
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 January 2024
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1322233
This article is part of the Research Topic New Approaches to Neoadjuvant Therapy and Translational Resection in Hepatobiliary Malignancies View all 9 articles
 Xiao Xu1,2†
Xiao Xu1,2† Ming-Da Wang3†
Ming-Da Wang3† Jia-Hao Xu3†
Jia-Hao Xu3† Zhong-Qi Fan1†
Zhong-Qi Fan1† Yong-Kang Diao3†
Yong-Kang Diao3† Zhong Chen4
Zhong Chen4 Hang-Dong Jia5
Hang-Dong Jia5 Fu-Bao Liu6
Fu-Bao Liu6 Yong-Yi Zeng7
Yong-Yi Zeng7 Xian-Ming Wang8
Xian-Ming Wang8 Han Wu3
Han Wu3 Wei Qiu1
Wei Qiu1 Chao Li3
Chao Li3 Timothy M. Pawlik9
Timothy M. Pawlik9 Wan Yee Lau3,10
Wan Yee Lau3,10 Feng Shen3
Feng Shen3 Guo-Yue Lv1*
Guo-Yue Lv1* Tian Yang1,3*
Tian Yang1,3*Background & aims: The effectiveness of adjuvant immunotherapy to diminish recurrence and improve long-term prognosis following curative-intent surgical resection for hepatocellular carcinoma (HCC) is of increased interest, especially among individuals at high risk of recurrence. The objective of the current study was to investigate the impact of adjuvant immunotherapy on long-term recurrence and survival after curative resection among patients with intermediate/advanced HCC.
Methods: Using a prospectively-collected multicenter database, patients who underwent curative-intent resection for Barcelona Clinic Liver Cancer (BCLC) stage B/C HCC were identified. Propensity score matching (PSM) analysis was used to compare recurrence-free survival (RFS) and overall survival (OS) between patients treated with and without adjuvant immune checkpoint inhibitors (ICIs). Multivariate Cox-regression analysis further identified independent factors of RFS and OS.
Results: Among the 627 enrolled patients, 109 patients (23.3%) received adjuvant immunotherapy. Most ICI-related adverse reactions were grading I-II. PSM analysis created 99 matched pairs of patients with comparable baseline characteristics between patients treated with and without adjuvant immunotherapy. In the PSM cohort, the median RFS (29.6 vs. 19.3 months, P=0.031) and OS (35.1 vs. 27.8 months, P=0.036) were better among patients who received adjuvant immunotherapy versus patients who did not. After adjustment for other confounding factors on multivariable analyzes, adjuvant immunotherapy remained independently associated with favorable RFS (HR: 0.630; 95% CI: 0.435-0.914; P=0.015) and OS (HR: 0.601; 95% CI: 0.401-0.898; P=0.013). Subgroup analyzes identified potentially prognostic benefits of adjuvant immunotherapy among patients with intermediate-stage and advanced-stage HCC.
Conclusion: This real-world observational study demonstrated that adjuvant immunotherapy was associated with improved RFS and OS following curative-intent resection of intermediate/advanced HCC. Future randomized controlled trials are warranted to establish definitive evidence for this specific population at high risks of recurrence.
Hepatocellular carcinoma (HCC) poses a significant global health challenge, ranking as the sixth most prevalent cancer and the fourth leading cause of cancer-related death across the globe (1, 2). The Barcelona Clinic Liver Cancer (BCLC) staging system is a widely accepted tool for prognostic prediction and treatment allocation in HCC (3–5). This system stratifies HCC patients into five stages based on tumor burden, liver function, and patient performance status, recommending specific treatments for each stage including surgical resection, liver transplantation, local ablation, transarterial chemoembolization (TACE), or systemic therapy (3, 6). Despite the BCLC’s widespread use, management of intermediate/advanced HCC (BCLC stage B/C) remains contentious (7–12). Almost all Western guidelines caution against surgical resection for intermediate/advanced HCC (BCLC stage B/C) due to high morbidity and mortality associated with significant liver dysfunction and tumor burden, instead advocating for TACE or systemic therapy (4, 11, 13, 14). In the real world, surgical resection is still performed by surgeons worldwide, particularly in many Asian countries where the incidence of HCC is highest (9, 15–22). Many previous studies have revealed that long-term prognosis following surgical resection for intermediate/advanced HCC may be superior to TACE or systemic therapy (16, 19, 20, 23–28). The high rate of postoperative recurrence remains a significant bottleneck to improve surgical outcomes in this specific population (29–31). As such, there is an urgent need to explore and develop efficacious neoadjuvant and/or adjuvant therapies aimed at enhancing the long-term survival outcomes for this patient cohort who are at high risks of recurrence after resection.
Adjuvant immunotherapy, especially with immune checkpoint inhibitors (ICIs), has emerged as a promising approach to reduce recurrence and improve survival for various malignant tumors (32–39). ICIs represent a category of immunotherapeutic agents designed to selectively target immune regulatory pathways, thereby reinstating the anti-tumor immune response (40, 41). Pembrolizumab (42, 43), tirellizumab (44), sintilimab (45), camrelizumab (46), atezolizumab (47), tremelimumab plus durvalumab (48) have demonstrated efficacy in the management of advanced-stage HCC (41). However, the real-world effectiveness of adjuvant immunotherapy to enhance the oncological prognosis following curative-intent HCC resection, especially among patients at high risks of postoperative recurrence, remains to be elucidated.
The present study aims to explore the impact of adjuvant immunotherapy on long-term recurrence and survival after surgical resection for intermediate-/advanced-stage HCC. Utilizing a prospectively-collected multicenter database, propensity score matching (PSM) analysis was employed to compare recurrence-free survival (RFS) and overall survival (OS) between patients who did and did not receive postoperative adjuvant immunotherapy. The findings may provide valuable insights into the potential benefits of adjuvant immunotherapy in this high-risk population and guide future therapeutic strategies.
Using a prospectively collected multicenter database, patients who underwent curative-intent resection for intermediate/advanced (BCLC stage B/C) HCC from January 2018 to July 2022 at 7 Chinese hospitals (Eastern Hepatobiliary Surgery Hospital, First Hospital of Jilin University, the Affiliated Hospital of Nantong University, Zhejiang Provincial People’s Hospital, the First Affiliated Hospital of Anhui Medical University, Mengchao Hepatobiliary Hospital, and Qianfoshan Hospital of Shandong Province) were retrospectively identified. Patients were included in the analytic cohort who: 1) had pathologically confirmed HCC; 2) had intermediate/advanced HCC (BCLC stage B/C); 3) underwent curative-intent resection for HCC, which was defined as complete resection of all microscopic and macroscopic tumors (R0 resection), and with the first postoperative evaluation demonstrating absence of any residual or recurrent disease within 4~6 weeks after surgery. Patients were excluded who 1) were under 18 years of age; 2) had recurrent HCC; 3) had received preoperative anti-HCC treatments, including TACE, portal vein embolization, and systemic therapy (including chemotherapy, molecular targeted therapy, and immunotherapy); 4) had palliative liver resections, i.e. microscopically positive (R1 resection) or grossly positive (R2 resection) resection margins, or had residual or recurrent diseases at the first follow-up; 5) had adjuvant molecular targeted therapy after surgery; 6) were lost to follow-up within 90 days after surgery; and 7) had missing data on prognostic variables or follow-up information. Data in the study were censored on 31 December 2022. Written, informed consent for the data to be used for clinical research was obtained from all participating patients. This study was approved by the institutional review boards of all participating centers and conducted in accordance with the Declaration of Helsinki. Patient data were anonymized to ensure confidentiality.
