
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Immunol., 25 September 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1282155
This article is a correction to:
Zilucoplan, a macrocyclic peptide inhibitor of human complement component 5, uses a dual mode of action to prevent terminal complement pathway activation
 Guo-Qing Tang1*
Guo-Qing Tang1* Yalan Tang1
Yalan Tang1 Ketki Dhamnaskar2†
Ketki Dhamnaskar2† Michelle D. Hoarty3†
Michelle D. Hoarty3† Rohit Vyasamneni2†
Rohit Vyasamneni2† Douangsone D. Vadysirisack3†
Douangsone D. Vadysirisack3† Zhong Ma3†
Zhong Ma3† Nanqun Zhu3†
Nanqun Zhu3† Jian-Guo Wang1
Jian-Guo Wang1 Charlie Bu1
Charlie Bu1 Bestine Cong1
Bestine Cong1 Elizabeth Palmer1
Elizabeth Palmer1 Petra W. Duda1
Petra W. Duda1 Camil Sayegh3†
Camil Sayegh3† Alonso Ricardo2†
Alonso Ricardo2†by Tang G-Q, Tang Y, Dhamnaskar K, Hoarty MD, Vyasamneni R, Vadysirisack DD, Ma Z, Zhu N, Wang J-G, Bu C, Cong B, Palmer E, Duda PW, Sayegh C and Ricardo A (2023) Front. Immunol. 14:1213920. doi: 10.3389/fimmu.2023.1213920
In the published article, there were errors in Figure 3 as published. In part A, the binding site is the “zilucoplan binding site”, rather than the “eculizumab binding site”. In part D, “nonspecific proteinases” should be “nonspecific proteases”. The corrected Figure 3 and its caption appear below.
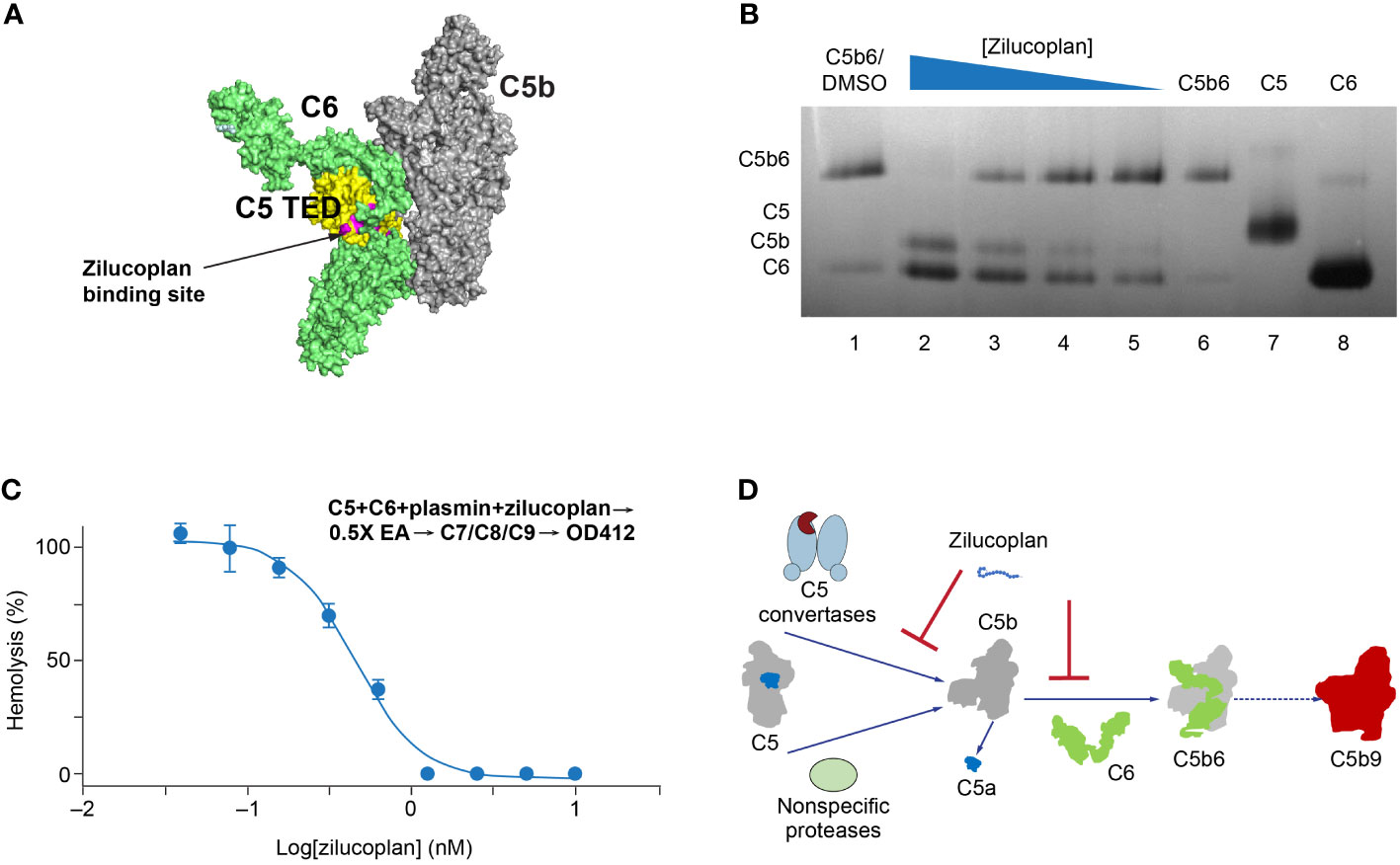
Figure 3 Zilucoplan destabilizes C5b6 and inhibits C5b6-mediated hemolysis lysis. (A) Schematic depiction (using PyMOL 2.4.0 based on PDB 4a5w) of the C5b6 complex. C5b is in gray except for the C5 TED in yellow, proposed binding sites for zilucoplan peptidyl portion based on a peptide analog (unpublished data) in pink, and C6 in lime. (B) Native gel analysis of the C5b6 complex in the presence and/or absence of zilucoplan. Lanes 1 and 6: C5b6 incubated at 37°C/h with (Lane 1) or without (Lane 6) 1% DMSO. Lanes 2–6: C5b6 after incubation with various concentrations of zilucoplan (in 1% DMSO) at 37°C for 1 h prior to gel loading. Lanes 7 and 8: C5 and C6 alone. (C) Zilucoplan inhibited the plasmin-mediated lysis of EA. Plasmin without or with increasing concentrations of zilucoplan was added to a mixture of C5 and C6 and incubated for 1 h. The plasmin reaction solution was then added to EA before mixing with purified C7, C8, and C9 to proceed with the lysis. More details are described in the Materials and Methods. Note that no sera were used to exclude any contribution by C3/C5 convertases. (D) A schematic diagram of the dual mechanism of action for zilucoplan: zilucoplan binds C5 (gray/dark blue) to prevent C5 cleavage into C5a (dark blue) and C5b (gray) by C5 convertases (light blue) and remains on C5b to prevent the formation of C5b6 (C6 in green) mediated by nonspecific proteases, both contributing to the blockade of MAC formation (red). TED, thioester-like domain; DMSO, dimethyl sulfoxide.
