- 1Leprosy Laboratory, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro, Brazil
- 2Department of Neurology, Antonio Pedro University Hospital/Fluminense Federal University, Niteroi, Brazil
- 3Post-Graduate Program in Neurology, Federal University of the State of Rio de Janeiro, Rio de Janeiro, Rio de Janeiro, Brazil
- 4Department of Neurology, Pedro Ernesto University Hospital/Rio de Janeiro State University, Rio de Janeiro, Rio de Janeiro, Brazil
Introduction: Pure Neural Leprosy (PNL) is a form of this long time known disease that affects only the peripheral nervous system. Since it is a rare form of the disease, its pathophisiology is still poorly understood.
Objective: Describe the cytokines profile in patients with PNL.
Methods: 30 Patients diagnosed with PNL in the Souza Araujo Outpatient Clinic and with cytokines evaluated were selected. They were evaluated by neurologists and diagnosed after a nerve biopsy. Serum levels of IL-1 β, IL-6, IL-10, IL-17, TNF, CCL-2/MCP-1, IFN-ϒ, CXCL-10/IP-10 and TGF-β were evaluates at the moment of the diagnosis.
Results: Neural thickening was a common clinical finding in this groups of patients. Small and medium sensitive fibers signs and symptoms were present in 92% of the patients and motor involvement in 53%. 43% of patients presented neuropathic pain and no one had neuritis TGF-beta, IL-17, CCl-2 and IP-10. CCL-2 levels were associated with demyelinating patters and IP-10 and IL-1o were associated with axonal patterns at NCS.
Discussion: PNL patients’ cytokine profile appears to be different of other clinical forms of leprosy, with the presence of cytokines described in both tuberculoid and lepromatous leprosy. High levels of CCl-2 may be related to the presence of silent neuritis as well as the presence of IL-10. PNL is unique a form of leprosy, therefore, understanding its immunological profiles essential to better understand the disease itself.
1 Introduction
Leprosy is an infectious disease that affects both the skin and peripheral nerves (1). Mycobacterium leprae, an intracellular pathogen that infects macrophages and Schwann cells, is the causative agent. Most of the disability related to the disease can be attributed to neuropathy (1–4).
The interaction between the innate and adaptive immune response, environmental factors, and genetic predisposition is responsible for the diverse clinical presentations of leprosy (5, 6). In terms of the host immune response, a strong or diminished cell-mediated immune response to the bacilli may occur, giving rise to the tuberculoid and lepromatous forms of the disease, respectively (7). Borderline groups (borderline tuberculoid, borderline borderline, and borderline lepromatous) lie between the two aforementioned polar groups (7, 8).
The borderline forms are considered immunologically unstable forms of the disease and patients with these forms are more susceptible to leprosy reactions (6). Type 1 leprosy reactions are determined by a cell-mediated immune response and clinically characterized by an inflammatory response in the skin or nerve. Type 2 leprosy reactions are mediated by antibody or immune complexes and clinically characterized by painful nodes and systemic symptoms. Neural damage and neuritis may occur in both types of leprosy reactions (6, 9). It is still unknown whether the damage to the peripheral nerve is caused by the M. leprae itself or the inflammatory response that it causes (10). It has been suggested that both mechanisms play a role in the clinical spectrum of the disease (11).
Cytokines are small glycoproteins that mediate the communication between cells of the immune system and play important roles in the immunopathology of leprosy (7).The cytokine profiles of the polar forms are distinct, with a predominance of Th1 cytokines in the tuberculoid pole and Th2 cytokines in the lepromatous pole (2, 12, 13).
The pure neural leprosy (PNL) is a rare form of the disease, where only neurological deficits manifest, without skin lesions (1, 14). PNL pathophysiology is still poorly understood (1, 9, 15). The objective of this study is to describe the cytokine profile found in patients with PNL.
2 Methods
The study was conducted in the Souza Araújo Outpatient Clinic and was previously approved by the Research Ethics Committee of the institution. Thirty patients with PNL, as diagnosed by nerve biopsy, and for which the cytokine profile had been evaluated at the moment of diagnosis, were selected and included in the study.
