
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 03 January 2024
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1325343
This article is part of the Research Topic Peripheral Blood-Based Biomarkers for Immune Monitoring of Cancer and Cancer Therapy View all 19 articles
 Anupam Kotwal1,2
Anupam Kotwal1,2 Michael P. Gustafson3,4
Michael P. Gustafson3,4 Svetlana Bornschlegl3
Svetlana Bornschlegl3 Allan B. Dietz3,5,6
Allan B. Dietz3,5,6 Danae Delivanis2
Danae Delivanis2 Mabel Ryder2,7*
Mabel Ryder2,7*Background: Exploring the immune interface of follicular cell-derived thyroid cancer has prognostic and therapeutic potential. The available literature is lacking for comprehensive immunophenotyping in relation to clinical outcomes. In this study, we identify circulating immunophenotypes associated with thyroid cancer prognosis.
Methods: We conducted a pilot observational study of adults with follicular cell-derived thyroid cancer who underwent surgery at our tertiary care referral center and had consented for flow cytometry on peripheral blood collected at the time of thyroidectomy.
Results: Of the 32 included subjects, 20 (62%) had well differentiated, 5 (16%) had poorly differentiated, and 7 (22%) had anaplastic thyroid cancer. The most frequent AJCC stage was 4 (59%) and the ATA risk of recurrence category was high (56%). Patients with AJCC stage 3/4 demonstrated fewer circulating mononuclear cells (CD45+), more monocytes (CD14+), fewer total lymphocytes (CD14-), fewer T cells (CD3+), fewer CD4+ T cells, fewer gamma-delta T cells, fewer natural killer (NK) T-like cells, more myeloid-derived suppressor cells (MDSCs; Lin-CD33+HLADR-), and more effector memory T cells but similar CD8+ T cells compared to stage1/2. Immunophenotype comparisons by ATA risk stratification and course of thyroid cancer were comparable to those observed for stage, except for significant differences in memory T cell subtypes. The median follow-up was 58 months.
Conclusions: Aggressive follicular cell-derived thyroid cancer either at presentation or during follow-up is associated with down-regulation of the T cell populations specifically CD4+ T cells, gamma-delta T cells, and NK T-like cells but up-regulation of MDSCs and altered memory T cells. These immunophenotypes are potential prognostic biomarkers supporting future investigation for developing targeted immunotherapies against advanced thyroid cancer.
The incidence of thyroid cancer has been steadily increasing. Follicular cell-derived differentiated thyroid cancers have a favorable prognosis with conventional treatment including thyroidectomy with or without radioactive iodine. However, at least 10% of patients develop radioactive iodine-refractory metastases to lung, bone, and other sites. In such cases, 5-year survival can be a dismal 15.3% (1). While aggressive multi-modality approaches and tyrosine kinase inhibitors have demonstrated improved outcomes, such therapies have toxicities and usually partial responses (2, 3). This has led to the exploration of immunotherapies for advanced thyroid cancer and stimulated an interest in understanding the effect of the immune system on thyroid cancer. The ligand for immune checkpoint programmed cell death protein 1 (PD-1) has been demonstrated on malignant thyroid cells (4, 5), thus leading to the trial of PD-1 inhibitors in anaplastic thyroid cancer (6). However, the effect of these therapies on advanced thyroid cancer has been unpredictable or poor (7). This could be due to the resurgence of an immunosuppressive tumor microenvironment as shown in a murine model of thyroid cancer (8).
The association between immune-mediated inflammation and follicular cell-derived thyroid cancer has been reported (9) as evidenced by a mixture of cytokines, chemokines, and immune cells in the tumor microenvironment. While an association between autoimmune thyroid disease and thyroid cancer has been reported in a database study (10), the impact of chronic lymphocytic thyroiditis on thyroid cancer prognosis remains unclear (11–14). To address this issue, studies have identified the increased immune suppressor cells, regulatory T cells (Tregs) (15), PD1+ T cells (15), myeloid-derived suppressor cells (MDSCs) (16), in circulation, and infiltrating the tumor of pathologically aggressive differentiated thyroid cancer (5, 17–19), while the association with effector CD8+ T cells has been mixed (20, 21). However, comprehensive examination of the relationship between circulating immunophenotypes and clinicopathologic outcomes in thyroid cancer patients remains limited. Our group has previously identified circulating immune cell profiles in thyroiditis caused by immune checkpoint inhibitors (ICIs) (22), as well as healthy and other malignancy patients (23, 24); and more immune activator T cell subpopulations in the thyroid glands of patients developing PD-1/PD-L1 inhibitor-induced thyroiditis (25). These studies highlight our ability to comprehensively analyze immunophenotypes in relation to clinically key factors. Based on the available literature and our previous work, we hypothesized that the circulating leukocyte populations, specifically suppressor (Tregs, MDSCs, effector memory T cells) and effector cells (CD4+ T cells, CD8+ T cells, gamma-delta T cells, NK cells, central memory T cells), in patients with high-risk thyroid cancer would be different from those in low-risk thyroid cancer. Hence, we aimed to identify immunophenotypes associated with follicular cell-derived thyroid cancer prognosis to recognize patients with aggressive thyroid cancer that could benefit from personalized management including novel immunotherapies.
We performed an institutional review board–approved pilot prospective cohort study of 32 adults with follicular cell-derived thyroid cancer who underwent initial or subsequent surgical management at a tertiary care cancer center. Informed consent was obtained from each participant and the research was completed in accordance with the Declaration of Helsinki as revised in 2013. We excluded patients with medullary thyroid cancer. Peripheral blood was collected at the time of surgery in tubes containing K2EDTA anticoagulant. The electronic medical record was utilized to gather clinical, radiographic, laboratory, and pathologic data.
Participants were categorized into low, intermediate, and high risk for recurrence groups according to the 2015 revised American Thyroid Association (ATA) guidelines (26); into tumor node metastasis (TNM) stage 1, 2, 3 or 4 according to the 8th American Joint Committee on Cancer (AJCC) edition (27); and according to the presence or absence of loco-regional and distant recurrence or progression during follow-up. Circulating immunophenotypes were compared among each of these groups.
This was performed using a 10-color flow cytometer panel of antibodies for quantification of all major leukocyte populations (Supplementary Table 1) as previously published (22–25). Samples were run on the Beckman Coulter Gallios 3-laser, 10-color flow cytometer that was calibrated per the manufacturer’s recommendations each day of use. List mode data (LMD) files were analyzed using Kaluza software version 1.2. Leukocyte populations of interest were colored by the representative gate or “backgated” using histograms of selected stained cell populations. Kaluza software was used to create radar plots. Leukocytes were quantified as: Cell count/microL = count (“Phenotype”) X (Flow-Count Flourospheres/microL)/count. This allowed quantification of the absolute number and percentage of immune cells. The gating strategy is demonstrated in Figure 1.
