- 1College of Acupuncture and Massage, Henan University of Chinese Medicine, Zhengzhou, China
- 2Tuina Department, The Third Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 3Rehabilitation Department, Jiaozuo Coal Industry (Group) Co. Ltd., Central Hospital, Jiaozuo, China
- 4College of Computer Science, Xidian University, Xian, China
- 5College of Acupuncture and Massage, Shanghai University of Chinese Medicine, Shanghai, China
Background: With the continuous development of clinical medicine, an increasing number of non-pharmacological interventions have been applied for the treatment of knee osteoarthritis (KOA), with the results of several recent randomized controlled trials (RCTs) showing that a variety of externally-applied, non-pharmacological interventions (EANPI) can improve symptoms and inflammation in patients with KOA. However, the relative benefits and disadvantages of non-drug therapies remain uncertain, and an optimal treatment strategy has not yet been determined.
Objective: This study applied network meta-analysis (NMA) to compare and rank the effectiveness of EANPI on the short- and long-term clinical symptoms and inflammatory cytokine levels in patients with KOA.
Methods: Two independent researchers searched online databases and performed manual retrieval of related citations to identify RCTs that met the selection criteria for the network meta-analysis. These researchers retrieved studies indexed from database inception to August 2023 and performed data extraction and assessment of the risk of bias.
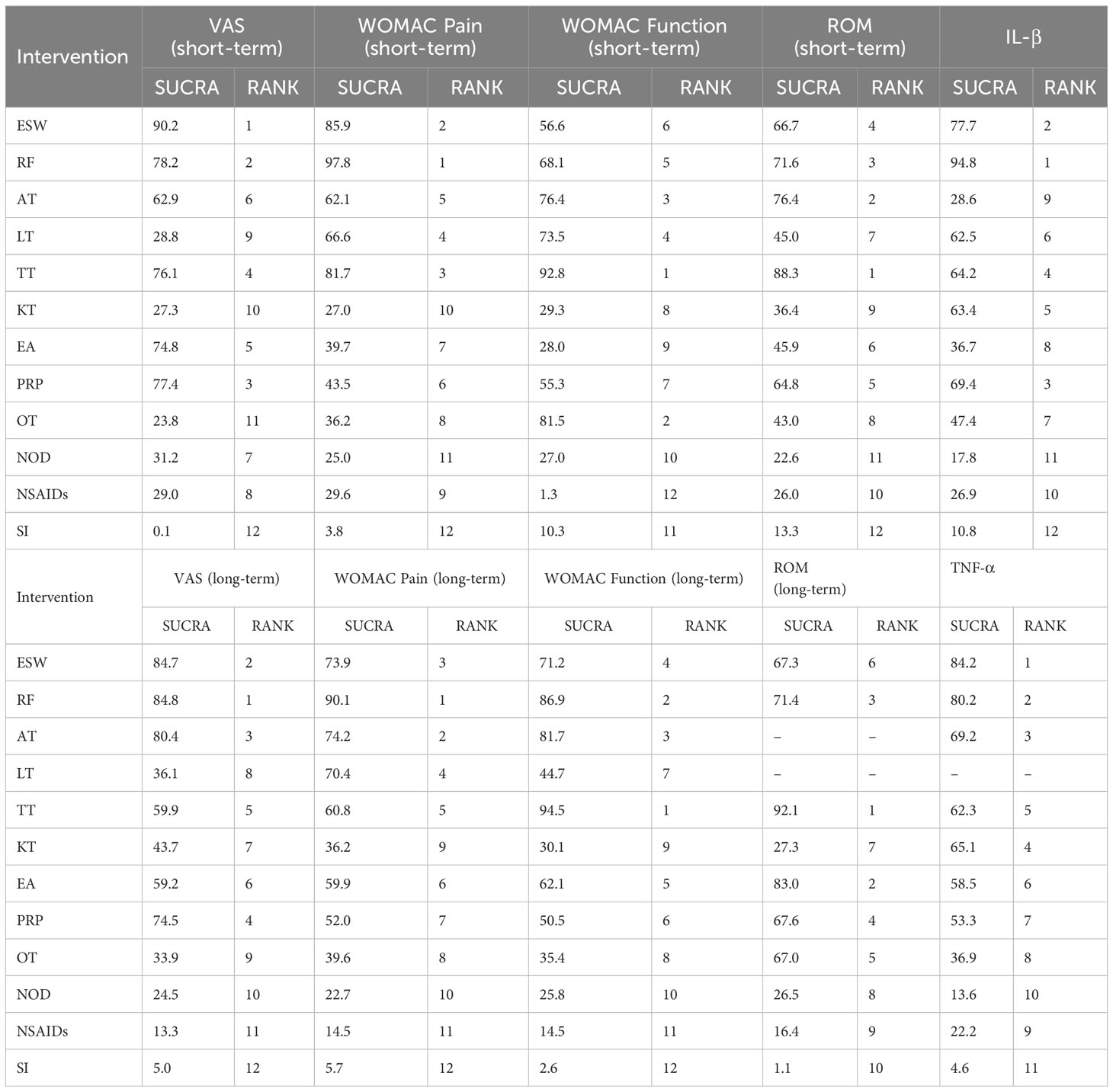
Results: The analysis included 80 RCTs involving 8440 participants and nine externally-applied, non-pharmacological therapies, namely extracorporeal shock wave, radiofrequency, acupotomy, laser therapy, Tuina therapy, kinesio taping, electroacupuncture, platelet-rich plasma injection, and ozone therapy. The treatment courses ranged from 1 to 12 weeks, with follow-up periods ranging from 4 to 24 weeks. The results of the NMA indicated that each non-drug therapy was superior to sham intervention in improving all outcome indicators. Except for the visual analog scale (VAS) and Western Ontario MacMaster (WOMAC) pain outcomes, all non-drug therapies had better efficacy than pharmacological treatments. For short-term VAS and tumor necrosis factor-alpha (TNF-α), extracorporeal shock wave performed better than other therapies (90.2% and 85.2% respectively). Radiofrequency therapy may be the most promising method to reduce long-term VAS, short- and long-term WOMAC pain, and interleukin (IL)-1β level (84.8%, 97.8%, 90.1%, 94.8% respectively). Tuina therapy may be a significant choice for short- and long-term outcomes of WOMAC function and range of motion (ROM).
Conclusions: The results of the comprehensive comparison of the outcome indicators in 9 different EANPI indicated that radiofrequency and Tuina therapy were more effective and consistently ranked high in improving clinical symptoms in the short and long term. Radiofrequency is effective at relieving pain, and Tuina therapy can be given priority for treatment when hypofunction is the main symptom. EANPI to improve pain symptoms may be related to the regulation of inflammatory cytokine levels, which may be a potential mechanism of action.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?, identifier CRD42023464177.
1 Introduction
Knee osteoarthritis (KOA) is the most common type of osteoarthritis and mainly manifests as knee pain, swelling, and unfavorable flexion and extension (1, 2). Globally, KOA is the 11th leading cause of disability, affecting approximately 3.8% of the population (3). Owing to the increase in work pressures and acceleration in the pace of life, the annual incidence of KOA has increased rapidly (4). The pathogenesis of KOA is complex and involves several inflammatory cytokines. Inflammatory factors are involved in processes such as chondrocyte damage, extracellular matrix degradation, and bone redundancy, which play important roles in KOA development (5, 6). Drug therapy can prevent or reduce joint damage and maintain normal joint function (7, 8). Although various types of drug therapies have been used for the treatment of KOA, including non-steroidal anti-inflammatory drugs (NSAIDs), sodium hyaluronate injection, and topical voltaline, the shortcomings include adverse reactions, poor long-term efficacy, and easily reached treatment bottleneck (9, 10). Therefore, the optimization of KOA treatment strategies is a major concern for clinicians.
Concerns regarding the safety and bottlenecking of drug treatments have increased the focus on non-drug therapies. Non-drug treatments for KOA have the advantages of significantly higher efficacy, lasting effects, and few adverse reactions, and have become a hot research topic in recent years (11, 12). Several guidelines and consensuses (13–15) list non-drug therapies as recommended interventions for the clinical treatment of KOA. However, the various types of non-pharmacological interventions include radiofrequency, extracorporeal shock wave, kinesio taping, and massage, and a direct comparison of the curative effects of different non-drug therapies is lacking. Therefore, the choice of non-drug therapy for KOA remains controversial.
While several traditional meta-analyses (16–19) have demonstrated the advantages of non-drug treatment of KOA, these analyses have focused on the comparison of a single non-drug therapy with drugs or another non-drug therapy and have not compared multiple non-pharmacological interventions simultaneously. As the number of alternative treatment options increases, comparative effectiveness studies will be necessary. To date, no meta-analysis has comprehensively compared and evaluated the efficacy of multiple types of non-drug therapies. Thus, the intervention measures with the best effects are unknown. In addition, most systematic reviews have focused only on short-term changes in clinical symptom indicators and have failed to explore long-term outcomes and changes in inflammatory cytokine levels in patients with KOA treated with non-pharmacological interventions. Therefore, a network meta-analysis (NMA) was performed to simultaneously analyze both direct and indirect evidence from different studies, estimate the relative effectiveness of all interventions, and rank the order of interventions (20, 21). This study systematically evaluated the effects of non-pharmacological therapies on short- and long-term outcomes and inflammatory cytokines in patients with KOA to provide evidence for choosing the best plans for the clinical treatment of patients with KOA.
2 Methods
2.1 Study protocol and registration
The NMA and systematic review were conducted strictly in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-NMA) guidelines (22) (see Supplementary Table S1). The study protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42023464177).
2.2 Inclusion criteria
2.2.1 Research type
Only randomized controlled trials (RCTs) were included and were not restricted to any language.
2.2.2 Research objects
All studies met the recognized diagnostic criteria for KOA, regardless of age, sex, or race.
2.2.3 Interventions
The patients in the treatment group received only externally-applied, non-pharmacological interventions. Patients in the control group were treated with a sham intervention, conventional medicine, or any non-pharmacological intervention in the treatment group. The inclusion of intervention drugs in the control group was based on accepted guidelines or consensus (23, 24). Conventional medicines are divided into oral and non-oral drugs (NOD); oral drugs are only included as NSAIDs.
2.2.4 Outcome indicators
(1) Pain: Visual analog scale (VAS), Western Ontario MacMaster (WOMAC) pain score (2); Function: WOMAC function score, Joint range of motion (ROM) (3); Inflammatory cytokine: Interleukin-1β (IL-1β), tumor necrosis factor (TNF-α). All outcome measures were analyzed after treatment to determine short-term efficacy. In addition, the long-term effects of non-pharmacological therapies on pain and functional indicators were analyzed during follow-up.
2.3 Exclusion criteria
The exclusion criteria were (1) patients with other inflammatory diseases (2), repeated publications, (3) more than one therapy, (4) no reference or homemade diagnostic criteria, (5) unavailability of full texts and outcomes, and (6) serious complications.
2.4 Literature search strategy
The Cochrane Library, Embase, PubMed, Web of Science, Chinese Biomedical Database (CBM), VIP, Chinese National Knowledge Infrastructure (CNKI), and Wanfang databases were searched for relevant studies. Grey literature was manually searched, and the reference catalogs included in each study and related systematic reviews were consulted. The retrieval strategy used a combination of subject headings and free words. The databases were searched from their inception to August 20, 2023. An example of the PubMed search strategy is shown in Supplementary Figure S1.
2.5 Literature screening and data extraction
Two researchers (WY and ZL) independently screened the studies based on the inclusion criteria. EndNote software was used to check for duplicate studies. The investigators screened the titles and abstracts of each study and excluded studies that did not meet the inclusion criteria. Subsequently, the investigators read the full texts of the remaining studies to decide whether to include them. Disagreements were resolved through consultations with a third party (LX). Two reviewers (LM and WZ) separately extracted the data from each eligible RCT using a standardized form. The extracted data included the study characteristics (author, country, and publication date), patient characteristics (sample size, disease duration, sex, and age), research site, methodology, intervention measures, treatment course, follow-up, and outcome indicators.
2.6 Risk of bias assessment
The risk of bias in the included studies was evaluated by two separate researchers (WY and ZH) using the RCT Bias Risk Assessment Tool of the Cochrane System Review Manual, version 6.1.0 (25). A third investigator (XH) assisted in resolving differences in assessments between the two researchers. The evaluation items included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete data, selective reporting, and other biases. Finally, the included studies were categorized as having low, high, or unclear risks of bias.
