- 1Department of Pulmonary and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 2School of Clinical Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
With the widespread use of immune checkpoint inhibitors to treat various cancers, pulmonary toxicity has become a topic of increasing concern. Anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibodies are strongly associated with rapidly progressive interstitial lung disease (RP-ILD) in patients with clinically amyopathic dermatomyositis. However, anti-MDA5 antibody expression has not been reported in patients with immune-related adverse events. We present the case of a 74-year-old man with lung adenocarcinoma who developed RP-ILD after treatment with immune checkpoint inhibitors. Further investigation revealed multiple autoantibodies, including anti-MDA5 antibodies. He initially responded to systemic glucocorticoids, immunosuppressants, and tocilizumab but eventually died from worsening pneumomediastinum. This case is the first one to suggest that checkpoint inhibitor pneumonitis can present as RP-ILD with positive anti-MDA5 antibodies, which may be predictive of a poor prognosis.
1 Introduction
Immune checkpoint inhibitors (ICIs) can have various adverse effects, including checkpoint inhibitor pneumonitis (CIP), which can be life-threatening. Myositis and dermatomyositis (DM) are considered part of the spectrum of immune-related adverse events (irAEs). The relationship between anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibodies and clinically amyopathic dermatomyositis was first described by Sato et al. in a Japanese cohort (1). Anti-MDA5 antibodies are considered markers of poor prognosis for rapidly progressive interstitial lung disease (RP-ILD) which refers to a course with measurable progression within a short period of time since onset of interstitial lung disease (ILD). However, anti-MDA5 antibodies have not been reported in patients with irAEs until now. Herein, we report the first case of a patient with CIP who tested positive for anti-MDA5 antibody.
