- 1Department of Hematology & Oncology, Jiujiang University Affiliated Hospital, Jiujiang, Jiangxi, China
- 2Graduate Department, Gannan Medical University, Ganzhou, Jiangxi, China
- 3Department of Oncology, First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 4Department of Social Medicine and Public Health, School of Basic Medicine, Jiujiang University, Jiujiang, Jiangxi, China
The phenomenon of histological transformation has been widely reported in advanced non-small cell lung cancer (NSCLC) with EGFR mutations following the failure of EGFR-TKI treatment. Recent evidence suggests that similar histological changes can also occur in advanced NSCLC without driver gene mutations after developing resistance to immunotherapy. In this review, it was found that 66.7% of cases with immunotherapy-induced histological transformation were classified as lung squamous cell carcinoma (LSCC), while histological conversion into lung adenocarcinoma (LUAD) without EGFR or ALK gene mutations has rarely been reported. There have been sporadic reports on the occurrence of mutual transformation between LUAD and LSCC. The histological conversion from NSCLC into small cell lung cancer (SCLC) appears to be significantly underestimated, likely due to the infrequency of re-biopsy following the development of immunotherapy resistance. Several studies have reported a close association between the transformation and mutations at TP53 and the RB1 splice site, as well as the loss of an FBXW7 mutation. However, the exact mechanisms underlying this conversion remain unclear. Currently, there is a lack of guidelines for the management of transformed SCLC from NSCLC following immunotherapy, with chemotherapy being the most commonly employed treatment approach.
Introduction
According to the most recent cancer statistics, lung cancer ranked second in incidence and first in mortality in 2023. Pathologically, lung cancer is divided into non-small cell lung cancer (NSCLC, about 85% of cases) and small cell lung cancer (SCLC, about 15% of cases) (1). Molecular targeted therapy (represented by EGFR-TKI and ALK-TKI) as first-line therapy has significantly improved the survival and prognosis of patients with advanced NSCLC harboring driver gene alterations. Meanwhile, immunotherapy (represented by PD-1/PD-L1 inhibitors) has revolutionized the anticancer treatment for advanced drive gene–negative NSCLC. Nevertheless, acquired drug resistance inevitably occurs in both targeted therapy and immunotherapy, which is a major clinical problem in advanced NSCLC (2, 3).
The mechanisms responsible for EGFR-TKI resistance are primarily the emergence of a second mutation (e.g., T790M mutation) and bypass pathway activation (e.g., MET) (4). The histological transformation of NSCLC into SCLC has been reported as an important mechanism of EGFR-TKI-resistance that occurs in 2%–15% of NSCLC patients after EGFR-TKI failure (5). Re-biopsy is frequently performed in patients with advanced NSCLC after EGFR-TKI resistance. Similarly, approximately 17% of prostate adenocarcinoma patients experience histological conversion into small cell carcinoma upon androgen-deprivation therapy. However, re-biopsy is not routinely carried out in patients with advanced NSCLC upon immunotherapy failure. Recently, there have been reports that immunotherapy resistance is also related to the histological conversion of NSCLC into SCLC. In the present article, we discuss the role of histological transformation in immunotherapy resistance in NSCLC and highlight a potential therapeutic strategy.