2.7 Statistical analysis
All outcome indicators were analyzed using random- or fixed-effects models, based on the level of heterogeneity. The P-values of the chi-square test and the I2 index in the heterogeneity test were used to indicate the level of statistical heterogeneity (26). When the level of heterogeneity was low, the data were analyzed using the fixed-effects model (P ≥0.1 and I2 <50%); otherwise, the random-effects model (P <0.1 or I2 <50%) was used (27). As the indicators to be analyzed were all continuous variables, we chose the standardized mean difference (SMD) as the effect scale. All results are presented as 95% confidence intervals (CIs).
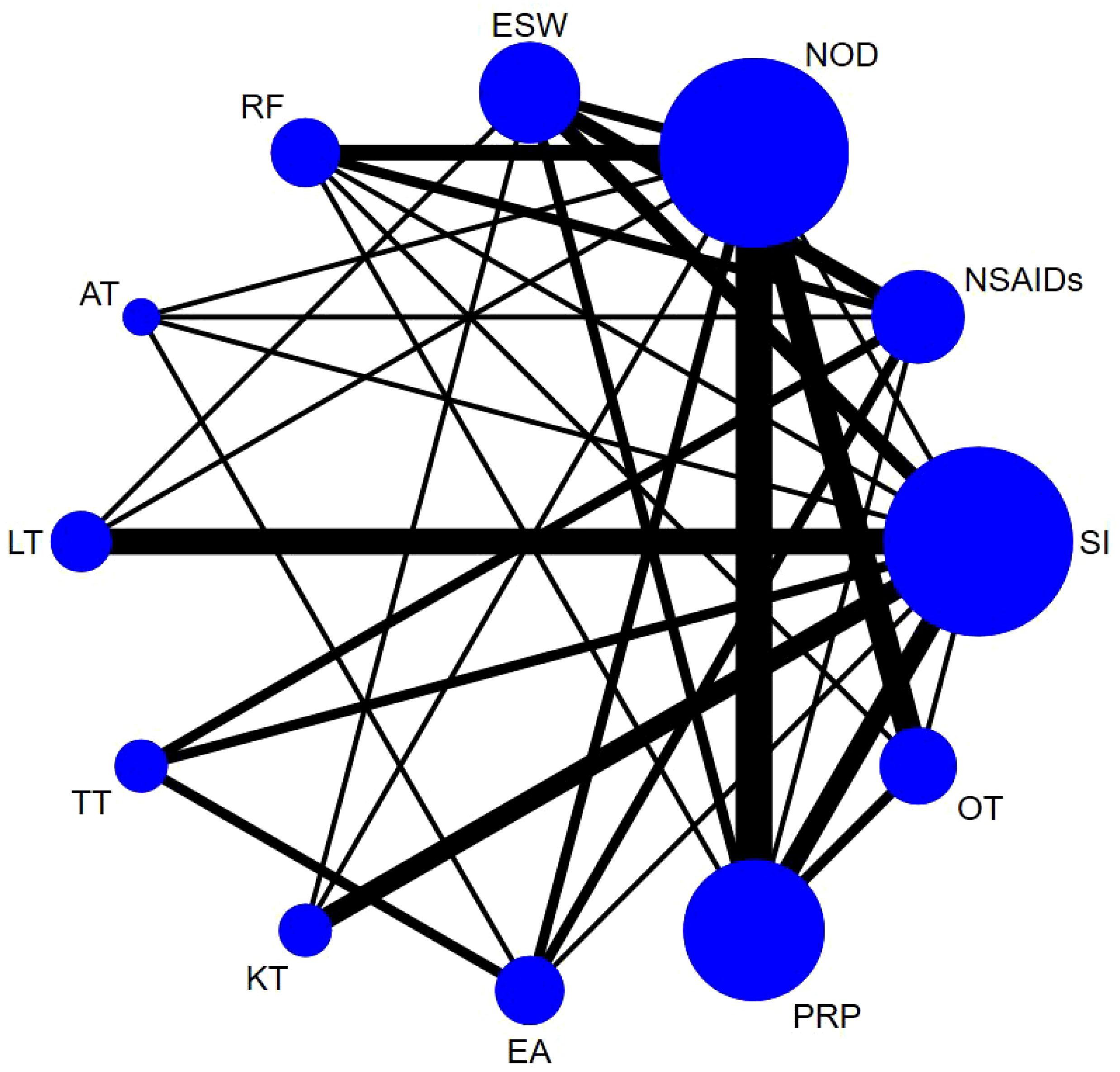
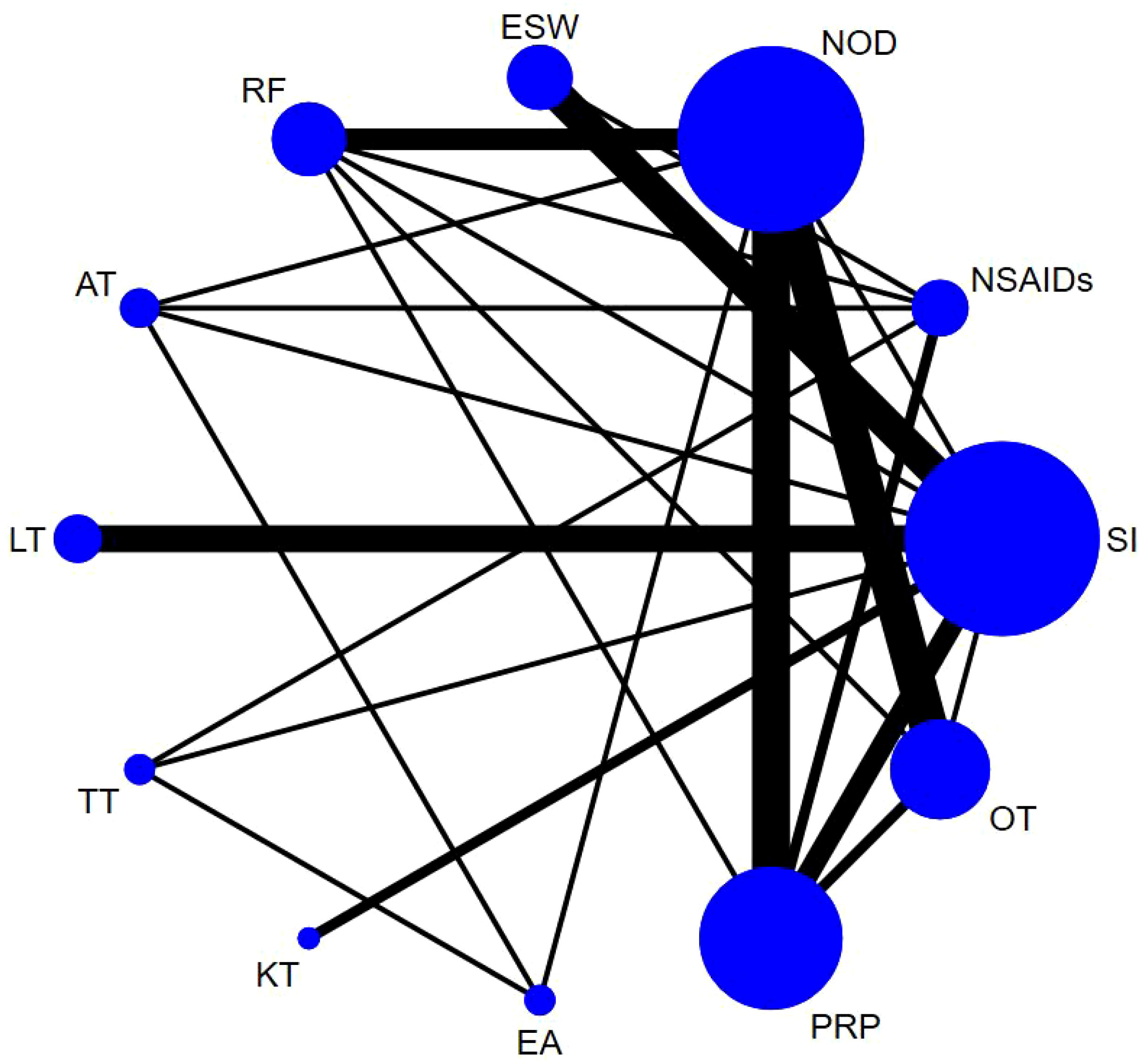
Based on the Bayesian model, Stata software (version 16.0, StataCorp LLC, College Station, TX, USA) was used to perform the network meta-analysis. The data were preprocessed using the network group command, and an evidence network diagram was drawn for each indicator. The curative effects of the indicators were sorted to obtain the surface under the cumulative ranking curve (SUCRA), and the probability sorting was plotted. The dots in the evidence network diagram represent an intervention; the larger the area, the greater the number of patients receiving the intervention. The line connecting the two dots indicates a direct comparison between the two interventions, while the thickness of the line represents the number of included studies (28, 29). SUCRA was expressed as a percentage, with a larger percentage indicating that the intervention has the highest probability of becoming the preferred option, and a value of zero indicating that the intervention may be completely ineffective (30). When a closed loop existed, the node-splitting method was used to check for inconsistencies. When >10 studies assessed the outcome indicator, funnel plots were drawn to determine the possibility of a small sample effect (31). To test the robustness of the findings, some factors that might have influenced the level of precision of the main outcome were removed and a sensitivity analysis was performed. Subgroup analyses were performed based on different treatment courses and follow-up cycles. Finally, the quality of the literature was evaluated using Review Manager 5.4 software.
3 Results
3.1 Literature screen results
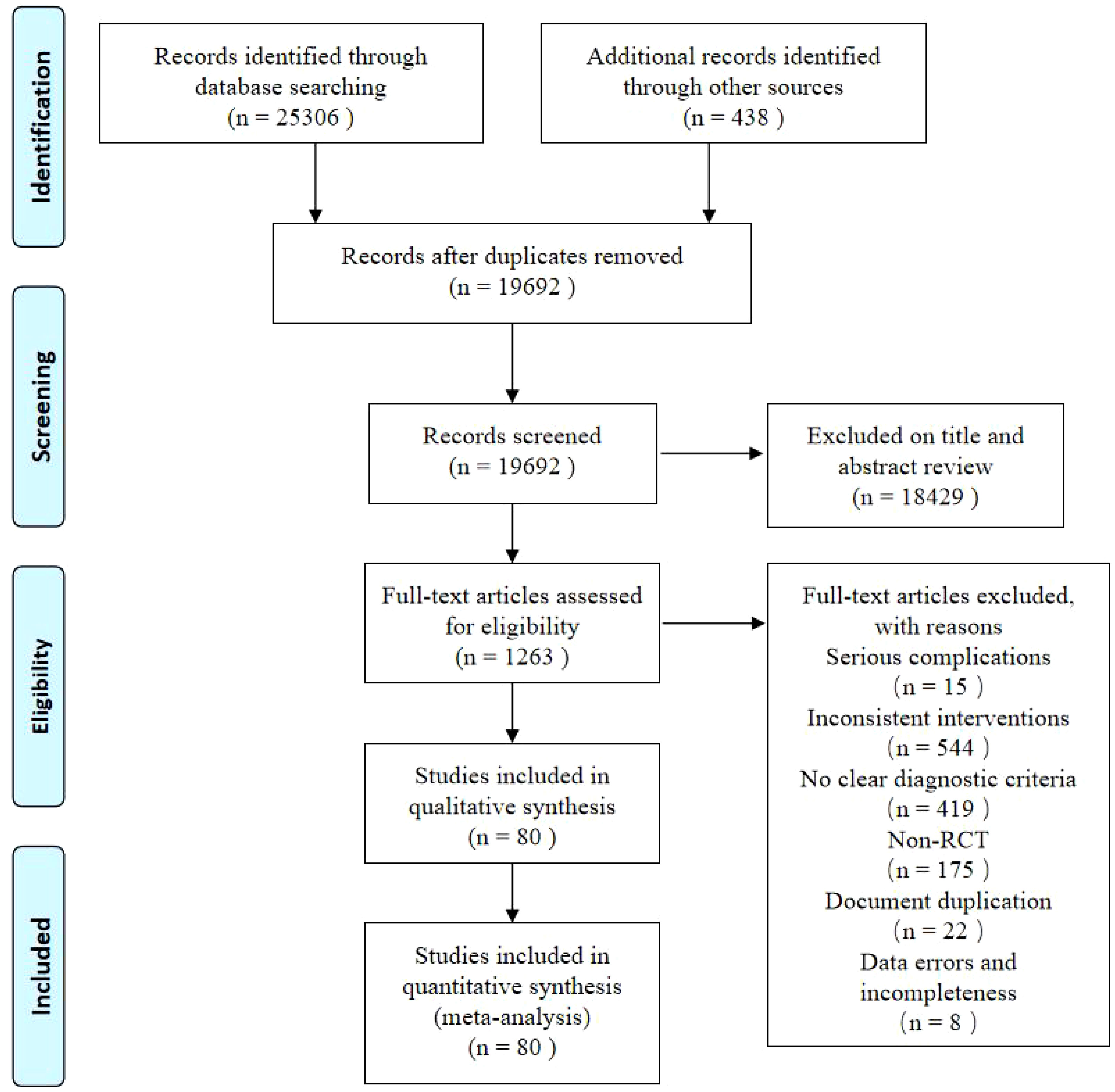
Of the 25,744 potentially relevant references identified (25,306 from each database and 438 from supplementary searches), 19692 articles were left after removing duplicates. A total of 1263 studies were subjected to full-text screening after title and abstract screening. Finally, the NMA included 80 RCTs (32–111). The screening flowchart is shown in Figure 1.
