- 1Department of Biomedical Sciences, Humanitas University, Milan, Italy
- 2Nephrology and Dialysis Unit, IRCCS Humanitas Research Hospital, Milan, Italy
- 3Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 4Clinical Rheumatology Unit, ASST Pini-CTO, Milan, Italy
Objectives: To evaluate the prevalence, incidence, and predictors of herpes zoster (HZ) development in lupus nephritis (LN).
Methods: This retrospective study included 292 LN patients to determine HZ incidence during the last decades and its correlation with LN activity. LN patients with HZ were matched with LN patients without HZ in a 1:2 ratio based on sex, age, year of LN diagnosis, and LN histological class at kidney biopsy to assess HZ risk factors. Statistical tests included t-test, U-test, and Fisher’s test. Univariate and multivariate logistic regression analyses were conducted to identify potential risk factors.
Results: HZ occurred after LN diagnosis in 66 patients (prevalence 22.6%) with an average of 8.7 years (range 0.2–28.4 years). Although with the potential limitations of the retrospective nature and the extensive duration of the study, the incidence of HZ was 15.6/1,000 person-years, increasing from 6.9 before 1980 to 16.0 in the 1990s and 43.9 after 2010. HZ onset was unrelated to LN activity. LN was active in 43% of cases and quiescent in the other 57% of cases at HZ diagnosis. The percentage of patients who developed lupus flares during the year after HZ (18.9%) was not different from that which occurred during the year before HZ (17.2%, p = 0.804). After excluding confounding factors through matching, the univariate analysis suggested that cyclosporin during induction therapy (p = 0.011) and higher cumulative doses of glucocorticoids (GCs; >50 g, p = 0.004), cyclophosphamide (CYC; >5 g, p = 0.001), and mycophenolate mofetil (MMF > 1,000 g, p = 0.007) predisposed patients to HZ. Univariate and multivariate analyses revealed a protective role of azathioprine (p = 0.008) and methylprednisolone pulses (p = 0.010) during induction therapy.
Conclusions: HZ occurs unpredictably throughout the course of LN, underscoring the importance of continuous monitoring for these patients. In addition, the incidence of HZ seems to have increased in recent decades. Induction therapy with azathioprine and methylprednisolone pulses appears to provide protection, while higher cumulative doses of GCs, CYC, and MMF increase susceptibility.
1 Introduction
Lupus nephritis (LN) is the most frequent and severe organ manifestation of systemic lupus erythematosus (SLE) (1). The spectrum of clinical presentation of LN is wide, ranging from silent urinary abnormalities to rapidly progressive renal failure, and the clinical course is characterized by phases of remission alternated by renal or extrarenal reactivation of SLE, called flares (2). The treatment almost always consists of glucocorticoids (GCs) and immunosuppressants (ISs), such as mycophenolate mofetil (MMF) and cyclophosphamide (CYC), and recently biological drugs were also employed (3).
Infections, triggered by immunosuppressive treatments and the impaired immune system during active LN, were in the past a substantial cause of mortality but today represent a concern (4, 5). Frequent bacterial infections include urinary tract infections, pneumonia, and bacteremia without known focus (6).
Herpes zoster (HZ) is considered one of the most frequent viral infections in patients with SLE (7, 8). Varicella zoster virus (VZV), or human herpes virus 3 (HHV3), is a neurotropic human herpes virus belonging to the genus Alphaherpesvirinae. VZV is distributed worldwide and is responsible for varicella (chickenpox) and herpes zoster (shingles) (9). Varicella is the clinical presentation of primary infection and usually infects young individuals. HZ represents a reactivation of VZV in the host and is typically characterized by the appearance of painful inflammation and vesiculation in the dermatome correlated to the ganglionic neurons in which the VZV has become latent after primary infection (10). However, the HZ clinical picture is variable, and some complications may occur, such as myelitis, cranial nerve palsies, meningitis, vasculopathy, retinitis, and gastroenterological infections (11). The incidence of HZ in the general population ranges from 1.2 to 4.9 cases per 1,000 person-years (12, 13). VZV reactivation is triggered by a reduction of cell-mediated immunity, as it occurs in advancing age or immunosuppressive states (cancer, human immunodeficiency virus, and bone marrow or solid organ transplantation and during immunosuppressive therapy in autoimmune diseases) (11).
The epidemiological data on HZ in SLE are scanty and contradictory. In the literature, the prevalence ranges from 3.6% to 30.5%, and the incidence is between 6.4 and 37.7 per 1,000 person-years (7, 14–18). These discrepancies may be attributed to the different sample sizes and ethnicity of patients and the duration of the observation period. Moreover, HZ diagnosis is still nowadays a clinical diagnosis, with the risk of underdiagnosis or misdiagnosis.
Data on HZ in LN are even more scanty. In a retrospective study on 251 Chinese patients with LN, 55 episodes of HZ were observed during the first 2 years after LN diagnosis, determining a prevalence of 18.0% and an incidence of 8.84 per 100 person-years (8).
The timing of HZ onset in relation to SLE activity is not defined. Chen et al. demonstrated a higher risk of HZ within 3–6 months following SLE diagnosis (18), while in the study by Kwan et al., a significant number of cases occurred more than 10 years after SLE diagnosis (7).
To the best of our knowledge, there are no studies in LN that have evaluated the change in the incidence of HZ in LN over the last decades, as well as the relationship between the phase of clinical activity of LN and HZ onset.
To cover this lack of knowledge, we have performed this study with the aims: a) to investigate the changes in the epidemiology of HZ in LN during the last decades, b) to evaluate the correlation between HZ occurrence after LN diagnosis and the phases of clinical activity of LN, c) to establish the impact of HZ on subsequent renal flares and chronic kidney disease (CKD) development, and d) to identify potential risk factors for HZ in LN population.
2 Materials and methods
We retrospectively analyzed the outcome of 292 LN patients followed up at IRCCS Humanitas Research Hospital and included them in the LN database from 1970 onwards. Inclusion criteria were age > 18 years, diagnosis of SLE according to American College of Rheumatology (ACR) criteria (19), and the presence of clinical or biopsy-proven LN. Exclusion criteria were a follow-up shorter than 12 months and the presence of incomplete clinical records.
