
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 November 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1288632
Background: Although numerous studies demonstrated a link between plasma homocysteine (Hcy) levels and psoriasis, there still exists a certain level of controversy. Therefore, we conducted a Mendelian randomization study to investigate whether homocysteine plays a causative role in the development or exacerbation of psoriasis.
Methods: A two-sample Mendelian randomization (MR) analysis was conducted. Summary-level data for psoriasis were acquired from the latest R9 release results from the FinnGen consortium (9,267 cases and 364,071 controls). Single nucleotide polymorphisms (SNPs) robustly linked with plasma Hcy levels at the genome-wide significance threshold (p < 5 × 10−8) (18 SNPs) were recognized from the genome-wide meta-analysis on total Hcy concentrations (n = 44,147 participants) in individuals of European ancestry. MR analyses were performed utilizing the random-effect inverse variance-weighted (IVW), weighted median, and MR-Egger regression methods to estimate the associations between the ultimately filtrated SNPs and psoriasis. Sensitivity analyses were conducted to evaluate heterogeneity and pleiotropy.
Results: MR analyses revealed no causal effects of plasma Hcy levels on psoriasis [IVW: odds ratio (OR) = 0.995 (0.863–1.146), p = 0.941; weighed median method: OR = 0.985 (0.834–1.164), p = 0.862; MR-Egger regression method: OR = 0.959 (0.704–1.305), p = 0.795]. The sensitivity analyses displayed no evidence of heterogeneity and directional pleiotropy, and the causal estimates of Hcy levels were not influenced by any individual SNP.
Conclusion: Our study findings did not demonstrate a causal effect of genetically determined circulating Hcy levels on psoriasis.
Psoriasis is a common inflammatory skin disease manifested as distinct red patches covered in silvery scales (1). Globally, over 60 million people suffer from psoriasis with varying prevalence across different regions. It has been reported that Oceania had the highest prevalence, followed by Western Europe, Central Europe, North America, and East Asia (2). Research also found that white individuals exhibited higher morbidity rates compared with other ethnic populations (3, 4). Children were reported to have lower prevalence and incidence rates than adults (5, 6). The differences in psoriasis prevalence and morbidity can be attributed primarily to the genetic makeup of individuals (7). Through the latest genome-wide association studies (GWASs), researchers have recognized more than 80 psoriasis susceptibility loci, which accounted for approximately 30% of the overall heritability (8).
Actually, psoriasis is not only a skin disease but is also associated with the occurrence of many other diseases including rheumatological, cardiovascular, and inflammatory bowel diseases and metabolic syndrome (9). In recent years, the relationship between psoriasis and its associated disorders has gradually become the research focus, especially for cardiovascular diseases like ischemic stroke, heart failure, myocardial infarction, and atrial fibrillation (10–13). Increasing attention has been paid to homocysteine (Hcy), with its elevated levels in the blood being identified as an independent risk factor for the occurrence of cardiovascular diseases (14, 15). Accordingly, the association between Hcy and psoriasis has drawn considerable attention.
Previous studies have found that patients with psoriasis tended to exhibit significantly higher plasma Hcy levels compared with controls (16–26), some of which also uncovered the association between the severity of psoriasis and circulating Hcy levels, possibly by affecting inflammation and oxidative stress pathways (17, 22, 23). However, some studies demonstrated that no significant differences in Hcy levels existed between patients with psoriasis and healthy individuals (27–30). It is important to note that research in this field is not entirely conclusive and the exact nature of the relationship between Hcy and psoriasis is not fully understood. It is of great interest and significance to investigate whether there is a link between hyperhomocysteinemia and psoriasis. More studies are required and reliable methods need to be employed in research to establish a definitive link and determine the role of Hcy in the development of psoriasis.
Mendelian randomization (MR), a novel epidemiological research method, can be adopted to investigate the causal relationship between exposure and outcome. It has been demonstrated as a dependable approach employing genetic variants to effectively and comparably group individuals based on their genotypes, akin to a randomized trial’s random allocation process (31, 32). Consequently, the challenges posed by confounding and reverse causation, commonly encountered in observational studies, can be circumvented, which enables the estimation of the causal impact of a risk factor on a particular outcome of interest (31–33). Previous research has explored the causal association between psoriasis and alcohol consumption (34), smoking (34), body mass index, vitamin D levels (35, 36), serum lipids (37, 38), COVID-19 (39), inflammatory bowel diseases (40), cardiovascular diseases (10, 41), non-alcoholic liver diseases (42), ankylosing spondylitis (43), and lung cancer (44, 45) via the MR approach. However, there is a scarcity of research investigating the causal effect of plasma Hcy levels on psoriasis. Therefore, we conduct this study adopting MR methods to investigate whether homocysteine contributes causally to the development or exacerbation of psoriasis.
