- 1Department of Hematology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Pathology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
- 3Department of Nuclear Medicine, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
Background: Follicular lymphoma (FL), a common indolent B-cell lymphoma, has the potential to transform into an aggressive lymphoma, such as diffuse large B-cell lymphoma (DLBCL). The outcome of patients with transformed follicular lymphoma (tFL) is poor, especially in patients with transformed lymphoma after chemotherapy and patients with progression within 24 months (POD24). Chimeric antigen receptor (CAR) T-cell therapy combined with autologous stem cell transplantation (ASCT) has promising antitumor efficacy.
Case presentation: Here, we described a 39-year-old male patient who was initially diagnosed with FL that transformed into DLBCL with POD24, CD20 negativity, TP53 mutation, and a bulky mass after 3 lines of therapy, all of which were adverse prognostic factors. We applied a combination approach: CD19 CAR T-cell infusion following ASCT. Ibrutinib was administered continuously to enhance efficacy, DHAP was administered as a salvage chemotherapy, and ICE was administered as a bridging regimen. The patient underwent BEAM conditioning on days -7~ -1, a total of 3.8 × 106/kg CD34+ stem cells were infused on days 01~02, and a total of 108 CAR T cells (relmacabtagene autoleucel, relma-cel, JWCAR029) were infused on day 03. The patient experienced grade 2 cytokine release syndrome (CRS), manifesting as fever and hypotension according to institutional standards. There was no immune effector cell-associated neurotoxicity syndrome (ICANS) after CAR T-cell infusion. Finally, the patient achieved CMR at +1 month, which has been maintained without any other adverse effects.
Conclusion: This case highlights the amazing efficacy of CD19 CAR T-cell therapy following ASCT for R/R tFL, thus providing new insight on therapeutic strategies for the future.
Introduction
Follicular lymphoma (FL) is a common indolent B-cell lymphoma, and the expected overall survival potentially extends beyond 20 years (1). However, this disease is highly heterogeneous, and some FLs have the potential to transform into aggressive lymphomas; one such example of transformed follicular lymphoma (tFL) is diffuse large B-cell lymphoma (DLBCL) (2, 3). Transformed DLBCL has a lower complete remission (CR) rate and shorter progression-free survival (PFS) than de novo DLBCL after conventional chemotherapy (4). Progression of tFL within 24 months (POD24) and TP53 mutation are also strongly correlated with a poor outcome (3, 5, 6). There are no standard therapeutic approaches for tFL, and the outcome is poor, especially in those whose transformation occurs early after chemotherapy (2, 7).
Although high-dose chemotherapy combined with autologous stem cell transplantation (HDT-ASCT) is the standard strategy for refractory and relapse (R/R) DLBCL patients who achieve efficacy above partial remission, approximately 50% of patients will not benefit from such treatment (8–10). In recent years, chimeric antigen receptor (CAR) T-cell therapy has emerged as a promising treatment for R/R non-Hodgkin lymphoma (NHL) (11–14). However, the 1-year OS in TP53-altered LBCL was significantly lower than that in TP53 wild-type LBCL (44% versus 76%, P =0.012) treated with CD19 CAR T cells (15). Moreover, primary resistance and relapse after CAR T-cell therapy remain major challenges. The efficacy of ASCT or CAR T-cell therapy alone still needs further improvement. A recent study found that for R/R B-NHL patients with TP53 alterations treated with CAR19/22 T-cell therapy combined with ASCT, the estimated 2-year PFS and OS rates were 77.5% and 89.3%, respectively (5). This suggests that the combination of CAR T-cell therapy with ASCT is worthy of further clinical application due to its high effectiveness, good safety, and beneficial outcomes (5, 16, 17).
Here, we present a patient with R/R CD20-negative tFL with POD24, TP53 mutation and a bulky mass. Complete metabolic remission (CMR) was achieved +1 month after autologous CD19 CAR T-cell infusion following ASCT, thus providing a meaningful combination treatment strategy for R/R tFL.
Case presentation
A 39-year-old Chinese male was diagnosed with FL (grade 3b, stage III, group A, FLIPI was 3, high risk and FLIPI-2 was 2, intermediate risk) in January 2020. Immunohistochemical results for lymphoma cells of left cervical lymph nodes were as follows: BCL6 (+), MUM1 (+), Ki-67 (80%+), CD20 (+), Pax5 (+), BCL2 (+), CD10 (+), CyclinD1 (-), and sparsely positive for CD3, CD5, CD15, CD30, CD68, and C-MYC. EBER by in situ hybridization test was negative in the lymphoma cells. Multiple lymphadenopathies on both sides of the mediastinum with high uptake of 18F-fluorodeoxyglucose (FDG) were revealed by positron emission tomography (PET-CT). The patient obtained CMR by PET-CT evaluation after 3 cycles of R-CHOP (rituximab 375 mg/m2 day 0, cyclophosphamide 750 mg/m2 day 1, pirarubicin 50 mg/m2 day 1, vincristine 1.4 mg/m2 day 1, and prednisone 100 mg day 1-5). After receiving additional 3 cycles of R-CHOP and 1 cycle of maintenance R therapy, PET-CT indicated an enlarged lymph node with high uptake in the right pelvic cavity (SUVmax was 7, Deauville was 5), and the original disease relapsed early. As the patient had no symptoms at the time, he refused further biopsy and treatment.
