
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 19 October 2023
Sec. T Cell Biology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1286251
This article is part of the Research TopicRegulatory T cells in Immune-mediated diseasesView all 14 articles
 Takemichi Fukasawa1,2
Takemichi Fukasawa1,2 Takashi Yamashita1
Takashi Yamashita1 Atsushi Enomoto3
Atsushi Enomoto3 Yuta Norimatsu1
Yuta Norimatsu1 Satoshi Toyama1
Satoshi Toyama1 Asako Yoshizaki-Ogawa1
Asako Yoshizaki-Ogawa1 Shoko Tateishi4
Shoko Tateishi4 Hiroko Kanda4
Hiroko Kanda4 Kiyoshi Miyagawa3
Kiyoshi Miyagawa3 Shinichi Sato1
Shinichi Sato1 Ayumi Yoshizaki1,2,4*
Ayumi Yoshizaki1,2,4*Introduction: As a form of precision medicine, this study aimed to investigate the specific patient population that would derive the greatest benefit from tildrakizumab, as well as the mechanism of action and efficacy of tildrakizumab in reducing the occurrence of psoriatic arthritis (PsA).
Methods: To achieve this, a multi-center, prospective cohort study was conducted, involving a population of 246 psoriasis patients who had not received any systemic therapy or topical finger therapy between January 2020 and April 2023. Two independent clinicians, who were blinded to the study, analyzed nailfold capillary (NFC) abnormalities, such as nailfold bleeding (NFB) and enlarged capillaries, as well as the incidence of new PsA. Additionally, the factors that determined the response of psoriasis after seven months of tildrakizumab treatment were examined. The study also examined the quantity and role of regulatory T cells (Tregs) and T helper 17 cells both pre- and post-treatment.
Results: The severity of psoriasis, as measured by the Psoriasis Area and Severity Index (PASI), was found to be more pronounced in the tildrakizumab group (n=20) in comparison to the topical group (n=226). At 7 months after tildrakizumab treatment, multivariate analysis showed that those 65 years and older had a significantly better response to treatment in those achieved PASI clear or PASI 2 or less (Likelihood ratio (LR) 16.15, p<0.0001; LR 6. 16, p=0.01). Tildrakizumab improved the number and function of Tregs, which had been reduced by aging. Tildrakizumab demonstrated significant efficacy in improving various pathological factors associated with PsA. These factors include the reduction of NFB, enlargement of capillaries, and inhibition of PsA progression. The hazard ratio for progression to PsA was found to be 0.06 (95% confidence interval: 0.0007-0.46, p=0.007), indicating a substantial reduction in the risk of developing PsA.
Discussion: Tildrakizumab's effectiveness in improving skin lesions can be attributed to its ability to enhance the number and function of Tregs, which are known to decline with age. Furthermore, the drug's positive impact on NFB activity and capillary enlargement, both of which are recognized as risk factors for PsA, further contribute to its inhibitory effect on PsA progression.
Psoriasis is a prevalent chronic inflammatory skin condition that is characterized by various treatment options (1). The field of psoriasis treatment has seen significant advancements, with the availability of at least 11 biologics specifically designed for its management (2). These treatment options encompass both oral and topical medications. The therapeutic targets for psoriasis are diverse and include the utilization of anti-tumor necrosis factor-alpha (TNF-α) antibodies, anti-interleukin (IL)-12/23p40 antibodies, and anti-IL-17 antibodies. Among such a wide variety of treatment options, treatment is often difficult because each patient responds differently to the same therapeutic agent, and what works well in one patient is often ineffective in another. Therefore, it is required to bring the most promising or most effective treatment for an individual patient. Predicting treatment response in advance and providing the most promising or most effective treatment for an individual patient is called precision medicine (3), but at present, it is difficult to predict these treatment responses in advance. In particular, among anti-IL-23 antibodies, there are more than three biologics, each of which differs in whether it is a fully human or humanized antibody, and its affinity also differs in each drug (4). Tildrakizumab, a humanized monoclonal antibody that specifically targets IL-23p19, has shown potential therapeutic effects. However, the specific patient population that would benefit from tildrakizumab remains unknown. Previous clinical trials have not provided sufficient evidence to determine the efficacy of tildrakizumab in elderly patients, likely due to the limited number of participants in this age group (5). Therefore, the objective of this study was to investigate the effectiveness of tildrakizumab in patients as part of a precision medicine approach.
