- 1Department of oncology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
- 2The Comprehensive Cancer Center of Drum Tower Hospital, Clinical College of Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, China
- 3National Institute of Healthcare Data Science, Nanjing University, Nanjing, China
- 4State Key Laboratory of Neurology and Oncology Drug Development Jiangsu Simcere Diagnostics Co, Ltd, Nanjing, China
Immune checkpoint inhibitors have limited efficacy in metastatic pancreatic cancer due to the complex tumor immune microenvironment (TIME). Studies have shown that radiotherapy can cause cell lesions to release tumor antigens and then take part in the remodeling of the tumor environment and the induction of ectopic effects via regional and systemic immunoregulation. Here, we reported a case of advanced metastatic pancreatic cancer treated with immunotherapy combined with chemotherapy and radiotherapy and a sharp shift of the TIME from T3 to T2 was also observed. One hepatic metastasis within the planning target volume (PTV) was evaluated complete response (CR), the other one was evaluated partial response (PR) and 2 hepatic metastases outside the PTV were surprisingly considered PR. In the study, we found that immunotherapy combined with chemotherapy and radiotherapy achieved significant therapeutic benefits, which may provide a new strategy for the treatment of advanced pancreatic cancer.
1 Introduction
Pancreatic cancer has very poor prognosis with a 5-year survival rate of only 8% (1). About 50% of patients with pancreatic cancer are diagnosed at an advanced stage (2) and there is no clear consensus on the second-line treatment when first-line treatment based on gemcitabine fails.
In recent years, immune checkpoint inhibitors (ICIs) have achieved decisive breakthroughs in many solid tumors (3–5), but the efficacy of ICIs in pancreatic cancer is still confronted with challenges. The complex TIME of pancreatic cancer limits the effectiveness of ICIs (6), but more and more clinical studies and experiments have proved that radiotherapy combined with immunotherapy can regulate the TIME, so as to strengthen the control of tumor (7, 8).
Here, we presented an advanced pancreatic cancer case with robust survival benefit from immunotherapy combined with chemotherapy and radiotherapy, while obvious TIME remodeling and an ectopic effect were also observed. Briefly, this comprehensive treatment mode remodulated pancreatic cancer from “cold” tumors to “hot” tumors in our case.
2 Case presentation