Incidence of histological conversion of NSCLC into SCLC upon immunotherapy
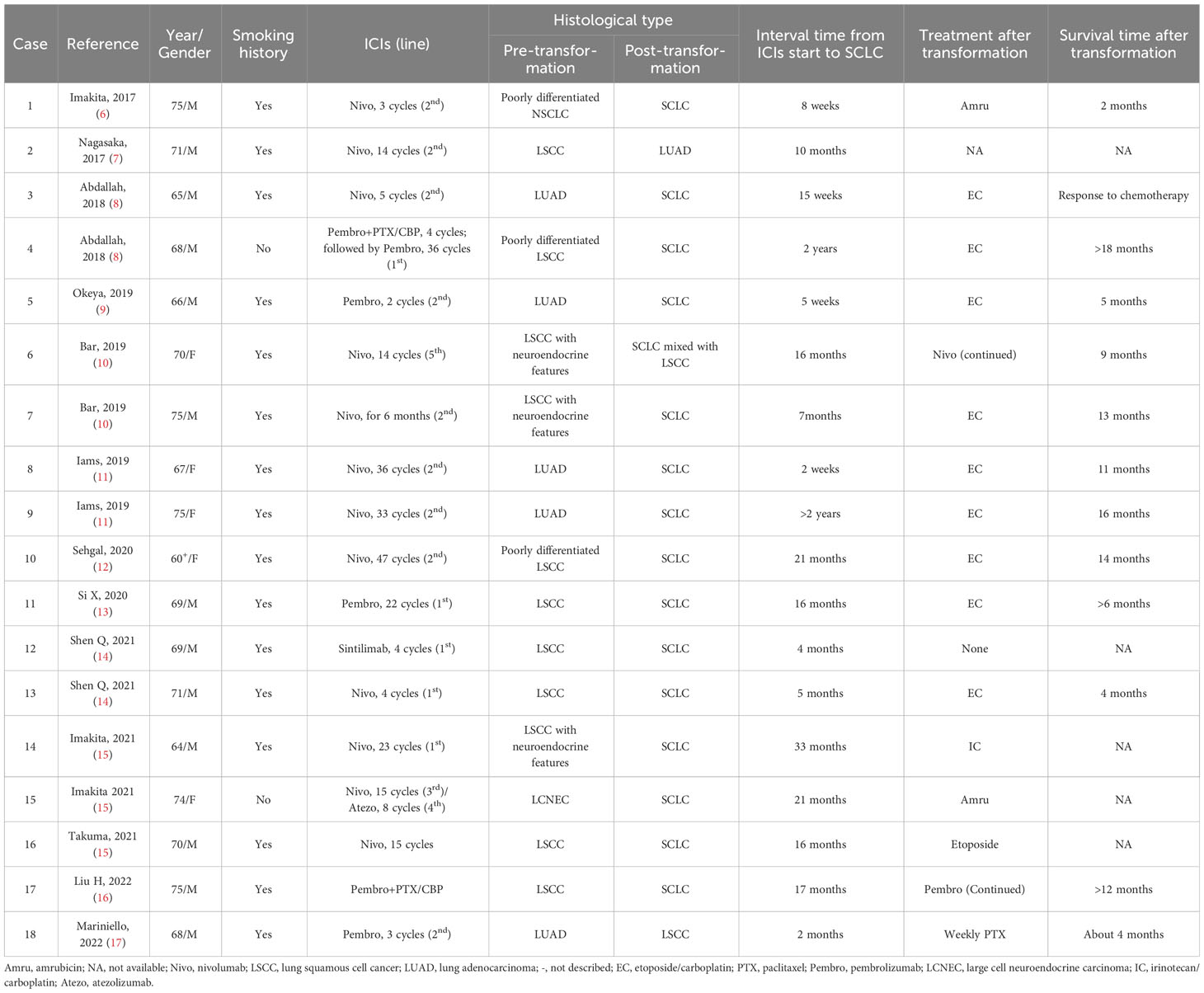
In 2017, Takuma et al. first reported that a patient with advanced NSCLC, who was initially diagnosed with poorly differentiated carcinoma and without EGFR gene mutation, experienced histological transformation into SCLC after immunotherapy resistance to nivolumab (6). Since then, there have been many similar case reports (shown in Table 1) (7–17). It is clear from this that increasing attention is paid to the concept of SCLC conversion as a result of immunotherapy in advanced NSCLC.
For advanced lung squamous cell cancer (LSCC), chemotherapy has been the sole treatment due to the lack of available molecular targets. Recently, PD-1/PD-L1 inhibitors have brought new hope to this type of patient. The NSCLC guidelines of the Chinese Society of Clinical Oncology (CSCO) (version 2023) recommends use of a PD-1/PD-L1 inhibitor alone (e.g., atezolizumab or pembrolizumab) and PD-1 inhibitor–containing combination therapy (e.g., nivolumab and sintilimab) as first- and second-line therapies for advanced LSCC, representing the primary foundation of immunotherapy-based therapy.
In the present review, 66.7% of patients (12/18) developed histological conversion from LSCC into SCLC after immunotherapy resistance (in Table 1). Among them, there were two cases that were initially diagnosed as LSCC with neuroendocrine features; one then transformed into SCLC and the other transformed into SCLC mixed with LSCC. Except for two patients without a smoking history, the other 10 patients had a history of tobacco smoking, and except for one patient receiving sintilimab therapy, the other 11 patients were receiving nivolumab or pembrolizumab therapy. The median interval time of transformation was 16.4 months, ranging from 4 to 33 months.