In March 2021, the patient complained of pain in the back and lower extremities, and further core needle aspiration biopsy of the right inguinal lymph node revealed grade 2 FL. The patient received 1 cycle of BR (rituximab 375 mg/m2 day 0 and bendamustine 90 mg/m2 day 1,2). Although pain relief and thrombosis occurred in his right lower limb, the disease progressed with further enlargement of the soft tissue mass in the bilateral inguinal and right paravascular iliac arteries. Subsequently, 2 cycles of GB ( ortuzumab 1000 mg day 1, 8, 15 for the first cycle and day 1 for the second cycle, bendamustine 90 mg/m2 day 1, 2 for each cycle) were administered. Unfortunately, the disease showed no response to treatment with salvage immunochemotherapy. Then, PET-CT scans showed a bulky mass (a maximum diameter of 84 mm) from the right psoas major muscle to the right pelvic wall with increased FDG uptake (SUVmax was 42.1) and a soft tissue mass in the right groin with increased FDG uptake (SUVmax was 21.2) (Figure 1A). The third pathological investigation of the right paravascular iliac lesion indicated that the lymphoma had transformed into DLBCL (GCB subtype) that was negative for CD20 and positive for Bcl-6 (90%+), MUM1 (90%+), Ki-67 (80%+), Bcl-2 (90%+), c-myc (60%+), CD10 (+), and CD19(+) (Figure 1B). A negative rearrangement of MYC and a positive rearrangement of BCL2 were demonstrated by fluorescence in situ hybridization (FISH). Next-generation sequencing (NGS) of the paraffin-embedded lymphoma tissues was performed using the Illumina high-throughput sequencing platform technology. The results showed that TP53 mutations were always present in 3 pathological specimens from different sites (Table 1). Moreover, there were more than 10 kinds of gene mutations, including TP53, KMT2D, MS4A1, CD83, DUSP2, EZH2, FOXO1, HIST1H1C, HIST1H1E, NFKBIA, SGK1, SOCS1, STAT3, and TBL1XR1.
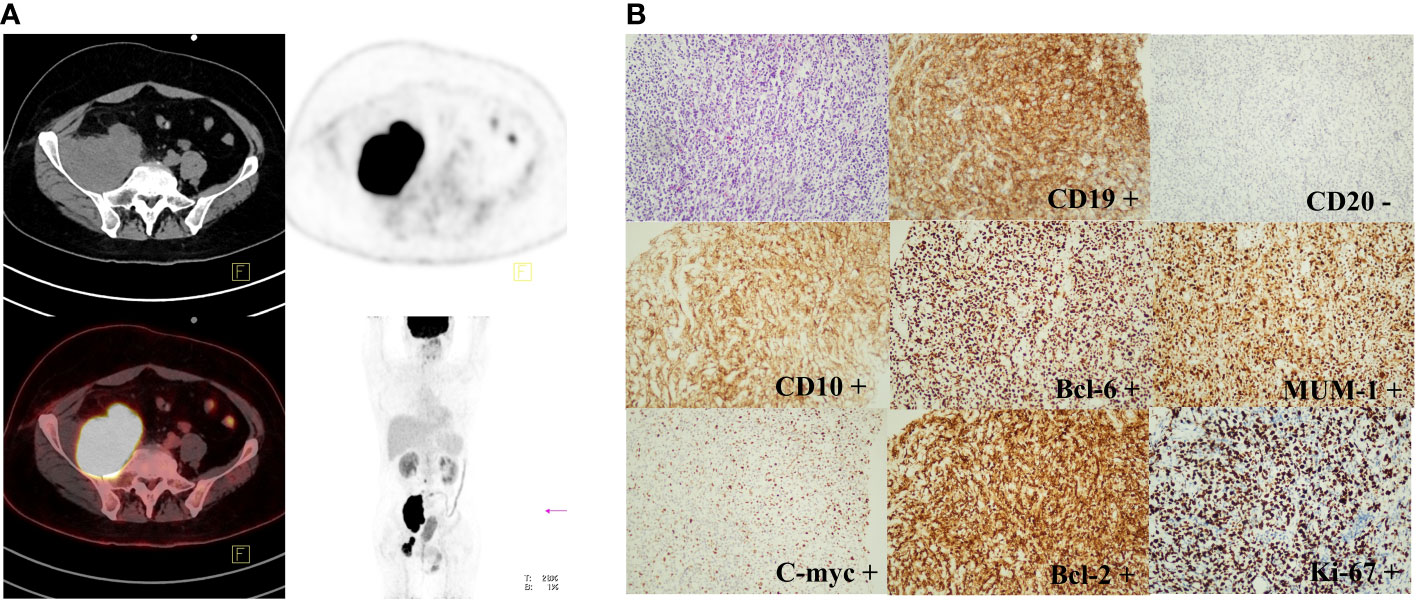
Figure 1 PET-CT scan and immunohistochemical staining results of FL transforming into DLBCL (GCB subtype). (A): PET-CT imaging showed a bulky mass from the right psoas major muscle to the right pelvic wall (SUVmax was 42.1) and a soft tissue mass in the right groin (SUVmax was 21.2). (B): The immunohistochemical staining results indicated that FL had transformed into DLBCL (GCB subtype) with CD20 negative, Bcl-6 (90%+), MUM1 (90%+), Ki-67 (80%+), Bcl-2 (90%+), c-myc (60%+), CD10 (+), and CD19 (+). (original magnification, 200×). The thin arrows represent the original comparable lesion locations.
