- 1Hospices Civils de Lyon, Hôpital Femme-Mère-Enfant, Service de Réanimation Pédiatrique, Bron, France
- 2Hospices Civils de Lyon, Hôpital Edouard Herriot, Laboratoire d’Immunologie, Lyon, France
- 3Hospices Civils de Lyon, Hôpital Femme-Mère-Enfant, Service de Néphrologie et Rhumatologie Pédiatrique, Centre de Référence RAISE (Rhumatismes Inflammatoires et Maladies Auto-Immunes Systémiques Rares de l’Enfant), ERN RITA (European Reference Network for Immunodeficiency, Autoinflammatory, Autoimmune and Paediatric Rheumatic Diseases), Bron, France
Introduction: Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening condition, and its diagnosis may be challenging. In particular, some cases show close similarities to sepsis (fever, organ failure, and high ferritin), but their treatment, while urgent, differ: prompt broad-spectrum antibiotherapy for sepsis and immunosuppressive treatment for HLH. We questioned whether monocyte human leucocyte antigen (mHLA)–DR could be a diagnostic marker for secondary HLH (sHLH).
Methods: We retrospectively reviewed data from patients with a sHLH diagnosis and mHLA-DR quantification. mHLA-DR data from healthy children and children with septic shock, whose HLA-DR expression is reduced, from a previously published study were also included for comparison.
Results: Six patients with sHLH had mHLA-DR quantification. The median level of monocyte mHLA-DR expression in patients with sHLH [79,409 antibodies bound per cell (AB/C), interquartile range (IQR) (75,734–86,453)] was significantly higher than that in healthy children and those with septic shock (29,668 AB/C, IQR (24,335–39,199), and 7,493 AB/C, IQR (3,758–14,659), respectively). Each patient with sHLH had a mHLA-DR higher than our laboratory normal values. Four patients had a second mHLA-DR sampling 2 to 4 days after the initial analysis and treatment initiation with high-dose corticosteroids; for all patients, mHLA-DR decreased to within or close to the normal range. One patient with systemic juvenile idiopathic arthritis had repeated mHLA-DR measurements over a 200-day period during which she underwent four HLH episodes. mHLA-DR increased during relapses and normalized after treatment incrementation.
Conclusion: In this small series, mHLA-DR was systematically elevated in patients with sHLH. Elevated mHLA-DR could contribute to sHLH diagnosis and help earlier distinction with septic shock.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening condition associated with unregulated and deleterious T-lymphocyte and macrophage activation. Various genetic and environmental factors may cause HLH through a defective immune regulation (1); these are commonly separated between primary/genetic HLH and secondary forms [sHLH; associated with rheumatologic diseases termed macrophage activation syndrome (MAS), malignancies, viral infections, and primary immunodeficiencies but also drug-induced (2, 3)]. HLH diagnosis may be challenging because of heterogeneity in cause and clinical presentation (4, 5), and this has led to the development and validation of scores adapted to specific populations (HLH-2004, 2016 EULAR/ACR, HScore) that are based on common parameters but with different thresholds (1, 6, 7).
Recent reports have explored markers of T-cell activation and found that HLH was characterized by strongly elevated activated CD8+ T cells. Specifically, CD38high HLA-DR+ CD8+ T cells above 7% (among CD8+ T cells) could help differentiate HLH from sepsis (8). Similarly, sHLH is associated with a proportion of CD4dim CD8+ T cells (that are highly activated and cytolytic CD8+ mature T cells) above 1.45% that reliably discriminate sHLH from other causes of systemic inflammation such as flare of systemic juvenile idiopathic arthritis (sJIA) (9). HLA-DR expression on T cells could also help to identify primary from sHLH with a higher expression in primary forms (10). However, no marker of monocyte activation has been described in HLH, although medullar hemophagocytosis, elevated Interferon-γ (IFN-γ), and serum biomarker profile (11–13) suggest a central role of macrophage stimulation in HLH pathogenesis. The two main actions of IFN-γ are, on the one hand, to enhance CD8+ T-cell cytotoxicity and T-helper 1 (Th1) response; and, on the other, to increase monocyte/macrophage activity and their expression of MHC surface molecules together with an enhancement of their phagocytic capacity. HLA-DR expression on monocytes reflects IFN-γ plasma levels (14). In 2018, we published the case of a young adolescent female patient suspected of septic shock (15), who was included in a prospective cohort of sepsis immunomonitoring: PedIRIS (16). The unexpectedly high level of monocyte human leucocyte antigen (mHLA)–DR (137,021 antibodies bound per cell, AB/C) compared to the usual low value of patients with septic shock (below 10,000 AB/C) helped in the diagnosis of sHLH caused by drug-induced hypersensitivity syndrome (15).
This raises the question of whether mHLA-DR could contribute to sHLH diagnosis, and following this case mHLA-DR was added to the usual lymphocyte phenotyping conducted upon sHLH suspicion in patients admitted to the pediatric departments of our institution. Herein, we retrospectively analyzed the clinical and laboratory data of these patients to describe mHLA-DR levels among children with sHLH diagnosis.