3.2 Characteristics of the included studies
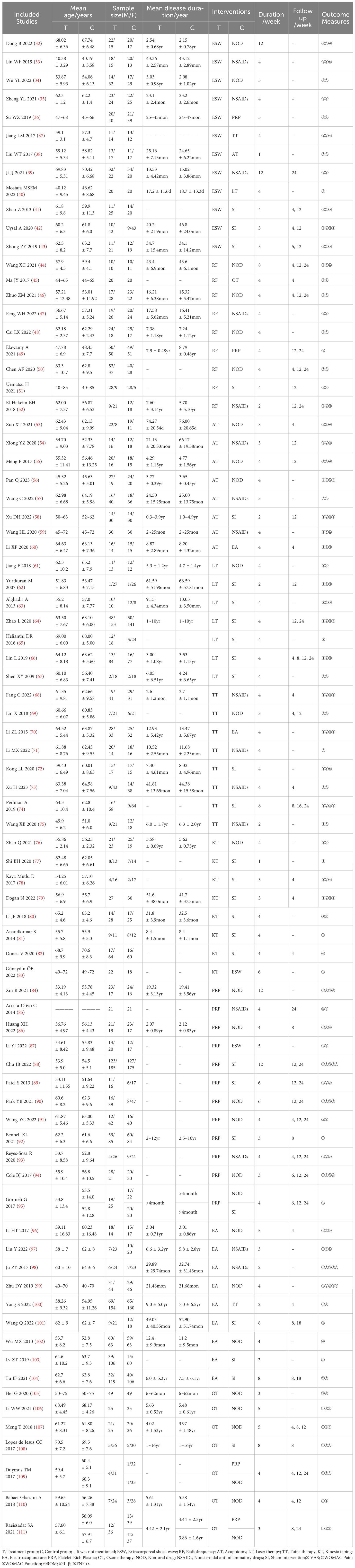
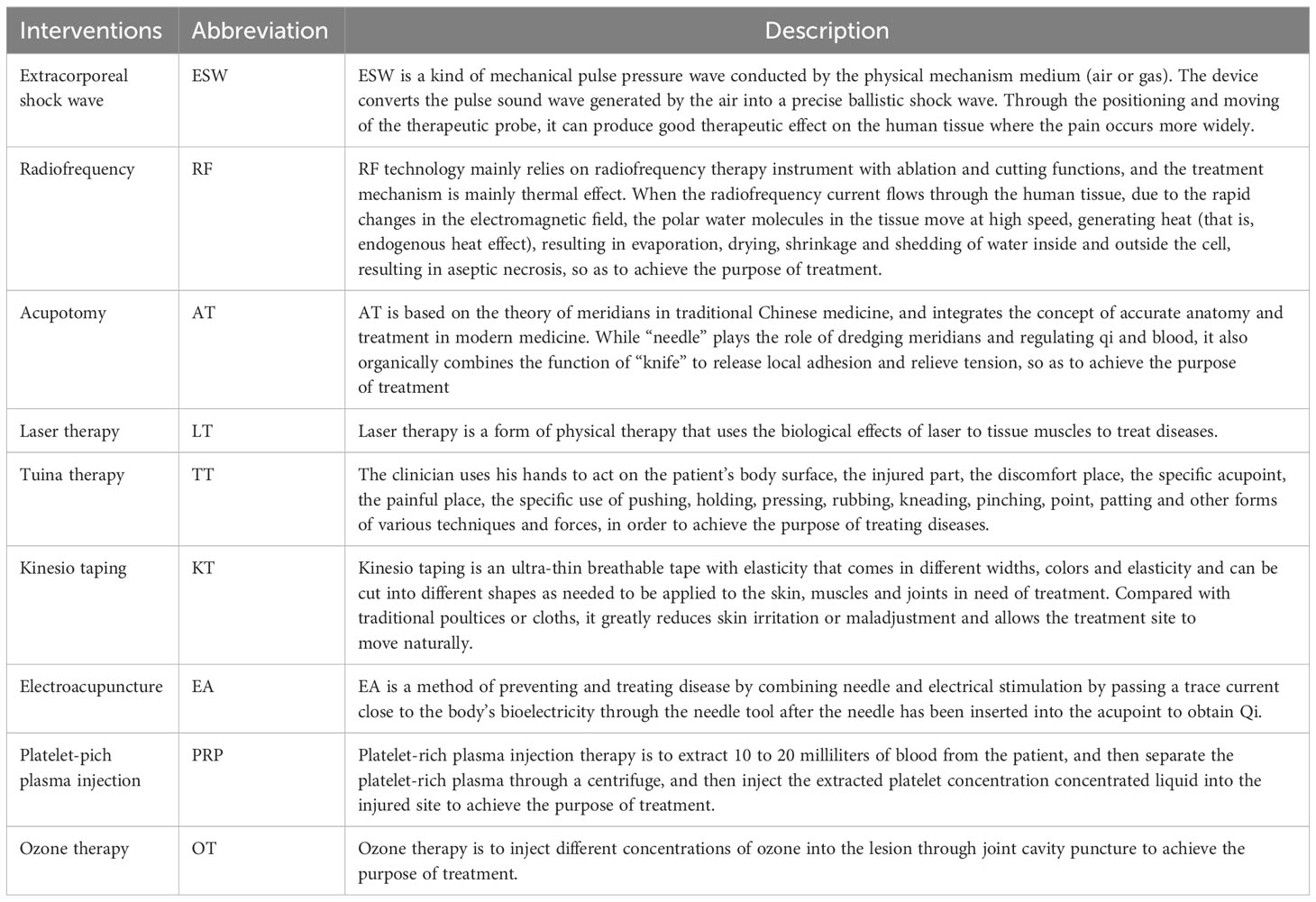
The basic characteristics of the included studies (n=80) are presented in Table 1. A total of 8440 subjects were included in this study, with 4242 in the treatment group and 4198 in the control group, respectively. Thirteen studies (43, 50, 64, 66, 73, 87, 91, 99, 102–104, 108, 111) were multi-center trials and the rest were single-center studies. The sample sizes of the included studies ranged from 39 to 615. The intervention durations ranged from 1 to 12 weeks. Forty-five studies (39, 41, 43–46, 49–55, 58, 60, 62, 64–66, 68, 69, 73, 74, 78, 79, 82, 85, 86, 88–96, 100, 101, 104, 107–111) reported follow-up durations ranging from 4 to 24 weeks. The nine non-drug therapies included extracorporeal shock waves (ESW), radiofrequency (RF), acupotomy (AT), laser therapy (LT), Tuina therapy (TT), kinesio taping (KT), electroacupuncture (EA), platelet-rich plasma injection (PRP), and ozone therapy (OT). The descriptions of each non-pharmacological intervention are presented in Table 2.
3.3 Risk of bias assessments
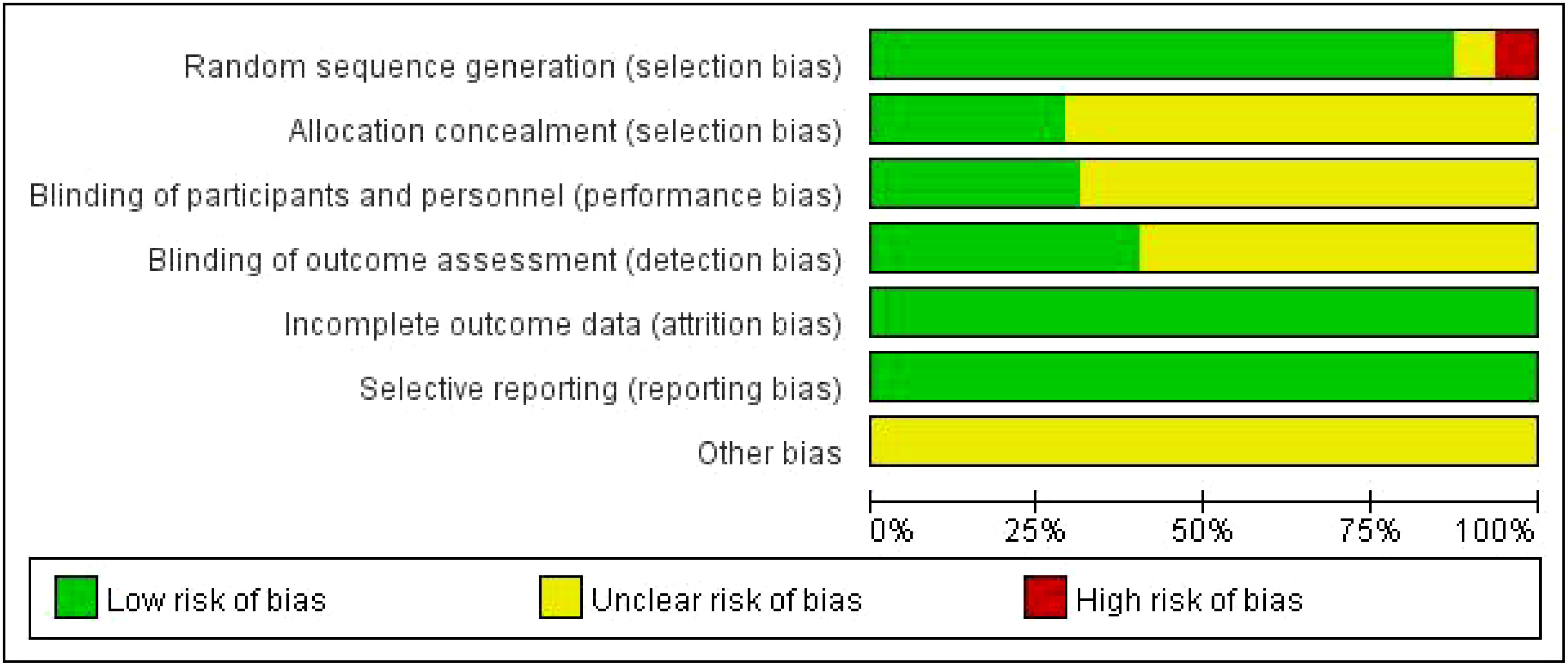
Of the 80 RCTs, 75 reported the generation of random sequences, whereas the remainder mentioned only random assignments. Thirty-three studies (34–36, 39, 41, 43–47, 53–56, 59–61, 64, 69, 71, 72, 75, 77, 80, 86, 89, 96, 98, 105–107) using random number tables, 21 (42, 49, 50, 63, 65, 66, 70, 74, 78, 82, 88, 90, 92–95, 100, 101, 103, 104, 109) using computer allocation randomization, 15 (40, 51, 57, 58, 67, 73, 79, 81, 83, 91, 97, 99, 108, 110, 111) using the envelope method, and one (102) study using the lottery method were all rated as low risk. Three (32, 33, 76) studies randomly selected patients according to different treatment methods, one (87) according to the patient’s wishes, and one (84) according to the order of admission, all of which were rated as high risk. Thirty-five studies mentioned blinding: 14 (40–42, 49, 50, 52, 57, 63, 73, 97, 101, 103, 110, 111) were single-blinded, 21 (43, 51, 58, 62, 64–66, 74, 78, 79, 81, 82, 88–92, 94, 95, 104, 108) were double-blinded, and 22 (40, 51, 57, 58, 62, 64, 66, 67, 73, 79, 81, 83, 91, 92, 94, 97, 99, 101, 103, 108, 110, 111) used allocation concealment rated as low risk. The remaining studies did not mention blinding or allocation concealment. All 80 studies reported the outcome indicators used in this study and did not identify falsified or incomplete data, with incomplete reporting and early discontinuation of trials rated as low risk. No other biases were mentioned in any study. The results are shown in Figure 2, while a summary of the risk of bias is shown in Supplementary Figure S2.
3.4 Network meta-analysis
The results of the heterogeneity test showed high heterogeneity for all outcome indicators (P < 0.05, I2 > 50%). Therefore, a random-effects model was used for all meta-analyses in this study. Except for long-term ROM, the evidence network diagrams of the outcome indicators were a closed loop. The node-splitting method showed good consistency with no heterogeneity emerging between the studies (P > 0.05). The results of the node-splitting tests are presented in Supplementary Tables S2-10.
