- The Department of Otolaryngology-Head and Neck Surgery, Nanchong Central Hospital, Second Clinical Medical College of North Sichuan Medical College, Nanchong, Sichuan, China
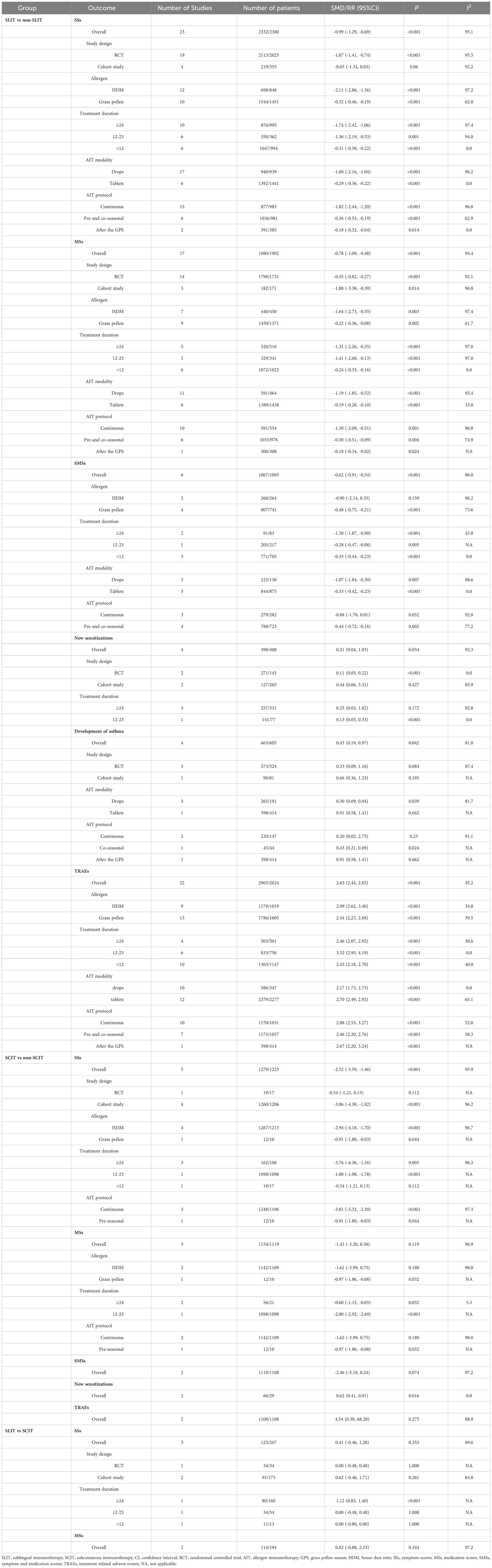
Aim: To systematically compare the efficacy and safety of subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) in children with allergic rhinitis (AR).
Methods: PubMed, Embase, Cochrane Library, and Web of Science were searched from inception to March 2, 2023. Outcomes included symptom scores (SSs), medication scores (MSs), symptom and medication scores (SMSs), new sensitizations, development of asthma, improvement, and treatment-related adverse events (TRAEs). The quality of the included studies was assessed by the modified Jadad scale and Newcastle-Ottawa scale (NOS). Meta-regression was carried out to explore the source of heterogeneity. Subgroup analysis was further conducted in terms of study design [randomized controlled trials (RCTs), cohort studies], allergen [house dust mites (HDMs), grass pollen], treatment duration (≥ 24, 12-23 or < 12 months), allergen immunotherapy (AIT) modality (drops or tablets), and AIT protocol [continuous, pre-seasonal, co-seasonal, or after the grass pollen season (GPS)]. Sensitivity analysis was conducted for all outcomes. A Bayesian framework and a Monte Carlo Markov Chain (MCMC) model were developed for indirect comparison.
Results: Totally 50 studies with 10813 AR children were included, with 4122 treated with SLIT, 1852 treated with SCIT, and 4839 treated with non-SLIT or non-SCIT therapy. For direct comparison, the SLIT group had a similar SS to the SCIT group [pooled standardized mean difference (SMD): 0.41, 95% confidence interval (CI): -0.46, 1.28, P = 0.353]. Comparable MSs were observed in the SLIT and SCIT groups (pooled SMD: 0.82, 95%CI: -0.88, 2.53, P = 0.344). For indirect comparison, no significant differences were found in SSs (pooled SMD: 1.20, 95% credibility interval (CrI): -1.70, 4.10), MSs (pooled SMD: 0.57, 95%CrI: -1.20, 2.30), SMSs (pooled SMD: 1.80, 95%CrI: -0.005, 3.60), new sensitizations [pooled relative risk (RR): 0.34, 95%CrI: 0.03, 3.58], and development of asthma (pooled RR: 0.68, 95%CrI: 0.01, 26.33) between the SLIT and SCIT groups; the SLIT group illustrated a significantly lower incidence of TRAEs than the SCIT group (pooled RR: 0.17, 95%CrI: 0.11, 0.26).
Conclusion: Considering both efficacy and safety, SLIT might be a more favorable AIT than SCIT in the treatment of pediatric AR, which may serve as a decision-making reference for clinicians.
Systematic review registration: PROSPERO (CRD42023460693).
Introduction
Allergic rhinitis (AR), an upper airway disease, is a health concern worldwide, with growing prevalence in the world (1, 2). It affects up to 50% of the global population (3), and often develops in children and adolescents (4). Typical symptoms comprise sneezing, runny nose, itchy nose, and nasal obstruction (5). This disorder influences the quality of life of patients and is related to severe comorbidities such as asthma, sinusitis and conjunctivitis, thus leading to a huge health burden (6). AR was also associated with great economic costs via impacts on education, productivity, and medical resources (7).
Pharmacotherapy is still the standard care for AR treatment (8). When pharmacotherapy is ineffective, allergen immunotherapy (AIT), as a major disease-modifying method, should be taken into account (9). AIT had a long-term disease-modifying effect, and can be administered through a subcutaneous (SCIT) or sublingual (SLIT) route (10). SLIT has been developed as a potential alternative to SCIT (11). Both routes are demonstrated to be clinically effective and safe by existing evidence (12, 13). Nevertheless, most current studies have assessed SCIT or SLIT respectively (14–17), and there is a paucity of studies on the direct comparison of these two routes (18, 19). A previous meta-analysis evaluated the effectiveness and adverse events of SCIT and SLIT in seasonal AR among both children and adults (20). Kim et al. (21) conducted a network meta-analysis to compare the efficacy of SCIT and SLIT for pediatric and adult patients with house dust mite allergy-related AR. A recent meta-analysis investigated the roles of SCIT and SLIT for adults with AR using indirect comparison (22). At present, no meta-analysis of SCIT versus SLIT has been performed specifically for AR children, which necessitates comprehensive research to facilitate AR management in the pediatric population.
This study intended to evaluate and compare the efficacy and safety of SCIT and SLIT in children with AR via a meta-analysis using direct and indirect comparisons, in order to provide a reference for clinical decision-making between SCIT and SLIT.
Methods
Search strategy
A comprehensive search was independently conducted by two investigators (JM Yang, SH Lei) on the following four databases from inception to March 2, 2023: PubMed, Embase, Cochrane Library, and Web of Science. Disagreements were settled via discussion. The English search terms were “Rhinitis, Allergic” OR “Allergic Rhinitides” OR “Allergic Rhinitis” OR “Pollen Allerg*” OR “Pollinos*” OR “Hay Fever” OR “Hayfever Rhinoconjunctivitis” OR “Rhinitis” OR “Rhinitides” OR “Dust Mite Allergy” OR “Dust Mite Hypersensitivit*” OR “Dermatophagoides pteronyssinus Allerg*” OR “Dermatophagoides farinae Allerg*” AND “Sublingual immunotherap*” OR “SLIT” OR “Subcutaneous immunotherap*” OR “SCIT” OR “Allergen immunotherapy” OR “Immunotherap*” OR “Immunologic Desensitization” OR “Hyposensitization Therapy” OR “Allergy Shot” OR “Immunosuppression Therap*” OR “Anti Rejection Therap*” OR “Antirejection Therap*” OR “Immunosuppressive Therap*” OR “Immunosuppression*” AND “Child” OR “Children” OR “Pediatric” OR “Pediatrics” OR “Childhood” OR “Adolescen*” OR “Teenager*” OR “Teen” OR “Teens” OR “Youth” OR “Youths”. The retrieved studies were first screened via titles and abstracts, and subsequently via full texts. This systematic review and meta-analysis was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA), and was registered in PROSPERO (registration number: CRD42023460693).
Inclusion and exclusion criteria
The inclusion criteria included (a) population: studies on children with AR (aged ≤ 18 years); (b) intervention and comparator: studies with SLIT versus non-SLIT, SCIT versus non-SCIT, or SLIT versus SCIT; (c) outcome: studies with any of the following outcomes: symptom scores (SSs), medication scores (MSs), symptom and medication scores (SMSs), new sensitizations, development of asthma, improvement, and treatment-related adverse events (TRAEs); and (d) study design: open or blind controlled trials, cohort studies, or case-control studies.