In recent years, especially in Western countries and Japan, society as a whole has been aging, and the question of how to provide medical care to the elderly has become a social issue (6). Regulatory T cells (Treg) are known to be decreased in psoriasis patients, and their suppressive capacity is also known to be reduced (7). As one of the mechanisms, IL-23 is known to act on Tregs and cause pathogenic conversion to IL-17 producing cells (8). Therefore, tildrakizumab, an anti-IL-23 antibody, is expected to reverse this process. It is also known that the number of Tregs is decreased and their suppressive capacity is also decreased in the elderly (9), but there are no reports yet on an elderly patient with psoriasis.
Psoriasis is often accompanied by a range of complications that have a substantial impact on the prognosis of patients (10). One significant comorbidity associated with psoriasis is psoriatic arthritis (PsA), which results in osteoclastic arthritis and significantly diminishes patients’ quality of life (10). Dermatologists have a crucial role in diagnosing PsA as skin symptoms typically manifest years before the onset of PsA-related symptoms (10). Prompt treatment is particularly vital for PsA, as delays in treatment can lead to a decline in quality of life (11). Consequently, it is imperative to impede the progression to PsA. However, there are no reports on treatment effect of tildrakizumab for risk factors of progression to PsA, which includes NFB or enlarged capillaries (12, 13).
In recent years, biologics have been reported to inhibit the progression to PsA (14). However, the agents that have been reported primarily consist of antibodies targeting TNF-α, IL-12/23p40, and IL-17. The impact of anti-IL-23p19 antibodies remains uncertain. This study aims to examine the therapeutic response and mechanism of action of tildrakizumab, assess its influence on nailfold capillary changes, and evaluate its potential to impede the progression to PsA.
Data for this prospective study were collected from patients diagnosed with psoriasis vulgaris, who provided informed consent at the University of Tokyo Hospital, Takahashi clinic, or MisatoKenwa Hospital and MisatoKenwa clinic between January 2020 and April 2023. The study sample consisted of 246 patients with psoriasis vulgaris (PsV) without arthritis. PsA patients were diagnosed by rheumatologists using the CASPAR criteria (15). Only patients who had not previously received any topical treatment for distal interphalangeal (DIP) joints and nails, or any systemic treatment, were included in this study. Exclusion criteria encompassed evidence of vascular disorders, hepatitis, collagen diseases, other skin diseases, infection, and drug abuse. The nailfolds of all fingers were examined for capillaroscopic changes, and the number and distribution of nailfold videocapillaroscopy (NVC) findings in each finger were recorded. This study received approval from the University of Tokyo Ethics Board. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
The nailfold capillaries were observed using the TOKU Capillaro-01 device (Toku Co., Tokyo, Japan). NVC examinations were conducted at each patient visit to determine their NVC findings. Prior to the test, patients were instructed to abstain from consuming caffeine for 12 hours. The patients were positioned in a supine position for 15 minutes at a room temperature of 22 to 25°C. Ten nailfolds were examined in each patient. The same dermatologist evaluated capillaroscopic parameters such as nailfold bleeding (NFB) and irregularly enlarged capillaries for each image. The evaluation methodology, items, and software used for the evaluation were consistent with previous descriptions (12).
