarian carcinoma. Anticancer Res (2006) 26:1715–8.
208. Wu L, Deng Z, Peng Y, Han L, Liu J, Wang L, et al. Ascites-derived IL-6 and IL-10 synergistically expand CD14 + HLA-DR -/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget (2017) 8:76843–56. doi: 10.18632/oncotarget.20164
209. Isobe A, Sawada K, Kinose Y, Ohyagi-Hara C, Nakatsuka E, Makino H, et al. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PloS One (2015) 10:e0118080. doi: 10.1371/journal.pone.0118080
210. Haque S, Morris JC. Transforming growth factor-β: A therapeutic target for cancer. Hum Vaccines Immunotherapeutics (2017) 13:1741–50. doi: 10.1080/21645515.2017.1327107
211. Rodriguez GC, Haisley C, Hurteau J, Moser TL, Whitaker R, Bast RC, et al. Regulation of invasion of epithelial ovarian cancer by transforming growth factor-beta. Gynecol Oncol (2001) 80:245–53. doi: 10.1006/gyno.2000.6042
212. Kao JY, Gong Y, Chen C-M, Zheng Q-D, Chen J-J. Tumor-derived TGF-β Reduces the efficacy of dendritic cell/tumor fusion vaccine1. J Immunol (2003) 170:3806–11. doi: 10.4049/jimmunol.170.7.3806
213. Natarajan S, Foreman KM, Soriano MI, Rossen NS, Shehade H, Fregoso DR, et al. Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res (2019) 79:2271–84. doi: 10.1158/0008-5472.CAN-18-2616
214. Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol (2011) 11:702–11. doi: 10.1038/nri3064
215. Jeong KJ, Park SY, Seo JH, Lee KB, Choi WS, Han JW, et al. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp Mol Med (2008) 40:607–16. doi: 10.3858/emm.2008.40.6.607
216. Benesch MGK, Ko YM, McMullen TPW, Brindley DN. Autotaxin in the crosshairs: Taking aim at cancer and other inflammatory conditions. FEBS Lett (2014) 588:2712–27. doi: 10.1016/j.febslet.2014.02.009
217. Lai D, Ma L, Wang F. Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int J Oncol (2012) 41:541–50. doi: 10.3892/ijo.2012.1475
218. McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology (2011) 43:420–32. doi: 10.1097/PAT.0b013e328348a6e7
219. Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol (2008) 109:370–6. doi: 10.1016/j.ygyno.2008.02.006
220. Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer (2000) 88:2584–9. doi: 10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5
221. Kuo K-T, Mao T-L, Jones S, Veras E, Ayhan A, Wang T-L, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol (2009) 174:1597–601. doi: 10.2353/ajpath.2009.081000
222. Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. New Engl J Med (2010) 363:1532–43. doi: 10.1056/NEJMoa1008433
223. Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol (2012) 188:4305–14. doi: 10.4049/jimmunol.1103568
224. Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol (2011) 186:5173–83. doi: 10.4049/jimmunol.1002050
225. Efimova OV, Kelley TW. Induction of granzyme B expression in T-cell receptor/CD28-stimulated human regulatory T cells is suppressed by inhibitors of the PI3K-mTOR pathway. BMC Immunol (2009) 10:59. doi: 10.1186/1471-2172-10-59
226. Lastwika KJ, Wilson W, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res (2016) 76:227–38. doi: 10.1158/0008-5472.CAN-14-3362
227. Wu L, Lv C, Su Y, Li C, Zhang H, Zhao X, et al. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol Endocrinol (2019) 35:251–6. doi: 10.1080/09513590.2018.1519787
228. Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med (2018) 24:556. doi: 10.1038/s41591-018-0012-z
229. Osuga Y, Koga K, Hirota Y, Hirata T, Yoshino O, Taketani Y. Lymphocytes in endometriosis. Am J Reprod Immunol (2011) 65:1–10. doi: 10.1111/j.1600-0897.2010.00887.x
230. Kocbek V, Vouk K, Bersinger NA, Mueller MD, Lanišnik Rižner T. Panels of cytokines and other secretory proteins as potential biomarkers of ovarian endometriosis. J Mol Diagn (2015) 17:325–34. doi: 10.1016/j.jmoldx.2015.01.006
231. Devlin M-J, Miller R, Laforets F, Kotantaki P, Garsed DW, Kristeleit R, et al. The tumor microenvironment of clear-cell ovarian cancer. Cancer Immunol Res (2022) 10:1326–39. doi: 10.1158/2326-6066.CIR-22-0407
232. Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, et al. Coexistent ARID1A–PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun (2015) 6:6118. doi: 10.1038/ncomms7118