The diagnosis of HZ was based on the history and physical findings and confirmed by an expert physician. First, we compared the clinical and histological presentation, as well as the therapy administered at the time of LN diagnosis, between patients who subsequently developed HZ and those who did not experience HZ. Then, patients with HZ (cases) were matched with a subgroup (controls) chosen according to sex, age (±5 years) at diagnosis of LN, year of LN diagnosis (±5 years), and histological class at kidney biopsy in a 1:2 case–control ratio.
The cumulative dose of GCs and ISs was calculated by adding the partial doses of the intravenous administration to the partial doses produced by each of the different periods during which the patient received drugs orally. For GCs, the cumulative dose is expressed as the total prednisone-equivalent dose in grams.
The study was approved by the Ethics Committee of IRCCS Humanitas Research Hospital, Milan, Italy (protocol code NEF0032023). All patients signed an informed consent for the scientific use of their data that were anonymized.
2.1 Definitions
SLE: classified according to the 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus (19). All patients diagnosed before 2019 were reclassified based on ACR 2019 criteria (19).
LN: diagnosed on clinical grounds or with kidney biopsies. Kidney biopsies were classified according to the 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) criteria, revised in 2018 (20, 21). Renal biopsies performed before 2002 were reclassified according to the last ISN/RPS classification (22). Class II and V were considered “non-proliferative forms”, while III and IV associated or not with class V were considered “proliferative forms”. Chronicity index (CI) and activity index (AI) were assessed according to Austin et al. (21, 23). In patients in which renal biopsy was not feasible, LN diagnosis was defined as impaired kidney function and/or urinary signs of renal involvement not explained by causes other than LN in the presence of a confirmed SLE diagnosis.
Normal kidney function: serum creatinine ≤1 mg/dL and estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Complete renal remission: normal kidney function, inactive urinary sediment (<5 red blood cells per higher power field and no erythrocyte casts) and proteinuria ≤0.5 g/day. Partial renal remission: ≥50% reduction in proteinuria to sub-nephrotic levels and normal or near normal eGFR (24).
CKD: GFR < 60 mL/min per 1.73 m2 by the CKD-EPI equation or markers of kidney damage for >3 months (25).
Proteinuria: measured by benzethonium chloride on the urine collected over 24 hours expressed as g/day.
SLE flare: defined as an increase in disease activity, both extrarenal and renal, requiring changes or intensification of therapy (26). Renal flares were defined as follows:
● Nephritic flare: increase in serum creatinine of at least 30% over the last value associated with nephritic urinary sediment, with or without increased proteinuria (27).
● Proteinuric flare: increase in proteinuria of at least 2 g/day if the previous proteinuria was <3.5 g/day or a doubling if the previous proteinuria was ≥3.5 g/day (27).
HZ therapy: according to best practice guidelines, HZ therapy consisted of acyclovir or valaciclovir (28). Dose and duration were determined considering the severity of the infection and renal function.
2.2 Statistical analysis
Demographic and clinical data are expressed as numbers or percentages in the case of discrete variables, whereas in the case of continuous variables, they are expressed as median and interquartile range (IQR) or average ± standard deviation (SD), where appropriate. The demographic characteristics were analyzed using Student’s t-test, the Mann–Whitney U-test, and Fisher’s test, where appropriate. Univariate and multivariate logistic regression models, testing clinically and histologically relevant variables, were performed to identify potential risk factors for HZ reactivation. The Kaplan–Meier estimate was used to draw survival curves, and the Mantel–Cox log-rank test was used to test their difference. Statistical significance was defined as p < 0.05. Data were analyzed using STATA version 16.1 (StataCorp).
3 Results
3.1 Patient cohort
A total of 292 patients were enrolled in this study (Table 1), of which 89% were female and 267 (91.4%) were White. The median duration of SLE before the diagnosis of LN was 3.4 years (IQR 0–4.4). Clinical presentation at the onset of LN encompassed normal renal function in 121 (41.4%) patients, nephrotic syndrome in 145 (49.7%), and arterial hypertension in 145 (49.7%).
Seventeen patients (5.8%) were not subjected to kidney biopsy. In 275 patients (94.2%), renal biopsy revealed 62 cases of class III or III+V, 147 cases of class IV or class IV+V, 55 cases of pure membranous LN (class V), 10 cases of class I or II LN, and one case of class VI.
At baseline, 226 patients received intravenous methylprednisolone pulses (MP pulses) in three subsequent days, followed by prednisone 0.5 mg/kg/day for 4 weeks, and then tapered to 7.5–5 mg/day. Another 47 patients were treated with prednisone 1 mg/kg/day for 4 weeks, and then the dosage was progressively tapered. In addition to GCs, immunosuppressive drugs were used in 227 (77.7%) of patients as induction therapy. None of the patients were treated with JAK inhibitors anifrolumab or baricitinib. The only biologic drug used was rituximab (RTX). Hydroxychloroquine was taken by 69 patients (23.6%) at baseline and in another 38% of patients during the follow-up.
After a median follow-up of 15.0 (IQR 6.1–24.9) years, 54 patients (18.7%) developed CKD. Of them, 19 (6.5%) developed end-stage kidney disease (ESKD), and 40 (13.7%) patients died.
None of the patients received HZ vaccination. At least one episode of HZ reactivation was present after LN diagnosis in the medical history of 66 patients (22.6%). In Supplementary Table 1, a comparison between the characteristics of patients who developed or did not develop HZ is reported.