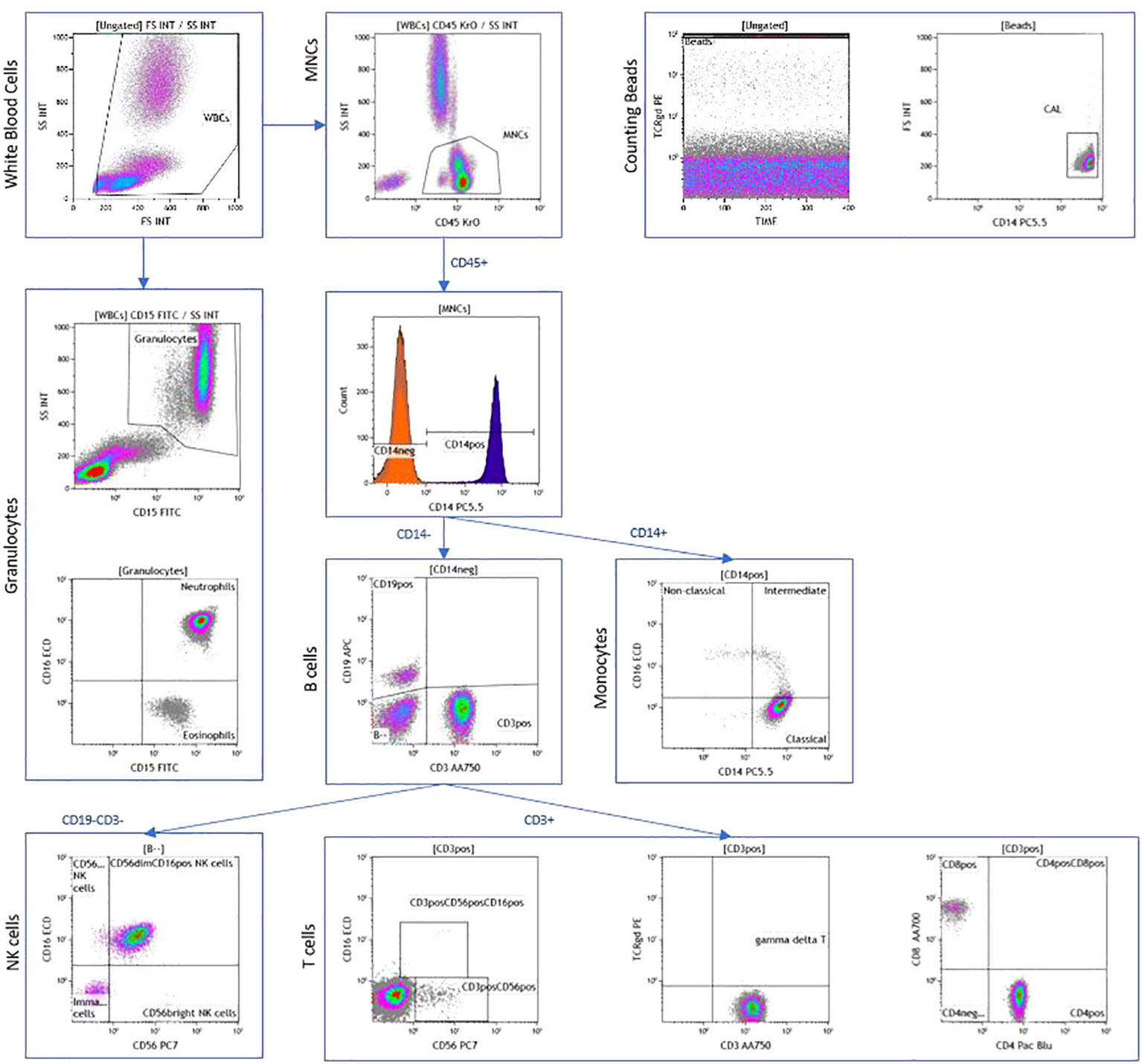
Figure 1 Peripheral blood immunophenotyping via flow cytometry demonstrating dot plots in a patient with AJCC TNM stage 4 and ATA high-risk follicular cell-derived thyroid cancer.
Descriptive statistics were used to determine mean and standard deviation or median and range for continuous variables, and number and percentage for categorical variables. Comparisons of immune cells between different cohorts were evaluated for statistical significance via the student t-test. A p-value <0.05 was used to classify statistically significant, and a p-value <0.001 to classify highly statistically significant differences. All graphical representations and statistical analyses were performed in Prism 9.4.0 (GraphPad, San Diego, CA).
In this cohort of 32 adult follicular cell-derived thyroid cancer patients followed for a median of 57.7 months from initial thyroid cancer surgery, the median age was 58 years (range 32, 85), all were non-Hispanic Caucasian, and 47% were females. Thyroid cancer was well-differentiated in 20 (62%), poorly differentiated in 5 (16%) and anaplastic in 7 (22%). The most frequent AJCC stage was 4 (59%) and ATA risk of recurrence category was high in 18 (56%). Radioactive iodine (RAI) treatment was provided to 17 patients, and tyrosine kinase inhibitor (TKI) to 6 patients after the initial thyroid cancer surgery and blood draw for flow cytometry. The median overall survival was 20 years. The mortality rate was 19%, of which four had distant metastasis at presentation while the other two had distant spread during follow-up (Table 1). Due to few patients with ATA low risk cancer (n=4), we combined those with ATA low and intermediate risk when comparing immunophenotypes to high ATA risk group.
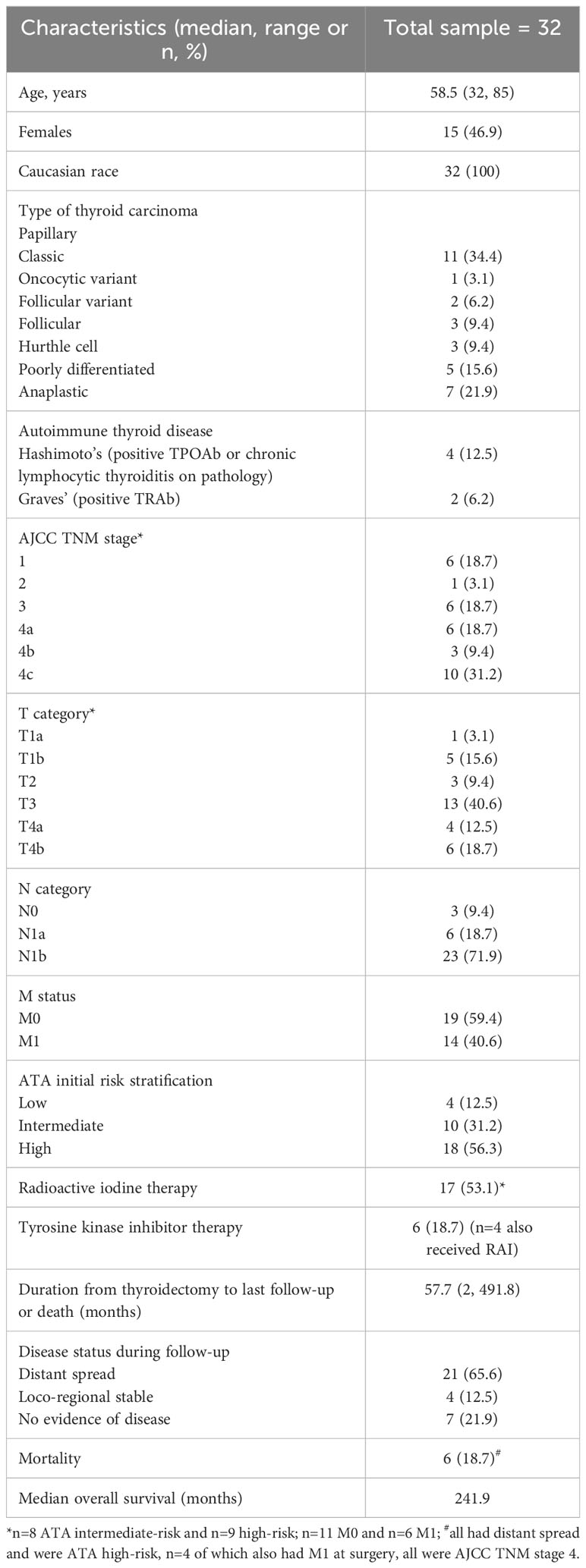
Table 1 Demographic and clinical characteristics of 32 adult patients with follicular cell-derived thyroid cancer that underwent immunophenotyping by peripheral blood flow cytometry.