We presented a case of a 59-year-old male who was hospitalized with intermittent upper abdominal pain in October 2021. Contrast-enhanced computed tomography (CT) scan showed a 2.2cm x 2cm mass at the neck of the pancreas with distal pancreatic duct dilatation (Figure 1A). The mass was closely related to the splenic vein. But after discussion, the Multiple Disciplinary Team (MDT) believed that the patient was also accompanied by superior mesenteric artery (SMA) invasion less than 180° (Figure 1B). But no distant metastasis was detected at that time. In addition, the baseline value of carbohydrate antigen 19-9 (CA19-9) was 12.99 U/ml. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was performed and subsequently cancer cells were verified pathologically (Figure 1C). The patient was definitely diagnosed with borderline resectable pancreatic cancer based on pathology and imaging. But the patient refused to consider the possibility of follow-up operation firmly at the very start.
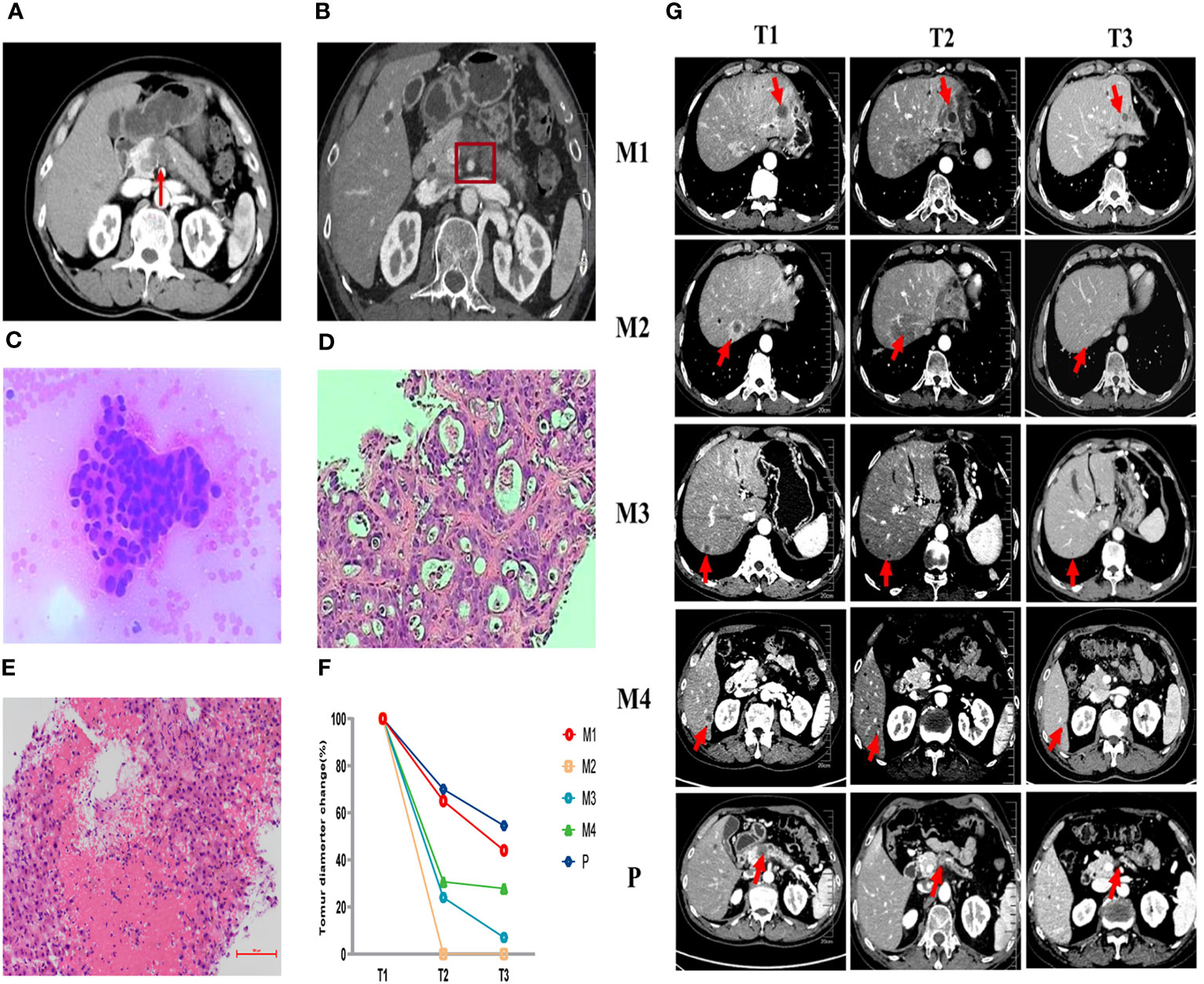
Figure 1 Pathological and imaging evaluations during the first-line (AG) and second-line (SOXPR) treatment. (A, B) A 2.2cm × 2cm mass at the neck of the pancreas was detected with superior mesenteric artery (SMA) invasion less than 180° on abdominal CT at baseline. (C) Cancer cells were observed in pancreas biopsy (×200) at T0. (D) Ultrasound guided biopsy of the hepatic mass (M2) was concordant with liver metastasis of pancreatic ductal adenocarcinoma (×200) at T1. (E) Pathology of M2 showed inflammatory cells and no residual tumor cells (×200) at T2. (F, G) Abdominal CT during second-line (SOXPR) treatment and the tumor diameter variation. T0, The baseline prior to first-line therapy; T1, The baseline prior to second-line therapy; T2, Obvious relief of TEN symptoms; T3,: Three additional cycles of SOX to end; M1-M4, 4 hepatic metastases; P, primary pancreatic cancer locus.
From November 2021 to March 2022, the patient received 5 cycles (21 days for one complete cycle) of gemcitabine 1000 mg/m2 and nab-paclitaxel 125 mg/m2 on day 1 and day 8 and the patient stayed a stable disease. After 5 cycles of the treatment, CA19-9 increased to 76.7U/ml. In addition, CT scan revealed that the size of pancreatic primary tumor had increased remarkably and four new hepatic masses appeared (Figure 1G-T1). Pathology for Ultrasound guided biopsy of the hepatic mass was concordant with liver metastasis of pancreatic ductal adenocarcinoma (Figure 1D). The patient was assessed progressive disease (PD).
Subsequently, S-1 plus oxaliplatin combined with immunotherapy and radiotherapy were used in second-line treatment. In detail, the patient received 3 cycles of S-1 80mg/day on day1-14 plus oxaliplatin 130mg/m2 on day 1 and Sintilimab 200mg on day 1 (21 days for a complete cycle) while 8Gy*3 fractions radiotherapy of liver metastases within PTV was conducted before Cycle2 started (Figure 2A).
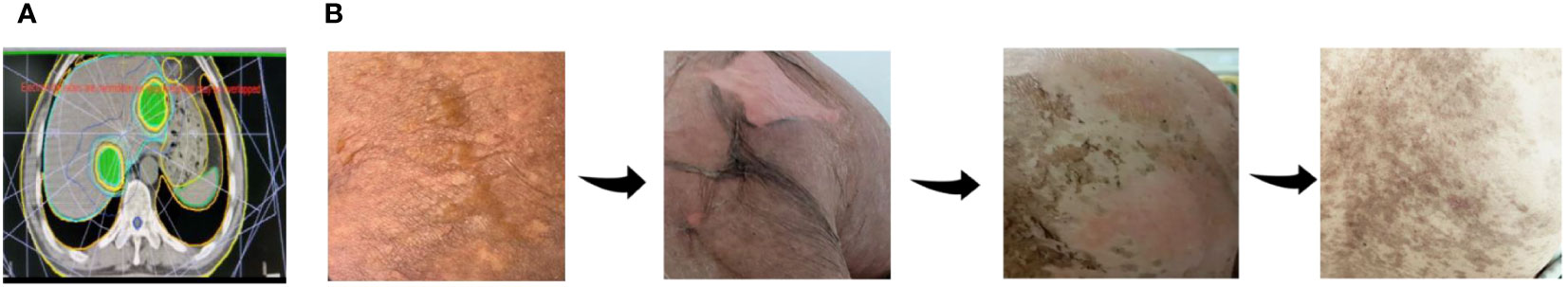
Figure 2 Occurrence of TEN after SOXPR. (A) Planning target volume of hepatic metastases radiotherapy. (B) Skin changes during treatment of TEN.