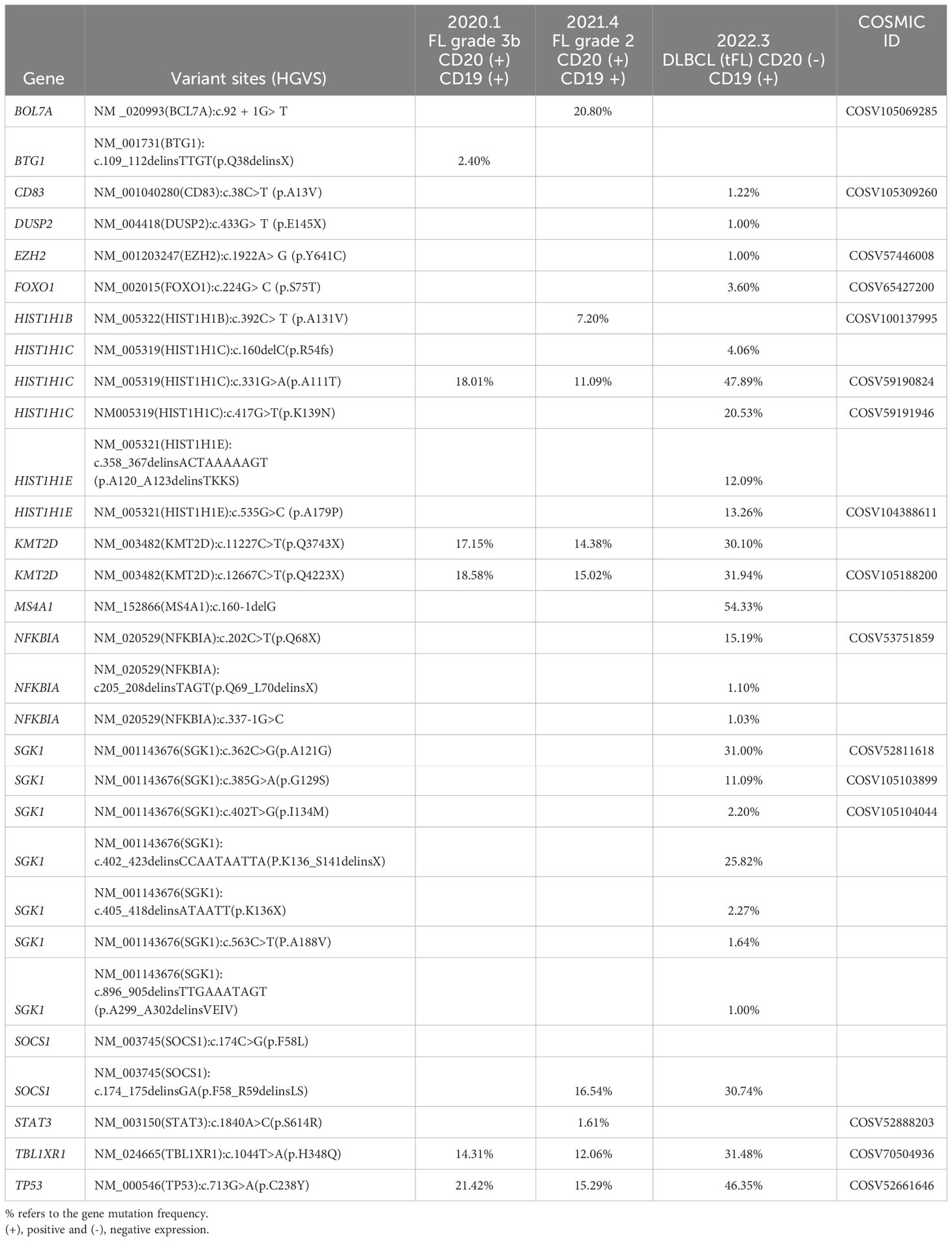
Table 1 Gene mutations revealed by next-generation sequencing (NGS) of the paraffin-embedded lymphoma tissues in 3 pathological specimens of the present patient.
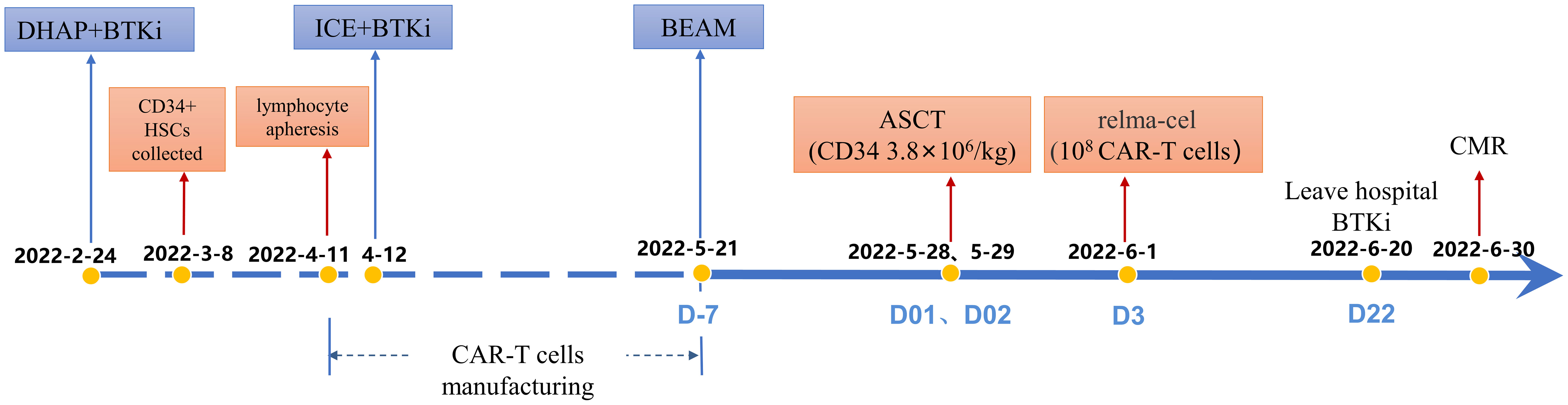
The patient received CD19 CAR T-cell infusion following ASCT therapy. Auto hematopoietic stem cells were collected after 1 cycle of DHAP (dexamethasone 40 mg day 1-4, cisplatin 100 mg/m2 day 1, cytarabine 2 g/m2 q12h day 2) with the BTK inhibitor ibrutinib (560 mg/day) administered continuously as salvage chemotherapy. One month later, autolymphocyte apheresis was performed to manufacture CAR T cells. During the production of CD19 CAR T cells, 1 cycle of ICE (ifosfamide 5000 mg/m2 day 2, carboplatin 737 mg day 2, etoposide 100 mg/m2 day 1-3) was administered as a bridging regimen. PET-CT evaluation before ASCT and CAR T-cell infusion indicated PD with a novel intraperitoneal tumor mass near the posterior abdominal wall and other remaining masses (Figure 2A). The patient underwent BEAM conditioning (carmustine 300 mg/m2 day -7, etoposide 200 mg/m2 day -6~-3, cytarabine 200 mg/m2 q12h day -6~-3, and melphalan 140 mg/m2 day -2) and ASCT following CD19 CAR T-cell infusion. On May 28, 2022 (day 01), and May 29, 2022 (day 02), a total of 3.8 × 106/kg CD34+ stem cells were infused. The patient received an infusion of CD19 CAR T cells (relmacabtagene autoleucel, relma-cel, JWCAR029, 2.7 ml containing 108 CAR T cells) on June 1, 2022 (day 3). Platelets were 35×109/L, implanted on June 18, 2022 (day 20); neutrophils were 1.52×109/L implanted on June 19, 2022 (day 21); lymphocytes were 1.6×109/L on July 7, 2022 (day 39). CD4 was 374/ul, IgG 5.57g/L, IgA 0.25g/L, IgM 0.12g/L on July 7, 2022 (day 39).