We conducted a two-sample Mendelian randomization (TSMR) analysis to evaluate the causal relationship between circulating Hcy levels and psoriasis. In MR analysis, multiple single nucleotide polymorphisms (SNPs) representing genetic variation chosen as instrumental variables (IVs) should satisfy three crucial assumptions (Figure 1): Firstly, IVs are directly related to the exposure of interest; secondly, there exist no confounding variables between IVs and outcomes; and thirdly, IVs only influence outcomes via exposure but not through other pathways (46, 47).
There are publicly accessible databases resulting from genome-wide association studies, one of which is the FinnGen research project that offers genetic insights from a well-phenotyped isolated population (48). Summary-level data for psoriasis were acquired from the most recent R9 release results from the FinnGen consortium (https://r9.finngen.fi/). The database comprised 9,267 cases with a diagnosis of psoriasis based on the ICD-10 (International Classification of Diseases) diagnostic criteria and 364,071 controls.
SNPs robustly associated with circulating Hcy levels at the genome-wide significance threshold (p < 5 × 10−8) (18 SNPs) were identified from the genome-wide meta-analysis on total Hcy concentrations (n = 44,147 participants) in individuals of European ancestry (Supplementary Table S1) (49). These SNPs explained approximately 6.0% of the variability observed in Hcy levels. To ensure the independence of the selected SNPs, the PLINK clumping approach was used to exclude SNPs in linkage disequilibrium at a threshold of r2 <0.001 within a window size of 10,000 kb in 1000 Genomes data (Europeans) (50). Each of the remaining SNPs was subsequently checked for secondary phenotypes in PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk) to assess whether these SNPs were in relation to other traits that may be potential confounders at a genome-wide significance level (p < 5 × 10−8) (51). The SNPs were then extracted from the GWAS data of the outcome (psoriasis). In this step, the SNPs related to psoriasis at genome-wide significance (p < 5 × 10−8) and those absent in the outcome data (only one SNP) were discarded. After that, the exposure–outcome datasets were harmonized to remove any palindromic SNPs with minor allele frequencies above 0.3 and any incompatible SNPs. Finally, the F-statistics of the ultimately selected SNPs were calculated to assess the strengths of the selected IVs using the formula F = R2 (n − 1 − k)/(1 − R2)k. Here, “n” denotes the sample size, “k” refers to the count of IVs, and “R2” represents the proportion of variation explained by the SNPs. IVs with an F-statistic greater than 10 were regarded to be strongly associated with the exposure of interest (52). Figure 2 illustrates the flowchart of IV selection.
The random-effect inverse-variance weighted (IVW) method was employed as the primary analysis to evaluate the cause relationship between circulating Hcy levels and psoriasis, supplemented by the weighted median and MR-Egger regression methods. The weighted median method provides reliable evidence when no more than 50% of instruments are invalid (53). The MR-Egger regression approach allows a consistent causal estimate even if the genetic IVs are all invalid (54). For MR analyses, statistical significance was set at a two-sided p-value less than 0.05. To assess any bias of the MR assumptions, sensitivity analyses were performed for the recognized significant MR estimates (IVW p < 0.05). Cochrane’s Q value was adopted to evaluate the heterogeneity among the estimates of SNPs (55). Cochrane’s Q-derived p < 0.05 and I2 > 25% were considered heterogeneous. Additionally, the MR pleiotropy residual sum and outlier (MR-PRESSO) test was also used to detect the heterogeneity. To assess horizontal pleiotropy, the intercept in MR-Egger regression was utilized (56). Furthermore, leave-one-out analysis was employed to detect whether there existed any SNP having an independent influence on the overall results.
All statistical analyses were executed adopting the “TwoSampleMR” package (version 0.5.7) (https://github.com/MRCIEU/TwoSampleMR) and the “MR-PRESSO” package (version 1.0) in R software (version 4.3.1). No ethical approval was required since the data used in this study were publicly available.
Originally, 18 SNPs robustly associated with plasma Hcy levels were identified from the genome-wide meta-analysis on circulating Hcy concentrations (49). After clumping, five SNPs in linkage disequilibrium were excluded. Checking each of the remaining 13 SNPs in PhenoScanner, we removed one SNP (rs548987) with potential confounding secondary phenotypes of body mass index and whole body fat mass since there has been an established association between obesity and psoriasis (57, 58). Extracting the residual SNPs from the GWAS data of psoriasis yielded a total of 11 SNPs with 1 SNP (rs234709) discarded because of the absence of this SNP in data of the outcome of psoriasis. All SNPs displayed F-statistics exceeding 10, suggesting they were strong instruments. Table 1 shows the characteristics of the total 11 SNPs included in our analysis.
Figure 3 shows that no significant association existed between Hcy and psoriasis utilizing all 11 SNPs as IVs via MR analysis. As indicated by the random-effect IVW method, the odds ratio (OR) and 95% confidence interval (CI) for each unit rise in circulating Hcy levels within psoriasis were 0.995 (0.863–1.146), p = 0.941, which was consistent with the results of the weighted median method [OR = 0.985 (0.834–1.164), p = 0.862] and the MR-Egger regression method [OR = 0.959 (0.704–1.305), p = 0.795]. A scatter plot depicting the effect sizes of SNPs for Hcy is shown in Figure 4.
