- 1Department of Respiratory Medicine, Children’s Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 2Department of Pediatrics, Suqian Hospital Affiliated to Xuzhou Medical University, Suqian, Jiangsu, China
Introduction: Mycoplasma pneumoniae pneumonia (MPP) may lead to various significant outcomes, such as necrotizing pneumonia(NP) and refractory MPP (RMPP). We investigated the potential of the peripheral blood neutrophil-to-lymphocyte ratio (NLR) to predict outcomes in patients with MPP.
Methods and materials: This was a prospective study of patients with MPP who were admitted to our hospital from 2019 to 2021. Demographic and clinical data were collected from patient records and associated with the development of NP and RMPP and other outcome measures.
Results: Of the 1,401 patients with MPP included in the study, 30 (2.1%) developed NP. The NLR was an independent predictor of NP (odds ratio 1.153, 95% confidence interval 1.022–1.300, P=0.021). The probability of NP was greater in patients with a high NLR (≥1.9) than in those with a low NLR (<1.9) (P<0.001). The NLR was also an independent predictor of RMPP (odds ratio 1.246, 95% confidence interval 1.102–1.408, P<0.005). Patients with a high NLR were more likely to develop NP and RMPP and require intensive care, and had longer total fever duration, longer hospital stays, and higher hospitalization expenses than those with a low NLR (all P<0.005).
Discussion: The NLR can serve as a predictor of poor prognosis in patients with MPP. It can predict the occurrence of NP, RMPP, and other poor outcomes. The use of this indicator would allow the simple and rapid prediction of prognosis in the early stages of MPP, enabling the implementation of appropriate treatment strategies.
1 Introduction
Mycoplasma pneumoniae (MP) is a common pathogen that causes lower respiratory tract infections in children. MP pneumonia (MPP) accounts for approximately 32.4% of community-acquired pneumonia in Chinese children (1) and may be accompanied by various extrapulmonary complications. Incidences of severe MPP and refractory MPP (RMPP) have been increasingly reported in recent years (2, 3), which can lead to necrotizing pneumonia (NP) and a poor prognosis (4, 5). NP, which is diagnosed by radiography or computed tomography (CT), is characterized by multiple low-density, thin-walled cavities based on lung consolidation, sometimes containing a fluid level (6, 7). RMPP is defined as MPP with progressive exacerbation of clinical symptoms, persistent fever, and deterioration as determined by lung imaging after standard treatment with macrolide drugs for ≥7 d (8). Therefore, it is important to find early indicators to predict the severity of MPP, which can provide guidance for early clinical treatment.
Currently, known laboratory indicators that can predict the severity of MPP include peripheral blood neutrophil count, C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin-6, interleukin-17A, and D-dimer (9–12). Models predicting poor prognosis in MPP have also been established and evaluated (6, 13). However, it is well-known that neutrophil counts and CRP levels can be influenced by bacterial infection, and measuring other biomarkers requires invasive vein puncture. The neutrophil-to-lymphocyte ratio (NLR) in peripheral blood is a simple, rapid, and widely available indicator that has been reported to predict poor outcomes in various diseases, such as diabetes mellitus (14), cardiovascular disease (15), and cancer (16). In respiratory diseases, the NLR has been shown to be associated with poor prognosis in idiopathic pulmonary fibrosis (17), chronic obstructive pulmonary disease (14), and coronavirus disease-2019 (18). However, there is a lack of large cohort studies on the predictive value of the NLR for poor prognosis in children with MPP. In addition, there are few long-term follow-up studies on NP in the prognostic evaluation of MPP.
Therefore, we explored the relationship between the NLR and outcomes in children with MPP. We aimed to prospectively evaluate the association between the baseline NLR and prognosis in 1,401 children with MPP enrolled over a 3-year period. This may establish the NLR as a simple and rapid method for predicting poor prognosis in MPP, to guide early treatment and monitor disease progression.
2 Materials and methods
2.1 Participants
Patients with MPP who were admitted to the Respiratory Department of Children’s Hospital of Nanjing Medical University between January 1, 2019, and December 31, 2021, were included in this cohort study. We prospectively collected information on demographic data, clinical characteristics, laboratory tests, chest imaging findings, and clinical outcomes from electronic medical records. The study was approved by the Research Ethics Committee of our institution (approval number: 201812257-1; approval date: November 29, 2018). The requirement for informed consent was waived for this anonymized data.
2.2 Inclusion and exclusion criteria
Inclusion criteria: 1) aged ≥28 d and <18 y; 2) presence of respiratory symptoms or fever; 3) chest imaging confirmed pneumonia; and 4) MP-DNA positive nasopharyngeal secretions (≥1×103 copies/mL) and positive serology. Exclusion criteria: 1) disease course ≥4 weeks prior to admission; 2) presence of other respiratory diseases, such as bronchial asthma, bronchopulmonary dysplasia, tuberculosis, primary ciliary dystrophy, cystic fibrosis, bronchial foreign body, pulmonary tumor, and non-infectious interstitial pulmonary disease; 3) presence of immunodeficiency, congenital heart disease, or heredity neurological disorders; 4) evidence of coinfection with other pathogens, as determined by sputum, blood, alveolar lavage fluid, pleural effusion culture, and virus testing; and 5) incomplete clinical data.
