
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 03 January 2024
Sec. Nutritional Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1288537
This article is part of the Research TopicDiet and Exercise-Induced InflammationView all 7 articles
 Mousa Khalafi1
Mousa Khalafi1 Aref Habibi Maleki2
Aref Habibi Maleki2 Michael E. Symonds3
Michael E. Symonds3 Mohammad Hossein Sakhaei4
Mohammad Hossein Sakhaei4 Sara K. Rosenkranz5
Sara K. Rosenkranz5 Mahsa Ehsanifar2
Mahsa Ehsanifar2 Mallikarjuna Korivi6*
Mallikarjuna Korivi6* Yubo Liu6*
Yubo Liu6*Purpose: Interlukin-15 (IL-15) is an inflammatory cytokine that plays a vital role in immunology and obesity-associated metabolic syndrome. We performed this systematic review and meta-analysis to investigate whether exercise promotes circulating IL-15 concentrations in adults.
Methods: We searched PubMed, Web of Science, and Scopus from inception to May, 2023 and identified original studies that investigated the effectiveness of acute and/or chronic exercise on serum/plasma IL-15 levels in adults. Standardized mean differences (SMD) and 95% confidence intervals (CI) were calculated using random effect models. Subgroup analyses were performed based on type of exercise, and training status, health status and body mass indexes (BMI) of participants.
Results: Fifteen studies involving 411 participants and 12 studies involving 899 participants were included in the acute and chronic exercise analyses, respectively. Our findings showed that acute exercise increased circulating IL-15 concentrations immediately after exercise compared with baseline [SMD=0.90 (95% CI: 0.47 to 1.32), p=0.001], regardless of exercise type and participants’ training status. Similarly, acute exercise was also associated with increased IL-15 concentrations even one-hour after exercise [SMD=0.50 (95% CI: 0.00 to 0.99), p=0.04]. Nevertheless, chronic exercise did not have a significant effect on IL-15 concentrations [SMD=0.40 (95% CI: -0.08 to 0.88), p=0.10].
Conclusion: Our results confirm that acute exercise is effective in increasing the IL-15 concentrations immediately and one-hour after exercise intervention, and thereby playing a potential role in improving metabolism in adults.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=445634, identifier CRD42023445634.
Cytokines comprise a large family of polypeptide signaling molecules that are produced by a variety of immune and non-immune cells (1). Cytokines regulate a large number of biological processes, including growth, differentiation, and pro- or anti-inflammatory signaling pathways upon release in response to stimulus (2). Accumulating evidence suggest that skeletal muscle produce and secret several cytokines known as ‘myokines’ which exert endocrine, autocrine or paracrine functions (3–5). Cytokines intrinsically involved in crosstalk between skeletal muscle and other organs, such as adipose tissue, brain, liver, immune system components, as well as within muscle itself (3–5). During exercise, skeletal muscle secreted myokines are involved in lipid and glucose metabolism, chronic low-grade inflammation, skeletal muscle hypertrophy, tumor growth, and cytokine production in tissues and organs (3, 6). Owing to the multi-system involvement of myokines, interventions that can alter circulating myokines could be novel strategies to treat chronic inflammatory diseases and promote health (7, 8).
Interlukin-15 (IL-15) is a 14-15 KDa member of the four α-helix bundle cytokine, with a primary role in the development, survival, and activation of natural killer cells (9). IL-15 is produced by a variety of tissues (skeletal muscle, placenta, heart and kidney) and cell types (epithelial, monocytes and macrophages (9). IL-15 stimulates immune cell responses primarily through specific IL-15 receptor alpha subunits (IL-15Rα), as well as through beta subunits (shared with IL-2) and gamma subunits (shared with IL-2, IL4, IL-7, IL-9 and IL-21) (10, 11). IL-15 that is expressed in skeletal muscle is reported to possess additional non-immune metabolic roles (12–14). Highly expressed IL-15 in skeletal muscle might be a response to muscular contractions during exercise (15). IL-15 promotes myoblast differentiation, skeletal muscle fiber hypertrophy, and inhibits protein degradation (16–18), suggesting a potential target for maintaining of healthy skeletal muscle, and treating muscle wasting disorders (19). In addition, IL-15 also exhibits anti-diabetic and anti-obesity properties, facilitating glucose metabolism in skeletal muscle, modulating adipose tissue deposition and improving insulin sensitivity (20–23). These immuno-metabolic effects of IL-15 suggest it may be a potential therapeutic target for treating chronic metabolic diseases.
Exercise promotes health and can prevent some chronic diseases where there is crosstalk between skeletal muscle and other organs and/or tissues that may act through cytokines (3, 24). Several systematic reviews have suggested that acute and chronic exercise modulate circulating cytokines, including pro- and anti-inflammatory cytokines, such as IL-6, tumor necrosis factor alpha (TNF-α), and IL-10 (25–30). However, currently there is no published comprehensive meta-analysis on IL-15. An increased expression and secretion of IL-15 is one potential mediator of the effect of exercise on skeletal muscle function although the response is inconsistent (31–38). In addition, there are several moderators that affect how acute or chronic exercise influences IL-15 concentrations. The extent to which exercise duration may modify the IL-15 response is unclear. Therefore, we performed this comprehensive meta-analysis to investigate the effect of acute and chronic exercise interventions on circulating IL-15 concentrations in adults.
The current systematic review and meta-analysis was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (39) and the Cochrane Handbook of Systematic Reviews of Interventions (40). The protocol was registered prospectively with ID: CRD42023445634.
A comprehensive literature search was performed in three main electronic databases including PubMed, Web of Science, and Scopus. Searches were conducted from inception to May 1, 2023 by two independent reviewers (A H M and M H S). The search strategy was performed using the Boolean operators “AND” and “OR”, and a combination of the following key words: “exercise”, “exercise training”, “physical activity”, “aerobic training”, “aerobic exercise”, “resistance training”, “resistance exercise”, “combined training”, “combined exercise”, “interval training”, “interval exercise”, “endurance training”, “endurance exercise”, “strength training”, “strength exercise”, “IL-15”, “IL15”, “Interleukin 15”, and Interleukin-15”. Additional filters included English language, human participants, and article/document type when they were available in electronic databases. In addition, searches in Google Scholar, as well as manual searches of the reference lists of all included studies, was conducted to obtain any relevant records that may have been missed.