In the published article, there was an error. The Introduction incorrectly states that zilucoplan is composed of a 15-amino acid macrocyclic peptide including three unnatural amino acids. Zilucoplan is composed of a 15-amino acid macrocyclic peptide including four unnatural amino acids.
A correction has been made to Introduction, paragraph 4. This sentence previously stated:
“It is composed of a 15-amino acid macrocyclic peptide, including three unnatural amino acids, designed to inhibit TCC activation”
The corrected sentence appears below:
“It is composed of a 15-amino acid macrocyclic peptide, including four unnatural amino acids, designed to inhibit TCC activation”
The authors apologize for these errors and state that these do not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: complement activation, C5 cleavage, C5 R885 variants, C5b6, MAC formation, macrocyclic peptide inhibitor, RBC hemolysis, permeability
Citation: Tang G-Q, Tang Y, Dhamnaskar K, Hoarty MD, Vyasamneni R, Vadysirisack DD, Ma Z, Zhu N, Wang J-G, Bu C, Cong B, Palmer E, Duda PW, Sayegh C and Ricardo A (2023) Corrigendum: Zilucoplan, a macrocyclic peptide inhibitor of human complement component 5, uses a dual mode of action to prevent terminal complement pathway activation. Front. Immunol. 14:1282155. doi: 10.3389/fimmu.2023.1282155
Received: 23 August 2023; Accepted: 08 September 2023;
Published: 25 September 2023.
Edited and Reviewed by:
Marie-Agnes Dragon-Durey, Université Paris Cité, FranceCopyright © 2023 Tang, Tang, Dhamnaskar, Hoarty, Vyasamneni, Vadysirisack, Ma, Zhu, Wang, Bu, Cong, Palmer, Duda, Sayegh and Ricardo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Qing Tang, R3VvUWluZy5UYW5nQHVjYi5jb20=
†Present addresses: Ketki Dhamnaskar, Nurix Therapeutics, San Francisco, CA, United States
Michelle D. Hoarty, Dyne Therapeutics, Waltham, MA, United States
Douangsone D. Vadysirisack, Dianthus Therapeutics, Waltham, MA, United States
Zhong Ma, Mariana Oncology, Watertown, MA, United States
Rohit Vyasamneni, BioNTech SE, Cambridge, MA, United States
Nanqun Zhu, Avilar Therapeutics, Waltham, MA, United States
Camil Sayegh, Mitochondria Emotion Inc., Cambridge, MA, United States
Alonso Ricardo, Mariana Oncology, Watertown, MA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.