3.2 Severe forms of HZ
Only two out of the 66 patients (3%) developed a severe form of HZ that required hospitalization. A 60-year-old woman presented with cutaneous HZ in the gluteal region 3 years after LN diagnosis and subsequently developed transverse myelitis at the D6–D7 level with a motor deficit in the right lower limb. HZ myelitis was confirmed by polymerase chain reaction (PCR) for varicella zoster virus in the cerebrospinal fluid. The patient was receiving treatment with 15 mg/day of prednisone (cumulative dose 6.9 g) and 1.5 g/day of MMF (cumulative dose 689.0 g). Hydroxychloroquine was not prescribed due to the presence of glucose-6-phosphate dehydrogenase deficiency. For HZ, she was treated initially with valaciclovir and shifted to acyclovir after the diagnosis of transverse myelitis. Despite the resolution of HZ reactivation, the patient had residual difficulty in walking. The second patient was a 27-year-old woman; 11 months after the diagnosis of LN, she presented with widespread and extensive cutaneous HZ involvement in the right lower limb that required hospitalization due to functional impotence of the right leg accompanied by paresthesia. The patient was receiving treatment with 20 mg/day of prednisone (cumulative dose 3.3 g), 2.0 g/day of MMF (cumulative dose 631.5 g), and 200 mg/day of hydroxychloroquine. Of note, 2 months after HZ, an IgG-Kappa monoclonal gammopathy of low-intermediate risk developed. In all the other patients, the clinical course of HZ was not complicated. During the acute HZ phase, MMF was withdrawn in both patients.
3.3 Prevalence and incidence of HZ during the last five decades
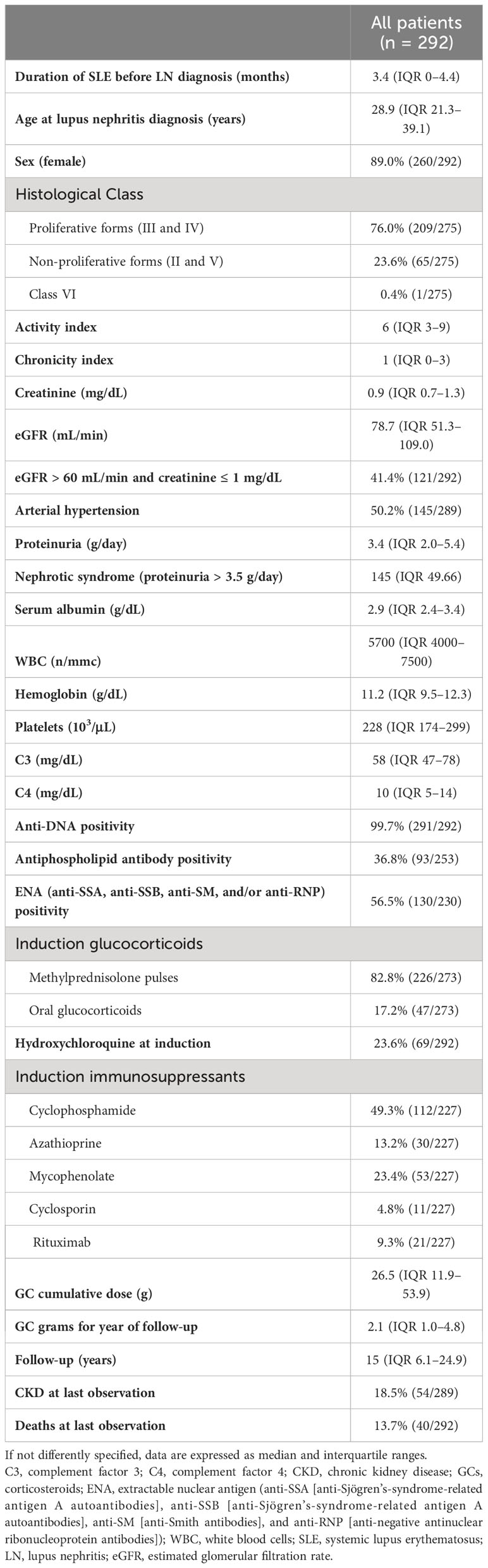
The prevalence of HZ in our cohort was 22.6%, and the incidence was 15.6 per 1,000 person-years, increasing from 6.9 before 1980 to 16.0 in the 1990s and 43.9 after 2010 (Figure 1A). The average time between LN diagnosis and HZ was 8.7 years (IQR 1.8–14.7), with 14% of patients developing HZ within 1 year after the LN diagnosis (10% within 6 months), 19% between 1 and 3 years, 12% between 3 and 5 years, and the remaining 55% after 5 years from the LN diagnosis (Figure 1B).
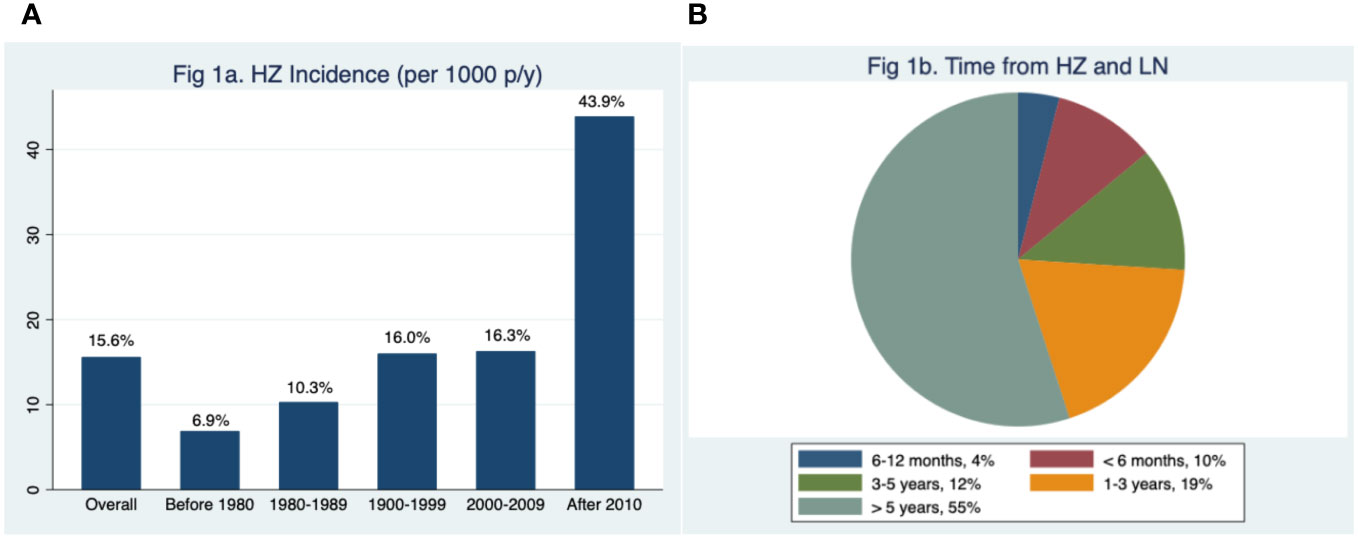
Figure 1 Incidence through decades of herpes zoster (HZ) reactivation in lupus nephritis (LN) (A). Time from LN diagnosis and first HZ reactivation episode. p/y, person-year (B).