2 Case presentation
A 74-year-old man was admitted to the Peking Union Medical College Hospital’s respiratory intensive care unit for a rash that lasted for one month and dyspnea that lasted for 2 weeks. He had been diagnosed with lung adenocarcinoma (Figures 1A, B) four months before admission. He had declined surgery due to severe comorbidities. Four weeks before admission, he was started on camrelizumab (200 mg, day 1), an ICI, combined with chemotherapy with pemetrexed (700 mg, day 1) and carboplatin (260 mg, day 1). He developed a pruritic maculopapular rash on his neck, chest wall, and sacrum. Two weeks before reporting to the hospital, he started experiencing shortness of breath on exertion, which gradually progressed. Chest computed tomography (CT) showed new bilateral subpleural opacities, which were more severe in the right lung (Figures 1C, D). CIP was suspected. Intravenous methylprednisolone 80 mg/day was administered for 5 days, tapered to 60 mg/day for 5 days, and maintained at 40 mg/day until admission. His rash improved but the dyspnea worsened. His medical history included hypertension, hyperlipidemia, cerebral infarction, and myocardial infarction. The patient had no history of ILD.
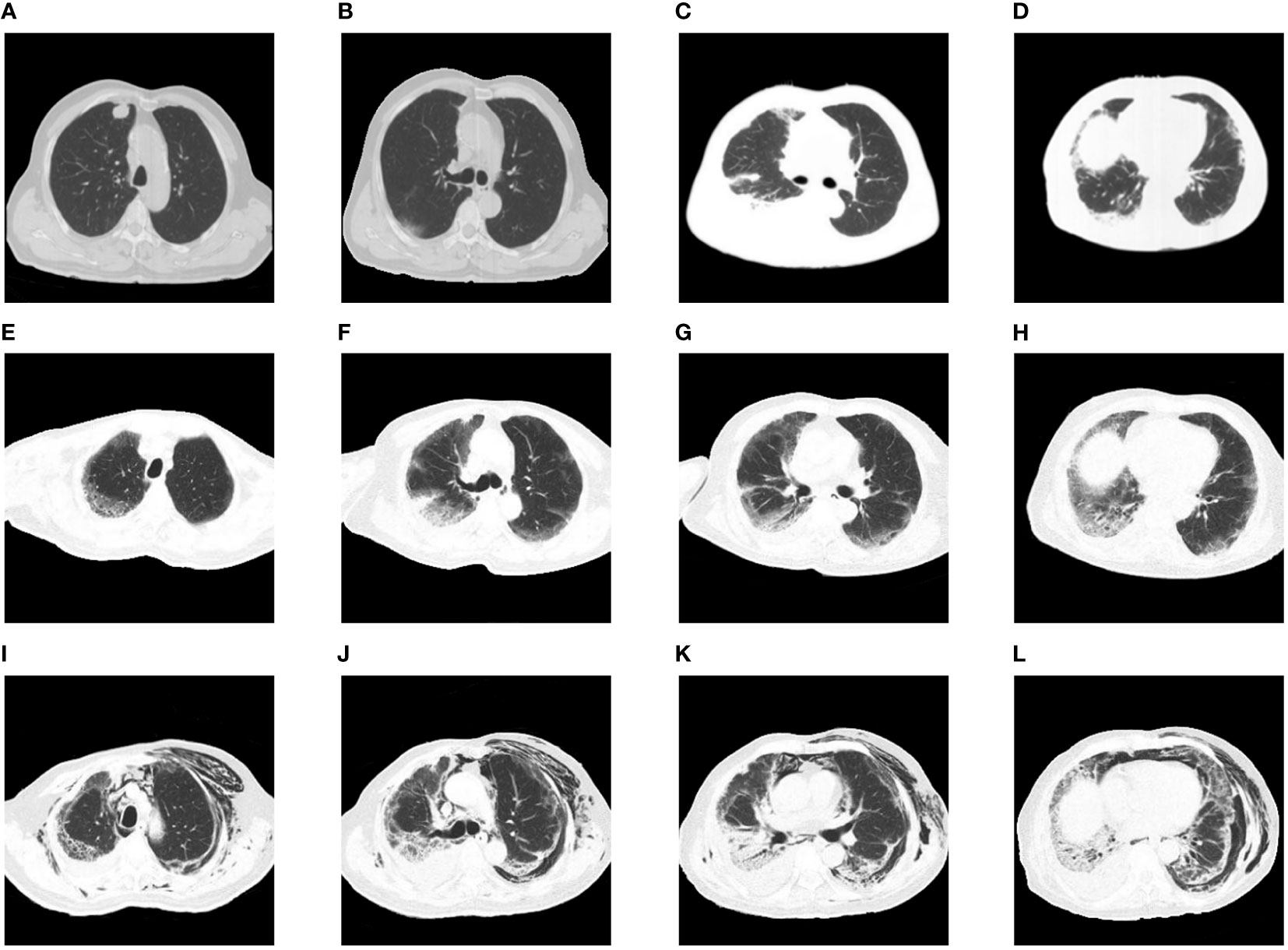
Figure 1 Chest images during the clinical episode. (A, B) The baseline chest CT before initiating therapy showed a nodule in the right upper lobe without any interstitial change. (C, D) Chest CT after onset of dyspnea showed new bilateral subpleural opacities, which were more severe in the right lung. (E–H) Chest CT on intubation day showed bilateral ground-glass opacities and consolidation with reticulation. (I–L) Chest CT after extubation revealed severe subcutaneous and mediastinal empysema, along with bilateral opacities consistent with interstitial pneumonitis.