On immunophenotyping in the T cell, B cell, and NK cell panel, patients with AJCC stage 3/4 demonstrated overall fewer circulating mononuclear cells (CD45+) as compared to stage 1/2 (2210 vs. 2855 cells/microL; p=0.04). They also had more monocytes (CD14+) (579 cells/microL vs. 442 cells/microL; p=0.04) but fewer total lymphocytes (CD14-) (1632 cells/microL vs. 2413 cells/microL; p=0.01). Within the lymphocyte compartment, differences in lymphocyte populations were specific to the T cell compartment. Patients with stage 3/4 demonstrated fewer T cells (CD3+) (1111 cells/microL vs. 1770 cells/microL; p=0.007). In sub-populations of T cells, they exhibited fewer CD4+ T cells (646 cells/microL vs. 1165 cells/microL; p=0.002) gamma-delta T cells (40.8 cells/microL vs. 124 cells/microL; p=0.007) and natural killer (NK) T-like cells (CD3+CD56+) (3.43 cells/microL vs. 38.2 cells/microL; p=0.009), but there were no differences in CD8+ T cells or NK cells when compared to stage 1/2 (Figures 2A, B).
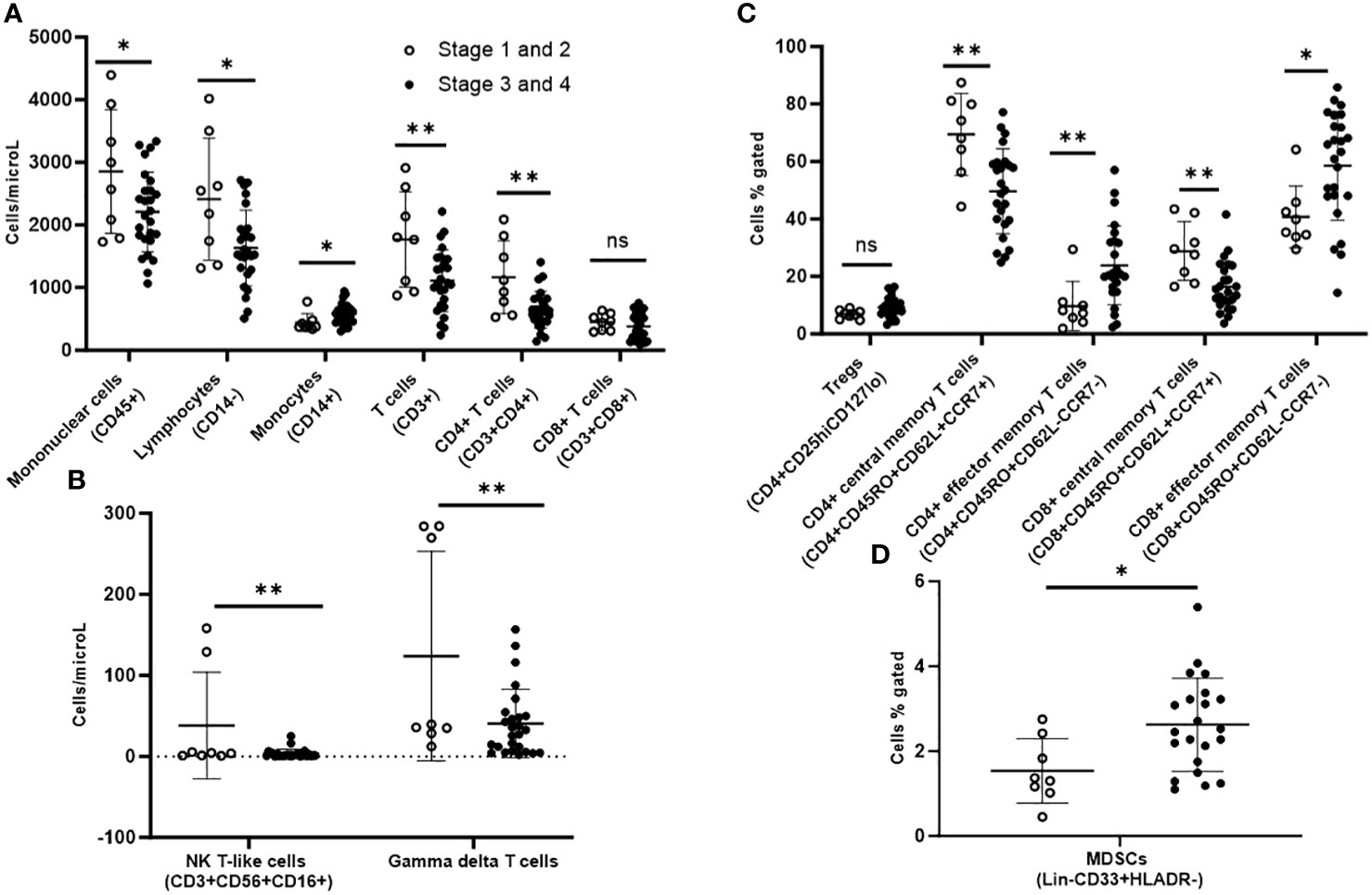
Figure 2 Peripheral blood immunophenotyping via flow cytometry comparing patients with AJCC TNM stages 3 and 4 versus stages 1 and 2 follicular cell-derived thyroid cancer. (A) shows immunophenotyping in the T cell, B cell and NK cell panel. (B) shows the NK T-like and gamma delta subpopulations of T cells. (C) shows further subtyping of T cells to characterize T regs and memory T cells. (D) shows MDSCs. *p<0.05; **p<0.01. NK cell: natural killer cell; Treg: T regulatory cell; MDSC: myeloid-derived suppressor cell.