3.4 Relationship between HZ activity and outcome of LN
Figure 2, which reports the occurrence of HZ in the different phases of LN activity, demonstrates that the infection can develop both during active and quiescent LN. Out of the 66 patients with at least one episode of HZ, 29 patients (43.9%) developed HZ during an active phase of the disease (4 [6.1%] during a nephritic flare, 10 [15.1%] during a proteinuric flare, and 15 [22.7%] during partial remission of LN), 36 patients (54.6%) during complete remission, and one (1.5%) after the development of CKD. Considering the ongoing LN therapies at the time of HZ infection (Supplementary Table 2), the percentage of patients taking GCs did not differ between patients in the active phase and patients in remission (89.7% vs. 78.4%, p = 0.224), while the GC dose was significantly higher in patients in the active phase (prednisone 11.5 ± 7.0 vs. 6.2 ± 6.3 mg, p = 0.001). No significant difference was observed in the percentage of patients taking hydroxychloroquine or ISs.
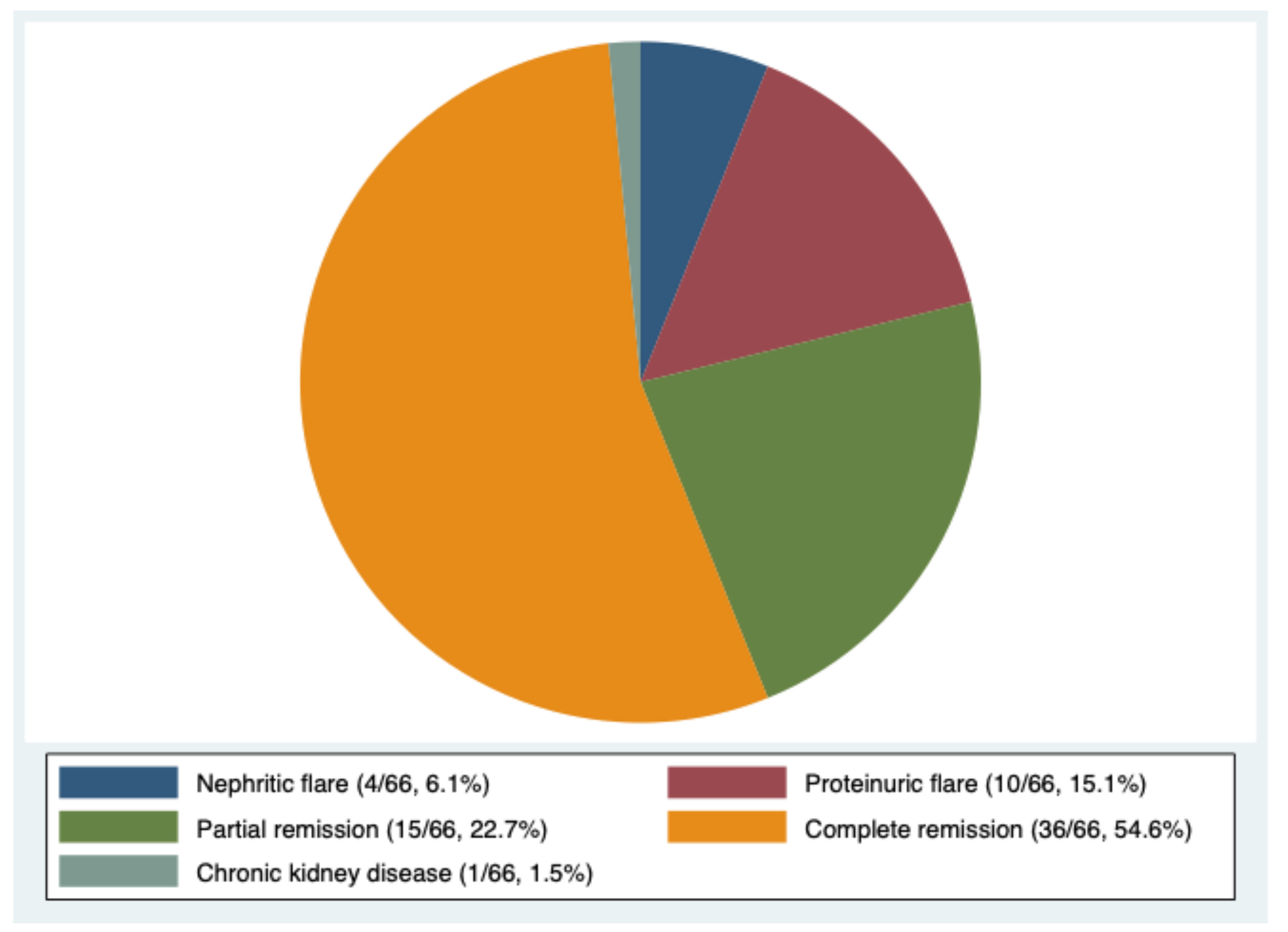
Figure 2 Correlation between lupus nephritis phases and herpes zoster reactivation (percentage of patients).
The development of HZ does not seem to have worsened the renal outcome of LN. During the first year following HZ reactivation, 18.9% of patients in complete or partial remission experienced a renal flare. However, this rate of flare is not different from that observed during the year before HZ (17.2%, p = 0.804). At the end of the observation of a median of 15.0 years (IQR 6.1–24.9), 12.1% of patients who developed and 20.6% of those who did not develop HZ had CKD (p = 0.08). No difference was observed in mortality, which was overall 13.7% (13.6% in cases and 13.7% in controls, p = 1.00).