Four patients with lung adenocarcinoma (LUAD) who carried no EGFR/ALK mutations histologically evolved into SCLC. These patients had a history of active smoking and received nivolumab or pembrolizumab treatment before transformation into SCLC. The conversion time ranged from 2 weeks to 2 years (Table 1).
Of particular concern is the mutual transformation between LUAD and LSCC. Nagasaka et al. reported a case of a man with stage IVA LSCC who had received paclitaxel/carboplatin for four cycles and nivolumab treatment for 10 months. After nivolumab failure, the patient’s re-biopsy revealed a transformation into LUAD. Repeated examination of the previous specimen found no evidence of LUAD (7). In contrast, Mariniello et al. described a patient with recurrent LUAD (harboring BRAF mutation on exon 11 of p.G469A) who had undergone concomitant chemo-radiotherapy and pembrolizumab for three cycles. The disease progressed, and the second biopsy confirmed the histology of LSCC with a retained previous BRAF mutation on exon 11 (p.G469A), strongly indicating histological transformation (17).
Due to the limitation of small scientific reports, the prevalence of transformed SCLC remains unclear in patients with advanced NSCLC who progress after immunotherapy. There are at least two points to be considered. First, there are currently no guidelines that recommend routine re-biopsy when immunotherapy resistance develops in patients with advanced NSCLC (18). In contrast, for patients who receive EGFR-TKI treatment, repeated biopsy is the standard procedure recommended by the NSCLC guideline of the National Comprehensive Cancer Network (NCCN) and CSCO (19, 20). Second, no molecular targets have been clearly defined for the treatment of immunotherapy-resistant advanced NSCLC (18). In contrast, approximately 50% of NSCLC patients who progress after EGFR-TKI failure harbor secondary and concomitant gene mutations, indicating that they have the opportunity to undergo other targeted therapies (e.g., savolitinib for MET mutation) (4). Thus, the real-world frequency of NSCLC-into-SCLC transformation with immunotherapy resistance is likely underestimated (12). The exact frequency of SCLC transformation remains to be validated in future clinical practice.
Potential mechanisms for histological transformation from NSCLC into SCLC induced by immunotherapy
Histological transformation of lung cancer was first reported in a female NSCLC patient with EGFR exon 19 deletion, who converted into SCLC after gefitinib resistance in 2006 (21). For EGFR-mutant NSCLC transformation into SCLC, there are many responsible additional gene alterations, such as RB1 loss, TP53 mutations, PIK3CA, BRAF, WNK1, and ETV1 mutations, SPP1 upregulation, and REST inactivation (22–29). Notably, the status of RB1 loss and TP53 mutations in EGFR TKI-treated NSCLC have been considered as an important predictor of SCLC transformation (28, 29). However, only recently has the histological transformation of NSCLC into SCLC upon immunotherapy been gradually recognized. Regarding the pathogenesis of the conversion, albeit still uncertain, two kinds of possible transformation mechanisms are proposed.
One is the selection doctrine of combined ingredients, i.e., that the initial tumor comprises NSCLC and SCLC components. The NSCLC cells are decreased or even disappear after immunotherapy, while the immunotherapy-resistant SCLC cells survive as predominant clones. Clinical analysis revealed that the mixed-histology subtype accounted for approximately 5% of all lung cancer (30). Tang et al., performed whole-exome sequencing and microarray profiling in 9 lung cancer patients with mixed histology, and found that the histologic phenotype of lung cancers was possibly determined by the transcriptomic features rather than the genomic characteristics (31). In 2015, the combined SCLC (c-SCLC) as a subtype of SCLC was characterized by an admixture of elements of SCLC with NSCLC, with the incidence of 1%~3% of all SCLC cases (32). Men et al. reported that among 114 cases with c-SCLC, the most common combined component was LSCC (52.6%), followed by LUAD (32.5%) and large cell cancer (11.4%) (33), all of whom were newly-diagnosed and untreated (34).
As indicated in Table 1, there were only a few cases where the pre-transformation tumor exhibited neuroendocrine features in histology (e.g., Cases 6–7, 14–15). Among them, only Case 6 had the mixed histology of SCLC and LSCC after transformation, but no such combination existed before transformation (Table 1). Of note, no component of SCLC was found in the pre-transformation biopsy from these patients (Table 1). A possible reason may be related with the insufficiency of needle biopsy or bronchoscopic lung biopsy, which probably results in the missing SCLC component. However, whether combined-ingredients hypothesis holds true for the histological transformation upon immunotherapy still remains to be confirmed in a large-sample and real-world clinical study.