The exclusion criteria included (a) studies on mixed population, such as patients of all ages or patients with AR or asthma; (b) studies with incomplete data or of which data could not be extracted; (c) meta-analyses, reviews, conference abstracts, animal tests, case reports; or (d) non-English studies.
Outcome measures
The outcomes in this analysis included SSs, MSs, SMSs, new sensitizations, development of asthma, improvement, and TRAEs. Concerning SSs, the higher the SS, the more severe the symptom (23). Symptoms included itchy nose, sneezing, running nose, blocked nose, itchy eyes, etc. (17, 24, 25), and the Visual Analogue Scale (VAS) was also used for evaluation (15, 26). Some studies recorded daily values (25, 27), while some recorded scoring results over a period of time (28, 29). For MSs, the higher the MS, the more medication was used (14). Some studies recorded average daily dosages (27, 30), while some recorded dosages over a period of time (28, 29). The SMS referred to the combination of the SS and MS.
Improvement was defined as “slight to moderate improvement” and “marked improvement” (31), improvement rates of 26–65% and ≥ 66% based on a scale of 1 to 3 (30), a reduction of over 1 point for symptoms (32), overall treatment effect (33), or self-reported clinical improvement (19).
Data collection and quality assessment
Two independent investigators (JM Yang, SH Lei) collected the following data from eligible studies: first author, year of publication, country, study design, AR diagnosis, group, group division, treatment, sample size, sex (male/female), age (years), duration of AR (years), allergen, mono-/poly-sensitization status, AIT modality, AIT protocol, product type/name (manufacturer), comorbidity, treatment duration (months), dropout rate (%), quality assessment (QA), and outcome.
The quality of randomized controlled trials (RCTs) was assessed by the modified Jadad scale from random sequence generation, randomization concealment, blinding, and withdrawal and dropout, which had a total score of 7, with 1-3 as low quality and 4-7 as high quality (34). The quality of cohort studies was estimated using the Newcastle-Ottawa scale (NOS) from population selection, intergroup comparability, and result measurement, which had a total score of 9, with 0-3 as poor quality, 4-6 as fair quality, and 7-9 as good quality (35).
Statistical analysis
Statistical analysis was performed using the Gemtc 1.0.1 package from Stata 15.1 (Stata Corporation, College Station, TX, USA) and R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). Measurement data were reported as standardized mean differences (SMDs) and 95% confidence intervals (CIs). When different studies utilize different rating instruments or different measurement units for the same outcome, standardized mean differences (SMDs) can be used in such cases (36). In this study, SMDs were used to handle variations in SS scales or individual SSs even for the same indicators among different studies. Counting data were shown as relative risks (RRs) and 95%CIs. Heterogeneity tests were conducted for each outcome. If the heterogeneity statistic I2 ≥ 50%, the random-effects model was used for analysis, and otherwise, the fixed-effects model was applied. Meta-regression was carried out to explore the source of heterogeneity. Subgroup analysis was further conducted in terms of study design (RCTs, cohort studies), allergen [house dust mites (HDMs), grass pollen], treatment duration (≥ 24, 12-23 or < 12 months), AIT modality (drops or tablets), and AIT protocol [continuous, pre-seasonal, co-seasonal, or after the grass pollen season (GPS)]. Sensitivity analysis was conducted for all outcomes by deleting one study at a time and comprehensively assessing the remaining studies.
Indirect comparison refers to indirectly obtaining the relative effect of A versus B through the results of A versus C and B versus C, with C as a common comparator (37). The indirect comparison of A and B is provided by the direct comparison of A and C and the direct comparison of B versus C with (38). The distinction between direct and indirect comparisons is that the direct comparison (i.e. head-to-head comparison) of A and B is directly provided by A versus B trials. The rationales for choosing indirect comparison are as follows: first, there are no studies for the direct comparison of A and B, but each is compared with a common comparator (e.g. C); second, there are studies for direct comparison, but the number or quality of these studies is relatively small or low. The most essential difference between the frequentist method and the Bayesian method lies in their different interpretations of probability. The Bayesian method has a prior distribution, and it treats unknown parameters as random variables, while frequentist statistics treat them as fixed but unknown values. The Bayesian inference allows the probability to be associated with an unknown parameter; the Bayesian interpretation also allows researchers to maintain their own understanding of specific parameter settings; the Bayesian result can be a posterior probability distribution obtained from experiments or research regarding parameters. The conclusion of frequency statistics is to accept or reject hypothesis testing or to see whether the results are included in the confidence interval under a certain sample inference (39). Compared with the frequentist analysis, the Bayesian analysis has the following advantages: (1) the Bayesian approach can not only effectively integrate data and flexibly build models, but also use the obtained posterior probability to rank all interventions participating in the comparison and distinguish comparative advantages and disadvantages, while the frequentist method can only rely on the effect size and its 95%CI obtained by pairwise comparison in ranking; (2) since the frequentist approach uses the maximum likelihood method in parameter estimation, which estimates the maximum likelihood function through continuous iteration, it is prone to instability and biased results, while the Bayesian approach does not have this problem, so its estimated values are more accurate than those of the frequentist approach (39). Then the data were converted into a relative data format. A Bayesian framework and a Monte Carlo Markov Chain (MCMC) model were developed for indirect comparison, the model has a chain number of 4, an initial iteration number of 20000, and a further updated iteration number of 50000, with a step size of 1. Indirect effect sizes and 95% credibility intervals (CrIs) were reported for different outcomes. The difference was statistically significant when P<0.05.
Results
Characteristics of the included studies
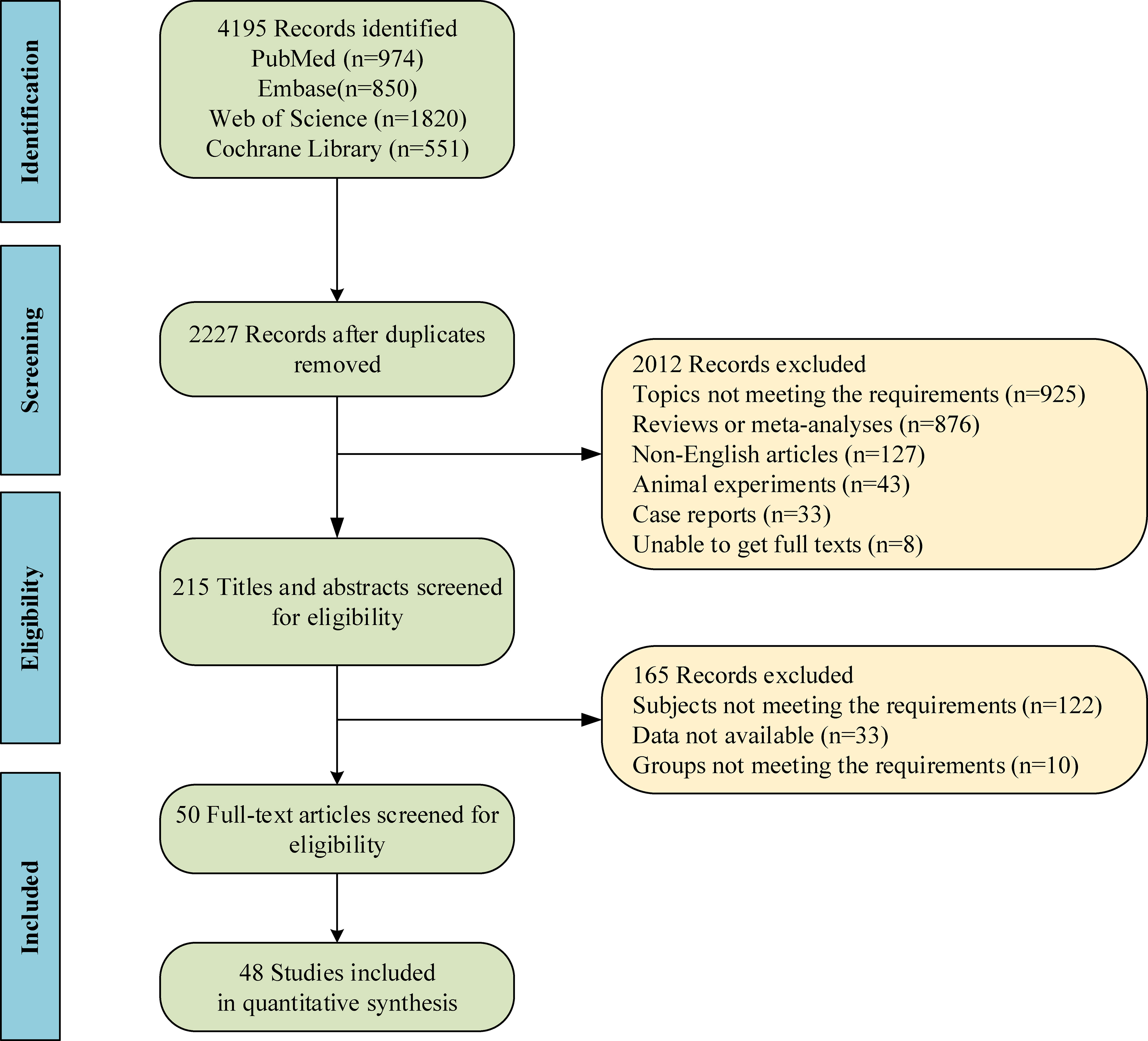
A total of 4195 studies were retrieved through database search, and then 1968 duplicates were removed. Based on screening with titles and abstracts, and subsequently with full texts, 50 studies (14–19, 23–33, 40–72) were included in the end, with 48 studies included for quantitative analysis. Figure 1 shows the screening process of qualified studies. These eligible studies included 10813 patients, with 4122 treated with SLIT, 1852 treated with SCIT, and 4839 treated with non-SLIT or non-SCIT therapy. The year of publication ranged from 1998 to 2023. Six studies (18, 19, 61, 63, 71, 72) made direct comparison between SLIT and SCIT. The features of the included studies are exhibited in Supplementary Table S1. Among the included studies, 35 studies were RCTs, of which 8 had low quality and 27 had high quality; 15 were cohort studies, of which 13 had medium quality and 2 had high quality. In RCTs, patients were randomly assigned to the SLIT group or the SCIT group. For cohort studies, the decision for SCIT or SLIT was made based on medical records or parental preference in 6 studies, and 9 studies did not report the grouping basis.