After finishing 3 cycles of this treatment, the patient developed toxic epidermal necrolysis (TEN) and after the Multiple Disciplinary Team (MDT) discussion, the experts unanimously assessed TEN as immune-related adverse event (irAE). After methylprednisolone, anti-infection, fluid infusion treatment, his symptoms quickly relieved (Figure 2B) and tumor marker CA19-9 decreased to 19.2 U/ml by the TIME. CT scan revealed that the primary pancreatic tumor and hepatic metastases had both shrunk remarkably (Figure 1G-T2). Surprisingly, a hepatic metastasis within the scope of radiotherapy had disappeared in CT scan. Obvious inflammatory cell infiltration was confirmed by pathology and no cancer cells was found in the biopsy tissues (Figure 1E). One hepatic metastasis within the scope of radiotherapy was assessed CR, the other one was evaluated PR, and other two hepatic metastases outside the scope of radiotherapy were also considered PR according to the Response Evaluation Criteria in Solid Tumors (RECIST1.1) criteria.
We conducted the SOX regimen for another 3 cycles when symptoms related to TEN were greatly relieved. At the time, CA19-9 decreased to 6.94U/ml and all tumors continued to shrink as CT indicated (Figure 1G-T3). Tumor diameter changes were demonstrated in Figure 1F. The concomitant changes of CA199 and timeline of events were demonstrated in Figure 3 in details.
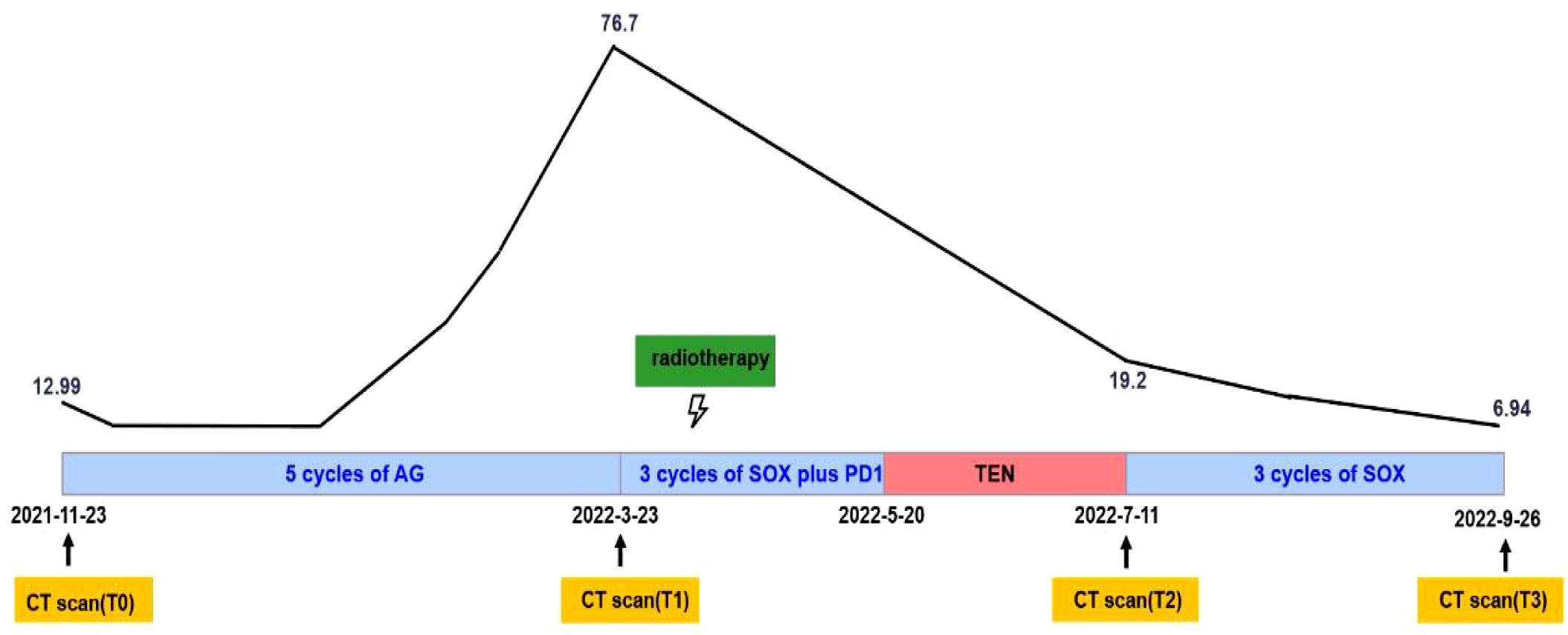
Figure 3 Systemic illustrations of clinical therapy flow chart. Broken Line indicates CA19-9 levels of this patient during the treatment.