3.4.1 Short-term VAS
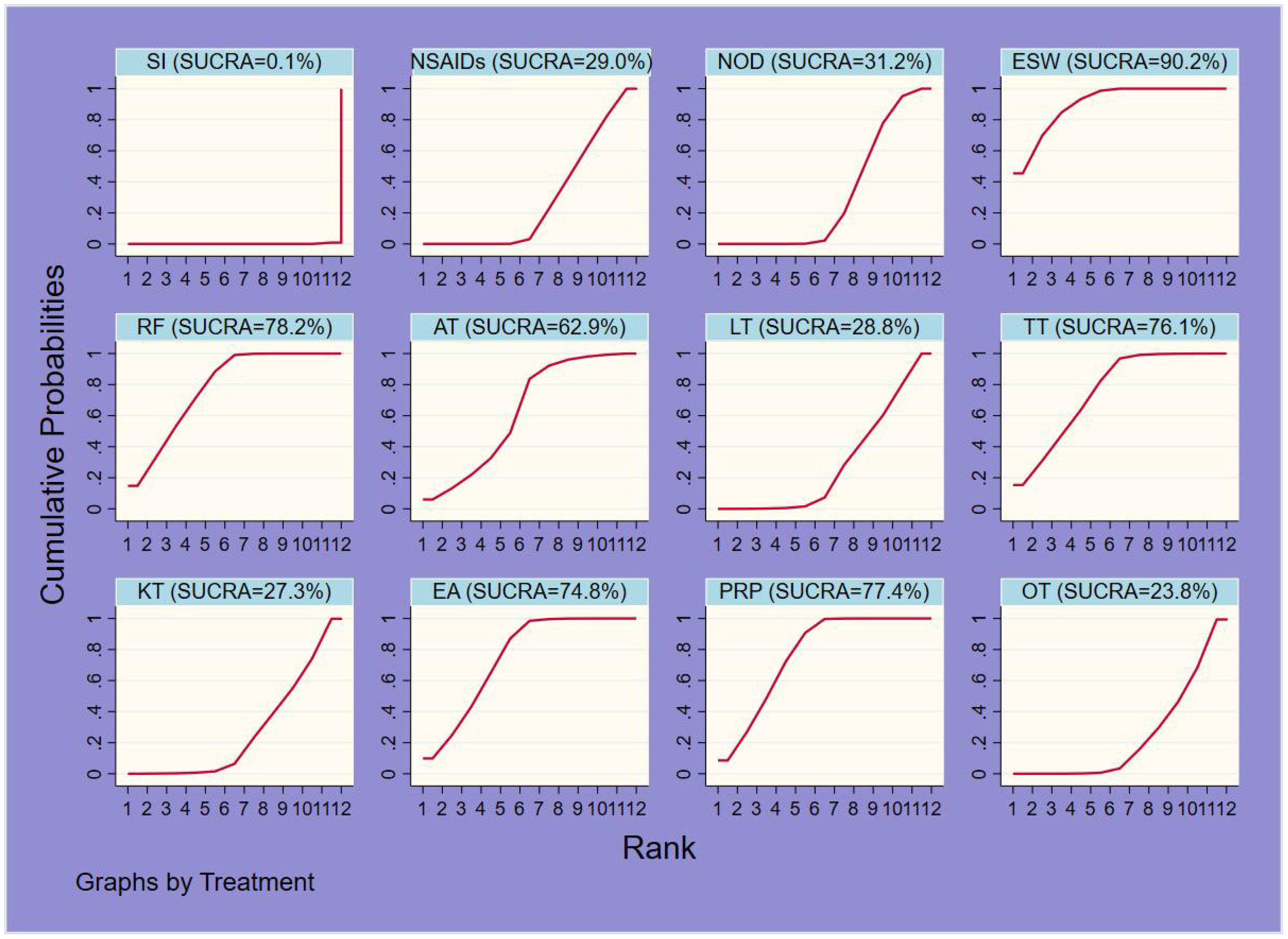
Sixty-one studies (32–36, 39–49, 51–54, 58, 60–66, 68, 70, 72, 74–79, 81, 83, 84, 86–90, 92–100, 103, 106–111) involving 6,489 participants reported short-term post-treatment VAS scores. Twelve interventions were considered, and 66 two-by-two comparisons were performed. The evidence network generally centered on NOD, thereby forming 22 closed loops (Figure 3). Compared to SI, all non-drug and drug therapies had a better effect on the VAS score (P < 0.05). ESW, RF, PRP, TT, and EA significantly reduced the VAS score compared with NOD, NSAIDs, LT, KT, and OT (P < 0.05) (Figure 4).
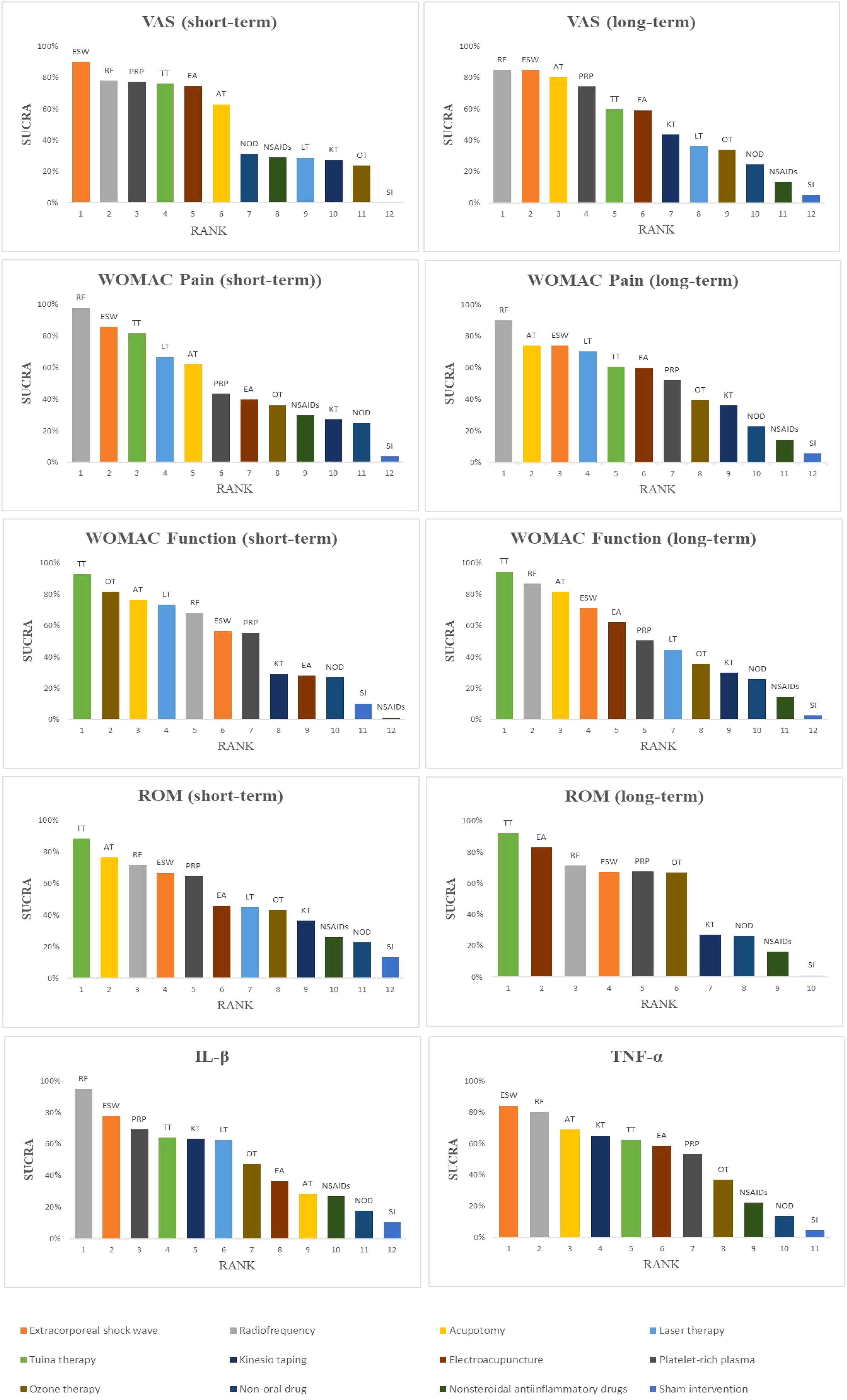
The probability ranking results of reducing short-term VAS were as follows: ESW (SUCRA=90.2%) > RF (78.2%) > PRP (77.4%) > TT (76.1%) > EA (74.8%) > AT (62.9%) > NOD (31.2%) > NSAIDs (29.0%) > LT (28.8%) > KT (27.3%) > OT (23.8%) > SI (0.1%) (Figure 5, 6, Table 3).
The bold font indicates that there was a statistically significant difference between the two treatments.
3.4.2 Long-term VAS
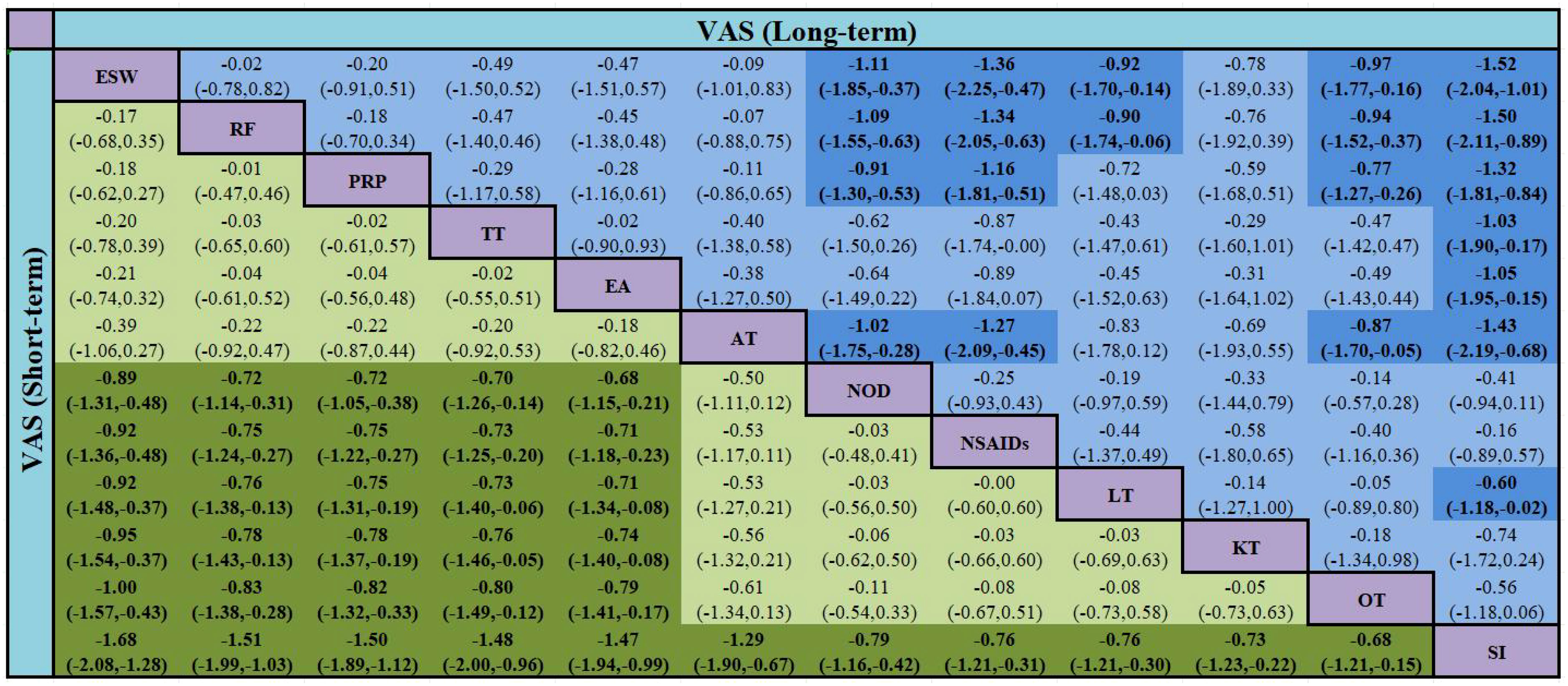
Thirty-six studies (39, 41–46, 49, 51–54, 58, 60, 62, 64, 66, 68, 74, 78, 79, 81, 83, 84, 86, 88–90, 92–96, 100, 107–111), involving 5,925 participants reported long-term VAS score. Twelve interventions were considered and 66 two-by-two comparisons were performed. The overall evidence network centered on SI, thereby forming 14 closed loops (Figure 7). The results of the meta-analysis indicated that compared with LT, RF (SMD = -0.90, 95% CI [-1.74, -0.06]) and ESW (SMD = -0.92, 95% CI [-1.70, -0.14]) significantly reduced the VAS score. RF, ESW, AT, and PRP were superior to OT, NOD, NSAIDs, and SI in terms of performance. Compared to SI, TT, EA, and LT were more effective in decreasing the long-term VAS scores. All of the above-mentioned differences were statistically significant (P < 0.05) (Figure 4).