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples using HetaSep (Stem Cell Technologies Inc., Vancouver, Canada) through gradient centrifugation. Following Fc blocking (I-4506, Sigma-aldrich, MO, USA), the cells were labeled with PE-labeled CD4 (MHCD0404, Thermo Fisher, Waltham, MA, USA), FITC-labeled CD25 (11-0257-42, Thermo Fisher), PE/Cy7-CD127 (25-1278-42, Thermo Fisher), APC-CD3 (17-0032-82, Thermo Fisher), PE/Cy7-CCR6 (25-1969-42, Thermo Fisher), or VioBright FITC-CXCR3 (130-106-009, Miltenyi-Biotec, Bergisch-Gladbach, Germany). The samples were then incubated at room temperature in the dark for 30 minutes. Subsequently, the samples were washed twice with PBS. After membrane staining, the cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences). Flow cytometry analysis was performed using a BD FACSVerseTM flow cytometer with BD FACSuiteTM software (BD Biosciences, Germany). All analyses were conducted using fresh blood samples.
CD4+CD25+ regulatory T cells were isolated following the protocol provided by the manufacturer (130-091-301, Miltenyi-Biotec). The proliferative capacity of the isolated CD4+CD25+ or CD4+CD25- T cells was assessed using the protocol provided by the manufacturer (130-092-909, Miltenyi-Biotec). The suppressor capacity of T cells was investigated through co-culture assays. The proliferative capacity of isolated CD4+CD25+ or CD4+CD25- T cells (5 x 104) was analyzed by bromodeoxyuridine (BrdU), after stimulation with anti-CD3/CD28-coated beads (Invitrogen, Breda, the Netherlands) with or without exogenously added recombinant human IL-2 (12.5 U ml-1). The suppressor capacity of T cells was studied in co-culture assays. In brief, CD4+CD25- (5 x 104) T cells were stimulated with anti-CD3/CD28-coated beads in the absence and presence of decreasing numbers of CD4+CD25+ or CD4+CD25- T cells. Cell proliferation was analyzed at day 4 of the cultures by specific anti-BrdU ELISA (Roche, Meylan, France).
To examine the factors associated with predicting the response to PASI or the development of PsA, a logistic regression model or Cox regression analysis was constructed. This analysis included PsA risk factors such as involvement of the scalp, nails, and buttocks, while adjusting for age, sex, psoriasis severity, and body mass index. Logistic regression analysis was employed to identify the key factors that coexisted with PsA, while Cox regression analysis was used to identify the key factors that predicted the development of PsA. The significance of covariate effects was determined using a two-sided Wald’s test with a p-value threshold of less than 0.05. Following the univariate analysis, the significant factors were further analyzed in a multivariate analysis. Other statistical significance was assessed using various tests, including the Mann-Whitney U-test, Wilcoxon signed-rank test, student’s t-test, paired t-test, χ2 test, Log-rank test, and Spearman’s rank correlation test. All statistical analyses were conducted using JMP Pro 14 software. A p-value of less than 0.05 was considered statistically significant.
A total of 246 patients diagnosed with Psoriasis Vulgaris (PsV) were enrolled in the present study, as indicated in Table 1. Among these patients, twenty individuals received treatment with tildrakizumab, while the remaining participants were solely treated with topical agents. It was observed that patients who received tildrakizumab exhibited significantly elevated PASI scores (7.5 ± 8.6 vs. 2.3 ± 3.1, p < 0.0001), significantly more scalp lesions (18 (90.0%) vs. 109 (48.2%), p = 0.0003), and significantly more buttock lesions (18 (90.0%) vs. 32 (14.2%), p < 0.0001), a significantly higher proportion with NFB (19 (95.0%) vs. 81 (35.8%), p < 0.0001), a significantly higher proportion with enlarged capillaries (19 (95.0%) vs. 59 (26.1%), p < 0.0001) compared with topical group. Among the risk factors for the development of PsA (16–18), a significantly higher percentage had scalp, buttocks, NFB and enlarged capillaries. It was suggested that those at higher risk of developing PsA were more likely to be treated with tildrakizumab.