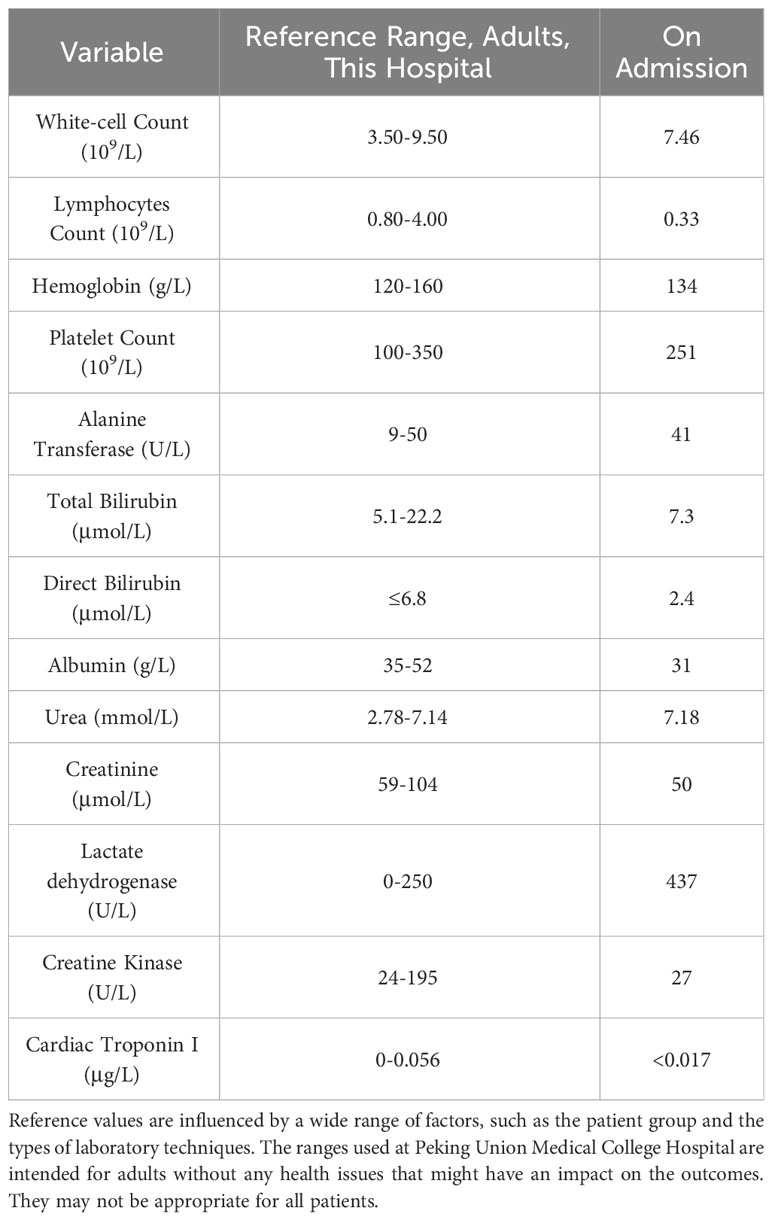
On physical examination, his body temperature was 36.5˚C, blood pressure was 147/56 mmHg, heart rate was 97 beats per minute, respiratory rate was 25 per minute, and oxygen saturation was 92% while he was receiving supplementary oxygen via a non-rebreathing mask at a flow rate of 12 liters per minute. Inspiratory tri-concave signs and scattered desquamated rashes were observed on inspection, and Velcro rales were heard bilaterally, but were more prominent in the right lung. Arterial blood gas (fraction of inspired oxygen was 70%) showed elevated pH (7.506), partial pressure of oxygen (76.6 mmHg; 83−108 mmHg), partial pressure of carbon dioxide (27 mmHg; 35−45 mmHg), and lactose concentration (2.3 mmol/L; 0.5−1.6 mmol/L). Elevated concentrations of C-reactive protein (69.8 mg/L; 0−3 mg/L), ferritin (1124 ng/ml; 24−336 ng/ml), and interleukin-6 (77 pg/ml; 0−5.9 pg/ml) were also documented. The rheumatology panel showed an elevated rheumatoid factor concentration of 57 IU/ml (0−20 IU/ml) and weak positivity for anti-nuclear antibody (1:160; normal range< 1:80 by ELISA), anti-mitochondrial M2 antibody (17; normal range< 15 by Western Blot), anti-MDA5 antibody (+; “+++” represents the strongest by Western Blot), and anti-Ro 52 antibody (+; “+++” represents the strongest by Western Blot). The other laboratory findings are presented in Table 1.
CIP (grade 4) was diagnosed, and the methylprednisolone dose was increased to 80 mg twice daily for 3 days before being tapered slowly. Tocilizumab (480 mg/week) was administered for 7 weeks, intravenous immunoglobulin was administered at 20 g/day for 3 days. Cyclophosphamide and tacrolimus were also administered. The patient was intubated and mechanically ventilated due to worsening respiratory distress. Chest CT (Figures 1E–H) showed bilateral ground-glass opacities and consolidation with reticulation. The patient improved and was successfully weaned from ventilation to a high-flow nasal cannula 11 days after intubation. However, he received a second round of intravenous methylprednisolone and intravenous immunoglobulin (20 g/day) due to exacerbation of his chest CT findings. Anti-MDA5 antibodies turned negative 20 days after the first report. His respiratory support level stabilized with the high-flow nasal cannula (flow rate of 30 L/min; fraction of inspired oxygen, 35−50%) until severe mediastinal and subcutaneous emphysema developed, which involved the neck and chest and extended to the scrotum and lower extremities (Figures 1I–L). Two months after admission, he strongly insisted on returning home. He died 2 days later due to severe dyspnea. The details of his treatment are summarized in Figure 2.