Materials and methods
Patients
We included all patients admitted to the pediatric intensive care unit or the pediatric nephrology and rheumatology department (Hospices Civils de Lyon, Lyon, France) who had a diagnosis of sHLH according to the 2016 EULAR/ACR classification criteria (6) and at least one mHLA-DR quantification between March 2018 and June 2022. The patient previously reported (15) was also included in the present study.
Clinical and laboratory data
We recorded for each patient their medical history, the date of sHLH diagnosis, their clinical data (fever, presence of splenomegaly, previous treatments, as well as those for the current episode), and the following laboratory data: ferritin, triglycerides, fibrinogen, aspartate amino-transferase (ASAT), hemoglobin, platelet count, white blood cell count, and evidence of bone marrow hemophagocytosis.
The values for all presented variables correspond to those on the day of maximum ferritin for each patient; in cases where these data were not available, the values obtained within a 24-h period (3 days for bone marrow aspiration) were recorded (and in case of multiple tests, the closest was considered). Expression of mHLA-DR was measured using flow cytometry following a previously published protocol and expressed as AB/C (17). In addition mHLA-DR data from healthy children and children with septic shock from a study previously published (16) were also included for comparison. Healthy children had a median age of 2.8 years [interquartile range (IQR) (1.5–5.9)], and 8 (27%) were female patients; children with septic shock had a median age of 2.4 years [IQR (0.4–4.4)], and 24 (52%) were female patients.
mHLA-DR flow cytometry measurement
The number of HLA-DR molecules per monocyte was determined using the BD quantibrite standardized method. Briefly, 25 μL of whole blood were stained with 10 μL of QuantiBrite HLA-DR/Monocyte mixture [QuantiBrite anti–HLA-DR Phycoerythrin (PE) (clone L243)/anti-monocytes (CD14) PerCP-Cy5.5 (clone MϕP9), Becton Dickinson San Jose, CA, USA] and 5µl of antiCD19 Pacific blue (PB) and kept at room temperature for 30 min in a dark chamber. Samples were then lysed with Fluorescence-Activated Cell Sorting (FACS) Lysing solution (Becton Dickinson) for 15 min. After a washing step, cells were analyzed on Navios Cytometer (Beckman Coulter). After leukocyte isolation on a Forward Scatter/Side Scatter biparametric chart to exclude possible red cells, monocytes and B lymphocytes were gated out, respectively, on the basis of CD14 and CD19 expression. HLA-DR expression on monocyte was expressed on a monoparametric chart, and its mean fluorescence intensity (MFI) was measured. B lymphocytes were removed from the gated monocytes because of their high HLA-DR expression to avoid faulty increase of mHLA-DR MFI.
To enable standardized results, mHLA-DR MFI was converted into the number of anti–HLA-DR antibody per cell (AB/C), using calibration beads (QuantibriteH, Becton Dickinson). Results were then expressed as the number of anti–HLA-DR antibodies per cell (AB/C). We ran monthly assays with calibration beads for conversion from MFI to AB/C (18, 19).