The probability ranking results for reducing the long-term VAS scores were as follows: RF (84.8%) > ESW (84.7%) > AT (80.4%) > PRP (74.5%) > TT (59.9%) > EA (59.2%) > KT (43.7%) > LT (36.1%) > OT (33.9%) > NOD (24.5%) > NSAIDs (13.3%) > SI (5.0%) (Figure 6, Table 3).
3.4.3 WOMAC pain (short-term)
Thirty-six studies (37, 38, 41–43, 50, 52, 54–56, 58, 60, 62–64, 66–70, 73, 74, 79, 88–91, 93, 94, 96, 98, 99, 104, 108, 109, 111) involving 4207 patients investigated short-term WOMAC pain score. Twelve types of interventions were considered, and 66 two-by-two comparisons were performed. The evidence network was generally centered on the SI, thereby forming 12 closed loops (Supplementary Figure S3). Compared with OT, RF [SMD = -4.79, 95% CI (-7.98, -1.60)] and ESW [SMD = -2.93, 95% CI (-5.59, -0.26)] significantly reduced the short-term WOMAC pain score (P < 0.05). RF, ESW, and TT were better interventions than PRP, EA, NSAIDs, NOD, and SI (P < 0.05). Compared with AT and KT, RF was more effective in reducing the WOMAC pain score (P < 0.05). LT, AT, PRP, and EA were better than SI (P < 0.05) (Supplementary Figure S11).
RF (97.8%) may be the most effective intervention in improving the short-term WOMAC pain score, followed by ESW (85.9%), TT (81.7%), LT (66.6%), AT (62.1%), PRP (43.5%), EA (39.7%), OT (36.2%), NSAIDs (29.6%), KT (27.0%), NOD (25.9%), and SI (3.8%) (Figure 6, Table 3).
3.4.4 WOMAC pain (long-term)
Twenty-nine studies (41–43, 50, 52, 54, 55, 58, 60, 62, 64, 66, 68, 69, 73, 74, 79, 88–91, 93, 94, 96, 104, 108, 109, 111) involving 4804 patients investigated long-term WOMAC pain score. Twelve types of interventions were considered and 66 two-by-two comparisons were performed. The evidence network was generally centered on SI, thereby forming eight closed loops (Supplementary Figure S4). Compared with NOD, RF [SMD = -4.70, 95% CI (-7.60, -1.81)], AT [SMD = -3.22, 95% CI (-6.13, -0.32)], and ESW [SMD = -3.19, 95% CI (-6.36, -0.02)] significantly reduced the long-term WOMAC pain score. RF, AT, ESW, LT, TT, and PRP were better interventions than NSAIDs and SI. RF was a superior intervention to OT. Compared with SI, EA was more effective in decreasing long-term WOMAC pain scores. All the above-mentioned differences were statistically significant (P < 0.05) (Supplementary Figure S11).
RF may be the most effective therapy for long-term WOMAC pain score reduction (90.1%), followed by AT (74.2%), ESW (73.9%), LT (70.4%), TT (60.8%), EA (59.9%), PRP (52.0%), OT (39.6%), KT (36.2%), NOD (22.7%), NSAIDs (14.5%), and SI (5.7%) (Figure 6, Table 3).
3.4.5 WOMAC function (short-term)
Thirty-seven studies (37, 38, 41–43, 50, 52–56, 58, 60, 62–64, 66–70, 73, 74, 78, 79, 88–91, 93, 96, 98, 99, 104, 108, 109, 111) involving 4,207 participants reported short-term WOMAC function score. Twelve interventions were considered and 66 two-by-two comparisons were performed. The overall evidence network centered on SI, thereby forming 14 closed loops (Supplementary Figure S5). Except for KT, all non-drug therapies and NOD were superior to NSAIDs in reducing short-term WOMAC function (P < 0.05). Compared to NOD and SI, TT, OT, AT, LT, RF, ESW, and PRP were more effective in decreasing WOMAC function (P < 0.05). TT was a better intervention than PRP, KT, and EA (P < 0.05). OT, AT, and LT significantly reduced the WOMAC function score compared with EA (P < 0.05) (Supplementary Figure S12).
The probability ranking results of improving short-term WOMAC function scores were as follows: TT (92.8%) > OT (81.5%) > AT (76.4%) > LT (73.5%) > RF (68.1%) > ESW (56.6%) > PRP (55.3%) > KT (29.3%) > EA (28.0%) > NOD (27.0%) > SI (10.3%) > NSAIDs (1.3%) (Figure 6, Table 3).
3.4.6 WOMAC function (long-term)
Twenty-nine studies (41–43, 50, 52–55, 58, 60, 62, 64, 66, 68, 69, 73, 74, 78, 79, 88–91, 93, 96, 104, 108, 109, 111) involving 4705 patients investigate long-term WOMAC function. Twelve types of interventions were considered, and 66 two-by-two comparisons were performed. The evidence network was generally centered on the SI, thereby forming eight closed loops (Supplementary Figure S6). The results of the meta-analysis indicated that except for KT, all non-drug therapies and NOD were superior to SI in improving long-term WOMAC function (P < 0.05). Compared with NOD and NSAIDs, TT, RF, AT, and PRP were more effective in reducing WOMAC function scores (P < 0.05). AT was superior to PRP [SMD = -4.28, 95% CI (-8.49, -0.07)] and OT [SMD = -5.92, 95% CI (-10.96, -0.89)] (P < 0.05). TT and RF were superior to PRP, OT, and KT. Compared to LT, TT significantly reduced WOMAC function (P < 0.05) (Supplementary Figure S12).
TT (94.5%) may be the most effective intervention in reducing the long-term WOMAC function score, followed by RF (86.9%), AT (81.7%), ESW (71.2%), EA (62.1%), PRP (50.5%), LT (44.7%), OT (35.4%), KT (30.1%), NOD (25.8%), NSAIDs (14.5%), and SI (2.6%) (Figure 6, Table 3).
3.4.7 ROM (short-term)
Twenty-one studies (36, 37, 42, 45, 51, 58, 59, 61, 65, 68, 70, 74, 76, 78, 79, 82, 84, 86, 87, 100, 101, 110) involving 2369 patients reported on short-term ROM. Twelve types of interventions were considered and 66 two-by-two comparisons were performed. The evidence network was generally centered on the SI, thereby forming four closed loops (Supplementary Figure S7). Compared to SI, TT [SMD = 14.65, 95% CI (6.70, 22.60)], AT [SMD = 12.29, 95% CI (1.66, 22.93)], RF [SMD = 11.10, 95% CI (0.44, 21.75)], and ESW [SMD = 9.91, 95% CI (1.24, 18.58)] were more effective in increasing short-term ROM. TT was a better intervention than KT, NSAIDs, or NOD (P < 0.05) (Supplementary Figure S13).
TT (88.3%) may be the most effective treatment for improving short-term ROM, followed by AT (76.4%), RF (71.6%), ESW (66.7%), PRP (64.8%), EA (45.9%), LT (45.0%), OT (43.0%), KT (36.4%), NSAIDs (26.0%), NOD (22.6%), and SI (13.3%) (Figure 6, Table 3).
3.4.8 ROM (long-term)
Thirteen studies (42, 45, 51, 58, 68, 74, 78, 79, 82, 86, 100, 101, 110) involving 1372 participants and 10 interventions reported long-term ROM as an outcome measure. Thus, 45 two-by-two comparisons were made, with an overall evidence network centered on the SI (Supplementary Figure S8). The results of the NMA indicated that all non-pharmacological interventions and NOD were superior to SI in improving long-term ROM (P < 0.05). Compared to NOD, PRP (SMD = 5.07, 95% CI (1.62, 8.52)] significantly increased the long-term ROM (P < 0.05). TT was a better intervention than ESW [SMD = 5.40, 95% CI (1.13, 9.66)] (P < 0.05). Compared with KT, NOD, and NSAIDs, TT, EA, RF, and OT were more effective in improving the long-term ROM (Supplementary Figure S13).
The probability ranking results for improving long-term ROM were as follows: TT (92.1%) > EA (83.0%) > RF (71.4%) > PRP (67.6%) > OT (67.0%) > ESW (47.3%) > KT (27.6%) > NOD (26.5%) > NSAIDs (16.4%) > SI (1.1%) (Figure 6, Table 3).
3.4.9 IL-1β
Twenty-three studies (32–34, 44, 48, 56, 57, 59, 64, 71, 72, 80, 84, 85, 88, 90, 94, 97–99, 105–107) involving 2674 patients investigated IL-1β levels. Twelve types of interventions were considered, and 66 two-by-two comparisons were performed. The evidence network was generally centered on NOD, thereby forming three closed loops (Supplementary Figure S9). RF, ESW, and PRP were better interventions than NSAIDs, NOD, and SI. Compared to OT [SMD = -15.14, 95% CI (-27.74, -2.55)], EA [SMD = -17.61, 95% CI (-31.58, -3.64)], and OT [SMD = -19.28, 95% CI (-32.96, -5.60)], RF significantly decreased IL-1β levels (P < 0.05). ESW significantly reduced IL-1β levels compared with AT (P < 0.05) (Supplementary Figure S14).
The results showed that RF (94.8%) may be the most effective therapy for reducing IL-1β levels, followed by ESW (77.7%), PRP (69.4%), TT (64.2%), KT (63.4%), LT (62.5%), OT (47.4%), EA (36.7%), AT (28.6%), NSAIDs (26.9%), NOD (17.8%), and SI (10.8%) (Figure 6, Table 3).
3.4.10 TNF-α
Twenty-seven studies (32, 33, 35, 39, 44, 46, 47, 53, 55–57, 59, 72, 75, 80, 84–86, 88, 94, 97–99, 102, 105–107) involving 2825 patients assessed TNF-α levels. Eleven types of interventions were considered, and 55 two-by-two comparisons were performed. The evidence network was generally centered on NOD, thereby forming four closed loops (Supplementary Figure S10). The NMA results showed that except for OT, all non-drug therapies were superior to SI in reducing TNF-α levels (P < 0.05). Compared to NOD, ESW, RF, AT, EA, and PRP significantly improved TNF-α levels (P < 0.05). ESW, RF, and AT were better than NSAIDs (P < 0.05) (Supplementary Figure S14).
ESW (84.2%) may be the most effective intervention in reducing TNF-α levels, followed by RF (80.2%), AT (69.2%), KT (65.1%), TT (62.3%), EA (58.5%), PRP (53.3%), OT (36.9%), NSAIDs (22.2%), NOD (13.6%), and SI (4.6%) (Figure 6, Table 3).
3.5 Publication bias
The indicators included in this study were tested for publication bias (Supplementary Figure S15-24). The indicators for WOMAC pain (long-term) and TNF-α were asymmetric in the funnel plots, suggesting a publication bias or small sample effect, which may have affected the results of the corresponding indicators. The funnel plots for the other indicators were symmetrical, suggesting a low possibility of publication bias in the current study.
3.6 Sensitivity analysis
To test the stability and reliability of the NMA results, we performed sensitivity analyses for short- and long-term VAS and WOMAC pain and function. First, five papers that were evaluated as high-risk in terms of literature quality were excluded (32, 33, 76, 84, 87), and sensitivity analyses were performed before and after exclusion. Second, as RCTS with small sample sizes may have affected the accuracy of the results, 11 studies with sample sizes of <50 were excluded from the sensitivity analysis (40, 44, 45, 61, 63, 67, 77, 78, 81, 83, 85).. The results showed little difference between the results before and after the exclusion of the two sensitivity analyses, indicating that the quality of the literature was good and that the results of the network meta-analysis were solid and stable. The results of the sensitivity analysis are shown in Supplementary Figure S25-31 and Table S11.
3.7 Subgroup analysis
To reduce heterogeneity caused by inconsistent treatment and follow-up cycles, two subgroup analyses were performed. First, the study population was divided into two subgroups according to the treatment duration (<4 weeks and ≥4 weeks). Second, the follow-up period was divided into two subgroups (<12 weeks and ≥12 weeks). Regarding the outcomes of this analysis, only NMA of partial outcomes (VAS, WOMAC pain, and function) could be performed.