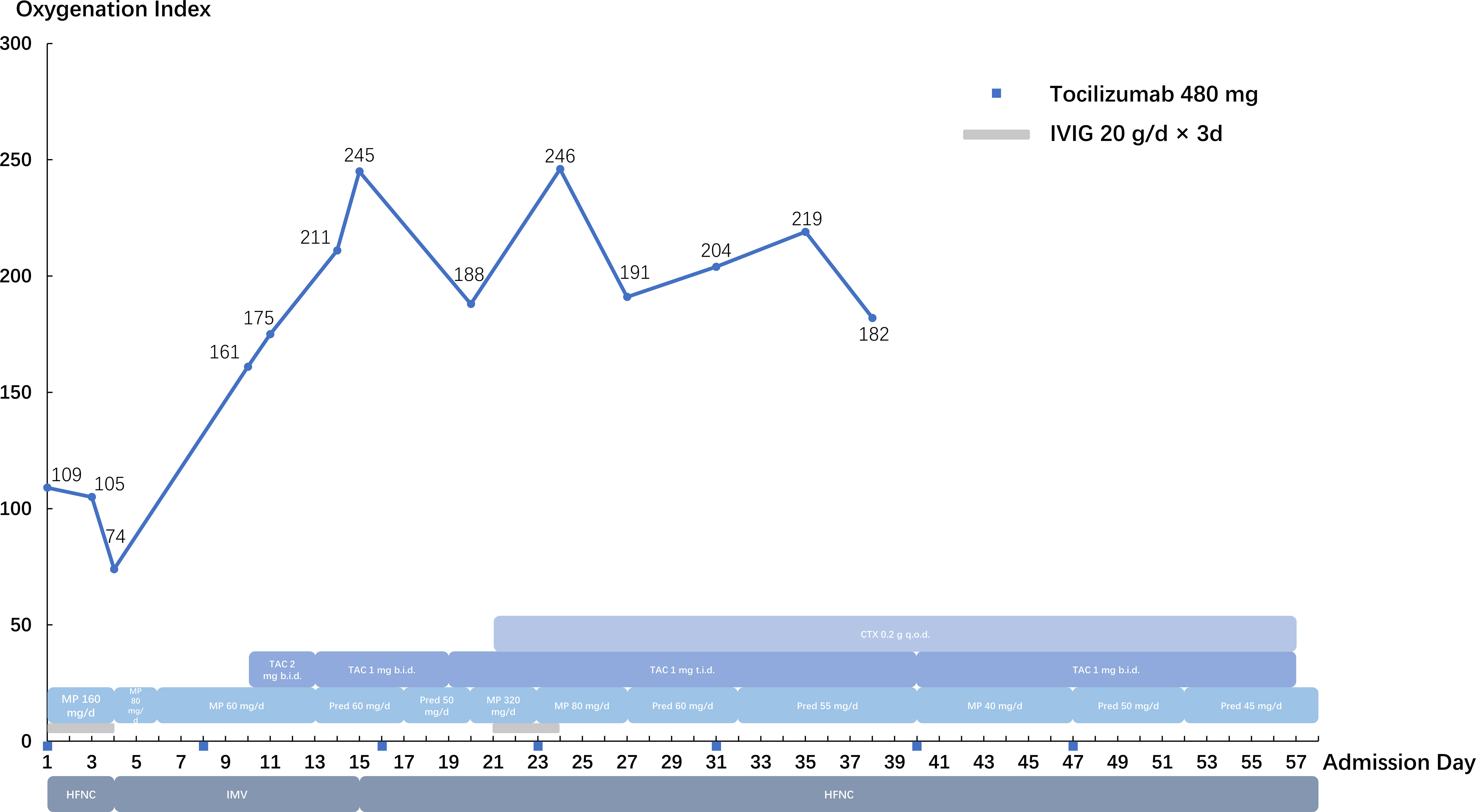
Figure 2 Summary of the clinical course. IVIG, intravenous immunoglobulin; HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation; MP, methylprednisolone; Pred, prednisone; TAC, tacrolimus; CTX, cyclophosphamide.