233. Kim SR, Cloutier BT, Leung S, Cochrane D, Britton H, Pina A, et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecologic Oncol (2020) 158:3–11. doi: 10.1016/j.ygyno.2020.04.043
234. Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer (2011) 117:554–62. doi: 10.1002/cncr.25460
235. Mackenzie R, Kommoss S, Winterhoff BJ, Kipp BR, Garcia JJ, Voss J, et al. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer (2015) 15:415. doi: 10.1186/s12885-015-1421-8
236. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol (2020) 38:1–10. doi: 10.1200/JCO.19.02105
237. Meagher NS, Hamilton P, Milne K, Thornton S, Harris B, Weir A, et al. Profiling the immune landscape in mucinous ovarian carcinoma. Gynecologic Oncol (2023) 168:23–31. doi: 10.1016/j.ygyno.2022.10.022
238. Matsuo K, Machida H, Matsuzaki S, Grubbs BH, Klar M, Roman LD, et al. Evolving population-based statistics for rare epithelial ovarian cancers. Gynecol Oncol (2020) 157:3–11. doi: 10.1016/j.ygyno.2019.11.122
239. Singer G, Oldt R, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst (2003) 95:484–6. doi: 10.1093/jnci/95.6.484
240. Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res (2015) 3:926–35. doi: 10.1158/2326-6066.CIR-14-0239
241. Ciucci A, Zannoni GF, Buttarelli M, Martinelli E, Mascilini F, Petrillo M, et al. Ovarian low and high grade serous carcinomas: hidden divergent features in the tumor microenvironment. Oncotarget (2016) 7:68033–43. doi: 10.18632/oncotarget.10797
242. Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase 2 KEYNOTE-100 study. Ann Oncol (2019) 30(7):1080–7. doi: 10.1093/annonc/mdz135
243. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol (2015) 33:4015–22. doi: 10.1200/JCO.2015.62.3397
244. Hamanishi J, Takeshima N, Katsumata N, Ushijima K, Kimura T, Takeuchi S, et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA). J Clin Oncol (2021) 39:3671–81. doi: 10.1200/JCO.21.00334
245. Zamarin D, Burger RA, Sill MW, Powell DJ, Lankes HA, Feldman MD, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. JCO (2020) 38:1814–23. doi: 10.1200/JCO.19.02059
246. Banerjee S, Imbimbo M, Roxburgh P, Kim J-W, Kim MH, Plummer R, et al. 529MO Phase II study of olaparib plus durvalumab with or without bevacizumab (MEDIOLA): Final analysis of overall survival in patients with non-germline BRCA-mutated platinum-sensitive relapsed ovarian cancer. Ann Oncol (2022) 33:S788–9. doi: 10.1016/j.annonc.2022.07.657
247. Drew Y, de JM, SH H, YH P, Wolfer A, Brown J, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated (gBRCAm) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecologic Oncol (2018) 149:246–7. doi: 10.1016/j.ygyno.2018.04.555
248. Harter P, Bidziński M, Colombo N, Floquet A, Rubio Pérez MJ, Kim J-W, et al. DUO-O: A randomized phase III trial of durvalumab (durva) in combination with chemotherapy and bevacizumab (bev), followed by maintenance durva, bev and olaparib (olap), in newly diagnosed advanced ovarian cancer patients. JCO (2019) 37:TPS5598–TPS5598. doi: 10.1200/JCO.2019.37.15_suppl.TPS5598
249. Tan DSP, Choi CH, Ngoi N, Sun H, Heong V, Ow SGW, et al. A multicenter phase II randomized trial of durvalumab (D) versus physician’s choice chemotherapy (PCC) in patients (pts) with recurrent ovarian clear cell adenocarcinoma (MOCCA/APGOT-OV2/GCGS-OV3). JCO (2022) 40:5565–5. doi: 10.1200/JCO.2022.40.16_suppl.5565
250. Kristeleit R, Clamp A, Gourley C, Roux R, Hall M, Devlin M-J, et al. 521MO Efficacy of pembrolizumab monotherapy (PM) for advanced clear cell gynaecological cancer (CCGC): Phase II PEACOCC trial. Ann Oncol (2022) 33:S783. doi: 10.1016/j.annonc.2022.07.649
251. Li R, Liu X, Song C, Zhang W, Liu J, Jiao X, et al. Sintilimab combined with bevacizumab in relapsed/persistent ovarian clear cell carcinoma (INOVA): an investigator-initiated, multicentre clinical trial-a study protocol of clinical trial. BMJ Open (2022) 12:e058132. doi: 10.1136/bmjopen-2021-058132
252. Shen J, Zhao W, Ju Z, Wang L, Peng Y, Labrie M, et al. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res (2019) 79:311–9. doi: 10.1158/0008-5472.CAN-18-1003
253. Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res (2003) 63:6076–83.
