- 1Department of Gynecology and Obstetrics, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China
- 2Liver Disease Center, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China
- 3Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China
- 4International Peace Maternity & Child Health Hospital Affiliated to Jiaotong University, Shanghai, China
- 5Department of Fetal Medicine & Prenatal Diagnosis Center, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, China
- 6Scientific Research Center, Shanghai Public Health Clinical Center, Shanghai, China
- 7Department of Infectious Disease, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China
- 8Department of Respiratory, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China
Background: Large sample of pregnant women vaccinated with COVID-19 vaccine has not been carried out in China. The objective of this study was to evaluate the safety and effectiveness of COVID-19 inactivated vaccine in pregnant women infected with the SARS-CoV-2 Omicron variant.
Methods: A total of 1,024 pregnant women and 120 newborns were enrolled in this study. 707 pregnant women received one to three doses of the inactivated COVID-19 vaccine, and 317 unvaccinated patients served as the control group. A comparison was made between their clinical and laboratory data at different stages of pregnancy.
Results: The incidence rate of patients infected with Omicron variant in the first, the second, and the third trimesters of pregnancy was 27.5%, 27.0%, and 45.5% in patients during, respectively. The corresponding length of hospital stay was 8.7 ± 3.3 days, 9.5 ± 3.3 days, and 11 ± 4.3 days, respectively. The hospitalization time of pregnant women who received 3 doses of vaccine was (8.8 ± 3.3) days, which was significantly shorter than that of non-vaccinated women (11.0 ± 3.9) days. (P<0.0001). The positive rate of SARS-CoV-2 IgG in patients in the early stage of pregnancy was 28.8%, while that in patients in the late stage of pregnancy was 10.3%. However, three-doses of vaccination significantly increased the SARS-CoV-2 IgG positive rate to 49.5%. The hospitalization time of SARS-CoV-2 IgG-positive patients was shorter than that of negative patients (9.9 ± 3.5 days), which was 7.4 ± 2.0 days. 12.2% of vaccinated women experienced mild adverse reactions, manifested as fatigue (10.6%) and loss of appetite (1.6%). The vaccination of mother did not affect her choice of future delivery mode and the Apgar score of their newborn. All newborns tested negative for SARS-CoV-2 nucleic acid, as well as for IgG and IgM antibodies.
Conclusions: Women in the third trimester of pregnancy are highly susceptible to infection with the Omicron strain. The vaccination of pregnant women with COVID-19 vaccine can accelerate the process of eliminating SARS-CoV-2 virus, and is considered safe for newborns. The recommended vaccination includes three doses.
Introduction
Since the 2019 coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection was first announced in January 2020, the number of confirmed cases and their related incidence rate and mortality have increased rapidly (1). According to the COVID-19 Excess Mortality Collaborators’ modeling, from January 1, 2020 to December 31, 2021, about 18 million people around the world have died of COVID-19, which is three times the official report (2). Since the emergence of the D614G variant, SARS-CoV-2 has continued to mutate, resulting in the development of various mutated strains. The World Health Organization (WHO) declared these variants as Variant of Concern (VOC) due to their increased public threat. Among them, Delta and Omicron strains are the most concerned. In particular, the Omicron variant has emerged as a global epidemic virus and exhibits higher infectivity than other variants, but with less virulence (3). As of July 2, 2022, the Omicron variant has caused 649,657 infections in Shanghai, China (4). The phylogenetic characteristics of the SARS-CoV-2 genomes from 129 patients confirmed the current Omicron BA.2.2 sub-lineage pandemic in Shanghai (5).
Pregnant women have always been considered a high-risk group, as they are particularly susceptible to respiratory pathogens and severe pneumonia during pregnancy due to the physiological and immunological changes, such as diaphragm elevation, increased oxygen consumption, and airway mucosal edema. For example, the 1918 influenza pandemic was the most serious pandemic before COVID-19, with a total mortality rate of 2.6% and a maternal mortality rate of 37% (6). Similarly, in 2003, the SARS pandemic led to a mortality rate of 25%, and about 50% of infected pregnant women were treated in the intensive care units (ICUs), and 33% required mechanical ventilation (7). In contrast, pregnant women with COVID-19 were three times more likely to need ICU care, 2.4 times more likely to need extracorporeal membrane oxygenation (ECMO), and 1.7 times more likely to die (8). Therefore, pregnancy serves as a risk factor for severe COVID-19.
Studies have confirmed that vaccination of COVID-19 vaccine can reduce the risk of severe illness caused by COVID-19. At present, the approved vaccines of COVID-19 include mRNA vaccine, adenovirus vector vaccine, and inactivated vaccine. Inactivated vaccines, also known as whole-virus vaccines, have lost their pathogenic ability but retain their immunogenicity. Due to their relative stability and safety, they have become the most frequently used COVID-19 vaccine in China. However, data on the protective effect of COVID-19 vaccine are mainly based on the general population. The vaccination rate of pregnant women is significantly lower than that of non-pregnant women of childbearing age, and no large-scale population studies have been conducted on women who are conceiving, pregnant, or breastfeeding (9). As such, the safety and effectiveness of COVID-19 vaccine for pregnant and lactating women and their offspring are still uncertain.
The purpose of this study was to evaluate the safety and efficacy of inactivated COVID-19 vaccines in a sample of pregnant women infected with the Omicron strain of COVID-19, as well as their newborns. We discovered that patients who received vaccination had rapid clearance of the virus, mild clinical symptoms, and short hospitalization time. In addition, their newborns tested negative for COVID-19. Only a small proportion experienced mild adverse reactions to vaccination. Therefore, we conclude that inactivated vaccines are safe and effective for pregnant women and their newborns, and it is recommended to follow the three-dose vaccination regimen as it is more effective.
Materials and methods
Patients
We conducted a retrospective cohort study of a total of 1024 pregnant women diagnosed with COVID-19 Omicron strain infection by the Shanghai Center for Disease Control and Prevention and hospitalized at the Shanghai Public Health Clinical Center in Shanghai, China from February 28, 2022 to May 3, 2022. All pregnant women included in this study were infected with COVID-19 Omicron strain for the first time due to the zero clearing policy implemented in Shanghai. Most pregnant women in this study were vaccinated first and infected with COVID-19 later. This study was conducted in accordance with the protocol (2022-S067-01) approved by the Ethics Committee of the Shanghai Public Health Clinical Center. According to the Diagnosis and Treatment Plan for Novel Coronavirus Pneumonia (Trial Ninth Edition), the patient’s epidemiological history, clinical manifestations, and laboratory tests were comprehensively evaluated and diagnosed. COVID-19 infection was confirmed by positive results of nucleic acid test. Other patients with lung infections were excluded from the study.
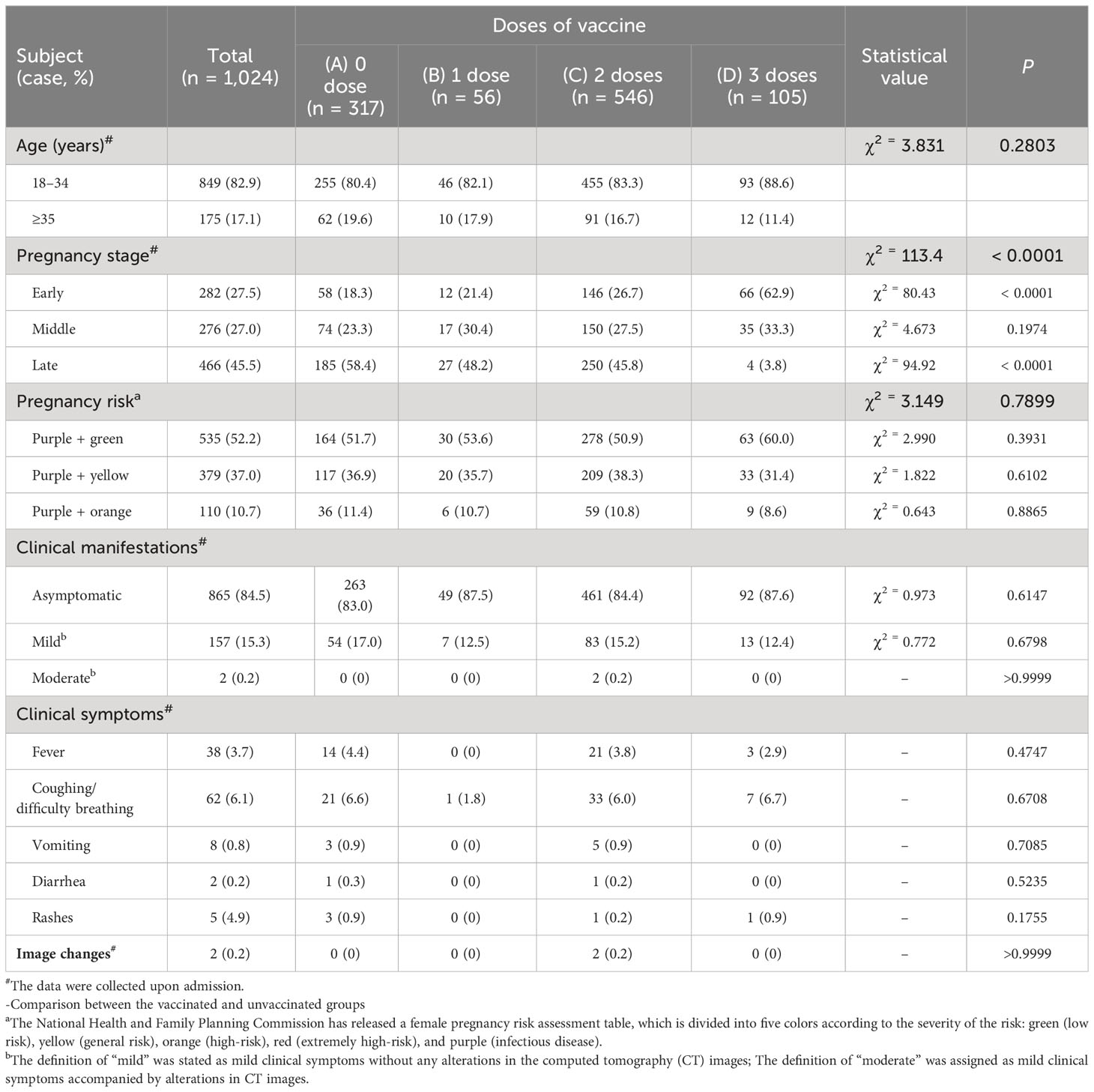
Of the 1,024 patients, 282 were in the early stage of pregnancy, 276 were in the second trimester, and 466 were in the third trimester. Among all cases, 865 were asymptomatic, 157 had mild symptoms, two patients had moderate symptoms, and no patients had severe or critical symptoms. Because of the zero clearing policy implemented in Shanghai, the discharge standard of patients is that COVID-19 nucleic acid turns negative. Patients were discharged if they met all four criteria (1): body temperature remains normal for more than 3 days (2), respiratory symptoms significantly improve (3), lung imaging shows significant improvement in acute exudative lesions, and (4) respiratory specimens have two consecutive negative viral nucleic acid tests (sampling time interval of at least 24 hours) and PCR computed tomography (CT) values of N and ORF genes are ≥35.