Statistical analysis
Patient characteristics and laboratory values were described by frequency, median, and IQR. Comparisons between sHLH, healthy children, and those with septic shock were conducted using Mann–Whitney two-sided unpaired test. Data analysis was performed using R software for Windows, version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
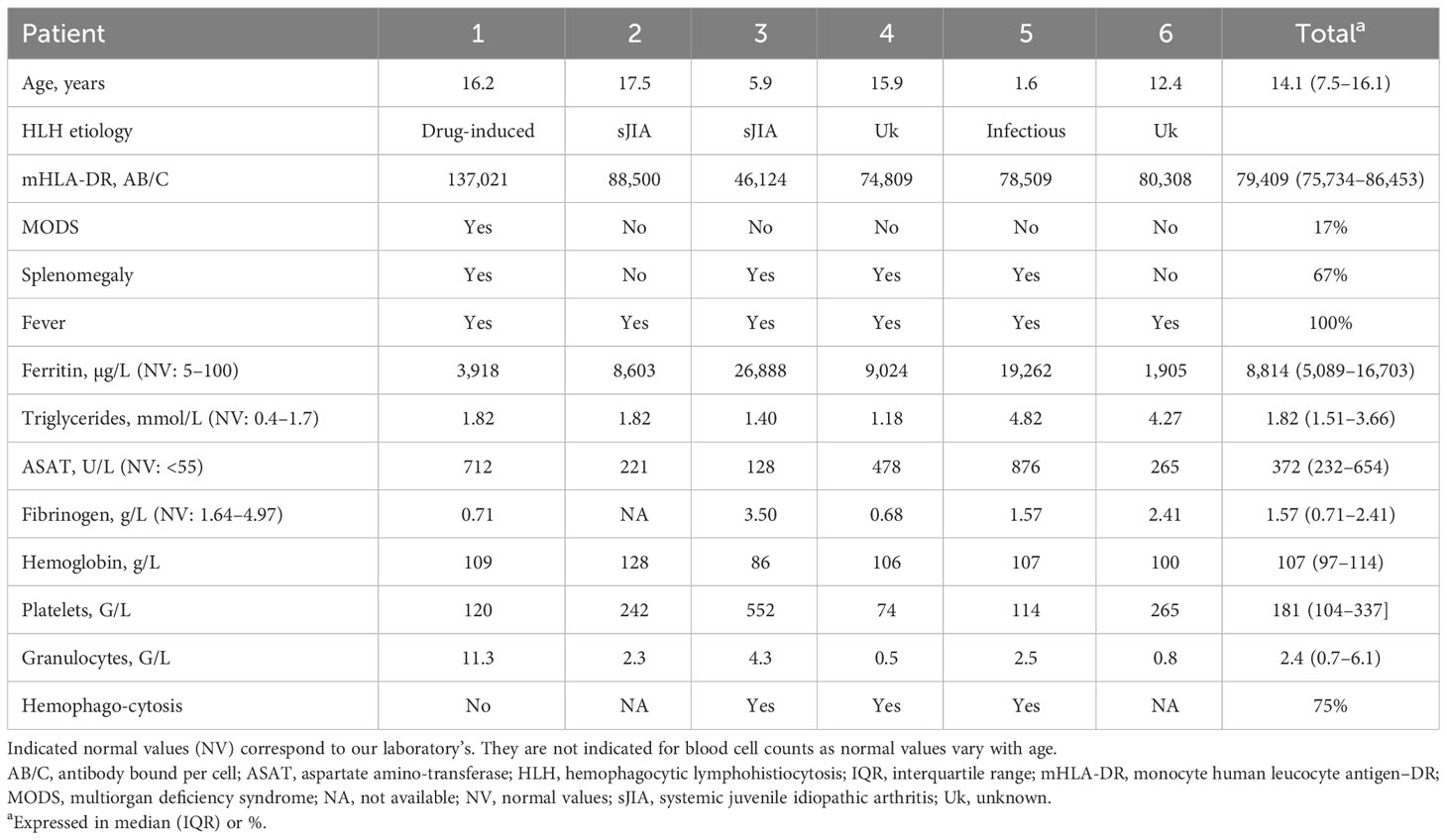
A total of six patients were included. The median age was 14.1 years [IQR (7.5–16.1)], and the etiology of sHLH was Epstein-Barr virus–induced for one patient, rheumatological in the course of sJIA for two patients, drug-induced for one patient, and unknown for the remaining two patients (Table 1). Both the latter had a history of persistent unexplained fever, for which possible infections had been ruled out (2016 EULAR/ACR criteria). Symptomatology resolved in these patients, and laboratory parameters normalized, but the cause of sHLH was not documented; no recurrence was observed during follow-up.
All patients presented with fever. Splenomegaly was found in four (67%) patients. One (Patient 1) had multiorgan deficiency syndrome (MODS) with hemodynamic instability requiring vasopressor therapy and respiratory distress with mechanical ventilation. Ferritin levels were elevated, and the median value was 8,814 µg/L [IQR (5,089–16,703)]; ASAT levels were also elevated, and the median value was 372 U/L [IQR (232–654)]. Triglyceride and fibrinogen levels were not abnormal in all patients, and the median triglycerides value was 1.82 mmol/L [IQR (1.51–3.66)] and that of fibrinogen was 1.57 g/L [IQR (0.71–2.41)]. Cytopenia was found in all patients, with monocytopenia for two patients, bicytopenia for two patients, and pancytopenia for the remaining two patients. Bone marrow aspiration found evidence of hemophagocytosis in three of the four patients for which a sample had been taken (Table 1).
Only the two patients with sJIA-associated HLH had an ongoing treatment prior to mHLA-DR measurement. Patient 2 had a systemic corticosteroid therapy for 3 months that was progressively tapered to 0.3 mg/kg at the time of sampling, and anti-Interleukin-1 (IL1) receptor agonist (anakinra), started 2 weeks earlier. Patient 3 had a high-dose intravenous immunoglobin treatment (twice 1 g/kg) in the preceding 2 weeks.
The median level of monocyte mHLA-DR expression in patients with sHLH [79,409 AB/C, IQR (75,734–86,453)] was significantly higher than in healthy children [29,668 AB/C, IQR (24,335–39,199), p < 0.01] and in children with septic shock [7,493 AB/C, IQR (3,758–14,659), p < 0.01]. Each patient with sHLH had a mHLA-DR higher than our laboratory normal values (15,000–43,000 AB/C). Only one patient with sHLH (Patient 3) had a mHLA-DR value (46,124 AB/C) overlapping with that of the healthy children (maximum of 67,255 AB/C; Figure 1).