Studies were eligible for inclusion if they met the following PICO (population, intervention, comparison, and outcome) criteria: (1) For population, human participants with mean age ≥ 18 years, regardless of biological sex, and health and fitness status, were included. Participants were classified into subgroups based on their training status (untrained or trained). For the untrained subgroup, studies recruited participants who had no history of regular exercise training for at least 6 months prior to the study. For the trained and athlete subgroup, studies recruited participants who reported at least five scheduled exercise sessions per week for at least one year. (2) For the intervention, studies with any mode of exercise (acute or chronic), irrespective of type (aerobic, resistance, or combined) were included. For acute exercise, studies with single session of exercise were included. For chronic exercise, studies with exercise duration ≥ 2 weeks were considered to include. Shorter exercise duration was considered in our analysis to distinguish any possible minimal adaptations to exercise. Recent meta-analyses have shown some metabolic adaptations to shorter-term chronic exercise training (41, 42). Nevertheless, the exercise duration in our included trials is five week or more, as there were no eligible studies with shorter exercise duration. There were no limitations for other exercise characteristics, such as intensity, frequency, and time. (3) To investigate the effects of acute exercise, post-exercise values versus pre-exercise values were required. For chronic exercise, the effects of training versus a non-exercise control group were required. (4) For outcomes, studies that reported serum or plasma levels of IL-15, measured using a fully validated method, were included.
Other inclusion criteria were that articles must have been peer-reviewed and published in English. Exclusion criteria were studies with animal models, conference abstracts and non-original studies. Chronic exercise studies with non-randomized trials or studies without control groups were excluded. Two authors (A H M and M H S) independently performed study selection, and any discrepancies between these authors were resolved through consensus with other authors (M Kh and M K). For study selection, all retrieved articles were exported to Endnote (version 20.21). After removing the duplicate articles, the titles and abstracts were screened, followed by full-texts assessment of the remaining articles.
The following data were extracted from all included articles: (1) study characteristics. including publication year, and study design (2) characteristics of participants including sample size, biological sex, age, body mass index (BMI), and health and training status, (3) exercise training characteristics i.e. mode, duration, intensity, time, and frequency, and (4) data of outcome variable and assessment methods. To calculate the effect sizes (ES) for acute exercise, means and standard deviations (SD) or mean changes (post-values – pre-values) and their SD, for the exercise group only, for pre-exercise, immediate post-exercise, and one-hour post-exercise outcomes were extracted. To calculate the effect sizes for chronic exercise, means and SDs, or mean changes (post-values – pre-values) and their SDs, for exercise and non-exercise groups were extracted. When required, means and SDs were calculated from standard errors, medians, ranges and IQRs (40, 43, 44). In addition, when required, the data were extracted from figures using Getdata Graph Digitizer. If a study had several exercise arms, all were included. To obtain any missing or additional data from the studies that were published within recent 5 years, we contacted respective corresponding authors. Data extraction was performed independently by two authors (A H M and M H S), and potential discrepancies between the authors were resolved through consensus with other authors (M Kh and M K).
Study quality was assessed using the Physiotherapy Evidence Database (PEDro) scale, which includes ratings for 11 methodological issues (45). However, two of the items, including blinding of participants, and blinding of intervention providers, were excluded as they are typically not possible in exercise interventions. Finally, study quality was determined using the 9 items listed in Supplementary Table 1, rated from 0 (lowest) to 9 (highest). Two authors (A H M and M H S) assessed the quality of included studies, and any disagreements were resolved via consensus with other authors (M Kh and M K).
All statistical analyses were performed using version 3 of Comprehensive Meta-analysis Software (CMA3, Biostat Inc., Englewood, NJ, USA). Three separate analyses were performed to calculate standardized mean differences (SMD) and 95% confidence intervals (CIs) using random effects models as follows: 1) effects of acute exercise on immediate post-exercise IL-15 levels using the data from immediately post-exercise versus pre-exercise, 2) effects of acute exercise on IL-15 changes during post-exercise recovery using data from one-hour post-exercise versus pre-exercise, and 3) effects of chronic exercise on IL-15 levels using data from exercise training versus non-exercise control groups. In addition, several sub-group analyses were performed based on types of exercise (aerobic, resistance, and combined training) and training status of participants (un-trained, trained and athletes) for acute exercise analyses. Subgroup analyses for chronic exercise studies, included BMIs (< 30 kg/m2 vs. ≥ 30 kg/m2), ages (< 50 years vs. ≥ 50 years), health status of participants (without cardio-metabolic diseases vs. with cardio-metabolic diseases), intervention durations (< 16 weeks vs. ≥ 16 weeks), and exercise types (aerobic, resistance, and combined training). Subgroup analyses were performed only when there was enough number of studies for each subgroup (at last 5 trials). The magnitude of effect sizes was interpreted using the Cochrane guidelines: trivial (< 0.2), small (0.2 to < 0.5), moderate (0.5 to 0.8), and large (> 0.8). The I2 statistic was used to assess heterogeneity according to the Cochrane guidelines: low (25%), moderate (50%), and high (75%). Publication bias was assessed using visual interpretation of funnel plots and Egger’s tests where p values were <0.10. To ensure that the results were not influenced by a single study, sensitivity analysis was conducted by omitting individual studies, as well as omitting studies with very large effect sizes. The trim and fill method was used to address any potential effects of publication bias, when present in visual interpretation of funnel plots. -
The initial database searches identified 1,059 records. After removing duplicates, 594 articles remained for screening based on titles and abstracts. After screening the titles and abstracts, 553 irrelevant records were excluded and 41 articles were remained for the full-text assessment. Of 41 articles, 13 were excluded with the reasons presented in Figure 1, and 4 articles cannot include in the meta-analysis due to insufficient data. Finally remaining 24 articles were included in the meta-analysis. Among the included studies, 12 studies (31, 31, 46–49, 53–55) investigated the acute exercise effect, nine (58–66) investigated the chronic exercise effect, and three (36, 56, 57) investigated both acute and chronic exercise effects on IL-15.
For acute exercise, a total of 411 participants with mean ages and BMIs ranging from ~19 to 84 years and 20 to 35 kg/m2, respectively, were included. Of the 15 included studies, four studies consisted of females (31, 51, 56, 57), six studies consisted of males (36, 46, 48, 50, 52, 54), and the remaining studies consisted of both males and females (47, 49, 55). Two studies did not clearly report the biological sex of participants (35, 53). For chronic exercise, 899 participants with mean ages ranging from ~37 to 90 years, and BMIs ranging from 23 to 35 kg/m2 were included. Among the 12 studies, seven studies included females (56–58, 61, 63–65), two studies included males (36, 60), and three studies included both males and females (59, 62, 66). Participants had a wide range of health conditions and diseases, such as overweight, obesity, diabetes, and high risk of breast cancer.