To comprehensively assess the alteration of TIME before and after treatment, we performed multiplexed immunofluorescence histochemical (mIHC) analysis at the protein level and gene expression analysis at the RNA level on M2 hepatic metastasis prior and post treatment, respectively. The spatial immune microenvironments of tumor tissues prior (Figure 4A) and post treatment (Figure 4B) were shown by mIHC assay. The relative values of CD8+, CD68+, CD163+, Foxp3+, and PD-L1+ were 7.74, 3.9, 1.91, 2.84, and 0.69 respectively, showing a high infiltration of immune cells and low expression of PD-L1 (subtype TIME-3, immune escape type). After 3 cycles of immunotherapy combined chemoradiotherapy, the relative values of CD8+, CD68+, CD163+, Foxp3+, and PD-L1+ were 19.02, 6.06, 30.44, 8.11, and 21.76 respectively, showing a high infiltration of immune cells and high expression of PD-L1 (subtype TIME-2, immune response type). The RNA-level expression assay of TIME was detected by 289 immune-related genes (NanoString Technologies, Seattle, USA) at Jiangsu Simcere Diagnostics Co., Ltd, and the selection of immune-related genes is shown in Supplementary Materials. The abundance of immune cells related with the tumor microenvironment was shown in Figure 4C, and the abundances of all immune cells were elevated to different degrees, and other immune signatures in Figure 4D. For example, the CD8+ T cell score increased from 4.13 to 7.48, and the Macrophage gene score improved from 6.05 to 8.07, and the Treg gene score improved from 3.32 to 5.36. In addition, the mIHC results also revealed an increase in Treg cells and M2 macrophages, consistent with previous study that the effect of radiotherapy on the tumor microenvironment may be dual, inducing both an immunostimulatory effect (recruitment of T cells) and an immunosuppressive effect (expansion of Treg cells) (9). Therefore, we hypothesized that this coexistence of immunostimulatory and immunosuppressive effect in radiotherapy leads to stabilization of the patient’s disease and may provide opportunities for immunomodulation (10). Scores of other signatures or markers also increased, such as the scores of IFNγ from 5.29 to 8.51, cytotoxic T lymphocyte from 4.43 to 7.64. Interestingly, the change in the scores for B7-H3 showed a decreasing trend in contrast to the other scores, and previous studies have also shown a negative correlation between its high expression and treatment response. Additional immune scores were shown in Supplementary Table 1. Both mIHC, as well as TIME assays at the RNA level, reveal that immunotherapy combined with chemoradiotherapy enhances immune cell infiltration, which may be responsible for promoting the immune response and benefiting patient’s clinical response.
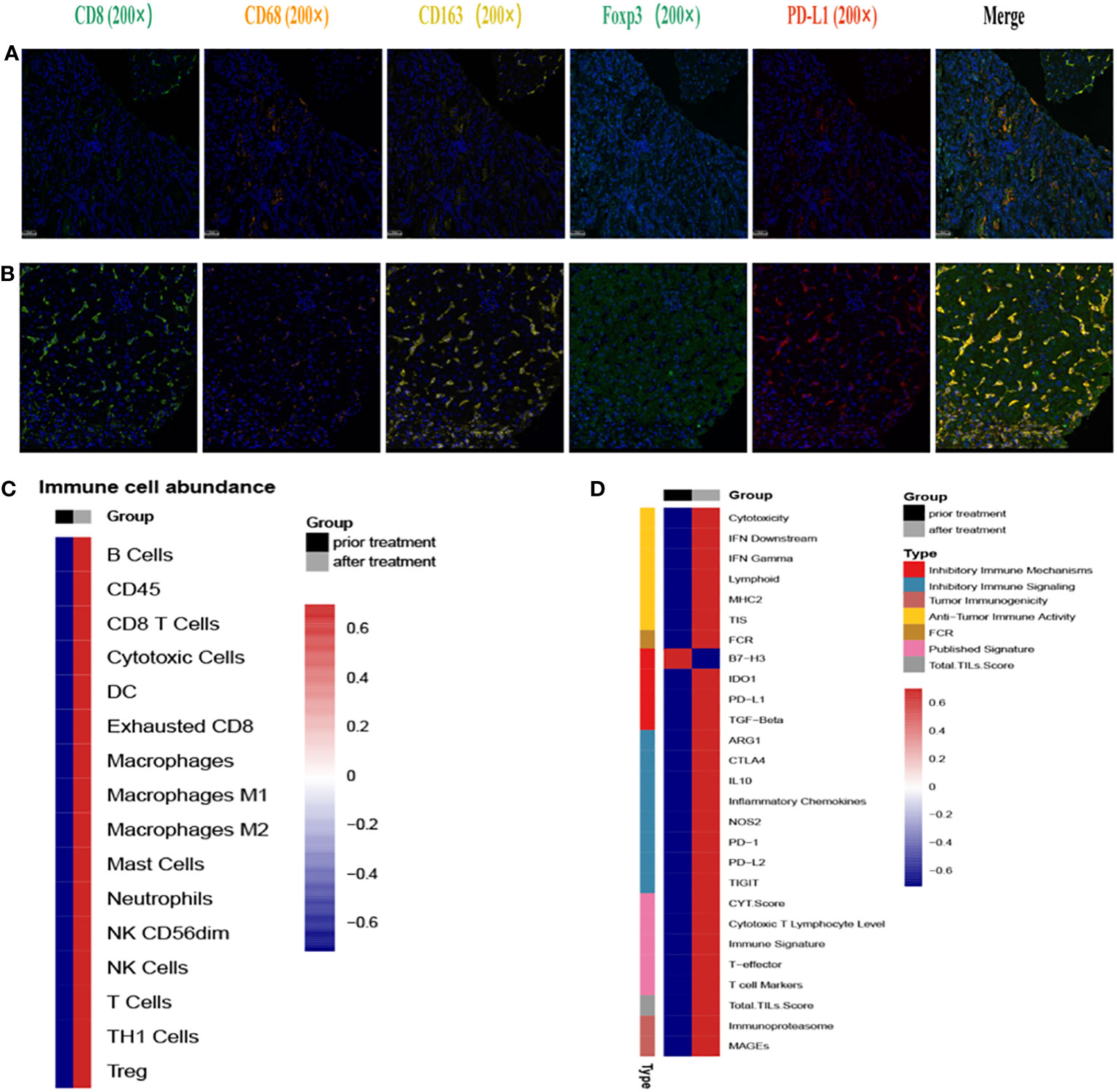