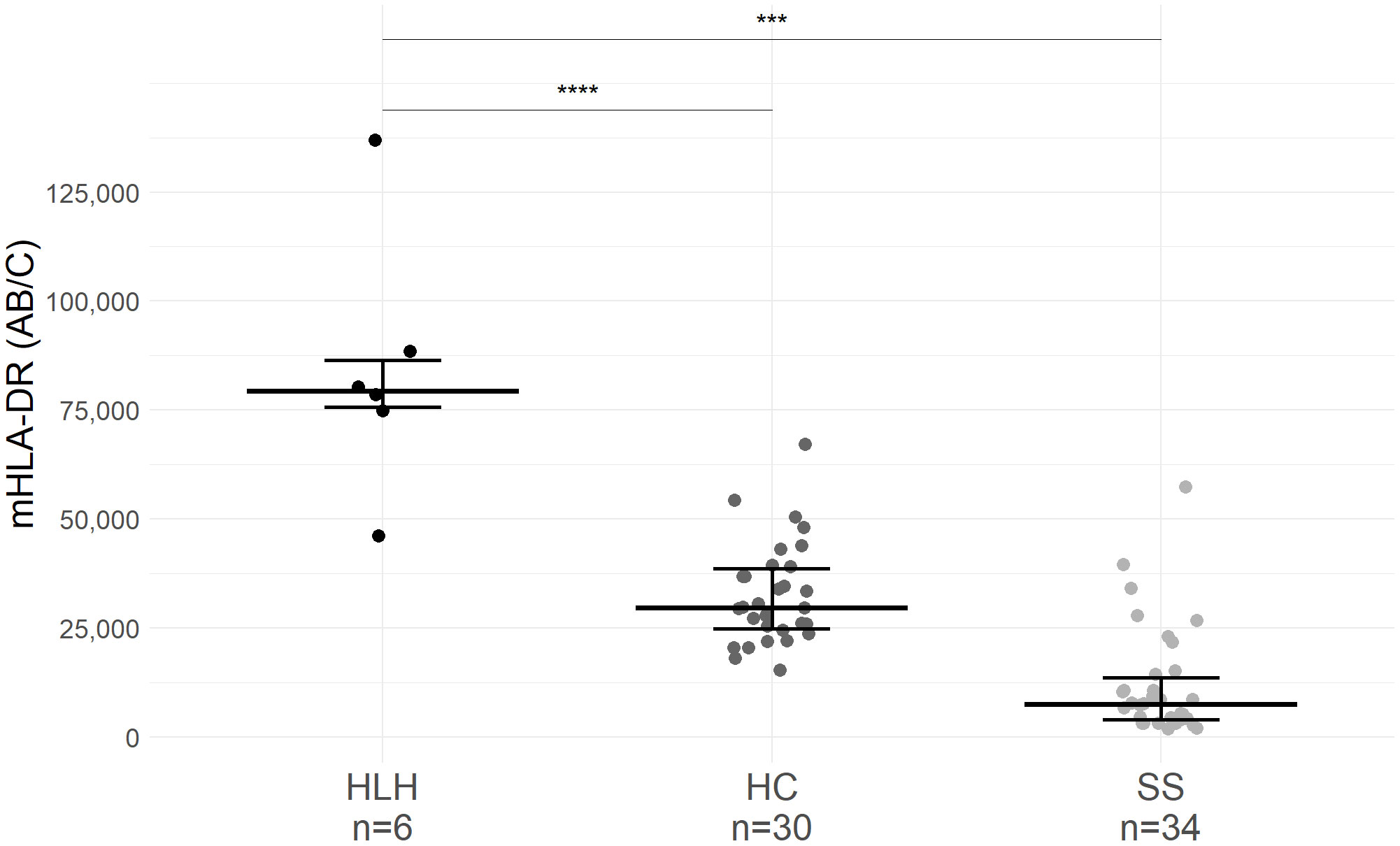
Figure 1 mHLA-DR expression in patients with hemophagocytic lymphohistiocytosis (HLH), healthy children (HC), and septic shock (SS). Only the first mHLA-DR value was retained for each patient. Values of mHLA-DR from HC and patients with SS originated from a previous study conducted in our institution [NCT02848144 (14)]. mHLA-DR expression corresponds to the number of antibodies bound per cell (AB/C). Wide lines correspond to the median value and narrow lines to first and third quartiles. Median age of HC was 2.8 years old [IQR (1.5–5.9)], and median age of SS children was 2.4 years old [IQR (0.4–4.4)]. Dots correspond to individual values. *** p<0.01, **** p<0.001.
Four patients (Patients 1, 3, 4, and 5) had a second mHLA-DR measurement 2 to 4 days after the initial analysis and treatment initiation with high-dose corticosteroids; for all four patients, the values decreased to within or close to the normal range (Figure 2A). Ferritin paralleled this favorable course, although values remained above the normal range (Figure 2B). Normalization of all HLH laboratory criteria was achieved for each patient within 30 days.