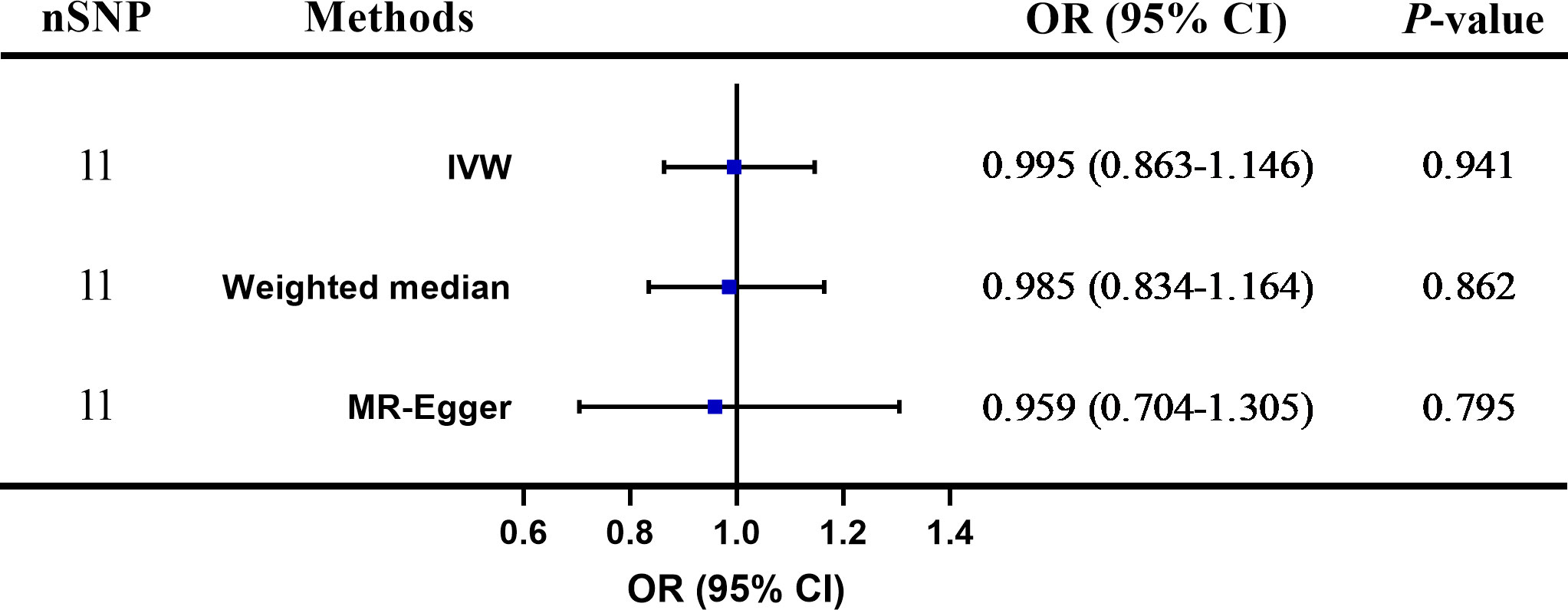
Figure 3 The two-sample Mendelian randomization (TSMR) of plasma homocysteine (Hcy) levels and psoriasis. SNP, single nucleotide polymorphism; IVW, inverse-variance weighted; OR, odds ratio; CI, confidence interval.
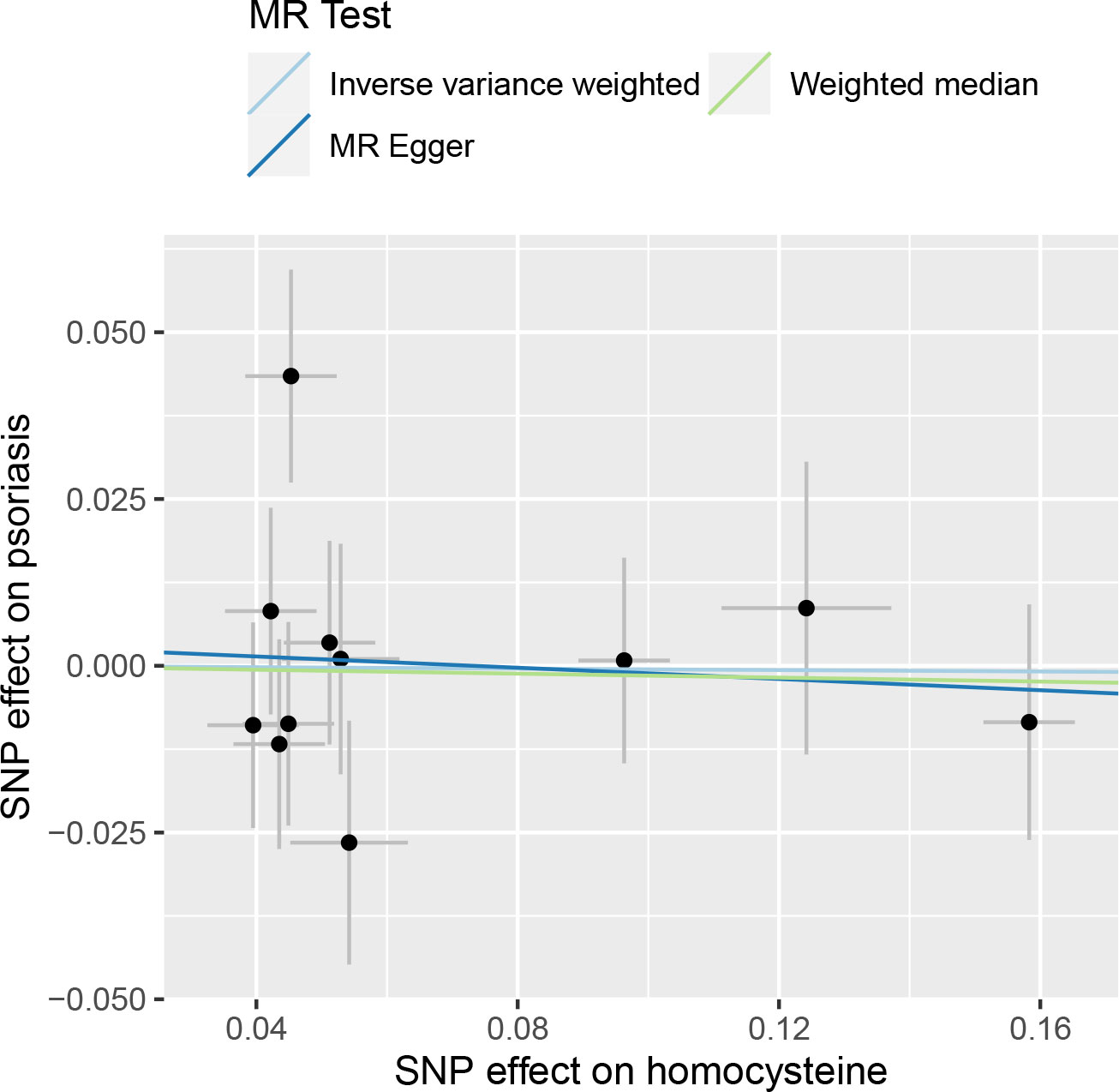
Figure 4 Scatter plot of the causal relationships between homocysteine and psoriasis using different MR methods.
Table 2 presents the results of the sensitivity analysis. Cochrane’s Q test and MR-PRESSO test showed no evidence of heterogeneity. The p-value (p > 0.05) for the MR-Egger intercept indicated no directional pleiotropy. Leave-one-out analysis revealed that the causal estimations of Hcy levels were not influenced by any individual SNP, further validating the lack of association between Hcy and psoriasis (Figure 5).
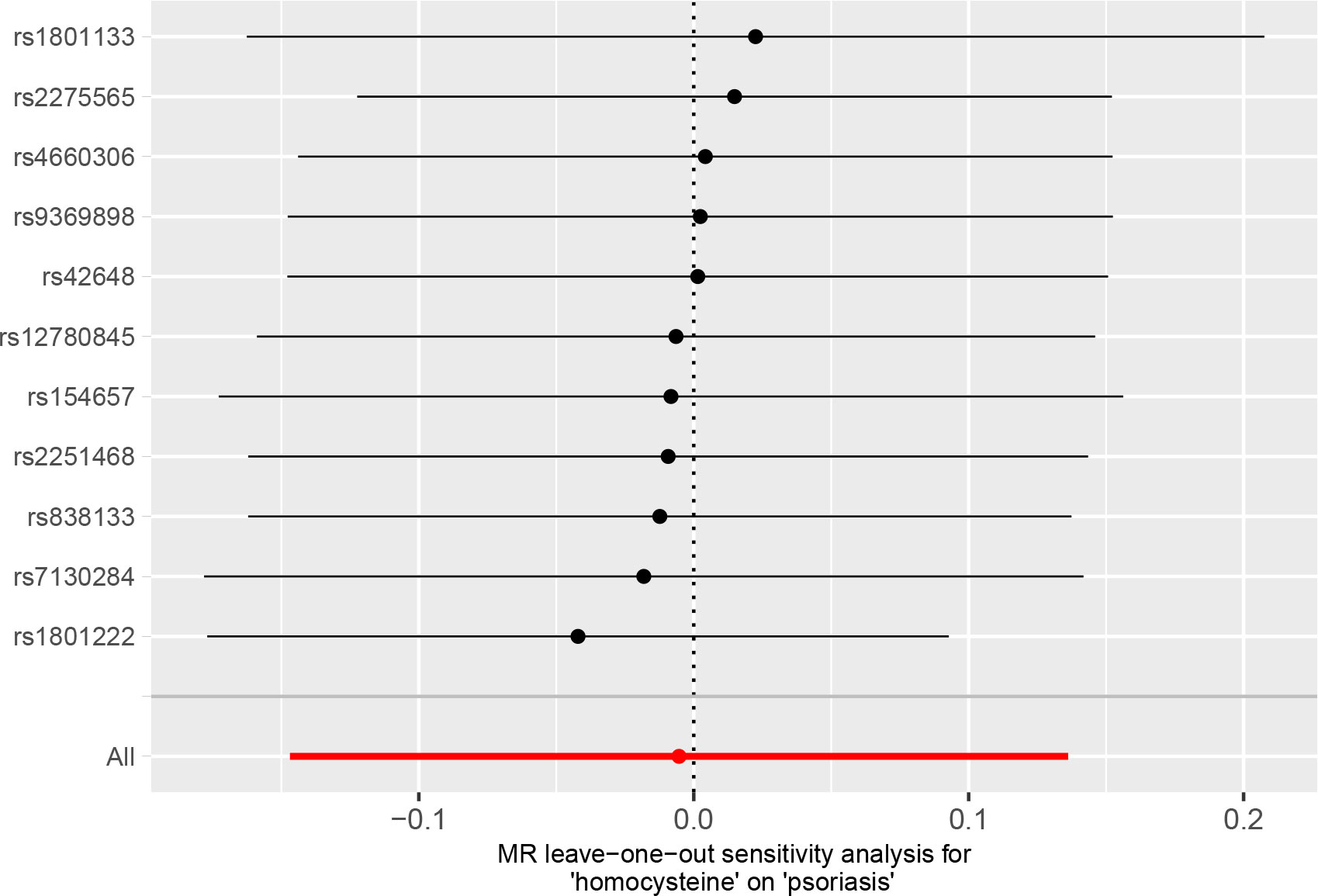
Figure 5 Leave-one-out analysis for the association of plasma homocysteine (Hcy) level with psoriasis.
To our knowledge, this is the first study to investigate the causal effect of plasma Hcy levels on psoriasis from a genetic view through MR analysis methods. Although randomized controlled trials (RCTs) represent the most potent approach for establishing causal hypotheses in epidemiological research, their implementation demands a more stringent research design and incurs higher costs, thereby rendering their execution challenging. MR is a novel epidemiological research method to explore the causal relationship between exposure and outcome. Integrating MR into traditional