3.5 Comparison between patients who developed HZ (cases) and matched control group (controls)
Table 2 shows the demographic and clinical characteristics of patients after the matching. We did not observe significant differences in the initial clinical presentation and the activity and chronicity indexes at kidney biopsy between the two groups. We found a trend of more frequent use of oral GCs and less frequent use of MP pulses as induction therapy in HZ patients in comparison to controls (p = 0.106). Moreover, patients treated with oral GCs at induction tended to develop HZ reactivation earlier and more frequently in comparison to those who received MP pulses (p = 0.125), as shown by the Kaplan–Meier plot in Figure 3A. AZA was more commonly used as induction therapy in controls (19.4% controls vs. 4.2% cases, p = 0.02), while cyclosporin (CsA) was more frequently employed in patients who developed HZ (10.4% cases vs. 1.1% controls, p = 0.02). The Kaplan–Meier plot in Figure 3B shows the development of HZ reactivation according to the ISs chosen for the induction. Although not statistically significant, patients treated with MMF and CsA tended to develop HZ reactivation earlier compared to those treated with CYC, AZA, and RTX.
Table 2 Comparison between demographic and clinical characteristics after matching of cases (patients who developed HZ) and controls (patients who did not develop HZ).
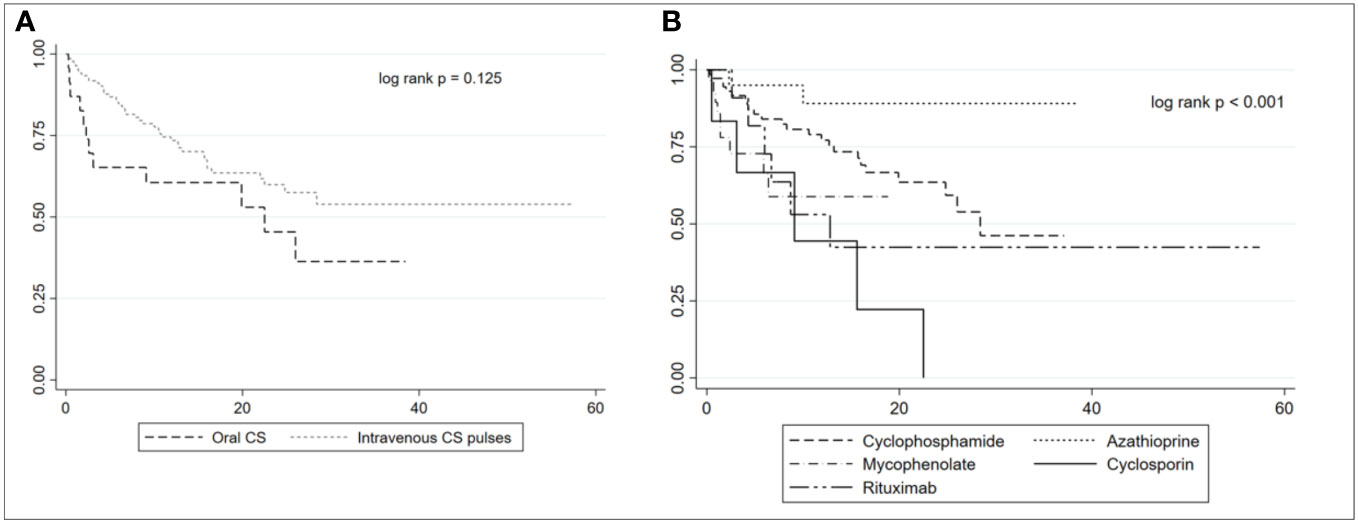
Figure 3 Kaplan–Meier plot of herpes zoster reactivation in patients treated with oral corticosteroids (CSs) or intravenous CS pulses (A). Kaplan–Meier plot of herpes zoster reactivation according to the immunosuppressants used at induction (B).
3.6 Predictors of HZ occurrence at univariate and multivariate analyses
The univariate analysis (Table 3) identified that the use of CsA (p = 0.011) and more than 50 g of GCs (p = 0.004), 5 g of CYC (p = 0.001), and 1,000 g of MMF (p = 0.007) emerged as predisposing factors for the development of HZ. On the contrary, the use of MP pulses (p = 0.010) and AZA (p = 0.008) at induction seemed to exert a protective effect. The multivariate analysis (Table 3), although not statistically significant, suggested the protective effect of MP pulses and AZA at induction, while higher GCs and MMF cumulative dose seemed to predispose to HZ.