All of the patients were first evaluated by dermatologists who excluded the presence of skin lesions. The patients were then referred to a neurologist and submitted to a neurological examination focused on the peripheral nerve system, as well as nerve conduction studies (NCS) to confirm the presence of peripheral neuropathy. The neurological examination and NCS were conducted as described by Vital et al. (2012) (16). All of the selected patients has multiplex mononeuropathy and were evaluated by neurologists with experience in peripheral neuropathy and screened for other causes of multiplex mononeuropathy. The patient was considered to have neuritis when presented with acute nerve function impairment associated with demyelinating features in the NCS. Patients were considered to have neuropathic pain when presented with pain in the territory of a clinically and neurophisiological affected nerve without nerve tenderness and demyelinating features at NCS.
If leprosy persisted as a differential diagnosis, the patients were submitted to a sensory nerve biopsy to confirm the diagnosis. The diagnostic criteria and methodology used for histopathological analysis were those described by Antunes et al. (2012) (15). All of the selected patients had PNL diagnosis confirmed by the sensory nerve biopsy.
Serum cytokine levels were assessed in samples collected at the moment of the diagnosis and stored at -70°C and evaluated by ELISA as described by Ansgt et al. (2020) (17).
Statistical analysis was performed using the Mann–Whitney test with a significance level of 5%. The Statistical Packagefor the Social Sciences (SPSS) v16.0 for Windows was used. The GraphPad Prism 9.0 were used to design the figures.
3 Results
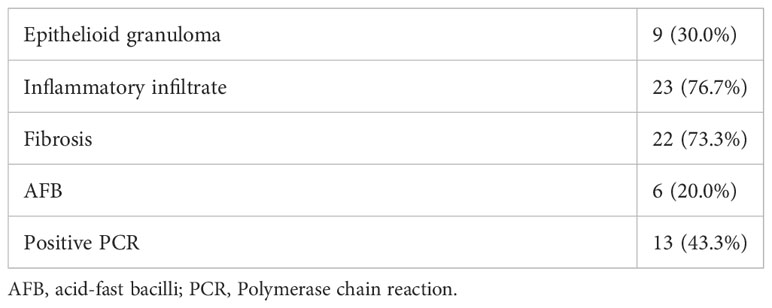