2.3 Data collection
Clinical information was prospectively collected from patient records. Age, sex, clinical symptoms and signs, extrapulmonary complications, preadmission fever duration, total fever duration, length of hospital stay, and hospitalization expenses were recorded. Allergy histories, including eczema, food and drug allergies, allergic rhinitis, allergic dermatitis, and allergic conjunctivitis, were recorded. Laboratory findings on admission, including white blood cell (WBC) counts, the NLR, CRP levels (BC-7500[NR]CS; Mindray, Shenzhen, China), alanine transaminase (ALT) levels, creatine kinase-MB levels, LDH levels (C502; Roche Diagnostics GmbH, Mannheim, Germany), blood coagulation function, and D-dimer levels (ACLTOP750; Werfen, Bedford, MA, USA), were recorded. Chest imaging findings were collected. Chest CT (Brilliance iCT; Philips Medical Systems, Cleveland, OH, USA) was performed when: 1) the clinical manifestations were inconsistent with the chest radiograph; 2) airway and lung malformations were suspected; 3) serious complications occurred; or 4) patients did not respond to treatment or had other diseases that needed to be excluded.
2.4 Clinical outcomes
Follow-up data were collected from electronic medical records, including primary (development of NP and RMPP) and secondary (total fever duration, length of hospital stay, hospitalization expenses, and referral to intensive care) outcomes.
The diagnosis of MP infection was based on MP-DNA positive nasopharyngeal secretions (≥1×103 copies/mL) and positive serology (anti-MP IgM ≥1.0 COI). MP-DNA was detected by fluorogenic quantitative PCR (5700 Automatic PCR Analyzer; Perkin Elmer Inc., Wellesley, MA, USA). Anti-MP IgM was detected by chemiluminescent immunoassay (iFlash 3000; YHLO, Shenzhen, China).
2.5 Statistical analysis
SPSS software (version 25.0; IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Normally distributed data are reported as mean ± SD; non-normally distributed data are reported as median (25th–75th percentile). Categorical variables are expressed as frequencies and percentages. The groups were compared using a two-sample Student’s t-test (normally distributed data) or the Mann–Whitney U test (non-normally distributed data). Statistical significance was determined using the chi-square test and Fisher’s exact test. Logistic regression analysis was used to identify risk factors for MP-associated NP or RMPP. Multivariate analysis was performed using clinical characteristics with P<0.05 in the univariate analysis. Receiver operating characteristic (ROC) curves were plotted and the areas under the curve (AUCs) were calculated to compare the prognostic value of each variable. Survival curves were plotted using the Kaplan–Meier method and compared using the log-rank test. P<0.05 was considered statistically significant.
3 Results
3.1 Association between the NLR and NP
In total, 9,661 patients with community-acquired pneumonia were considered for this study. Among them, 2,826 (29.3%) were MP-DNA positive and had positive serology. A total of 1,401 patients with MPP were included in the final analysis. All enrolled patients underwent chest X-ray or CT, which determined that 30 (2.1%) had developed NP. The study flowchart is presented in Figure 1.
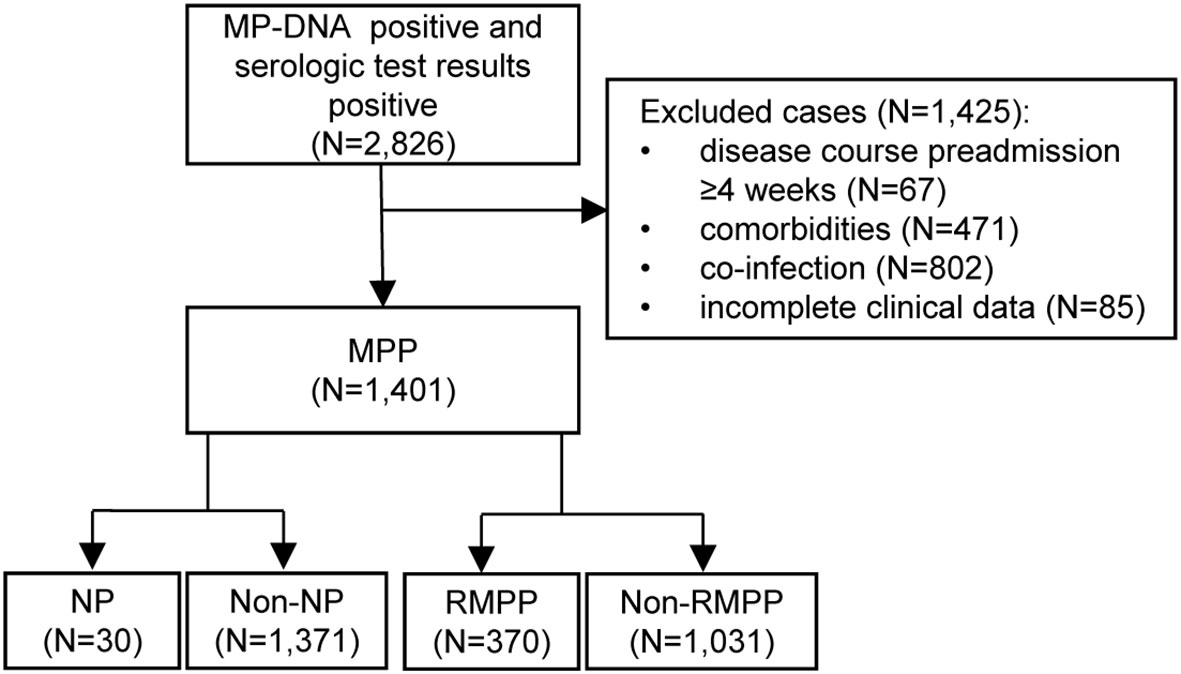
Figure 1 Flow chart of the study. MP, Mycoplasma pneumoniae; MPP, Mycoplasma pneumoniae pneumonia; NP, necrotizing pneumonia; RMPP, refractory Mycoplasma pneumoniae pneumonia.
