
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Immunol. , 20 December 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1290459
This article is part of the Research Topic Intercellular Metabolic Crosstalk in Gastrointestinal Tumor Microenvironment: Implications for Cancer Immune Escape View all articles
This article is a commentary on:
PFKFB3 overexpression in monocytes of patients with colon but not rectal cancer programs pro-tumor macrophages and is indicative for higher risk of tumor relapse
 Nikolay Shakhpazyan1*
Nikolay Shakhpazyan1* Liudmila Mikhaleva1
Liudmila Mikhaleva1 Arcady Bedzhanyan2
Arcady Bedzhanyan2 Nikolay Sadykhov1
Nikolay Sadykhov1 Konstantin Midiber1,3
Konstantin Midiber1,3 Alexander Orekhov1,4,5
Alexander Orekhov1,4,5By Larionova I, Patysheva M, Iamshchikov P, Kazakova E, Kazakova A, Rakina M, Grigoryeva E, Tarasova A, Afanasiev S, Bezgodova N, Kiselev A, Dobrodeev A, Kostromitskiy D, Cherdyntseva N and Kzhyshkowska J (2023) Front. Immunol. 13:1080501. doi: 10.3389/fimmu.2022.1080501
The evolution and progression of colon cancer, akin to other solid neoplasms, constitute an elaborate nexus of multifarious biological interactions and dependencies. A notable exemplar is metabolic reprogramming, characterized by enhanced glycolytic activity coupled with diminished oxidative phosphorylation. Larionova et al. have convincingly illustrated that monocytes, isolated from the peripheral blood of patients with colon cancer, demonstrate pronounced upregulation of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), a key enzyme in the glycolytic cascade (1). In our assessment, this seminal work paves the way for extensive future research into immunogenesis and inflammation in colon cancer.
In line with recent findings by Larionova et al., which highlighted the marked upregulation of the glycolytic enzyme PFKFB3 in circulating monocytes derived from colorectal cancer patients, our study serves to augment and elaborate upon the current scientific understanding of these metabolic abnormalities. Larionova et al. posited that dysregulated monocyte metabolism plays a significant role in the pathogenesis of colorectal cancer. They further hypothesized a potential correlation between elevated PFKFB3 expression and altered immune functions, substantiated by a transcriptomic analysis that revealed perturbations in several genes, notably those governing the functions of TNF-α and IL-1β cytokines.
These results make us look at our study from a new perspective. Our research was conducted on a more limited cohort, comprising 12 patients diagnosed with colorectal cancer and 9 healthy controls. Employing enzyme-linked immunosorbent assays (ELISA) in cultured monocytes, we identified aberrant secretion patterns of pro-inflammatory cytokines. Specifically, upon a 24-hour stimulation with lipopolysaccharide (LPS), monocytes from colorectal cancer patients displayed elevated secretion levels of TNF-α and IL-1β in the culture medium. Intriguingly, when LPS stimulation was repeated after 7 days, there was a marked reduction in TNF-α secretion compared to the healthy controls, while IL-1β secretion remained consistent (2). Our observations are consistent with previously published data (3, 4).
The divergence in the secretion profiles of TNF-α and IL-1β, regulated predominantly through the NF-κB and NLRP3 inflammasome pathways respectively, suggests an underlying mechanistic dichotomy that merits further investigation. The reduction in TNF-α secretion upon repeated stimulation could indicate an altered metabolic state in monocytes, consistent with the metabolic paradigms proposed by Larionova et al., particularly concerning glycolytic pathways modulated by PFKFB3.
Our data, in synergy with the seminal work of Larionova et al., provide a robust foundation for further, large-scale investigations. Notably, future research could advantageously employ dual LPS stimulation protocols to emulate chronic inflammatory conditions, a pivotal determinant in colorectal cancer pathogenesis. The application of advanced immunological and proteomic methodologies, as contrasted with transcriptomic techniques, is poised to yield a more nuanced depiction of cellular cytokine secretion patterns, thereby contributing to a more comprehensive understanding of immune responses in colorectal cancer.