The other is the transformation doctrine of common precursor, i.e., that NSCLC and SCLC share a common cancer stem cell. NSCLC cells may turn into SCLC under the pressure of immunotherapy. Regarding EGFR-mutant LUAD conversion into SCLC, most oncologists consider EGFR-mutant LUAD and SCLC to originate from the same alveolar type II cells (5). Sehgal et al. found that a patient with poorly differentiated LSCC (Case 10 in Table 1) preserved the original genetic alterations after immunotherapy-triggered conversion into SCLC, i.e., TP53 mutation (p. R283fs*62), CDKN2A mutation (R58), SOX2 amplification, and PIK3CA amplification (12). These results support the doctrine of a common precursor rather than the doctrine of two ingredients. More importantly, the second gene mutations, e.g., TP53 mutation, RB1 splice site mutation, and FBXW7 mutation (Arg441Phe) loss, also occur in the histological conversion from immunotherapy-resistant NSCLC into SCLC, as described by Iams et al. (11), Bar et al. (10), and Si et al. (13), respectively. These foundations highly suggest that immunotherapy may remodel the immune milieu, which triggers additional genetic alterations and ultimately contributes to histological transformation. As a result, the second theory of a common precursor seems more convincing. In addition, the specific mechanisms governing mutual conversion between LUAD and LSCC remain unclear due to sporadic reports. Mariniello et al. reported that an NSCLC patient (Case 18 in Table 1) had the same BRAF mutation on exon 11 (p. G469A) before and after transformation from LUAD into LSCC after immunotherapy resistance (17), indicating the possibility of a common cell or origin.
Taken together, most of the literature supports the second doctrine (i.e., common precursor) (Figure 1), as the unique phenomenon that transformed SCLC still retains the same gene alterations as the original histology of either LSCC or EGFR-mutant LUAD. However, the potential mechanisms governing the histological conversion, and in particular that for immunotherapy-induced transformation from NSCLC into SCLC, remain to be thoroughly investigated in the future.
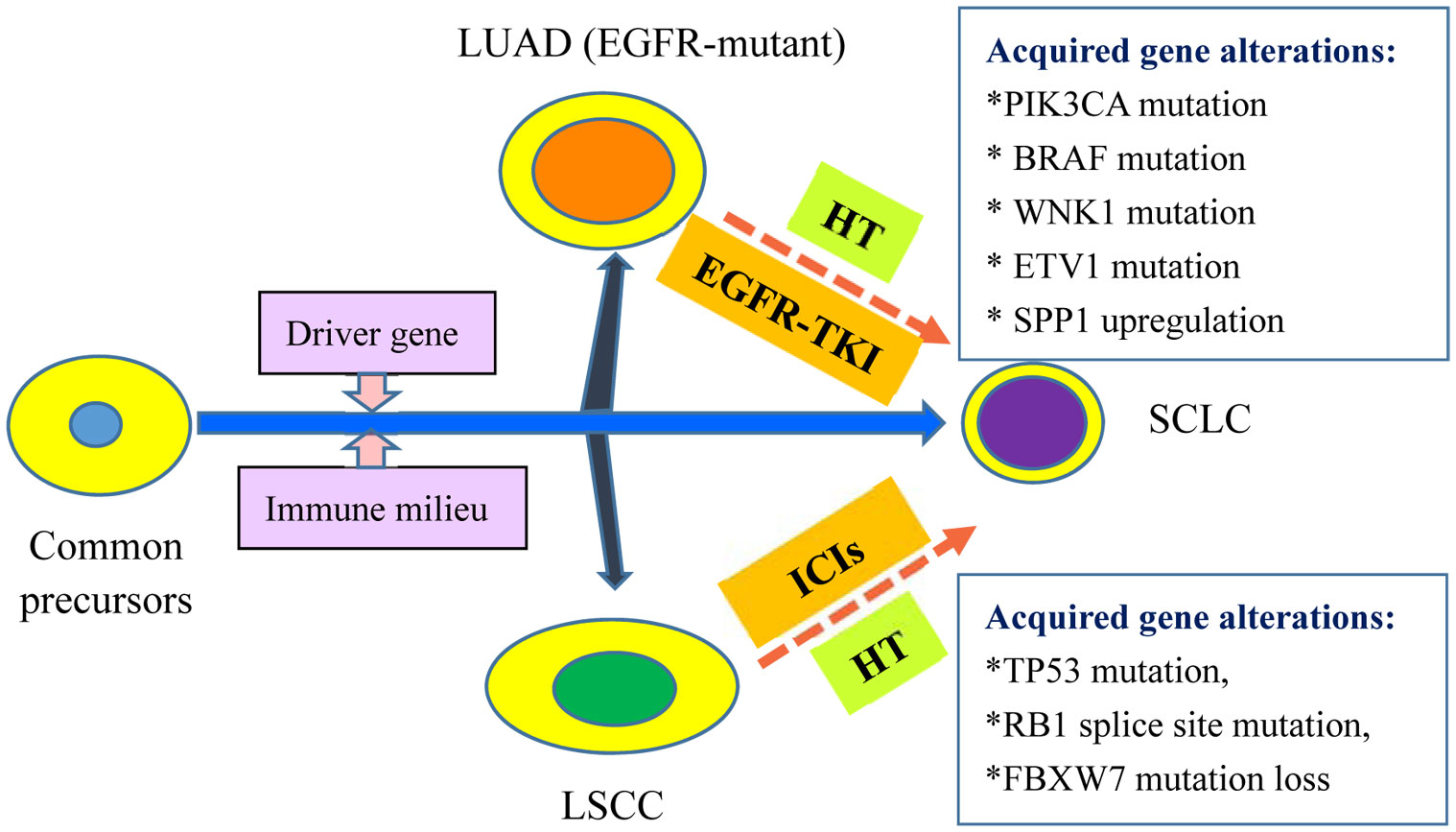