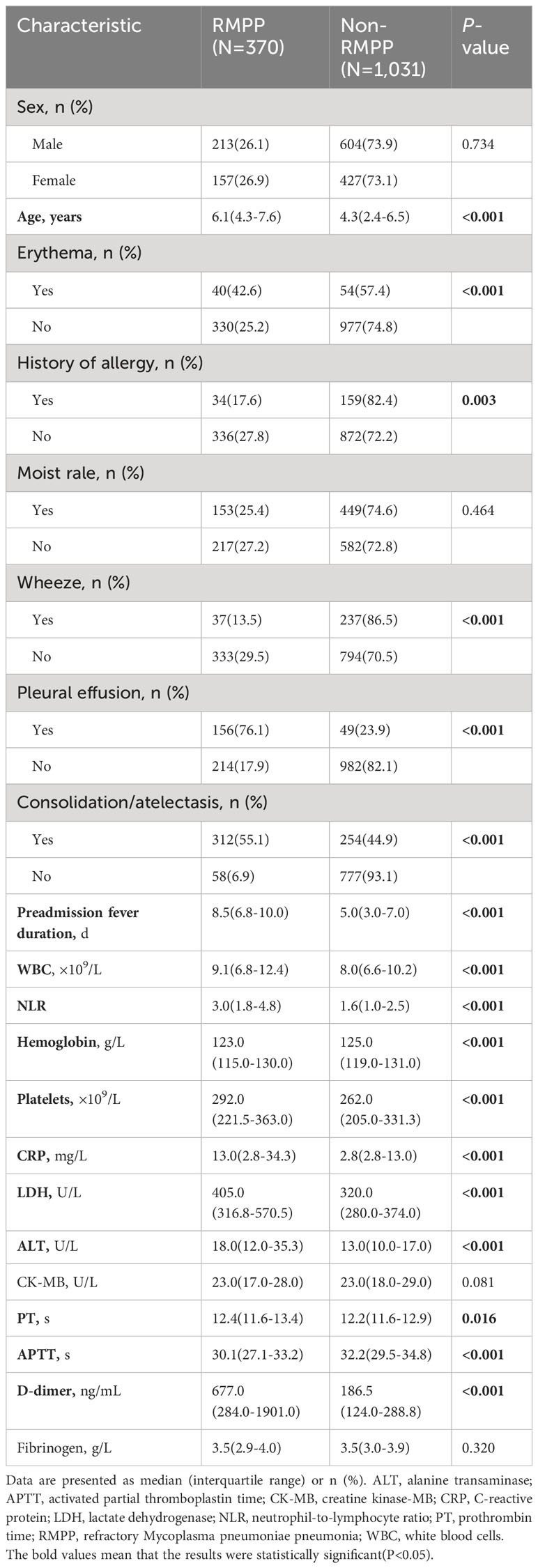
The demographic and baseline clinical characteristics of the 1,401 patients are presented in Table 1. The patients were prospectively monitored for 60 d from the time of admission. There was no significant difference in the sex ratio between the NP and non-NP groups (P=0.191). Patients in the NP group were older than those in non-NP group (P=0.002). Patients with NP were more likely to have erythema, pleural effusion, and consolidation/atelectasis than those without NP (all P<0.05). Preadmission fever duration, WBC counts, the NLR, and CRP, LDH, ALT, and D-dimer levels were all significantly different between the NP and non-NP groups (all P<0.05).
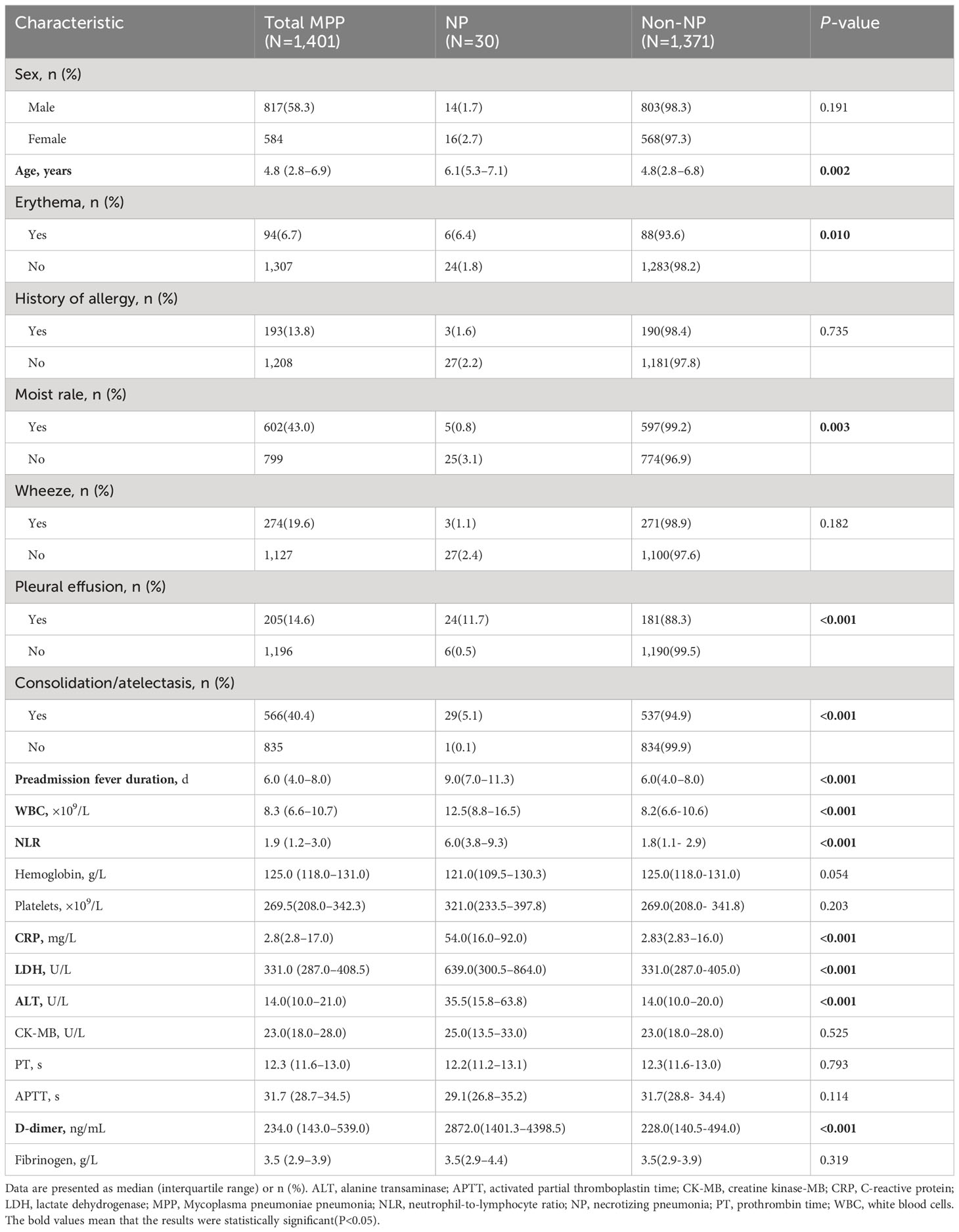
Table 1 Demographic and baseline clinical characteristics of patients with MPP, with and without NP.