254. Magid Diefenbach CS, Sabbatini P, Hensley ML, Konner JA, Tew WP, Ritter G, et al. A phase I study of NY-ESO-1 overlapping peptides with or without incomplete Freund’s adjuvant and poly-ICLCL vaccination of ovarian cancer patients in second or third clinical remission. JCO (2010) 28:TPS174–4. doi: 10.1200/jco.2010.28.15_suppl.tps174
255. Gray HJ, Benigno B, Berek J, Chang J, Mason J, Mileshkin L, et al. Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. J Immunother Cancer (2016) 4:34. doi: 10.1186/s40425-016-0137-x
256. Block MS, Dietz AB, Gustafson MP, Kalli KR, Erskine CL, Youssef B, et al. Th17-inducing autologous dendritic cell vaccination promotes antigen-specific cellular and humoral immunity in ovarian cancer patients. Nat Commun (2020) 11:5173. doi: 10.1038/s41467-020-18962-z
257. Sabbatini P, Harter P, Scambia G, Sehouli J, Meier W, Wimberger P, et al. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a phase III trial of the AGO OVAR, COGI, GINECO, and GEICO–the MIMOSA study. J Clin Oncol (2013) 31:1554–61. doi: 10.1200/JCO.2012.46.4057
258. Pietragalla A, Duranti S, Daniele G, Nero C, Ciccarone F, Lorusso D, et al. Oregovomab: an investigational agent for the treatment of advanced ovarian cancer. Expert Opin Investigational Drugs (2021) 30:103–10. doi: 10.1080/13543784.2021.1868436
259. Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol (2009) 27:418–25. doi: 10.1200/JCO.2008.17.8400
260. Caro AA, Deschoemaeker S, Allonsius L, Coosemans A, Laoui D. Dendritic cell vaccines: A promising approach in the fight against ovarian cancer. Cancers (Basel) (2022) 14:4037. doi: 10.3390/cancers14164037
261. DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature (2012) 482:405–9. doi: 10.1038/nature10803
262. Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature (2019) 574:696–701. doi: 10.1038/s41586-019-1671-8
263. Zhou S, Liu M, Ren F, Meng X, Yu J. The landscape of bispecific T cell engager in cancer treatment. biomark Res (2021) 9:38. doi: 10.1186/s40364-021-00294-9
264. Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y, et al. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol (2015) 33:1079–86. doi: 10.1038/nbt.3349
265. Yeku OO, Rao TD, Laster I, Kononenko A, Purdon TJ, Wang P, et al. Bispecific T-cell engaging antibodies against MUC16 demonstrate efficacy against ovarian cancer in monotherapy and in combination with PD-1 and VEGF inhibition. Front Immunol (2021) 12:663379. doi: 10.3389/fimmu.2021.663379
266. Stadler CR, Bähr-Mahmud H, Plum LM, Schmoldt K, Kölsch AC, Türeci Ö, et al. Characterization of the first-in-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6. Oncoimmunology (2015) 5:e1091555. doi: 10.1080/2162402X.2015.1091555
267. Avanzino BC, Prabhakar K, Dalvi P, Hartstein S, Kehm H, Balasubramani A, et al. A T-cell engaging bispecific antibody with a tumor-selective bivalent folate receptor alpha binding arm for the treatment of ovarian cancer. OncoImmunology (2022) 11:2113697. doi: 10.1080/2162402X.2022.2113697
268. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer (2018) 6:56. doi: 10.1186/s40425-018-0343-9
269. Stein AS, Schiller G, Benjamin R, Jia C, Zhang A, Zhu M, et al. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann Hematol (2019) 98:159–67. doi: 10.1007/s00277-018-3497-0
270. Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res (1995) 1:501–7.