Upon further subtyping of T cells, there was a trend towards more Tregs (CD4+CD25hiCD127lo) among patients with AJCC stage 3/4 as compared to stage 1/2, but the difference was not statistically significant (p=0.06). We observed more circulating CD4+ effector memory T cells (CD4+CD45RO+CD62L-CCR7-) (23.9% vs. 9.7%; p=0.009) and CD8+ effector memory T cells (CD8+CD45RO+CD62L-CCR7-) (58.5% vs. 40.7%; p=0.02), but fewer CD4+ central memory T cells (CD4+CD45RO+CD62L+CCR7+) (49.6 vs. 69.5; p=0.002) and CD8+ central memory T cells (CD8+CD45RO+CD62L+CCR7+) in patients with stage 3/4 versus stage 1/2 disease (Figure 2C).
Stage 3/4 thyroid cancer patients also had more circulating myeloid-derived suppressor cells (MDSCs; Lin-CD33+HLADR-) as subset of mononuclear cells (2.63% vs. 1.54%; p=0.02) compared with stage1/2 (Figure 2D).
On immunophenotyping in the T cell, B cell, and NK cell panel, patients with ATA high-risk thyroid cancer demonstrated overall fewer circulating mononuclear cells (CD45+) compared with ATA low/intermediate risk. They also had fewer total lymphocytes, and within the lymphocyte compartment, fewer T cells (CD3+) but no difference in B cells (CD19+). They also had fewer CD4+ T cells and gamma-delta T cells, but there were no differences in CD8+ T cells or NK cells compared with ATA low/intermediate-risk (Figures 3A, B).
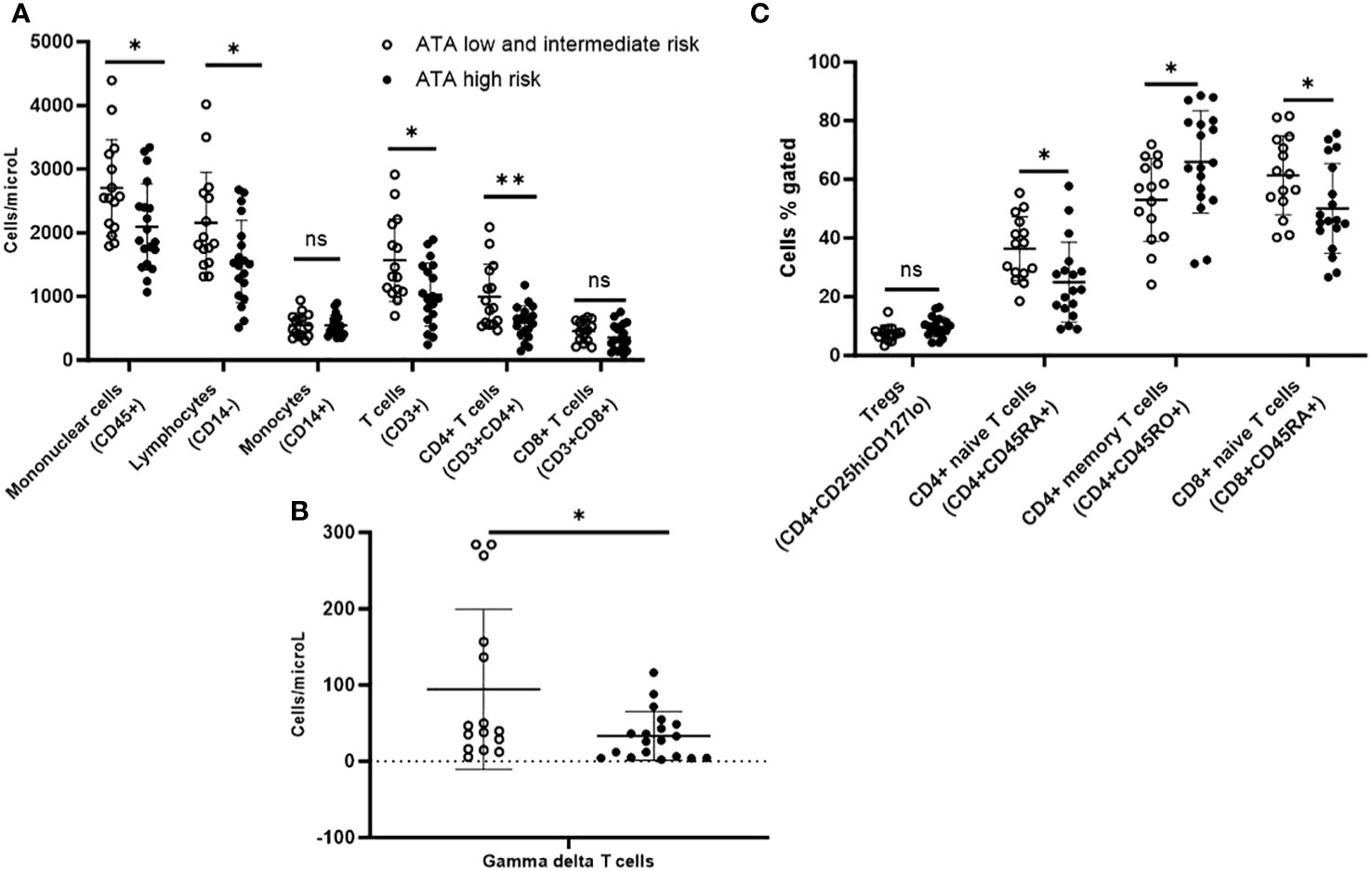
Figure 3 Peripheral blood immunophenotyping via flow cytometry comparing patients with ATA high-risk versus low/intermediate-risk of recurrence follicular cell-derived thyroid cancer. (A) shows immunophenotyping in the T cell, B cell and NK cell panel. (B) shows gamma delta subpopulation of T cells. (C) shows further subtyping of T cells to characterize T regs and memory T cells. *p<0.05; **p<0.01. NK cell: natural killer cell; Treg: T regulatory cell.
Upon further subtyping of T cells, there was a trend towards more Tregs (CD4+CD25hiCD127lo) among patients with ATA high compared to ATA low/intermediate risk, but the difference was not statistically significant (p=0.06). We observed more circulating CD4+ memory T cells (CD4+CD45RO+) (65.9% vs. 53.1%; p=0.03) and a non-significant trend for more CD8+ memory T cells (CD8+CD45RO+) (45.5% vs. 36.1%; p=0.06), but fewer CD4+ naive T cells (CD4+CD45RA+) (25% vs. 36.4%; p=0.014) and CD8+ naive T cells (CD8+CD45RA+) (50.1% vs. 61.4%; p=0.03) in ATA high-risk compared with ATA low/intermediate-risk patients (Figure 3C). There was a trend towards more MDSCs in ATA high-risk patients, but the difference was not statistically significant.