Figure 1 Current Hypothesis of Common Precursors: Histology Transformation to SCLC from NSCLC. The common precursors evolve to LUAD, LSCC and SCLC in different scenarios including driver gene and immune milieu. For EGFR-mutant LUAD treated with EGFR-TKI, acquired gene alterations might be required for the HT to SCLC including PIK3CA mutation, BRAF mutation, WNK1 mutation, ETV1 mutation, SPP1 upregulation, and REST inactivation. For LSCC without available molecular targets, ICIs treatment also result in HT to SCLC. Some acquired gene alterations have been observed in the evolution, including TP53 mutation, RB1 splice site mutation and FBXW7 mutation loss. LUAD, lung adenocarcinoma; EGFR, epidermal growth factor receptor; EGFR-TKI: EGFR- tyrosine kinase inhibitors; HT: histological transformation; LSCC: lung squamous cell cancer; ICIs: immune checkpoint inhibitors; SCLC: small cell lung cancer.
Therapeutic strategies for SCLC transformed from NSCLC after immunotherapy
For extensive-stage de novo SCLC, combination chemotherapy with etoposide/carboplatin (EC) has been the standard regimen since last two decades. Since 2019, chemotherapy plus immunotherapy (e.g., a PD-L1 inhibitor, such as atezolizumab, durvalumab, or adebrelimab, and a PD-1 inhibitor, such as serplulimab) has been recommended as the first-line treatment, with median overall survival (mOS) reaching 12.3–15.4 months (34–37). However, there is currently a lack of guidelines for managing SCLC transformed from NSCLC after immunotherapy failure.
According to the literature (Table 1), the majority of transformed SCLC cases were treated with combination chemotherapy using EC or irinotecan/carboplatin (IC). The overall survival (OS) after transformation was 11.8 ± 4.51 months, ranging from 6 to 18+ months, and the longest OS was more than 18 months. Only a few cases were managed using amurubicin (Cases 1 and 15) or paclitaxel alone (Case 18), but the OS was only approximately 2 to 4 months. The reports indicate that combination chemotherapy achieves better efficacy than single-agent chemotherapy.
The second therapeutic strategy is the continuation of immunotherapy alone. As described in Table 1, Cases 6 and 17 received continued treatment of nivolumab or pembrolizumab, and achieved an OS of 9 months and of over 12 months, respectively. In spite of the small sample size, the two reports suggested the potential feasibility of prolonged immunotherapy. However, this needs to be further verified by expanding the sample size.