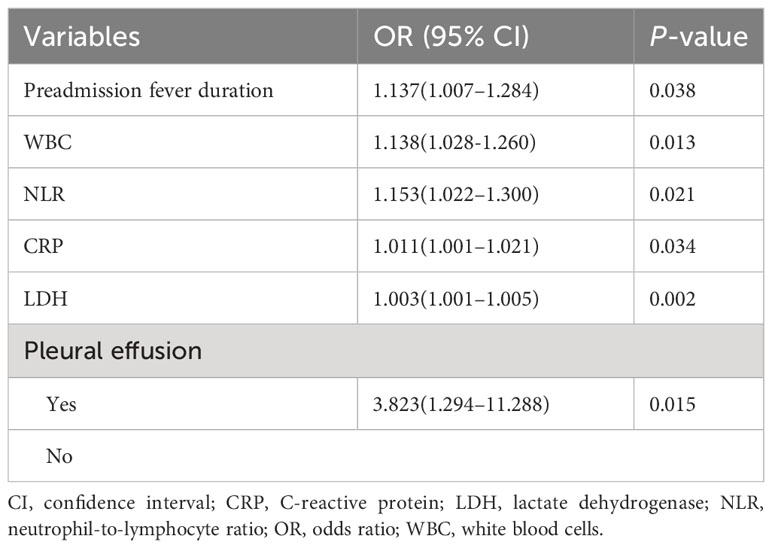
Multivariate analysis showed that the NLR was associated with the development of NP after adjusting for other variables (odds ratio 1.153, 95% confidence interval 1.022–1.300, P=0.021) (Table 2). The NLR was, therefore, considered an independent risk factor for NP.
ROC curves were generated for the variables found to be significant in the logistic regression analysis. The results showed that the NLR was a more effective predictor of NP than pleural effusion, CRP levels, preadmission fever duration, LDH levels, and WBC counts, with AUCs of 0.888, 0.834, 0.827, 0.784, 0.729, and 0.725, respectively (Figure 2A). The median NLR of the entire cohort (1.9) was used to divide patients into two groups: low (<1.9; N=708) and high (≥1.9; N=693) NLR groups. NP developed earlier, and the incidence of NP was significantly higher, in the high NLR group than in the low NLR group (P<0.001; Figure 2B).
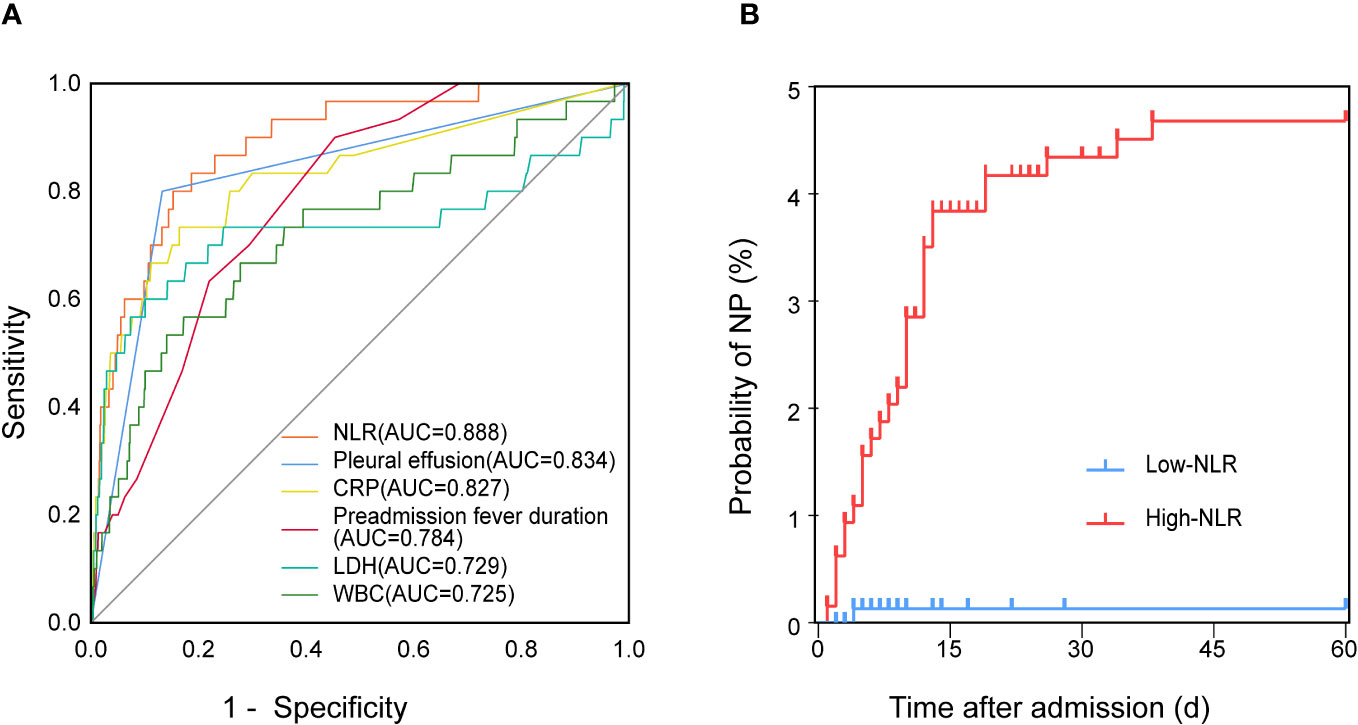
Figure 2 Relationship between the NLR and NP in patients with MPP. (A) ROC curves of the NLR, pleural effusion, CRP levels, preadmission fever duration, LDH levels, and WBC counts for predicting NP. (B) Kaplan–Meier curves of the incidence of NP in patients with a low (<1.9; N=708) and high (≥1.9; N=693) NLR at baseline. AUC, area under the curve; CRP, C-reactive protein; LDH, lactate dehydrogenase; MPP, Mycoplasma pneumoniae pneumonia; NLR, neutrophil-to-lymphocyte ratio; NP, necrotizing pneumonia; ROC, receiver operating characteristic; WBC, white blood cells.