Figure 4 Immunofluorescence and RNA immune-related panel sequencing prior and post second-line treatment. (A) The representative immunofluorescent images of CD8, CD68, CD163, FoxP3 and PD-L1 of tumor tissues prior second-line treatment. (B) The representative immunofluorescent images of CD8, CD68, CD163, FoxP3 and PD-L1 of tumor tissues post second-line treatment. (C) Immune cell-related gene expression prior and after second-line treatment. Black indicates pre-treatment, gray indicates post-treatment; blue indicates decreased expression, red indicates increased expression. (D) Immune signature-related gene expression prior and after second-line treatment. Black indicates pre-treatment, gray indicates post-treatment; blue indicates decreased expression, red indicates increased expression.
3 Discussion
At present, chemotherapy is still the main treatment for advanced pancreatic cancer. With the deepening understanding of the pathogenesis of pancreatic cancer, immunotherapy based on remodeling TIME has become a hot topic of pancreatic cancer treatment (11). However, the specific and complex TIME of pancreatic cancer limits the effectiveness of immune checkpoint inhibitors therapy (12–15). Studies have shown that nearly 50% of the stroma cellular component of pancreatic cancer tissue is immune-related cells, but only a few are anti-tumor-related effector cells (16). Single-agent immunotherapy rarely works in second-line therapy in advanced pancreatic cancer, but we made breakthroughs and achieved unexpected clinical efficacy by a cocktail therapy consisted of immunotherapy combined with chemotherapy and radiotherapy in our case.
To better predict the response of immunotherapy in solid tumors, researchers divided the TIME into 4 subtypes based on PDL1 expression and the presence of tumor-infiltrating lymphocytes (TILs): T1 (PDL1−, TIL−), T2 (PDL1+, TIL+), T3 (PDL1−, TIL+), and T4 (PDL1+, TIL−) (17). T2 are considered to be the type to better predict the immune response. Radiation upregulated the expression of PD-L1 (8, 18, 19)and increased the infiltration of CD8+ T cells (20, 21), which changed the TIME from type 3 to type 2 in our case. Hot tumors were remodeled in this way to achieve enhanced clinical efficacy.
On one hand, radiation accelerates tumor cell lesions and death to promote the exposure and presentation of tumor associated antigens. On the other hand, high infiltration of CD8+ T cell is influenced by chemokines such as CXCL9 and CXCL10 (22, 23). Radiotherapy induces the production of these chemokines, and promote the recruitment of T cells to tumor tissues (24–26).T cells infiltration and antigens exposure activate T cell response to release IFN- γ and IFN- γ stimulates the upregulation of PD-L1 (27, 28). Besides, radiotherapy can also up-regulate PDL1 by activating cGAS-STING (cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon gene) pathway to lay basis for use of ICIs (29, 30). In addition, radiotherapy has been reported to induce normalization of blood vessels to achieve T cell infiltration, but the exact mechanism has not been fully elucidated (31).