For the acute exercise protocol, aerobic or resistance exercise were used in majority of the studies, while maximal incremental, submaximal exercise tests and marathon running were used in other studies. Exercise duration was ranged from 3 to 360 minutes, while the intensity was widely differ for each mode of exercise. For resistance exercise, the intensity was ranged from 30 to 80% 1RM, and for aerobic exercise the intensity was ranged from 50 to 70% HRmax. In addition, long-distance trail running or standard exercise test were performed in several studies. For chronic exercise studies, trials used aerobic, resistance, combined and sprint, and high intensity interval trainings. Briefly, for chronic exercise, intervention duration was ranged from 5 weeks to 6 months, and weekly exercise sessions ranged from 1–3 to 5 sessions. Most of the chronic exercise was performed under supervision. Full details of exercise intensity and duration with the mode of exercise are summarized in Tables 1, 2.
Based on 24 intervention arms, acute exercise significantly increased circulating IL-15 levels immediately after exercise compared with baseline [SMD=0.90 (95% CI: 0.47 to 1.32), p=0.001] (Figure 2). There was significant and high heterogeneity among the included studies (I2 = 88.60, p=0.001). Visual interpretation of the funnel plots suggested publication bias, but the Egger’s test did not (p=0.40). The Trim and fill method identified four missing studies from the right side of the plots. When accounting for these missing studies, the overall effect size was [SMD=1.21 (95% CI: 0.77 to 1.64)]. Sensitivity analysis by omitting individual studies did not change the significance or the direction of the effect. In addition, sensitivity analysis by omitting studies with very large effect size did not change the significance or direction of any effect. Subgroup analysis based on type of exercise showed that both aerobic [SMD=0.86 (95% CI: 0.37 to 1.34), p=0.001] and resistance [SMD=1.05 (95% CI: 0.23 to 1.79), p=0.01] exercise led to increased circulating IL-15 immediately following acute exercise. In addition, subgroup analysis based on training status of participants showed that acute exercise led to increased circulating IL-15 in both athletes [SMD=2.32 (95% CI: 0.86 to 3.78), p=0.001] and untrained [SMD=0.86 (95% CI: 0.35 to 1.37), p=0.001] individuals, but not in trained individuals [SMD=0.00 (95% CI: -0.27 to 0.27), p=1.00] immediately after exercise.
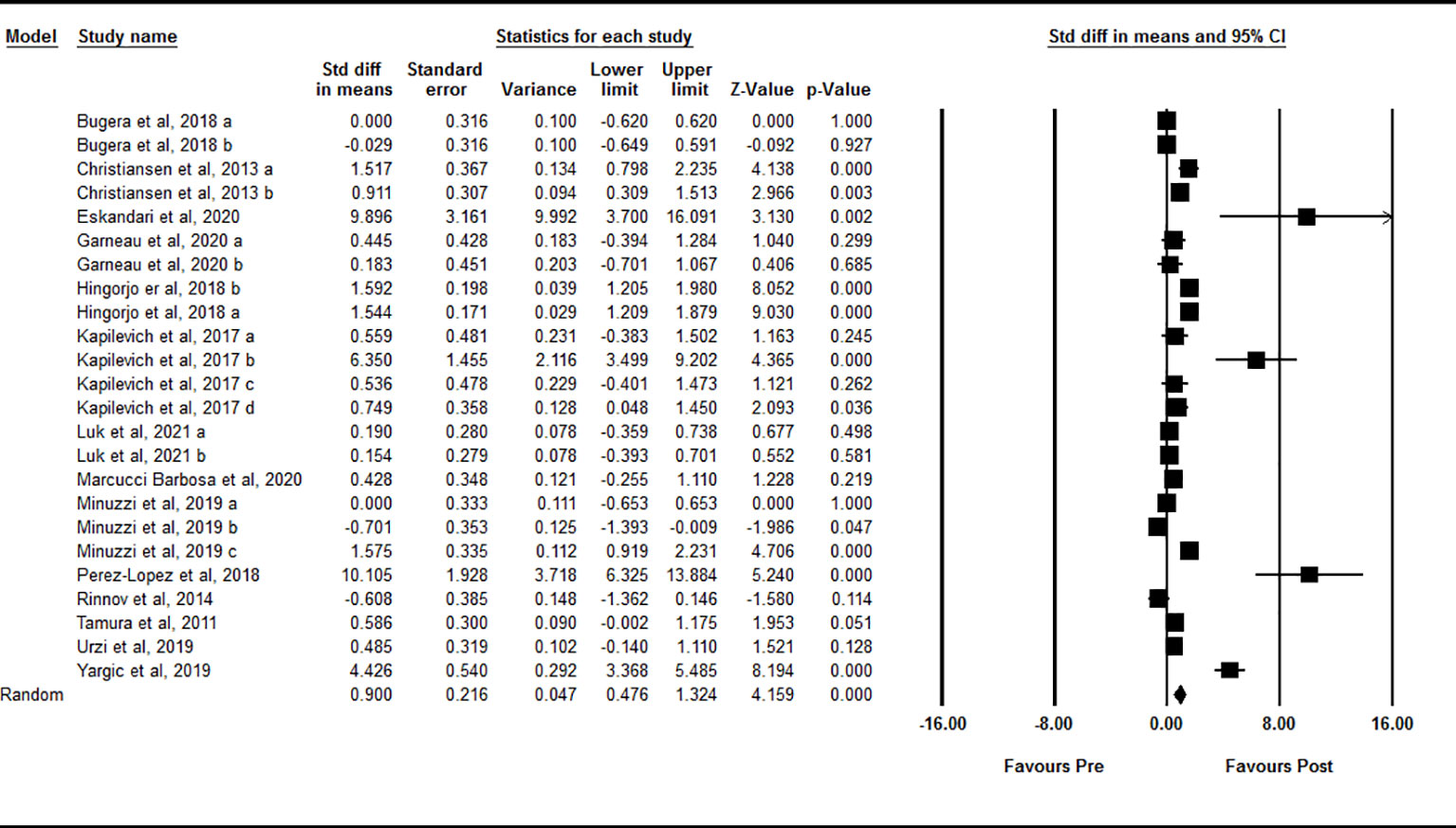
Figure 2 Forest plot of the effects of acute exercise on immediate post-exercise IL-15. Data are reported as SMD (95% confidence limits). SMD, standardized mean difference.