Here, age ≥ 65 is defined as “elderly.” The number of elderly patients in the tildrakizumab group was 11. The differences in background factors between the two groups of patients are shown in Supplementary Table S1. There were no significant differences other than age. We first attempted to identify the clinical characteristics of the 20 patients treated with tildrakizumab who achieved PASI clear or PASI ≤ 2 at 7 months post-treatment (Table 2). In logistic regression analysis, we compared age, gender, BMI, duration of skin lesions, PASI, scalp, nail, and buttock lesions, NFB, and presence of enlarged capillaries. As a result, age≥65 was the only significant variable in the multivariate analysis. Similarly, we identified clinical characteristics of patients who were able to achieve PASI ≤ 2 (Table 3). Similarly, age≥65 was the only significant variable. In summary, the results suggest that age is an important factor in predicting treatment responsiveness of skin lesions after 7 months of tildrakizumab.
The above analysis suggests that age is an important factor in determining response to treatment with tildrakizumab. We focused on Treg as one factor explaining this phenomenon. Since it has been reported that the number of Tregs is decreased and Treg function is impaired in psoriasis and in the elderly (9), we first examined differences in the number of Tregs and T helper 17 (Th17) cells with age in psoriasis patients (Figure 1A). The number of Treg was significantly decreased in the elderly, whereas that of Th17 showed no age-related differences (Figure 1A). Analysis of the reduced Treg function also revealed that the suppressive capacity of Tregs was also reduced (Figure 1B). Therefore, we examined these Tregs and Th17 before and after treatment with tildrakizumab (Figure 1C). Tregs were significantly increased and Th17 was significantly decreased after treatment with Tildrakizumab (Figure 1C). The suppressive function of Treg was also improved by treatment with tildrakizumab (Figure 1D). These findings suggest that one factor explaining the higher efficacy of tildrakizumab in the elderly may be its ability to improve the number and function of reduced Tregs.
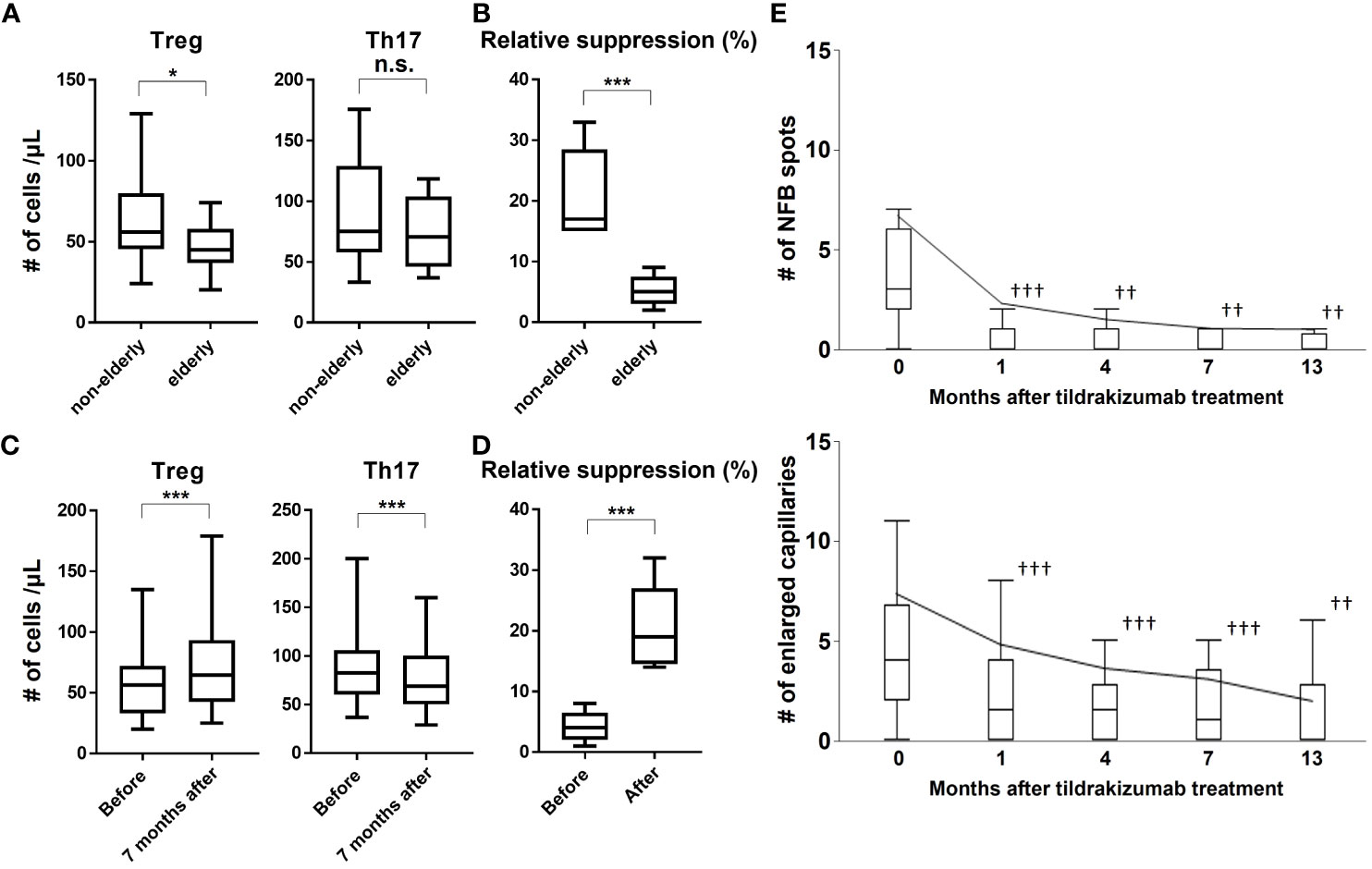
Figure 1 Treg number and function are reduced in the elderly, and tildrakizumab improves them and also NFB and enlarged capillaries, risk factors for progression to PsA. We examined the numbers of Treg and Th17 in the peripheral blood of elderly and non-elderly subjects (A) and their suppressive ability (B). We also examined the change in the number of Tregs and Th17 (C) and their suppressive capacity before and after Tildrakizumab treatment (D). The number of NFBs and enlarged capillaries were examined before and at 1, 4, 7, and 13 months after treatment with tildrakizumab (E). Box plot shows median, 25th, and 75th percentile, and whiskers show the standard deviation. *p<0.05, ***p<0.005. ††p<0.01, †††p<0.005 vs. before treatment. n.s. = not significant.