epidemiology offers a clever solution to address the limitations of conventional research in uncovering causation, including issues related to confounding factors and uncertain causal pathways. This amalgamation opens up novel perspectives and methodologies for investigating the origins of diseases in epidemiological research (59). As the genetic makeup of offspring is inherited in a random manner from their parents, utilizing SNP as a genetic variable tool becomes a highly dependable approach for inferring causal relationships between two factors (31, 32). Consequently, in recent years, some researchers have regarded MR research as the most favorable substitute for RCTs (60). In our research, we investigated the causation between plasma Hcy levels and psoriasis using datasets from large-scale GWASs. The outcomes of our TSMR analysis did not demonstrate a detrimental effect of genetically increased Hcy levels on psoriasis risk. Our results were robust, achieved through the utilization of diverse instrumental variables and analytical methods.
Numerous studies have exhibited a link between elevated plasma Hcy levels and psoriasis. A case–control study found that the average levels of serum total Hcy were elevated in patients with psoriasis and suggested that increased plasma Hcy concentrations might significantly contribute to the emergence of atherothrombotic complications in individuals with psoriasis (16). Brazzelli et al. additionally documented a notably greater occurrence of hyperhomocysteinemia in individuals with psoriasis compared with the healthy controls (19). Similarly, the same findings were discovered in a study conducted by Malerba et al., where a connection between Hcy levels and disease severity was also reported. They suggested that individuals suffering from psoriasis could be potentially inclined to experience hyperhomocysteinemia, a condition that might make them more susceptible to increased cardiovascular risks (17). Additionally, two studies also indicated the association of plasma Hcy concentrations with the severity of psoriasis (22, 23). However, using an MR analysis approach, our findings did not demonstrate a direct causal effect of plasma Hcy levels on psoriasis. Consistent with our results, it was reported by Uslu et al. that no statistically significant differences existed between psoriatic patients and the control group in terms of plasma Hcy levels (p > 0.05) (27). Similar results were also found in a case–control study conducted by Liew et al. (26). Additionally, including individuals with moderate to severe plaque psoriasis (in the patient group) and those with non-psoriatic dermatological conditions (as controls), Akcali et al. documented the absence of any disparity in homocysteine levels between these two groups (29). Furthermore, two more studies by Cakmak et al. and Erturan et al., involving patients with a shared ethnic background, consistently reported the absence of a significant elevation in plasma Hcy levels among individuals with psoriasis (28, 30). These research works confirmed our research results.