3.2 Association between the NLR and RMPP
A total of 370 (26.4%) patients were diagnosed with RMPP. Patients in the RMPP group were older than those in the non-RMPP group (P<0.001). Patients with RMPP were more likely to have erythema, pleural effusion, and consolidation/atelectasis than those without RMPP (all P<0.005). Preadmission fever duration, WBC counts, the NLR, hemoglobin levels, platelet counts, prothrombin time, activated partial thromboplastin time, and CRP, LDH, ALT, and D-dimer levels were all significantly different between the RMPP and non-RMPP groups (all P<0.05; Table 3).
Multivariate analysis showed that the NLR was associated with the development of RMPP after adjusting for other variables (odds ratio 1.246, 95% confidence interval 1.102–1.408, P<0.001) (Table 4). The NLR was, therefore, considered an independent risk factor for RMPP.
ROC curves were generated for the variables found to be significant in the logistic regression analysis. The results showed that the NLR effectively predicted RMPP, with an AUC of 0.736. The AUCs for consolidation/atelectasis, preadmission fever duration, LDH levels, pleural effusion, ALT levels, and WBC counts were 0.798, 0.763, 0.705, 0.688, 0.678, and 0.576, respectively (Figure 3).
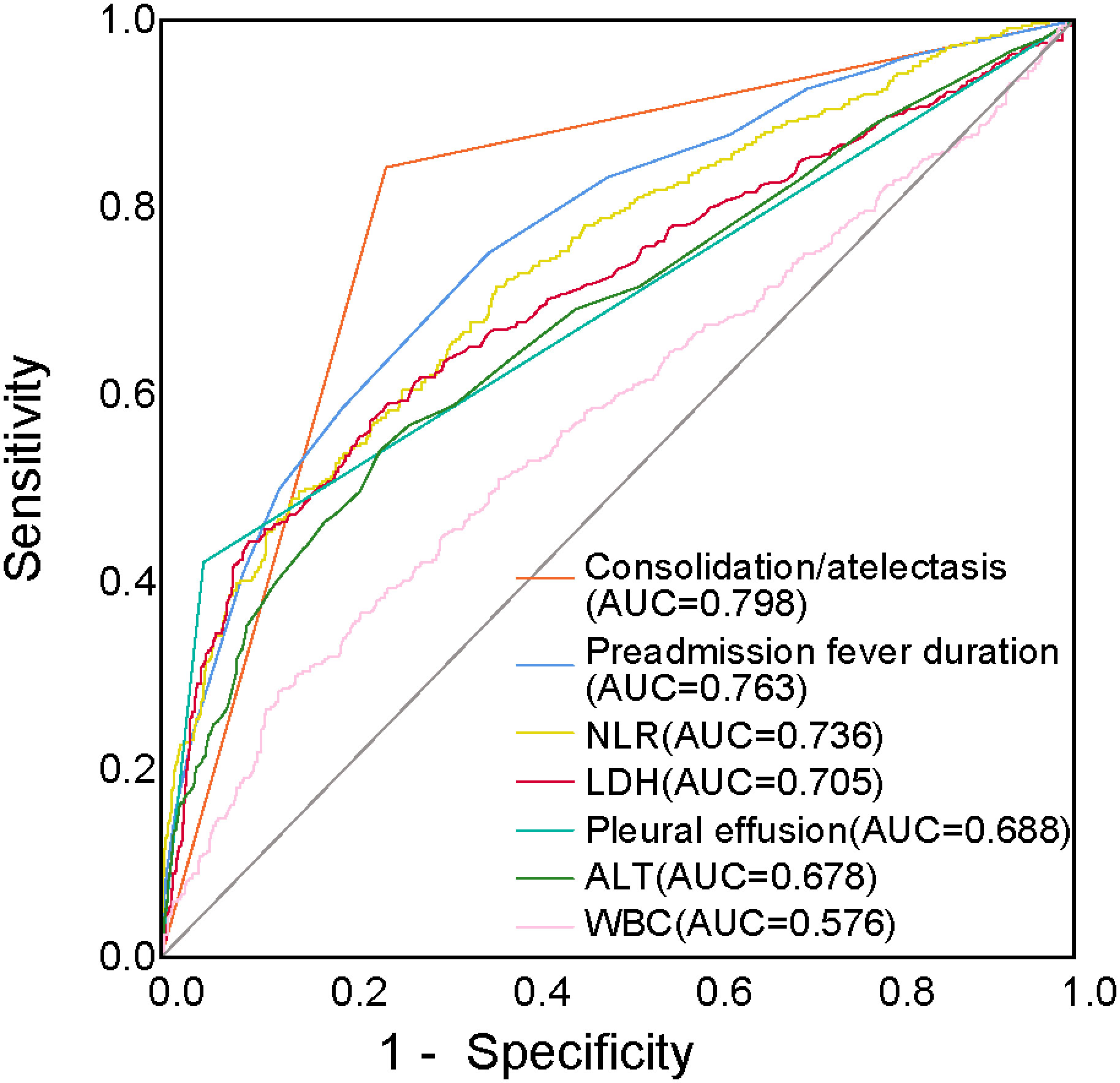
Figure 3 ROC curves of the NLR, consolidation/atelectasis, preadmission fever duration, LDH levels, pleural effusion, ALT levels, and WBC counts for predicting RMPP. ALT, alanine transaminase; AUC, area under the curve; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; RMPP, refractory Mycoplasma pneumoniae pneumonia; ROC, receiver operating characteristic; WBC, white blood cells.