Thirty patients were included in the study, 15 were male and 15 were female. The mean age was 46 years. Two patients (6.7%) had leprosy reactions represented by acute neuritis and 13 patients (43.3%) had neuropathic pain at diagnosis. Upon neurological evaluation, 21 patients (70.0%) had neural thickening. The results of the neurological examinations, NCS, and histopathological findings are given in Tables 1 and 2.
The cytokine levels in the serum were evaluated and the medium values of IL-1β and IL-17 were associated with neurophatic pain. The results are shown in Figure 1.
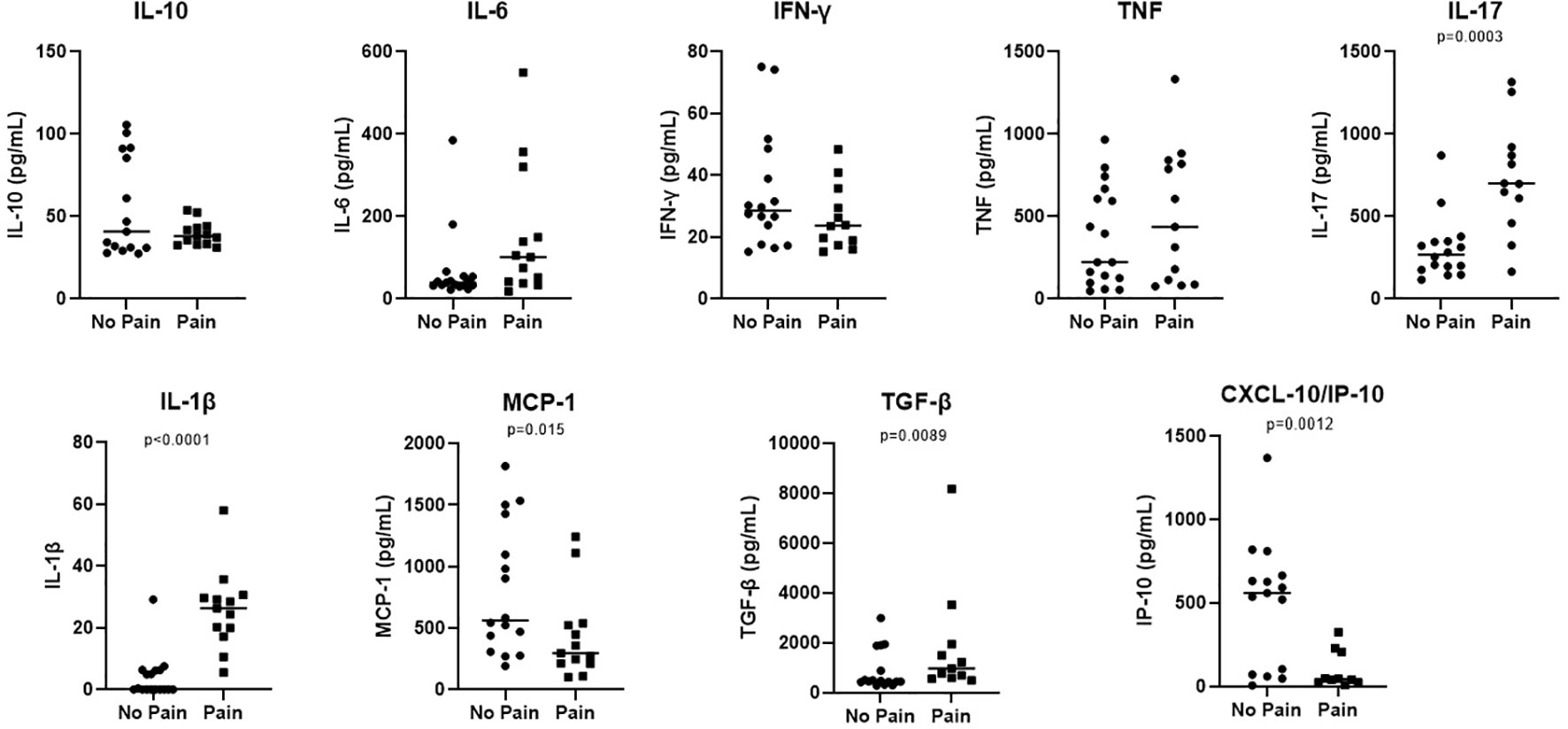
Figure 1 Increased levels of IL-1β and IL-17 are associated with Pain in patients with neural pure form of the disease. Samples from neural pure patients were according the presence or not of pain. Serum levels of IL-10, IL-1β, IL-6, TNF, IL-17, MCP-1, IFN-ϒ, IP-10 and, TGF-β were evaluated by ELISA. The results were showed in a Scatter plot with the median of each group. Significance of the difference between the two groups evaluated were considered when p < 0.05.
Statistically significant correlations between the serum cytokine levels and the clinical, histopathological, or NCS findings are given in Table 3.
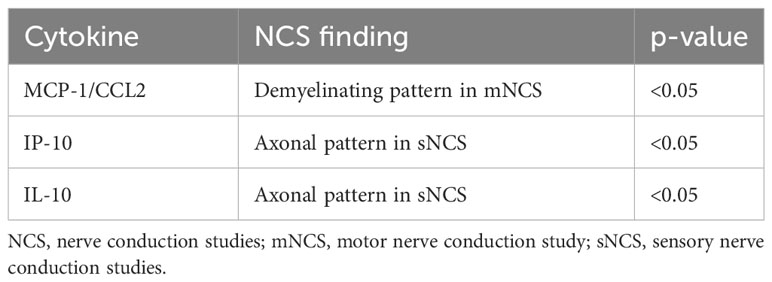
Table 3 Statistically significant differences between the serum cytokine levels and the clinical, histopathological, or nerve conduction study findings of patients with pure neural leprosy.
4 Discussion
PNL is still a poorly understood form of leprosy (9). Cytokines are produced as a response of the immune system to antigens (7) and, although the cytokine profile has been investigated for other forms of leprosy, few studies have evaluated cytokine levels in patients with PNL.