With regard to the observational studies where high plasma homocysteine levels were noticed in patients with psoriasis, caution should be observed when interpreting the research findings especially the relationship between Hcy and psoriasis. Hcy, an amino acid derived from methionine, is catabolized to cysteine with the contribution of vitamin B12 and folate (22). Previous studies suggested that high levels of homocysteine in patients with psoriasis were attributed to deficiencies in folate and vitamin B12 rather than a direct effect of psoriasis (17, 19). Decreased folate absorption may result from vitamin consumption by the skin or intestinal inflammation (17). Tobin et al. suggested that keratinocytes of patients with psoriasis regenerated faster, during which folate may be utilized for methylating DNA and undergoing mitotic activity, thus increasing homocysteine levels (20, 24). Additionally, previous research found that homocysteine might facilitate the progression of psoriasis by driving immune inflammatory processes through some pathways, such as activating Th1 and Th17 cells and inducing the activation of nuclear factor κB (25). This kind of possible promotion of psoriasis progression may not mean that Hcy could causally give rise to the occurrence of psoriasis. Although some studies showed the correlation between Hcy and psoriasis, the causal relationship between them has not been proven. Whether Hcy exerts a causative role in the initiation of psoriasis remains unclear.
Furthermore, considering that Hcy has been documented as an independent risk factor for cardiovascular diseases (14, 15), caution should be observed when performing observational studies to explore the association between plasma Hcy levels and psoriasis. It is important to note that psoriatic patients with cardiovascular diseases or risk factors may not be included in the research. The issue of the association and causation between Hcy and psoriasis requires further study.
There were several limitations in our study. First, although we have employed the PhenoScanner to check for secondary phenotypes of potential confounders and excluded them, there still existed a possibility that we did not entirely eliminate the impact of potential pleiotropy, through which the IVs influence the outcome of psoriasis. Second, the predominant population included in our analysis is of European ancestry. Thus, caution should be observed when interpreting the results among different ancestries. Third, we investigated the causal relationship between Hcy and psoriasis from a genetic view. To comprehensively understand the pathogenesis of psoriasis, environmental factors should also be taken into account.
To conclude, our study findings did not demonstrate a causal effect of genetically determined circulating Hcy levels on psoriasis utilizing a genetic approach. Confirmation of the findings from this study necessitates a further MR analysis, utilizing a more extensive dataset from GWASs and incorporating a greater number of genetic instruments. Future research is needed to further explore the mechanism of the association between Hcy and psoriasis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CC: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. SL: Data curation, Writing – original draft, Investigation. JL: Formal Analysis, Visualization, Writing – original draft. ZZ: Formal Analysis, Visualization, Writing – original draft. YZ: Software, Validation, Writing – review & editing. ZL: Software, Validation, Writing – review & editing. YL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Guangdong Province (2022A1515012413).
We would like to thank the investigators for sharing the summary data on GWAS for homocysteine and the participants and investigators of the FinnGen study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1288632/full#supplementary-material
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet (London England) (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
2. Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ (Clinical Res ed) (2020) 369:m1590. doi: 10.1136/bmj.m1590
3. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol (2013) 133(2):377–85. doi: 10.1038/jid.2012.339
4. Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun (2010) 34(3):J314–21. doi: 10.1016/j.jaut.2009.12.001
5. Cantarutti A, Donà D, Visentin F, Borgia E, Scamarcia A, Cantarutti L, et al. Epidemiology of frequently occurring skin diseases in italian children from 2006 to 2012: A retrospective, population-based study. Pediatr Dermatol (2015) 32(5):668–78. doi: 10.1111/pde.12568
6. Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol (2010) 62(6):979–87. doi: 10.1016/j.jaad.2009.07.029
7. Nestle FO, Kaplan DH, Barker J. Psoriasis. New Engl J Med (2009) 361(5):496–509. doi: 10.1056/NEJMra0804595
8. Tsoi LC, Stuart PE, Tian C, Gudjonsson JE, Das S, Zawistowski M, et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat Commun (2017) 8:15382. doi: 10.1038/ncomms15382
9. Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol (2012) 132(3 Pt 1):556–62. doi: 10.1038/jid.2011.365
10. Gao N, Kong M, Li X, Zhu X, Wei D, Ni M, et al. The association between psoriasis and risk of cardiovascular disease: A mendelian randomization analysis. Front Immunol (2022) 13:918224. doi: 10.3389/fimmu.2022.918224
11. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: A comprehensive review. Adv Ther (2020) 37(5):2017–33. doi: 10.1007/s12325-020-01346-6
12. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol (2018) 9:579. doi: 10.3389/fimmu.2018.00579
13. Orlando G, Molon B, Viola A, Alaibac M, Angioni R, Piaserico S. Psoriasis and cardiovascular diseases: an immune-mediated cross talk? Front Immunol (2022) 13:868277. doi: 10.3389/fimmu.2022.868277
14. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J (2015) 14:6. doi: 10.1186/1475-2891-14-6
15. Jensen MK, Bertoia ML, Cahill LE, Agarwal I, Rimm EB, Mukamal KJ. Novel metabolic biomarkers of cardiovascular disease. Nat Rev Endocrinol (2014) 10(11):659–72. doi: 10.1038/nrendo.2014.155
16. Vanizor Kural B, Orem A, Cimşit G, Uydu HA, Yandi YE, Alver A. Plasma homocysteine and its relationships with atherothrombotic markers in psoriatic patients. Clinica chimica acta; Int J Clin Chem (2003) 332(1-2):23–30. doi: 10.1016/S0009-8981(03)00082-2
17. Malerba M, Gisondi P, Radaeli A, Sala R, Calzavara Pinton PG, Girolomoni G. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis. Br J Dermatol (2006) 155(6):1165–9. doi: 10.1111/j.1365-2133.2006.07503.x
18. Karabudak O, Ulusoy RE, Erikci AA, Solmazgul E, Dogan B, Harmanyeri Y. Inflammation and hypercoagulable state in adult psoriatic men. Acta dermato-venereologica (2008) 88(4):337–40. doi: 10.2340/00015555-0456
19. Brazzelli V, Grasso V, Fornara L, Moggio E, Gamba G, Villani S, et al. Homocysteine, vitamin B12 and folic acid levels in psoriatic patients and correlation with disease severity. Int J immunopathol Pharmacol (2010) 23(3):911–6. doi: 10.1177/039463201002300327
20. Tobin AM, Hughes R, Hand EB, Leong T, Graham IM, Kirby B. Homocysteine status and cardiovascular risk factors in patients with psoriasis: a case-control study. Clin Exp Dermatol (2011) 36(1):19–23. doi: 10.1111/j.1365-2230.2010.03877.x
21. Bilgiç Ö, Altınyazar HC, Baran H, Ünlü A. Serum homocysteine, asymmetric dimethyl arginine (ADMA) and other arginine-NO pathway metabolite levels in patients with psoriasis. Arch Dermatol Res (2015) 307(5):439–44. doi: 10.1007/s00403-015-1553-3
22. Giannoni M, Consales V, Campanati A, Ganzetti G, Giuliodori K, Postacchini V, et al. Homocysteine plasma levels in psoriasis patients: our experience and review of the literature. J Eur Acad Dermatol Venereol JEADV. (2015) 29(9):1781–5. doi: 10.1111/jdv.13023
23. Das K, Sarkar S, Dawn I, Das M. Plasma homocysteine levels in patients with Psoriasis. Asian J Med Sci (2017) 8(5):4–7. doi: 10.3126/ajms.v8i5.17136
24. Tsai TY, Yen H, Huang YC. Serum homocysteine, folate and vitamin B(12) levels in patients with psoriasis: a systematic review and meta-analysis. Br J Dermatol (2019) 180(2):382–9. doi: 10.1111/bjd.17034
25. Lin X, Meng X, Song Z. Homocysteine and psoriasis. Biosci Rep (2019) 39(11):5284. doi: 10.1042/BSR20190867
26. Liew SC, Das-Gupta E, Wong SF, Lee N, Safdar N, Jamil A. Association of methylentetraydrofolate reductase (MTHFR) 677 C > T gene polymorphism and homocysteine levels in psoriasis vulgaris patients from Malaysia: a case-control study. Nutr J (2012) 11:1. doi: 10.1186/1475-2891-11-1
27. Uslu M, Karul A, Gokbulut C, Kozaci D, Savk E, Karaman G, et al. Investigation of the plasma homocysteine levels in patients with psoriasis. J Am Acad Dermatol (2013) 68(4):AB204–AB. doi: 10.1016/j.jaad.2012.12.843
28. Erturan I, Köroğlu BK, Adiloğlu A, Ceyhan AM, Akkaya VB, Tamer N, et al. Evaluation of serum sCD40L and homocysteine levels with subclinical atherosclerosis indicators in patients with psoriasis: a pilot study. Int J Dermatol (2014) 53(4):503–9. doi: 10.1111/ijd.12397
29. Akcali C, Buyukcelik B, Kirtak N, Inaloz S. Clinical and laboratory parameters associated with metabolic syndrome in Turkish patients with psoriasis. J Int Med Res (2014) 42(2):386–94. doi: 10.1177/0300060513502891
30. Cakmak SK, Gül U, Kiliç C, Gönül M, Soylu S, Kiliç A. Homocysteine, vitamin B12 and folic acid levels in psoriasis patients. J Eur Acad Dermatol Venereol JEADV. (2009) 23(3):300–3. doi: 10.1111/j.1468-3083.2008.03024.x
31. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
32. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32(1):1–22. doi: 10.1093/ije/dyg070
33. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol (2013) 37(7):658–65. doi: 10.1002/gepi.21758
34. Wei J, Zhu J, Xu H, Zhou D, Elder JT, Tsoi LC, et al. Alcohol consumption and smoking in relation to psoriasis: a Mendelian randomization study. Br J Dermatol (2022) 187(5):684–91. doi: 10.1111/bjd.21718