Figure 2 PET-CT evaluation before and after ASCT and CAR T-cell infusion. (A): PET-CT examination demonstrated PD before ASCT and CAR T-cell infusion. (B): PET-CT examination demonstrated CMR in the +1 month after ASCT and CAR T-cell therapy. (C): PET-CT examination demonstrated sustained CMR in the +3 month after ASCT and CAR T-cell therapy. The thick arrows represent the positive lesions; thin arrows represent the original comparable lesion locations.
On the 4th day after ASCT (the 1st day after CAR T-cell infusion), the patient’s oral mucosa showed pseudomembranes and ulcers, and he had diarrhea 3-5 times a day but was able to eat and swallow liquid diet. Therefore, a diagnosis of mucositis grade 2 was made, which lasted for 6 days and gradually recovered. Additionally, on the 2nd day after CAR T-cell infusion, the patient developed a fever with a temperature of approximately 38.5°C and was not effectively treated with nonsteroidal antipyretic analgesic drugs and antibiotics. Repeated bacterial blood cultures were negative. On the 4th day after CAR T-cell infusion, the patient’s blood pressure was 88/49 mmHg (the IL-6 level was 659.7 pg/ml) and was not effectively treated with rapid fluid replenishment. The patient was diagnosed with CRS grade 2 and treated with one dose of 8 mg/kg tocilizumab. His blood pressure gradually returned to normal within a few hours, and his temperature gradually returned to normal within 2 days. There was no ICANS after CAR T-cell infusion.
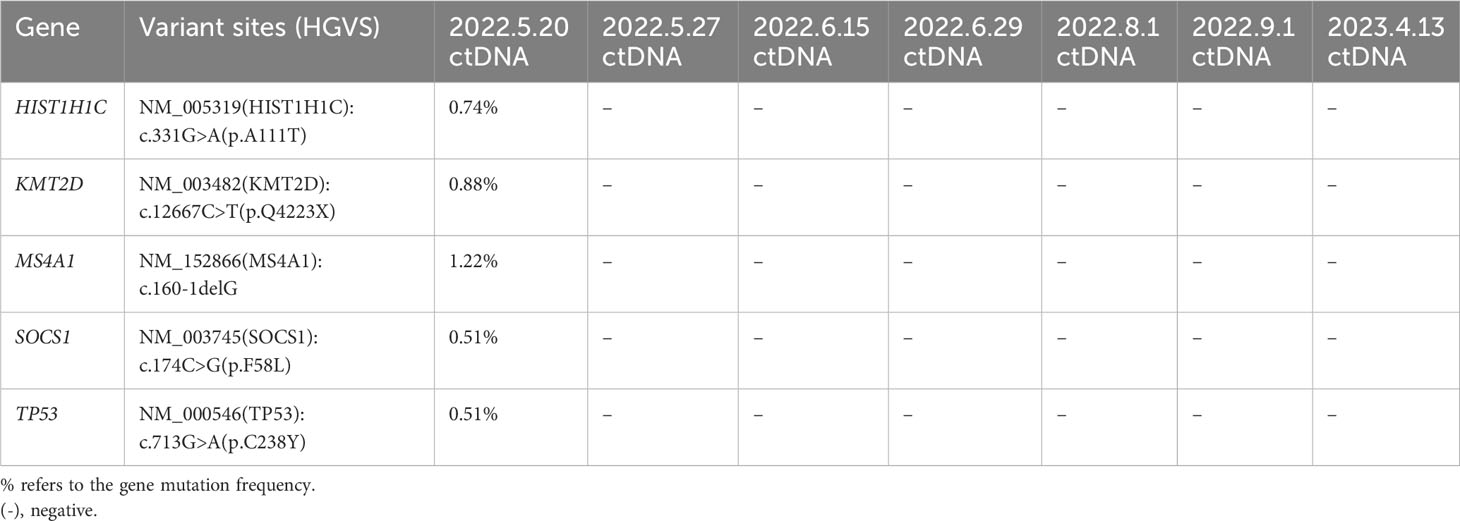
Surprisingly, PET-CT examination demonstrated CMR in the +1 month after CD19 CAR T-cell infusion following ASCT (Figure 2B). At the last follow-up, the patient received ibrutinib as maintenance therapy and remained in CMR (Figure 2C). Importantly, the circulating tumor DNA (ctDNA) results indicated that the mutations of TP53, KMT2D, MS4A1, SOCS1 and HIST1H1C were persistently negative after CD19 CAR T-cell infusion following ASCT (Table 2). The timeline of treatment is shown in Figure 3.