3.3 Association between the NLR and other outcomes
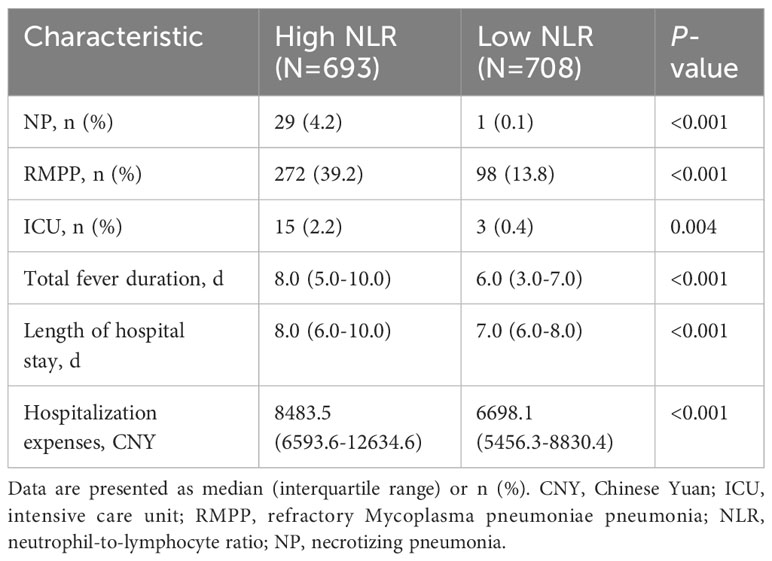
The incidences of NP and RMPP, intensive care unit (ICU) admission, total fever duration, length of hospital stay, and hospitalization expenses were compared between the high (≥1.9; N=693) and low (<1.9; N=708) NLR groups. The high NLR group had higher incidences of NP and RMPP and ICU admission, longer total fever duration and hospital stay, and higher hospitalization expenses than those in the low NLR group (all P<0.005; Table 5).
4 Discussion
The present study prospectively investigated the association between the NLR in peripheral blood and poor outcomes in patients with MPP who were admitted to our department from 2019 to 2021. We set the occurrence of NP as a major adverse outcome. Of the 1,401 patients with MPP, 30 (2.1%) developed NP. This is consistent with the proportion reported by Luo and Wang (6), in a retrospective study of 3,872 children with MPP, 84 (2.2%) of whom developed NP. NP is a rare complication of community-acquired pneumonia, and is most commonly associated with Streptococcus pneumoniae and Staphylococcus aureus (19). However, since Oermann et al. (20) first reported NP resulting from MP infection in 1997, an increasing number of cases have been reported. NP does not usually occur in the early stages of MPP, and diagnosis requires monitoring by chest CT. NP causes considerable lung damage in patients with MPP and recovery is slow. Therefore, early and easy-to-measure prognostic biomarkers are necessary for a better understanding of the clinical course and risk stratification of patients with MPP, which would also permit the early use of glucocorticoids, to improve the poor prognosis.
In our study, patients with erythema, pleural effusion, and consolidation/atelectasis had higher incidences of NP and RMPP than those without these complications, which are associated with the systemic inflammatory response to MP infection (21). We also found that preadmission fever duration, WBC counts, the NLR, and CRP, LDH, ALT, and D-dimer levels were higher in patients with NP or RMPP than those without NP or RMPP. High WBC counts, NLR, and CRP, LDH, and D-dimer levels are indicators of inflammation that reflect a stronger systemic inflammatory response in patients with NP or RMPP than in those without NP or RMPP. These findings indicate that excessive inflammatory and immune responses by the host play important roles in the pathogenesis of NP and RMPP. Our study showed that patients with NP or RMPP were older than those without NP or RMPP.
Our study indicates that the NLR is an independent risk factor for NP and RMPP caused by MP. ROC analysis showed that the NLR predicted the incidence of NP with greater accuracy than pleural effusion, preadmission fever duration, WBC counts, and CRP and LDH levels. The NLR and neutrophil counts are recognized parameters of MPP disease progression. A retrospective observational study (10) reported that a WBC count >12.3×109/L, a neutrophil count >73.9%, and D-dimer levels >1,367.5 ng/mL were risk factors for pulmonary necrosis caused by MP. Another retrospective study (9) concluded that an NLR >3.92 may have important predictive value for RMPP in children over 6 years. However, few studies have focused on the predictive value of the NLR in NP and other adverse outcomes of MPP in children. Our study showed that NP developed earlier, and the incidence of NP was significantly higher, in the high NLR group (≥1.9) than in the low NLR (<1.9) group. Patients with a high NLR were more likely to develop NP or RMPP and require ICU admission than those with a low NLR. They also had a longer total fever duration and hospital stay and higher hospitalization expenses.