A total of 707 patients received one to three doses of inactivated COVID-19 vaccine. The manufacturers of vaccines received by our study cohort include China’s Biological Products Research Institute Co., Ltd., Wuhan Institute of Biological Products Co., Ltd., and Sinovac Biotech Co., Ltd. According to the information provided by the three manufacturers, their production of vaccines proceeded with similar procedures, including the usage of African green monkey kidney Vero cells for virus amplification, the subsequent inactivation step subjecting viruses to effective inactivation while retaining the immunogenicity of the antigen components, and the addition of adjuvant-aluminum hydroxide to boost the immunogenicity of the vaccine. Given that our study subjects received the same type of vaccine produced following a similar manufacturing process, we did not distinguish them according to their manufacturers. As a retrospective study, we also did not use the interval between vaccinations as a distinguishing factor. Within the study cohort, the intervals between the second and first shots had an approximate range of 3-6 weeks, and those between the third and second shots were about 6-12 months. We only grouped patients according to the total number of SARS-CoV-2 vaccines they received. Of the 707 vaccinated patients, 685 were vaccinated before becoming pregnancy, and 22 were vaccinated without knowing they were pregnant. According to the vaccination record, the patients were divided into four groups: unvaccinated group (n = 317), one-dose vaccine group (n = 56), two-dose vaccine group (n = 546), and three-dose vaccine group (n = 105). The time of sample collection was the first blood draw and nucleic acid test during the patient’s admission to the hospital.
Data analysis
All pregnant women infected with the SARS-CoV-2 Omicron strain received standard treatment according to the guidelines provided by the World Health Organization (WHO). Clinical data, including symptoms, chest CT images, and laboratory test results, were collected and compared, as follows: (1) clinical presentations at admission, SARS-CoV-2 nucleic acid PCR CT value, SARS-CoV-2-specific IgG and IgM antibody levels, and other blood biochemical indicators such as transaminase, creatinine, etc. (2) clinical presentation of Omicron-infected patients at different stages of pregnancy; (3) length of hospitalization. In order to determine whether maternal vaccination affects newborns, and whether Omicron infection is transmitted through vertically transmission or breastfeeding, we will analyze the clinical characteristics of their newborns. A multiple linear regression analysis was conducted to examine factors that may affect maternal hospitalization time. (Figures 1A, B).
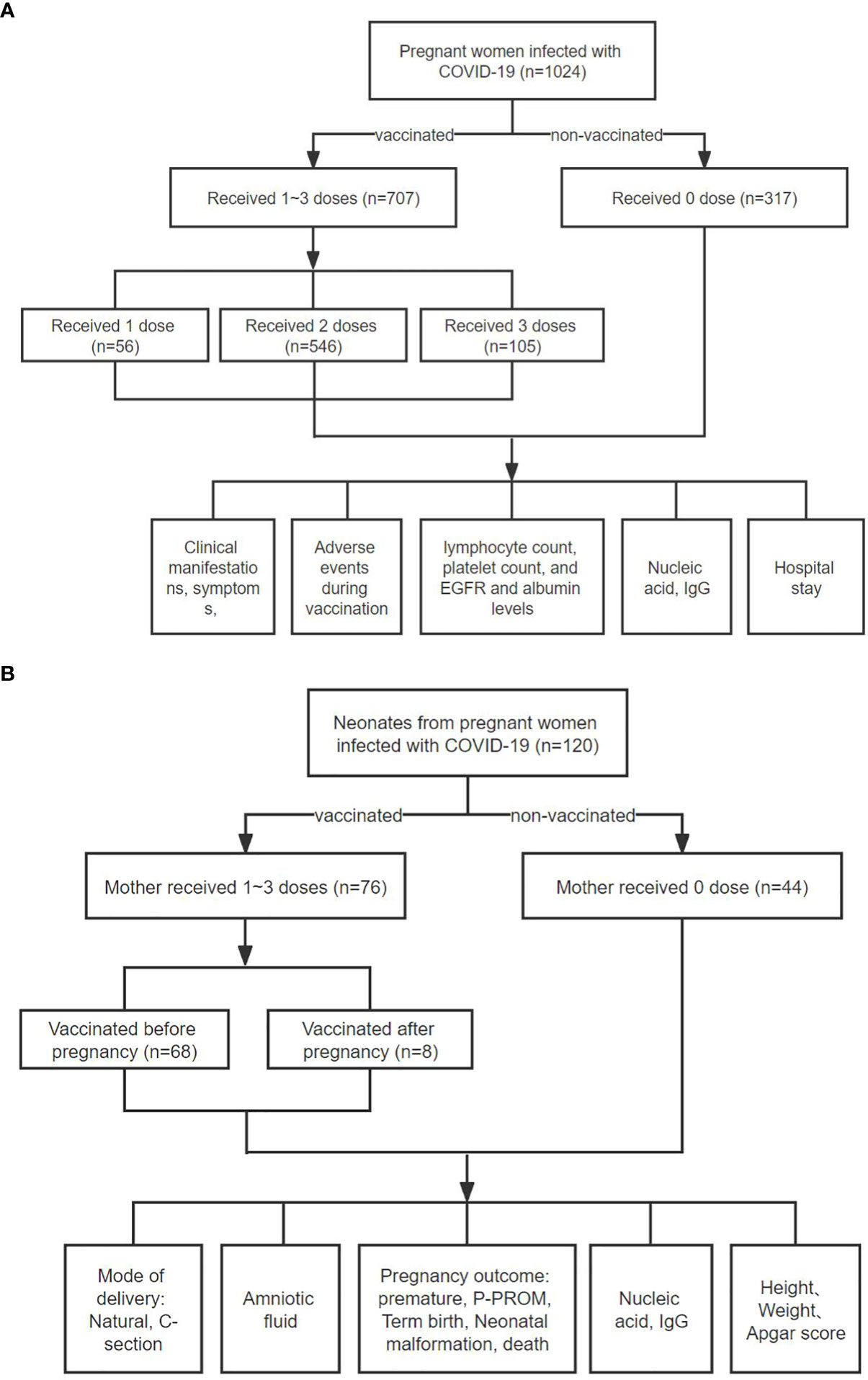
Figure 1 Flowchart of this study. (A) A total of 1,024 pregnant women diagnosed with COVID-19 Omicro infection were selected. According to the number of doses administered, patients were divided into four groups: unvaccinated group (n = 317), one dose vaccine group (n = 56), two-dose vaccine group (n = 546), and three-dose vaccine group (n = 105). Subsequently, we compared various clinical indicators of these groups of pregnant women. (B) A total of 118 COVID-19 patients gave birth to 120 newborns during hospitalization. Among them, 76 patients were vaccinated with inactivated COVID-19 vaccine, and 42 patients were not vaccinated. Among 76 vaccinated patients, 68 were vaccinated before pregnancy and 8 were vaccinated during pregnancy. Subsequently, we compared the pregnancy and neonatal outcomes between the two groups of patients, such as mode of delivery, newborn’s weight and height, etc. Here, the term “Mother” is conveniently used throughout the flow chart, although at the initial vaccination, the subjects were either mothers-to-be or not yet pregnant.