During median follow-up of 57.7 years since initial thyroid cancer surgery, 21 patients demonstrated distant spread, of which 14 patients already had distant metastases during initial presentation. Due to the small sample of 6 patients that developed new distant metastases during follow-up, we compared 21 patients with any distant spread to 11 patients who had no evidence of disease or locoregional stable disease during this follow-up duration (Table 1). On immunophenotyping in the T cell, B cell, and NK cell panel, patients who demonstrated distant metastases during their disease course demonstrated overall fewer circulating mononuclear cells (CD45+) compared to those who had no evidence of disease or locoregional stable disease. They also had fewer total lymphocytes, and within the lymphocyte compartment, fewer T cells (CD3+) but no difference in B cells (CD19+). They had fewer CD4+ T cells and gamma-delta T cells, but there were no differences in CD8+ T cells or NK cells when compared to those with no evidence of disease or locoregional stable disease (Figures 4A, B).
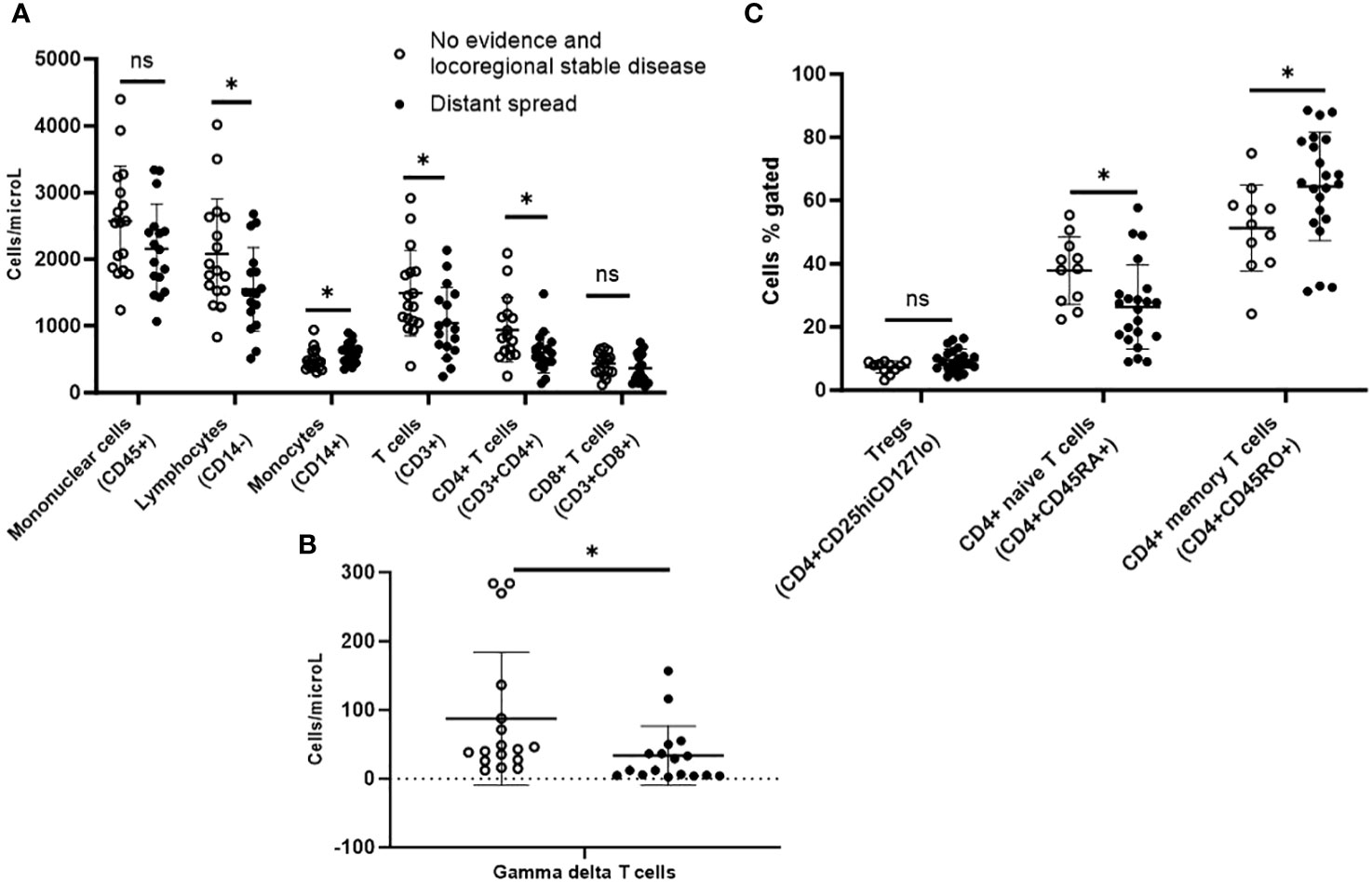
Figure 4 Peripheral blood immunophenotyping via flow cytometry comparing patients with distant metastases versus no recurrence or only loco-regional disease during follow-up follicular cell-derived thyroid cancer. *p<0.05; **p<0.01. (A) shows immunophenotyping in the T cell, B cell and NK cell panel. (B) gamma delta subpopulation of T cells. (C) shows further subtyping of T cells to characterize T regs and memory T cells. *p<0.05; **p<0.01. NK cell: natural killer cell; Treg: T regulatory cell.
Upon further subtyping of T cells, there was a trend towards more Tregs (CD4+CD25hiCD127lo) among patients who developed distant metastases during their disease course compared with those who had no evidence of disease or locoregional stable disease, but the difference was not statistically significant (p=0.06). We also observed more circulating CD4+ memory T cells (CD4+CD45RO+) (64.4% vs. 51.3%; p=0.03) but fewer CD4+ naive T cells (CD4+CD45RA+) (26.3% vs. 37.9%; p=0.02) in patients who developed distant metastases during their disease course compared with those who had no evidence of disease or locoregional stable disease (Figure 4C). There was a trend towards more MDSCs in these patients, but the difference was not statistically significant.
There were no significant differences in the circulating immunophenotypes between patients treated when comparing patients based on age (55 years as cut-off), sex (male versus female), RAI treatment status (17 received versus the rest), TKI treatment status (6 received versus the rest). However, our sample of 32 subjects is not large enough to provide adequately powered conclusions from these negative results.
In this study of 32 adults with follicular cell-derived thyroid cancer, we demonstrated that circulating immunophenotypes serve as prognostic biomarkers. Overall, aggressive thyroid cancer at presentation or during follow-up was characterized by more immune suppressor cells (MDSCs and trend for Tregs) but fewer immune effector cells (CD4+ T cells, gamma-delta T cells and NK T-like cells) and altered memory T cell subtypes compared to less aggressive thyroid cancer. The immunophenotypes were not different based on sex or age of the patients. These findings prove our hypothesis that suppressor circulating immunophenotypes and altered T cell signaling portend a worse thyroid cancer prognosis.