Data from 17 intervention arms showed that acute exercise significantly increased circulating IL-15 at one-hour after exercise compared with baseline [SMD=0.50 (95% CI: 0.00 to 0.99), p=0.04] (Figure 3). We found a significant and high heterogeneity among included studies (I2 = 85.37, p=0.001). Visual interpretation of the funnel plots and the Egger’s test revealed publication bias (p=0.001). The Trim and fill method identified one missing study from the right side of the plots. When accounting for this missing study, the overall effect size was [SMD=0.66 (95% CI: 0.11 to 1.20)]. Sensitivity analysis by omitting individual studies changed the significance. In addition, sensitivity of analysis by omitting studies with very large effect size changed the significance [SMD=0.12 (95% CI: -0.25 to 0.49), p=0.52]. Subgroup analysis based on type of acute exercise showed that both aerobic [SMD=0.42 (95% CI: -0.18 to 1.02), p=0.17] and resistance [SMD=0.68 (95% CI: -0.10 to 1.46), p=0.09] exercise increased circulating IL-15, but the change was not statistically significant. Subgroup analysis for the training status of participants revealed that increased circulating IL-15 at one-hour during post-exercise was seen only in athletes [SMD=2.27 (95% CI: 0.75 to 3.79), p=0.003], but not in untrained [SMD=0.33 (95% CI: -0.35 to 1.02), p=0.34] and trained [SMD=-0.09 (95% CI: -0.38 to 0.19), p=0.53] individuals.
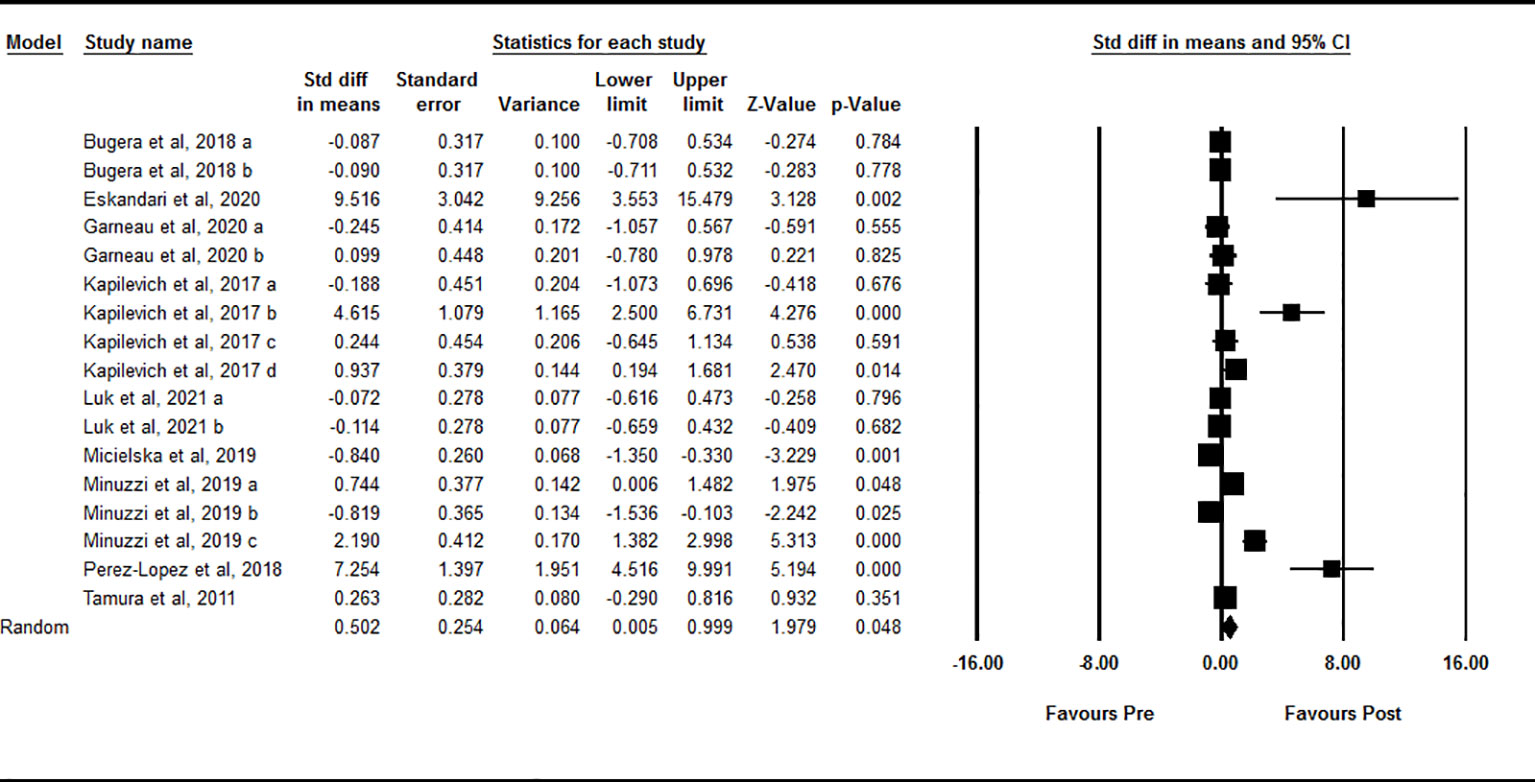
Figure 3 Forest plot of the effects of acute exercise on one-hour post-exercise IL-15. Data are reported as SMD (95% confidence limits). SMD, standardized mean difference.
Based on 18 intervention arms, chronic exercise did not significantly change circulating IL-15 compared with control [SMD=0.002 (95% CI: -0.51 to 0.51), p=0.99] (Figure 4). There was significant and high heterogeneity among included studies (I2 = 87.25, p=0.001). Visual interpretation of the funnel plots suggested publication bias, but the Egger’s test did not showed the bias (p=0.79). The Trim and fill method identified five missing studies from the right side of the plots. When accounting for these missing studies, the overall effect size increased [SMD=0.65 (95% CI: 0.08 to 1.21)]. Sensitivity analysis by omitting individual studies did not change the significance. Subgroup analysis based on type of exercise training showed that chronic aerobic [SMD=-0.63 (95% CI: -1.42 to 0.15), p=0.11], resistance [SMD=0.46 (95% CI: -0.21 to 1.15), p=0.18], and combined [SMD=0.91 (95% CI: -1.24 to 1.46), p=0.40] exercise training did not significantly change circulating IL-15. Subgroup analysis based on the health status of participants showed that exercise training did not significantly change circulating IL-15 in healthy individuals [SMD=-0.02 (95% CI: -0.69 to 0.64), p=0.93] or participants with chronic diseases [SMD=0.12 (95% CI: -0.60 to 0.85), p=0.74]. Subgroup analysis based on BMI showed that chronic exercise training did not significantly change circulating IL-15 in individuals with [SMD=-0.08 (95% CI: -1.23 to 1.07), p=0.88] or without [SMD=0.08 (95% CI: -0.38 to 0.55), p=0.71] obesity. Subgroup analysis based on the intervention duration showed that exercise training did not significantly change circulating IL-15 with medium-term (durations < 16 weeks) [SMD=-0.32 (95% CI: -0.93 to 0.28), p=0.29] or long-term (durations ≥ 16 weeks) [SMD=0.93 (95% CI: -0.23 to 2.09), p=0.11] interventions.