Statistics
The statistical analysis was carried out using the SPSS 25.0 software. The skewed distribution of the measurement data was denoted as M (Q1, Q3). The Mann–Whitney U test was utilized to analyze inter-group comparisons of continuous numerical variables with non-normal distribution. Counting data was expressed as the number of examples (percentages). The categorical variables were compared between groups using the χ2 test or the Fisher exact probability method. The Spearman correlation test was used to analyze the correlation between the length of hospital stay and various influencing factors. Multifactor analysis was carried out through the use of multivariate linear regression analysis. The survival curve was plotted using GraphPad 9.3.1. All the tests were conducted on both sides. P<0.05 was considered statistically significant.
Results
Clinical manifestations of pregnant women infected with the Omicron variant of SARS-CoV-2
The 1024 pregnant women infected with the Omicron BA.2.2 subline virus were aged between 18 and 46 years, with a median age of 30 years (range: 27-33 years). (Table 1) Among them, 82.9% were in the normal pregnancy age range (18–34 years), while 17.1% were in the advanced maternal age group (≥35 years). The main symptoms at admission were fever (3.7%), cough and shortness of breath (6.1%), vomiting (0.8%), diarrhea (0.2%), and rash (4.9%), while the remaining (84.6%) were asymptomatic. The clinical manifestations were not affected by vaccination or vaccination dose (P>0.05) (Table 1).
A total of 466 patients, accounting for 45.5% of the patients, were in the third trimester of pregnancy, which was significantly more than the first and second trimesters, with 282 cases (27.5%) and 276 cases (27.0%), respectively. (Supplementary Table S1). About 13.5% of patients in the third trimester of pregnancy had high-risk factors (orange/high-risk), which was significantly higher than the first and second trimester, with 7.1% and 9.8%, respectively (P = 0.0190). The hospitalization time for the third trimester population was 11 ± 4.3 days, which was significantly longer than the first trimester population’s 8.7 ± 3.3 days (P<0.0001) and the second trimester population’s 9.5 ± 3.3 days (P = 0.0004). (Supplementary Table S1).
The effect of vaccine dose on COVID-19 in pregnant women
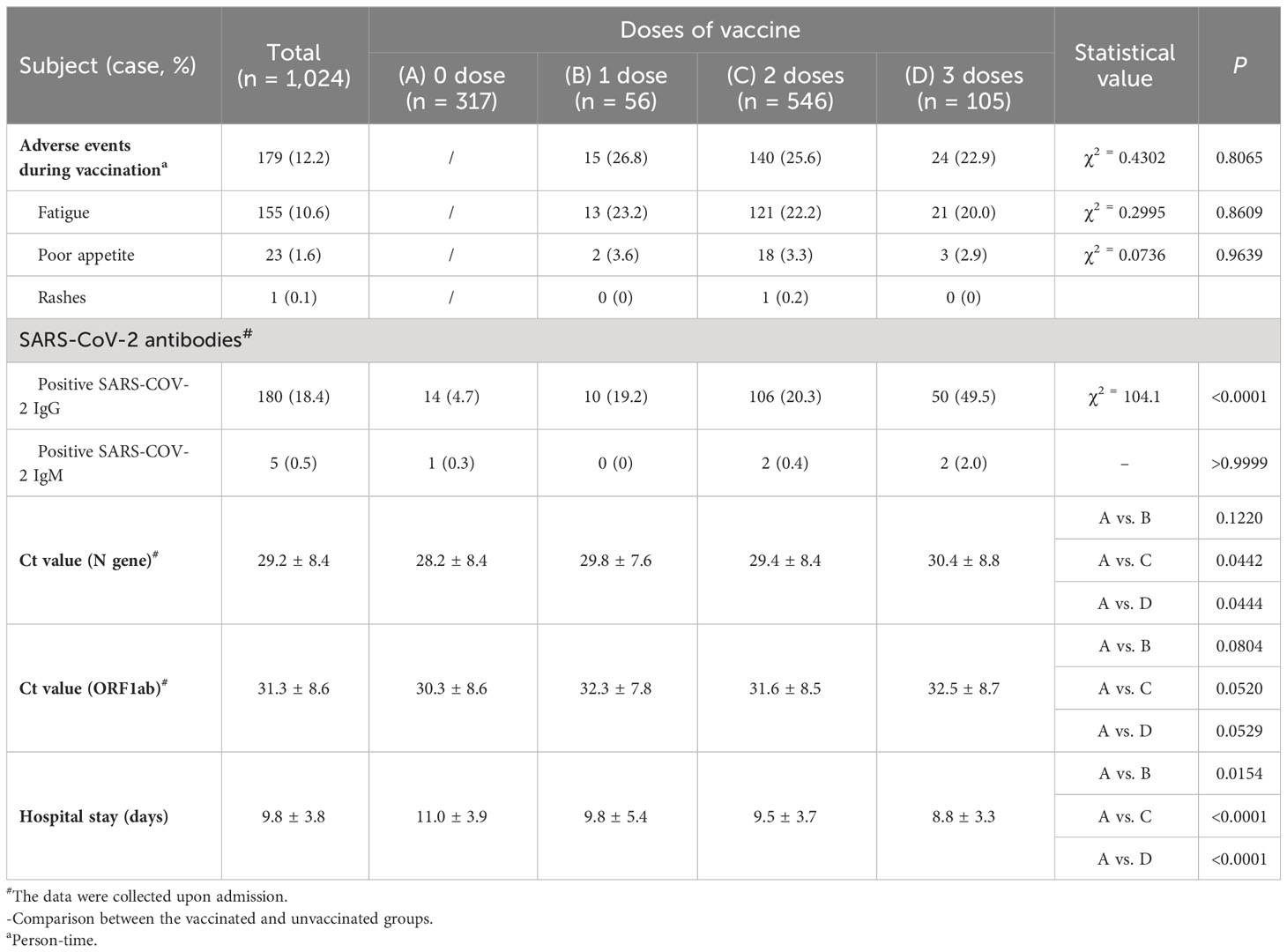
Among 1,024 patients, 707 (69.0%) have received 1-3 doses of inactivated COVID-19 vaccine. Their interval between vaccinations were not fixed. The interval between the second and first shots was about 3-6 weeks, and the interval between the third and second shots was about 6-12 months. 12.3% of pregnant women who received the vaccine experienced mild adverse reactions, mainly including fatigue (10.6%), decreased appetite (1.6%), and rash (0.1%). The occurrence of adverse reactions was not related to vaccine dose (P = 0.8065). (Table 2).
Because of the zero clearing policy implemented in Shanghai, the discharge standard of patients is that COVID-19 nucleic acid turns negative. The average hospitalization time of pregnant women who received three doses of vaccine was 8.8 ± 3.3 days, significantly shorter than 11.0 ± 3.9 days of unvaccinated patients (P<0.0001). This difference was significantly higher than 28.2 ± 8.4 of unvaccinated patients (P=0.0444). (Table 2).
Overall, about 10.3% of late pregnancy population tested positive for SARS-CoV-2 IgG, significantly lower than 28.8% of early pregnancy population (P<0.0001). (Supplementary Table S1). However, about 49.5% of pregnant women tested positive for SARS-CoV-2 IgG after receiving three doses of vaccine, significantly higher than 4.7% of the unvaccinated group, 19.2% of the one-dose vaccine group, and 20.3% of the two-dose vaccine group (P<0.0001). (Table 2). The average PCR Ct value of N gene in pregnant women who received three doses of vaccine at the time of admission was 30.4 ± 8.8. (Table 2). The average hospitalization time of pregnant women who received three doses of vaccine was 8.8 ± 3.3 days, significantly shorter than 11.0 ± 3.9 days of unvaccinated patients (P<0.0001). This difference was significantly higher than 28.2 ± 8.4 of unvaccinated patients (P = 0.0444). (Table 2).
At admission, patients who had received three doses of vaccine had higher lymphocyte (Median: 1.575*10^9/L) compared to patients who had not been vaccinated (Median: 1.300*10^9/L) (P<0.05) (Figure 2A), higher platelet counts (Median: 210.5*10^9/L) compared to patients who had not been vaccinated (Median: 190.5*10^9/L) (P<0.05) (Figure 2B), higher eGFR (Median: 153.5ml/(min*1.73m2) compared to patients who had not been vaccinated (Median: 148.4ml/(min*1.73m2) (P<0.05) (Figure 2D), and higher albumin levels (Median: 38g/L) compared to patients who had not been vaccinated (Median: 35g/L) (P<0.05) (Figure 2F), Patients who had received three doses of vaccine had lower creatinine levels (Median: 44umol/L) compared to patients who had not been vaccinated (Median: 45.4umol/L) (P<0.05) (Figure 2C), and lower aspartate aminotransferase levels (Median: 17U/L) compared to patients who had not been vaccinated (Median: 19U/L) (P<0.05) (Figure 2E), However, there were no statistically significant differences in the levels of IL-6, IL-8, interferon alpha, interferon gamma, CD4+T lymphocytes, CD8+ T lymphocytes, D-dimer, or fibrin degradation products between the vaccinated and unvaccinated groups (P>0.05). (Figure 2).