At present, the therapeutic strategy of antiangiogenesis targeting VEGF has become an indispensable strategy for cancer treatment. In 2019, CSCO recommended anlotinib as the only antiangiogenic agent for refractory extensive-stage de novo SCLC in China based on the results of the ALTER 1202 study, which compared the efficacy of anlotinib versus placebo, namely, median progression-free survival (mPFS) (4.1 vs. 0.7 months, P < 0.0001) and mOS (7.3 vs. 4.9 months, P = 0.0029) (38). Furthermore, the ACTION-2 study prospectively reported that the first-line treatment with EP plus anlotinib for extensive-stage de novo SCLC achieved an overall response rate (ORR) of 87.2%, a disease control rate (DCR) of 97.7%, an mPFS of 9.0 months, and an mOS of 19.0 months (39). In our retrospective study, transformed SCLC after EGFR-TKI failure was treated with EP plus anlotinib, reaching 9.0 months of mPFS and 14.0 months of mOS (40). At present, there are no reports about anlotinib treatment for the transformed SCLC from NSCLC on immunotherapy. According to the clinical outcome of de novo SCLC and EGFR-TKI–induced transformed SCLC, we boldly speculate that the combination regimen of anlotinib with EC chemotherapy may be considered a potential therapeutic strategy. However, this treatment still needs to be tested in real-world clinical practice.
Conclusions
With the widespread application of immunotherapy, there has been an increasing histological transformation of NSCLC without targetable driver genes. Because re-biopsy is not routinely taken after immunotherapy resistance, the true incidence of histological conversion is almost certainly underestimated in patients with advanced NSCLC, and in particular those with LSCC. There have been a few reports describing the close relation with additional gene alterations, including TP53 mutation, RB1 splice site mutation, and loss of FBXW7 mutation (Arg441Phe). However, this might be just the tip of the iceberg, and the exact mechanism of histological transformation resulting from immunotherapy remains to be clarified. Regarding therapeutic strategy, no guidelines are available for transformed SCLC from immunotherapy-resistant NSCLC. EC chemotherapy is the most common treatment, and the combination of EC with anlotinib may be a potential treatment strategy instead of chemotherapy alone. Of course, the small and insufficient literatures are the primary limitation in our review. We believe that the problem will be gradually resolved with the continuing attention on immunology-induced histological transformation from clinical oncologists.
Author contributions
JZ: Investigation, Methodology, Writing – original draft. XD: Data curation, Investigation, Writing – original draft. JD: Conceptualization, Data curation, Supervision, Writing – review & editing. XW: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This paper received funding from the Key project of Jiangxi Natural Science Foundation (No. 20224ACB206038), China.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adams SJ, Stone E, Baldwin DR, Vliegenthart R, Lee P, Fintelmann FJ. Lung cancer screening. Lancet (2023) 401:390–408. doi: 10.1016/S0140-6736(22)01694-4
2. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer (2023) 22:40. doi: 10.1186/s12943-023-01740-y
3. Tan AC, Tan D. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol (2022) 40:611–25. doi: 10.1200/JCO.21.01626
4. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd−generation egfr−tki resistance in advanced non−small cell lung cancer (review). Int J Oncol (2021) 59:90. doi: 10.3892/ijo.2021.5270
5. Xu J, Xu L, Wang B, Kong W, Chen Y, Yu Z. Outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after egfr tyrosine kinase inhibitors resistance: a systematic review and pooled analysis. Front Oncol (2021) 11:766148. doi: 10.3389/fonc.2021.766148
6. Imakita T, Fujita K, Kanai O, Terashima T, Mio T. Small cell lung cancer transformation during immunotherapy with nivolumab: a case report. Respir Med Case Rep (2017) 21:52–5. doi: 10.1016/j.rmcr.2017.03.019