In addition, the mIHC results showed an increase in Foxp3+ regulatory T cells (Foxp3+ Treg), which was consistent with previous studies. In bladder and liver cancer, increased accumulation of Treg cells was observed in tumor tissues after radiotherapy, which was shown to be related to radiation-induced Akt pathway activation (32, 33); In prostate cancer, radiotherapy provides a growth and survival advantage for Tregs by inducing TGF-β (34). However, our study hasn’t explored the mechanism by which radiation therapy increases Foxp3+ Treg cells yet, which need to be explained deeply.
In previous studies, some researchers have paid attention to the ectopic effect of radiotherapy, and the specific mechanism of ectopic effect of radiotherapy is attributed to immune effect (35–37). Radiotherapy can induce immune cells to infiltrate into tumor tissue, produce a large number of reactive oxygen species, activate cytotoxic T lymphocytes (CTLs), and lead to apoptosis of tumor cells (38), this was also confirmed by our results of multiple immunofluorescence histochemistry and tumor microenvironment detection. Therefore, we conclude that the synergistic effect of ectopic radiotherapy and immunotherapy enhances the immune response and provides a new therapeutic strategy for advanced pancreatic cancer.
However, not all patients can benefit from radiotherapy combined with immunotherapy. It is well-known that the timing of radiotherapy and the dose of radiotherapy will affect the effect of immunotherapy. There is no consensus of the best time for radiotherapy, but existing studies have found that simultaneous administration of radiotherapy and immunotherapy or timely immunotherapy after radiotherapy is beneficial to the clinical outcome (39, 40). Taking two factors into consideration, we chose to introduce radiotherapy in the middle course of ICIs usage. One point, immunotherapy enhances the tumor’s sensitivity to radiotherapy by cellular pathways. The other point, radiotherapy upregulated PD-L1 to better response to subsequent ICIs. Up to the optimal dose for radiotherapy, studies have shown that both low-dose and high-dose radiotherapy can affect the efficacy of immunotherapy by inflaming tumors, but the reason is not clear (39, 41). Whether it is related to the type of cancer needs to be further explored. Therefore, our patient benefited from synchronous radiotherapy and chemotherapy combined with immunotherapy, and benefited from high-dose radiotherapy((8Gy*3f). Our patient also benefited from the sensitizing effect of radiotherapy on chemotherapeutic drugs. Clinical studies have shown that S-1 and oxaliplatin can be used as radiosensitizers in the treatment of solid tumors (42–44). S-1 can inhibit the repair of radiation-induced DNA damage and oxaliplatin can inhibit DNA replication and transcription.
More and more studies have shown that immune-related adverse events(irAEs) are related to better therapeutic effects (45, 46). Researchers believe that severity of irAEs are bystander effect from activated T cells (47). Thus, patients who experience more severe irAEs may acquire better clinical outcomes, but this conclusion needs to be supported by more clinical data. Our patient developed TEN after treatment with PD1, and CT scan showed a good tumor regression after remission of symptoms.
In conclusion, we provide a potential treatment strategy for the use of immunotherapy combined with chemotherapy and radiotherapy in patients with advanced pancreatic cancer. We consider that this is a typical case that comprehensive treatment mode can convert pancreatic cancer from “cold” tumors to “hot” tumors. More randomized clinical trials are needed to verify the safety and efficacy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Drum Tower Hospital Affiliated to Nanjing University Medical School. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FT: Writing – original draft. YS: Writing – original draft. YZ: Formal analysis, Writing – review & editing. HS: Formal analysis, Writing – review & editing. JN: Writing – review & editing, Data curation. LQ: Data curation, Writing – review & editing. QG: Writing – review & editing, Methodology. CZ: Writing – review & editing, Methodology. WX: Writing – review & editing, Formal analysis. BL: Writing – review & editing, Conceptualization, Methodology. WK: Conceptualization, Writing – review & editing. JD: Conceptualization, Writing – review & editing, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the following grants: National Natural Science Foundation of China (82072926); National Key Research and Development Program of China (2020YFA0713804); Special Fund of Health Science and Technology Development of Nanjing (YKK20080). Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2023-LCYJ-PY-29). the National Natural Science Foundation of China (Nos. 82373280 and 82072926).