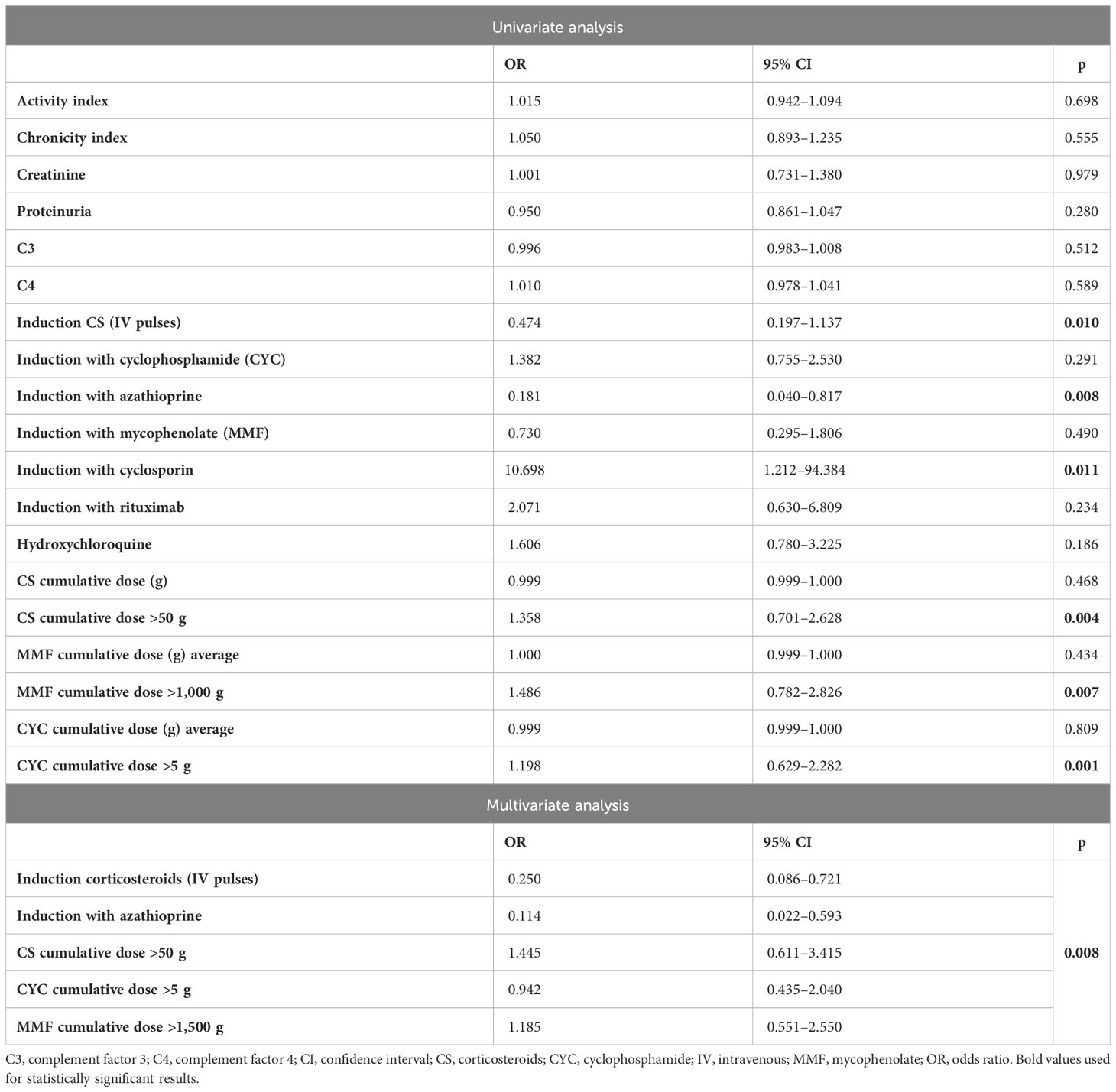
Table 3 Univariate and multivariate logistic regression analyses for the risk of herpes zoster (HZ) development.
4 Discussion
In our LN patients, we found a prevalence of HZ of 22.6% and an incidence of 15.6 per 1,000 person-years; these results are in keeping with those reported in SLE patients (7, 8, 14, 15) and significantly higher compared to those of the general population (incidence 1.2 to 4.9 per 1,000 person-years) (12, 13). Nevertheless, it is essential to acknowledge that the data could be influenced by the study’s retrospective nature, its prolonged duration, and the reliance on clinical reports for HZ diagnosis, potentially introducing biases. There are limited data about the differences in the epidemiology of HZ reactivation between patients with SLE and LN. However, two separate studies on SLE have observed that patients experiencing HZ reactivation have a higher likelihood of having previously encountered severe complications such as LN (14, 15). This finding may be explained by the severe immunosuppressive condition caused by the need for aggressive treatment in LN to induce remission (29) and by the impaired immunity that characterizes the active LN phases. In fact, it has been suggested that dysfunction in T cells, B cells, and NK cells may contribute to infection development in SLE patients (30). Contrary to expectations, we did not observe a higher incidence of HZ during the active phase of LN. Approximately half of the patients in our cohort developed HZ during a period of stable remission of LN, with about a quarter of them receiving no immunosuppressive therapy at the time. These findings suggest that other factors than immunosuppressive therapy and impaired immunological status may predispose HZ.
Our data show a progressive increase in HZ incidence during the last 50 years, with a peak incidence rate after 2010 of 43.9 per 1,000 person-years. These results, which should nevertheless be confirmed by larger and preferably prospective cohort studies, may suggest that HZ is a significant issue in LN patients. The increased HZ incidence during the last decades could be attributed to the increasing use of combined immunosuppressive drugs, including biological drugs, in the treatment of LN, as well as by the progressively more frequent use of ISs in maintenance therapy (29). As a matter of fact, treatment with high-dose GCs (7, 8, 18), ISs (14, 15), or concomitant use of both drugs (16) has been reported as risk factors for HZ in SLE patients (16). In a case–control study in which 65 SLE patients with HZ were matched with 130 SLE patients without HZ, ISs use and GC use emerged as risk factors for HZ (31). In the study by Mok et al., higher doses of prednisolone at induction therapy, peak daily MMF dose, and cumulative CYC dose during induction therapy were significantly associated with HZ reactivation in LN (8). Nonetheless, this study evaluated only the HZ risk during the first 2 years following the diagnosis of LN. A meta-analysis of 17 randomized controlled trials that evaluated the treatments of LN found that the risk of HZ was increased with the use of CYC, MMF, or tacrolimus in combination with GCs, compared with GCs alone (32). To thoroughly explore the role of the treatments in HZ, we matched the patients according to sex, age at diagnosis of LN, year of diagnosis of LN, and histological class at kidney biopsy to exclude confounding factors. At univariate analysis, the use of CsA and higher cumulative dosage of GCs, CYC, and MMF was associated with an increased risk of HZ reactivation. However, at multivariate analysis, CsA did not emerge as an independent predictor probably because of the small number of patients treated with this drug. At univariate analysis, the use of MP pulses was protective against HZ. This trend might be attributed to the fact that the oral GC doses following MP pulses are typically lower compared to the doses used when induction therapy commences with oral GCs. Patients treated with oral GCs as induction therapy showed, at the Kaplan–Meier curves, not only a higher but also an early occurrence of HZ than those receiving induction with MP pulses. Furthermore, we have found that the use of AZA during induction therapy may be protective against HZ at univariate analysis. Unfortunately, multivariate analysis was unable to identify independent predictors of HZ.