7. Nagasaka M, Pansare RS, Abdulfatah E, Guan H, Tranchida P, Gadgeel SM. Histologic transformation in nsclc with pd-1 therapy. J Thorac Oncol (2017) 12:e133–34. doi: 10.1016/j.jtho.2017.04.026
8. Abdallah N, Nagasaka M, Abdulfatah E, Shi D, Wozniak AJ, Sukari A. Non-small cell to small cell lung cancer on pd-1 inhibitors: two cases on potential histologic transformation. Lung Cancer (Auckl) (2018) 9:85–90. doi: 10.2147/LCTT.S173724
9. Okeya K, Kawagishi Y, Muranaka E, Izumida T, Tsuji H, Takeda S. Hyperprogressive disease in lung cancer with transformation of adenocarcinoma to small-cell carcinoma during pembrolizumab therapy. Intern Med (2019) 58:3295–98. doi: 10.2169/internalmedicine.2892-19
10. Bar J, Ofek E, Barshack I, Gottfried T, Zadok O, Kamer I, et al. Transformation to small cell lung cancer as a mechanism of resistance to immunotherapy in non-small cell lung cancer. Lung Cancer (2019) 138:109–15. doi: 10.1016/j.lungcan.2019.09.025
11. Iams WT, Beckermann KE, Almodovar K, Hernandez J, Vnencak-Jones C, Lim LP, et al. Small cell lung cancer transformation as a mechanism of resistance to pd-1 therapy in kras-mutant lung adenocarcinoma: a report of two cases. J Thorac Oncol (2019) 14:e45–48. doi: 10.1016/j.jtho.2018.11.031
12. Sehgal K, Varkaris A, Viray H, VanderLaan PA, Rangachari D, Costa DB. Small cell transformation of non-small cell lung cancer on immune checkpoint inhibitors: uncommon or under-recognized? J Immunother Cancer (2020) 8:e697. doi: 10.1136/jitc-2020-000697
13. Si X, You Y, Zhang X, Wang H, Wang M, Zhang L. Histologic transformation of lung cancer during pembrolizumab therapy: a case report. Thorac Cancer (2020) 11:793–96. doi: 10.1111/1759-7714.13312
14. Shen Q, Qu J, Sheng L, Gao Q, Zhou J. Case report: transformation from non-small cell lung cancer to small cell lung cancer during anti-pd-1 therapy: a report of two cases. Front Oncol (2021) 11:619371. doi: 10.3389/fonc.2021.619371
15. Imakita T, Fujita K, Kanai O, Okamura M, Hashimoto M, Nakatani K, et al. Small cell transformation of non-small cell lung cancer under immunotherapy: case series and literature review. Thorac Cancer (2021) 12:3062–67. doi: 10.1111/1759-7714.14180
16. Liu H, Chen LH, Zhang ZH, Wang N, Zhuang SH, Chen H, et al. Histomorphological transformation from non-small cell lung carcinoma to small cell lung carcinoma after targeted therapy or immunotherapy: a report of two cases. Front Oncol (2022) 12:1022705. doi: 10.3389/fonc.2022.1022705
17. Mariniello A, Righi L, Morrone A, Carnio S, Bironzo P. Squamous cell histological transformation in a lung adenocarcinoma patient (hyper) progressing upon immunotherapy. Tumori (2022) 108:NP15–19. doi: 10.1177/03008916221080487
18. Giaccone G, He Y. Current knowledge of small cell lung cancer transformation from non-small cell lung cancer. Semin Cancer Biol (2023) 94:1–10. doi: 10.1016/j.semcancer.2023.05.006
19. Commitee CSOC. Consensus on application of third-generation egfr-tki in egfr mutated nsclc (2022 version). Zhongguo Fei Ai Za Zhi (2022) 25:627–41. doi: 10.3779/j.issn.1009-3419.2022.101.47
20. Cho BC, Loong H, Tsai CM, Teo M, Kim HR, Lim SM, et al. Genomic landscape of non-small cell lung cancer (nsclc) in east asia using circulating tumor dna (ctdna) in clinical practice. Curr Oncol (2022) 29:2154–64. doi: 10.3390/curroncol29030174
21. Zakowski MF, Ladanyi M, Kris MG. Egfr mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med (2006) 355:213–15. doi: 10.1056/NEJMc053610
22. Masawa M, Sato-Yazawa H, Kashiwagi K, Ishii J, Miyata-Hiramatsu C, Iwamoto M, et al. Rest inactivation and coexpression of ascl1 and pou3f4 are necessary for the complete transformation of rb1/tp53-inactivated lung adenocarcinoma into neuroendocrine carcinoma. Am J Pathol (2022) 192:847–61. doi: 10.1016/j.ajpath.2022.03.007
23. Zhou Y, Bai H, Xia J, Xu WY, Cheng L, Xiong L. Novel etv1 mutation in small cell lung cancer transformation resistant to egfr tyrosine kinase inhibitors. Ann Transl Med (2021) 9:1150. doi: 10.21037/atm-21-2625