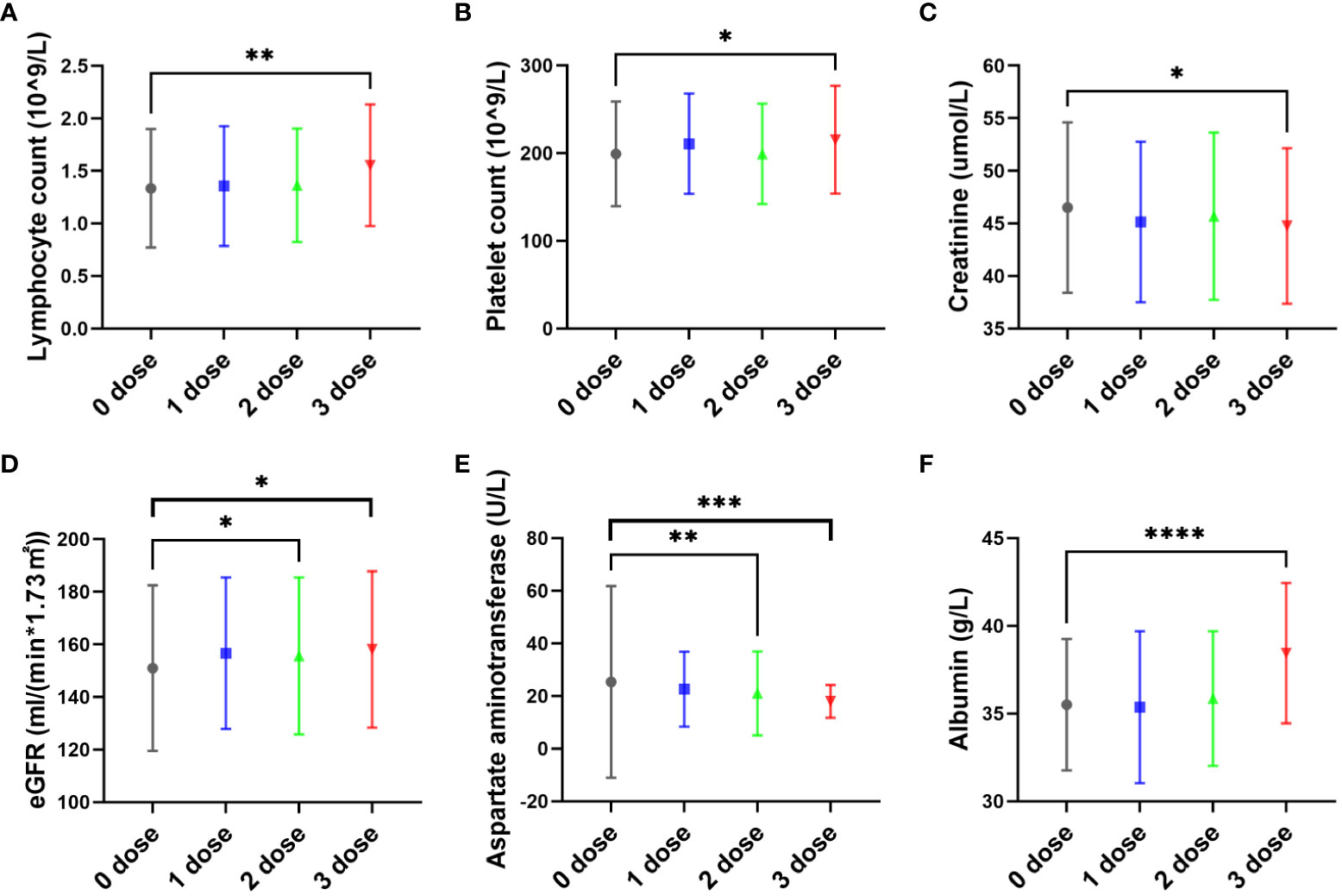
Figure 2 The effect of vaccine dose on pregnant women with COVID-19 infection. The pregnant women in this study had received zero, one, two, or three doses of inactivated SARS-CoV-2 vaccines before or after pregnancy prior to infection with the SARS-CoV-2 Omicron variant. Most pregnant women in this study were vaccinated first and infected with COVID-19 later. At admission, patients who had received three doses of vaccine had higher lymphocyte (Median: 1.575*10^9/L) compared to patients who had not been vaccinated (Median: 1.300*10^9/L) (P<0.05) (A), higher platelet counts (Median: 210.5*10^9/L) compared to patients who had not been vaccinated (Median: 190.5*10^9/L) (P<0.05) (B), higher eGFR (Median: 153.5ml/(min*1.73m2) compared to patients who had not been vaccinated (Median: 148.4ml/(min*1.73m2) (P<0.05) (D), and higher albumin levels (Median: 38g/L) compared to patients who had not been vaccinated (Median: 35g/L) (P<0.05) (F), Patients who had received three doses of vaccine had lower creatinine levels (Median: 44umol/L) compared to patients who had not been vaccinated (Median: 45.4umol/L) (P<0.05) (C), and lower aspartate aminotransferase levels (Median: 17U/L) compared to patients who had not been vaccinated (Median: 19U/L) (P<0.05) (E). * P<0.05, **P<0.01, ***P<0.001, and ***P<0.0001.
Patients who were positive for SARS-CoV-2 IgG or had been vaccinated at the time of admission had significantly shorter hospital stays, which was not related to vaccine dose (P<0.0001). (Figure 3).
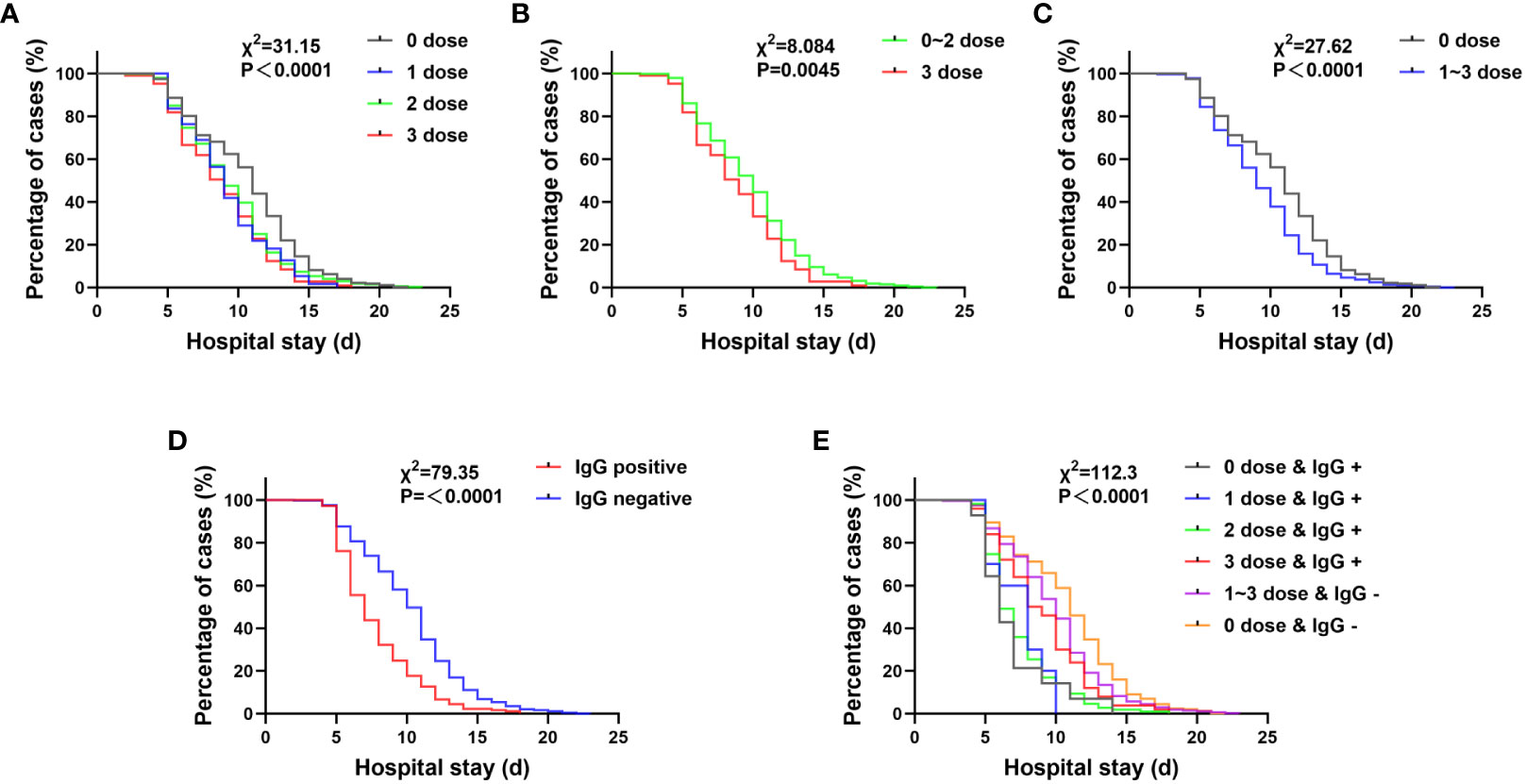
Figure 3 The influence of various factors on the hospitalization time of pregnant women with COVID-19. (A–C) The hospitalization time of pregnant women after vaccination is shortened. 0 dose vs 3 doses: χ2 = 21.99, P< 0.0001; 0 dose vs 2 doses: χ2 = 20.76, P< 0.0001; 0 dose vs 1 dose: χ2 = 9.83, P = 0.0017. (D) The length of hospitalization for pregnant women with SARS-CoV-2 IgG positive at admission was significantly shortened.(P< 0.0500). (E) (a) When reinfected, individuals previously infected with SARS-CoV-2 (0 dose & IgG +) had the shortest hospitalization time. 0 dose & IgG + vs 3 doses & IgG +: χ2 = 4.650, P = 0.0311; 0 dose & IgG + vs 0 dose & IgG -: χ2 = 20.03, P< 0.0001; 0 dose & IgG + vs 1~3 doses & IgG -: χ2 = 12.65, P = 0.0004; (b) Even if only one dose of vaccine is administered, as long as the SARS-CoV-2 IgG test is positive, the hospitalization time is shorter than that of patients who received three doses of vaccine but have a negative IgG response. 1 dose & IgG + vs 1~3 doses & IgG -: χ2 = 9.865, P = 0.0017; 2 doses & IgG + vs 1~3 doses & IgG -: χ2 = 56.2, P< 0.0001; (c) Although the IgG test result after vaccination was negative, the hospitalization time of those who were vaccinated and had a negative IgG test result was shorter than that of those who were not vaccined and had a negative IgG test result. 1~3 doses & IgG – vs 0 dose & IgG -: χ2 = 15.06, P= 0.0001.