The NLR is an inexpensive, widely available, and easily measured biomarker. Neutrophils and lymphocytes are essential components of the immune system. Neutrophils, as part of the first line of defense against infections, play a crucial role in the immune response to MP (22), and their numbers in peripheral blood (23), bronchoalveolar lavage fluid (24), and lung tissue (25) increase after MP infection. Neutrophils interact with other cells, including endothelial cells and platelets, to form a link between inflammation and thrombosis (26); overactivation can lead to tissue damage (27). However, excessive inflammation may induce the apoptosis of lymphocytes, resulting in a decrease in their numbers (28).
Our results indicate that the NLR at admission can predict the prognosis of MPP, providing early treatment guidance. As an inexpensive, easily accessible indicator that is independently associated with NP, RMPP, and other adverse outcomes of MPP, the NLR is a promising prognostic biomarker. It is also easily obtained in primary hospitals prior to CT, allowing early identification and referral of critically ill patients. Future large, prospective, multicenter, cohort studies are needed to confirm the predictive value of the NLR for MPP.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical approval was granted by the research ethics committee of Children’s Hospital of Nanjing Medical University (approval number: 201812257-1). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
DL: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. HG: Funding acquisition, Writing – review & editing, Investigation, Data curation, Project administration. LC: Investigation, Writing – review & editing. RW: Investigation, Writing – review & editing. YJ: Formal analysis, Writing – review & editing. XH: Software, Writing – review & editing. DZ: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. FL: Funding acquisition, Supervision, Writing – review & editing, Formal analysis, Investigation, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China [grant numbers 82270008 and 82200008] and Nanjing Health Bureau Medical Science and Technology Development Foundation [grant number YKK22162].
Acknowledgments
The authors thank all the nursing staff working in our department for maintaining detailed patient records, which contributed greatly to this research. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Oumei H, Xuefeng W, Jianping L, Kunling S, Rong M, Zhenze C, et al. Etiology of community-acquired pneumonia in 1500 hospitalized children. J Med Virol (2018) 90(3):421–8. doi: 10.1002/jmv.24963
2. Lee KL, Lee CM, Yang TL, Yen TY, Chang LY, Chen JM, et al. Severe mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010-2019. J Formos Med Assoc (2021) 120(1 Pt 1):281–91. doi: 10.1016/j.jfma.2020.08.018
3. Zhu Z, Zhang T, Guo W, Ling Y, Tian J, Xu Y. Clinical characteristics of refractory mycoplasma pneumoniae pneumonia in children treated with glucocorticoid pulse therapy. BMC Infect Dis (2021) 21(1):126. doi: 10.1186/s12879-021-05830-4
4. Wang X, Zhong LJ, Chen ZM, Zhou YL, Ye B, Zhang YY. Necrotizing pneumonia caused by refractory mycoplasma pneumonia pneumonia in children. World J Pediatr (2018) 14(4):344–9. doi: 10.1007/s12519-018-0162-6
5. Yang B, Zhang W, Gu W, Zhang X, Wang M, Huang L, et al. Differences of clinical features and prognosis between mycoplasma pneumoniae necrotizing pneumonia and non-mycoplasma pneumoniae necrotizing pneumonia in children. BMC Infect Dis (2021) 21(1):797. doi: 10.1186/s12879-021-06469-x
6. Luo Y, Wang Y. Risk prediction model for necrotizing pneumonia in children with mycoplasma pneumoniae pneumonia. J Inflammation Res (2023) 16:2079–87. doi: 10.2147/JIR.S413161
7. Lemaitre C, Angoulvant F, Gabor F, Makhoul J, Bonacorsi S, Naudin J, et al. Necrotizing pneumonia in children: report of 41 cases between 2006 and 2011 in a french tertiary care center. Pediatr Infect Dis J (2013) 32(10):1146–9. doi: 10.1097/INF.0b013e31829be1bb
8. Zhou Y, Wang J, Chen W, Shen N, Tao Y, Zhao R, et al. Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory mycoplasma pneumoniae pneumonia. BMC Infect Dis (2020) 20(1):2. doi: 10.1186/s12879-020-05356-1
9. Ling Y, Ning J, Xu Y. Explore the predictive value of peripheral blood cell parameters in refractory mycoplasma pneumoniae pneumonia in children over 6 years old. Front Pediatr (2021) 9:659677. doi: 10.3389/fped.2021.659677
10. Zheng B, Zhao J, Cao L. The clinical characteristics and risk factors for necrotizing pneumonia caused by mycoplasma pneumoniae in children. BMC Infect Dis (2020) 20(1):391. doi: 10.1186/s12879-020-05110-7
11. Yang M, Meng F, Wang K, Gao M, Lu R, Li M, et al. Interleukin 17a as a good predictor of the severity of mycoplasma pneumoniae pneumonia in children. Sci Rep (2017) 7(1):12934. doi: 10.1038/s41598-017-13292-5