Thus, tildrakizumab acts at the cellular level and improves the skin lesions of psoriasis. Th17 cells produce inflammatory cytokines, which have also been implicated in nailfold capillary abnormalities (12). In fact, it was discovered that treatment with tildrakizumab resulted in significant improvements in NFB and enlarged capillaries, both of which are risk factors for the development of PsA. These improvements were observed as early as one month after treatment initiation and were sustained throughout the study period (Figure 1E). During the course of the study, 36 patients in the topical group and 1 patient in the tildrakizumab group developed PsA. A multivariate analysis using a Cox proportional hazards model identified age, nail lesions, NFB, and enlarged capillaries as risk factors for the progression to PsA. However, treatment with tildrakizumab was found to be effective in preventing the progression to PsA (Table 4). In conclusion, these findings demonstrate that tildrakizumab not only alleviates psoriasis at the cellular level but also improves NFB and enlarged capillaries, which are known risk factors for the development of PsA, ultimately inhibiting the progression to PsA.
In this study, a significantly higher percentage of patients in the tildrakizumab group had scalp, buttocks, NFB and enlarged capillaries, and those at higher risk of progressing to PsA were treated with more tildrakizumab compared to the topical group (Table 1). Among those treated with tildrakizumab, those who achieved better skin lesions at 7 months post-treatment were older (Tables 2 and 3). One factor that may explain this is the importance of Tregs, which were found to be more reduced and less functional in elderly patients with psoriasis (Figures 1A, B). These abnormalities improved with treatment with tildrakizumab (Figures 1C, D). One factor that may explain the higher efficacy of tildrakizumab in elderly patients is that it may be more effective in improving the number and function of reduced Tregs. Tildrakizumab also improved NFB and enlarged capillaries, one of the risk factors for progression to PsA (Figure 1E), and inhibited progression to PsA (Table 4). These results suggest that tildrakizumab improved psoriasis at the cellular level, ameliorated NFB and enlarged capillaries, one of the risk factors for progression to PsA, and inhibited progression to PsA.