Patients who were only vaccinated with one dose of vaccine and found to be SARS-CoV-2 IgG-positive at admission had a significantly shorter hospitalization time of 7.4 ± 2.0 days while patients who were vaccinated with three doses of vaccine but were SARS-CoV-2 IgG-negative had a hospitalization time of 9.9 ± 3.5 days (P = 0.0017). (Figure 3). This hospitalization time was still shorter than that of patients who were not vaccinated and were SARS-CoV-2 IgG-negative, which was 10.9 ± 3.8 days (P = 0.0001). Even without vaccination, patients who were SARS-CoV-2 IgG-positive at admission had the shortest hospitalization time of 6.9 ± 2.7 days. These patients may have been infected with SARS-CoV-2 in the past and have developed immunity against SARS-CoV-2, or the test results could be false positive. (Figure 3). A possibility can be abortive infection. Another, after infection these individuals might have developed immunity for reinfection (10–12).
Other factors also influence the length of stay of pregnant women infected with COVID-19
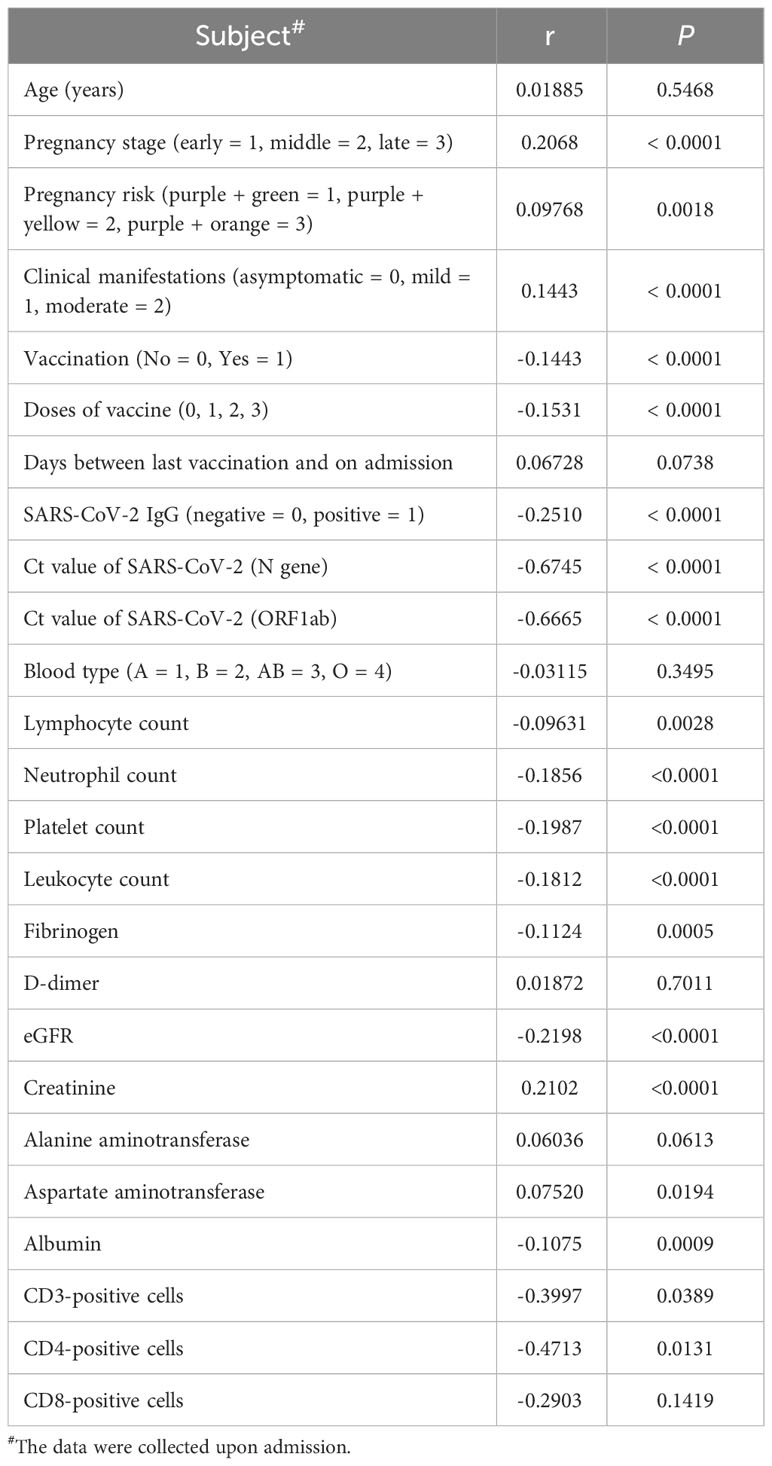
In addition to vaccination, several other factors that may affect hospitalization time were analyzed through Spearman’s test, including pregnancy, pregnancy risk factors, diagnostic classification (asymptomatic = 0, mild = 1, moderate = 2), the interval between the last vaccination and hospitalization, SARS-CoV-2 specific IgG level, SARS-CoV-2 nucleic acid PCR Ct value, blood type, and common laboratory indicators. (Table 3). Important indicators with positive correlation were screened. Multilinear regression analysis showed that six factors were associated with hospitalization time. SARS-CoV-2 infection in late pregnancy was positively correlated with disease severity, creatinine level, and hospitalization time. It was found that the PCR Ct value of SARS-CoV-2 N gene, lymphocyte count, and fibrinogen level were negatively correlated with hospitalization time (P<0.050) (Table 4). In order to predict the hospitalization time of pregnant women with COVID-19-infection, we developed a model as follows: Y = 12.99 + 0.3844*P + 0.5743*D - 0.1511*N - 1.414*LYM - 0.3886*Fg + 0.08461*Cr, where Y represents hospitalization time, P represents pregnancy, D represents diagnostic classification, N represents SARS-CoV-2 CT value (N gene), LYM represents lymphocyte count, Fg represents fibrinogen, and Cr represents creatinine (Figure 4).