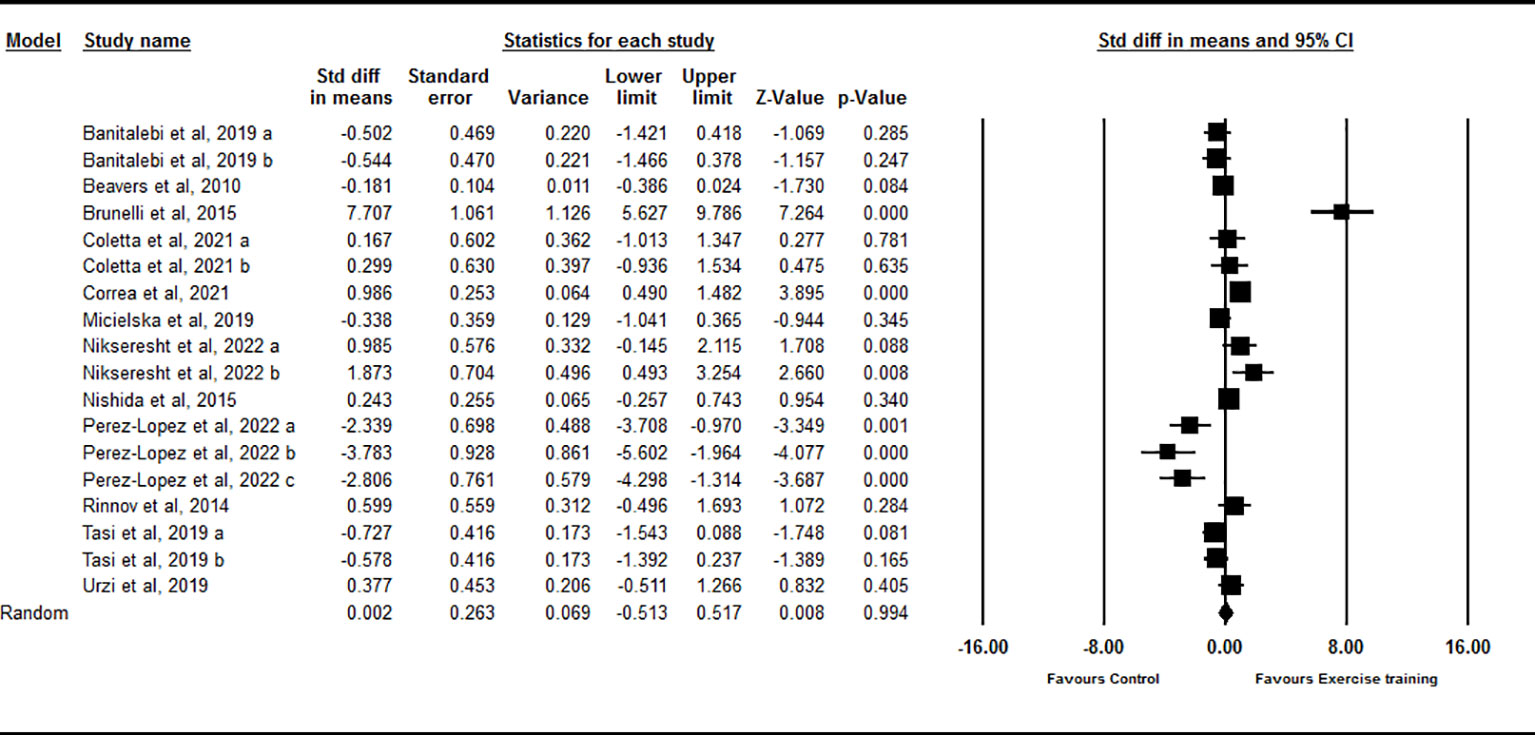
Figure 4 Forest plot of the effects of chronic exercise on IL-15. Data are reported as SMD (95% confidence limits). SMD, standardized mean difference.
The overall quality of the included studies is summarized in Supplementary Table 1. For acute exercise, the PEDro scores ranged from three to seven, out of a possible score of nine. For chronic exercise, the PEDro scores ranged from five to seven, out of a possible score of nine.
Our findings demonstrated that acute exercise increased circulating IL-15 concentration immediately after exercise, and this response was independent of exercise type, but appears to be modulated by the training status of participants. However, the increased IL-15 at one-hour post-exercise was inconsistent with additional sensitivity analyses were required to show the significance and effect size. Chronic exercise was not effective in altering the circulating IL-15. These findings suggest that increased IL-15 is a transient response to exercise in untrained and trained individuals. Although we confirmed increased IL-15 concentrations immediately after exercise, the potential mechanism is not clear given that secretion of IL-15 occurs from several tissues, and the regulation of IL-15 occurs by several receptor isoforms (56, 67). Skeletal muscle may be the main source for exercise-induced circulating IL-15 (32, 33) for which a positive correlation has been shown with the protein content of muscle (35). Exercise promotes gene expression of IL-15 in human skeletal muscle, but it is unclear whether it translates to increase circulating IL-15 concentrations (68–70). The secretion of IL-15 from muscle is not necessarily dependent on transcriptional changes in its mRNA, and the intramuscular pool of secretable IL-15 could be the primary source of IL-15 following exercise (32, 35, 71). It is considered that IL-15 is a promising target in the treatment of metabolic disorders through improvements in lipid and glucose metabolism, and pharmacologically beneficial in cancer immunotherapy through anti-tumor activity (20, 72, 73). Since IL-15 is a myokine with significant metabolic, anabolic, and immune functions (20, 74), exercise-induced changes might be beneficial for prevention and management of metabolic disorders in humans.
Based on our analyses, IL-15 increases immediately after exercise, with an inconsistent increase during the recovery period (within one-hour), that is not unexpected given the relatively short half-life of IL-15 (about 30 min) (15, 31). In addition, the effect size was larger in athletes compared to trained and untrained individuals, and was significant only in athletes at one-hour post-exercise. This larger effect size may be due to an adaption to chronic training, which leads to increased expression of IL-15 and accumulation in skeletal muscle potentially enhancing its secretion (35, 36, 53). Previous human studies have shown that acute exercise can elevate circulating IL-15 concentrations in 10 min after exercise and it may sustain until 120 min following exercise (32, 54). Nevertheless, the increased IL-15 concentrations subsequently returned to pre-exercise level at 3-h during post-exercise recovery (54). We further demonstrated that exercise increased circulating IL-15 regardless of exercise type. It appears that both aerobic and resistance exercise, lead to stimulate IL-15 secretion through skeletal muscle contraction and metabolic training adaptations, particularly in the immediate post-exercise period.