Direct comparison
SLIT versus non-SLIT
SSs
SSs were evaluated in 23 studies (15, 17, 24–31, 33, 41–45, 53, 55, 58, 60, 65–67), including 2332 patients in the SLIT group and 2380 in the non-SLIT group. Pooled analysis illustrated that compared with the non-SLIT group, the SLIT group had a significantly lower SS (pooled SMD: -0.99, 95%CI: -1.29, -0.69, I2 = 95.1%, P < 0.001) (Supplementary Figure 1). Subgroup analysis based on study design, allergen, treatment duration, AIT modality, and AIT protocol found significant differences in SSs between the SLIT and non-SLIT groups when the included studies were RCTs, allergens were HDMs or grass pollen, treatment duration was ≥ 24, 12-23 or < 12 months, AIT modality was drops or tablets, or AIT protocol was continuous, pre- and co-seasonal, or after the GPS (all P < 0.05) (Table 1).
MSs
MSs were assessed in 17 studies (15, 24–31, 41–44, 53, 55, 60, 65), including 1980 patients in the SLIT group and 1902 in the non-SLIT group. Pooled analysis demonstrated that the SLIT group had a significantly lower MS than the non-SLIT group (pooled SMD: -0.78, 95%CI: -1.09, -0.48, I2 = 94.4%, P < 0.001) (Supplementary Figure 2). Subgroup analysis based on study design, allergen, treatment duration, AIT modality, and AIT protocol found significant differences in MSs between the SLIT and non-SLIT groups in all subgroups (all P < 0.05) (Table 1).
SMSs
Six studies (25, 27–29, 31, 43) provided data on SMSs, with 1067 patients in the SLIT group and 1005 in the non-SLIT group. Pooled analysis showed that the SLIT group had a significantly lower SMS than the non-SLIT group (pooled SMD: -0.62, 95%CI: -0.91, -0.34, I2 = 86.0%, P < 0.001) (Supplementary Figure 3). Subgroup analysis based on allergen, treatment duration, AIT modality, and AIT protocol found significant differences in SMSs between the SLIT and non-SLIT groups when the allergen was grass pollen, treatment duration was ≥ 24, 12-23 or < 12 months, AIT modality was drops or tablets, or AIT protocol was pre- or co-seasonal (all P < 0.05) (Table 1).
New sensitizations
Information on new sensitizations was reported by 4 studies (41, 59, 62, 67), with 398 patients in the SLIT group and 408 in the non-SLIT group. Pooled analysis illustrated that the SLIT group had a similar incidence of new sensitizations to the non-SLIT group (pooled RR: 0.21, 95%CI: 0.04, 1.03, I2 = 92.3%, P = 0.054) (Supplementary Figure 4). Subgroup analysis based on study design and treatment duration showed that the SLIT group had a significantly lower incidence of new sensitizations than the non-SLIT group when the included studies were RCTs or treatment duration was 12-23 months (both P < 0.05) (Table 1).
Development of asthma
Four studies (26, 41, 52, 59) investigated the development of asthma, including 663 patients in the SLIT group and 605 in the non-SLIT group. Pooled analysis exhibited a significantly lower incidence of developing asthma in the SLIT group versus the non-SLIT group (pooled RR: 0.43, 95%CI: 0.19, 0.97, I2 = 81.0%, P = 0.042) (Supplementary Figure 5). Subgroup analysis based on study design, AIT modality and AIT protocol showed that the SLIT group had a significantly decreased incidence of developing asthma than the non-SLIT group when the AIT modality was drops or AIT protocol was co-seasonal (both P < 0.05) (Table 1).
TRAEs
Twenty-two studies investigated TRAEs (24, 26–29, 31, 32, 42–44, 46, 47, 49, 50, 53, 55, 56, 60, 62, 64, 68, 69), with 2965 patients in the SLIT group and 2624 in the non-SLIT group. According to pooled analysis, the SLIT group had a significantly higher incidence of TRAEs than the non-SLIT group (pooled RR: 2.63, 95%CI: 2.44, 2.83, I2 = 45.2%, P < 0.001) (Supplementary Figure 6). Subgroup analysis based on allergen, treatment duration, AIT modality, and AIT protocol found significant differences in the incidence of TRAEs between the SLIT and non-SLIT groups in all subgroups (all P < 0.05) (Table 1).
Improvement
The percentage of patients evaluated as “improved” by patients/guardians was significantly higher in the SLIT group (78.8%) than in the placebo group (58.3%) (P < 0.0001), which was consistent with the percentage of patients evaluated as general improvement by researchers (67.5% vs 57.4%, P = 0.0348) (31). As shown by another study (30), the total effective rate of the SLIT group and the drug only group was 98.08% and 86.00%, respectively (P = 0.030). No significant difference was found by de Bot et al. (33) in the overall evaluation of treatment efficacy between the SLIT group and the placebo group (slightly better 33.3% vs 35.8%, much better 21.9% vs 27.5%, no complaints any more 2.9% vs 1.8%). The research of Yonekura et al. (32) illustrated that 33% of patients in the SLIT group improvement of symptoms, compared with 0% in the placebo group.
SCIT versus non-SCIT
SSs
SSs were evaluated in 5 studies (14, 23, 40, 54, 70), including 1279 patients in the SCIT group and 1223 in the non-SCIT group. Pooled analysis illustrated that compared with the non-SCIT group, the SCIT group had a significantly lower SS (pooled SMD: -2.52, 95%CI: -3.59, -1.46, I2 = 95.9%, P < 0.001) (Supplementary Figure 7). Subgroup analysis based on study design, allergen, treatment duration, and AIT protocol found significant differences in SSs between the SCIT and non-SCIT groups when the included studies were cohort studies, allergens were HDMs or grass pollen, treatment duration was ≥ 24 or 12-23 months, or AIT protocol was continuous or pre-seasonal (all P < 0.05) (Table 1).
MSs
MSs were assessed in 3 studies (14, 23, 54), including 1154 patients in the SCIT group and 1119 in the non-SCIT group. Pooled analysis demonstrated that the SCIT group had an equivalent MS to the non-SCIT group (pooled SMD: -1.42, 95%CI: -3.20, 0.36, I2 = 96.9%, P = 0.119) (Supplementary Figure 8). Subgroup analysis based on allergen, treatment duration, and AIT protocol found significant differences in MSs between the SCIT and non-SCIT groups when the allergen was grass pollen, treatment duration was ≥ 24 or 12-23 months, or AIT protocol was continuous or pre-seasonal (all P < 0.05) (Table 1).
SMSs
Two studies (14, 54) provided data on SMSs, with 1110 patients in the SCIT group and 1108 in the non-SCIT group. Pooled analysis showed that the SCIT group had a similar SMS to the non-SCIT group (pooled SMD: -2.46, 95%CI: -5.16, 0.24, I2 = 97.2%, P = 0.074) (Table 1; Supplementary Figure 9).
New sensitizations
Information on new sensitizations was reported by 2 studies (16, 51), with 66 patients in the SCIT group and 29 in the non-SCIT group. Pooled analysis illustrated that the SCIT group had a significantly lower incidence of new sensitizations than the non-SCIT group (pooled RR: 0.62, 95%CI: 0.41, 0.91, I2 = 0.0%, P = 0.016) (Table 1; Supplementary Figure 10).
Development of asthma
One study (57) investigated the development of asthma, including 64 patients in the SCIT group and 53 in the non-SCIT group. The SCIT group had a significantly lower incidence of developing asthma than the non-SCIT group (RR: 0.55, 95%CI: 0.33, 0.93, P = 0.024).