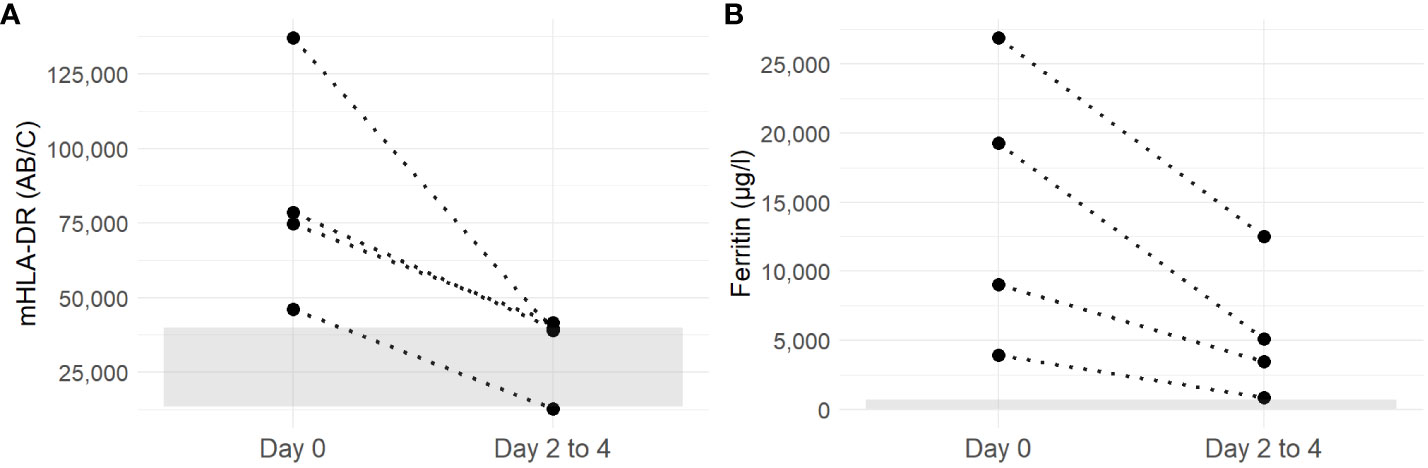
Figure 2 Evolution of mHLA-DR (A) and ferritin (B) of patients with sHLH between day 0 (before corticosteroid initiation) and days 2 to 4 (after corticosteroid treatment). Discontinuous lines link values from each individual patient. The gray ribbon corresponds to the laboratory normal values for mHLA-DR and ferritine (mHLA-DR: 15,000–43,000 AB/C).
One patient (Patient 2) had repeated mHLA-DR measurements over a period of over 200 days, during which she underwent four episodes of rheumatologic-associated MAS (Figure 3). She was a 17-year-old female patient with a history of sJIA, diagnosed 1 month before the first occurrence of MAS. She was treated with anakinra (IL1 receptor antagonist, 100 mg/day) and oral corticosteroid therapy that was being tapered (0.3 mg/kg/day at the time of MAS). The first MAS episode was suspected clinically because of persistent fever and fatigue. Her ferritin level was elevated (8,603 µg/L), and she had a mild hypertriglyceridemia (1.82 mmol/L) and hepatic cytolysis (ASAT: 221 U/L); mHLA-DR was markedly elevated (88,500 AB/C). After treatment with three consecutive corticosteroid pulses at 10 mg/kg, increase of anakinra to 200 mg/day and of oral corticosteroids, a strong decrease in both ferritin and mHLA-DR was observed. Fever and fatigue resolved. A month and a half later while corticosteroids were being tapered, despite a favorable clinical course, ferritin started to rise slowly and mHLA-DR had risen dramatically. To avoid excessive cumulative corticosteroid treatment, cyclosporine was started at 5 mg/kg/day with a favorable effect on ferritin and mHLA-DR. Less than 3 months after cyclosporine initiation, after a decrease of oral corticosteroids to 0.2 mg/kg/day and of anakinra to 100 mg/day, fever resumed along with fatigue and joint pain. Ferritin had risen to 714 µg/l, as did mHLA-DR but below the upper normal range (41,731AB/C). An increase in oral corticosteroid treatment to 1 mg/kg/day was sufficient to improve clinical and laboratory signs of disease activity. Similarly, 2 months later, and in the absence of cyclosporine and while anakinra and corticosteroid treatment were being tapered, she experienced a fourth MAS episode. She suffered from abnormal fatigue and fever for 7 days. Analyses found signs of MAS: ferritin at 2256 µg/L and ASAT at 101U/L; mHLA-DR was elevated (86,005 AB/C). After treatment with one corticosteroid pulse and a moderate increase in oral corticosteroids, both clinical and laboratory signs of MAS normalized. She did not experience another episode of MAS, but corticodependance remained elevated thereafter.