Several meta-analyses have provided evidence that chronic exercise can modulate circulating cytokines (30, 75, 76), further studies are required to determine whether exercise training leads to adaptations in circulating IL-15. Inconsistent reports have shown increased, decreased and non-significant effects on IL-15 following chronic exercise training (26, 57–66). Our findings also showed that chronic exercise had no effect on circulating IL-15. It is worth noting that subgroup analyses indicated variable effect sizes according to exercise type. Effect sizes were negative, medium, and non-significant with aerobic training, but positive, small and non-significant with resistance training, and large with combined training, suggesting that exercise type may contribute to heterogeneity and introduce bias in interpreting the results. IL-15 is an anabolic growth factor that is expressed in skeletal muscle and acts in an endocrine fashion (18), that may be dependent on acute changes (38). Furthermore, chronic exercise may increase the intramuscular supply of IL-15, thereby contributing to the larger effect sizes in trained participants following acute exercise. In addition, we showed large and medium effect sizes following resistance and combined training, indicating that the higher circulating values of IL-15 could be a result of training adaptations as compared to aerobic exercise training. Subgroup analyses were also performed based on the health status and BMI of participants with chronic exercise training, and results indicated that these factors had no effect on IL-15.
The current meta-analysis has several limitations that should be considered when interpreting our results. For acute exercise, we included data at the one-hour time point after exercise, and the available data did not allow for closer examination of longer responses. There was significant heterogeneity indicated in all of the primary analyses, which may be partly explained by exercise type and training status of participants. Additionally, publication bias was detected with visual interpretation of funnel plots, but the Trim and fill method confirmed that adding missing studies either did not change the effect sizes, or increased them. In addition, the variation in exercise protocols (acute or chronic), lack of adequate information provided in some studies, and reporting of exercise intensity using different criteria (such as the percentage of 1RM, or 10-RM, HRmax, VO2max, BRPE), did not allow us to perform sub-group analyses based on exercise characteristics, including intensity and duration. These issues should be considered in future studies to address the effect of exercise on IL-15 concentrations.
Our systematic review and meta-analysis demonstrated that acute, but not chronic exercise training, increased circulating IL-15 concentrations immediately after exercise. Chronic exercise may increase IL-15 muscle content, leading to an acute increase in circulating IL-15 concentrations in subsequent periods of exercise.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
MKh: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AM: Data curation, Investigation, Methodology, Software, Writing – original draft. MES: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing. MHS: Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. SR: Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Writing – review & editing. ME: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. MKo: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. YL: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1288537/full#supplementary-material
1. Saha B, Silvestre R. Cytokines in the immunity and immunopathogenesis in leishmaniases. Cytokine (2021) 145:155320. doi: 10.1016/j.cyto.2020.155320
2. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J cancer. (2019) 120(1):6–15. doi: 10.1038/s41416-018-0328-y
3. Severinsen MCK, Pedersen BK. Muscle–organ crosstalk: the emerging roles of myokines. Endocrine Rev (2020) 41(4):594–609. doi: 10.1210/endrev/bnaa016
4. Di Felice V, Coletti D, Seelaender M. Myokines, adipokines, cytokines in muscle pathophysiology. Front Media SA; (2020) p:592856. doi: 10.3389/978-2-88966-272-2
5. Delezie J, Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front neurology. (2018) 9:698. doi: 10.3389/fneur.2018.00698
6. Pedersen BK, Åkerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (2007) 103(3):1093–9. doi: 10.1152/japplphysiol.00080.2007
7. Lightfoot AP, Cooper RG. The role of myokines in muscle health and disease. Curr Opin Rheumatol (2016) 28(6):661–6. doi: 10.1097/BOR.0000000000000337
8. Hoffmann C, Weigert C. Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harbor Perspect Med (2017) 7(11):a029793. doi: 10.1101/cshperspect.a029793
9. Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin Cancer Res (2014) 20(8):2044–50. doi: 10.1158/1078-0432.CCR-12-3603
10. Sindaco P, Pandey H, Isabelle C, Chakravarti N, Brammer JE, Porcu P, et al. The role of interleukin-15 in the development and treatment of hematological Malignancies. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1141208
11. Hangasky JA, Waldmann TA, Santi DV. Interleukin 15 pharmacokinetics and consumption by a dynamic cytokine sink. Front Immunol (2020) 11:1813. doi: 10.3389/fimmu.2020.01813
12. Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science (1994) 264(5161):965–8. doi: 10.1126/science.8178155
13. Quinn LS, Strait-Bodey L, Anderson BG, Argilés JM, Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int (2005) 29(6):449–57. doi: 10.1016/j.cellbi.2005.02.005
14. Pierce JR, Maples JM, Hickner RC. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: local effects of IL-15 on adipose tissue lipolysis. Am J Physiology-Endocrinology Metab (2015) 308(12):E1131–E9. doi: 10.1152/ajpendo.00575.2014
15. Nadeau L, Aguer C. Interleukin-15 as a myokine: mechanistic insight into its effect on skeletal muscle metabolism. Appl Physiology Nutrition Metab (2019) 44(3):229–38. doi: 10.1139/apnm-2018-0022
16. Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology (1995) 136(8):3669–72. doi: 10.1210/endo.136.8.7628408
17. Busquets S, Figueras MT, Meijsing S, Carbó N, Quinn LS, Almendro V, et al. Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol Med (2005) 16(3):471–6. doi: 10.3892/ijmm.16.3.471