It has been hypothesized that the different forms of leprosy mainly arise owing to factors of the host rather than to variations of the M. leprae (2). The classical classification of leprosy was proposed by Ridley and Jopling in 1966 and continues to be used nowadays. It proposes a spectrum with two poles, tuberculoid and lepromatous, and a borderline range in between (8). The cytokine profile is different between the two poles of the disease, with higher levels of IL-7 and IL-15 in the tuberculoid pole and higher IL-4, IL-5, and IL-10 levels in the lepromatous pole (2). Furthermore, high levels of IL-17 have been found in patients of the tuberculoid pole and with leprosy reactions (2). Our patients had high serum levels of both IL-17, which has been related to limited leprosy, and TGF-β, which has been related to disseminated leprosy (2). This indicates that PNL could be a unique form of the disease. As PNL does not fit any of the Ridley–Jopling classifications, it is likely that the cytokine profile of patients with this form may also differ from those with the other clinical forms.
Monocyte chemoattractant protein-1 (MCP-1/CCL2) is a chemokine that regulates the migration and infiltration of macrophages (18). Macrophages are considered one of the major sources of MCP-1/CCL2. Demyelinating patterns in NCS can be suggestive of acute neuritis as well as the beginning of the infection (19).MCP-1/CCL2 was found in high levels in patients with PNL and was also significantly associated with demyelinating patterns in NCS. Most of the patients of our cohort did not have clinical neuritis at diagnosis. Furthermore, silent neuritis has been proposed to play an important role in PNL (9). These data suggest that the function of macrophages may be associated with the beginning of the infection and clinical or silent neuritis.
It has been proposed that M. leprae induces Schwann cell death by a pathway that involves proinflammatory cytokines, and TNF-α has already been identified in the nerve biopsies of patients with neuritis (10). TNF-α has been related to demyelination and axonal degeneration as well as immune cell recruitment to the injury site and, in leprosy, it has been detected in reactional states (3). Teles et al. (2007) reported that the levels of TNF-α were higher in patients with PNL especially when AFB were found in the nerve biopsy (20). Although the patients of our cohort did not have clinical neuritis and most of them did not have reactional episodes, the presence of TNF-α in the serum may suggest that it plays a role in the pathogenesis of leprosy neuropathy.
IP-10/CXCL10 has been linked to inflammatory processes, including viral and bacterial infectious diseases (21). Levels of this cytokine were significantly associated with axonal damage in the present study, suggesting that an inflammatory process is involved in the nerve damage observed in PNL. In our sample, levels of this cytokine were also associated with neural pain, which may be a result of the nerve damage.
IL-10 is considered an anti-inflammatory mediator (22) and, in the present study, levels of this cytokine were significantly associated with axonal loss. IL-10 has also been described in the lepromatous pole of leprosy (2). This suggests that patients with PNL these patients may have a form of the disease related to the multibacillary forms, when silent neuritis plays an important role (23).
Cytokines are considered important components of leprosy reactions (7). Type 1 leprosy reactions are characterized by an exacerbated cellular immune response, which can lead to neural damage. In these episodes, high levels of TNF-α, IFN-γ, IL-12, IL-1β, and IL-2 occur (2). Patients with PNL are considered to have fewer reactional episodes when compared to those with other clinical forms of leprosy (9). Only two patients in our group had leprosy reactions at diagnosis, which could explain the lower levels of these cytokines.
Type 2 leprosy reactions are characterized by a systemic process related to immune complexes and high levels of TNF-α, IL-2, IL-4, IL-5, IL-6, and IL-10 (2).Type 2 leprosy reactionsare even more rare that type 1 leprosy reaction in patients with PNL (9)and this may be related to the lower levels of IL-10 found in our sample, suggesting that the immunological profile of patients with PNL is different to that usually found in patients with type 2 leprosy reactions.
Proinflammatory cytokines such as TNF-α and IL-1β can directly stimulate and sensitize Aδ fibers and C-type fibers (17). The spontaneous discharge of these fibers has already been related to neuropathic pain (24). The majority of our cohort did not have neural pain at diagnosis, which could explain the lower levels of these cytokines when compared to others.