271. Kverneland AH, Pedersen M, Westergaard MCW, Nielsen M, Borch TH, Olsen LR, et al. Adoptive cell therapy in combination with checkpoint inhibitors in ovarian cancer. Oncotarget (2020) 11:2092–105. doi: 10.18632/oncotarget.27604
272. Yan W, Hu H, Tang B. Advances of chimeric antigen receptor T cell therapy in ovarian cancer. Onco Targets Ther (2019) 12:8015–22. doi: 10.2147/OTT.S203550
273. Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology (2015) 4:e994446. doi: 10.4161/2162402X.2014.994446
274. Chekmasova AA, Rao TD, Nikhamin Y, Park KJ, Levine DA, Spriggs DR, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res (2010) 16:3594–606. doi: 10.1158/1078-0432.CCR-10-0192
275. Schoutrop E, El-Serafi I, Poiret T, Zhao Y, Gultekin O, He R, et al. Mesothelin-specific CAR T cells target ovarian cancer. Cancer Res (2021) 81:3022–35. doi: 10.1158/0008-5472.CAN-20-2701
276. Murad JP, Kozlowska AK, Lee HJ, Ramamurthy M, Chang W-C, Yazaki P, et al. Effective targeting of TAG72+ Peritoneal ovarian tumors via regional delivery of CAR-engineered T cells. Front Immunol (2018) 9:2268. doi: 10.3389/fimmu.2018.02268
277. Domínguez-Prieto V, Qian S, Villarejo-Campos P, Meliga C, González-Soares S, Guijo Castellano I, et al. Understanding CAR T cell therapy and its role in ovarian cancer and peritoneal carcinomatosis from ovarian cancer. Front Oncol (2023) 13:1104547. doi: 10.3389/fonc.2023.1104547
278. Klapdor R, Wang S, Morgan MA, Zimmermann K, Hachenberg J, Büning H, et al. NK cell-mediated eradication of ovarian cancer cells with a novel chimeric antigen receptor directed against CD44. Biomedicines (2021) 9:1339. doi: 10.3390/biomedicines9101339
279. Yang Y, Yang Y, Yang J, Zhao X, Wei X. Tumor microenvironment in ovarian cancer: function and therapeutic strategy. Front Cell Dev Biol (2020) 8:758. doi: 10.3389/fcell.2020.00758
280. Colombo I, Karakasis K, Suku S, Oza AM. Chasing immune checkpoint inhibitors in ovarian cancer: novel combinations and biomarker discovery. Cancers (Basel) (2023) 15:3220. doi: 10.3390/cancers15123220
281. Kandalaft LE, Odunsi K, Coukos G. Immune therapy opportunities in ovarian cancer. Am Soc Clin Oncol Educ Book (2020) 40:1–13. doi: 10.1200/EDBK_280539
Keywords: ovarian cancer, immunotherapy, immune microenvironment, tumor-infiltrating lymphocytes, tumor-associated macrophages, adoptive cell therapy, cancer vaccine
Citation: Blanc-Durand F, Clemence Wei Xian L and Tan DSP (2023) Targeting the immune microenvironment for ovarian cancer therapy. Front. Immunol. 14:1328651. doi: 10.3389/fimmu.2023.1328651
Received: 27 October 2023; Accepted: 05 December 2023;
Published: 18 December 2023.
Edited by:
Ming Yi, Zhejiang University, ChinaReviewed by:
Siqi Chen, Washington University in St. Louis, United StatesSandra Cascio, University of Pittsburgh, United States
Copyright © 2023 Blanc-Durand, Clemence Wei Xian and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David S. P. Tan, ZGF2aWRfc3BfdGFuQG51aHMuZWR1LnNn
 Felix Blanc-Durand
Felix Blanc-Durand Lai Clemence Wei Xian
Lai Clemence Wei Xian David S. P. Tan
David S. P. Tan