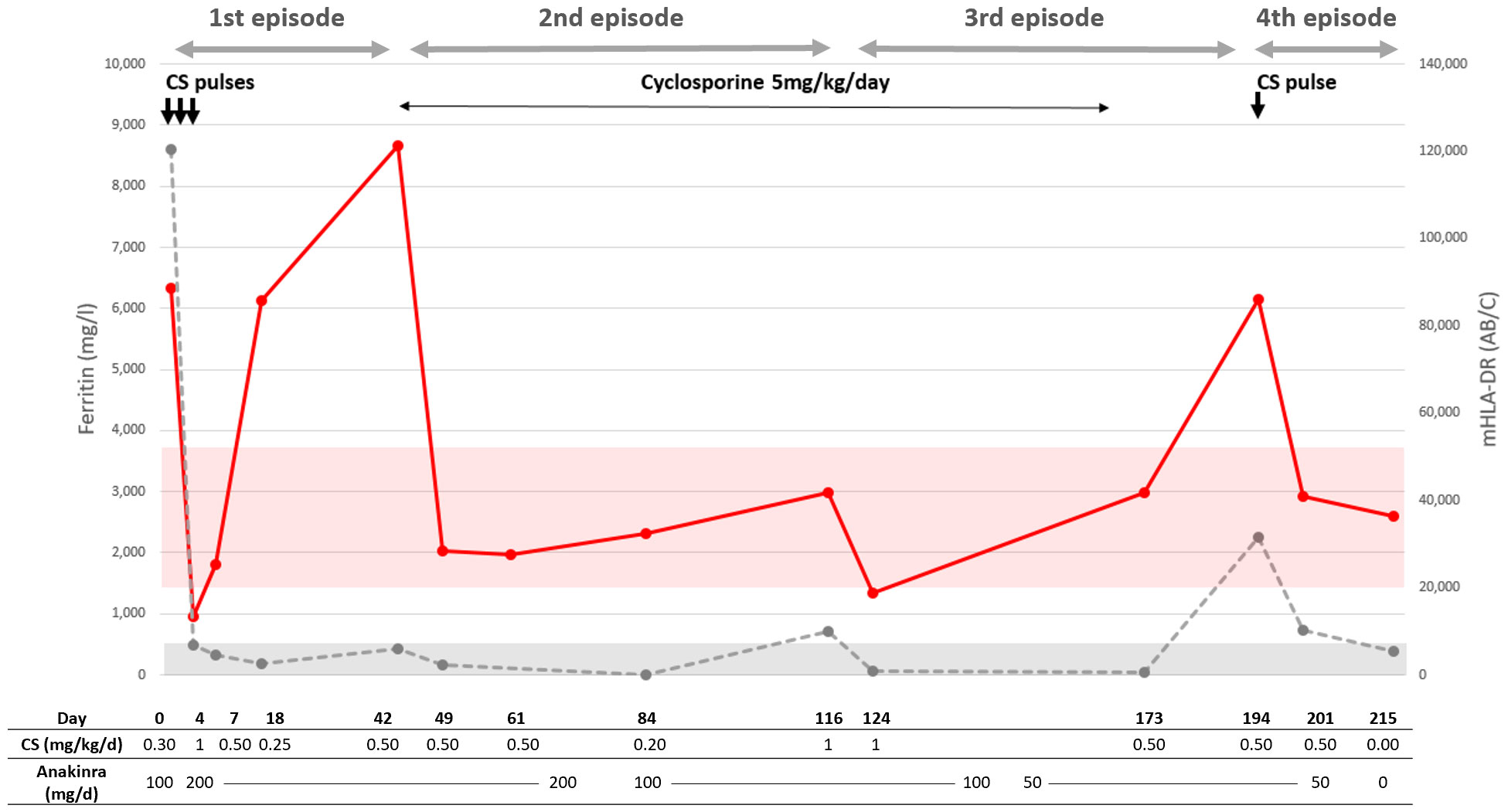
Figure 3 Course of Patient 1. Red dots and line correspond to mHLA-DR; the red ribbon corresponds to normal values. The gray dots and discontinuous line correspond to ferritin; the gray ribbon corresponds to normal values with an upper threshold of 500 µg/L. Each corticosteroid (CS) pulse arrow corresponds to methylprednisolone at 10mg/kg. The start of a new episode is defined by the relapse of sHLH symptoms and laboratory criteria.
Discussion
In the present study, mHLA-DR levels were systematically abnormally high in patients with sHLH, and, in contrast to other HLH biomarkers such as ferritin, mHLA-DR quickly normalized under corticosteroid treatment and conversely rose in case of sHLH relapse. In this context, mHLA-DR might be a useful tool for their differential diagnosis, reflecting the known physiopathology of HLH driven by IFN-γ production, leading to an enhanced and dysregulated activation of CD8+ cytotoxic T cells and monocytes. In addition, among patients with MODS, mHLA-DR could be able to better distinguish sHLH from those with another etiology than the majority of HLH markers. A low mHLA-DR level has been well established as a marker of monocyte anergy in adult patients who experience septic shock (20), severe trauma, and burns or who undergo cardiac surgery with extra-corporeal circulation (21), and similar results are reported in pediatric populations (16, 22–24); herein, the patient with MODS, initially considered as a septic shock, had extremely elevated mHLA-DR (three-fold), and it was later understood that the etiology was a drug reaction with eosinophilia and systemic symptoms associated to HLH, and treatment with corticosteroids was sufficient for a resolution of clinical symptoms and laboratory parameters (15).
sHLH is a complex and multifaceted syndrome, and its diagnosis remains a challenge to this day, overlapping with other cytokine storm such as sepsis (25–27). The validated scores adapted to specific populations are based on the same laboratory parameters (high ferritin, high triglycerides, low fibrinogen, presence of cytopenia, and elevated ASAT) but use different thresholds. For instance, analyses of the performance of HLH-2004 in the setting of adult ICU found that changing ferritin and fever cutoffs was necessary to take into account the other causes of lesser ferritin elevation and fever among ICU patients, such as those with sepsis (28), and which led to the development of the Hscore (7). It is of note that, despite being eponymous of sHLH, hemophagocytosis does not have either a good positive or a good negative predictive value as it is associated with many conditions (29), especially within a critical care setting, as highlighted by a postmortem evaluation of critically ill patients that found less than two-thirds of patients had hemophagocytosis (30). This issue is all the more important considering that, while sharing symptomatic treatment of organ dysfunction, etiologic treatment differ; a prompt and broad-spectrum antibiotherapy is required for sepsis, but an effective immunosuppression with high-dose corticosteroids, possibly in association with etoposide or cyclosporine, is required for sHLH (1, 31–33). The data presented herein therefore suggest that mHLA-DR could contribute to sHLH diagnosis. However, the study presents several limitations. Because of the retrospective nature of the study design, mHLA-DR and other laboratory values were not systematically determined on the same day. In addition, sHLH diagnosis and etiology required a post-hoc analysis to be confirmed which exposes to a risk of misclassification. In addition, because the pediatric hematology department is in another institution in Lyon, the cohort does not include hemopathy-associated HLH that may present with differences regarding mHLA-DR levels. A more general note is that the small sample included herein may not represent accurately HLH patients as a whole and precludes the investigation of a mHLA-DR cutoff for sHLH diagnosis. A prospective study is therefore warranted to further investigate mHLA-DR as a marker for sHLH, with a larger cohort of patients, and a balanced distribution between HLH etiologies, including primary HLH and hemopathy-associated HLH.