TRAEs
TRAEs were evaluated by 2 studies (48, 61), with 1108 patients in the SCIT group and 1108 in the non-SCIT group. Pooled analysis showed that no significant difference was found in the incidence of TRAEs between the SCIT and non-SCIT groups (pooled RR: 4.54, 95%CI: 0.30, 68.28, I2 = 88.9%, P = 0.275) (Table 1; Supplementary Figure 11).
Improvement
A study (16) showed that compared with the drug only group alone, the SCIT group also showed a greater improvement in SSs (P = 0.0009), with 78.44% of patients feeling “a good deal better” or “slightly better” following SCIT treatment versus 47.06% following drug only therapy.
SLIT versus SCIT
SSs
SSs were evaluated in 3 studies (18, 63, 72), including 125 patients in the SLIT group and 207 in the SCIT group. Pooled analysis illustrated that the SLIT group had a comparable SS to the SCIT group (pooled SMD: 0.41, 95%CI: -0.46, 1.28, I2 = 89.6%, P = 0.353) (Figure 2). Subgroup analysis based on study design and treatment duration found that the SS of the SLIT group was significantly higher than that of the SCIT group when the treatment duration was ≥ 24 months (SMD: 1.12, 95%CI: 0.83, 1.40, P < 0.001) (Table 1).
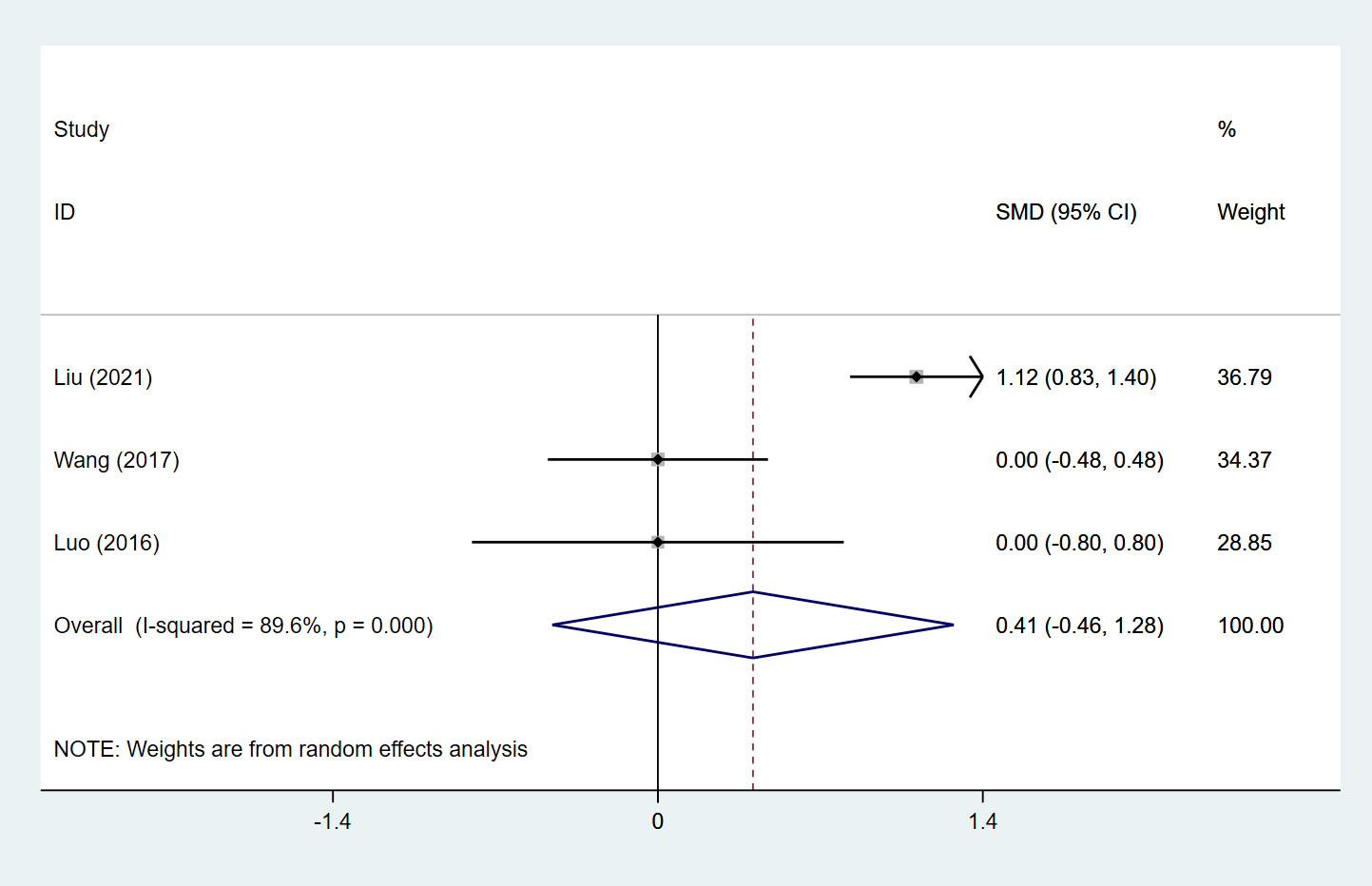
Figure 2 Forest plot for SSs in children receiving SLIT versus SCIT treatment. SLIT, sublingual immunotherapy; SCIT, subcutaneous immunotherapy; SMD, standardized mean differences; CI, confidence interval; SSs, symptom scores.
MSs
MSs were assessed in 2 studies (18, 72), including 114 patients in the SLIT group and 194 in the SCIT group. Pooled analysis demonstrated no significant difference in MSs between the SLIT and SCIT groups (pooled SMD: 0.82, 95%CI: -0.88, 2.53, I2 = 97.2%, P = 0.344) (Table 1, Figure 3).

Figure 3 Forest plot for MSs in children receiving SLIT versus SCIT treatment. SLIT, sublingual immunotherapy; SCIT, subcutaneous immunotherapy; SMD, standardized mean differences; CI, confidence interval; MSs, medication scores.
SMSs
One study (72) reported data on SMSs, with 80 patients in the SLIT group and 160 in the SCIT group. The SLIT group had a significantly higher SMS than the SCIT group (SMD: 0.88, 95%CI: 0.60, 1.16, P < 0.001).
TRAEs
One study (72) assessed TRAEs, with 80 patients in the SLIT group and 160 in the SCIT group. The incidence of TRAEs in the SLIT group was significantly lower than that in the SCIT group (RR: 0.45, 95%CI: 0.23, 0.88, P = 0.020).
Improvement
As shown by Özdoğru et al. (19), 53.3% of patients in the SCIT group and 61.9% in the SLIT group had self-reported clinical improvement.
Indirect comparison
SLIT versus non-SLIT
Compared with the non-SLIT group, the SLIT group had a significantly lower incidence of new sensitizations (pooled RR: 0.21, 95%CrI: 0.05, 0.83), and exhibited a significantly higher incidence of TRAEs (pooled RR: 3.40, 95%CrI: 2.10, 5.50) (Table 2).
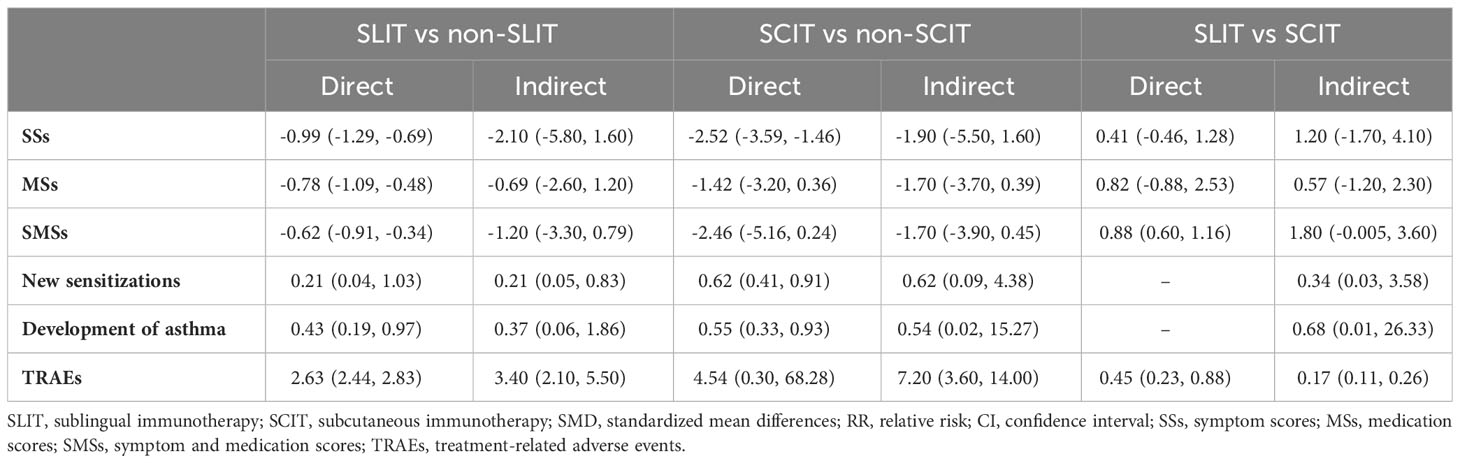
Table 2 Respective overall results of direct and indirect comparisons in different outcomes [SMD/RR (95%CI)].