Patient demographics, clinical characteristics, laboratory findings, radiological and pathological features, surgical data, adjuvant ICIs medication usage, and follow-up data were prospectively collected from the medical records at each participating center, and retrospectively studied. The advantage of prospective data collection is that the data collected aligns more closely with the actual circumstances. The following variables were analyzed: age, sex, ECOG performance status, hepatitis B virus (HBV) infection status, Child-Pugh grading, presence of liver cirrhosis, serum alpha-fetoprotein (AFP) levels, maximum tumor size, tumor number, presence of macrovascular invasion, presence of microvascular invasion, presence of satellite nodules, tumor encapsulation, blood loss, transfusion, extent of liver resection, resection margins, adjuvant transarterial chemoembolization (TACE) and adjuvant immunotherapy. Minor liver resection was defined as resection of fewer than three Couinaud liver segments, while major liver resection was defined as resection of three or more liver segments. Non-anatomical liver resection included limited resection or wedge resection; anatomical resections were defined by the Brisbane 2000 system.
Patients with intermediate/advanced-stage HCC would be recommended for adjuvant immunotherapy (ICIs) and/or TACE 4~6 weeks after surgery, and the decision ultimately depended on the patient’s wishes. Adjuvant ICIs included pembrolizumab, sintilimab, camrelizumab, toripalimab, and tislelizumab, which were administered for 12 months according to the recommended dosages starting from 4~6 weeks after surgery until HCC recurrence serious adverse events, patient automagical withdrawal, death or occurrence of other conditions necessitating treatment termination. Generally, 3 weeks treatment was taken as one course, and patients in the adjuvant immunotherapy group received at least 3 months of treatment. The selection of ICI agents, dosage regimens, and treatment duration were determined by the attending physicians at each of the collaborating centers. Intermittent or reduced dosage was allowed during treatment to reduce drug-related toxicities. Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The use of adjuvant ICI treatment and immune-related adverse events (irAEs) were recorded.
The primary endpoints of this study were RFS and OS. RFS was defined as the time from surgery to the detection of recurrence, death from any cause, or the last follow-up, whichever occurred first, while OS was defined as the time from surgery to death from any cause or the last follow-up. The secondary endpoint was the incidence of irAEs among patients who received adjuvant immunotherapy.
To mitigate potential biases and confounding factors in the comparative analysis of outcomes between the groups with and without adjuvant immunotherapy, a rigorous PSM analysis was conducted (1, 49–51). The propensity scores were calculated using a logistic regression model, based on the following covariates: age, sex, ECOG performance status, HBV infection status, Child-Pugh grading, liver cirrhosis, preoperative serum AFP levels, largest tumor diameter, tumor number, macrovascular invasion, microvascular invasion, satellite nodules, tumor encapsulation, intraoperative blood loss, intraoperative blood transfusion, extent of hepatectomy, and adjuvant TACE. Patients in both the adjuvant immunotherapy and non-adjuvant immunotherapy groups were meticulously matched in a 1:1 ratio using the nearest neighbor matching method, with a caliper width set at 0.05 times the standard deviation of the logit of the propensity score to ensure optimal comparability. The balance of the matched variables between the two groups was assessed using standardized mean differences (SMD), with an SMD of less than 0.2 indicating a negligible difference in the mean or prevalence of the covariates between the matched groups.
Descriptive statistics were employed to concisely summarize the baseline characteristics of the patients involved in this study. Continuous variables were reported as means with standard deviations (SD) or medians with interquartile ranges (IQR), as appropriate. Categorical variables were expressed as frequencies and percentages. The comparisons of continuous variables between groups were performed using the independent t-test or the Mann-Whitney U test, as appropriate. The chi-square test or Fisher’s exact test was used to compare categorical variables. Kaplan-Meier survival analysis was used to estimate RFS and OS, and the log-rank test was applied to compare the survival differences between the groups with and without adjuvant immunotherapy. Univariate and multivariate Cox proportional hazards regression models were used to identify the independent prognostic factors associated with RFS and OS. Variables with a P-value less than 0.10 in the univariate analysis were included in the multivariate analysis. The results were presented as hazard ratios (HR) with 95% confidence intervals (CI). Subgroup analyzes in the PSM cohort were performed to identify the potential prognostic benefit of adjuvant immunotherapy among patients with intermediate-stage and patients with advanced-stage HCC, respectively. All statistical analyzes were performed using the R software version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 26.0 (IBM Corp., Armonk, NY, USA). A two-sided P-value less than 0.05 was considered statistically significant.
A total of 1542 HCC patients were screened for eligibility, of which 627 patients with intermediate/advanced-stage HCC (BCLC stage B/C) met the inclusion criteria (Supplementary Figure 1). Among these individuals, 109 patients received adjuvant immunotherapy treatment (the adjuvant immunotherapy group) and 518 patients did not receive adjuvant immunotherapy treatment (the non-adjuvant immunotherapy group). PSM created 99 patient pairs who received and did not receive adjuvant immunotherapy. Patient clinical characteristics and operative variables between the two groups in the entire and PSM cohorts are shown in Table 1.
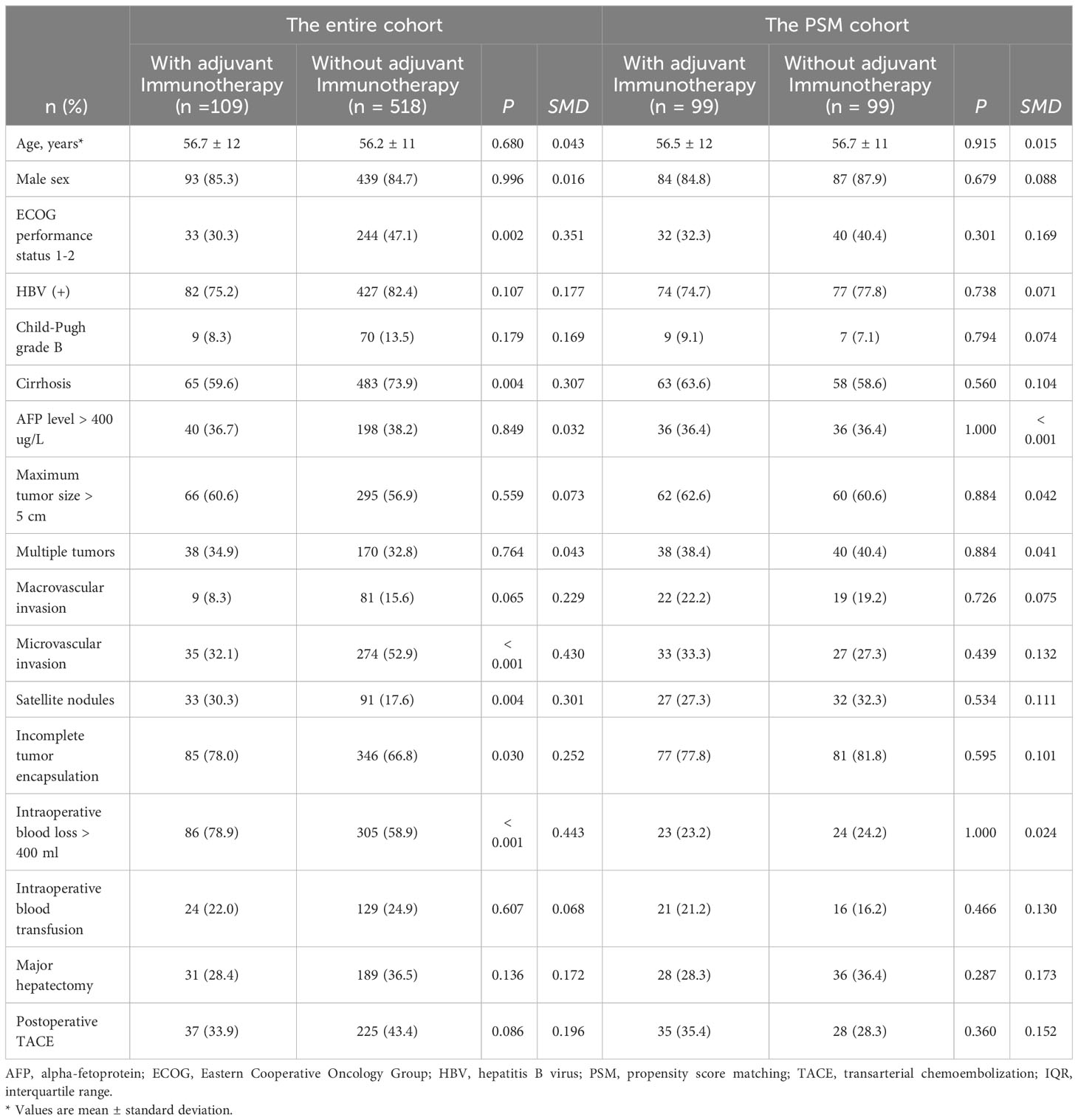
Table 1 Comparison of patient baseline characteristics and operative variables in the entire and PSM cohorts.