Discussion
Histologic transformation of FL to DLBCL occurs in 10%–70% of patients over time, with a risk of 2% - 3% per year; this transformation is associated with an increased rate of mortality, especially in patients who progress early after immunochemotherapy (2, 18, 19). However, prospective randomized studies or clinical trials always exclude patients with transformed lymphoma, and there is no standard therapy strategy to guide practice in the modern era. The treatment choice is often individualized depending on the previous treatment history (2). For patients who are anthracycline naïve, R-CHOP or other anthracycline-based therapy approaches are suggested, which could yield a similar response to patients with de novo DLBCL. Patients with anthracycline exposure could benefit from salvage chemotherapy and consolidative ASCT. However, the role of ASCT in tFL patients with bendamustine exposure remains unclear (2). Hence, the optimal treatment strategies to overcome the poor prognosis of these transformed patients have yet to be determined.
In our present study, the patient received R-CHOP as initial therapy and BR and GB as salvage chemotherapy, but no effects were observed. NGS of the lymphoma tissue showed an MS4A1 gene mutation that resulted in the loss of CD20 expression, which is most likely the reason that BR and GB failed (20). Moreover, the disease transformed to DLBCL with both anthracycline and bendamustine exposure, which poses challenges in selecting therapy approaches. First, in terms of clinical manifestations, the patient underwent POD24 and transformation early after the third line of therapies, with a bulky tumor mass and higher serum LDH level. All of these characteristics portended a poor prognosis (21). Second, in terms of pathological features, the disease transformed to DLBCL with double expression of Bcl-2 and c-myc and negative CD20 expression. The CD20 level was proven to be an independent factor of poor prognosis in newly diagnosed DLBCL patients (22). A study showed that for R/R DLBCL, 26.3% (5/19) of patients were confirmed to be CD20 negative according to posttreatment rebiopsy. The OS of all 5 patients was less than 11 months from CD20-negative transformation (23). The other study found that CD20 loss occurred in 16% of R/R FL patients, whose median OS was significantly shorter than that of CD20-positive patients (8.9 months vs. 28.3 months) (24). These results indicated that CD20 loss was related to a poor prognosis of B-NHL. Third, in genomic profiles, there were more than 10 gene mutations, including TP53, KMT2D, MS4A1, CD83, DUSP2, EZH2, FOXO1, HIST1H1C, HIST1H1E, NFKBIA, SGK1, SOCS1, STAT3, and TBL1XR1, when the disease underwent histologic transformation. Mutations in TP53, KMT2D, HIST1H1C and TBL1XR1 always existed from onset to transformation. TP53 confers lymphomas with poor outcomes, which cannot be overcome by chemotherapy or HSCT (5). Even in the era of cellular therapy, TP53 mutations and/or copy number alterations were independent factors correlated with lower CR and shorter OS for R/R DLBCL patients treated with CD19 CAR T cells. The 1-year OS was 44% in TP53-altered patients and 76% in TP53 wild-type patients (15). Therefore, the treatment strategy for our present case should be explored innovatively to improve the prognosis to the greatest extent.
For relapsed or refractory lymphomas, high-dose chemotherapy and ASCT (HDT-ASCT) following salvage chemotherapy is a common strategy, especially for those who achieve a partial remission (PR) after salvage chemotherapy (8). However, there are few therapeutic options for patients who are chemoresistant, resulting in lower response rates and shorter OS. In recent years, CAR T-cell therapy has emerged as a revolutionary treatment for R/R NHL to improve prognosis. CAR T cells are preferred as the first cellular immunotherapy with a lower nonrelapse mortality rate and similar relapse incidence, progression-free survival (PFS) and OS rates compared with allo-HCT for multiple R/R DLBCL (10). There have been three FDA-approved CAR T-cell products for R/R DLBCL or FL. For FL patients, the CR rates of CD19 CAR T-cell therapy were approximately 60%-65.4% (14, 25). The efficacy of CD19 CAR T-cell therapy for R/R DLBCL was inferior, with 44%-58% CR and 44%-65% 1-year PFS (11–13). Notably, for more than half of the enrolled patients with POD24, the CR rates (CRR) were 55% and 59% in the ZUMA-5 and ELARA trials, respectively (14, 25). Importantly, the JULIET, ZUMA-1 and TRANSCEND studies reported that patients with transformed follicular lymphoma achieved a notable duration of response. Compared with de novo/primary DLBCL, transformed/secondary DLBCL is associated with a more favorable outcome, including higher CRR, PFS, OS and lower mortality rates after CAR T-cell therapy (26). In the ZUMA-7 study, compared with standard care, axi-cel therapy led to significant improvements with higher response (83% vs. 50%) and CRR (65% vs. 32%), and it resulted in longer median event-free survival (EFS) in axi-cel therapy (8.3 months vs. 2.0 months), and the 24-month EFS was 41% and 16%, respectively (27). The real-world data suggested that the primary efficacy of CAR T-cell therapy in DLBCL with 32-66% CRR still has significant room for improvement (28). In addition, the durable remission rate is much lower at 30 to 40%. Furthermore, relapse after CAR T-cell therapy remains an important problem (28).