To date, factors determining response to treatment with tildrakizumab have not been identified. For the first time in this study, tildrakizumab restores the number and function of Tregs and is more effective, especially in the elderly. It has been reported that Treg function is reduced in the elderly (9). Some researchers have reported a decreased percentage of Tregs in peripheral blood of psoriasis patients (19–21), while others showed no difference in circulating Treg frequency (22–25). However, differences in Tregs by age have not been investigated in patients with psoriasis. This study suggested that Tregs may be reduced in number and function especially in the elderly psoriasis patients. Tildrakizumab, an anti-IL-23 antibody that targets IL-23, which is known to act on Tregs, as a therapeutic target, was thought to be more effective in the elderly by targeting them.
PsA is a disease in which immune abnormalities are strongly implicated; it has been suggested that in the development to PsA, the disease progresses to skin lesions, immune abnormalities, subclinical and clinical PsA (26). Tildrakizumab, an anti-IL-23 antibody, acts at the cellular level and is thought to improve the skin lesions of psoriasis by inhibiting the differentiation and proliferation of Th17 cells and the pathogenic conversion of Tregs to Th17 cells (27). IL-23 produced by macrophages, dendritic cells, and B cells (28) can act on Tregs, making them Th17-like and exacerbating the skin lesions of psoriasis (27). Treg functions include reducing inflammation and autoimmunity. In recent years, a number of reports have emerged that the use of biologics for skin lesions can inhibit their progression to PsA (14). However, only the types of antibodies were reported, such as anti-TNF-α, anti-IL-12/23p40, anti-IL-17, and anti-IL-23p19 antibodies, and there are few reports on whether individual antibodies inhibit progression to PsA. This study shows for the first time that tildrakizumab improves NFB and enlarged capillaries, which are risk factors for PsA, and reduces progression to PsA.
Capillary abnormalities such as NFB and enlarged capillaries are thought to be secondary to the systemic inflammation of psoriasis (12). Indeed, the correlation between serum cytokine levels and capillary abnormalities may support these views. Therefore, it is reasonable that tildrakizumab, which targets IL-23, an inflammatory cytokine, ameliorated nailfold capillary abnormalities. A strong association between inflammatory cytokines and capillary abnormalities is suggested by the reported efficacy of brodalumab, an anti-IL-17RA antibody, in the treatment of systemic sclerosis, one of the most common diseases showing nailfold capillary abnormalities (29–31).
Taken together, tildrakizumab is particularly effective in the elderly. Its high safety profile also makes it safe for use on the elderly (32). The mechanism of action was suggested to be targeting Tregs. Improvement of risk factors for progression to PsA and, indeed, inhibition of progression to PsA were revealed. The primary drawback of this study is its exclusive focus on Japanese patients. The potential influence of genetic variations between the Japanese population and other ethnic groups on treatment response cannot be overlooked. Another limitation of this study is the low patient number. To ascertain the efficacy of tildrakizumab in preventing PsA in psoriasis, it is anticipated that future large-scale clinical trials will encompass diverse ethnic populations.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the ethics committee of the University of Tokyo Graduate School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