The rankings of non-pharmacological therapies showed little variation between VAS and WOMAC pain, whereas the rankings of pharmacological therapies varied considerably. For treatment courses <4 weeks, the efficacy of the NOD and NSAIDs was better than that of some non-drug therapies; however, for treatment courses ≥4 weeks, the effect was inferior to all non-drug therapies. The <12-week and ≥12-week follow-up subgroups showed no significant difference.
In terms of WOMAC function, the comparison between the <4-week and ≥4-week subgroups showed that OT lost its ranking advantage over AT and LT, and no significant difference in the other comparisons. The NMA results and rankings for subgroup analyses based on the follow-up period remained consistent with those before subgrouping. The results of the subgroup analysis are shown in Supplementary Figure S32-34 and Table S12.
4 Discussion
There remains no consensus regarding the use of non-pharmacological interventions for the treatment of KOA. This study conducted NMA to generate a hierarchy of treatment rankings (112). The ranking probabilities for these treatment plans were calculated in terms of their clinical efficacy and inflammatory cytokine levels at various endpoints to provide a basis for making optimal choices.
This study included 80 RCTs that adopted nine non-drug interventions and included a total of 8440 individuals. VAS and WOMAC pain scores were used as pain indicators, while WOMAC function and ROM were used as functional indicators to evaluate the effect of non-pharmacological treatments on the improvement of short- and long-term symptoms in patients with KOA. The results of the NMA demonstrated that each non-drug therapy was superior to the sham intervention in terms of improving all efficacy indicators. Except for the short-term VAS and WOMAC pain outcomes, all non-drug therapies showed better efficacy than pharmacological treatments. An in-depth analysis of the indicators revealed that the immediate analgesic effect of NODs and NSAIDs was significant and superior to that of some nonpharmacological therapies, while their long-term analgesic efficacy was inferior to that of all nonpharmacological therapies. This may be related to drug resistance and other bottlenecks. Moreover, the short- and long-term effects of these drugs on improving joint function are poor. For short-term VAS, ESW therapy (90.2%) had the greatest likelihood of achieving the best efficacy among the treatment regimens, followed by RF therapy (78.2%) and PRP injection (77.4%). Radiofrequency therapy (84.8%, 97.8%, and 90.1%, respectively) may be the most promising method for reducing long-term VAS and short- and long-term WOMAC pain scores (84.8%, 97.8%, and 90.1%, respectively). Both ESW and RF were effective in improving short-term pain symptoms; however, radiofrequency was more effective for long-term analgesia. RF therapy may be a better choice in both the short and long term. The reason for the better effect of RF may be the inhibition of pain-sensing nerve C fibers and the promotion of endogenous opioid precursor mRNA transcription and related opioid peptide production (113, 114). TT can effectively improve WOMAC function and ROM in the short and long term, indicating that it can be given priority for treatment in patients with symptoms of functional dysfunction. Moreover, TT can regulate the interaction between interleukin-1β and the ERK1/2-nuclear transcription factor κB signaling pathway, thereby inhibiting excessive chondrocyte apoptosis, maintaining the stability of the internal environment of chondrocytes, and repairing injured cartilage tissue to restore functional activities of patients (115).
Inflammatory cytokines are important for maintaining the homeostasis of the internal environment of the knee (116). The representative inflammatory cytokines IL-β and TNF-α participate in chondrocyte apoptosis and proliferation and are closely related to KOA occurrence and development (117, 118). Additionally, the secretion of inflammatory factors is closely associated with pain symptoms (119). Our analysis of the levels of two outcome indicators, IL-β and TNF-α, showed that ESW and RF, which ranked higher in pain indicators, could also more effectively improve IL-β and TNF-α levels and showed a positive correlation, which may be the basis of its mechanism of action. Improvements in functional indicators correlated poorly with changes in inflammatory cytokine levels.
Our assessment of the quality of the literature showed that almost all studies used low-risk random assignment methods. The literature included in previous studies rarely mentioned blinding and allocation concealment, or described them inaccurately. In the present analysis, nearly half of the studies explicitly proposed blinded methods and allocation concealment and described specific implementation methods. Part of the study adopted multi-center and large-sample research, which improved its credibility. To enhance the strength of the evidence, we conducted two sensitivity analyses of the VAS: WOMAC pain and WOMAC function (short- and long-term). After excluding high-risk and small-sample studies, the overall results remained robust, indicating that the quality of the included literature was acceptable.
The results of subgroup analysis showed no significant difference between the <12-week and ≥12-week follow-up subgroups. The treatment course subgroups showed little change in the ranking of non-drug treatments, whereas there was a greater change in the ranking of drug treatments in VAS and WOMAC pain. For treatment courses <4 weeks, the efficacy of NOD and NSAIDs was better than that of some non-drug therapies; in contrast, for treatment courses ≥4 weeks, the effect was inferior to all non-drug therapies. This finding indicated that the immediate analgesic effect of the drug was better and the long-term effect was worse, which is consistent with the above follow-up ranking results. In terms of WOMAC function, the comparison between the <4-week and ≥4-week subgroups showed OT lost its ranking advantage over AT and LT, which may be related to the slow onset of AT and LT (120).
However, this study has some limitations. First, during literature selection in the present study, not all existing literature could be included because the original text for some studies could not be found and some studies used geometric means. Second, the sample sizes of the included studies were limited, which might have affected the accuracy of the results. Second, fewer studies in this analysis published pretrial protocols, which may have led to selective reporting bias. Finally, other non-drug therapies, such as wedge insoles and pulsed ultrasound, were lacking owing to the limited number of original studies, thus preventing the comparison of the effects of all non-pharmacological interventions.
5 Conclusion
The results of the comprehensive comparison of the outcome indicators of 9 different EANPI showed that radiofrequency was effective in relieving pain, and that tuina therapy can be given priority for treatment in patients with hypofunction as their main symptom. In clinical practice, an appropriate treatment method should be selected based on the actual situation. EANPI to improve pain symptoms may be related to the regulation of inflammatory cytokine levels, which may be a potential mechanism of action. Owing to the limitation of the quality and quantity of the included studies, more large-sample, multi-center, high-quality RCT studies are needed to verify our conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ZhenW: Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. HX: Funding acquisition, Investigation, Project administration, Writing – original draft, Writing – review & editing. ZhengW: Data curation, Formal Analysis, Investigation, Project administration, Supervision, Writing – review & editing. HZ: Conceptualization, Methodology, Project administration, Writing – review & editing. JD: Conceptualization, Formal Analysis, Visualization, Writing – review & editing. LZ: Formal Analysis, Investigation, Methodology, Writing – review & editing. YW: Conceptualization, Software, Supervision, Validation, Writing – review & editing. ML: Methodology, Software, Supervision, Writing – review & editing. YZ: Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Henan Province Chinese Medicine Scientific Research Special project (No. 2022ZY1108), the Central Plains Thousand Talents Program-Central Plains Famous Doctors (No. ZYQR201912120), the 2022 Central Plains Talent Plan (Talent Education Series)-Central Plains Youth Top Talent Project (No. Yu Talent Office [2022] No. 5), the Henan Province Science and Technology Research-Social Development Project (No. 222102310214), the Henan Provincial Science and Technology R&D Program Joint Fund (superior discipline cultivation category) (No. 222301420061), Henan Province key research and development and promotion special project (science and technology research)(No. 232102311203), and Henan Province Traditional Chinese Medicine “Double First-Class” Scientific Research Project (No. HSRP-DFCTCM-2023-7-09).
Acknowledgments
We would like to give my heartfelt thanks to my academic supervisor, Prof. Zhou Yunfeng, for his invaluable instruction and inspiration. This study could not have been conducted without his advice and guidance. Furthermore, we acknowledge their professors and classmates at the Acupuncture-Moxibustion and Tuina School of Henan University of Chinese Medicine for their generous guidance and assistance.
Conflict of interest
Author LZ was employed by Jiaozuo Coal Industry Group Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1309751/full#supplementary-material
References
1. Hussain SM, Neilly DW, Baliga S, Patil S, Meek R. Knee osteoarthritis: a review of management options. Scott Med J (2016) 61(1):7–16. doi: 10.1177/0036933015619588
2. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. (2011) 377(9783):2115–26. doi: 10.1016/S0140-6736(11)60243-2
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol (2018) 30(2):160–7. doi: 10.1097/BOR.0000000000000479
4. Culvenor AG, Ruhdorfer A, Juhl C, Eckstein F, Øiestad BE. Knee extensor strength and risk of structural, symptomatic, and functional decline in knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res (Hoboken). (2017) 69(5):649–58. doi: 10.1002/acr.23005
5. Dainese P, Wyngaert KV, De Mits S, Wittoek R, Van Ginckel A, Calders P. Association between knee inflammation and knee pain in patients with knee osteoarthritis: a systematic review. Osteoarthritis Cartilage. (2022) 30(4):516–34. doi: 10.1016/j.joca.2021.12.003
6. Xie J, Wang Y, Lu L, Liu L, Yu X, Pei F. Cellular senescence in knee osteoarthritis: molecular mechanisms and therapeutic implications. Ageing Res Rev (2021) 70:101413. doi: 10.1016/j.arr.2021.101413
7. Adatia A, Rainsford KD, Kean WF. Osteoarthritis of the knee and hip. Part I: aetiology and pathogenesis as a basis for pharmacotherapy. J Pharm Pharmacol (2012) 64(5):617–25. doi: 10.1111/j.2042-7158.2012.01458.x
8. Arai T, Suzuki-Narita M, Takeuchi J, Tajiri I, Inage K, Kawarai Y, et al. Analgesic effects and arthritic changes following intra-articular injection of diclofenac etalhyaluronate in a rat knee osteoarthritis model. BMC Musculoskelet Disord (2022) 23(1):960. doi: 10.1186/s12891-022-05937-y
9. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. (2019) 27(11):1578–89. doi: 10.1016/j.joca.2019.06.011
10. Yu SP, Hunter DJ. Managing osteoarthritis. Aust Prescr. (2015) 38(4):115–9. doi: 10.18773/austprescr.2015.039
11. Sari Z, Aydoğdu O, Demirbüken İ, Yurdalan SU, Polat MG. A better way to decrease knee swelling in patients with knee osteoarthritis: A single-blind randomised controlled trial. Pain Res Manage (2019) 2019:8514808. doi: 10.1155/2019/8514808
12. Güler T, Yurdakul FG, Önder ME, Erdoğan F, Yavuz K, Becenen E, et al. Ultrasound-guided genicular nerve block versus physical therapy for chronic knee osteoarthritis: a prospective randomised study. Rheumatol Int (2022) 42(4):591–600. doi: 10.1007/s00296-022-05101-8
13. Xu H, Xiao LB, Zhai WT. Expert consensus on diagnosis and treatment of knee osteoarthritis with integrated Chinese and Western Medicine. World Chin Med (2023) 18(07):929–35.
14. Pan JK, Han YH, Huang HT. Diagnosis and Treatment Guide of Combined Chinese and Western Medicine for knee osteoarthritis (2023 edition). Traditional Chin Med Bone Orthopaedics (2023) 35(06):1–10.
15. Wang SQ, Zhu LG, Zhan JW, Chen M, Zhao GD, Yan A, et al. Clinical practice Guide of Traditional Chinese Medicine rehabilitation·Knee osteoarthritis. J Rehabilitation. (2020) 30(03):177–82.
16. Rahimzadeh P, Imani F, Azad Ehyaei D, Faiz SHR. Efficacy of oxygen-ozone therapy and platelet-rich plasma for the treatment of knee osteoarthritis: A meta-analysis and systematic review. Anesth Pain Med (2022) 12(4):e127121. doi: 10.5812/aapm-127121
17. Ferreira RM, Torres RT, Duarte JA, Gonçalves RS. Non-pharmacological and non-surgical interventions for knee osteoarthritis: A systematic review and meta-analysis. Acta Reumatol Port (2019) 44(3):173–217.