The metabolic alterations evident in circulating monocytes, specifically the notable upregulation of PFKFB3, establish an auspicious landscape for interdisciplinary research at the intersection of oncology, immunology, and cellular metabolism. Pertinent avenues for exploration include the phenotypic ramifications of PFKFB3 upregulation, the implications of metabolic exhaustion arising from sustained glycolytic activity, and the modulation of inflammatory signaling pathways by these metabolic shifts (5). Additionally, the overlap between metabolic reprogramming and epigenetic alterations offers another compelling domain of inquiry. Metabolic intermediates may act as cofactors for epigenetic enzymes, thereby exerting an influence on the transcriptional regulation of cytokines or other immune-related genes (6). In general, the formation of a pathological circle is possible (Figure 1).
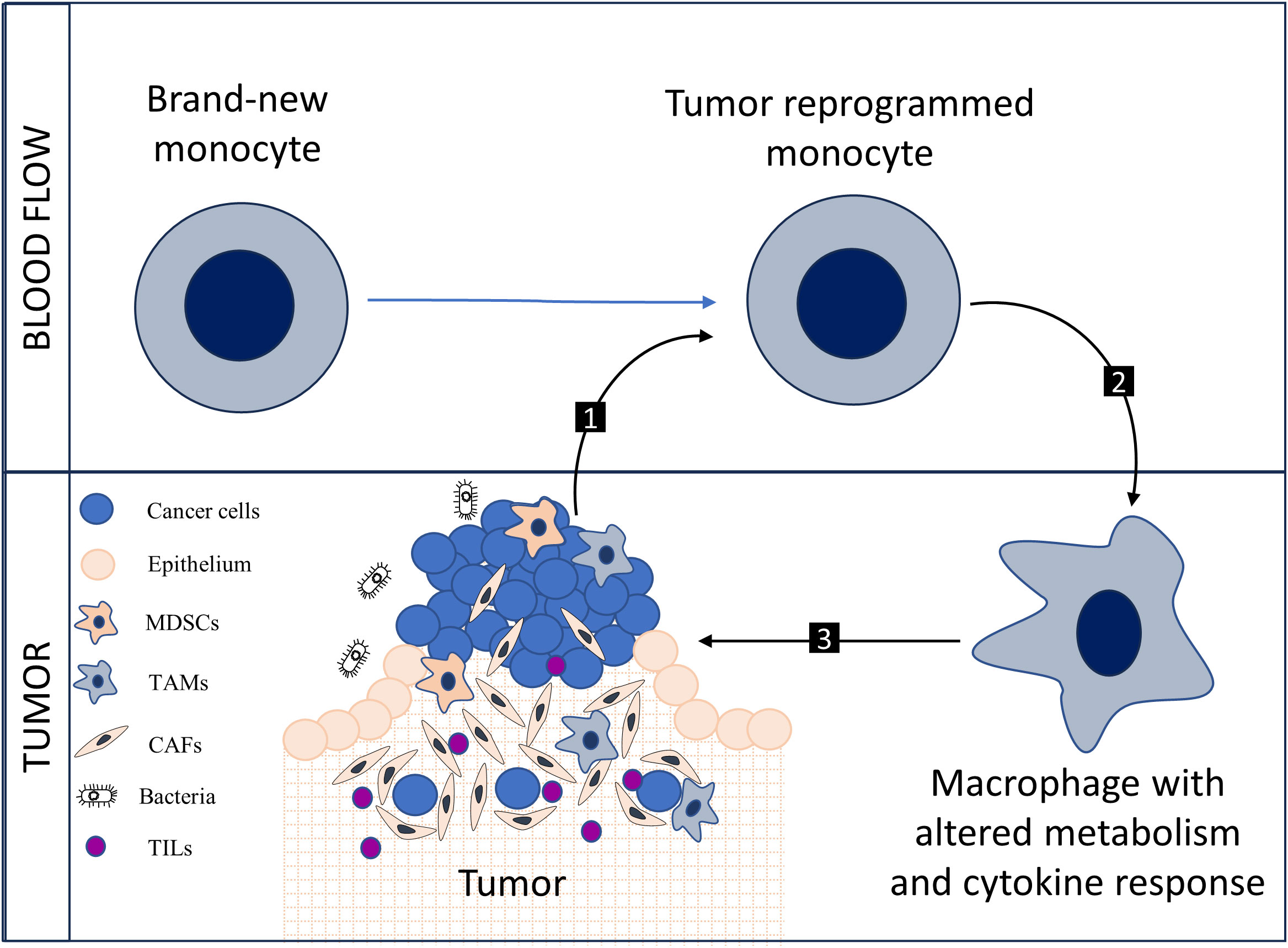
Figure 1 New monocytes undergo metabolic reprogramming under the influence of the tumor {1}, the shift towards glycolytic metabolism alters the cytokine potential of monocytes; monocytes transform into tumor-associated macrophages (TAMs) with modified metabolism and cytokine response {2}, a pro-tumoral interaction occurs between TAMs and components of the tumor stroma, including myeloid-derived suppressor cells (MDSCs), cancer-associated fibroblasts (CAFs), and tumor-infiltrating lymphocytes (TILs) {3}. Collectively, 1, 2, and 3 form a pathological cycle.