3 Discussion
ICIs are immunomodulatory antibodies that boost the immune system. Their main targets are programmed cell death receptor 1, programmed cell death ligand 1, and cytotoxic lymphocyte-associated antigen 4. ICIs have significantly improved the prognosis of patients with various advanced malignancies, including lung cancer. Immune-related adverse events (irAEs) are inflammatory responses caused by ICIs. They can involve the skin, gastrointestinal tract, endocrine system, lungs, and other organs (2). A study by Naidoo et al. showed that the overall incidence of CIP caused by anti-programmed cell death receptor 1 or programmed cell death ligand 1 treatment was 5%. The duration of treatment before pneumonitis onset varied with a median of 2.8 months (9 days to 19 months) (3). The clinical manifestations and imaging findings of CIP are nonspecific. Cough and dyspnea are the most common symptoms, but some patients may be asymptomatic. The signs and patterns on chest imaging include ground-glass opacity, consolidation, and diffuse alveolar damage. CIP is usually sensitive to glucocorticoid therapy (4). This patient developed a rash and pulmonary lesion soon after the administration of ICIs. Although his onset was rapid, immunotherapy-related skin and pulmonary toxicities should be considered first. Antineoplastic agent like pemetrexed can infrequently cause lung toxicities including ILD, with ground glass opacities the predominate CT pattern and good response to steroids, which should be differentiated in this case (5). However, pemetrexed is unlikely to cause the autoantibodies.
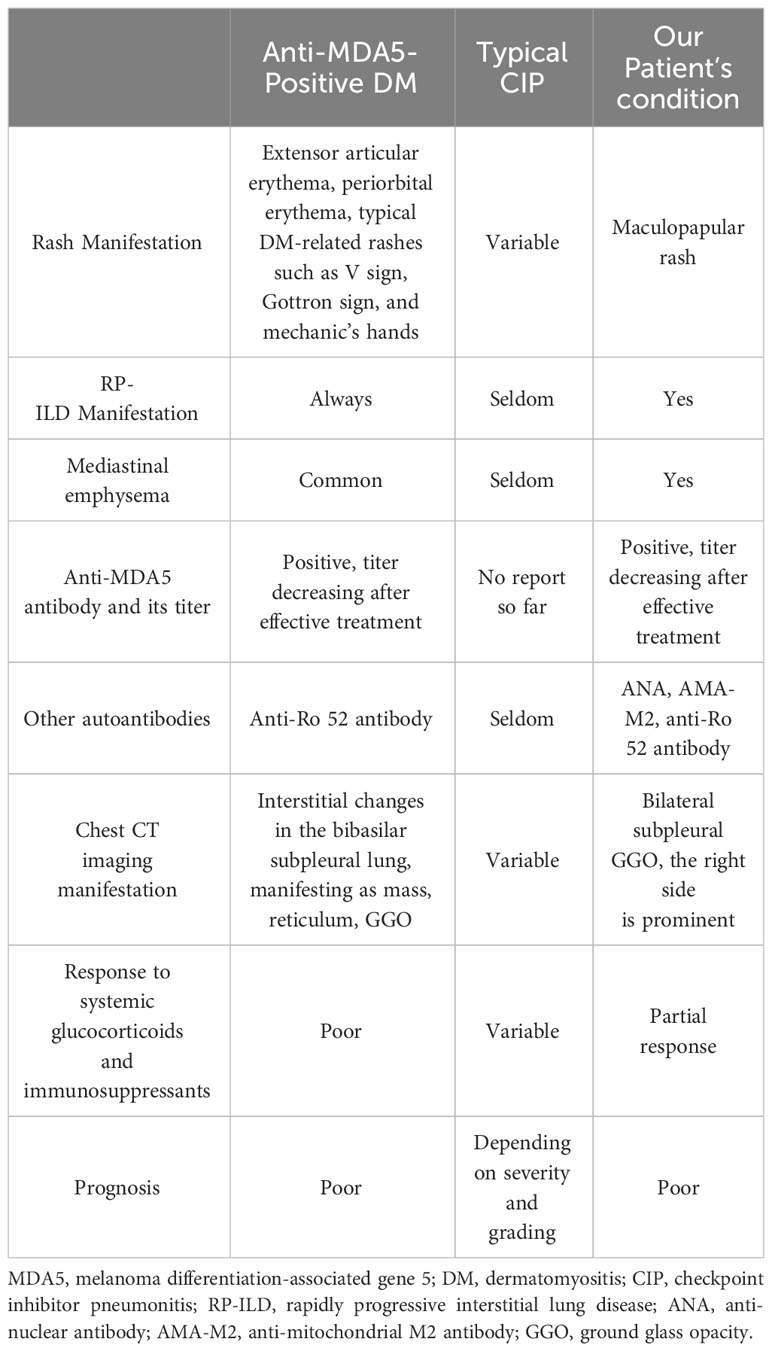
Anti-MDA5 antibodies are associated with clinically amyopathic dermatomyositis, which usually manifests as a characteristic rash and RP-ILD with high mortality. RP-ILD in anti-MDA5-positive DM is suggested to be defined as either worsening of dyspnea and CT progression within 1 month, or deterioration to respiratory failure within 3 months since respiratory symptom onset (6). According to the consensus of the 239th European Neuromuscular Centre meeting, DM can be diagnosed with the following criteria: typical DM-related rash, dermatopathological evidence of interface dermatitis, evidence of myositis, or positive DM-specific autoantibodies (7). Although this patient tested positive for anti-MDA5 antibodies, he did not have a typical DM-related rash or any evidence of myositis such as creatine kinase elevation; hence, DM could not be diagnosed. Moreover, he showed no signs of ILD on chest CT besides lung cancer at baseline. Anti-MDA5 