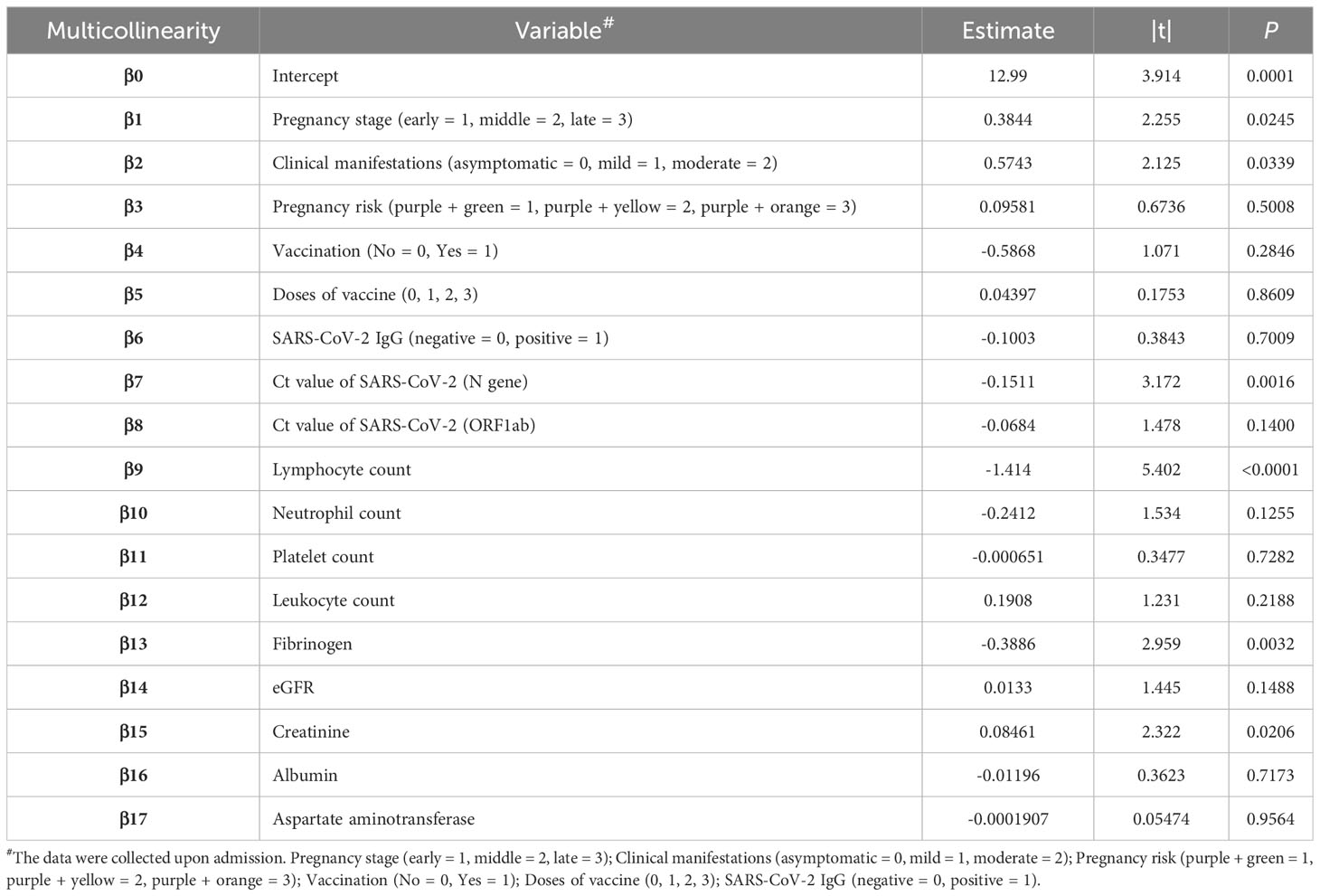
Table 4 Multivariate linear analysis of potential factors affecting the length of stay of pregnant women with COVID-19.
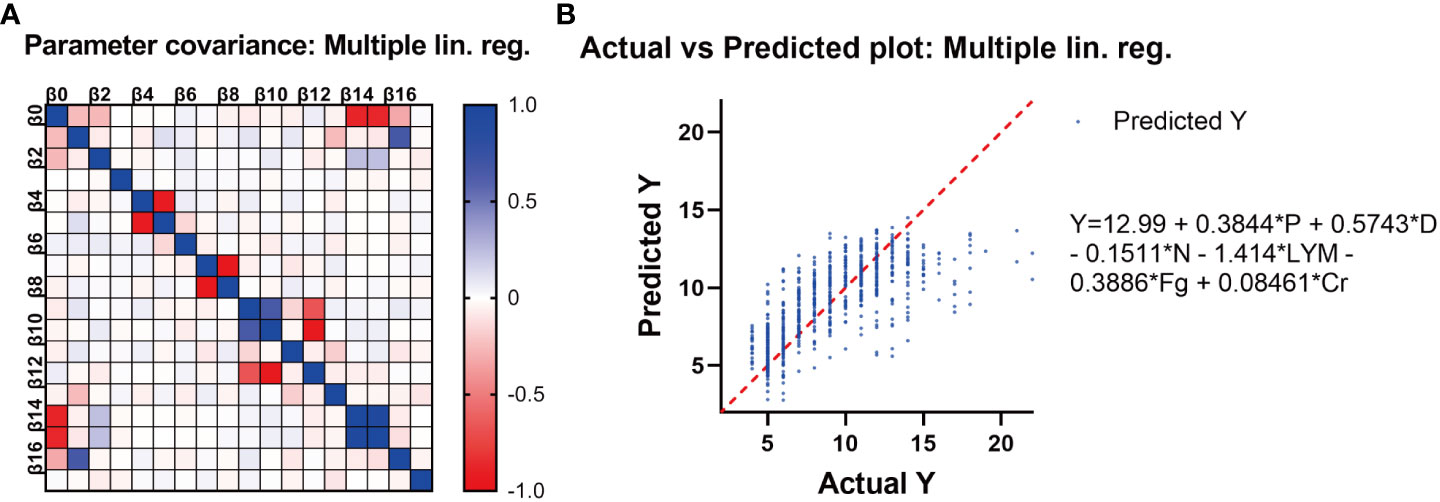
Figure 4 (A) The correlation between different variables. (B) Multivariate linear analysis of the potential factors affecting the length of stay of pregnant women with COVID-19. The length of hospitalization for pregnant women infected with SARS-CoV-2 Omicron variant is related to six factors, namely, Y: hospital stay, P: pregnancy stage, D: diagnostic type, N: Ct value (N gene), lym: lymphocyte count, FG: fibrinogen, and Cr: creatinine. Their relationship can be expressed as Y = 12.99 + 0.3844 * P + 0.5743 * D - 0.1511 * N - 1.414 * lym - 0.3886 * FG + 0.08461 * Cr.
Effect of vaccination on newborns born to mothers with COVID-19
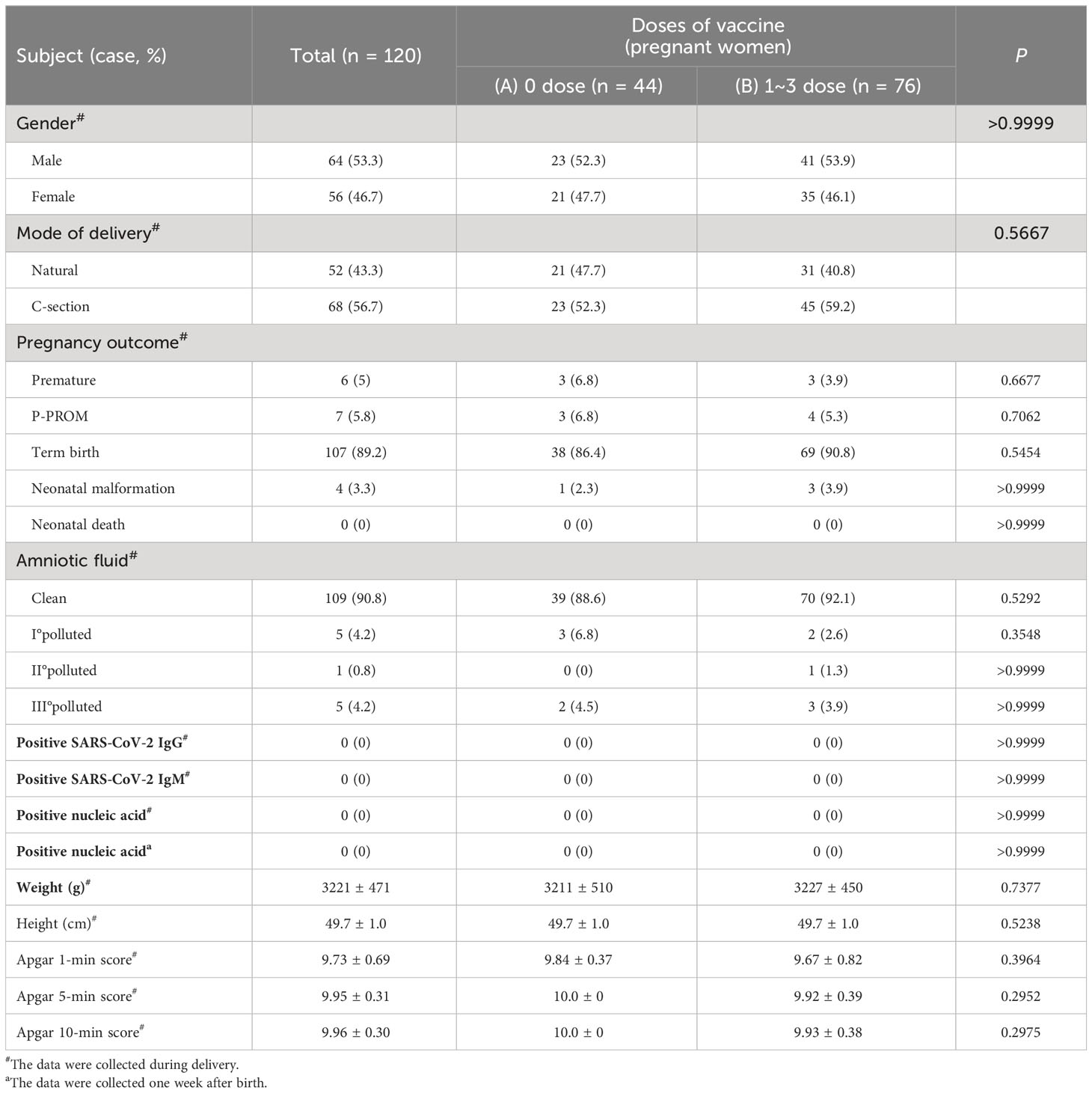
A total of 118 COVID-19 patients gave birth to 120 newborns during hospitalization. Among them, 76 patients were vaccinated with inactivated COVID-19 vaccines, and 42 patients were not vaccinated. (Table 5). The vaccination status before infection with SARS-CoV-2 did not affect the mode of delivery, pregnancy outcomes (premature delivery, premature rupture of membranes (P-PROM), full-term delivery (term birth), neonatal malformation, neonatal death), amniotic fluid contamination index, body weight and length, malformation rate, or Apgar score (P>0.05). (Table 5).