We posit that future research in this domain would be significantly enriched by the adoption of dual LPS stimulation protocols, designed to emulate the chronic inflammatory conditions that are integral to the pathogenesis of colorectal cancer. Furthermore, we advocate for the employment of advanced methodologies spanning immunology, metabolomics, epigenetics, and proteomics. These approaches, we argue, could furnish a broader and more direct representation of cellular cytokine secretion compared to traditional transcriptomic techniques, thereby providing a comprehensive and dynamically nuanced portrait of immune responses implicated in this malignancy.
In summary, these diverse research trajectories not only corroborate the invaluable observations initially made by Larionova et al., but also underscore the complexity and multifactorial nature of colorectal cancer pathogenesis. Such avenues furnish a fertile ground for comprehensive studies employing proteomic, metabolomic, and in vitro cell culture methodologies, aimed at disentangling the convoluted mechanisms of immune regulation in colorectal cancer. This, in turn, may pave the way for the development of innovative therapeutic strategies.
NSh: Conceptualization, Visualization, Writing – original draft. LM: Conceptualization, Supervision, Writing – review & editing. AB: Conceptualization, Supervision, Writing – review & editing. NSa: Funding acquisition, Writing – review & editing. KM: Validation, Writing – review & editing. AO: Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Russian Science Foundation, Grant number 23-25-00196.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Larionova I, Patysheva M, Iamshchikov P, Kazakova E, Kazakova A, Rakina M, et al. PFKFB3 overexpression in monocytes of patients with colon but not rectal cancer programs pro-tumor macrophages and is indicative for higher risk of tumor relapse. Front Immunol (2022) 13:1080501. doi: 10.3389/fimmu.2022.1080501
2. Shakhpazyan N, Mikhaleva L, Bedzhanyan A, Sadykhov N, Midiber K, Orekhov A. Altered Proinflammatory Cytokine Response in Colorectal Cancer Patients: Insights into Immune Dysregulation . Available at: https://www.researchsquare.com/article/rs-2956862/v1.
3. Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG. Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer (2000) 82(5):1009–12. doi: 10.1054/bjoc.1999.1034
4. Trejo YG, Bordenave RH, Beviacqua M, Zanoni L, Rumi LS. Tumor necrosis factor-alfa production by monocytes from lung and colorectal cancer patients. J Exp Clin Cancer Res (2001) 20(1):71–3.
5. Padoan A, Plebani M, Basso D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci (2019) 20(3):676. doi: 10.3390/ijms20030676
Keywords: colorectal cancer, inflammation, metabolism, monocytes, cytokines
Citation: Shakhpazyan N, Mikhaleva L, Bedzhanyan A, Sadykhov N, Midiber K and Orekhov A (2023) Commentary: PFKFB3 overexpression in monocytes of patients with colon but not rectal cancer programs pro-tumor macrophages and is indicative for higher risk of tumor relapse. Front. Immunol. 14:1290459. doi: 10.3389/fimmu.2023.1290459
Received: 07 September 2023; Accepted: 11 December 2023;
Published: 20 December 2023.
Edited by:
Qingmin Sun, Affiliated Hospital of Nanjing University of Chinese Medicine, ChinaReviewed by:
Sergei Kusmartsev, University of Florida, United StatesCopyright © 2023 Shakhpazyan, Mikhaleva, Bedzhanyan, Sadykhov, Midiber and Orekhov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolay Shakhpazyan, bnNoYWtocGF6eWFuQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.