24. Xie T, Li Y, Ying J, Cai W, Li J, Lee KY, et al. Whole exome sequencing (wes) analysis of transformed small cell lung cancer (sclc) from lung adenocarcinoma (luad). Transl Lung Cancer Res (2020) 9:2428–39. doi: 10.21037/tlcr-20-1278
25. Ferrer L, Giaj LM, Brevet M, Antoine M, Mazieres J, Rossi G, et al. A brief report of transformation from nsclc to sclc: molecular and therapeutic characteristics. J Thorac Oncol (2019) 14:130–34. doi: 10.1016/j.jtho.2018.08.2028
26. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to egfr inhibitors. Sci Transl Med (2011) 3:75ra26. doi: 10.1126/scitranslmed.3002003
27. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol (2015) 16:e165–72. doi: 10.1016/S1470-2045(14)71180-5
28. Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol (2017) 35:3065–74. doi: 10.1200/JCO.2016.71.9096
29. Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, et al. Rb loss in resistant egfr mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun (2015) 6:6377. doi: 10.1038/ncomms7377
30. Deng P, Hu C, Zhou L, Li Y, Huang L. Clinical characteristics and prognostic significance of 92 cases of patients with primary mixed-histology lung cancer. Mol Clin Oncol (2013) 1:863–68. doi: 10.3892/mco.2013.137
31. Tang M, Abbas HA, Negrao MV, Ramineni M, Hu X, Hubert SM, et al. The histologic phenotype of lung cancers is associated with transcriptomic features rather than genomic characteristics. Nat Commun (2021) 12:7081. doi: 10.1038/s41467-021-27341-1
32. Dagogo-Jack I, Saltos A, Shaw AT, Gray JE. Pathology issues in thoracic oncology: histologic characterization and tissue/plasma genotyping may resolve diagnostic dilemmas. Am Soc Clin Oncol Educ Book (2017) 37:619–29. doi: 10.1200/EDBK_175197
33. Men Y, Hui Z, Liang J, Feng Q, Chen D, Zhang H, et al. Further understanding of an uncommon disease of combined small cell lung cancer: clinical features and prognostic factors of 114 cases. Chin J Cancer Res (2016) 28:486–94. doi: 10.21147/j.issn.1000-9604.2016.05.03
34. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and pd-l1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (impower133). J Clin Oncol (2021) 39:619–30. doi: 10.1200/JCO.20.01055
35. Paz-Ares L, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from caspian. Esmo Open (2022) 7:100408. doi: 10.1016/j.esmoop.2022.100408
36. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (capstone-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23:739–47. doi: 10.1016/S1470-2045(22)00224-8
37. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the astrum-005 randomized clinical trial. Jama (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
38. Cheng Y, Wang Q, Li K, Shi J, Wu L, Han B, et al. Anlotinib for patients with small cell lung cancer and baseline liver metastases: a post hoc analysis of the alter 1202 trial. Cancer Med (2022) 11:1081–87. doi: 10.1002/cam4.4507
39. Zhang W, Deng P, Kong T, Zhang B, Qian F, Dong Y, et al. Safety and efficacy of anlotinib in combination with standard chemotherapy as first-line treatment for extensive-stage small cell lung cancer: a multi-center, prospective study (action-2). Lung Cancer (2022) 173:43–8. doi: 10.1016/j.lungcan.2022.09.003
Keywords: histological transformation, small cell lung cancer, resistance, immunotherapy, non-small cell lung cancer
Citation: Zeng J, Ding X, Ding J and Wang X (2023) Histological transformation into SCLC: An important resistance mechanism of NSCLC upon immunotherapy. Front. Immunol. 14:1275957. doi: 10.3389/fimmu.2023.1275957
Received: 10 August 2023; Accepted: 16 October 2023;
Published: 30 October 2023.
Edited by:
Paul Zarogoulidis, Euromedica General Clinic, GreeceReviewed by:
Jianjun Zhang, University of Texas MD Anderson Cancer Center, United StatesBingnan Zhang, University of Texas MD Anderson Cancer Center, in collaboration with reviewer JZ
Copyright © 2023 Zeng, Ding, Ding and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianghua Ding, ZG9jdG9yMDkyMkAxMjYuY29t
†These authors have contributed equally to this work
 Jiao Zeng1,2†
Jiao Zeng1,2† Jianghua Ding
Jianghua Ding