In conclusion, elevated mHLA-DR could contribute to sHLH diagnosis, but this requires further investigation in a larger study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Scientific and Ethical Committee of Hospices Civils de Lyon. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the data used originated from medical files and was made anonymous prior to analysis. Patients and legal guardians are informed during their stay that medical data may be used for retrospective analysis.
Author contributions
SyR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GM: Methodology, Supervision, Validation, Writing – review & editing. MG: Data curation, Methodology, Writing – review & editing. FV: Data curation, Methodology, Validation, Writing – review & editing. AB: Methodology, Supervision, Writing – review & editing. FZ: Data curation, Validation, Writing – review & editing. SoR: Data curation, Validation, Writing – review & editing. EJ: Conceptualization, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the institution employing the authors, as part of their usual duties.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer (2007) 48(2):124–31. doi: 10.1002/pbc.21039
2. Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med (2012) 63:233–46. doi: 10.1146/annurev-med-041610-134208
3. Jordan MB, Allen CE, Greenberg J, Henry M, Hermiston ML, Kumar A, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer (2019) 66(11):e27929. doi: 10.1002/pbc.27929
4. Chinn IK, Eckstein OS, Peckham-Gregory EC, Goldberg BR, Forbes LR, Nicholas SK, et al. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood. (2018) 132(1):89–100. doi: 10.1182/blood-2017-11-814244
5. Gurunathan A, Boucher AA, Mark M, Prus KM, O’Brien MM, Breese EH, et al. Limitations of HLH-2004 criteria in distinguishing Malignancy-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer (2018) 65(12):e27400. doi: 10.1002/pbc.27400
6. Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A european league against rheumatism/american college of rheumatology/paediatric rheumatology international trials organisation collaborative initiative. Ann Rheum Dis (2016) 75(3):481–9. doi: 10.1136/annrheumdis-2015-208982
7. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol (Hoboken NJ) (2014) 66(9):2613–20. doi: 10.1002/art.38690
8. Chaturvedi V, Marsh RA, Zoref-Lorenz A, Owsley E, Chaturvedi V, Nguyen TC, et al. T-cell activation profiles distinguish hemophagocytic lymphohistiocytosis and early sepsis. Blood. (2021) 137(17):2337–46. doi: 10.1182/blood.2020009499
9. De Matteis A, Colucci M, Rossi MN, Caiello I, Merli P, Tumino N, et al. Expansion of CD4dimCD8+ T cells characterizes macrophage activation syndrome and other secondary HLH. Blood. (2022) 140(3):262–73. doi: 10.1182/blood.2021013549
10. Ammann S, Lehmberg K, Zur Stadt U, Janka G, Rensing-Ehl A, Klemann C. Primary and secondary hemophagocytic lymphohistiocytosis have different patterns of T-cell activation, differentiation and repertoire. Eur J Immunol (2017) 47(2):364–73. doi: 10.1002/eji.201646686
11. Pachlopnik Schmid J, Ho CH, Chrétien F, Lefebvre JM, Pivert G, Kosco-Vilbois M, et al. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med (2009) 1(2):112–24. doi: 10.1002/emmm.200900009
12. Prencipe G, Caiello I, Pascarella A, Grom AA, Bracaglia C, Chatel L, et al. Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol (2018) 141(4):1439–49. doi: 10.1016/j.jaci.2017.07.021
13. Kessel C, Fall N, Grom A, de Jager W, Vastert S, Strippoli R, et al. Definition and validation of serum biomarkers for optimal differentiation of hyperferritinaemic cytokine storm conditions in children: a retrospective cohort study. Lancet Rheum (2021) 3(8):e563–e573. doi: 10.1016/S2665-9913(21)00115-6
14. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol (2018) 233(9):6425–40. doi: 10.1002/jcp.26429
15. Remy S, Gossez M, Belot A, Hayman J, Portefaix A, Venet F, et al. Massive increase in monocyte HLA-DR expression can be used to discriminate between septic shock and hemophagocytic lymphohistiocytosis-induced shock. Crit Care (2018) 22(1):213. doi: 10.1186/s13054-018-2146-2
16. Remy S, Kolev-Descamps K, Gossez M, Venet F, Demaret J, Javouhey E, et al. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: a pilot study. Ann Intensive Care (2018) 8(1):36. doi: 10.1186/s13613-018-0382-x
17. Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med (2006) 32(8):1175–83. doi: 10.1007/s00134-006-0204-8
18. Demaret J, Walencik A, Jacob MC, Timsit JF, Venet F, Lepape A, et al. Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytometry B Clin Cytom (2013) 84(1):59–62. doi: 10.1002/cyto.b.21043
19. Döcke WD, Höflich C, Davis KA, Röttgers K, Meisel C, Kiefer P, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem (2005) 51(12):2341–7. doi: 10.1373/clinchem.2005.052639
20. Venet F, Demaret J, Gossez M, Monneret G. Myeloid cells in sepsis-acquired immunodeficiency. Ann N Y Acad Sci (2020) 1499(1):3–17. doi: 10.1111/nyas.14333
21. Rol ML, Venet F, Rimmele T, Moucadel V, Cortez P, Quemeneur L, et al. The REAnimation Low Immune Status Markers (REALISM) project: a protocol for broad characterisation and follow-up of injury-induced immunosuppression in intensive care unit (ICU) critically ill patients. BMJ Open (2017) 7(6):e015734. doi: 10.1136/bmjopen-2016-015734
22. Chenouard A, Rimbert M, Joram N, Braudeau C, Roquilly A, Bourgoin P, et al. Monocytic human leukocyte antigen DR expression in young infants undergoing cardiopulmonary bypass. Ann Thorac Surg (2020) 111(5):1636–42. doi: 10.1016/j.athoracsur.2020.05.071
23. Boeddha NP, Kerklaan D, Dunbar A, van Puffelen E, Nagtzaam NMA, Vanhorebeek I, et al. HLA-DR expression on monocyte subsets in critically ill children. Pediatr Infect Dis J (2018) 37(10):1034–40. doi: 10.1097/INF.0000000000001990
24. Hall MW, Greathouse KC, Thakkar RK, Sribnick EA, Muszynski JA. Immunoparalysis in pediatric critical care. Pediatr Clin North Am (2017) 64(5):1089–102. doi: 10.1016/j.pcl.2017.06.008
25. Machowicz R, Janka G, Wiktor-Jedrzejczak W. Similar but not the same: Differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol (2017) 114:1–12. doi: 10.1016/j.critrevonc.2017.03.023
26. Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, Dimopoulos G, Pantazi A, Orfanos SE. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med (2017) 15(1):172. doi: 10.1186/s12916-017-0930-5
27. Raschke RA, Garcia-Orr R. Hemophagocytic lymphohistiocytosis: a potentially underrecognized association with systemic inflammatory response syndrome, severe sepsis, and septic shock in adults. Chest. (2011) 140(4):933–8. doi: 10.1378/chest.11-0619
28. Knaak C, Nyvlt P, Schuster FS, Spies C, Heeren P, Schenk T, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care (2020) 24(1):244. doi: 10.1186/s13054-020-02941-3
29. Gupta A, Tyrrell P, Valani R, Benseler S, Weitzman S, Abdelhaleem M. The role of the initial bone marrow aspirate in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer (2008) 51(3):402–4. doi: 10.1002/pbc.21564
30. Strauss R, Neureiter D, Westenburger B, Wehler M, Kirchner T, Hahn EG. Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients–a postmortem clinicopathologic analysis. Crit Care Med (2004) 32(6):1316–21. doi: 10.1097/01.CCM.0000127779.24232.15
31. Crayne C, Cron RQ. Pediatric macrophage activation syndrome, recognizing the tip of the Iceberg. Eur J Rheumatol (2019) 7(Suppl1):S13–S20. doi: 10.5152/eurjrheum.2019.19150
32. Henderson LA, Cron RQ. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: diagnosis and management. Paediatr Drugs (2020) 22(1):29–44. doi: 10.1007/s40272-019-00367-1
Keywords: hemophagocytic lymphohistiocytosis, monocyte HLA-DR, macrophage activation syndrome, lymphocyte activation, cytokine storm
Citation: Raimbault S, Monneret G, Gossez M, Venet F, Belot A, Zekre F, Remy S and Javouhey E (2023) Elevated monocyte HLA-DR in pediatric secondary hemophagocytic lymphohistiocytosis: a retrospective study. Front. Immunol. 14:1286749. doi: 10.3389/fimmu.2023.1286749
Received: 31 August 2023; Accepted: 08 November 2023;
Published: 24 November 2023.
Edited by:
Pietro Merli, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Maurizio Aricò, Azienda Sanitaria Locale, ItalySandra Ammann, University of Freiburg Medical Center, Germany
Copyright © 2023 Raimbault, Monneret, Gossez, Venet, Belot, Zekre, Remy and Javouhey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylvain Raimbault, c3lsdmFpbi5yYWltYmF1bHRAY2h1LWx5b24uZnI=
 Sylvain Raimbault
Sylvain Raimbault Guillaume Monneret
Guillaume Monneret Morgane Gossez
Morgane Gossez Fabienne Venet
Fabienne Venet Alexandre Belot
Alexandre Belot Franck Zekre
Franck Zekre Solene Remy
Solene Remy Etienne Javouhey
Etienne Javouhey