Among the 76 vaccinated patients, 68 were vaccinated before pregnancy, and 8 were vaccinated during pregnancy. (Table 6). The comparison between the two groups showed that the vaccination time did not affect the delivery mode, pregnancy outcome, amniotic fluid pollution level, body weight and length, malformation rate, and Apgar score (P>0.05). (Table 6).
To verify the possibility of vertical transmission of SARS-CoV-2, we tested 120 newborns for SARS-CoV-2 nucleic acid and SARS-CoV-2-specific IgG and IgM levels, and the results were all negative. (Table 5). All the newborns lived with their mothers and were breastfed. One week after birth, the nucleic acid test results for SARS-CoV-2 in the newborns were all negative. (Table 5).
Discussion
A recent study of nearly 1 million cases confirmed the safety of COVID-19 inactivated vaccines in China, although mild adverse reactions such as transient low fever, headache or fatigue were observed (13–15). These vaccines are theoretically safe for women who are preparing pregnancy or are pregnant. In 2021, the US Center for Disease Control and Prevention (CDC) suggested that any authorized COVID-19 vaccine can be used for pregnant or lactating women (16). In addition, the American College of Obstetrics and Gynecology and SMFM strongly recommend vaccination for pregnant and breastfeeding women. There are no restrictions on the type or dose of vaccine during pregnancy (17). However, due to the lack of maternal vaccination data in China, the impact of COVID-19 vaccine on pregnant women, fetuses, and newborns is still uncertain. In addition, China has not yet implemented large-scale vaccination for pregnant women.
Previous studies have confirmed that pregnancy increases the risk of serious illness associated with COVID-19. However, it is still unclear which specific pregnant women are more likely to be infected with SARS-CoV-2. In this study, we analyzed data of 1,024 pregnant women diagnosed with COVID-19. Our findings suggest that most patients were in the third trimester of pregnancy, which may be due to increased diaphragmatic muscle elevation, increased oxygen consumption, and impaired immune functions during this stage. The low level of SARS-CoV-2 IgG further confirms this evidence; Only 10.3% of late pregnancy population tested positive for SARS-CoV-2 IgG, which significantly lower compared to early pregnancy population (28.8%).
Pregnant women with COVID-19 are more likely to have mild symptoms on admission. However, only a small number of patients have developed symptoms such as fever, cough, shortness of breath, vomiting, diarrhea, and rash. The low toxicity of the Omicron strain may explain this. In addition, our study found that the status of vaccination or the number of vaccinations does not predict the clinical symptoms or severity of disease in pregnant women admitted to the hospital. Among pregnant women who had received three doses of vaccine, 49.5% were positive for SARS-CoV-2 IgG, significantly higher than 4.7% of those who had not received vaccine, 19.2% of those who had received one dose of vaccine, and 20.3% of those who had received two doses of vaccine. Such a positive correlation between SAR-COV-2 IgG positivity and the number of vaccinations were in line with what had been observed in general populations, strengthening the notion that more vaccinations with appropriate intervals could cultivate a more robust antibody memory response with higher titers and longer durations (10, 12, 18, 19). Based on our data, we conclude that, for pregnant women, multiple vaccinations during pregnancy or preparation for pregnancy conferred beneficial effects upon the later CoVID-19 infections, with the degree of protection positively correlated with the vaccine doses. It should be noted that this conclusion was drawn from comparing the effects of one, two, and three doses of vaccines. Whether more vaccinations beyond three doses could further enhance protection is an open question awaiting future investigation.
In addition, compared with patients who did not receive vaccination, patients who received three doses of the vaccine showed higher lymphocyte and platelet counts, as well as higher eGFR and albumin levels, while also exhibiting lower creatinine and aspartate aminotransferase levels. Vaccination may provide protection and reduce the severity of maternal disease when infected with SARS-CoV-2. The hospitalization time of pregnant women who received three doses of vaccine was only 8.8 ± 3.3 days, which was significantly shorter than that of pregnant women who did not receive the vaccine (11.0 ± 3.9 days). This finding shows that repeated vaccination of COVID-19 vaccine can speed up virus clearance and shorten the duration of disease in pregnant women. In Figure 2A, only three doses of vaccination significantly increased the lymphocyte count in the blood of pregnant women. We believe that this may be consistent with the positive SARS-CoV-2 specific IgG, and is also due to the more significant stimulation of pregnant women’s immune cells by more vaccinations, leading to immune cell proliferation and antibody secretion. However, as no pregnant women have received 4 or more doses of vaccine, it is unclear whether receiving 4 doses is better than receiving 3 doses. According to current data, the higher the number of vaccinations, the higher the antibody positive rate and the lymphocyte count.
Most importantly, even if only one dose of vaccine is administered, as long as the individual is positive for SARS-CoV-2 IgG, their hospitalization time (7.4 ± 2.0 days) is still shorter than that of individuals who have received three doses of vaccine but are negative for SARS-CoV-2 IgG (9.9 ± 3.5 days), with a statistically significant difference (P = 0.0017). Even if the IgG test is negative after vaccination, the hospitalization time (9.9 ± 3.5 days) is still shorter than that of individuals who have not been and who are negative for antibodies (10.9 ± 3.8 days), again with a statistically significant difference (P = 0.0001). These observations suggest that the three-dose vaccination regimen is more effective, and for those who hesitant about whether to receive multiple doses of vaccine, IgG testing was after vaccination is necessary.
Our study also showed that infection with SARS-CoV-2 in the third trimester of pregnancy and high viral nucleic acid PCR Ct values were positively correlated with disease severity, while high lymphocyte counts were negatively correlated with disease severity and length of hospital stay. These observations can help identify pregnant women with high-risk factors early and focus on preventing disease progression.
Regarding the safety of vaccines, previous studies conducted in other countries have shown that pregnant women experience slightly higher levels of pain at the injection site compared to non-pregnant women, and have slightly lower incidences of headache, muscle ache, chills, and fever. Among the 827 pregnant women in the Vsafe Pregnancy Registry, the incidence of adverse pregnancy and neonatal outcomes, such as miscarriage, premature delivery, small gestational age, and congenital anomalies, is comparable to published background rates. In addition, there were no reports of newborn deaths (20). Another retrospective cohort study has also found that vaccination of pregnant women was not associated with serious adverse events within 42 days (21). It is worth noting that rare adverse reactions, such as thrombocytopenia syndrome (TTS), have also been reported after vaccination with adenovirus vaccines (22, 23). For safety reasons, the United Kingdom prefers to use mRNA vaccines rather than adenovirus vaccines (24, 25). In our study, the only vaccine used for domestic vaccination is the inactivated COVID-19 vaccine. These vaccines retain the immunogenic components that trigger the immune response in the human body, while losing their infectivity and replication ability, thus ensuring safety and effectiveness.
A total of 118 patients gave birth to 120 newborns during their hospital stay. Our findings suggest that maternal vaccination is safe for newborns, as it does not affect delivery mode, pregnancy outcome, weight and length, malformation rate, or Apgar score, regardless of whether vaccination was given before or during pregnancy.