18. Dantas LO, Osani MC, Bannuru RR. Therapeutic ultrasound for knee osteoarthritis: A systematic review and meta-analysis with grade quality assessment. Braz J Phys Ther (2021) 25(6):688–97. doi: 10.1016/j.bjpt.2021.07.003
19. Ahmad MA, Hamid MS A, Yusof A. Effects of low-level and high-intensity laser therapy as adjunctive to rehabilitation exercise on pain, stiffness and function in knee osteoarthritis: a systematic review and meta-analysis. Physiotherapy. (2022) 114:85–95. doi: 10.1016/j.physio.2021.03.011
20. Seitidis G, Nikolakopoulos S, Hennessy EA, Tanner-Smith EE, Mavridis D. Network meta-analysis techniques for synthesizing prevention science evidence. Prev Sci (2022) 23(3):415–24. doi: 10.1007/s11121-021-01289-6
21. Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health (2017) 39:e2017047. doi: 10.4178/epih.e2017047
22. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med (2015) 162(11):777–84. doi: 10.7326/M14-2385
23. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. American college of rheumatology/Arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020 (2019) 72(2):149–62. doi: 10.1002/acr.24131
24. Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. (2008) 16(2):137–62. doi: 10.1016/j.joca.2007.12.013
25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol (2011) 11:41. doi: 10.1186/1471-2288-11-41
27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
28. Watt J, Del Giovane C. Network meta-analysis. Methods Mol Biol (2022) 2345:187–201. doi: 10.1007/978-1-0716-1566-9_12
29. Wang Z, Zhou Z, Zhang L, Li X, Li M, Pan Y, et al. Efficacy and safety of nonpharmacological strategies for the treatment of oligoasthenospermia: a systematic review and Bayesian network meta-analysis. Eur J Med Res (2023) 28(1):6. doi: 10.1186/s40001-022-00968-6
30. Uhlmann L, Jensen K, Kieser M. Hypothesis testing in Bayesian network meta-analysis. BMC Med Res Methodol (2018) 18(1):128. doi: 10.1186/s12874-018-0574-y
31. Page MJ, McKenzie JE, Higgins JPT. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open (2018) 8(3):e019703. doi: 10.1136/bmjopen-2017-019703
32. Dong B, Lei T. Study on the efficacy of extracorporeal acupoint shockwave in the treatment of early osteoarthritis of the knee. Modern Chin Med (2022) 42(01):108–10. doi: 10.13424/j.cnki.mtcm.2022.01.021
33. Liu WF. Analysis of short-term efficacy of extracorporeal shock wave therapy for early and middle stage knee osteoarthritis. China Med Equip Inf (2019) 25(8):61–2.
34. Wu YL, Zhong T, Tang F. Clinical effect observation of extracorporeal shock wave therapy for early and middle stage knee osteoarthritis. Clin J Pract Hospital. (2022) 19(2):62–4. doi: 10.3969/j.issn.1672-6170.2022.02.017
35. Zheng YL, Yang QS. Effect of extracorporeal shock wave therapy on clinical related indexes in patients with knee osteoarthritis. Chin J Clin (2011) 49(06):717–9.
36. Su WZ, Lin YJ, Wang GW, Geng Z, Wang ZY, Hou DL, et al. Prospective clinical comparative study of extracorporeal shock wave combined with platelet-rich plasma injection in the treatment of knee osteoarthritis. Chin J Prosthoplastic Reconstructive Surg (2019) 33(12):1527–31.
37. Jiang LM, Huang SJ, Yu XM, Tao Y, Shen LH. Effect of extracorporeal shock wave combined with joint loosening in the treatment of knee osteoarthritis. Chin J Bone Joint Injury (2017) 32(12):1299–301.
38. Liu WD, Xue KL, Tian M, Zhang F. Comparative study on the efficacy of extracorporeal shock wave and acupotomy in the treatment of knee osteoarthritis. J Neck Low Back Pain (2017) 38(01):64–7.
39. Ji JJ, Fang MQ, Zhu T, Wang YY, Chen XH. Effect and mechanism of radial extracorporeal shock wave acupoint therapy on elderly patients with knee osteoarthritis. Chin J geriatric multi-organ Dis (2020) 20(06):410–3.
40. Mostafa MSEM, Hamada HA, Kadry AM, Zahran SS, Helmy NA. Effect of high-power laser therapy versus shock wave therapy on pain and function in knee osteoarthritis patients: A randomized controlled trial. Photobiomodul Photomed Laser Surg (2022) 40(3):198–204. doi: 10.1089/photob.2021.0136
41. Zhao Z, Jing R, Shi Z, Zhao B, Ai Q, Xing G. Efficacy of extracorporeal shockwave therapy for knee osteoarthritis: a randomized controlled trial. J Surg Res (2013) 185(2):661–6. doi: 10.1016/j.jss.2013.07.004
42. Uysal A, Yildizgoren MT, Guler H, Turhanoglu AD. Effects of radial extracorporeal shock wave therapy on clinical variables and isokinetic performance in patients with knee osteoarthritis: a prospective, randomized, single-blind and controlled trial. Int Orthop (2020) 44(7):1311–9. doi: 10.1007/s00264-020-04541-w
43. Zhong Z, Liu B, Liu G, Chen J, Li Y, Chen J, et al. A randomized controlled trial on the effects of low-dose extracorporeal shockwave therapy in patients with knee osteoarthritis. Arch Phys Med Rehabil. (2019) 100(9):1695–702. doi: 10.1016/j.apmr.2019.04.020
44. Wang XC, Sun YZ, Ma JY, Shen QM, Zhang Y. Clinical observation of knee cavity pulse radiofrequency therapy for knee osteoarthritis. Chin J Pain Med (2016) 27(10):785–8.
45. Ma JY, Zhu LX, Cai YH, Wang LX, Ye YH, Lu K. Comparison of therapeutic effect of pulsed radiofrequency and ozone injection in the treatment of knee osteoarthritis. Hainan Med (2017) 28(21):3553–5.
46. Zhuo ZM, Xing S, Wang HJ, Chen LX, Wang Y. Effects of pulsed radiofrequency surgery on knee function and serum levels of IL-7R, TNF-α and IGF in patients with knee osteoarthritis. Modern Biomed Prog (2021) 21(13):2551–4.
47. Feng WH, Kang Z, Liu TH. Effects of pulsed radiofrequency surgery on levels of inflammatory factors and insulin-like growth factors in patients with knee osteoarthritis. Sichuan J Physiol Sci (2019) 44(3):457–60.
48. Cai LX. Effect of highly selective peripheral nerve radiofrequency ablation in the treatment of severe knee osteoarthritis and its influence on the level of inflammatory mediators [J]. Chin Sci Technol J Database Med Health (2022) 12):0001–3.
49. Elawamy A, Kamel EZ, Mahran SA, Abdellatif H, Hassanien M. Efficacy of genicular nerve radiofrequency ablation versus intra-articular platelet rich plasma in chronic knee osteoarthritis: A single-blind randomized clinical trial. Pain Physician. (2021) 24(2):127–34.
50. Chen AF, Khalouf F, Zora K, DePalma M, Kohan L, Guirguis M, et al. Cooled radiofrequency ablation provides extended clinical utility in the management of knee osteoarthritis: 12-month results from a prospective, multi-center, randomized, cross-over trial comparing cooled radiofrequency ablation to a single hyaluronic acid injection. BMC Musculoskelet Disord (2020) 21(1):363. doi: 10.1186/s12891-020-03380-5
51. Uematsu H, Osako S, Hakata S, Kabata D, Shintani A, Kawazoe D, et al. A double-blind, placebo-controlled study of ultrasound-guided pulsed radiofrequency treatment of the saphenous nerve for refractory osteoarthritis-associated knee pain. Pain Physician. (2021) 24(6):E761–9.
52. El-Hakeim EH, Elawamy A, Kamel EZ, Goma SH, Gamal RM, Ghandour AM, et al. Fluoroscopic guided radiofrequency of genicular nerves for pain alleviation in chronic knee osteoarthritis: A single-blind randomized controlled trial. Pain Physician. (2018) 21(2):169–77.
53. Zuo XT. Study on the effect of acupotomy on knee osteoarthritis and interleukin-6 and TNF-α. Nanjing Univ Chin Med (2022). doi: 10.27253/dcnki.Gnjzu.2021.000208
54. Xiong YZ, Zhu JC, Wang C, Zhi YL, Su Y, Li YC, et al. Clinical effect of acupotomy combined with celecoxib in the treatment of knee osteoarthritis. Chin J Orthopaedics Traumatology Traditional Chin Med (2020) 28(02):19–23.
55. Meng F, Yin Y, Wang TC, Feng L. Effect of small acupotomy on the expression of TNF-α and MMPs in synovial fluid of knee joint osteoarthritis. Hebei Med (2017) 39(02):168–72.
56. Pan Q. Effect of small acupotomy on knee osteoarthritis and its effect on surface electromyography of quadriceps [J]. J liaoning traditional Chin Med (2023) 50(03):174–7. doi: 10.13192/j.iSSN.1000-1719.2023.03.049
57. Wang C, Zhu JC, Zheng ZW, Xiong YZ, Ma XF, Gong YC, et al. Effect of acutomolysis of pain point on partial motor gait and serum TNF-α and IL-1 in patients with knee osteoarthritis. Chin J Bone Injury (2019) 35(09):848–52.
58. Xu DH, Lin YX, Wei J, Huang CH, Li MH, Yao TT, et al. Harmonizing Yin and Yang acupotomy for osteoarthritis of the knee: A randomized controlled trial. Chin J Acupuncture Moxibustion (2022) 42(12):1351–6. doi: 10.13703/j.0255-2930.20220126-k0002
59. Wang HL, Li ZX, Yi WJ, Li SB, Wang L. Clinical study on the effect of micro-acupotomy on the level of inflammatory factors in patients with knee osteoarthritis based on the theory of meridian tendon. J Clin Med Literature Electronic (2019) 7(56):74–6.
60. Li XP, Ren YY, Zhang WJ, Zhang XS, Bai XR, Zeng BX. Clinical effect of acupotomy on knee osteoarthritis based on muscle pain point theory. Western Chin Med (2020) 33(9):107–10. doi: 10.12174/j.issn.1004-6852.2020.09.31
61. Jiang F, Xu JW, Zhang L, Zhou ZH, Yuan LX, Fu SS. Clinical study of semiconductor laser acupoint irradiation combined with ligustrazine in treatment of knee osteoarthritis. Chin J Modern Med (2018) 28(34):48–53.
62. Yurtkuran M, Alp A, Konur S, Ozçakir S, Bingol U. Laser acupuncture in knee osteoarthritis: a double-blind, randomized controlled study. Photomed Laser Surg (2007) 25(1):14–20. doi: 10.1089/pho.2006.1093
63. Alghadir A, Omar MT, Al-Askar AB, Al-Muteri NK. Effect of low-level laser therapy in patients with chronic knee osteoarthritis: a single-blinded randomized clinical study. Lasers Med Sci (2014) 29(2):749–55. doi: 10.1007/s10103-013-1393-3
64. Zhao L, Cheng K, Wu F, Du J, Chen Y, Tan MT, et al. Effect of laser moxibustion for knee osteoarthritis: A multisite, double-blind randomized controlled trial. J Rheumatol (2021) 48(6):924–32. doi: 10.3899/jrheum.200217
65. Helianthi DR, Simadibrata C, Srilestari A, Wahyudi ER, Hidayat R. Pain reduction after laser acupuncture treatment in geriatric patients with knee osteoarthritis: a randomized controlled trial. Acta Med Indones. (2016) 48(2):114–21.
66. Lin L. The clinical effect of 10.6μm laser moxibustion on knee osteoarthritis and the behavior of inhibiting inflammatory pain. Shanghai Univ traditional Chin Med (2021). doi: 10.27320/,dcnki.Gszyu.2019.000043
67. Shen X, Zhao L, Ding G, Tan M, Gao J, Wang L, et al. Effect of combined laser acupuncture on knee osteoarthritis: a pilot study. Lasers Med Sci (2009) 24(2):129–36. doi: 10.1007/s10103-007-0536-9
68. Fang GG, Liang DB, Zhang HR, Xie S, Zeng ZC. Methods to ease the popliteus treatment of knee osteoarthritis clinical research. J modern traditional Chin Med (2022) 02):122–5.
69. Lin X, Wang JP, Chen B, Pang J, Zhang M, Wang HH, et al. A clinical study on the treatment of knee osteoarthritis by Shi's massage and correction technique combined with red cinnamon tincture rubbing. J Chin Med (2018) 4(01):23–6. doi: 10.13193/j.iSSN.1673-7717.2018.01.005
70. Li ZL, Wang JJ, Liu K. Short-term effect of deep kneading massage in the treatment of elderly knee osteoarthritis. Chin J Rehabil Med (2015) 30(06):580–4.
71. Li MX, Fan XH. Effect of Pingle bonesetting technique on knee osteoarthritis and its influence on pain degree and knee function. J Traditional Chin Med (2019) 35(12):35–9.