and other autoantibodies before ICI therapy were not measured, which is a limitation of this report, but we disregarded them as a marker of newly developed or exacerbated anti-MDA5-positive DM. Additionally, the pathophysiology was different from that of typical anti-MDA5-positive DM in the following respects. First, the pulmonary lesion was more prominent on the tumor side. Second, the patient responded better to large doses of glucocorticoids and immunosuppressants and was successfully weaned from invasive mechanical ventilation, which is rare for patients with anti-MDA5-positive DM. Third, the titer of anti-MDA5 antibodies was mismatched with the severity of lung disease, and the patient tested negative for them immediately after therapy. A detailed comparison of anti-MDA5-positive DM, typical CIP, and CIP in this patient is shown in Table 2.
ICIs can lead to various rheumatic irAEs such as arthritis and myositis, as well as presence of new autoantibodies. The incidence of ICI-induced myositis is approximately 0.6% (8). Ghosh et al. systematically reviewed the incidence of autoantibodies in patients with irAEs and found that 67 patients tested positive for myositis-associated antibodies, and 27% tested positive for at least one antibody, such as anti-Mi-2 antibody, anti-PM/Scl antibody, and anti-signal recognition particle (SRP) antibody (9). However, anti-MDA5 antibodies, which represent a type of myositis-associated antibodies, have not been previously reported after the administration of ICIs. In short, it is more reasonable to consider anti-MDA5 antibodies and other autoantibodies in this patient as byproducts of immunotherapy.
Anti-MDA5 antibodies are not found exclusively in patients with DM. Wang et al. reported that 48.2% of patients with coronavirus disease 2019 (COVID-19) tested positive for anti-MDA5 antibodies, and the non-survival group had a greater prevalence (10). Based on the shared features between this case and classical anti-MDA5-positive DM patients, we speculate that the anti-MDA5 antibodies may have partially accounted for the clinical characteristics justifying the use of immunosuppressive medication, and might be predictive of a poor prognosis. Tocilizumab, an interleukin-6 receptor monoclonal antibody, is licensed for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, and COVID-19. It has been reported to improve the outcomes of patients with irAEs (11) and is utilized as salvage treatment for anti-MDA5-positive DM with RP-ILD (12). In our case, the administration of tocilizumab may have contributed to clinical remission.