Another key question is whether COVID-19 will cross the placenta and directly harm the fetus. It is crucial to pay attention to this during pregnancy, childbirth, and breastfeeding. We tested 120 newborns for SARS-CoV-2 nucleic acid, IgG, and IgM levels, and found that all of them were negative, thus eliminating the possibility of vertical transmission. This is consistent with previous studies conducted in other countries, indicating that the prevalence of vertical transmission is low. This may be related to the low level of SARS-CoV-2 and the reduced co-expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) required for entry into placental cells (26, 27). The pregnant women in this study lived with breastfed newborns. No positive nucleic acid tests was detected in the newborn after one week of birth, indicating that maternal with the newborn is safe under the continuous use of surgical masks, hand hygiene, and breast cleaning. (Supplementary Figure S1). Many foreign guidelines also advocate allowing newborns to adapt to infected mothers, especially if the mother is asymptomatic (28, 29).
Our strategies for preventing neonatal infection with SARS-CoV-2 includes minimizing labor processes, avoiding invasive surgery during delivery, reducing the contact time between the newborn and the maternal birth canal, wiping away mucus and amniotic fluid as soon as possible after birth, and promptly transferring the newborn from the operating room or delivery room. A study conducted in the United States on nearly 99,000 pregnant women infected with SARS-CoV-2 found that 109 patients died, with a mortality rate of 0.1%. On the other hand, our study did not record any deaths among the 1,024 pregnant women, which may be due to our screening and timely intervention for high-risk patients (30).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Shanghai Public Health Clinical Center (2022-S067-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HD: Writing – original draft. YJ: Writing – original draft. MS: Writing – original draft. ML: Writing – review & editing. JS: Writing – review & editing. WQ: Writing – review & editing. GZ: Writing – review & editing. YXL: Writing – review & editing. TL: Writing – review & editing. YL: Writing – review & editing. XF: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1303058/full#supplementary-material
Supplementary Figure 1 | Shanghai’s experience in preventing vertical transmission of COVID-19.
Supplementary Table 1 | Clinical characteristics of COVID-19 infection in different stages of pregnancy.
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
2. COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet (2022) 399(10334):1513–36. doi: 10.1016/S0140-6736(21)02796-3
3. Christensen PA, Olsen RJ, Long SW, Snehal R, Davis JJ, Saavedra MO, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol (2022) 192(4):642–52. doi: 10.1016/j.ajpath.2022.01.007
4. Latest statistical table of epidemic situation data in Shanghai (2022). Available at: http://m.sh.bendibao.com/news/233243.html.
5. Zhang X, Zhang W, Chen S. Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet (2022) 399(10340):2011–2. doi: 10.1016/S0140-6736(22)00838-8
6. Gottfredsson M. Spaenska veikin á Islandi 1918. Laerdómur í laeknisfraedi og sögu [The Spanish flu in Iceland 1918. Lessons in medicine and history]. Laeknabladid (2008) 94(11):737–45.
7. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses (2020) 12(2):194. doi: 10.3390/v12020194
8. Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-COV-2 infection by pregnancy status— United States, January 22eOctober 3, 2020. MMWR Morb Mortal Wkly Rep (2020) 69:1641–7. doi: 10.15585/mmwr.mm6944e3
9. Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, et al. COVID-19 vaccination coverage among pregnant women during pregnancy—eight integrated health care organizations, United States, December 14, 2020eMay 8, 2021. MMWR Morb Mortal Wkly Rep (2021) 70:895–9. doi: 10.15585/mmwr.mm7024e2
10. Mattoo SS, Myoung J. A promising vaccination strategy against COVID-19 on the horizon: heterologous immunization. J Microbiol Biotechnol (2021) 31(12):1601–14. doi: 10.4014/jmb.2111.11026
11. Swadling L, Diniz MO, Schmidt NM, Amin OE, Chandran A, Shaw E, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. (2022) 601(7891):110–7. doi: 10.1038/s41586-021-04186-8
12. Mattoo SU, Myoung J. T cell responses to SARS-CoV-2 in humans and animals. J Microbiol (2022) 60(3):276–89. doi: 10.1007/s12275-022-1624-z
13. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis (2021) 21(2):181–92. doi: 10.1016/S1473-3099(20)30843-4
14. Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis (2021) 21(6):803–12. doi: 10.1016/S1473-3099(20)30987-7
15. Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung T, et al. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines (2021) 20(7):891–8. doi: 10.1080/14760584.2021.1925112
16. Centers for Disease Control and Prevention. Use of COVID-19 Vaccines in the United States. (November 3, 2023). Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
17. Huddleston HG, Jaswa EG, Lindquist KJ, Kaing A, Morris JR, Hariton E, et al. COVID-19 vaccination patterns and attitudes among American pregnant individuals[J]. Am J Obstet Gynecol MFM (2022) 4(1).
18. Grewal R, Nguyen L, Buchan SA, Wilson SE, Nasreen S, Austin PC, et al. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat Commun (2023) 14(1):2031. doi: 10.1038/s41467-023-36566-1
19. Intawong K, Chariyalertsak S, Chalom K, Wonghirundecha T, Kowatcharakul W, Thongprachum A, et al. Effectiveness of heterologous third and fourth dose COVID-19 vaccine schedules for SARS-CoV-2 infection during delta and omicron predominance in Thailand: a test-negative, case-control study. Lancet Reg Health Southeast Asia. (2023) 10:100121. doi: 10.1016/j.lansea.2022.100121
20. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med (2021) 384:2273–82. doi: 10.1056/NEJMoa2104983
21. Rasmussen SA, Jamieson DJ. Covid-19 vaccination during pregnancy - two for the price of one. N Engl J Med (2022) 387(2):178–9. doi: 10.1056/NEJMe2206730
22. MacIntyre CR, Veness B, Berger D, Hamad N, Bari N. Thrombosis with Thrombocytopenia Syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination - A risk-benefit analysis for people < 60 years in Australia. Vaccine (2021) 39(34):4784–7. doi: 10.1016/j.vaccine.2021.07.013
23. MacNeil JR, Su JR, Broder KR, Guh AY, Gargano JW, Wallace M, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep (2021) 70:651–6. doi: 10.15585/mmwr.mm7017e4
24. COVID-19 vaccines, pregnancy and breastfeeding FAQs. Available at: https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-COVID-19-pregnancyand-womenshealth/COVID-19-vaccines-and-pregnancy/covid19-vaccines-pregnancy-and-breastfeeding/ (Accessed 15, 2021).
25. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol (2021) 225(303):e1–17. doi: 10.1016/j.ajog.2021.03.023
26. Mofenson LM, Idele P, Anthony D, Requejo J, You D, Luo C, et al. The evolving epidemiologic and clinical picture of SARS-COV-2 and COVID-19 disease in children and young people. (2020). Available at: https://www.unicef-irc.org/publications/pdf/Working%20Paper_2020-07_Epidemiology%20of%20SARS%20CoV%202-COVID%2019%20and%20Children_Adolescents_August_update.pdf.
27. Ouyang Y, Bagalkot T, Fitzgerald W, Sadovsky E, Chu T, Martínez-Marchal A, et al. Term human placental trophoblasts express SARS-COV-2 entry factors ACE2, TMPRSS2, and Furin. mSphere (2021) 6:e00250–21. doi: 10.1128/mSphere.00250-21
28. Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health (2020) 4:721–7. doi: 10.1016/S2352-4642(20)30235-2
29. American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics[J]. Washington DC, American College of Obstetricians and Gynecologists. Available at: https://www.acog.org/clinical-information/physician-faqs/COVID-19-faqs-for-ob-gyns-obstetrics,dilihatpadatanggal. (2020) 13.
Keywords: COVID-19 vaccination, Omicron strain infection, pregnancy, newborns, breastfeeding, vertical transmission
Citation: Deng H, Jin Y, Sheng M, Liu M, Shen J, Qian W, Zou G, Liao Y, Liu T, Ling Y and Fan X (2024) Safety and efficacy of COVID-19 vaccine immunization during pregnancy in 1024 pregnant women infected with the SARS-CoV-2 Omicron virus in Shanghai, China. Front. Immunol. 14:1303058. doi: 10.3389/fimmu.2023.1303058
Received: 27 September 2023; Accepted: 21 December 2023;
Published: 16 January 2024.
Edited by:
Carlos Angulo, Centro de Investigación Biológica del Noroeste (CIBNOR), MexicoReviewed by:
Sidra Islam, Cleveland Clinic, United StatesAtika Dhar, National Institutes of Health (NIH), United States
Sameer -ul-Salam Mattoo, The Ohio State University, United States
Copyright © 2024 Deng, Jin, Sheng, Liu, Shen, Qian, Zou, Liao, Liu, Ling and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Fan, ZmFueGlhb2hvbmdAc2hwaGMub3JnLmNu; Yun Ling, eXVuLmxpbmdAc2hwaGMub3JnLmNu
†These authors have contributed equally to this work
 Hongmei Deng1†
Hongmei Deng1† Yinpeng Jin
Yinpeng Jin Yixin Liao
Yixin Liao Tiefu Liu
Tiefu Liu Yun Ling
Yun Ling