SCIT versus non-SCIT
The incidence of TRAEs in the SCIT group was significantly higher than that in the non-SCIT group (pooled RR: 7.20, 95%CrI: 3.60, 14.00) (Table 2).
SLIT versus SCIT
In contrast to the SCIT group, the SLIT group illustrated a significantly lower incidence of TRAEs (pooled RR: 0.17, 95%CrI: 0.11, 0.26) (Table 2).
Source of heterogeneity and sensitivity analysis
The result of meta-regression showed that treatment duration was a source of heterogeneity in assessing SLIT versus non-SLIT for SMSs (P=0.006) (Supplementary Table S2). According to sensitivity analysis, one-study deletion did not have a significant impact on the pooled results, suggesting that the findings of this meta-analysis were stable and robust.
Discussion
To the best of our knowledge, this systematic review and meta-analysis is the first to compare SCIT and SLIT for SSs, MSs, SMSs, new sensitizations, development of asthma, improvement, and TRAEs in children with AR through both direct and indirect comparisons. It was found that SCIT and SLIT may have comparable effects on SSs, MSs, SMSs, new sensitizations, and development of asthma. For safety, patients undergoing SLIT may exhibit a significantly lower incidence of TRAEs than those undergoing SCIT. These findings indicated that considering both efficacy and safety, SLIT might be superior to SCIT in the treatment of pediatric AR. Clinicians may make AIT choices for AR in children based on the above findings.
Several studies have been conducted to systematically evaluate the efficacy of SCIT versus SLIT among patients with AR. The meta-analysis of Dretzke et al. (20) on the general population with seasonal AR obtained inconclusive results for the superiority of SCIT or SLIT over the other treatment in terms of SSs, MSs, SMSs, and quality-of-life scores. Another meta-analysis demonstrated that patients with seasonal AR to grass pollen receiving SCIT had better control of symptoms and less use of medications than those receiving SLIT (73). Tie et al. (22) compared SCIT and SLIT in adult AR patients via a meta-analysis, and reported similar effects of the two immunotherapies regarding SSs, MSs and SMSs. The current study paid attention to children with AR, and made direct and indirect comparisons between SCIT and SLIT in terms of SSs, MSs, SMSs, new sensitizations, development of asthma, improvement, and TRAEs. With head-to-head comparison, pooled analysis showed that compared with non-SLIT treatment, SLIT was more effective concerning SSs, MSs, SMSs, development of asthma, and TRAEs; AR patients receiving SCIT had lower SSs and reduced new sensitizations than those receiving non-SCIT treatment, suggesting that SCIT and SLIT were superior to non-SCIT and non-SLIT therapy for pediatric AR. In seasonal AR, SLIT was shown to relieve symptoms by 30 to 40% and prescription use (74). A previous review found that AR patients treated with SLIT had reduced symptoms and need for medications than those receiving placebo, and indicated that SLIT causes notable changes in allergen-specific IgG and IgG4 antibodies, which is consistent with clinical responses regarding SSs and MSs (75). The efficacy of SCIT among individuals with AR was identified by prior meta-analyses (76, 77). SCIT was reported to be effective in terms of SSs compared with the placebo control in seasonal AR (78). Besides, Alvaro-Lozano et al. (79) demonstrated that SCIT could lower the occurrence of new allergen sensitization in asthmatic children. SCIT and SLIT may be preferred in the treatment of pediatric AR to non-SCIT and non-SLIT treatment, while close attention should be paid to TRAEs during SCIT or SLIT.
Of the 50 included studies (14–19, 23–33, 40–72), merely 6 studies (18, 19, 61, 63, 71, 72) directly compared SLIT and SCIT, no studies directly compared the effects of SLIT and SCIT on new sensitizations and development of asthma, and only one study (72) provided the direct comparison of SLIT and SCIT for SMSs and TRAEs despite significant differences between these two treatments. Thus, indirect comparison was conducted to further assess the efficacy and safety of SLIT versus SCIT. According to direct and indirect evidence, regarding efficacy, SLIT and SCIT may display the equivalent effects on SSs, MSs, SMSs, new sensitizations, and development of asthma. Since limited studies qualified for the direct comparison of SLIT and SCIT in pediatric AR, more well-designed studies are required to directly compare the two routes of administration in the future, which may strengthen the results of this meta-analysis. Besides, two included studies provided qualitative evidence for SCIT versus SLIT in terms of SSs and MSs: Proctor et al. (71) showed that after 3 years of intervention, patients with pollen SCIT, pollen SLIT, or HDM SLIT improved their VAS scores by about 50%, with no significant difference among the three groups. The study by Yukselen et al. (61) reported similar effects of SCIT and SLIT in the reduction of rhinitis symptoms (P=0.28) or MSs related to rhinitis (P=0.18) and asthma (P=0.31). Compared with the SLIT group, only asthma symptoms were significantly reduced in the SCIT group (P=0.01). Since the inconsistent definition of improvement in the included studies, and the difficulty in pooled analysis, qualitative descriptions was made for this outcome. Relevant data under a unified definition are needed to quantitatively explore the role of these two treatments in improvement. A prior review illustrated that both SLIT and SCIT played an effective role in reducing symptoms and need for additional medication for AR patients (11). Nelson et al. (80) similarly reported comparable decreases in allergic symptoms and rescue medication intake after using SLIT tablets and SCIT for grass pollen allergies. Concerning the possible similar effectiveness of SLIT and SCIT, these two routes of therapy may function under similar mechanisms. For example, SLIT and SCIT may cause similar generation of IgG antibodies, activity of FOXP3+ CD25+ Treg cells, and allergen-specific tolerance in pediatric AR (76). Specific underlying mechanisms of SLIT and SCIT in children with AR are worth further exploration. Restoring immunological tolerance to allergens is the main objective of AIT’s mechanism of action, which has been demonstrated to entail many immunologic pathways and the interaction of the innate and adaptive immune responses (79, 81). Of note, concerning safety, a lower incidence of TRAEs were found after SLIT versus SCIT in the current analysis, indicating that SLIT appears to have a better safety profile than SCIT. Likewise, Ji et al. (82) reported that patients undergoing SLIT had fewer adverse reactions. Another study showed that adverse responses were more common with SCIT compared with SLIT (83). SCIT can cause serious adverse events and even anaphylactic shock, while SLIT can be safely self-administered, and local adverse reactions (primarily limited to oral discomfort) caused by SLIT are often mild and subside without treatment (11, 82, 84, 85).
Apart from efficacy and safety, the convenience and cost-effectiveness of these two treatment methods also need to be considered in clinical treatment choices. Subcutaneous injection (SCIT) is regarded as a time-consuming and invasive therapeutic method (86). As a self-administered alternative to SCIT, SLIT offers the advantages of AIT without the expense and inconvenience of frequent office visits or the discomfort of injections (87, 88). Meadows et al. (89) showed that both SCIT and SLIT may be cost-effective compared with symptomatic therapy after about 6 years (threshold of £20000-30000 per quality-adjusted life-year) in AR. As exhibited by previous studies, SCIT was more cost-effective than SLIT in children and adults with AR, with slightly higher patient adherence and lower pharmacological expenditures (90–92). However, Hardin et al. (93) demonstrated that in contrast to SCIT, SLIT is financially beneficial, and should be seen as an economically conscious choice for patients with >40% treatment compliance. SLIT provides the benefit over SCIT in that it does not require injections (94). In young children, injections are less acceptable (95). Due to inconsistent evidence, future investigations are necessary to compare the cost-effectiveness of SLIT and SCIT, which may depend on the local health system.
This meta-analysis comprehensively compared the efficacy and safety of SLIT and SCIT in children with AR using the direct and indirect evidence. In clinical practice, SLIT and SCIT may both be applied to manage symptoms, medication use and development of new sensitizations and asthma related to AR, while SLIT may be preferred as regards adverse events. Convenience and cost-effectiveness as well as relevant clinical experience, patient preference and adherence should also be taken into account by clinicians in the treatment of children with AR. Some limitations should be acknowledged. First, the heterogeneity of the results was high. The meta-regression found that treatment duration was a source of heterogeneity. There may be other sources of heterogeneity, such as the drugs used, dosage administered, etc., which necessitates future research to further assess the source of heterogeneity. Second, the diagnostic criteria for AR in the included studies were not entirely consistent, while most studies were based on skin prick tests or immunoglobulin E (IgE) levels for diagnosis. Due to almost not exactly the same diagnostic methods and limited data, the subgroup analysis cannot be achieved. Studies in the future should standardize the diagnostic criteria for AR to improve equivalence between patients for pooled analysis. Besides, the demographic data of the included patients were inadequate for assessing the role of demographic factors on the outcome of each treatment, indicating that future investigations should improve the reporting of demographic data to facilitate relevant assessment. Third, the lack of studies on some outcomes (e.g. new sensitizations, development of asthma) may have affected the stability of the results. Finally, there were a relatively small number of studies on direct comparison between SLIT and SCIT, and more head-to-head studies are needed in the future to enhance the reliability of the findings.