35. Ren Y, Liu J, Li W, Zheng H, Dai H, Qiu G, et al. Causal associations between vitamin D levels and psoriasis, atopic dermatitis, and vitiligo: A bidirectional two-sample mendelian randomization analysis. Nutrients (2022) 14(24):5284. doi: 10.3390/nu14245284
36. Bohmann P, Stein MJ, Konzok J, Tsoi LC, Elder JT, Leitzmann MF, et al. Relationship between genetically proxied vitamin D and psoriasis risk: a Mendelian randomization study. Clin Exp Dermatol (2023) 48(6):642–7. doi: 10.1093/ced/llad095
37. Zhang ZY, Jian ZY, Tang Y, Li W. The relationship between blood lipid and risk of psoriasis: univariable and multivariable Mendelian randomization analysis. Front Immunol (2023) 14:1174998. doi: 10.3389/fimmu.2023.1174998
38. Xiao Y, Jing D, Tang Z, Peng C, Yin M, Liu H, et al. Serum lipids and risk of incident psoriasis: A prospective cohort study from the UK biobank study and mendelian randomization analysis. J Invest Dermatol (2022) 142(12):3192–9 e12. doi: 10.1016/j.jid.2022.06.015
39. Gu X, Chen X, Shen M. Association of psoriasis with risk of COVID-19: A 2-sample Mendelian randomization study. J Am Acad Dermatol (2022) 87(3):715–7. doi: 10.1016/j.jaad.2022.01.048
40. Li Y, Guo J, Cao Z, Wu J. Causal association between inflammatory bowel disease and psoriasis: A two-sample bidirectional mendelian randomization study. Front Immunol (2022) 13:916645. doi: 10.3389/fimmu.2022.916645
41. Zhang L, Wang Y, Qiu L, Wu J. Psoriasis and cardiovascular disease risk in European and East Asian populations: evidence from meta-analysis and Mendelian randomization analysis. BMC Med (2022) 20(1):421. doi: 10.1186/s12916-022-02617-5
42. Naslund-Koch C, Bojesen SE, Gluud LL, Skov L, Vedel-Krogh S. Non-alcoholic fatty liver disease is not a causal risk factor for psoriasis: A Mendelian randomization study of 108,835 individuals. Front Immunol (2022) 13:1022460. doi: 10.3389/fimmu.2022.1022460
43. Tian D, Zhou Y, Chen Y, Wu Y, Wang H, Jie C, et al. Genetically predicted ankylosing spondylitis is causally associated with psoriasis. Front Immunol (2023) 14:1149206. doi: 10.3389/fimmu.2023.1149206
44. Wang X, Wang X, Wang H, Yang M, Dong W, Shao D. Association between psoriasis and lung cancer: two-sample Mendelian randomization analyses. BMC Pulm Med (2023) 23(1):4. doi: 10.1186/s12890-022-02297-0
45. Luo Q, Chen J, Qin L, Luo Y, Zhang Y, Yang X, et al. Psoriasis may increase the risk of lung cancer: a two-sample Mendelian randomization study. J Eur Acad Dermatol Venereol JEADV. (2022) 36(11):2113–9. doi: 10.1111/jdv.18437
46. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (Clinical Res ed) (2018) 362:k601. doi: 10.1136/bmj.k601
47. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
48. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature (2023) 613(7944):508–18. doi: 10.1038/s41586-022-05473-8
49. van Meurs JB, Pare G, Schwartz SM, Hazra A, Tanaka T, Vermeulen SH, et al. Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr (2013) 98(3):668–76. doi: 10.3945/ajcn.112.044545
50. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet (2007) 81(3):559–75. doi: 10.1086/519795
51. Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinf (Oxford England) (2016) 32(20):3207–9. doi: 10.1093/bioinformatics/btw373
52. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol (2016) 40(7):597–608. doi: 10.1002/gepi.21998
53. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
54. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
55. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med (2015) 34(21):2926–40. doi: 10.1002/sim.6522
56. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
57. Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr diabetes (2012) 2(12):e54. doi: 10.1038/nutd.2012.26
58. Aune D, Snekvik I, Schlesinger S, Norat T, Riboli E, Vatten LJ. Body mass index, abdominal fatness, weight gain and the risk of psoriasis: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol (2018) 33(12):1163–78. doi: 10.1007/s10654-018-0366-z
59. Badsha MB, Fu AQ. Learning causal biological networks with the principle of mendelian randomization. Front Genet (2019) 10:460. doi: 10.3389/fgene.2019.00460
Keywords: homocysteine, psoriasis, Mendelian randomization, casual effect, genome-wide association studies
Citation: Chen C, Liu S, Liu J, Zheng Z, Zheng Y, Lin Z and Liu Y (2023) No causal effect of genetically determined circulating homocysteine levels on psoriasis in the European population: evidence from a Mendelian randomization study. Front. Immunol. 14:1288632. doi: 10.3389/fimmu.2023.1288632
Received: 04 September 2023; Accepted: 23 October 2023;
Published: 08 November 2023.
Edited by:
Seik-Soon Khor, Nanyang Technological University, SingaporeReviewed by:
Takemichi Fukasawa, The University of Tokyo, JapanCopyright © 2023 Chen, Liu, Liu, Zheng, Zheng, Lin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuchun Liu, eXVjaHVubGl1X2dtY0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.