Upon initial analysis, there was a down-regulation of T cell populations specifically in the CD4+ T cell compartment in patients with aggressive thyroid cancer defined as: i) AJCC TNM stage 3 or 4, ii) high risk of recurrence by ATA criteria, or iii) demonstrating distant metastases during follow-up. Available literature evaluating the presence of chronic lymphocytic thyroiditis in the tumor microenvironment without further identifying the specific cell types has demonstrated contradictory results in terms of its association with prognosis (11–14). Hence, we performed a more in-depth analysis of subsets of T cells and NK cells. There are conflicting data regarding the role of CD8+ cytotoxic T cells with one study demonstrating good (20) while another poor prognosis (21). In our study, the lower ratio of CD4/CD8, with significantly fewer CD4+ T cells but no significant difference in CD8+ T cells in patients with more aggressive disease supports the role of CD4+ cytotoxic and helper T cells in mediating immune response against cancer cells.
The lower number of circulating gamma-delta T cells in those with more aggressive thyroid cancer has not been reported in previous thyroid cancer studies of either the tumor microenvironment or peripheral blood. T lymphocytes expressing the gamma-delta form of the T-cell receptor are a distinct functional class whose physiologic role is not completely understood. Their activation results in cell proliferation, proinflammatory cytokine and chemokine secretion, and alteration of cell surface phenotypes (28). It has been postulated that they contribute more to immunoregulation and tissue repair than to immunoprotection (29). They contribute to the pathogenesis of autoimmune disorders, including autoimmune (Hashimoto’s) thyroiditis (30). In addition, they have demonstrated an anti-tumor role via their antigen-presenting cell-like effects in gastric cancer (31) but to our knowledge, have not been evaluated in thyroid cancer. This significant finding of our study supports the hypothesis that the immune suppressor phenotype is associated with thyroid cancer aggressiveness. These cells should be investigated for avenues of immune upregulation.
Another important focus of our study was to identify MDSCs, which were significantly higher in those with stage 3 and 4 thyroid cancer and there was also a similar but non-significant trend in those with ATA high-risk and distant metastases on follow-up. MDSCs are a heterogeneous cell population that suppresses T cell and NK cell function. They arise from myeloid progenitor cells that do not differentiate into mature dendritic cells, granulocytes, or macrophages (32–34). They play a major role in immune evasion and tumor progression, however, a clear consensus on which phenotypes are most relevant in cancer patients has not been reached (34). Studies have demonstrated their immunosuppressive role in cancers including squamous head and neck cancer (35), breast cancer, and non-small cell lung cancer (36). We identified these cells as CD33+ and lineage negative (Lin-) meaning CD3-, CD14-, CD19-, and CD57- like previous studies (36). To our knowledge, only one study has evaluated circulating MDSCs in thyroid cancer patients demonstrating their association with clinicopathologically advanced thyroid cancer (16). Our results validate these findings suggesting that MDSCs are novel biomarkers for predicting aggressiveness of thyroid cancer at diagnosis and should be investigated as therapeutic targets in advanced thyroid cancer.
Tregs (CD4+CD25hiCD127lo) inhibit the anti-tumor response by producing IL-10 and expressing immune checkpoints CTLA-4 and PD-1, hence higher number of these cells in the circulation is associated with more aggressive thyroid cancer (15). The same study showed that PD-1+ T cells (15) were also elevated in more aggressive thyroid cancer. In our study, there was a trend towards more Tregs among patients with AJCC stage 3/4, ATA high-risk, and those that developed distant metastases, but the difference was not statistically significant. Due to the limited sample size, we cannot conclude if Tregs are associated with aggressive thyroid cancer or not. However, our findings support further investigation of their role in a larger cohort of thyroid cancer patients because they could be a potential target for immunotherapy that functions in an antagonistic manner.
In the literature, certain immunoregulatory subtypes of NK cells (CD3-CD16-CD56+)are reported to be associated with pathologically aggressive thyroid cancer (37), however, the main immune effector subtype of NK cells have been shown to clear cancerous cells with low MHC expression. We did not find significant differences in NK cells between the thyroid cancer subgroups; however, this could be a limitation of our sample size and characteristics. The NK T-like cells (CD3+CD56+) combine the characteristics of T (CD3+) and NK (CD56+) cells. The exact pathophysiological role of these cells remains unknown although literature has reported on their effector role in autoimmune diseases (38), and cytotoxic role against infectious diseases (39) and cancer (40, 41). They are reduced in circulation in patients with metastatic as compared to non-metastatic colorectal cancer (42) but have not been investigated in thyroid cancer. Their ability to clear cancerous cells with low MHC expression supports our investigation of their role in thyroid cancer. In our study, NK T-like cells were reduced in patients with advanced clinicopathologic thyroid cancer suggesting their cytotoxic role against tumor cells. Future studies should investigate ways for upregulating these cells as a novel form of therapy against advanced cancer.
In our study, advanced stage (III/IV) thyroid cancer at presentation was characterized by more effector memory T cells but fewer central memory T cells. These differences in subtypes of memory T cells were not significant when comparing by ATA risk or course during follow-up, but in general, there were more memory T cells and fewer naïve T cells in ATA high risk compared to low/intermediate risk and among patients who developed distant metastases during their disease course compared with those who had no evidence of disease or locoregional stable disease. Since central memory T cells express the chemokine receptor CCR7, they traffic to lymph nodes and interact with dendritic cells. Effector memory T cells lacking CCR7 expression migrate to areas of inflamed tissue and display immediate effector function (43, 44). Studies have shown that in chronic infectious processes, there is a gradual shift in the composition of the memory T cell pool from an effector to a central memory phenotype (45, 46). Effector memory cells present an immediate, but not sustained, defense at pathogen sites of entry, whereas central memory T cells sustain the response by proliferating in the secondary lymphoid organs and producing a supply of new effectors (47–49) In addition, effector memory T cells are less efficient than central memory T cells at mediating recall responses in terms of proliferation and accumulation at inflammatory sites (46, 50). Hence, even though not definitively certain, the differences in these memory T cells observed in our study are consistent with the immune suppressor phenotype observed in aggressive thyroid cancer. CCR7 expression has been shown to be lower in poorly differentiated compared with differentiated thyroid cancer (51) which fits with more effector memory T cells (less CCR7) in advanced stages of thyroid cancer in our study. While previous studies have evaluated pathological aggressiveness, our study is novel in investigating this in a cohort predominantly comprised of differentiated thyroid cancer patients with adequate follow-up. Our results suggest that T cell trafficking is altered in advanced stages of thyroid cancer, thus shedding light on both the biology and potentially prognostic applications.