Conclusion
SCIT and SLIT may have similar effects on SSs, MSs, SMSs, new sensitizations, and development of asthma, while SLIT may be superior to SCIT in terms of TRAEs in children with AR. Considering both efficacy and safety, SLIT might be a more favorable AIT than SCIT in the treatment of pediatric AR. Future large-scale studies for head-to-head comparisons of SCIT and SLIT are warranted to verify our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JY: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1274241/full#supplementary-material
Supplementary Figure 1 | Forest plot for SSs in children receiving SLIT versus non-SLIT treatment. SLIT, sublingual immunotherapy; SMD, standardized mean differences; CI, confidence interval; SSs, symptom scores.
Supplementary Figure 2 | Forest plot for MSs in children receiving SLIT versus non-SLIT treatment. SLIT, sublingual immunotherapy; SMD, standardized mean differences; CI, confidence interval; MSs, medication scores.
Supplementary Figure 3 | Forest plot for SMSs in children receiving SLIT versus non-SLIT treatment. SLIT, sublingual immunotherapy; SMD, standardized mean differences; CI, confidence interval; SMSs, symptom and medication scores.
Supplementary Figure 4 | Forest plot for new sensitizations in children receiving SLIT versus non-SLIT treatment. SLIT, sublingual immunotherapy; SMD, standardized mean differences; CI, confidence interval.
Supplementary Figure 5 | Forest plot for development of asthma in children receiving SLIT versus non-SLIT treatment. SLIT, sublingual immunotherapy; RR, relative risk; CI, confidence interval.
Supplementary Figure 6 | Forest plot for TRAEs in children receiving SLIT versus non-SLIT treatment. SLIT, sublingual immunotherapy; RR, relative risk; CI, confidence interval; TRAEs, treatment-related adverse events.
Supplementary Figure 7 | Forest plot for SSs in children receiving SCIT versus non-SCIT treatment. SCIT, subcutaneous immunotherapy; SMD, standardized mean differences; CI, confidence interval; SSs, symptom scores.
Supplementary Figure 8 | Forest plot for MSs in children receiving SCIT versus non-SCIT treatment. SCIT, subcutaneous immunotherapy; SMD, standardized mean differences; CI, confidence interval; MSs, medication scores.
Supplementary Figure 9 | Forest plot for SMSs in children receiving SCIT versus non-SCIT treatment. SCIT, subcutaneous immunotherapy; SMD, standardized mean differences; CI, confidence interval; SMSs, symptom and medication scores.
Supplementary Figure 10 | Forest plot for new sensitizations in children receiving SCIT versus non-SCIT treatment. SCIT, subcutaneous immunotherapy; RR, relative risk; CI, confidence interval.
Supplementary Figure 11 | Forest plot for TRAEs in children receiving SCIT versus non-SCIT treatment. SCIT, subcutaneous immunotherapy; RR, relative risk; CI, confidence interval; TRAEs, treatment-related adverse events.
References
1. Meng Y, Wang C, Zhang L. Advances and novel developments in allergic rhinitis. Allergy (2020) 75(12):3069–76. doi: 10.1111/all.14586
2. Zhang Y, Lan F, Zhang L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy (2022) 77(11):3309–19. doi: 10.1111/all.15454
3. Wise SK, Damask C, Roland LT, Ebert C, Levy JM, Lin S, et al. International consensus statement on allergy and rhinology: Allergic rhinitis - 2023. Int Forum Allergy Rhinol (2023) 13(4):293–859. doi: 10.1002/alr.23090
4. Schuler Iv CF, Montejo JM. Allergic rhinitis in children and adolescents. Pediatr Clin North Am (2019) 66(5):981–93. doi: 10.1016/j.pcl.2019.06.004
5. Schuler Iv CF, Montejo JM. Allergic rhinitis in children and adolescents. Immunol Allergy Clin North Am (2021) 41(4):613–25. doi: 10.1016/j.iac.2021.07.010
6. Siddiqui ZA, Walker A, Pirwani MM, Tahiri M, Syed I. Allergic rhinitis: diagnosis and management. Br J Hosp Med (Lond) (2022) 83(2):1–9. doi: 10.12968/hmed.2021.0570
7. Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, et al. Allergic rhinitis. Nat Rev Dis Primers (2020) 6(1):95. doi: 10.1038/s41572-020-00227-0
9. Linton S, Burrows AG, Hossenbaccus L, Ellis AK. Future of allergic rhinitis management. Ann Allergy Asthma Immunol (2021) 127(2):183–90. doi: 10.1016/j.anai.2021.04.029
10. Field K, Blaiss MS. Sublingual versus subcutaneous immunotherapy for allergic rhinitis: what are the important therapeutic and real-world considerations? Curr Allergy Asthma Rep (2020) 20(9):45. doi: 10.1007/s11882-020-00934-4
11. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol (2016) 137(2):339–349.e310. doi: 10.1016/j.jaci.2015.12.1298
12. Kakli HA, Riley TD. Allergic rhinitis. Prim Care (2016) 43(3):465–75. doi: 10.1016/j.pop.2016.04.009
13. Drazdauskaitė G, Layhadi JA, Shamji MH. Mechanisms of allergen immunotherapy in allergic rhinitis. Curr Allergy Asthma Rep (2020) 21(1):2. doi: 10.1007/s11882-020-00977-7
14. Endaryanto A, Nugraha RA. Indonesia-based study of the clinical and cost-saving benefits of subcutaneous allergen immunotherapy for children with allergic rhinitis in private practice. Cells (2021) 10(7):1841. doi: 10.3390/cells10071841
15. Wang J, Qiu L, Chen Y, Chen M. Sublingual immunotherapy increases Treg/Th17 ratio in allergic rhinitis. Open Med (Wars) (2021) 16(1):826–32. doi: 10.1515/med-2021-0285
16. Kim CK, Callaway Z, Park JS, Kwon E. Efficacy of subcutaneous immunotherapy for patients with asthma and allergic rhinitis in Korea: effect on eosinophilic inflammation. Asia Pac Allergy (2021) 11(4):e43. doi: 10.5415/apallergy.2021.11.e43
17. Wang X, Shen Y, Hong S, Kang H, Ke X. Changes in type 2 innate lymphoid cells and serum cytokines in sublingual immunotherapy in pediatric patients with allergic rhinitis. BMC Pediatr (2023) 23(1):13. doi: 10.1186/s12887-022-03788-z
18. Wang ZX, Shi H. Single-allergen sublingual immunotherapy versus multi-allergen subcutaneous immunotherapy for children with allergic rhinitis. J Huazhong Univ Sci Technolog Med Sci (2017) 37(3):407–11. doi: 10.1007/s11596-017-1748-2
19. Özdoğru EE, Sancakli Ö, Tuncel T. Evaluation of children with allergic rhinitis and asthma who have completed allergen immunotherapy: 19 years of real-life data, single-center study. Asthma Allergy Immunol (2022) 20(1):48–54. doi: 10.21911/AAI.670
20. Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol (2013) 131(5):1361–6. doi: 10.1016/j.jaci.2013.02.013
21. Kim JY, Jang MJ, Kim DY, Park SW, Han DH. Efficacy of subcutaneous and sublingual immunotherapy for house dust mite allergy: A network meta-analysis-based comparison. J Allergy Clin Immunol Pract (2021) 9(12):4450–4458.e4456. doi: 10.1016/j.jaip.2021.08.018
22. Tie K, Miller C, Zanation AM. Subcutaneous versus sublingual immunotherapy for adults with allergic rhinitis: A systematic review with meta-analyses. Laryngoscope (2022) 132(3):499–508. doi: 10.1002/lary.29586
23. Liu J, Hu M, Tao X, He J, Wang J, Song Z, et al. Salivary igG4 levels contribute to assessing the efficacy of dermatophagoides pteronyssinus subcutaneous immunotherapy in children with asthma or allergic rhinitis. J Clin Med (2023) 12(4):1665. doi: 10.3390/jcm12041665
24. Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol (2009) 123(1):160–166.e163. doi: 10.1016/j.jaci.2008.10.009
25. Chen WB, Shen XF, Li Q, Zhou WC, Cheng L. Efficacy of a 3-year course of sublingual immunotherapy for mite-induced allergic rhinitis with a 3-year follow-up. Immunotherapy (2020) 12(12):891–901. doi: 10.2217/imt-2020-0006
26. Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sørensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol (2018) 141(2):529–538.e513. doi: 10.1016/j.jaci.2017.06.014
27. Nolte H, Bernstein DI, Nelson HS, Ellis AK, Kleine-Tebbe J, Lu S. Efficacy and safety of ragweed SLIT-tablet in children with allergic rhinoconjunctivitis in a randomized, placebo-controlled trial. J Allergy Clin Immunol Pract (2020) 8(7):2322–2331.e2325. doi: 10.1016/j.jaip.2020.03.041
28. Stelmach I, Kaluzinska-Parzyszek I, Jerzynska J, Stelmach P, Stelmach W, Majak P. Comparative effect of pre-coseasonal and continuous grass sublingual immunotherapy in children. Allergy (2012) 67(3):312–20. doi: 10.1111/j.1398-9995.2011.02758.x
29. Wahn U, Klimek L, Ploszczuk A, Adelt T, Sandner B, Trebas-Pietras E, et al. High-dose sublingual immunotherapy with single-dose aqueous grass pollen extract in children is effective and safe: a double-blind, placebo-controlled study. J Allergy Clin Immunol (2012) 130(4):886–893.e885. doi: 10.1016/j.jaci.2012.06.047
30. Yu WB, Mao LF, Pan QC, He T, Yu M. Efficacy of sublingual administration of dermatophagoides farinae drops for treatment of pediatric allergic rhinitis accompanied by adenoid hypertrophy and improvement of immune function. Med Sci Monitor (2019) 25:333–40. doi: 10.12659/msm.911982
31. Okamoto Y, Fujieda S, Okano M, Hida H, Kakudo S, Masuyama K. Efficacy of house dust mite sublingual tablet in the treatment of allergic rhinoconjunctivitis: A randomized trial in a pediatric population. Pediatr Allergy Immunol (2019) 30(1):66–73. doi: 10.1111/pai.12984
32. Yonekura S, Okamoto Y, Sakurai D, Horiguchi S, Hanazawa T, Nakano A, et al. Sublingual immunotherapy with house dust extract for house dust-mite allergic rhinitis in children. Allergol Int (2010) 59(4):381–8. doi: 10.2332/allergolint.10-OA-0200
33. de Bot CM, Moed H, Berger MY, Röder E, Hop WC, de Groot H, et al. Sublingual immunotherapy not effective in house dust mite-allergic children in primary care. Pediatr Allergy Immunol (2012) 23(2):150–8. doi: 10.1111/j.1399-3038.2011.01219.x
34. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
35. Wells GA, Shea BJ, O'Connell D, Peterson J, Tugwell P. The newcastle–ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. (2000).
36. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry (2020) 81(5):20f13681. doi: 10.4088/JCP.20f13681
37. Jansen JP, Trikalinos T, Cappelleri JC, Daw J, Andes S, Eldessouki R, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health (2014) 17(2):157–73. doi: 10.1016/j.jval.2014.01.004
38. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health (2011) 14(4):429–37. doi: 10.1016/j.jval.2011.01.011
39. Tian JH, Li L, Zhao Y, Ge L. Writing and reporting of network meta-analysis. Chin J Drug Eval (2013) 30(6):4. doi: 10.3969/j.issn.2095-3593.2013.06.001
40. Cengizlier R, Saraclar Y, Tomac N. Evaluation of immunotherapy by nasal antigen challenge. J Otolaryngol (1999) 28(4):185–8.
41. Acquistapace F, Agostinis F, Castella V, Kantar A, Novembre E, Perrone MR, et al. Efficacy of sublingual specific immunotherapy in intermittent and persistent allergic rhinitis in children: an observational case-control study on 171 patients. The EFESO-children multicenter trial. Pediatr Allergy Immunol (2009) 20(7):660–4. doi: 10.1111/j.1399-3038.2009.00860.x
42. Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol (2009) 123(1):167–173.e167. doi: 10.1016/j.jaci.2008.10.044
43. Blaiss M, Maloney J, Nolte H, Gawchik S, Yao R, Skoner DP. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol (2011) 127(1):64–71, 71.e61-64. doi: 10.1016/j.jaci.2010.11.034
44. Aydogan M, Eifan AO, Keles S, Akkoc T, Nursoy MA, Bahceciler NN, et al. Sublingual immunotherapy in children with allergic rhinoconjunctivitis mono-sensitized to house-dust-mites: a double-blind-placebo-controlled randomised trial. Respir Med (2013) 107(9):1322–9. doi: 10.1016/j.rmed.2013.06.021
45. Chen Y, Zhou L, Yang Y. Effect of sublingual immunotherapy on platelet activity in children with allergic rhinitis. Braz J Otorhinolaryngol (2017) 83(2):190–4. doi: 10.1016/j.bjorl.2016.03.006
46. Biedermann T, Kuna P, Panzner P, Valovirta E, Andersson M, de Blay F, et al. The SQ tree SLIT-tablet is highly effective and well tolerated: Results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol (2019) 143(3):1058–1066.e6. doi: 10.1016/j.jaci.2018.12.1001
47. Demoly P, Corren J, Creticos P, De Blay F, Gevaert P, Hellings P, et al. A 300 IR sublingual tablet is an effective, safe treatment for house dust mite-induced allergic rhinitis: An international, double-blind, placebo-controlled, randomized phase III clinical trial. J Allergy Clin Immunol (2021) 147(3):1020–1030.e10. doi: 10.1016/j.jaci.2020.07.036
48. Endaryanto A, Nugraha RA. Safety Profile and issues ofsubcutaneous immunotherapy in the treatment of children with allergic rhinitis. Cells (2022) 11(9):1584. doi: 10.3390/cells11091584
49. Vourdas D, Syrigou E, Potamianou P, Carat F, Batard T, André C, et al. Double-blind, placebo-controlled evaluation of sublingual immunotherapy with standardized olive pollen extract in pediatric patients with allergic rhinoconjunctivitis and mild asthma due to olive pollen sensitization. Allergy (1998) 53(7):662–72. doi: 10.1111/j.1398-9995.1998.tb03952.x
50. La Rosa M, Ranno C, André C, Carat F, Tosca MA, Canonica GW. Double-blind placebo-controlled evaluation of sublingual-swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol (1999) 104(2 Pt 1):425–32. doi: 10.1016/s0091-6749(99)70388-x
51. Eng PA, Reinhold M, Gnehm HE. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy: Eur J Allergy Clin Immunol (2002) 57(4):306–12. doi: 10.1034/j.1398-9995.2002.1o3264.x
52. Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol (2004) 114(4):851–7. doi: 10.1016/j.jaci.2004.07.012
53. Rolinck-Werninghaus C, Wolf H, Liebke C, Baars JC, Lange J, Kopp MV, et al. A prospective, randomized, double-blind, placebo-controlled multi-centre study on the efficacy and safety of sublingual immunotherapy (SLIT) in children with seasonal allergic rhinoconjunctivitis to grass pollen. Allergy (2004) 59(12):1285–93. doi: 10.1111/j.1398-9995.2004.00627.x
54. Eng PA, Borer-Reinhold M, Heijnen I, Gnehm HPE. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy (2006) 61(2):198–201. doi: 10.1111/j.1398-9995.2006.01011.x
55. Valovirta E, Jacobsen L, Ljorring C, Koivikko A, Savolainen J. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy (2006) 61(10):1177–83. doi: 10.1111/j.1398-9995.2006.01190.x
56. Ibañez MD, Kaiser F, Knecht R, Armentia A, Schöpfer H, Tholstrup B, et al. Safety of specific sublingual immunotherapy with SQ standardized grass allergen tablets in children. Pediatr Allergy Immunol (2007) 18(6):516–22. doi: 10.1111/j.1399-3038.2007.00556.x
57. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy (2007) 62(8):943–8. doi: 10.1111/j.1398-9995.2007.01451.x
58. Röder E, Berger MY, Hop WC, Bernsen RM, de Groot H, Gerth van Wijk R. Sublingual immunotherapy with grass pollen is not effective in symptomatic youngsters in primary care. J Allergy Clin Immunol (2007) 119(4):892–8. doi: 10.1016/j.jaci.2006.12.651
59. Marogna M, Tomassetti D, Bernasconi A, Colombo F, Massolo A, Businco AD, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol (2008) 101(2):206–11. doi: 10.1016/s1081-1206(10)60211-6
60. Tseng SH, Fu LS, Nong BR, Weng JD, Shyur SD. Changes in serum specific IgG4 and IgG4/ IgE ratio in mite-sensitized Taiwanese children with allergic rhinitis receiving short-term sublingual-swallow immunotherapy: a multicenter, randomized, placebo-controlled trial. Asian Pac J Allergy Immunol (2008) 26(2-3):105–12.
61. Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol (2012) 157(3):288–98. doi: 10.1159/000327566
62. Shao J, Cui YX, Zheng YF, Peng HF, Zheng ZL, Chen JY, et al. Efficacy and safety of sublingual immunotherapy in children aged 3-13 years with allergic rhinitis. Am J Rhinol Allergy (2014) 28(2):131–9. doi: 10.2500/ajra.2014.28.4006
63. Luo X, Hong H, Tang J, Wu X, Lin Z, Ma R, et al. Increased expression of miR-146a in children with allergic rhinitis after allergen-specific immunotherapy. Allergy Asthma Immunol Res (2016) 8(2):132–40. doi: 10.4168/aair.2016.8.2.132
64. Maloney J, Prenner BM, Bernstein DI, Lu S, Gawchik S, Berman G, et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol (2016) 116(1):59–65. doi: 10.1016/j.anai.2015.10.024
65. Wang C, Wang K, Liu S, Qin X, Chen K, Zhang T. Decreased level of osteopontin in children with allergic rhinitis during sublingual immunotherapy. Int J Pediatr Otorhinolaryngol (2016) 81:15–20. doi: 10.1016/j.ijporl.2015.12.001
66. Yin GQ, Jiang WH, Wu PQ, He CH, Chen RS, Deng L. Clinical evaluation of sublingual administration of dust mite drops in the treatment of allergic asthma and allergic rhinitis of children. Eur Rev Med Pharmacol Sci (2016) 20(20):4348–53.
67. Lim JH, Kim JY, Han DH, Lee CH, Hong SN, Wee JH, et al. Sublingual immunotherapy (SLIT) for house dust mites does not prevent new allergen sensitization and bronchial hyper-responsiveness in allergic rhinitis children. PloS One (2017) 12(8):e0182295. doi: 10.1371/journal.pone.0182295
68. Matsuoka T, Bernstein DI, Masuyama K, Nolte H, Okamiya K, Seitzberg D, et al. Pooled efficacy and safety data for house dust mite sublingual immunotherapy tablets in adolescents. Pediatr Allergy Immunol (2017) 28(7):661–7. doi: 10.1111/pai.12747
69. Masuyama K, Okamoto Y, Okamiya K, Azuma R, Fujinami T, Riis B, et al. Efficacy and safety of SQ house dust mite sublingual immunotherapy-tablet in Japanese children. Allergy (2018) 73(12):2352–63. doi: 10.1111/all.13544
70. Song Y, Long J, Wang T, Xie J, Wang M, Tan G. Long-term efficacy of standardised specific subcutaneous immunotherapy in children with persistent allergic rhinitis due to multiple allergens including house dust mites. J Laryngol Otol (2018) 132(3):230–5. doi: 10.1017/s0022215117002547
71. Proctor T, Morrough E, Fenske O, Allatt S, Hughes SM, Sharma V, et al. Impact on quality of life and safety of sublingual and subcutaneous immunotherapy in children with severe house dust mite and pollen-associated allergic rhinoconjunctivitis. Clin Transl Allergy (2020) 10:10. doi: 10.1186/s13601-020-00315-0
72. Liu W, Zeng Q, He C, Chen R, Tang Y, Yan S, et al. Compliance, efficacy, and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr Allergy Immunol (2021) 32(1):86–91. doi: 10.1111/pai.13332
73. Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol (2012) 130(5):1097–1107.e1092. doi: 10.1016/j.jaci.2012.08.012
74. Alamri RA, Aljabri GH, Tahlawi R, Aljabri HA. Immunotherapy in the treatment of allergic rhinitis in children. Cureus (2022) 14(12):e32464. doi: 10.7759/cureus.32464
75. Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev (2010) 2010(12):Cd002893. doi: 10.1002/14651858.CD002893.pub2
76. Viswanathan RK, Busse WW. Allergen immunotherapy in allergic respiratory diseases: from mechanisms to meta-analyses. Chest (2012) 141(5):1303–14. doi: 10.1378/chest.11-2800
77. Dhami S, Kakourou A, ASamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy (2017) 72(12):1825–48. doi: 10.1111/all.13208
78. Frew AJ, Powell RJ, Corrigan CJ, Durham SR. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol (2006) 117(2):319–25. doi: 10.1016/j.jaci.2005.11.014
79. Alvaro-Lozano M, Akdis CA, Akdis M, Alviani C, Angier E, Arasi S, et al. EAACI allergen immunotherapy user’s guide. Pediatr Allergy Immunol (2020) 31 Suppl 25(Suppl 25):1–101. doi: 10.1111/pai.13189
80. Nelson H, Cartier S, Allen-Ramey F, Lawton S, Calderon MA. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract (2015) 3(2):256–266.e253. doi: 10.1016/j.jaip.2014.09.018
81. Shamji MH, Sharif H, Layhadi JA, Zhu R, Kishore U, Renz H. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J Allergy Clin Immunol (2022) 149(3):791–801. doi: 10.1016/j.jaci.2022.01.016
82. Ji Z, Jiang F. Efficacy and safety of sublingual immunotherapy for allergic rhinitis: A network meta-analysis. Front Immunol (2023) 14:1144816. doi: 10.3389/fimmu.2023.1144816
83. Ji DX, Tan JR, Yu HW. Efficacy, safety and compliance of immunotherapy in the treatment of allergic rhinitis: a Meta-analysis. Chin J otorhinolaryngology Head Neck Surg (2019) 54(12):894–901. doi: 10.3760/cma.j.issn.1673-0860.2019.12.003
84. Passalacqua G, Garelli V, Sclifò F, Canonica GW. Sublingual immunotherapy for allergic rhinitis and conjunctivitis. Immunotherapy (2013) 5(3):257–64. doi: 10.2217/imt.12.157
85. Roux M, Devillier P, Yang WH, Montagut A, Abiteboul K, Viatte A, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts: Results of a dose-ranging study in an environmental exposure chamber. J Allergy Clin Immunol (2016) 138(2):451–8. doi: 10.1016/j.jaci.2016.03.039
86. Meteran H, Backer V. SQ house dust mite sublingual immunotherapy for the treatment of adults with house dust mite-induced allergic rhinitis. Expert Rev Clin Immunol (2019) 15(11):1127–33. doi: 10.1080/1744666X.2020.1676731
87. Brunton S, Nelson HS, Bernstein DI, Lawton S, Lu S, Nolte H. Sublingual immunotherapy tablets as a disease-modifying add-on treatment option to pharmacotherapy for allergic rhinitis and asthma. Postgrad Med (2017) 129(6):581–9. doi: 10.1080/00325481.2017.1308208
88. Waserman S, Shah A, Avilla E. Recent development on the use of sublingual immunotherapy tablets for allergic rhinitis. Ann Allergy Asthma Immunol (2021) 127(2):165–75. doi: 10.1016/j.anai.2021.05.020
89. Meadows A, Kaambwa B, Novielli N, Huissoon A, Fry-Smith A, Meads C, et al. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess (2013) 17(27):vi–322. doi: 10.3310/hta17270
90. Brüggenjürgen B, Reinhold T. Cost-effectiveness of grass pollen subcutaneous immunotherapy (SCIT) compared to sublingual immunotherapy (SLIT) and symptomatic treatment in Austria, Spain, and Switzerland. J Med Econ (2018) 21(4):374–81. doi: 10.1080/13696998.2017.1419959
91. Farraia M, Paciência I, Castro Mendes F, Cavaleiro Rufo J, H Shamji M, Agache I, et al. Cost-effectiveness analysis of house dust mite allergen immunotherapy in children with allergic asthma. Allergy (2022) 77(9):2688–98. doi: 10.1111/all.15321
92. Farraia M, Paciência I, Castro Mendes F, Cavaleiro Rufo J, Delgado L, Moreira A. Cost-effectiveness analysis of grass pollen specific immunotherapy in children with allergic rhinitis compared to the standard of care symptomatic treatment in Portugal. Eur Ann Allergy Clin Immunol (2023) 55(5):212–28. doi: 10.23822/EurAnnACI.1764-1489.240
93. Hardin FM, Eskander PN, Franzese C. Cost-effective analysis of subcutaneous vs sublingual immunotherapy from the payor’s perspective. OTO Open (2021) 5(4):2473974X211052955. doi: 10.1177/2473974X211052955
94. Lombardi C, Melli V, Incorvaia C, Ridolo E. Pharmacoeconomics of sublingual immunotherapy with the 5-grass pollen tablets for seasonal allergic rhinitis. Clin Mol Allergy (2017) 15:5. doi: 10.1186/s12948-017-0058-3
Keywords: subcutaneous immunotherapy, sublingual immunotherapy, allergic rhinitis, efficacy, safety, meta-analysis
Citation: Yang J and Lei S (2023) Efficacy and safety of sublingual versus subcutaneous immunotherapy in children with allergic rhinitis: a systematic review and meta-analysis. Front. Immunol. 14:1274241. doi: 10.3389/fimmu.2023.1274241
Received: 08 August 2023; Accepted: 17 November 2023;
Published: 15 December 2023.
Edited by:
Md Asiful Islam, University of Birmingham, United KingdomReviewed by:
Jorge Agustin Luna-Pech, Universidad de Guadalajara, MexicoJeyasakthy Saniasiaya, University of Malaya, Malaysia
Lu Tan, Renmin Hospital of Wuhan University, China
Copyright © 2023 Yang and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sihong Lei, bGxzaWhvbmdAMTYzLmNvbQ==
 Jiumei Yang
Jiumei Yang Sihong Lei
Sihong Lei