12. Huang X, Li D, Liu F, Zhao D, Zhu Y, Tang H. Clinical significance of D-dimer levels in refractory mycoplasma pneumoniae pneumonia. BMC Infect Dis (2021) 21(1):14. doi: 10.1186/s12879-020-05700-5
13. Xie Q, Zhang X, Cui W, Pang Y. Construction of a nomogram for identifying refractory mycoplasma pneumoniae pneumonia among macrolide-unresponsive mycoplasma pneumoniae pneumonia in children. J Inflammation Res (2022) 15:6495–504. doi: 10.2147/JIR.S387809
14. Wan H, Wang Y, Fang S, Chen Y, Zhang W, Xia F, et al. Associations between the neutrophil-to-lymphocyte ratio and diabetic complications in adults with diabetes: A cross-sectional study. J Diabetes Res (2020) 2020:6219545. doi: 10.1155/2020/6219545
15. Angkananard T, Anothaisintawee T, Ingsathit A, McEvoy M, Silapat K, Attia J, et al. Mediation effect of neutrophil lymphocyte ratio on cardiometabolic risk factors and cardiovascular events. Sci Rep (2019) 9(1):2618. doi: 10.1038/s41598-019-39004-9
16. Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol (2012) 19(1):217–24. doi: 10.1245/s10434-011-1814-0
17. Mikolasch TA, George PM, Sahota J, Nancarrow T, Barratt SL, Woodhead FA, et al. Multi-center evaluation of baseline neutrophil-to-lymphocyte (Nlr) ratio as an independent predictor of mortality and clinical risk stratifier in idiopathic pulmonary fibrosis. EClinicalMedicine (2023) 55:101758. doi: 10.1016/j.eclinm.2022.101758
18. Dymicka-Piekarska V, Dorf J, Milewska A, Lukaszyk M, Kosidlo JW, Kaminska J, et al. Neutrophil/lymphocyte ratio (Nlr) and lymphocyte/monocyte ratio (Lmr) - risk of death inflammatory biomarkers in patients with covid-19. J Inflammation Res (2023) 16:2209–22. doi: 10.2147/JIR.S409871
19. Spencer DA, Thomas MF. Necrotising pneumonia in children. Paediatr Respir Rev (2014) 15(3):240–5. doi: 10.1016/j.prrv.2013.10.001
20. Oermann C, Sockrider MM, Langston C. Severe necrotizing pneumonitis in a child with mycoplasma pneumoniae infection. Pediatr Pulmonol (1997) 24(1):61–5. doi: 10.1002/(sici)1099-0496(199707)24:1<61::aid-ppul11>3.0.co;2-8
21. Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev (2017) 30(3):747–809. doi: 10.1128/CMR.00114-16
22. Zhang Z, Wan R, Yuan Q, Dou H, Tu P, Shi D, et al. Cell damage and neutrophils promote the infection of mycoplasma pneumoniae and inflammatory response. Microb Pathog (2022) 169:105647. doi: 10.1016/j.micpath.2022.105647
23. Choi YJ, Jeon JH, Oh JW. Critical combination of initial markers for predicting refractory mycoplasma pneumoniae pneumonia in children: A case control study. Respir Res (2019) 20(1):193. doi: 10.1186/s12931-019-1152-5
24. Guo L, Liu F, Lu MP, Zheng Q, Chen ZM. Increased T cell activation in balf from children with mycoplasma pneumoniae pneumonia. Pediatr Pulmonol (2015) 50(8):814–9. doi: 10.1002/ppul.23095
25. Tamiya S, Yoshikawa E, Ogura M, Kuroda E, Suzuki K, Yoshioka Y. Neutrophil-mediated lung injury both via tlr2-dependent production of il-1alpha and il-12 P40, and tlr2-independent cards toxin after mycoplasma pneumoniae infection in mice. Microbiol Spectr (2021) 9(3):e0158821. doi: 10.1128/spectrum.01588-21
26. Blanch-Ruiz MA, Ortega-Luna R, Martinez-Cuesta MA, Alvarez A. The neutrophil secretome as a crucial link between inflammation and thrombosis. Int J Mol Sci (2021) 22(8):2–3. doi: 10.3390/ijms22084170
27. Tsai CY, Hsieh SC, Liu CW, Lu CS, Wu CH, Liao HT, et al. Cross-talk among polymorphonuclear neutrophils, immune, and non-immune cells via released cytokines, granule proteins, microvesicles, and neutrophil extracellular trap formation: A novel concept of biology and pathobiology for neutrophils. Int J Mol Sci (2021) 22(6):9. doi: 10.3390/ijms22063119
28. Tezol O, Bozlu G, Sagcan F, Tuncel Daloglu F, Citak C. Value of neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio and red blood cell distribution width in distinguishing between reactive lymphadenopathy and lymphoma in children. Bratisl Lek Listy (2020) 121(4):287–92. doi: 10.4149/BLL_2020_045
Keywords: neutrophil to lymphocyte ratio (NLR), Mycoplasma pneumoniae pneumonia (MPP), necrotizing pneumonia (NP), refractory Mycoplasma pneumoniae pneumonia (RMPP), outcomes
Citation: Li D, Gu H, Chen L, Wu R, Jiang Y, Huang X, Zhao D and Liu F (2023) Neutrophil-to-lymphocyte ratio as a predictor of poor outcomes of Mycoplasma pneumoniae pneumonia. Front. Immunol. 14:1302702. doi: 10.3389/fimmu.2023.1302702
Received: 26 September 2023; Accepted: 05 December 2023;
Published: 19 December 2023.
Edited by:
Marina De Bernard, University of Padua, ItalyReviewed by:
Shaoyi Zhang, University of California, San Francisco, United StatesKatarzyna Dudek, National Veterinary Research Institute (NVRI), Poland
Copyright © 2023 Li, Gu, Chen, Wu, Jiang, Huang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Liu, YXhzbGl1QDE2My5jb20=; Deyu Zhao, emhhb2RleXU5OEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Dan Li1†
Dan Li1† Haiyan Gu
Haiyan Gu Lei Chen
Lei Chen Xia Huang
Xia Huang Feng Liu
Feng Liu