Our study is limited by its small sample size which precludes comparing immunophenotype differences between several types of follicular cell-derived thyroid cancer, stage 3 and 4 patients, and ATA intermediate and high-risk patients. The overall few events of distant progression do not allow us to evaluate the relationship between time to cancer progression and various immune phenotypes in this study. All patients being non-Hispanic Caucasian prevented us from analyzing differences by race or ethnicity. Additionally, the lack of significant differences in circulating immunophenotypes based on treatment with RAI or TKI, and lack of significant differences in Tregs or NK cells between various clinicopathologic subgroups could be due to inadequately powered sample size, hence should not be inferred as definitive lack of difference. Importantly, circulating immune phenotypes may not reflect the in-situ tumor microenvironment, including spatial relationships among immune cells. Tumor-associated macrophages are associated with poor outcomes such as lymph node metastases (52), larger tumor size (53) and reduced survival (54, 55), but are not enough in circulation to perform comparisons, hence these and other tumor-infiltrating immune cells should be the focus of further research on tumor and tumor-adjacent tissue to characterize the thyroid cancer tumor microenvironment. Also, we did not perform an investigation of intracellular factors or functionality of immune cells in our study, did not serially analyze the immunophenotypes over the course of disease or compare them to inflammatory thyroid diseases. The heterogeneity in our results and the overlap in immunophenotypes between the compared groups despite significant differences are also limitations. Hence, future studies with larger cohorts of thyroid cancer patients are required to investigate these important factors in thyroid cancer prognostication. Our pilot study’s strengths include its prospective nature, comprehensiveness of immunophenotyping, evaluation of clinically important outcomes, being the first to demonstrate fewer gamma-delta T cells and NK T-like cells, and only the second to demonstrate more circulating MDSCs amongst patients with advanced thyroid cancer. These findings provide a strong basis for further investigation into the immune phenotypes in circulation and tumor microenvironment in a larger cohort of patients with thyroid cancer.
In conclusion, we have demonstrated that aggressive follicular cell-derived thyroid cancer either at presentation or during the disease course is associated with circulating suppressor immunophenotypes characterized by fewer CD4+ T cells, gamma-delta T cells, and NK T-like cells but more MDSCs; and altered memory T cell subtypes. These immunophenotypes serve as prognostic biomarkers for advanced thyroid cancer. Future studies with larger cohorts should evaluate the changes in circulating and tumor-infiltrating immunophenotypes with thyroid cancer progression and investigate the role of immunotherapies antagonistic to MDSCs and Tregs while upregulating NK T-like and gamma-delta T cells as well as influencing T cell signaling in advanced thyroid cancer.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Mayo Clinic Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AK: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MG: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. SB: Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing. AD: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing. DD: Data curation, Methodology, Visualization, Writing – review & editing. MR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH); and Mayo Foundation Small Grants Program. The abstract of this study was presented as an oral presentation at ENDO 2021, the annual meeting of the Endocrine Society.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1325343/full#supplementary-material
1. Wang LY, Palmer FL, Nixon IJ, Thomas D, Patel SG, Shaha AR, et al. Multi-organ distant metastases confer worse disease-specific survival in differentiated thyroid cancer. Thyroid (2014) 24(11):1594–9. doi: 10.1089/thy.2014.0173
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
3. Albero A, Lopéz JE, Torres A, de la Cruz L, Martín T. Effectiveness of chemotherapy in advanced differentiated thyroid cancer: a systematic review. Endocr Relat Cancer (2016) 23(2):R71–84. doi: 10.1530/ERC-15-0194
4. Lubin D, Baraban E, Lisby A, Jalali-Farahani S, Zhang P, Livolsi V. Papillary thyroid carcinoma emerging from hashimoto thyroiditis demonstrates increased PD-L1 expression, which persists with metastasis. Endocr Pathol (2018) 29(4):317–23. doi: 10.1007/s12022-018-9540-9
5. Ahn S, Kim TH, Kim SW, Ki CS, Jang HW, Kim JS, et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer (2017) 24(2):97–106. doi: 10.1530/ERC-16-0421
6. Iyer PC, Dadu R, Gule-Monroe M, Busaidy NL, Ferrarotto R, Habra MA, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J ImmunoTherapy Cancer (2018) 6(1):68. doi: 10.1186/s40425-018-0378-y
7. Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin C-C, Prawira A, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer (2019) 19(1):196–6. doi: 10.1186/s12885-019-5380-3
8. Gunda V, Gigliotti B, Ndishabandi D, Ashry T, McCarthy M, Zhou Z, et al. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br J Cancer (2018) 119(10):1223–32. doi: 10.1038/s41416-018-0296-2
9. Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab (1995) 80(12):3421–4.
10. McLeod DSA, Bedno SA, Cooper DS, Hutfless SM, Ippolito S, Jordan SJ, et al. Pre-existing thyroid autoimmunity and risk of papillary thyroid cancer: A nested case-control study of US active-duty personnel. J Clin Oncol (2022) 40(23):2578–87. doi: 10.1200/JCO.21.02618
11. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab (1999) 84(2):458–63. doi: 10.1210/jcem.84.2.5443
12. Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid (1998) 8(3):197–202. doi: 10.1089/thy.1998.8.197
13. Gupta S, Patel A, Folstad A, Fenton C, Dinauer CA, Tuttle RM, et al. Infiltration of differentiated thyroid carcinoma by proliferating lymphocytes is associated with improved disease-free survival for children and young adults. J Clin Endocrinol Metab (2001) 86(3):1346–54. doi: 10.1210/jc.86.3.1346
14. Pellegriti G, Belfiore A, Giuffrida D, Lupo L, Vigneri R. Outcome of differentiated thyroid cancer in Graves' patients. J Clin Endocrinol Metab (1998) 83(8):2805–9. doi: 10.1210/jc.83.8.2805
15. French JD, Kotnis GR, Said S, Raeburn CD, McIntyre RC Jr, Klopper JP, et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab (2012) 97(6):E934–43. doi: 10.1210/jc.2011-3428
16. Angell TE, Lechner MG, Smith AM, Martin SE, Groshen SG, Maceri DR, et al. Circulating myeloid-derived suppressor cells predict differentiated thyroid cancer diagnosis and extent. Thyroid (2016) 26(3):381–9. doi: 10.1089/thy.2015.0289
17. Means C, Clayburgh DR, Maloney L, Sauer D, Taylor MH, Shindo ML, et al. Tumor immune microenvironment characteristics of papillary thyroid carcinoma are associated with histopathological aggressiveness and BRAF mutation status. Head Neck (2019) 41(8):2636–46. doi: 10.1002/hed.25740
18. Ahn J, Jin M, Song E, Ryu YM, Song DE, Kim SY, et al. Immune profiling of advanced thyroid cancers using fluorescent multiplex immunohistochemistry. Thyroid (2021) 31(1):61–7. doi: 10.1089/thy.2020.0312
19. Giannini R, Moretti S, Ugolini C, Macerola E, Menicali E, Nucci N, et al. Immune profiling of thyroid carcinomas suggests the existence of two major phenotypes: an ATC-like and a PDTC-like. J Clin Endocrinol Metab (2019) 104(8):3557–75. doi: 10.1210/jc.2018-01167
20. Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, et al. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) (2012) 77(6):918–25. doi: 10.1111/j.1365-2265.2012.04482.x
21. Cunha LL, Marcello MA, Nonogaki S, Morari EC, Soares FA, Vassallo J, et al. CD8+ tumour-infiltrating lymphocytes and COX2 expression may predict relapse in differentiated thyroid cancer. Clin Endocrinol (Oxf) (2015) 83(2):246–53. doi: 10.1111/cen.12586
22. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab (2017) 102(8):2770–80. doi: 10.1210/jc.2017-00448
23. Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, et al. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PloS One (2015) 10(3):e0121546. doi: 10.1371/journal.pone.0121546
24. Bornschlegl S, Gustafson MP, Delivanis DA, Ryder M, Liu MC, Vasmatzis G, et al. Categorisation of patients based on immune profiles: a new approach to identifying candidates for response to checkpoint inhibitors. Clin Transl Immunol (2021) 10(4):e1267. doi: 10.1002/cti2.1267
25. Kotwal A, Gustafson MP, Bornschlegl S, Kottschade L, Delivanis DA, Dietz AB, et al. Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid (2020) 30(10):1440–50. doi: 10.1089/thy.2020.0075
26. Wells SA Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid Off J Am Thyroid Assoc (2015) 25(6):567–610. doi: 10.1089/thy.2014.0335
27. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. New York: Springer (2017).