Compared with individuals who did not receive adjuvant immunotherapy, patients who received adjuvant immunotherapy had a lower proportion of performance status 1-2 (30.3% vs. 47.1%, P = 0.002), cirrhosis (59.6% vs. 73.9%, P = 0.004), and microvascular invasion (32.1% vs. 52.9%, P < 0.001), yet a higher proportion of satellite nodules (30.3% vs. 17.6%, P = 0.004), incomplete tumor encapsulation (78.0% vs. 66.8%, P = 0.030), and intraoperative blood transfusion > 400 ml (78.9% vs. 58.9%, P < 0.001). Of note, there were no significant differences among patients who did versus those who did not receive adjuvant immunotherapy for any covariate after matching (all P > 0.05, SMD < 0.2) (Figure 1).
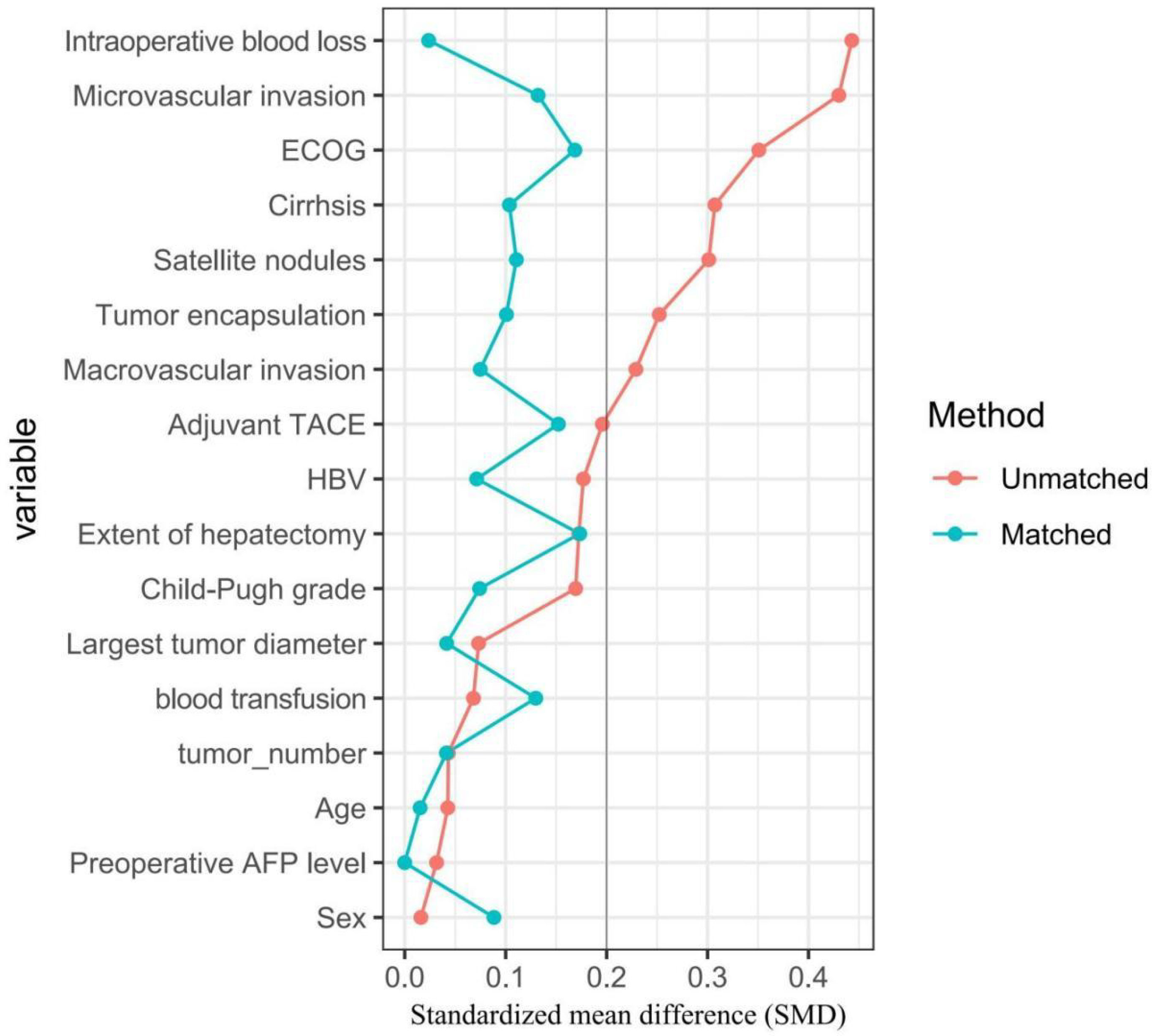
Figure 1 Comparisons of standardized mean difference of clinical variables between patients with and without adjuvant immunotherapy in the entire cohort (red dots), in the PSM cohort (green dots), respectively.
The ICI agents used among patients who received adjuvant immunotherapy (n=109) were tislelizumab (51.4%, n=56), sintilimab (29.3%, n=32), camrelizumab (9.2%, n=10), pembrolizumab (6.4%, n=7), and toripalimab (3.7%, n=4). All patients receiving adjuvant immunotherapy completed at least 3 months of ICI treatment, with no patients discontinuing the treatment due to irAEs. The overall incidence of irAEs among patients in the adjuvant immunotherapy group was 93.6% (99/109), and the most common irAEs were anorexia (84.8%), followed by fatigue (64.2%), allergic reactions (50.4%), gastrointestinal symptoms (e.g., vomiting or diarrhea; 44.0%), liver dysfunction (22.9%), proteinuria (7.3%), and anemia (5.5%). All of these irAEs were grade I-II, being manageable, and reversible with appropriate interventions, including dose adjustment, symptomatic treatment, or temporary discontinuation of ICI therapy.
