A Commentary on
Tissue factor as a potential coagulative/vascular marker in relapsing-remitting multiple sclerosis
by Koudriavtseva T, Lorenzano S, Cellerino M, Truglio M, Fiorelli M, Lapucci C, D’Agosto G, Conti L, Stefanile A, Zannino S, Filippi MM, Cortese A, Piantadosi C, Maschio M, Maialetti A, Galiè E, Salvetti M and Inglese M (2023) Front. Immunol. 14:1226616. doi: 10.3389/fimmu.2023.1226616
Koudriavtseva and colleagues (1) measured levels of different biomarkers in healthy controls, patients with remitting multiple sclerosis (MS), and patients with relapsing MS. The Quantikine enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, Cat. No. DCF300) was used to measure plasma tissue factor (TF) (S. Lorenzano, personal communication). The authors found that patients with relapsing MS had lower levels of plasma TF compared to either patients with remitting MS or the control group. It was concluded that plasma TF is a promising biomarker and possible therapeutic target in relapsing-remitting MS.
A previous clinical study called Comparison of Acute Treatments in Cancer Hemostasis (CATCH) used the same Quantikine ELISA to measure plasma TF levels in 805 patients with cancer and acute symptomatic venous thromboembolism treated with anticoagulants (2). The mean and median values for TF were 72.5 pg/mL and 50.3 pg/mL, respectively. Plasma TF was identified as a biomarker of recurrent venous thromboembolism in this group of patients. We were invited to write a commentary on the paper (understanding the pathway), in which we raised concerns about the ability of the Quantikine ELISA to accurately measure levels of TF in human plasma (3).
Recently, we assessed the ability of four commercial human TF ELISAs, including the Quantikine ELISA, to detect TF in various biological samples, including human plasma (4). Two of the ELISAs (Quantikine and Imubind) detected recombinant TF and TF in extracellular vesicles from a TF-expressing human pancreatic cell line. However, the ELISAs failed to detect TF in plasma that contained TF-positive extracellular vesicles (1.2–2.6 pg/mL) (determined using a factor Xa generation assay). The only plasma samples that had a signal above the background of plasma from healthy controls were from three patients with acute leukemia, who had very high levels of extracellular vesicle TF activity (151–696 pg/mL). We concluded that, except for some patients with leukemia, the Quantikine ELISA could not detect TF in human plasma.
There are two significant challenges with measuring TF in human plasma. First, the concentration of this potent procoagulant protein is very low. Secondly, plasma contains multivalent substances, such as heterotypic antibodies, that can generate signals in ELISAs independently of the target protein by simply bridging assay antibodies. Such heterotypic antibodies may increase in different disease states. The datasheet for the Quantikine ELISA states that the mean value for plasma from healthy controls is 33.3 pg/mL (Table 1). We and others (4–7) have observed similar low values for healthy individuals (mean, 23.5–45.1 pg/mL; median, 39.1–52.4 pg/mL) (Table 1). In contrast, Koudriavtseva and colleagues (1) report much higher values of TF in healthy controls (mean, 110.85 pg/mL; median, 120.69 pg/mL) (Table 1). Patients with remitting MS had similarly high values (mean, 94.06 pg/mL; median, 99.23 pg/mL), whereas patients with relapsing MS had low values (mean, 45.03 pg/mL; median, 22.72 pg/mL). These low values are more consistent with healthy controls than a disease group.