Cytokines are produced as a response of the immune system to antigens (7) In PNL the M. leprae antigens are confined within the Schwann cell in the peripheral nerve and this may explain why the cytokines usually related to leprosy and leprosy reactions are not found in high levels in the serum of these patients. Furthermore, patients with PNL may present with a wide variety of clinical phenotypes. As the cytokine profile appears to be different from other clinical forms of leprosy, this may suggest that PNL is a different form of the disease that cannot be classified in the classical Ridley–Jopling classification system.
This is one of the first studies to address the cytokine profile in patients with PNL. Although the number of patients of the sample is a limitation of the present study, we believe that the results could provide new information and raise new questions about the immunological pattern and pathophysiology of PNL.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Fundação Oswaldo Cruz Ethics in Research Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IP: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. DA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. RP: Conceptualization, Investigation, Writing – review & editing. JV: Data curation, Investigation, Writing – review & editing. CS: Investigation, Writing – review & editing. LA: Investigation, Writing – review & editing. LC: Investigation, Writing – review & editing. MH: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. ES: Conceptualization, Investigation, Writing – review & editing. MJ: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Our gratitude goes to the team of neurologists, dermatologists, nurses and technicians at the Fiocruz Outpatient Clinic and the Leprosy Laboratory that collaborate to this study. We would also like to thank Amy Goundry for editing the English version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jardim MR, Antunes SLG, Santos AR, Nascimento OJM, Nery JAC, Sales AM, et al. Criteria for diagnosis of pure neural leprosy. J Neurol (2003) 250(7):806–9. doi: 10.1007/s00415-003-1081-5
2. Froes LAR, Trindade MAB, Sotto MN. Immunology of leprosy. Int Rev Immunol (2020) 41(2):72–83. doi: 10.1080/08830185.2020.1851370
3. Andrade PR, Jardim MR, Da Silva ACC, Manhaes PS, Antunes SLG, Vital R, et al. Inflammatory cytokines are involved in focal demyelination in leprosy neuritis. J Neuropathol Exp Neurol (2016) 75(3):272–83. doi: 10.1093/jnen/nlv027
4. Pitta IJR, Hacker MAV, Andrade LR, Spitz CN, Vital RT, Sales AM, et al. Follow-up assessment of patients with Pure Neural Leprosy in a reference center in Rio de Janeiro—Brazil. PloS Negl Trop Dis (2022) 16(1):1–10. doi: 10.1371/journal.pntd.0010070
5. Pinheiro RO, Salles JS, Sarno EN, Sampaio EP. Mycobacterium leprae-host-cell interactions and genetic determinants in leprosy: an overview. Future Microbiol (2011) 6(2):217–30. doi: 10.2217/fmb.10.173
6. van Hooij A, Geluk A. In search of biomarkers for leprosy by unraveling the host immune response to Mycobacterium leprae. Immunol Rev (2021) 301(1):175–92. doi: 10.1111/imr.12966
7. Madan NK, Agarwal K, Chander R. Serum cytokine profile in leprosy and its correlation with clinico-histopathological profile. Lepr Rev (2011) 82(4):371–82. doi: 10.47276/lr.82.4.371
8. Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis (1966) 34(3):255–73.
9. Pitta IJR, Hacker MA, Vital RT, Andrade LR, Spitz CN, Sales AM, et al. Leprosy reactions and neuropathic pain in pure neural leprosy in a reference center in rio de janeiro – Brazil. Front Med (2022) 9(March):1–6. doi: 10.3389/fmed.2022.865485
10. Pinheiro RO, Schmitz V, de Andrade Silva BJ, Dias AA, de Souza BJ, de Mattos Barbosa MG, et al. Innate immune responses in leprosy. Front Immunol (2018) 9(MAR):1–15. doi: 10.3389/fimmu.2018.00518