Comparison of survival outcomes among patients who did and did not receive adjuvant immunotherapy in the entire and PSM cohorts are noted in Table 2. In the entire cohort, the median RFS for patients who received adjuvant immunotherapy was not significantly better than that for patients who did not receive (29.6 months [95% CI 22.4 to 36.8 months] vs. 19.4 months [95% CI 16.0 to 22.8 months]; P = 0.079, Figure 2A); the median OS was also comparable (35.1 months [95% CI 29.9 to 40.3 months] vs. 37.1 months [95% CI 31.3 to 42.9 months]; P = 0.406, Figure 2B).
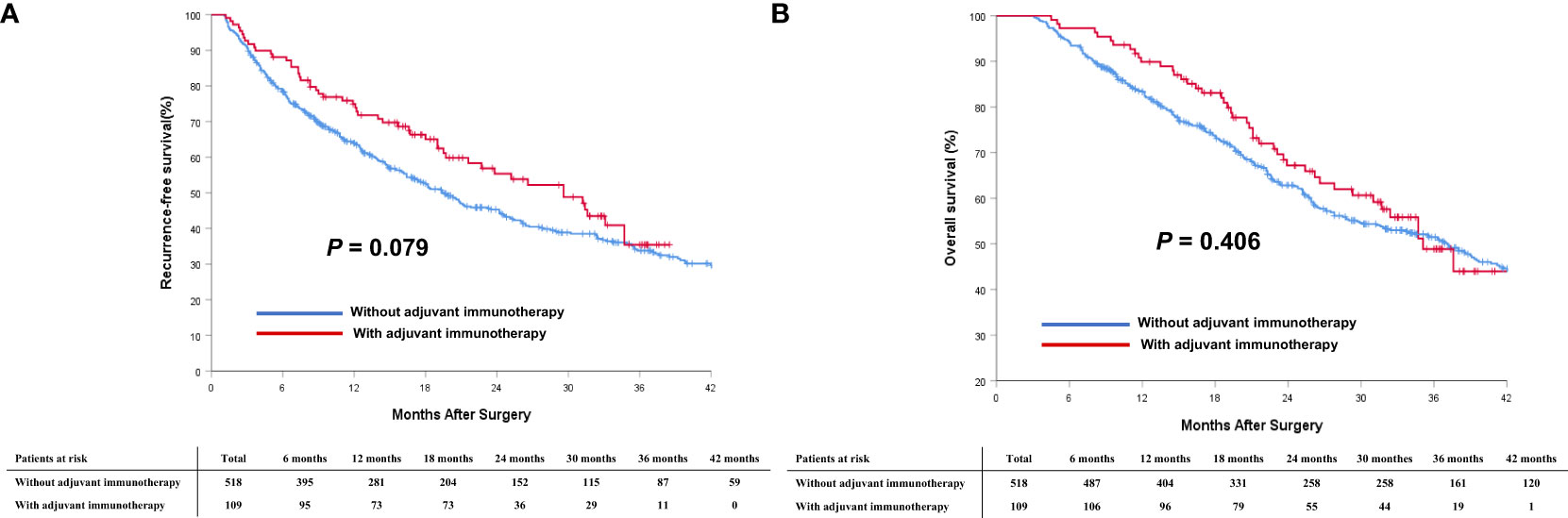
Figure 2 Comparisons of cumulative incidence of recurrence-free survival (RFS, A) and overall survival (OS, B) curves between patients with and without adjuvant immunotherapy in the entire cohort.
In the PSM cohort, the median RFS for patients who received adjuvant immunotherapy was significantly longer than those patients who did not receive adjuvant immunotherapy (29.6 months [95% CI 21.7 to 37.5] vs. 19.3 months [95% CI 13.1 to 25.5]; P < 0.001, Figure 3A). Similarly, the median OS was significantly more favorable in the adjuvant immunotherapy group than in the non-adjuvant immunotherapy group (35.1 months [95% CI 29.3 to 40.9] vs. 27.8 months [95% CI 22.6 to 33.0]; P < 0.001, Figure 3B).

Figure 3 Comparisons of cumulative incidence of recurrence-free survival (RFS, A) and overall survival (OS, B) curves between patients with and without adjuvant immunotherapy in the PSM cohort.
Univariate and multivariate Cox-regression analyzes to identify independent factors associated with RFS and OS in the PSM cohort are shown in Tables 3, 4, respectively. On multivariate analysis, after adjusting for other confounding factors, postoperative adjuvant immunotherapy remained independently associated with more favorable RFS (HR: 0.630, 95% CI 0.435-0.914, P = 0.015) and OS (HR: 0.601, 95% CI 0.401-0.898, P = 0.013) after surgical resection for intermediate/advanced-stage HCC.
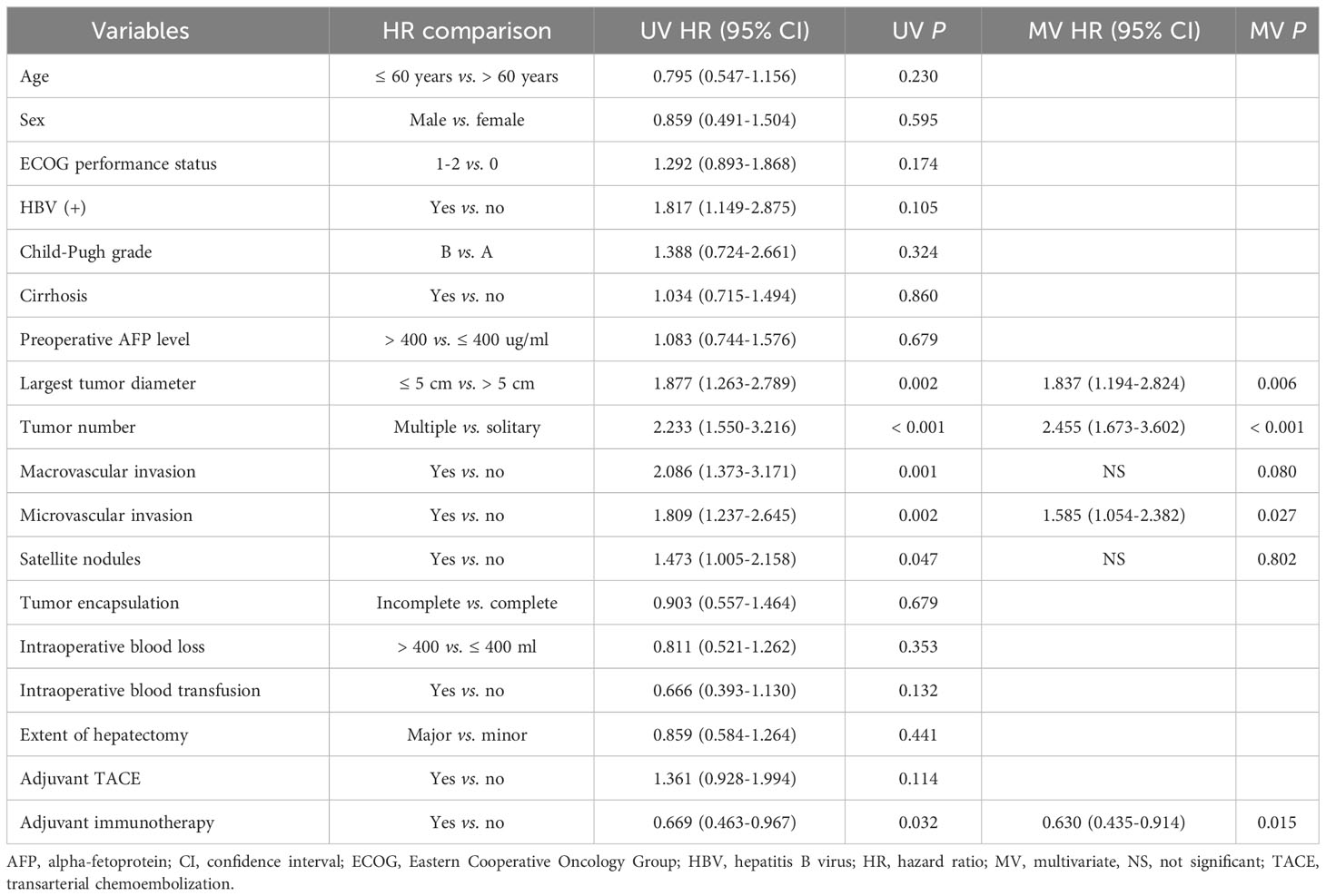
Table 3 Univariate and multivariate Cox-regression analyzes predicting recurrence-free survival in the PSM cohort.