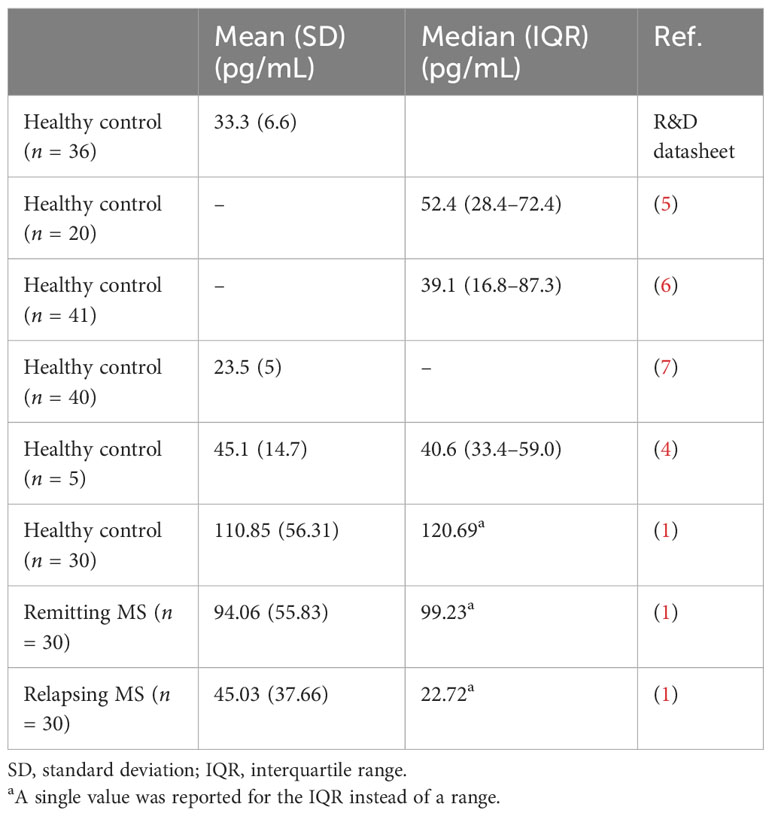
Table 1 Measurement of the levels of plasma tissue factor in healthy controls and patients with multiple sclerosis using the quantikine TF ELISA.
We found that the Quantikine ELISA could not detect low levels of TF in human plasma. We believe that additional methods should be used to demonstrate changes in TF levels in the plasma, such as measuring extracellular vesicle TF activity, before concluding that plasma TF is a biomarker of relapsing-remitting MS.
Author contributions
NM: Writing – original draft. AS: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Koudriavtseva T, Lorenzano S, Cellerino M, Truglio M, Fiorelli M, Lapucci C, et al. Tissue factor as a potential coagulative/vascular marker in relapsing-remitting multiple sclerosis. Front Immunol (2023) 14:1226616. doi: 10.3389/fimmu.2023.1226616
2. Khorana AA, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, et al. Tissue factor as a predictor of recurrent venous thromboembolism in Malignancy: biomarker analyses of the CATCH trial. J Clin Oncol (2017) 35(10):1078–85. doi: 10.1200/JCO.2016.67.4564
3. Ay C, Mackman N. Tissue factor: catch me if you can! J Clin Oncol (2017) 35(10):1128–30. doi: 10.1200/JCO.2016.70.6788
4. Sachetto ATA, Archibald SJ, Bhatia R, Monroe D, Hisada Y, Mackman N. Evaluation of four commercial ELISAs to measure tissue factor in human plasma. Res Pract Thromb Haemostasis (2023) 100133. doi: 10.1016/j.rpth.2023.100133
5. Talar-Wojnarowska R, Woźniak M, Borkowska A, Cypryk K, Olakowski M, Małecka-Panas E. Procoagulant disorders in patients with newly diagnosed pancreatic adenocarcinoma. Medicina (2020) 56(12):677. doi: 10.3390/medicina56120677
6. Kobayashi S, Koizume S, Takahashi T, Ueno M, Oishi R, Nagashima S, et al. Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer. Cancer Sci (2021) 112(11):4679–91. doi: 10.1111/cas.15106
Keywords: multiple sclerosis, tissue factor, inflammation, biomarker, coagulation
Citation: Mackman N and Sachetto ATA (2023) Commentary: Tissue factor as a potential coagulative/vascular marker in relapsing-remitting multiple sclerosis. Front. Immunol. 14:1320179. doi: 10.3389/fimmu.2023.1320179
Received: 11 October 2023; Accepted: 15 November 2023;
Published: 01 December 2023.
Edited by:
Uday Kishore, United Arab Emirates University, United Arab EmiratesReviewed by:
Paolo Ragonese, University of Palermo, ItalyCopyright © 2023 Mackman and Sachetto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nigel Mackman, bm1hY2ttYW5AbWVkLnVuYy5lZHU=
 Nigel Mackman
Nigel Mackman Ana T.A. Sachetto
Ana T.A. Sachetto