4 Conclusion
We report the first case of a patient with CIP with anti-MDA5 antibody positivity, who transiently responded to treatment with glucocorticoids, immunosuppressants, and tocilizumab. However, he eventually died from dyspnea. Therefore, screening for anti-MDA5 antibodies is warranted in patients with CIP who present with RP-ILD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SP: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. HX: Data curation, Validation, Writing – review & editing. LW: Data curation, Validation, Writing – review & editing. YW: Validation, Writing – review & editing. MZ: Data curation, Resources, Writing – review & editing. YX: Writing – review & editing. XT: Supervision, Validation, Writing – review & editing. JF: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. JW: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National High Level Hospital Clinical Research Funding (grant numbers 2022-PUMCH-A-129, 2022-PUMCH-C-054). These funding sources had no role in the study design or execution, analyses, interpretation of the data, or decision to submit results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum (2005) 52:1571–76. doi: 10.1002/art.21023
2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
3. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
4. Wang H, Guo X, Zhou J, Li Y, Duan L, Si X, et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer (2020) 11:191–97. doi: 10.1111/1759-7714.13240
5. Tomii K, Kato T, Takahashi M, Noma S, Kobashi Y, Enatsu S, et al. Pemetrexed-related interstitial lung disease reported from post marketing surveillance (malignant pleural mesothelioma/non-small cell lung cancer). Jpn J Clin Oncol (2017) 47:350–56. doi: 10.1093/jjco/hyx010
6. Wu W, Guo L, Fu Y. et al.Interstitial lung disease in anti-MDA5 positive dermatomyositis. Clin Rev Allergy Immunol (2021) 60:293–304. doi: 10.1007/s12016-020-08822-5
7. Mammen AL, Allenbach Y, Stenzel W, Benveniste O. 239th ENMC international workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14-16 December 2018. Neuromuscul Disord (2020) 30:70–92. doi: 10.1016/j.nmd.2019.10.005
8. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6:38. doi: 10.1038/s41572-020-0160-6
9. Ghosh N, Chan KK, Jivanelli B, Bass AR. Autoantibodies in patients with immune-related adverse events from checkpoint inhibitors: A systematic literature review. J Clin Rheumatol (2022) 28:e498–505. doi: 10.1097/RHU.0000000000001777
10. Wang G, Wang Q, Wang Y, Liu C, Wang L, Chen H, et al. Presence of anti-MDA5 antibody and its value for the clinical assessment in patients with COVID-19: A retrospective cohort study. Front Immunol (2021) 12:791348. doi: 10.3389/fimmu.2021.791348
11. Campochiaro C, Farina N, Tomelleri A, Ferrara R, Lazzari C, De Luca G, et al. Tocilizumab for the treatment of immune-related adverse events: a systematic literature review and a multicentre case series. Eur J Intern Med (2021) 93:87–94. doi: 10.1016/j.ejim.2021.07.016
Keywords: immune checkpoint inhibitors, immune-related adverse events, pneumonitis, rapidly progressive interstitial lung disease, anti-MDA5 antibodies
Citation: Pan S, Xie H, Wang L, Wang Y, Zou M, Xu Y, Tian X, Fan J and Wang J (2024) Case report: Checkpoint inhibitor pneumonitis with positive anti-melanoma differentiation-associated gene 5 antibodies in a patient with lung cancer. Front. Immunol. 14:1309531. doi: 10.3389/fimmu.2023.1309531
Received: 08 October 2023; Accepted: 20 December 2023;
Published: 12 January 2024.
Edited by:
Xuanye Cao, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Wen Jiang, AstraZeneca, United StatesXiang Zhang, Soochow University, China
Koji Sakamoto, Nagoya University, Japan
Copyright © 2024 Pan, Xie, Wang, Wang, Zou, Xu, Tian, Fan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junping Fan, ZmFuanVucGluZ0BwdW1jaC5jbg==; Jinglan Wang, d2FuZ2ppbmdsYW5AYWxpeXVuLmNvbQ==
 Siqi Pan
Siqi Pan Huaiya Xie1
Huaiya Xie1 Yuanzhuo Wang
Yuanzhuo Wang Menglian Zou
Menglian Zou Yan Xu
Yan Xu Junping Fan
Junping Fan