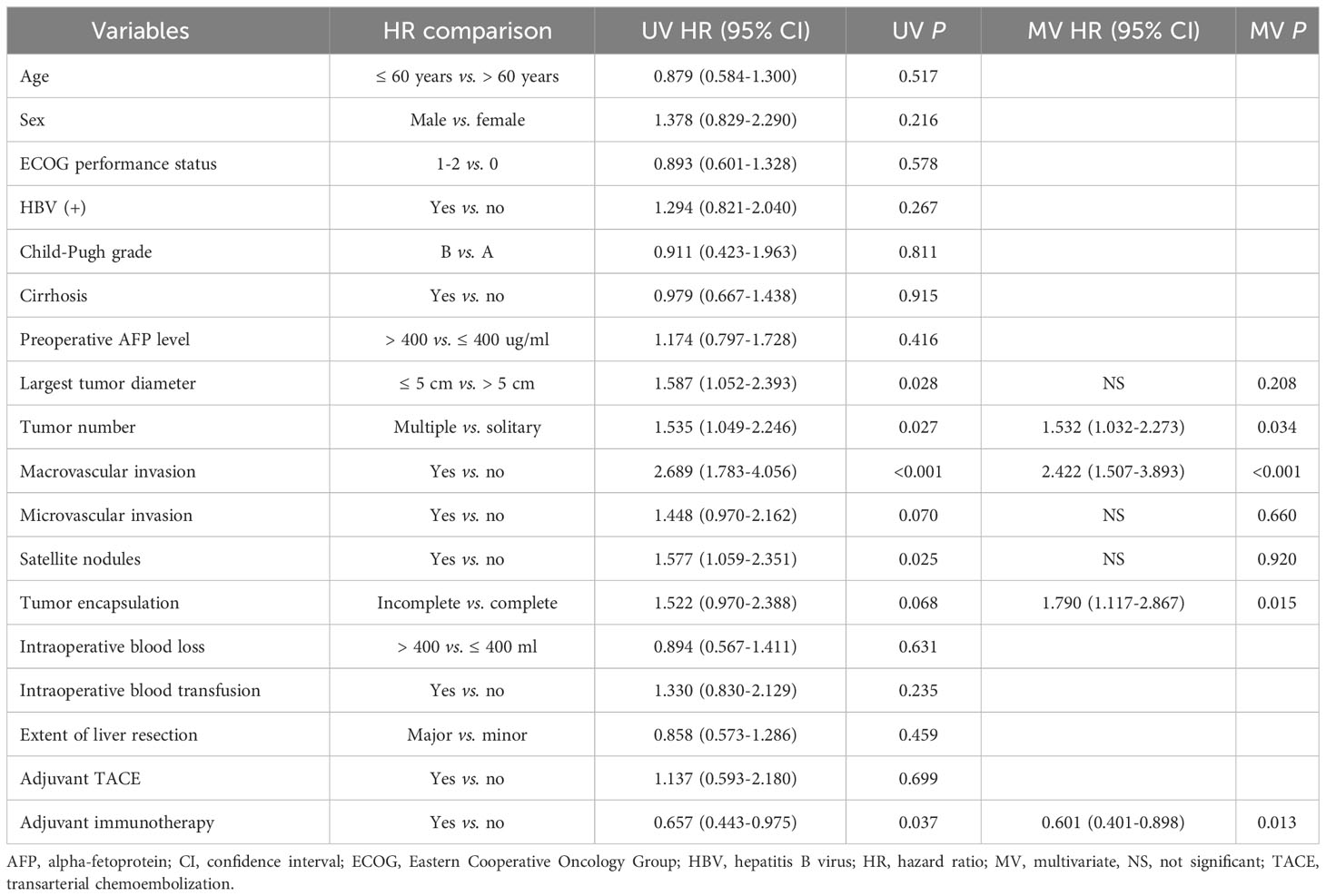
Table 4 Univariate and multivariate Cox-regression analyzes predicting overall survival in the PSM cohort.
To understand better the potential effectiveness of adjuvant immunotherapy, analyzes stratified by different BCLC tumor stages, subgroup analyzes of patients with intermediate-stage (BCLC stage B) HCC and advanced-stage (BCLC stage C) HCC were performed. In the PSM cohort, 53 (66.3%) patients with intermediate-stage HCC and 46 (39.0%) with advanced-stage HCC received adjuvant immunotherapy after surgery. As shown in Figure 4, in the cohort of patients with intermediate-stage HCC, there was a trend toward better RFS among patients who received adjuvant immunotherapy versus individuals who did not receive adjuvant immunotherapy (2-year RFS rates: 41.5% vs. 29.6%; P = 0.383). There was a trend toward better OS among patients who received adjuvant immunotherapy versus individuals who did not receive adjuvant immunotherapy (2-year OS rates: 58.5% vs. 51.9%; P = 0.509). A similar trend was noted in the cohort of patients with advanced-stage HCC (Figure 5). There was a trend toward better RFS among patients who did versus those did not receive adjuvant immunotherapy (2-year RFS rates: 34.7% vs. 23.9%; P = 0.035). There was a trend toward better OS among patients who received adjuvant immunotherapy versus those who did not receive adjuvant immunotherapy (2-year OS rates: 59.7% vs. 43.5%; P = 0.036).