Conflict of interest
Authors CZ and WX were employed by Oncology Drug Development Jiangsu Simcere Diagnostics Co,Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1277810/full#supplementary-material
Abbreviations
TIME, tumor immune microenvironment; CR, complete response; PR, partial response; ICIs, immune checkpoint inhibitors; CT, contrast-enhanced computed tomography; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration biopsy; PD, progressive disease; TEN, toxic epidermal necrolysis; MDT, multiple Disciplinary Team; irAE, immune-related adverse event; RECIST, Response Evaluation Criteria in Solid Tumors; mIHC, multiplexed immunofluorescence histochemical.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
3. Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol (2017) 18(5):587–98. doi: 10.1016/S1470-2045(17)30239-5
4. Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist (2017) 22(1):81–8. doi: 10.1634/theoncologist.2016-0189
5. Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol (2016) 17(6):717–26. doi: 10.1016/S1470-2045(16)00175-3
6. Ullman NA, Burchard PR, Dunne RF, Linehan DC. Immunologic strategies in pancreatic cancer: making cold tumors hot. J Clin Oncol (2022) 40(24):2789–805. doi: 10.1200/JCO.21.02616
7. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. New Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
8. Tang Z, Wang Y, Liu D, Wang X, Xu C, Yu Y, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun (2022) 13(1):6807. doi: 10.1038/s41467-022-34403-5
9. Zhai D, An D, Wan C, Yang K. Radiotherapy: Brightness and darkness in the era of immunotherapy. Transl Oncol (2022) 19:101366. doi: 10.1016/j.tranon.2022.101366
10. Weichselbaum RR, Liang H, Deng L, Fu Y-X. PMID: 28094262. Nat Rev Clin Oncol (2017) 14(6):365–79. doi: 10.1038/nrclinonc.2016.211
11. Huber M, Brehm CU, Gress TM, Buchholz M, Alashkar Alhamwe B, von Strandmann EP, et al. The immune microenvironment in pancreatic cancer. Int J Mol Sci (2020) 21(19):7307. doi: 10.3390/ijms21197307
12. Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg (2018) 267(5):936–45. doi: 10.1097/SLA.0000000000002234
13. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet (2020) 395(10242):2008–20. doi: 10.1016/S0140-6736(20)30974-0
14. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res (2014) 74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155
15. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell (2015) 27(4):450–61. doi: 10.1016/j.ccell.2015.03.001
16. Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res (2007) 67(19):9518–27. doi: 10.1158/0008-5472.CAN-07-0175
17. Zhang Y, Chen L. Classification of advanced human cancers based on tumor immunity in the microEnvironment (TIME) for cancer immunotherapy. JAMA Oncol (2016) 2(11):1403–4. doi: 10.1001/jamaoncol.2016.2450
18. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. (2014) 124(2):687–95. doi: 10.1172/JCI67313
19. Yoshino H, Sato Y, Nakano M. KPNB1 inhibitor importazole reduces ionizing radiation-increased cell surface PD-L1 expression by modulating expression and nuclear import of IRF1. Curr Issues Mol Biol (2021) 43(1):153–62. doi: 10.3390/cimb43010013
20. Chen HY, Xu L, Li LF, Liu XX, Gao JX, Bai YR. Inhibiting the CD8(+) T cell infiltration in the tumor microenvironment after radiotherapy is an important mechanism of radioresistance. Sci Rep (2018) 8(1):11934. doi: 10.1038/s41598-018-30417-6
21. Miyauchi S, Sanders PD, Guram K, Kim SS, Paolini F, Venuti A, et al. HPV16 E5 mediates resistance to PD-L1 blockade and can be targeted with rimantadine in head and neck cancer. Cancer Res (2020) 80(4):732–46. doi: 10.1158/0008-5472.CAN-19-1771
22. Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell (2019) 35(6):885–900. doi: 10.1016/j.ccell.2019.05.004
23. Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res (2009) 69(7):3077–85. doi: 10.1158/0008-5472.CAN-08-2281
24. Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol (2008) 181(5):3099–107. doi: 10.4049/jimmunol.181.5.3099
25. Meng Y, Mauceri HJ, Khodarev NN, Darga TE, Pitroda SP, Beckett MA, et al. Ad.Egr-TNF and local ionizing radiation suppress metastases by interferon-beta-dependent activation of antigen-specific CD8+ T cells. Mol Ther (2010) 18(5):912–20. doi: 10.1038/mt.2010.18
26. Wang C-L, Ho A-S, Chang C-C, Sie Z-L, Peng C-L, Chang J, et al. Radiotherapy enhances CXCR3highCD8+ T cell activation through inducing IFNγ-mediated CXCL10 and ICAM-1 expression in lung cancer cells. Cancer Immunol Immunother (2023) 72(6):1865–80. doi: 10.1007/s00262-023-03379-6
27. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med (2002) 8(8):793–800. doi: 10.1038/nm730
28. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med (2012) 4(127):127ra37. doi: 10.1126/scitranslmed.3003689