In addition to immunosuppression, in this study, we did not find other possible predictors of HZ reactivation among the clinical, histological, and immunological features at diagnosis of LN. Moreover, the fact that more than half of HZ cases occur after 5 years confirms that HZ reactivation is difficult to predict in LN. HZ usually has a mild clinical presentation (10); however, also severe complications may occur (11). Only two patients in our cohort had a severe form of HZ with sequelae. Therefore, considering the unpredictability of HZ occurrence and the possibility of a severe presentation, vaccination should be recommended. The effectiveness of HZ vaccines, notably the recombinant zoster vaccine, in preventing HZ has been demonstrated in both healthy older individuals and selected immunocompromised populations (33, 34). Kidney Disease: Improving Global Outcomes (KDIGO) guidelines already encourage vaccination in LN (35), and, more recently, ACR guidelines recommend vaccinations in subjects with rheumatic disease (36). Since in patients who are receiving strong immunosuppression there is a risk of infection after the administration of live attenuated vaccines and a possible reduction of immunogenicity of vaccines (37, 38), vaccination should be performed with recombinant zoster vaccine during periods of disease quiescence in which immunosuppression is minimal or before starting IS treatment.
A limitation of this study is the retrospective nature, and we cannot exclude that we may have underestimated the number of HZ cases in the early years of the study. Furthermore, we were unable to assess the real impact of hydroxychloroquine therapy on the potential development of HZ. In fact, not all our patients received hydroxychloroquine at the time of their LN diagnosis, as suggested by guidelines nowadays (3), especially for those diagnosed in the 1970s and 1980s. This could be attributed to the limited utilization of this medication in nephrology units during the early years of our study.
Finally, while the relatively small sample size in this study calls for multi-center studies with larger sample sizes to better explore the predisposing factors of HZ in LN, a strength of this study is the long follow-up of the patients, which allowed for analysis of the long-term outcome in patients with LN who developed HZ.
5 Conclusions
This study confirmed that HZ, with a prevalence of 22.6% and an incidence of 15.6 per 1,000 person-years, represents a more common complication among individuals with LN compared to the general population. Furthermore, with more than half of the cases occurring 5 years from the diagnosis of LN, regardless of the disease stages, HZ is an unpredictable complication. Taking into consideration these observations and the fact that severe forms are possible, vaccination should be advocated. However, establishing the timing of vaccination is not easy. If it has not been conducted before the diagnosis of LN, it is reasonable to suggest its use during a quiescent phase when the dose of immunosuppressants is minimal. The use of AZA and MP pulses at induction turned out to be protective. However, higher cumulative doses of CS, CYC, and MMF seemed to be predisposing factors. However, multi-center studies on a larger sample size are required to better explore the predisposing factors of HZ in LN and to develop a tailored approach to this complication.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of IRCCS Humanitas Research Hospital, Milano, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SC: Data curation, Investigation, Writing – review & editing. FT: Conceptualization, Data curation, Formal analysis, Writing – review & editing. MC: Investigation, Supervision, Writing – review & editing. LL: Investigation, Visualization, Writing – review & editing. MG: Conceptualization, Supervision, Writing – review & editing. NP: Conceptualization, Supervision, Writing – review & editing. GM: Conceptualization, Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was partially supported by “Ricerca Corrente” funding from Italian Ministry of Health to IRCCS Humanitas Research Hospital.
Acknowledgments
The authors thank Professor Renato Sinico for reviewing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EB declared a shared parent affiliation with the authors MG and NP to the handling editor at the time of review.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1293269/full#supplementary-material
References
1. Anders H-J, Saxena R, Zhao M, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers (2020) 6:1–25. doi: 10.1038/s41572-019-0141-9
2. Gasparotto M, Gatto M, Binda V, Doria A, Moroni G. Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatol (Oxford) (2020) 59(Suppl5):v39–v51. doi: 10.1093/rheumatology/keaa381
3. Fanouriakis A, Kostopoulou M, Cheema K, Anders H-J, Aringer M, Bajema I, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis (2020) 79:713–23. doi: 10.1136/annrheumdis-2020-216924
4. Singh JA, Hossain A, Kotb A, Wells G. Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis. BMC Med (2016) 14:137. doi: 10.1186/s12916-016-0673-8
5. Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med (1976) 60:221–5. doi: 10.1016/0002-9343(76)90431-9
6. Bosch X, Guilabert A, Pallarés L, Cervera R, Ramos-Casals M, Bové A, et al. Infections in systemic lupus erythematosus: a prospective and controlled study of 110 patients. Lupus (2006) 15:584–9. doi: 10.1177/0961203306071919
7. Kwan A, Rayes HA, Lazova T, Anderson N, Bonilla D, Su J, et al. Herpes zoster in SLE: prevalence, incidence and risk factors. Lupus Sci Med (2022) 9:e000574. doi: 10.1136/lupus-2021-000574
8. Mok CC, Tse SM, Chan KL, Ho LY. Prevalence and risk factors of herpes zoster infection in patients with biopsy proven lupus nephritis undergoing immunosuppressive therapies. Lupus (2020) 29:836–44. doi: 10.1177/0961203320923739
9. Patil A, Goldust M, Wollina U. Herpes zoster: A review of clinical manifestations and management. Viruses (2022) 14:192. doi: 10.3390/v14020192
10. Rajbhandari L, Shukla P, Jagdish B, Mandalla A, Li Q, Ali MA, et al. Nectin-1 is an entry mediator for varicella-zoster virus infection of human neurons. J Virol (2021) 95:e0122721. doi: 10.1128/JVI.01227-21
11. Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. Varicella zoster virus infection. Nat Rev Dis Primers (2015) 1:1–18. doi: 10.1038/nrdp.2015.16
12. Jih J-S, Chen Y-J, Lin M-W, Chen Y-C, Chen T-J, Huang Y-L, et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm Venereol (2009) 89:612–6. doi: 10.2340/00015555-0729
13. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis (2004) 4:26–33. doi: 10.1016/s1473-3099(03)00857-0
14. Kahl LE. Herpes zoster infections in systemic lupus erythematosus: risk factors and outcome. J Rheumatol (1994) 21:84–6.