TF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TY: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AE: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YN: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. StT: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AY: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SkT: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HK: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. KM: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SS: Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software. AY: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Maiko Enomoto for technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1286251/full#supplementary-material
1. Radtke MA, Reich K, Blome C, Rustenbach S, Augustin M. Prevalence and clinical features of psoriatic arthritis and joint complaints in 2009 patients with psoriasis: Results of a German national survey. J Eur Acad Dermatol Venereol (2009) 23(6):683–91. doi: 10.1111/j.1468-3083.2009.03159.x
2. Sbidian E, Chaimani A, Garcia-Doval I, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev (2022) 2022(5):CD011535. doi: 10.1002/14651858.CD011535.PUB5/INFORMATION/EN
3. Fritzsche MC, Buyx AM, Hangel N. Mapping ethical and social aspects of biomarker research and its application in atopic dermatitis and psoriasis: a systematic review of reason. J Eur Acad Dermatol Venereol (2022) 36(8):1201–13. doi: 10.1111/JDV.18128
4. Graier T, Weger W, Jonak C, Sator P, Zikeli C, Prillinger K, et al. Real-world effectiveness of anti-interleukin-23 antibodies in chronic plaque-type psoriasis of patients from the Austrian Psoriasis Registry (PsoRA). Sci Rep (2022) 12(1):15078. doi: 10.1038/S41598-022-18790-9
5. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet (London England) (2017) 390(10091):276–88. doi: 10.1016/S0140-6736(17)31279-5
6. Tsugane S. Why has Japan become the world’s most long-lived country: insights from a food and nutrition perspective. Eur J Clin Nutr 2020 756 (2020) 75(6):921–8. doi: 10.1038/s41430-020-0677-5
7. Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol (2021) 184(1):14–24. doi: 10.1111/BJD.19380
8. Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med (2014) 20(1):62–8. doi: 10.1038/nm.3432
9. Palatella M, Guillaume SM, Linterman MA, Huehn J. The dark side of Tregs during aging. Front Immunol (2022) 13:940705/BIBTEX. doi: 10.3389/FIMMU.2022.940705/BIBTEX
10. Baek HJ, Yoo CD, Shin KC, Lee YJ, Kang SW, Lee EB, et al. Spondylitis is the most common pattern of psoriatic arthritis in Korea. Rheumatol Int (2000) 19(3):89–94. doi: 10.1007/s002960050109
11. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis (2015) 74(6):1045–50. doi: 10.1136/ANNRHEUMDIS-2013-204858
12. Fukasawa T, Toyama S, Enomoto A, Yoshizaki-Ogawa A, Norimatsu Y, Tateishi S, et al. Utility of nailfold capillary assessment for predicting psoriatic arthritis based on a prospective observational cohort study. Rheumatol (Oxford) (2022) 62(7):2418–25. doi: 10.1093/RHEUMATOLOGY/KEAC664
13. Fukasawa T, Takashi Y, Enomoto A, Toyama S, Yoshizaki-Ogawa A, Tateishi S, et al. Utility of nailfold capillary assessment for predicting pustulotic arthro-osteitis in palmoplantar pustulosis based on a prospective cohort study. J Am Acad Dermatol (2023) 28:S0190-9622(23)02406-4. doi: 10.1016/j.jaad.2023.07.1014
14. Acosta Felquer ML, Logiudice L, Galimberti ML, Rosa J, Mazzuoccolo L, Soriano ER. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann Rheum Dis (2022) 81(1):74–9. doi: 10.1136/ANNRHEUMDIS-2021-220865
15. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheumatol (2006) 54(8):2665–73. doi: 10.1002/ART.21972
16. Yamamoto T, Ohtsuki M, Sano S, Igarashi A, Morita A, Okuyama R, et al. Epidemiological analysis of psoriatic arthritis patients in Japan. J Dermatol (2016) 43(10):1193–6. doi: 10.1111/1346-8138.13342
17. Koga H. Dermoscopic evaluation of melanonychia. J Dermatol (2017) 44(5):515–7. doi: 10.1111/1346-8138.13863