Recently, the efficacy of CAR T-cell therapy combined with HDT-ASCT has been confirmed in several clinical studies. Compared with CAR T cells alone, the ORR, CRR and long-term outcome were significantly improved after CAR T-cell infusion following ASCT therapy (5, 29). Compared with ASCT alone, significantly higher CRR and 3-year PFS rates and lower rates of 3-year relapse/progression were achieved after CAR T-cell infusion following ASCT (16). TP53 genomic alterations have been shown to be associated with inferior CRR and OS rates in multivariable regression models among patients with LBCL treated with CD19 CAR T-cell therapy (15). However, combining CAR T cells with ASCT is effective for R/R aggressive B-NHL with TP53 alterations, leading to long-term outcomes (5). Sandwich therapy of CAR T cells combined with ASCT could lead to long-term survival in patients with R/R Burkitt’s lymphoma with TP53 mutations (30). An open-label single-arm prospective clinical study demonstrated that ASCT and sequential CD19/CD22 CAR T-cell cocktail therapy showed high CRR in R/R aggressive B-NHL, which was ineffective with chemotherapy (17). The safety of CAR T-cell therapy combined with ASCT was proven not only in case report studies but also in larger sample studies (5, 17, 30). In a single-arm study, grade 3 CRS occurred in only 2 patients (2/42), and grade 3 neurotoxicity occurred in 5% of patients (17). Compared with CAR19/22 T-cell cocktail treatment, CAR19/22 T-cell treatment in combination with ASCT reduced the occurrence of severe CRS (10.7% vs. 37.5%) and similar incidence rates of ICANS (9.1% vs. 19.3%) (5). These results indicated that the adverse events in these studies were manageable and reversible for most patients.
Relma-cel is a CD19-targeted, second-generation CAR T-cell product with a 4-1BB costimulatory domain manufactured in China. A multicenter trial conducted in China demonstrated its efficacy and safety in R/R DLBCL patients (31). Surprisingly, the patient received Relma-cel following ASCT and achieved CR at +1 month. There are several potential synergistic mechanisms of the CAR T cell-therapy combined with ASCT strategy. First, ASCT could eradicate lymphoma cells nonselectively to reduce tumor load. Second, myeloablative conditioning improves the bone marrow microenvironment by restraining immunosuppressive elements such as monocytes and macrophages (32). Third, the effect of ASCT on lymphodepletion is superior to that of traditional fludarabine and cyclophosphamide, and CAR T cells that are highly activated during hematopoietic reconstitution could further eliminate residual tumors (5, 33). The patient experienced grade 2 CRS and no ICANS, which indicates the good safety of this treatment strategy (34). The findings of the present case study suggest the high efficacy and good safety of combined ASCT and CAR T-cell therapies, which provide a novel approach for such patients. However, the follow-up time was relatively short, and long-term observation is needed to confirm the enduring efficacy of the combination therapy.
In some literature, the synergistic effect of ibrutinib and CAR-T cells has been studied, which indicated that the previous or concurrent ibrutinib treatment might overcome the resistance of CAR-T therapy (35, 36). Ibrutinib could effectively restore the numbers and function of T cells via inhibition of interleukin-2 inducible T cell kinase, which has a positive effect on anti-CD19/CD3 bispecific antibodies and CAR T cells (37–39). In chronic lymphocytic leukemia, ibrutinib increased the proportion of CAR T cells with less-differentiated naïve-like phenotype and also inhibited the expression of exhaustion markers to enhance CAR T cell function (40, 41). In R/R NHL or CLL, supplement of ibrutinib or acalabrutinib also improved the CAR T function, and ibrutinib causing the emergence of type 1 T-helper memory-like T-cell phenotype, which was not found with acalabrutinib (41, 42). Furthermore, ibrutinib could inhibit malignant B cells homing to spleens and lymph nodes through reducing homing chemokines CXCR4, which resulted tumor cells moving into the circulation and are killed by CAR T cells (43). The addition ibrutinib to CART for mantle cell lymphoma led to better response and longer survival in a xenograft mice model (44, 45). Take the above into consideration, we supplemented ibrutinib to combinatorial ASCT and CAR T therapies in our patient with a surprising efficacy.