29. Du S-S, Chen G-W, Yang P, Chen Y-X, Hu Y, Zhao Q-Q, et al. Radiation Therapy Promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING Activation. Int J Radiat Oncol Biol Phys (2022) 112(5):1243–55. doi: 10.1016/j.ijrobp.2021.12.162
30. Jiang M, Jia K, Wang L, Li W, Chen B, Liu Y, et al. Alterations of DNA damage response pathway: Biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharm Sin B (2021) 11(10):2983–94. doi: 10.1016/j.apsb.2021.01.003
31. Hauth F, Ho AY, Ferrone S, Duda DG. Radiotherapy to enhance chimeric antigen receptor T-cell therapeutic efficacy in solid tumors: A narrative review. JAMA Oncol (2021) 7(7):1051–9. doi: 10.1001/jamaoncol.2021.0168
32. Wang M, Gou X, Wang L. Protein kinase B promotes radiation-induced regulatory T cell survival in bladder carcinoma. Scand J Immunol (2012) 76(1):70–4. doi: 10.1111/j.1365-3083.2012.02707.x
33. Li C-G, He M-R, Wu F-L, Li Y-J, Sun A-M. Akt promotes irradiation-induced regulatory T-cell survival in hepatocellular carcinoma. Am J Med Sci (2013) 346(2):123–7. doi: 10.1097/MAJ.0b013e31826ceed0
34. Wu C-T, Hsieh C-C, Yen T-C, Chen W-C, Chen M-F. TGF-β1 mediates the radiation response of prostate cancer. J Mol Med (Berl). (2015) 93(1):73–82. doi: 10.1007/s00109-014-1206-6
35. Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res (2013) 1(6):365–72. doi: 10.1158/2326-6066.CIR-13-0115
36. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev (2015) 41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011
37. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature (2015) 520(7547):373–7. doi: 10.1038/nature14292
38. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol (2016) 13(8):516–24. doi: 10.1038/nrclinonc.2016.30
39. Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol (2019) 5(9):1276–82. doi: 10.1001/jamaoncol.2019.1478
40. Woody S, Hegde A, Arastu H, Peach MS, Sharma N, Walker P, et al. Survival is worse in patients completing immunotherapy prior to SBRT/SRS compared to those receiving it concurrently or after. Front Oncol (2022) 12:785350. doi: 10.3389/fonc.2022.785350
41. Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov (2022) 12(1):108–33. doi: 10.1158/2159-8290.CD-21-0003
42. Xu J, Li X, Lv X. Effect of oxaliplatin combined with 5-fluorouracil on treatment efficacy of radiotherapy in the treatment of elderly patients with rectal cancer. Exp Ther Med (2019) 17(3):1517–22. doi: 10.3892/etm.2018.7119
43. Lee EM, Hong YS, Kim K-P, Lee J-L, Kim SY, Park YS, et al. Phase II study of preoperative chemoradiation with S-1 plus oxaliplatin in patients with locally advanced rectal cancer. Cancer Sci (2013) 104(1):111–5. doi: 10.1111/cas.12041
44. Tang M, Lu X, Zhang C, Du C, Cao L, Hou T, et al. Downregulation of SIRT7 by 5-fluorouracil induces radiosensitivity in human colorectal cancer. Theranostics (2017) 7(5):1346–59. doi: 10.7150/thno.18804
45. Dupont R, Bérard E, Puisset F, Comont T, Delord JP, Guimbaud R, et al. The prognostic impact of immune-related adverse events during anti-PD1 treatment in melanoma and non-small-cell lung cancer: a real-life retrospective study. Oncoimmunology (2020) 9(1):1682383. doi: 10.1080/2162402X.2019.1682383
46. Schweizer C, Schubert P, Rutzner S, Eckstein M, Haderlein M, Lettmaier S, et al. Prospective evaluation of the prognostic value of immune-related adverse events in patients with non-melanoma solid tumour treated with PD-1/PD-L1 inhibitors alone and in combination with radiotherapy. Eur J Cancer (2020) 140:55–62. doi: 10.1016/j.ejca.2020.09.001
Keywords: immune checkpoint inhibitors, tumor immune microenvironment, radiotherapy, metastatic pancreatic cancer, second-line treatment
Citation: Tong F, Sun Y, Zhu Y, Sha H, Ni J, Qi L, Gu Q, Zhu C, Xi W, Liu B, Kong W and Du J (2023) Making “cold” tumors “hot”- radiotherapy remodels the tumor immune microenvironment of pancreatic cancer to benefit from immunotherapy: a case report. Front. Immunol. 14:1277810. doi: 10.3389/fimmu.2023.1277810
Received: 15 August 2023; Accepted: 07 December 2023;
Published: 20 December 2023.
Edited by:
Gulderen Yanikkaya Demirel, Yeditepe University, TürkiyeReviewed by:
Yanxun Han, First Affiliated Hospital of Anhui Medical University, ChinaBin Li, Shanghai Jiao Tong University, China
Copyright © 2023 Tong, Sun, Zhu, Sha, Ni, Qi, Gu, Zhu, Xi, Liu, Kong and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Du, ZHVqdWFuZ2x5eUAxNjMuY29t; Weiwei Kong, a29uZ3Z2QDEyNi5jb20=; Baorui Liu, YmFvcnVpbGl1QG5qdS5lZHUuY24=
†These authors have contributed equally to this work
 Fan Tong
Fan Tong Yi Sun
Yi Sun Yahui Zhu1
Yahui Zhu1 Huizi Sha
Huizi Sha Liang Qi
Liang Qi Chan Zhu
Chan Zhu Baorui Liu
Baorui Liu Juan Du
Juan Du