11. Rambukkana A. Mycobacterium leprae-induced demyelination: A model for early nerve degeneration. Curr Opin Immunol (2004) 16(4):511–8. doi: 10.1016/j.coi.2004.05.021
12. Marçal PHF, Gama RS, Pereira de Oliveira LB, Martins-Filho OA, Pinheiro RO, Sarno EN, et al. Functional biomarker signatures of circulating T-cells and its association with distinct clinical status of leprosy patients and their respective household contacts. Infect Dis Poverty (2020) 9(1):1–11. doi: 10.1186/s40249-020-00763-7
13. Marçal PHF, De Souza MLM, Gama RS, De Oliveira LBP, Gomes MDS, Do Amaral LR, et al. Algorithm design for a cytokine release assay of antigen-specific in vitro stimuli of circulating leukocytes to classify leprosy patients and household contacts. Open Forum Infect Dis (2022) 9(3):1–9. doi: 10.1093/ofid/ofac036
14. Kumar B, Kaur I, Dogra S, Kumaran MS. Pure neuritic leprosy in India: An appraisal. Int J Lepr Other Mycobact Dis (2004) 72(3):284–90. doi: 10.1489/0020-7349(2004)72<284:PNLIIA>2.0.CO;2
15. Luiz S, Antunes G, Chimelli L, Jardim MR, Vital RT, Augusto J, et al. Histopatologiahansen. Memorias do Instituo Oswaldo Cruz (2012) 107(March):246–53. doi: 10.1590/S0074-02762012000200015
16. Vital RT, Illarramendi X, Nascimento O, Hacker MA, Sarno EN, Jardim MR. Progression of leprosy neuropathy: A case series study. Brain Behav (2012) 2(3):249–55. doi: 10.1002/brb3.40
17. Bartzen D, Angst M, Pinheiro RO, Soares J. Cytokine levels in neural pain in leprosy. Front Immunol (2020) 11(January):1–9. doi: 10.3389/fimmu.2020.00023
18. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interf Cytokine Res (2009) 29(6):313–25. doi: 10.1089/jir.2008.0027
19. Jardim MR, Vital R, Hacker MA, Nascimento M, Balassiano SL, Sarno EN, et al. Leprosy neuropathy evaluated by NCS is independent of the patient’s infectious state. Clin Neurol Neurosurg (2015) 131:5–10. doi: 10.1016/j.clineuro.2015.01.008
20. Teles RMB, Antunes SLG, Jardim MR, Oliveira AL, Nery JAC, Sales AM, et al. Expression of metalloproteinases (MMP-2, MMP-9, and TACE) and TNF-α in the nerves of leprosy patients. J Peripher Nerv Syst (2007) 12(3):195–204. doi: 10.1111/j.1529-8027.2007.00139.x
21. Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev (2011) 22(3):121–30. doi: 10.1016/j.cytogfr.2011.06.001
22. Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med (2020) 217(1):1–19. doi: 10.1084/jem.20190418
23. Penna PS, De Souza SAL, De Lacerda PGLN, Rodrigues Pitta IJ, Spitz CN, Sales AM, et al. Evidencing leprosy neuronal inflammation by 18-Fluoro-deoxy-glucose. PloS Negl Trop Dis (2023) 17(6):e0011383. doi: 10.1371/journal.pntd.0011383
Keywords: pure neural leprosy, leprosy neuropathy, cytokine profile, leprosy reactions, leprosy nerve conduction studies
Citation: Pitta IJR, Angst DBM, Pinheiro RO, Vieira JSdS, Spitz CN, Andrade LR, Carvalho LB, Hacker MA, Sarno EN and Jardim MR (2023) Cytokines profile in pure neural leprosy. Front. Immunol. 14:1272471. doi: 10.3389/fimmu.2023.1272471
Received: 04 August 2023; Accepted: 20 November 2023;
Published: 05 December 2023.
Edited by:
Dario S. Zamboni, University of São Paulo, BrazilReviewed by:
Tarun Narang, Postgraduate Institute of Medical Education and Research, IndiaUtpal Sengupta, The Leprosy Mission Trust India, India
Copyright © 2023 Pitta, Angst, Pinheiro, Vieira, Spitz, Andrade, Carvalho, Hacker, Sarno and Jardim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Izabela Jardim R. Pitta, aXphYmVsYXBpdHRhQGlkLnVmZi5icg==
 Izabela Jardim R. Pitta
Izabela Jardim R. Pitta Debora Bartzen Moraes Angst1
Debora Bartzen Moraes Angst1 Roberta Olmo Pinheiro
Roberta Olmo Pinheiro Clarissa Neves Spitz
Clarissa Neves Spitz Ligia Rocha Andrade
Ligia Rocha Andrade Mariana Andrea Hacker
Mariana Andrea Hacker Marcia Rodrigues Jardim
Marcia Rodrigues Jardim