In summary, we presented the first successful case of combination therapy with ASCT and CAR T cells for the treatment of relapsed and refractory CD20-negative tFL with TP53 mutation and a bulky mass. Our study highlights the potential therapeutic strategy in tFL, which deserves further investigation in a large population in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First Affiliated Hospital of China Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Writing – original draft. DC: Writing – original draft. RG: Writing – review & editing. YM: Data curation, Writing – review & editing. YC: Data curation, Writing – review & editing. ZL: Investigation, Writing – review & editing. HZ: Investigation, Writing – review & editing. XY: Supervision, Writing – review & editing. NS: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cartron G, Trotman J. Time for an individualized approach to first-line management of follicular lymphoma. Haematologica (2022) 107(1):7–18. doi: 10.3324/haematol.2021.278766
2. Smith S. Transformed lymphoma: what should I do now? Hematol Am Soc Hematol Educ Program (2020) 2020(1):306–11. doi: 10.1182/hematology.2020000115
3. Casulo C, Herold M, Hiddemann W, Iyengar S, Marcus RE, Seymour JF, et al. Risk factors for and outcomes of follicular lymphoma histological transformation at first progression in the GALLIUM study. Clin lymphoma myeloma leukemia (2023) 23(1):40–8. doi: 10.1016/j.clml.2022.09.003
4. Desai S, Chaturvedi M, Hameed R, Baez-Sosa V, Shenoy AG. Single-center analysis of characteristics and outcomes of de novo, concurrent, and transformed diffuse large B-cell lymphoma. oncologist (2021) 26(9):e1660–e3. doi: 10.1002/onco.13846
5. Wei J, Xiao M, Mao Z, Wang N, Cao Y, Xiao Y, et al. Outcome of aggressive B-cell lymphoma with TP53 alterations administered with CAR T-cell cocktail alone or in combination with ASCT. Signal transduction targeted Ther (2022) 7(1):101. doi: 10.1038/s41392-022-00924-0
6. Kridel R, Chan FC, Mottok A, Boyle M, Farinha P, Tan K, et al. Histological transformation and progression in follicular lymphoma: A clonal evolution study. PloS Med (2016) 13(12):e1002197. doi: 10.1371/journal.pmed.1002197
7. Alonso-Álvarez S, Magnano L, Alcoceba M, Andrade-Campos M, Espinosa-Lara N, Rodríguez G, et al. Risk of, and survival following, histological transformation in follicular lymphoma in the rituximab era. A retrospective multicentre study by the Spanish GELTAMO group. Br J haematol (2017) 178(5):699–708. doi: 10.1111/bjh.14831
8. Shah NN, Ahn KW, Litovich C, He Y, Sauter C, Fenske TS, et al. Is autologous transplant in relapsed DLBCL patients achieving only a PET+ PR appropriate in the CAR T-cell era? Blood (2021) 137(10):1416–23. doi: 10.1182/blood.2020007939
9. Shadman M, Pasquini M, Ahn KW, Chen Y, Turtle CJ, Hematti P, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood (2022) 139(9):1330–9. doi: 10.1182/blood.2021013289
10. Dreger P, Dietrich S, Schubert ML, Selberg L, Bondong A, Wegner M, et al. CAR T cells or allogeneic transplantation as standard of care for advanced large B-cell lymphoma: an intent-to-treat comparison. Blood advances (2020) 4(24):6157–68. doi: 10.1182/bloodadvances.2020003036
11. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
12. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. New Engl J Med (2019) 380(1):45–56. doi: 10.1056/NEJMoa1804980
13. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet (London England) (2020) 396(10254):839–52. doi: 10.1016/S0140-6736(20)31366-0
14. Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol (2022) 23(1):91–103. doi: 10.1016/S1470-2045(21)00591-X
15. Shouval R, Alarcon Tomas A, Fein JA, Flynn JR, Markovits E, Mayer S, et al. Impact of TP53 genomic alterations in large B-cell lymphoma treated with CD19-chimeric antigen receptor T-cell therapy. J Clin Oncol (022) 40(4):369–81. doi: 10.1200/JCO.21.02143
16. Wang T, Xu L, Gao L, Tang G, Chen L, Chen J, et al. Chimeric antigen receptor T-cell therapy combined with autologous stem cell transplantation improved progression-free survival of relapsed or refractory diffuse large B-cell lymphoma patients: A single-center, retrospective, cohort study. Hematological Oncol (2022) 40(4):637–44. doi: 10.1002/hon.2975
17. Cao Y, Xiao Y, Wang N, Wang G, Huang L, Hong Z, et al. CD19/CD22 chimeric antigen receptor T cell cocktail therapy following autologous transplantation in patients with relapsed/refractory aggressive B cell lymphomas. Transplant Cell Ther (2021) 27(11):910.e1–.e11. doi: 10.1016/j.jtct.2021.08.012
18. Fischer T, Zing NPC, Chiattone CS, Federico M, Luminari S. Transformed follicular lymphoma. Ann hematol (2018) 97(1):17–29. doi: 10.1007/s00277-017-3151-2
19. Rusconi C, Anastasia A, Chiarenza A, Marcheselli L, Cavallo F, Rattotti S, et al. Outcome of transformed follicular lymphoma worsens according to the timing of transformation and to the number of previous therapies. A retrospective multicenter study on behalf of Fondazione Italiana Linfomi (FIL). Br J haematol (2019) 185(4):713–7. doi: 10.1111/bjh.15816
20. Rushton CK, Arthur SE, Alcaide M, Cheung M, Jiang A, Coyle KM, et al. Genetic and evolutionary patterns of treatment resistance in relapsed B-cell lymphoma. Blood advances (2020) 4(13):2886–98. doi: 10.1182/bloodadvances.2020001696
21. Jacobsen E. Follicular lymphoma: 2023 update on diagnosis and management. Am J hematol (2022) 97(12):1638–51. doi: 10.1002/ajh.26737
22. Suzuki Y, Yoshida T, Wang G, Togano T, Miyamoto S, Miyazaki K, et al. Association of CD20 levels with clinicopathological parameters and its prognostic significance for patients with DLBCL. Ann hematol (2012) 91(7):997–1005. doi: 10.1007/s00277-012-1407-4