15. Manzi S, Kuller LH, Kutzer J, Pazin GJ, Sinacore J, Medsger TA, et al. Herpes zoster in systemic lupus erythematosus. J Rheumatol (1995) 22:1254–8.
16. Borba EF, Ribeiro ACM, Martin P, Costa LP, Guedes LKN, Bonfá E. Incidence, risk factors, and outcome of Herpes zoster in systemic lupus erythematosus. J Clin Rheumatol (2010) 16:119–22. doi: 10.1097/RHU.0b013e3181d52ed7
17. Chen H-H, Chen Y-M, Chen T-J, Lan J-L, Lin C-H, Chen D-Y. Risk of herpes zoster in patients with systemic lupus erythematosus: a three-year follow-up study using a nationwide population-based cohort. Clinics (Sao Paulo) (2011) 66:1177–82. doi: 10.1590/s1807-59322011000700009
18. Chen D, Li H, Xie J, Zhan Z, Liang L, Yang X. Herpes zoster in patients with systemic lupus erythematosus: Clinical features, complications and risk factors. Exp Ther Med (2017) 14:6222–8. doi: 10.3892/etm.2017.5297
19. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol (2019) 71:1400–12. doi: 10.1002/art.40930
20. Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol (2004) 15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d
21. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int (2018) 93:789–96. doi: 10.1016/j.kint.2017.11.023
22. Moroni G, Porata G, Raffiotta F, Quaglini S, Frontini G, Sacchi L, et al. Beyond ISN/RPS lupus nephritis classification: adding chronicity index to clinical variables predicts kidney survival. Kidney360 (2021) 3:122–32. doi: 10.34067/KID.0005512021
23. Austin HA, Boumpas DT, Vaughan EM, Balow JE. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int (1994) 45:544–50. doi: 10.1038/ki.1994.70
24. Moroni G, Gatto M, Tamborini F, Quaglini S, Radice F, Saccon F, et al. Lack of EULAR/ERA-EDTA response at 1 year predicts poor long-term renal outcome in patients with lupus nephritis. Ann Rheum Dis (2020) 79:1077–83. doi: 10.1136/annrheumdis-2020-216965
25. Levin A, Stevens PE, Bilous RW, Coresh J, Francisco ALMD, Jong PED, et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl (2013) 3:1–150. doi: 10.1038/kisup.2012.73
26. Sprangers B, Monahan M, Appel GB. Diagnosis and treatment of lupus nephritis flares–an update. Nat Rev Nephrol (2012) 8:709–17. doi: 10.1038/nrneph.2012.220
27. Moroni G, Quaglini S, Maccario M, Banfi G, Ponticelli C. “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int (1996) 50:2047–53. doi: 10.1038/ki.1996.528
28. Herpes zoster infection - Symptoms, diagnosis and treatment | BMJ Best Practice US. Available at: https://bestpractice.bmj.com/topics/en-us/23 (Accessed June 11, 2023).
29. Montigny PM, Houssiau FA. New treatment options in lupus nephritis. Arch Immunol Ther Exp (Warsz) (2022) 70:11. doi: 10.1007/s00005-022-00647-8
30. He J, Li Z. Dilemma of immunosuppression and infection risk in systemic lupus erythematosus. Rheumatology (2023) 62:i22–9. doi: 10.1093/rheumatology/keac678
31. Zamora LD, MaTM C, Navarra SV. Risk factors for herpes zoster infection among Filipinos with systemic lupus erythematosus. Int J Rheum Dis (2020) 23:197–202. doi: 10.1111/1756-185X.13725
32. Singh JA, Hossain A, Kotb A, Oliveira A, Mudano AS, Grossman J, et al. Treatments for lupus nephritis: A systematic review and network metaanalysis. J Rheumatol (2016) 43:1801–15. doi: 10.3899/jrheum.160041
33. Harbecke R, Cohen JI, Oxman MN. Herpes zoster vaccines. J Inf Dis (2021) 224:S429–42. doi: 10.1093/infdis/jiab387
34. Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis (2020) 79:39–52. doi: 10.1136/annrheumdis-2019-215882
35. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
36. Bass AR, Chakravarty E, Akl EA, Bingham CO, Calabrese L, Cappelli LC, et al. 2022 American college of rheumatology guideline for vaccinations in patients with rheumatic and musculoskeletal diseases. Arthritis Rheumatol (2023) 75:333–48. doi: 10.1002/art.42386
37. Kamei K. Live attenuated vaccines in patients receiving immunosuppressive agents. Pediatr Nephrol (2023) 38(12):3889–900. doi: 10.1007/s00467-023-05969-z
Keywords: herpes zoster, systemic lupus erythematosus, lupus nephritis, immunosuppressive therapy, vaccination
Citation: Reggiani F, Cardi S, Tumminello F, Calatroni M, Locatelli L, Gerosa M, Del Papa N and Moroni G (2023) Herpes zoster in lupus nephritis: experience on 292 patients followed up for 15 years. Front. Immunol. 14:1293269. doi: 10.3389/fimmu.2023.1293269
Received: 12 September 2023; Accepted: 30 October 2023;
Published: 22 November 2023.
Edited by:
Pier Paolo Sainaghi, University of Eastern Piedmont, ItalyReviewed by:
Emanuele Bizzi, ASST Fatebenefratelli Sacco, ItalyMatteo Piga, University of Cagliari, Italy
Copyright © 2023 Reggiani, Cardi, Tumminello, Calatroni, Locatelli, Gerosa, Del Papa and Moroni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Reggiani, ZnJhbmNlc2NvLnJlZ2dpYW5pQGh1bmltZWQuZXU=
 Francesco Reggiani
Francesco Reggiani Silvia Cardi1,2
Silvia Cardi1,2 Marta Calatroni
Marta Calatroni Maria Gerosa
Maria Gerosa Nicoletta Del Papa
Nicoletta Del Papa Gabriella Moroni
Gabriella Moroni