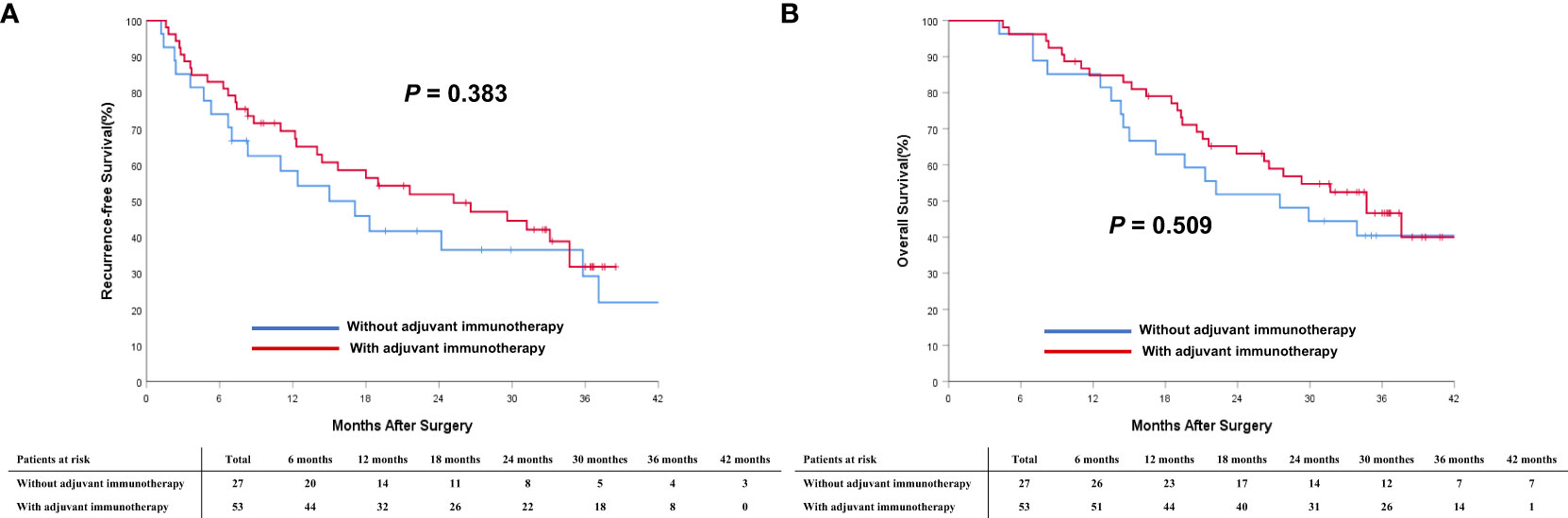
Figure 4 Comparisons of cumulative incidence of recurrence-free survival (RFS, A) and overall survival curves (OS, B) between patients with and without adjuvant immunotherapy in the BCLC B stage cohort.
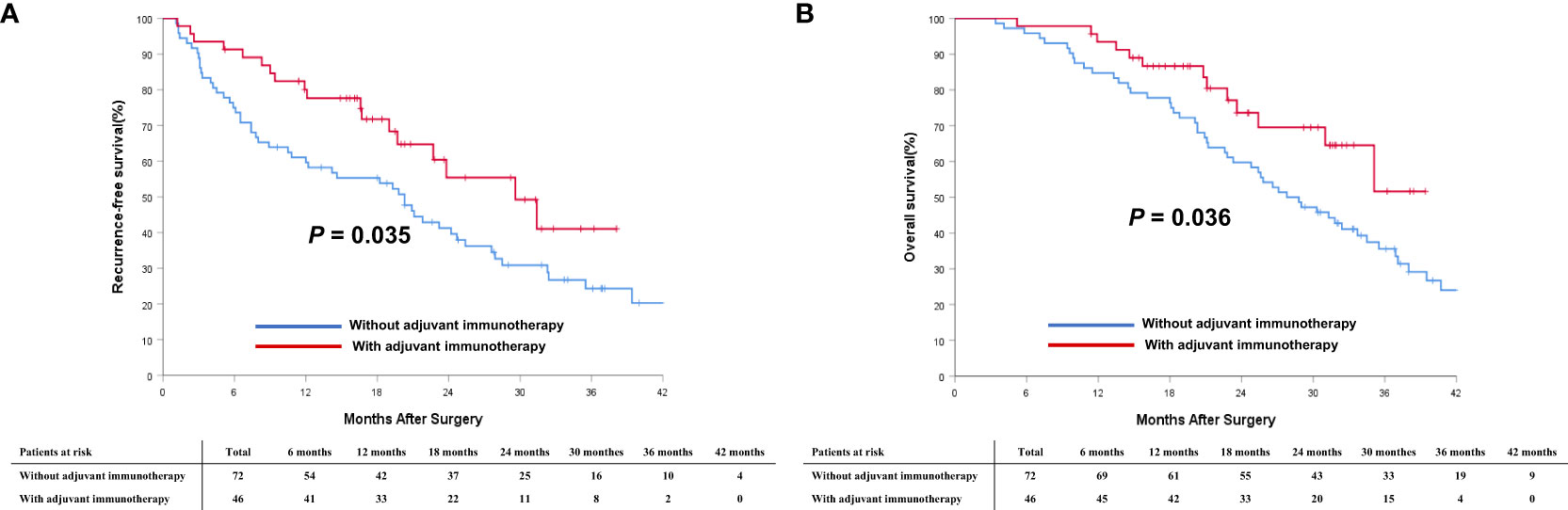
Figure 5 Comparisons of cumulative incidence of recurrence-free survival (RFS, A) and overall survival curves (OS, B) between patients with and without adjuvant immunotherapy in the BCLC C stage cohort.
Nevertheless, even in patients who are considered to be ideal candidates for curative treatment, the recurrence rates following resection or ablation have been documented to exceed 70% within five years (52). Furthermore, in patients with more advanced tumor burden undergoing curative-intent therapies, the risk of HCC recurrence is further amplified (53, 54). The present study focused on patients with intermediate and advanced-stage HCC who underwent curative-intent surgical resection, and attempted to highlight the potential benefits of adjuvant immunotherapy in a cohort of patients that Western guidelines might consider non-operable. This study is among the first to specifically investigate the impact of adjuvant immunotherapy in a real-world setting for HCC patients who are at high risks of tumor recurrence after curative-intent resection. Utilizing PSM analysis, the results of this multicenter real-world study provided aditional evidence to support the use of adjuvant immunotherapy in enhancing RFS and OS following curative-intent resection of intermediate-/advanced-stage HCC. These findings align with the growing body of literature that underscores the potential of adjuvant immunotherapy in the management of various malignancies (32, 34–37).
In the present study, adjuvant immunotherapy significantly improved RFS and OS after curative-intent surgical resection for intermediate/advanced-stage HCC. These findings are in agreement with outcomes obtained from contemporary clinical trials seeking to evaluate the therapeutic effectiveness of adjuvant immunotherapy among individuals diagnosed with HCC. The conclusive results of the IMbrave 050 trial have demonstrated that the combination of atezolizumab and bevacizumab demonstrated improved recurrence-free survival compared to active surveillance in patients with a high risk of HCC recurrence after curative-intent resection or ablation. To the best of our knowledge, the IMbrave 050 study is the first phase III trial evaluating adjuvant treatment for HCC to report positive outcomes. However, longer follow-up is still needed to comprehensively assess the survival benefit as well as potential risks of this regimen, particularly in terms of both recurrence-free and overall survival (55, 56).
In patients with HCC, the immune microenvironment manifests a pronounced abundance of immune cell infiltration, encompassing T cells, natural killer cells, macrophages, and dendritic cells (57–59). The PD-1/PD-L1 axis plays a crucial role in tumor immune evasion by suppressing the immune response (60–63). ICIs, by blocking the PD-1/PD-L1 axis, can restore the antitumor immune response and potentially eliminate residual tumor cells after curative HCC resection (64). Thus, there is an inherent theoretical advantage to employ adjuvant immunotherapy in HCC.
The notable irAEs observed in the present study, such as anorexia, fatigue, and allergic reactions, were aligned with known side effects of ICIs therapy (65). Fortunately, these adverse events were manageable and did not necessitate treatment discontinuation in any patients with adjuvant immunotherapy, underscoring the relative safety of these agents when monitored closely. Additionally, the subgroup analyzes further defined benefits of adjuvant immunotherapy across different BCLC tumor stages. While patients with intermediate-stage HCC was associated with a positive trend toward improved RFS and OS, patients with advanced-stage HCC derived the most benefit from ICI adjuvant therapy. This observation suggested that advanced-stage disease might harbor a more immunosuppressive microenvironment, which, when counteracted by ICIs, leads to a profound clinical response (62, 66–68).