18. Furmanczyk PS, Quinn LS. Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biol Int (2003) 27(10):845–51. doi: 10.1016/S1065-6995(03)00172-0
19. Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res (2002) 280(1):55–63. doi: 10.1006/excr.2002.5624
20. Busquets S, Figueras M, Almendro V, López-Soriano FJ, Argilés JM. Interleukin-15 increases glucose uptake in skeletal muscle An antidiabetogenic effect of the cytokine. Biochim Biophys Acta (BBA)-General Subjects. (2006) 1760(11):1613–7. doi: 10.1016/j.bbagen.2006.09.001
21. Barra N, Chew M, Holloway A, Ashkar A. Interleukin-15 treatment improves glucose homeostasis and insulin sensitivity in obese mice. Diabetes Obes Metab (2012) 14(2):190–3. doi: 10.1111/j.1463-1326.2011.01495.x
22. Fujimoto T, Sugimoto K, Takahashi T, Yasunobe Y, Xie K, Tanaka M, et al. Overexpression of Interleukin-15 exhibits improved glucose tolerance and promotes GLUT4 translocation via AMP-Activated protein kinase pathway in skeletal muscle. Biochem Biophys Res Commun (2019) 509(4):994–1000. doi: 10.1016/j.bbrc.2019.01.024
23. Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argilés JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiology-Endocrinology Metab (2009) 296(1):E191–202. doi: 10.1152/ajpendo.90506.2008
24. Leal LG, Lopes MA, Batista ML Jr. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front Physiol (2018) 9:1307. doi: 10.3389/fphys.2018.01307
25. Khalafi M, Symonds ME, Akbari A. The impact of exercise training versus caloric restriction on inflammation markers: a systemic review and meta-analysis. Crit Rev Food Sci Nutr (2022) 62(15):4226–41. doi: 10.1080/10408398.2021.1873732
26. Cerqueira É, Marinho DA, Neiva HP, Lourenço O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front Physiol (2020) 10:1550. doi: 10.3389/fphys.2019.01550
27. Brown WM, Davison GW, McClean CM, Murphy MH. A systematic review of the acute effects of exercise on immune and inflammatory indices in untrained adults. Sports medicine-open. (2015) 1(1):1–10. doi: 10.1186/s40798-015-0032-x
28. Cabral-Santos C, de Lima Junior EA, Fernandes I, Pinto RZ, Rosa-Neto JC, Bishop NC, et al. Interleukin-10 responses from acute exercise in healthy subjects: A systematic review. J Cell Physiol (2019) 234(7):9956–65. doi: 10.1002/jcp.27920
29. Khosravi N, Stoner L, Farajivafa V, Hanson ED. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain behavior immunity. (2019) 81:92–104. doi: 10.1016/j.bbi.2019.08.187
30. Hayashino Y, Jackson JL, Hirata T, Fukumori N, Nakamura F, Fukuhara S, et al. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism (2014) 63(3):431–40. doi: 10.1016/j.metabol.2013.08.018
31. Garneau L, Parsons SA, Smith SR, Mulvihill EE, Sparks LM, Aguer C. Plasma myokine concentrations after acute exercise in non-obese and obese sedentary women. Front Physiol (2020) 11:18. doi: 10.3389/fphys.2020.00018
32. Crane JD, MacNeil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell (2015) 14(4):625–34. doi: 10.1111/acel.12341
33. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol (2012) 8(8):457–65. doi: 10.1038/nrendo.2012.49
34. Tamura K, Satoh Y, Ikoma A, Miyazaki T. Pneumoperitoneum in a patient with lymphangiomyomatosis. Respiration (1991) 58(3-4):211–3. doi: 10.1159/000195929
35. Pérez-López A, McKendry J, Martin-Rincon M, Morales-Alamo D, Pérez-Köhler B, Valadés D, et al. Skeletal muscle IL-15/IL-15Rα and myofibrillar protein synthesis after resistance exercise. Scandinavian J Med Sci sports. (2018) 28(1):116–25. doi: 10.1111/sms.12901
36. Rinnov A, Yfanti C, Nielsen S, Åkerström TC, Peijs L, Zankari A, et al. Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine (2014) 45:271–8. doi: 10.1007/s12020-013-9969-z
37. Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, et al. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol (2007) 584(Pt 1):305–12. doi: 10.1113/jphysiol.2007.139618
38. Pérez-López A, Gonzalo-Encabo P, Pérez-Köhler B, García-Honduvilla N, Valadés D. Circulating myokines IL-6, IL-15 and FGF21 response to training is altered by exercise type but not by menopause in women with obesity. Eur J Sport Science. (2022) 22(9):1426–35. doi: 10.1080/17461391.2021.1939430
39. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J surgery. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
40. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons (2019).
41. Ashton RE, Tew GA, Aning JJ, Gilbert SE, Lewis L, Saxton JM. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: systematic review with meta-analysis. Br J sports Med (2020) 54(6):341–8. doi: 10.1136/bjsports-2017-098970
42. Fedewa MV, Hathaway ED, Ward-Ritacco CL, Williams TD, Dobbs WC. The effect of chronic exercise training on leptin: a systematic review and meta-analysis of randomized controlled trials. Sports Med (2018) 48:1437–50. doi: 10.1007/s40279-018-0897-1
43. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res methodology. (2014) 14:1–13. doi: 10.1186/1471-2288-14-135
44. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res methodology. (2005) 5(1):1–10. doi: 10.1186/1471-2288-5-13
45. De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiotherapy. (2009) 55(2):129–33. doi: 10.1016/S0004-9514(09)70043-1
46. Bugera EM, Duhamel TA, Peeler JD, Cornish SM. The systemic myokine response of decorin, interleukin-6 (IL-6) and interleukin-15 (IL-15) to an acute bout of blood flow restricted exercise. Eur J Appl Physiol (2018) 118(12):2679–86. doi: 10.1007/s00421-018-3995-8
47. Christiansen T, Bruun JM, Paulsen SK, Olholm J, Overgaard K, Pedersen SB, et al. Acute exercise increases circulating inflammatory markers in overweight and obese compared with lean subjects. Eur J Appl Physiol (2013) 113(6):1635–42. doi: 10.1007/s00421-013-2592-0
48. Eskandari A, Fashi M, Saeidi A, Boullosa D, Laher I, Ben Abderrahman A, et al. Resistance exercise in a hot environment alters serum markers in untrained males. Front Physiol (2020) 11:597. doi: 10.3389/fphys.2020.00597
49. Hingorjo MR, Zehra S, Saleem S, Qureshi MA. Serum Interleukin-15 and its relationship with adiposity Indices before and after short-term endurance exercise. Pak J Med Sci (2018) 34(5):1125–31. doi: 10.12669/pjms.345.15516
50. Kapilevich LV, Zakharova AN, Kabachkova AV, Kironenko TA, Orlov SN. Dynamic and static exercises differentially affect plasma cytokine content in elite endurance- and strength-trained athletes and untrained volunteers. Front Physiol (2017) 8:35. doi: 10.3389/fphys.2017.00035