72. Kong LL, Li WF, Tan RL, Li XL, Wang ZG, Tu Y. Aromatherapy massage the clinical curative effect of treating knee joint osteoarthritis. Jiangsu Med (2020) 46-48(10):1000–1003 + 1007. doi: 10.19460/j.carolcarrollnki.0253-3685.2020.10.008
73. Xu H, Zhao C, Guo G, Li Y, Xinyu A, Qiu G, et al. The effectiveness of tuina in relieving pain, negative emotions, and disability in knee osteoarthritis: A randomized controlled trial. Pain Med (2023) 24(3):244–57. doi: 10.1093/pm/pnac127
74. Perlman A, Fogerite SG, Glass O, Bechard E, Ali A, Njike VY, et al. Efficacy and safety of massage for osteoarthritis of the knee: a randomized clinical trial. J Gen Intern Med (2019) 34(3):379–86. doi: 10.1007/s11606-018-4763-5
75. Wang XB, Ding YX, Tan ZD, Pan JY, Zhang HT, Jiang DY. Clinical observation of "form-qi differentiation" massage therapy for knee osteoarthritis. J traditional Chin Med (2020) 13(8):55–9. doi: 10.19664/j.carolcarrollnki.1002-2392.200145
76. Zhao Q, Dai HY, Zhang CL, Li H, Zhao J. Effect of intramuscular adhesive Therapy on pain level of patients with knee osteoarthritis. J Logistics Coll Armed Police Force (Medical Edition) (2021) 30(11):20–2. doi: 10.16548/J.2095-3720.2021.11.072
77. Shi BH, Huang CM, Zhu Y, Chen Z, Li KP, Luo Y, et al. Short-term clinical effect of intramuscular adhesive on walking ability in patients with knee osteoarthritis. Clin Rev (2019) 35(12):1111–5.
78. Kaya Mutlu E, Mustafaoglu R, Birinci T, Razak Ozdincler A. Does kinesio taping of the knee improve pain and functionality in patients with knee osteoarthritis?: A randomized controlled clinical trial. Am J Phys Med Rehabil. (2017) 96(1):25–33. doi: 10.1097/PHM.0000000000000520
79. Dogan N, Yilmaz H, Ince B, Akcay S. Is kinesio taping effective for knee osteoarthritis? Randomised, controlled, double-blind study. J Coll Physicians Surg Pak (2022) 32(11):1441–7. doi: 10.29271/jcpsp.2022.11.1441
80. Li JF, Zhang J, Hei G, Dong TL, Zhuang ZG. Effect of intramuscular adhesive on the level of inflammatory factors in joint fluid of patients with early and middle stage knee osteoarthritis. Chin elderly Med J (2018) 10):1126–8. doi: 10.3760/cma.J.iSSN.0254-9026.2018.10.016
81. Anandkumar S, Sudarshan S, Nagpal P. Efficacy of kinesio taping on isokinetic quadriceps torque in knee osteoarthritis: a double blinded randomized controlled study. Physiother Theory Pract (2014) 30(6):375–83. doi: 10.3109/09593985.2014.896963
82. Donec V, Kubilius R. The effectiveness of Kinesio Taping for mobility and functioning improvement in knee osteoarthritis: a randomized, double-blind, controlled trial. Clin Rehabil. (2020) 34(7):877–89. doi: 10.1177/0269215520916859
83. Günaydin ÖE, Bayrakci Tunay V. Comparison of the added effects of kinesio taping and extracorporeal shockwave therapy to exercise alone in knee osteoarthritis. Physiother Theory Pract (2022) 38(5):661–9. doi: 10.1080/09593985.2020.1780657
84. Xin R, Quan D, Zhang B, Shi C, Lin YP, Zhang S, et al. Autologous platelet rich plasma for treatment of knee osteoarthritis. J Clin Med Res Pract (2021) 6(09):35–7. doi: 10.19347/j.carolcarrollnki.2096-1413.202109013
85. Acosta-Olivo C, Esponda-Colmenares F, Vilchez-Cavazos F, Lara-Arias J, Mendoza-Lemus O, Ramos-Morales T. Platelet rich plasma versus oral paracetamol for the treatment of early knee osteoarthritis. Preliminary study. Cir Cir. (2014) 82(2):163–9.
86. Huang XH, Zhong L, Jin HM. Platelet rich plasma treatment of patients with knee osteoarthritis clinical research. Chin J Clin Pharmacol (2022) 12):1316–1319 + 1324. doi: 10.13699/j.carolcarrollnki.1001-6821.2022.12.005
87. Li YJ, Duan JF, Sun C, Zhao ZB, Wang B, Wang YM. Clinical effect of platelet-rich plasma combined with extracorporeal shock wave in the treatment of knee osteoarthritis. Lab Med Clinic (2002) 19(02):240–3.
88. Chu J, Duan W, Yu Z, Tao T, Xu J, Ma Q, et al. Intra-articular injections of platelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. (2022) 30(12):4063–71. doi: 10.1007/s00167-022-06887-7
89. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med (2013) 41(2):356–64. doi: 10.1177/0363546512471299
90. Park YB, Kim JH, Ha CW, Lee DH. Clinical efficacy of platelet-rich plasma injection and its association with growth factors in the treatment of mild to moderate knee osteoarthritis: A randomized double-blind controlled clinical trial as compared with hyaluronic acid. Am J Sports Med (2021) 49(2):487–96. doi: 10.1177/0363546520986867
91. Wang YC, Lee CL, Chen YJ, Tien YC, Lin SY, Chen CH, et al. Comparing the efficacy of intra-articular single platelet-rich plasma(PRP) versus novel crosslinked hyaluronic acid for early-stage knee osteoarthritis: A prospective, double-blind, randomized controlled trial. Medicina (Kaunas). (2022) 58(8):1028. doi: 10.3390/medicina58081028
92. Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. (2021) 326(20):2021–30. doi: 10.1001/jama.2021.19415
93. Reyes-Sosa R, Lugo-Radillo A, Cruz-Santiago L, Garcia-Cruz CR, Mendoza-Cano O. Clinical comparison of platelet-rich plasma injection and daily celecoxib administration in the treatment of early knee osteoarthritis: A randomized clinical trial. J Appl Biomed (2020) 18(2-3):41–5. doi: 10.32725/jab.2020.012
94. Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: A prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med (2017) 45(2):339–46. doi: 10.1177/0363546516665809
95. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. (2017) 25(3):958–65. doi: 10.1007/s00167-015-3705-6
96. Li HT, Liu H, Yang FJ, Li YQ, Sun XW. Cupping treatment of knee osteoarthritis clinical efficacy analysis. J traditional Chin Med (2017) 01):110–3. doi: 10.19664/j.carolcarrollnki.1002-2392.2017.01.033
97. Liu Y, Wu H. Electroacupuncture for knee osteoarthritis and its influence on the levels of related inflammatory factors: a randomized clinical trial. World J Acupuncturity-Moxibustion (2022) 32(04):329–35. doi: 10.1016/j.wjam.2022.07.009
98. Gu ZY, Wang Y, Chen CL, Cui HS, Ding JY, Wang K. Electric acupuncture treatment of knee osteoarthritis clinical research. J Shanghai acupuncture magazine (2017) 4(9):1111–5. doi: 10.13460/jiSSN.1005-0957.2017.09.1111
99. Zhu DY. Clinical and basic research of electroacupuncture on inhibiting inflammation mediated cartilage degeneration in osteoarthritis. Fujian Univ Traditional Chin Med (2019).
100. Yang SS, Meng L, Zhao Y, Zhang W, Du GS, Long AG, et al. Clinical study of electroacupuncture combined with massage in the treatment of knee osteoarthritis. Chin J Acute Chin Med (2022) 31(07):1177–1180+1192.
101. Wang Q, Lv H, Sun ZT, Tu JF. Electricity for knee osteoarthritis joint function the influence of subjective and objective evaluation index. J Shanghai acupuncture magazine (2022) 9(7):713–9. doi: 10.13460/j.iSSN.1005-0957.2021.13.0048
102. Wu MX, Li XH, Lin N, Jia XR, Mu R, Wan WR, et al. Clinical study on the treatment of knee osteoarthritis of shen-sui insufficiency syndrome type by electroacupuncture. Chin J Integr Med (2010) 16(04):291–7. doi: 10.1007/s11655-010-0513-1
103. Lv ZT, Shen LL, Zhu B, Zhang ZQ, Ma CY, Huang GF, et al. Effects of intensity of electroacupuncture on chronic pain in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther (2019) 14(21) (1):120. doi: 10.1186/s13075-019-1899-6
104. Tu JF, Yang JW, Shi GX, Yu ZS, Li JL, Lin LL, et al. Efficacy of intensive acupuncture versus sham acupuncture in knee osteoarthritis: A randomized controlled trial. Arthritis Rheumatol (2021) 73(3):448–58. doi: 10.1002/art.41584
105. Hei G, Li JF, Zhang J, Zhuang ZG, Dong TL. Eaffect of ozone water on the treatment of knee osteoarthritis and its effect on cytokines in joint fluid. Chin J Pain Med (2019) 26(02):150–2.
106. Li WW. Effect and safety evaluation of ozone therapy in patients with chronic pain of knee osteoarthritis. Chin Sci Technol J Database (full-text edition) Med Health (2021) 9):0005–6.
107. Meng T, Su C. Clinical efficacy of sodium hyaluronate combined with ozone in the treatment of knee osteoarthritis. Chongqing Med J (2018) 47(24):3184–7.
108. Lopes de Jesus CC, Dos Santos FC, de Jesus LMOB, Monteiro I, Sant'Ana MSSC, Trevisani VFM. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PloS One (2017) 12(7):e0179185. doi: 10.1371/journal.pone.0179185
109. Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. (2017) 25(2):485–92. doi: 10.1007/s00167-016-4110-5
110. Babaei-Ghazani A, Najarzadeh S, Mansoori K, Forogh B, Madani SP, Ebadi S, et al. The effects of ultrasound-guided corticosteroid injection compared to oxygen-ozone (O2-O3) injection in patients with knee osteoarthritis: a randomized controlled trial. Clin Rheumatol (2018) 37(9):2517–27. doi: 10.1007/s10067-018-4147-6
111. Raeissadat SA, Ghazi Hosseini P, Bahrami MH, Salman Roghani R, Fathi M, Gharooee Ahangar A, et al. The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet Disord (2021) 22(1):134. doi: 10.1186/s12891-021-04017-x
112. Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med (2017) 12(1):103–11. doi: 10.1007/s11739-016-1583-7
113. Moffett J, Fray LM, Kubat NJ. Activation of endogenous opioid gene expression in human keratinocytes and fibroblasts by pulsed radiofrequency energy fields. J Pain Res (2012) 5:347–57. doi: 10.2147/JPR.S35076
114. Sluijter ME, Teixeira A, Serra V, Balogh S, Schianchi P. Intra-articular application of pulsed radiofrequency for arthrogenic pain–report of six cases. Pain Pract (2008) 8(1):57–61. doi: 10.1111/j.1533-2500.2007.00172.x
115. Zheng LL, Wang K, Li MZ, Wang JM, Qiao YJ, Li HD. Shutiaojing massage can maintain the stability of the internal environment of rabbit cartilage cells damaged by knee osteoarthritis. J Tissue Eng (2019) 27(35):5681–7.
116. Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, et al. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheumatol (2011) 63(5):1343–8. doi: 10.1002/art.30287
117. Mabey T, Honsawek S, Tanavalee A, Yuktanandana P, Wilairatana V, Poovorawan Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers. (2016) 21(7):639–44. doi: 10.3109/1354750X.2016.1171907
118. Bertazzolo N, Punzi L, Stefani MP, Cesaro G, Pianon M, Finco B, et al. Interrelationships between interleukin (IL)-1, IL-6 and IL-8 in synovial fluid of various arthropathies. Agents Actions. (1994) 41(1-2):90–2. doi: 10.1007/BF01986402
119. Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. (2018) 100(6):1292–311. doi: 10.1016/j.neuron.2018.11.009
Keywords: knee osteoarthritis, externally-applied, non-pharmacological interventions, short- and long-term, efficacy, inflammatory cytokine, network meta-analysis
Citation: Wang Z, Xu H, Wang Z, Zhou H, Diao J, Zhang L, Wang Y, Li M and Zhou Y (2023) Effects of externally-applied, non-pharmacological Interventions on short- and long-term symptoms and inflammatory cytokine levels in patients with knee osteoarthritis: a systematic review and network meta-analysis. Front. Immunol. 14:1309751. doi: 10.3389/fimmu.2023.1309751
Received: 08 October 2023; Accepted: 30 November 2023;
Published: 14 December 2023.
Edited by:
Stefania Croci, IRCCS Local Health Authority of Reggio Emilia, ItalyReviewed by:
Jehan J. El-Jawhari, Nottingham Trent University, United KingdomRoger David Adams, University of Canberra, Australia
Copyright © 2023 Wang, Xu, Wang, Zhou, Diao, Zhang, Wang, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunfeng Zhou, enlmNTY4MDE5OEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Zhen Wang
Zhen Wang Hui Xu
Hui Xu Zheng Wang
Zheng Wang Hang Zhou1
Hang Zhou1 Miaoxiu Li
Miaoxiu Li Yunfeng Zhou
Yunfeng Zhou