28. Ribot JC, debarros A, Silva-Santos B. Searching for "signal 2": costimulation requirements of γδ T cells. Cell Mol Life Sci (2011) 68(14):2345–55. doi: 10.1007/s00018-011-0698-2
29. Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol (2000) 18:975–1026. doi: 10.1146/annurev.immunol.18.1.975
30. Liu H, Zheng T, Mao Y, Xu C, Wu F, Bu L, et al. γδ T cells enhance B cells for antibody production in Hashimoto’s thyroiditis, and retinoic acid induces apoptosis of the γδ T cell. Endocrine (2016) 51(1):113–22. doi: 10.1007/s12020-015-0631-9
31. Mao C, Mou X, Zhou Y, Yuan G, Xu C, Liu H, et al. Tumor-activated TCRγδ+ T cells from gastric cancer patients induce the antitumor immune response of TCRαβ+ T cells via their antigen-presenting cell-like effects. J Immunol Res (2014) 2014:593562. doi: 10.1155/2014/593562
32. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol (2009) 9(3):162–74. doi: 10.1038/nri2506
33. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol (2012) 12(4):253–68. doi: 10.1038/nri3175
34. Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol (2014) 41(2):174–84. doi: 10.1053/j.seminoncol.2014.02.003
35. Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res (1995) 1(1):95–103.
36. Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol (2001) 166(1):678–89. doi: 10.4049/jimmunol.166.1.678
37. Gogali F, Paterakis G, Rassidakis GZ, Liakou CI, Liapi C. CD3(-)CD16(-)CD56(bright) immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid (2013) 23(12):1561–8. doi: 10.1089/thy.2012.0560
38. Lin S-J, Chen J-Y, Kuo M-L, Hsiao H-S, Lee P-T, Huang J-L. Effect of interleukin-15 on CD11b, CD54, and CD62L expression on natural killer cell and natural killer T-like cells in systemic lupus erythematosus. Med Inflamm (2016) 2016:9675861. doi: 10.1155/2016/9675861
39. Srivastava R, Aggarwal R, Bhagat MR, Chowdhury A, Naik S. Alterations in natural killer cells and natural killer T cells during acute viral hepatitis E. J Viral Hepatitis (2008) 15(12):910–6. doi: 10.1111/j.1365-2893.2008.01036.x
40. Almeida J-S, Couceiro P, López-Sejas N, Alves V, Růžičková L, Tarazona R, et al. NKT-like (CD3+CD56+) cells in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.02493
41. ZdrazilovaDubska L, Valik D, Budinska E, Frgala T, Bacikova L, Demlova R. NKT-like cells are expanded in solid tumour patients. cytokines (2012) 5:8.
42. Gharagozloo M, Rezaei A, Kalantari H, Bahador A, Hassannejad N, Maracy M, et al. Decline in peripheral blood NKG2D+CD3+CD56+ NKT cells in metastatic colorectal cancer patients. Bratisl Lek Listy (2018) 119(1):6–11. doi: 10.4149/BLL_2018_002
43. Marelli-Berg FM, Fu H, Vianello F, Tokoyoda K, Hamann A. Memory T-cell trafficking: new directions for busy commuters. Immunology (2010) 130(2):158–65. doi: 10.1111/j.1365-2567.2010.03278.x
44. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature (1999) 401(6754):708–12. doi: 10.1038/44385
45. Usherwood EJ, Hogan RJ, Crowther G, Surman SL, Hogg TL, Altman JD, et al. Functionally heterogeneous CD8(+) T-cell memory is induced by Sendai virus infection of mice. J Virol (1999) 73(9):7278–86. doi: 10.1128/JVI.73.9.7278-7286.1999
46. Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol (2003) 4(3):225–34. doi: 10.1038/ni889
47. Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J Immunol (2003) 171(4):2024–34. doi: 10.4049/jimmunol.171.4.2024
48. Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med (2002) 195(3):317–26. doi: 10.1084/jem.20011558
49. Hengel RL, Thaker V, Pavlick MV, Metcalf JA, Dennis G, Yang J, et al. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol (2003) 170(1):28–32. doi: 10.4049/jimmunol.170.1.28
50. Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med (2005) 202(1):123–33. doi: 10.1084/jem.20050137
51. Sancho M, Vieira JM, Casalou C, Mesquita M, Pereira T, Cavaco BM, et al. Expression and function of the chemokine receptor CCR7 in thyroid carcinomas. J Endocrinol (2006) 191(1):229–38. doi: 10.1677/joe.1.06688
52. Fang W, Ye L, Shen L, Cai J, Huang F, Wei Q, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis (2014) 35(8):1780–7. doi: 10.1093/carcin/bgu060
53. Kim BH. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab (Seoul) (2013) 28(3):178–9. doi: 10.3803/EnM.2013.28.3.178
54. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer (2008) 15(4):1069–74. doi: 10.1677/ERC-08-0036
Keywords: immune cell, flow cytometry, prognosis, thyroid carcinoma, T cell, immune markers
Citation: Kotwal A, Gustafson MP, Bornschlegl S, Dietz AB, Delivanis D and Ryder M (2024) Circulating immunophenotypes are potentially prognostic in follicular cell-derived thyroid cancer. Front. Immunol. 14:1325343. doi: 10.3389/fimmu.2023.1325343
Received: 21 October 2023; Accepted: 12 December 2023;
Published: 03 January 2024.
Edited by:
Raquel Tarazona, University of Extremadura, SpainReviewed by:
Sabine Hombach-Klonisch, University of Manitoba, CanadaCopyright © 2024 Kotwal, Gustafson, Bornschlegl, Dietz, Delivanis and Ryder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mabel Ryder, cnlkZXIubWFiZWxAbWF5by5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.