51. Luk HY, Jones MT, Vingren JL. Effect of rest period configurations on systemic inflammatory response in resistance-trained women. J Sports Sci (2021) 39(13):1504–11. doi: 10.1080/02640414.2021.1882725
52. Marcucci-Barbosa LS, Martins F, Lobo LF, Morais MG, Moreira JM, Vieira ELM, et al. 10 km running race induces an elevation in the plasma myokine level of nonprofessional runners. Sport Sci Health (2020) 16(2):313–21. doi: 10.1007/s11332-019-00608-3
53. Minuzzi LG, Chupel MU, Rama L, Rosado F, Munoz VR, Gaspar RC, et al. Lifelong exercise practice and immunosenescence: Master athletes cytokine response to acute exercise. Cytokine (2019) 115:1–7. doi: 10.1016/j.cyto.2018.12.006
54. Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J (2011) 58(3):211–5. doi: 10.1507/endocrj.K10E-400
55. Yargic MP, Torgutalp S, Akin S, Babayeva N, Torgutalp M, Demirel HA. Acute long-distance trail running increases serum IL-6, IL-15, and Hsp72 levels. Appl Physiol Nutr Metab (2019) 44(6):627–31. doi: 10.1139/apnm-2018-0520
56. Micielska K, Gmiat A, Zychowska M, Kozlowska M, Walentukiewicz A, Lysak-Radomska A, et al. The beneficial effects of 15 units of high-intensity circuit training in women is modified by age, baseline insulin resistance and physical capacity. Diabetes Res Clin Pract (2019) 152:156–65. doi: 10.1016/j.diabres.2019.05.009
57. Urzi F, Marusic U, Ličen S, Buzan E. Effects of elastic resistance training on functional performance and myokines in older women-A randomized controlled trial. J Am Med Dir Assoc (2019) 20(7):830–4.e2. doi: 10.1016/j.jamda.2019.01.151
58. Banitalebi E, Kazemi A, Faramarzi M, Nasiri S, Haghighi MM. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci (2019) 217:101–9. doi: 10.1016/j.lfs.2018.11.062
59. Beavers KM, Hsu FC, Isom S, Kritchevsky SB, Church T, Goodpaster B, et al. Long-term physical activity and inflammatory biomarkers in older adults. Med Sci Sports Exerc. (2010) 42(12):2189–96. doi: 10.1249/MSS.0b013e3181e3ac80
60. Brunelli DT, Chacon-Mikahil MPT, Gaspari AF, Lopes WA, Bonganha V, Bonfante ILP, et al. Combined training reduces subclinical inflammation in obese middle-age men. Med Sci Sports Exercise. (2015) 47(10):2207–15. doi: 10.1249/MSS.0000000000000658
61. Coletta AM, Agha NH, Baker FL, Niemiro GM, Mylabathula PL, Brewster AM, et al. The impact of high-intensity interval exercise training on NK-cell function and circulating myokines for breast cancer prevention among women at high risk for breast cancer. Breast Cancer Res Treat (2021) 187(2):407–16. doi: 10.1007/s10549-021-06111-z
62. Corrêa HL, Neves RVP, Deus LA, Souza MK, Haro AS, Costa F, et al. Blood flow restriction training blunts chronic kidney disease progression in humans. Med Sci Sports Exerc. (2021) 53(2):249–57. doi: 10.1249/MSS.0000000000002465
63. Mahmoud N, Mohammadreza HA, Abdolhosein TK, Mehdi N, Arent SM. Serum myokine levels after linear and flexible non-linear periodized resistance training in overweight sedentary women. Eur J Sport Sci (2022) 22(4):658–68. doi: 10.1080/17461391.2021.1895893
64. Nishida Y, Tanaka K, Hara M, Hirao N, Tanaka H, Tobina T, et al. Effects of home-based bench step exercise on inflammatory cytokines and lipid profiles in elderly Japanese females: A randomized controlled trial. Arch Gerontol Geriatr. (2015) 61(3):443–51. doi: 10.1016/j.archger.2015.06.017
65. Pérez-López A, Gonzalo-Encabo P, Pérez-Köhler B, García-Honduvilla N, Valadés D. Circulating myokines IL-6, IL-15 and FGF21 response to training is altered by exercise type but not by menopause in women with obesity. Eur J Sport Sci (2022) 22(9):1426–35. doi: 10.1080/17461391.2021.1939430
66. Tsai CL, Pai MC, Ukropec J, Ukropcová B. Distinctive effects of aerobic and resistance exercise modes on neurocognitive and biochemical changes in individuals with mild cognitive impairment. Curr Alzheimer Res (2019) 16(4):316–32. doi: 10.2174/1567205016666190228125429
67. Perez-Lopez A, Valadés D, Vázquez Martínez C, de Cos Blanco A, Bujan J, Garcia-Honduvilla N. Serum IL-15 and IL-15Rα levels are decreased in lean and obese physically active humans. Scandinavian J Med Sci Sports. (2018) 28(3):1113–20. doi: 10.1111/sms.12983
68. Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (2007) 103(5):1744–51. doi: 10.1152/japplphysiol.00679.2007
69. Nieman DC, Davis J, Brown VA, Henson DA, Dumke CL, Utter AC, et al. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol (2004) 96(4):1292–8. doi: 10.1152/japplphysiol.01064.2003
70. Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol (2003) 94(5):1917–25. doi: 10.1152/japplphysiol.01130.2002
71. Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol (1998) 160(9):4418–26. doi: 10.4049/jimmunol.160.9.4418
72. Cai M, Huang X, Huang X, Ju D, Zhu YZ, Ye L. Research progress of interleukin-15 in cancer immunotherapy. Front Pharmacol (2023) 14:1184703. doi: 10.3389/fphar.2023.1184703
73. Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R, et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab (2008) 93(11):4486–93. doi: 10.1210/jc.2007-2561
74. Almendro V, Busquets S, Ametller E, Carbó N, Figueras M, Fuster G, et al. Effects of interleukin-15 on lipid oxidation: disposal of an oral [14C]-triolein load. Biochim Biophys Acta (BBA)-Molecular Cell Biol Lipids. (2006) 1761(1):37–42. doi: 10.1016/j.bbalip.2005.12.006
75. García-Hermoso A, Ramírez-Vélez R, Díez J, González A, Izquierdo M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J Sport Health Sci (2022) 12(2):147–57. doi: 10.1016/j.jshs.2022.11.003
Keywords: exercise, interleukin 15, body mass index, myokine, inflammation
Citation: Khalafi M, Maleki AH, Symonds ME, Sakhaei MH, Rosenkranz SK, Ehsanifar M, Korivi M and Liu Y (2024) Interleukin-15 responses to acute and chronic exercise in adults: a systematic review and meta-analysis. Front. Immunol. 14:1288537. doi: 10.3389/fimmu.2023.1288537
Received: 04 September 2023; Accepted: 11 December 2023;
Published: 03 January 2024.
Edited by:
Mojtaba Kaviani, Acadia University, CanadaReviewed by:
Mahdieh Molanouri Shamsi, Tarbiat Modares University, IranCopyright © 2024 Khalafi, Maleki, Symonds, Sakhaei, Rosenkranz, Ehsanifar, Korivi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mallikarjuna Korivi, bWFsbGlrLms1QGdtYWlsLmNvbQ==; bWFsbGlrQHpqbnUuZWR1LmNu; Yubo Liu, bGl1eXVibzAxMjRAb3V0bG9vay5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.