While generally considered as the gold standard in clinical research, RCTs often have stringent inclusion and exclusion criteria, which may limit their generalizability to a broader patient population (69). Real-world studies, on the other hand, offer insights into the effectiveness of interventions in routine clinical practice, encompassing a more diverse patient population and may reflect more genuine clinical scenarios (70). For a considerable subset of HCC patients, especially in Asian contexts, who might find themselves at the crossroads of palliative care and aggressive intervention, our study offers a glimmer of hope. Not only do the data validate the decision for surgical resection, but also highlights the potential importance of adjuvant therapy for improving long-term outcomes. Moreover, multicenter studies, by virtue of their design, capture variations in practice patterns across different institutions, further enhancing the external validity of the findings. In addition, the use of PSM was to minimize the potential confounding effects inherent in observational studies. PSM ensures that the treated and untreated groups are balanced on observed covariates, thereby approximating the conditions of an RCT (50). This methodological approach strengthens the internal validity of our findings.
The current study has several limitations. As with all observational studies, there remains the potential for unmeasured confounding. While PSM can balance observed covariates, it cannot account for unobserved or unmeasured variables. In addition, the duration of follow-up in this study may not be sufficient to capture long-term outcomes and late complications associated with adjuvant immunotherapy. The heterogeneity in the types and regimens of ICIs used across different centers might introduce variability in outcomes. Of note, due to the wide range of PD-1/PD-L1 categories employed in our study and the small sample size, the number of patients receiving specific immunotherapy regimen within the subgroup analysis was relatively limited, leading to non-significant statistical results for subgroup analysis based on different drug types. Finally, the real-world nature of our study highly suggests that the decision to administer adjuvant immunotherapy was clinician-driven, potentially introducing selection bias. Our focus, while innovative, also necessitates multicenter validations, preferably through randomized controlled trials to further solidify the evidence. Future endeavors should aim to address these limitations by incorporating larger patient cohorts, diversified across various regions, and possibly introducing prospective study designs with using a single, standard immunotherapy regimen. Further explorations into the specific mechanisms by which adjuvant immunotherapy offers benefits in postoperative HCC patients could also pave the way for personalized therapeutic strategies.
In conclusion, this multicenter real-world PSM analysis provides promising evidence supporting the role of adjuvant immunotherapy to improve RFS and OS among patients with intermediate-/advanced-stage HCC who underwent curative-intent surgical resection. The findings underscore the potential of ICIs to enhance long-term outcomes in this specific population at high risks of recurrence. While our study offers valuable insights, future RCTs are essential to establish definitive evidence and further elucidate the optimal timing, duration, and regimen of adjuvant immunotherapy for HCC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Data in the study were censored on 31 December 2022. Written, informed consent for the data to be used for clinical research was obtained from all participating patients. This study was approved by the institutional review boards of all participating centers and conducted in accordance with the Declaration of Helsinki. Patient data were anonymized to ensure confidentiality. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XX: Methodology, Visualization, Conceptualization, Data curation, Formal analysis, Writing – original draft, Investigation. M-DW: Funding acquisition, Resources, Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. J-HX: Validation, Conceptualization, Data curation, Writing – original draft, Formal analysis. Z-QF: Conceptualization, Data curation, Writing – original draft, Formal analysis. Y-KD: Methodology, Software, Conceptualization, Data curation, Writing – original draft. ZC: Supervision, Writing – review & editing, Conceptualization, Data curation. H-DJ: Resources, Writing – original draft, Software, Conceptualization, Data curation. F-BL: Conceptualization, Data curation, Resources, Writing – original draft. Y-YZ: Funding acquisition, Supervision, Conceptualization, Data curation, Resources, Writing – original draft. X-MW: Methodology, Project administration, Conceptualization, Data curation, Writing – original draft. HW: Formal analysis, Validation, Conceptualization, Data curation, Writing – original draft. WQ: Resources, Software, Conceptualization, Data curation, Writing – original draft. CL: Conceptualization, Data curation, Writing – original draft. TP: Formal analysis, Supervision, Writing – review & editing, Conceptualization. WL: Writing – review & editing, Visualization, Conceptualization, Supervision. FS: Data curation, Conceptualization, Formal analysis, Supervision, Writing – review & editing. G-YL: Conceptualization, Data curation, Formal analysis, Supervision, Writing – review & editing. TY: Funding acquisition, Project administration, Writing – original draft, Conceptualization, Data curation, Formal analysis, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 81972726 and 82273074 for TY; 82372813 for M-DW, 82241223 and U20A20360 for G-YL), Dawn Project Foundation of Shanghai (No. 21SG36 for TY), Shanghai Health and Hygiene Discipline Leader Project (No. 2022XD001 for TY), Shanghai Outstanding Academic Leader Program (No. 23XD1424900 for TY), the Natural Science Foundation of Shanghai (No. 22ZR1477900 for M-DW) and Shanghai Science and Technology Committee Rising-Star Program (No. 22QA1411600 for M-DW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1322233/full#supplementary-material
Supplementary Figure 1 | Flow chart of participant population. HCC, hepatocellular carcinoma.
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; CT, computed tomography; CTCAE, Common Terminology Criteria for Adverse Events; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HR, hazard ratio; ICI, immune checkpoint inhibitors; IQR, interquartile range; MRI, magnetic resonance imaging; MV, multivariate; MVI, microvascular vascular invasion; MDT, Multi-Disciplinary Treatment; NS, not significant; OS, overall survival; PD-1, programmed death-1; RFS, recurrence-free survival; RCT, Randomized Controlled Trial; SD, standard deviation; UV, univariate.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology (2021) 73 Suppl 1(Suppl 1):4–13. doi: 10.1002/hep.31288
3. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
4. EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
5. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis (1999) 19:329–38. doi: 10.1055/s-2007-1007122
6. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
7. Golfieri R, Bargellini I, Spreafico C, Trevisani F. Patients with barcelona clinic liver cancer stages B and C hepatocellular carcinoma: time for a subclassification. Liver Cancer (2019) 8:78–91. doi: 10.1159/000489791
8. Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology (2020) 72:2206–18. doi: 10.1002/hep.31187
9. Zhang ZM, Guo JX, Zhang ZC, Jiang N, Zhang ZY, Pan LJ. Therapeutic options for intermediate-advanced hepatocellular carcinoma. World J Gastroenterol (2011) 17:1685–9. doi: 10.3748/wjg.v17.i13.1685
10. Kim H, Ahn SW, Hong SK, Yoon KC, Kim HS, Choi YR, et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg (2017) 104:1045–52. doi: 10.1002/bjs.10541
11. Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol (2017) 67:173–83. doi: 10.1016/j.jhep.2017.03.007
12. Sangiovanni A, Colombo M. Treatment of hepatocellular carcinoma: beyond international guidelines. Liver Int (2016) 36 Suppl 1:124–9. doi: 10.1111/liv.13028
13. Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved. Oncologist (2010) 15 Suppl 4:42–52. doi: 10.1634/theoncologist.2010-S4-42
14. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2019) 30(5):871–3. doi: 10.1093/annonc/mdy308
15. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer (2012) 12(4):298–306. doi: 10.1038/nrc3245
16. Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol (2020) 72(2):262–76. doi: 10.1016/j.jhep.2019.11.017
17. Ho MC, Hasegawa K, Chen XP, Nagano H, Lee YJ, Chau GY, et al. Surgery for intermediate and advanced hepatocellular carcinoma: A consensus report from the 5th asia-pacific primary liver cancer expert meeting (APPLE 2014). Liver Cancer (2016) 5:245–56. doi: 10.1159/000449336
18. Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol (2015) 41:1153–61. doi: 10.1016/j.ejso.2015.05.023
19. Chang WT, Kao WY, Chau GY, Su CW, Lei HJ, Wu JC, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection. Surgery (2012) 152:809–20. doi: 10.1016/j.surg.2012.03.024
20. Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology (2018) 68:977–93. doi: 10.1002/hep.29883
21. Han KH, Kudo M, Ye SL, Choi JY, Poon RT, Seong J, et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology (2011) 81 Suppl 1:158–64. doi: 10.1159/000333280
22. Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg (2013) 257(5):929–37. doi: 10.1097/SLA.0b013e31828329b8
23. Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, et al. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol (2012) 138:1121–9. doi: 10.1007/s00432-012-1188-0
24. Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg (2014) 260:329–40. doi: 10.1097/SLA.0000000000000236
25. Torzilli G, Donadon M, Marconi M, Palmisano A, Del Fabbro D, Spinelli A, et al. Hepatectomy for stage B and stage C hepatocellular carcinoma in the Barcelona Clinic Liver Cancer classification: results of a prospective analysis. Arch Surg (2008) 143:1082–90. doi: 10.1001/archsurg.143.11.1082
26. Tada T, Kumada T, Toyoda H, Tsuji K, Hiraoka A, Itobayashi E, et al. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci (2017) 108:1414–20. doi: 10.1111/cas.13257
27. Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol (2015) 62:617–24. doi: 10.1016/j.jhep.2014.10.037
28. Furukawa K, Shiba H, Horiuchi T, Shirai Y, Haruki K, Fujiwara Y, et al. Survival benefit of hepatic resection for hepatocellular carcinoma beyond the Barcelona Clinic Liver Cancer classification. J Hepatobil Pancreat Sci (2017) 24:199–205. doi: 10.1002/jhbp.436
29. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma[J]. Ann Surg (2000) 232(1):10–24. doi: 10.1097/00000658-200007000-00003
30. Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update[J]. Clin J Gastroenterol (2021) 14(3):699–713. doi: 10.1007/s12328-021-01394-7
31. Lu LC, Cheng AL, Poon RT. Recent advances in the prevention of hepatocellular carcinoma recurrence[J]. Semin Liver Dis (2014) 34(4):427–34. doi: 10.1055/s-0034-1394141
32. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell (2015) 161(2):205–14. doi: 10.1016/j.cell.2015.03.030
33. Attrill GH, Owen CN, Ahmed T, Vergara IA, Colebatch AJ, Conway JW, et al. Higher proportions of CD39+ tumor-resident cytotoxic T cells predict recurrence-free survival in patients with stage III melanoma treated with adjuvant immunotherapy. J Immunother Cancer (2022) 10(6):e004771. doi: 10.1136/jitc-2022-004771
34. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
35. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med (2021) 385(8):683–94. doi: 10.1056/NEJMoa2106391
36. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med (2017) 377(19):1824–35. doi: 10.1056/NEJMoa1709030
37. Eggermont A, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med (2018) 378(19):1789–801. doi: 10.1056/NEJMoa1802357
38. McKay RR. The promise of adjuvant immunotherapy in renal-cell carcinoma. N Engl J Med (2021) 385(8):756–8. doi: 10.1056/NEJMe2109354
39. Ilson DH. Adjuvant nivolumab in esophageal cancer - A new standard of care. N Engl J Med (2021) 384(13):1269–71. doi: 10.1056/NEJMe2101983
40. Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol (2019) 70(5):999–1007. doi: 10.1016/j.jhep.2019.01.027
41. Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: A review. JAMA Oncol (2021) 7(1):113–23. doi: 10.1001/jamaoncol.2020.3381
42. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6
43. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307
44. Qin S, Finn RS, Kudo M, et al. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol (2019) 15(16):1811–22. doi: 10.2217/fon-2019-0097
45. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
46. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol (2020) 21(4):571–80. doi: 10.1016/S1470-2045(20)30011-5
47. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
48. Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol (2021) 39(27):2991–3001. doi: 10.1200/JCO.20.03555
49. Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med (1998) 17(19):2265–81. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B
50. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res (2011) 46(3):399–424. doi: 10.1080/00273171.2011.568786
51. Landreneau RJ, Normolle DP, Christie NA, Awais O, Wizorek JJ, Abbas G, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol (2014) 32(23):2449–55. doi: 10.1200/JCO.2013.50.8762
52. Li L, Zhang J, Liu X, Li X, Jiao B, Kang T, et al. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol (2012) 27(1):51–8. doi: 10.1111/j.1440-1746.2011.06947.x
53. Chan A, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol (2018) 69(6):1284–93. doi: 10.1016/j.jhep.2018.08.027
54. Wee I, Moe F, Sultana R, Ang R, Quek P, Goh B, et al. Extending surgical resection for hepatocellular carcinoma beyond barcelona clinic for liver cancer (BCLC) stage A: A novel application of the modified BCLC staging system[. J Hepatocell Carcinoma (2022) 9:839–51. doi: 10.2147/JHC.S370212
55. Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol (2020) 16(15):975–89. doi: 10.2217/fon-2020-0162
56. Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet (2023) 402(10415):1835–47. doi: 10.1016/S0140-6736(23)01796-8
57. Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut (2015) 64(5):842–8. doi: 10.1136/gutjnl-2014-307990
58. Chaisaingmongkol J, Budhu A, Dang H, et al. Common molecular subtypes among asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell (2017) 32(1):57–70.e3. doi: 10.1016/j.ccell.2017.05.009
59. Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature (2016) 531(7593):253–7. doi: 10.1038/nature16969
60. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol (2018) 18(3):153–67. doi: 10.1038/nri.2017.108
61. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017
62. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
63. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discovery (2015) 14(8):561–84. doi: 10.1038/nrd4591
64. Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res (2009) 15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608
65. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
66. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
67. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res (2020) 30(8):660–9. doi: 10.1038/s41422-020-0343-4
68. Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell (2023) 41(3):450–65. doi: 10.1016/j.ccell.2023.02.014
69. Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet (2005) 365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8
Keywords: hepatocellular carcinoma, BCLC staging, recurrence, adjuvant therapy, immune checkpoint inhibitors, propensity matching analysis, recurrent-free survival, overall survival
Citation: Xu X, Wang M-D, Xu J-H, Fan Z-Q, Diao Y-K, Chen Z, Jia H-D, Liu F-B, Zeng Y-Y, Wang X-M, Wu H, Qiu W, Li C, Pawlik TM, Lau WY, Shen F, Lv G-Y and Yang T (2024) Adjuvant immunotherapy improves recurrence-free and overall survival following surgical resection for intermediate/advanced hepatocellular carcinoma a multicenter propensity matching analysis. Front. Immunol. 14:1322233. doi: 10.3389/fimmu.2023.1322233
Received: 16 October 2023; Accepted: 14 December 2023;
Published: 08 January 2024.
Edited by:
Luca Nespoli, University of Milano-Bicocca, ItalyReviewed by:
Hongyun Shi, Affiliated Hospital of Hebei University, ChinaCopyright © 2024 Xu, Wang, Xu, Fan, Diao, Chen, Jia, Liu, Zeng, Wang, Wu, Qiu, Li, Pawlik, Lau, Shen, Lv and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Yang, eWFuZ3RpYW5kZmdkQGhvdG1haWwuY29t; Guo-Yue Lv, bHZneUBqbHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.