18. Mease P, Goffe BS. Diagnosis and treatment of psoriatic arthritis. J Am Acad Dermatol (2005) 52(1):1–19. doi: 10.1016/J.JAAD.2004.06.013
19. Quaglino P, Ortoncelli M, Comessatti A, Ponti R, Novelli M, Bergallo M, et al. Circulating CD4+CD25 bright FOXP3+ T cells are up-regulated by biological therapies and correlate with the clinical response in psoriasis patients. Dermatology (2009) 219(3):250–8. doi: 10.1159/000238305
20. Ma L, Xue HB, Gao T, Gao ML, Zhang YJ. Notch1 signaling regulates the th17/Treg immune imbalance in patients with psoriasis vulgaris. Mediators Inflamm (2018) 2018:3069521. doi: 10.1155/2018/3069521
21. Karamehic J, Zecevic L, Resic H, Jukic M, Jukic T, Ridjic O, et al. Immunophenotype lymphocyte of peripheral blood in patients with psoriasis. Med Arch (Sarajevo Bosnia Herzegovina) (2014) 68(4):236–8. doi: 10.5455/MEDARH.2014.68.236-238
22. Furuhashi T, Saito C, Torii K, Nishida E, Yamazaki S, Morita A. Photo(chemo)therapy reduces circulating Th17 cells and restores circulating regulatory T cells in psoriasis. PLoS One (2013) 8(1):e54895. doi: 10.1371/JOURNAL.PONE.0054895
23. Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol (2010) 135(1):108–17. doi: 10.1016/J.CLIM.2009.11.008
24. Yun WJ, Lee DW, Chang SE, Yoon GS, Huh JR, Won CH, et al. Role of CD4CD25FOXP3 regulatory T cells in psoriasis. Ann Dermatol (2010) 22(4):397–403. doi: 10.5021/AD.2010.22.4.397
25. Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol (2005) 174(1):164–73. doi: 10.4049/JIMMUNOL.174.1.164
26. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol (2019) 15(3):153–66. doi: 10.1038/S41584-019-0175-0
27. Soler DC, McCormick TS. The dark side of regulatory T cells in psoriasis. J Invest Dermatol (2011) 131(9):1785. doi: 10.1038/JID.2011.200
28. Fukasawa T, Yoshizaki A, Ebata S, Fukayama M, Kuzumi A, Norimatsu Y, et al. Single-cell-level protein analysis revealing the roles of autoantigen-reactive B lymphocytes in autoimmune disease and the murine model. Elife (2021) 10:366–9. doi: 10.7554/ELIFE.67209
29. Fukasawa T, Yoshizaki A, Ebata S, Sato S. Interleukin-17 pathway inhibition with brodalumab in early systemic sclerosis: analysis of a single-arm, open-label, phase 1 trial. J Am Acad Dermatol (2023) 13:kead287. doi: 10.1016/J.JAAD.2023.02.061
30. Fukasawa T, Yoshizaki-Ogawa A, Yoshizaki A, Sato S. Impact of guselkumab on three cases of SSc accompanying psoriasis. Rheumatol (Oxford) (2023) 13:kead287. doi: 10.1093/RHEUMATOLOGY/KEAD287
31. Fukasawa T, Yoshizaki-Ogawa A, Enomoto A, Miyagawa K, Sato S, Yoshizaki A. Involvement of molecular mechanisms between T/B cells and IL-23: from palmoplantar pustulosis to autoimmune diseases. Int J Mol Sci (2022) 23(15):8261. doi: 10.3390/IJMS23158261
32. Thaci D, Piaserico S, Warren RB, Gupta AK, Cantrell W, Draelos Z, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2). Br J Dermatol (2021) 185(2):323–34. doi: 10.1111/BJD.19866
Keywords: psoriasis, psoriatic arthritis, nailfold capillary, risk factors, tildrakizumab, regulatory T cells, elderly, prospective study
Citation: Fukasawa T, Yamashita T, Enomoto A, Norimatsu Y, Toyama S, Yoshizaki-Ogawa A, Tateishi S, Kanda H, Miyagawa K, Sato S and Yoshizaki A (2023) The optimal use of tildrakizumab in the elderly via improvement of Treg function and its preventive effect of psoriatic arthritis. Front. Immunol. 14:1286251. doi: 10.3389/fimmu.2023.1286251
Received: 31 August 2023; Accepted: 10 October 2023;
Published: 19 October 2023.
Edited by:
Giang Tran, University of New South Wales, AustraliaReviewed by:
Thomas Graier, Medical University of Graz, AustriaCopyright © 2023 Fukasawa, Yamashita, Enomoto, Norimatsu, Toyama, Yoshizaki-Ogawa, Tateishi, Kanda, Miyagawa, Sato and Yoshizaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayumi Yoshizaki, YXl1eW9zaGlAbWUuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.