23. Hiraga J, Tomita A, Sugimoto T, Shimada K, Ito M, Nakamura S, et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood (2009) 113(20):4885–93. doi: 10.1182/blood-2008-08-175208
24. Michot JM, Buet-Elfassy A, Annereau M, Lazarovici J, Danu A, Sarkozy C, et al. Clinical significance of the loss of CD20 antigen on tumor cells in patients with relapsed or refractory follicular lymphoma. Cancer Drug resistance (Alhambra Calif) (2021) 4(3):710–8. doi: 10.20517/cdr.2020.109
25. Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med (2022) 28(2):325–32. doi: 10.1038/s41591-021-01622-0
26. Nydegger A, Novak U, Kronig MN, Legros M, Zeerleder S, Banz Y, et al. Transformed lymphoma is associated with a favorable response to CAR-T-cell treatment in DLBCL patients. Cancers (2021) 13(23):6073. doi: 10.3390/cancers13236073
27. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. New Engl J Med (2022) 386(7):640–54. doi: 10.1056/NEJMoa2116133
28. Xiao X, Wang Y, Zou Z, Yang Y, Wang X, Xin X, et al. Combination strategies to optimize the efficacy of chimeric antigen receptor T cell therapy in haematological Malignancies. Front Immunol (2022) 13:954235. doi: 10.3389/fimmu.2022.954235
29. Xue F, Zheng P, Liu R, Feng S, Guo Y, Shi H, et al. The autologous hematopoietic stem cells transplantation combination-based chimeric antigen receptor T-cell therapy improves outcomes of relapsed/refractory central nervous system B-cell lymphoma. J Oncol (2022) 2022:2900310. doi: 10.1155/2022/2900310
30. Zhang Q, Zhu X, Liu B, Zhang Y, Xiao Y. Case report: Sandwich therapy of CAR-T combined with ASCT: Sequential CAR-T cell therapy with ASCT after remission with CAR-T therapy caused long-term survival in a patient with relapsed/refractory Burkitt’s lymphoma with TP53 mutations. Front Immunol (2023) 14:1127868. doi: 10.3389/fimmu.2023.1127868
31. Ying Z, Yang H, Guo Y, Li W, Zou D, Zhou D, et al. Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China. Cancer Med (2021) 10(3):999–1011. doi: 10.1002/cam4.3686
32. Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol (2005) 17(2):195–201. doi: 10.1016/j.coi.2005.02.002
33. Amini L, Silbert SK, Maude SL, Nastoupil LJ, Ramos CA, Brentjens RJ, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol (2022) 19(5):342–55. doi: 10.1038/s41571-022-00607-3
34. Dong N, Lopes-Garcia LR, Viñal D, Bachmeier C, Shah BD, Nishihori T, et al. Outcomes of CD19-directed chimeric antigen receptor T-cell therapy for transformed non-follicular lymphoma. Transplant Cell Ther (2023) 29(6):349.e1–8. doi: 10.1016/j.jtct.2023.02.021
35. Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood (2020) 135(19):1650–60. doi: 10.1182/blood.2019002936
36. Liu M, Wang X, Li Z, Zhang R, Mu J, Jiang Y, et al. Synergistic effect of ibrutinib and CD19 CAR-T cells on Raji cells in vivo and in vitro. Cancer Sci (2020) 111(11):4051–60. doi: 10.1111/cas.14638
37. Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood (2013) 122(15):2539–49. doi: 10.1182/blood-2013-06-507947
38. Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood (2016) 127(9):1117–27. doi: 10.1182/blood-2015-11-679134
39. Mhibik M, Gaglione EM, Eik D, Kendall EK, Blackburn A, Keyvanfar K, et al. BTK inhibitors, irrespective of ITK inhibition, increase efficacy of a CD19/CD3-bispecific antibody in CLL. Blood (2021) 138(19):1843–54. doi: 10.1182/blood.2020009686
40. Fan F, Yoo HJ, Stock S, Wang L, Liu Y, Schubert ML, et al. Ibrutinib for improved chimeric antigen receptor T-cell production for chronic lymphocytic leukemia patients. Int J cancer (2021) 148(2):419–28. doi: 10.1002/ijc.33212
41. Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest (2017) 127(8):3052–64. doi: 10.1172/JCI89756
42. Qin JS, Johnstone TG, Baturevych A, Hause RJ, Ragan SP, Clouser CR, et al. Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J immunother (Hagerstown Md: 1997) (2020) 43(4):107–20. doi: 10.1097/CJI.0000000000000307
43. Chen SS, Chang BY, Chang S, Tong T, Ham S, Sherry B, et al. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia (2016) 30(4):833–43. doi: 10.1038/leu.2015.316
44. Ruella M, Kenderian SS, Shestova O, Fraietta JA, Qayyum S, Zhang Q, et al. The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T cells (CART19) improves responses against mantle cell lymphoma. Clin Cancer Res (2016) 22(11):2684–96. doi: 10.1158/1078-0432.CCR-15-1527
Keywords: transformed follicular lymphoma, diffuse large B-cell lymphoma, TP53 mutation, chimeric antigen receptor T-cell, autologous stem cell transplantation
Citation: Zhang J, Cai D, Gao R, Miao Y, Cui Y, Liu Z, Zhang H, Yan X and Su N (2023) Case Report: CD19 CAR T-cell therapy following autologous stem cell transplantation: a successful treatment for R/R CD20-negative transformed follicular lymphoma with TP53 mutation. Front. Immunol. 14:1307242. doi: 10.3389/fimmu.2023.1307242
Received: 04 October 2023; Accepted: 21 November 2023;
Published: 08 December 2023.
Edited by:
Ingo Schmidt-Wolf, University Hospital Bonn, GermanyReviewed by:
Joaquim Carreras, Tokai University, JapanKentaro Minagawa, The Pennsylvania State University, United States
Copyright © 2023 Zhang, Cai, Gao, Miao, Cui, Liu, Zhang, Yan and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojing Yan, eWFueGlhb2ppbmdfcHBAaG90bWFpbC5jb20=; Nan Su, YmVlcGVyY2hpbGRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jinjing Zhang
Jinjing Zhang Dali Cai
Dali Cai Ran Gao1
Ran Gao1 Yan Cui
Yan Cui Zhenghua Liu
Zhenghua Liu Xiaojing Yan
Xiaojing Yan Nan Su
Nan Su