- 1State Key Laboratory of Dairy Biotechnology, Shanghai Engineering Research Center of Dairy Biotechnology, Dairy Research Institute, Bright Dairy & Food Co., Ltd., Shanghai, China
- 2State Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, Wuxi, China
Helicobacter pylori (H. pylori) is a gram-negative bacterium exhibiting high pathogenicity. Traditional antibiotic treatments are considered ineffective as the H. pylori resistance has increased. Recently, a quadruple therapy strategy of probiotics and antibiotics to eliminate H. pylori was proposed. Probiotics play a therapeutic role as supplements in this process. The present research screened a probiotic strain (Lactobacillus crispatus FSCDJY67L3) that co-aggregates strongly with H. pylori. L. crispatus FSCDJY67L3 was demonstrated to significantly reduce H. pylori load (14C breath test) in clinical trials with H. pylori-positive patients. The Gastrointestinal Symptom Rating Scale (GSRS) score decreased, indicating improvement in the gastrointestinal discomfort of patients. Furthermore, L. crispatus FSCDJY67L3 showed no change in the structure of the intestinal flora of patients. Routine blood indices and blood biochemical indices related to liver and kidney function were also not affected in the patients. Therefore, L. crispatus FSCDJY67L3 may be used clinically as a supplement for the treatment of H. pylori.
Clinical Trial Registration: https://www.chictr.org.cn/, Chinese Clinical Trial Registry (ChiCTR2100053710).
1 Introduction
H. pylori, a gram-negative bacterium, is typically parasitic in human gastric mucosal tissue (1). It is claimed that more than 50% of the population is infected with H. pylori at the global level (2). In particular, infection rates are significantly higher in developing countries (70-90%) than in developed countries (25-50%) (3). H. pylori is highly pathogenic and is the main cause of active gastritis, gastrointestinal ulcers, and gastric lymphoma (4). Furthermore, it is identified as a group 1 carcinogen by the World Health Organization (WHO) (5). Therefore, the clearance of H. pylori is essential for the prevention and treatment of these diseases. In the 1990s, triple therapy with antibiotics was the standard of treatment in most parts of the world. However, with the increase in clarithromycin resistance, the success rate of triple therapy has rapidly decreased (6, 7). Furthermore, triple therapy is expensive and causes serious side effects (8). Therefore, it is necessary to explore novel alternative treatment options which address the H. pylori epidemic.
Numerous studies have focused on the prevention and treatment of H. pylori by probiotics in the past few years. Recently, H. pylori treatment guidelines issued by the European Helicobacter and Microbiota Study Group, an authoritative group on the subject, suggest that certain specific probiotics are worth considering as an add-on to protect vulnerable patients who are poorly tolerant to antibiotics (1, 5). Among these probiotics, Lactobacillus is considered to be potentially effective due to the capability of these bacteria to inhibit H. pylori, which has been demonstrated by numerous in vivo/in vitro experiments (9). Most significantly, the inhibition mechanism of H. pylori by Lactobacillus has been elucidated. In brief, the mechanism can be summarized by the following points: (i) the intake of Lactobacillus stabilizes the intestinal mucosal barrier by producing antimicrobial substances that bind to H. pylori adhesion receptors (e.g., Lactiplantibacillus pentosus SLC13) (10); (ii) Lactobacillus may prevent H. pylori colonization of the gastric mucosa by inhibiting its adhesion to epithelial cells (e.g., Lactobacillus gasseri Kx110A1) (11); and (iii) the mucosal barrier is strengthened by Lactobacillus through stimulation of the local lgA response, which decreases the inflammatory response associated with H. pylori infection (e.g., Lactobacillus Rhamnosus JB3) (12, 13). Based on this, Lactobacillus could potentially be an effective strategy for the eradication of H. pylori.
The colonization of H. pylori with gastric epithelial cells means that it is possible to escape removal by host clearance mechanisms (14). Hence, numerous researchers were focused on preventing the colonization of H. pylori. The contact of Lactobacillus with H. pylori to form a copolymer may be an effective method to stop the colonization of H. pylori. According to Holz et al. (15), colonization is reduced by Lactobacillus recognizing H. pylori and attaching to its surface, causing it to be excreted as a co-aggregate. Therefore, the screening of a lactobacillus that could effectively form a co-aggregation with H. pylori should be the focus of research. In this paper, we found that the L. crispatus FSCDJY67L3 strain is able to form a copolymer with H. pylori. The effect of L. crispatus FSCDJY67L3 on H. pylori-positive patients via clinical trials was evaluated, which aimed to clarify whether L. crispatus FSCDJY67L3 has the potential to prevent and treat H. pylori.
2 Materials and methods
2.1 Strains and cultivation
The strain was isolated from the feces of a 90-year-old woman in Du Jiang Yan City, Sichuan. 16 S-rDNA sequence analysis allows taxonomic identification of Lactobacillus strains to the species level (sequencing and taxonomic classification performed by Pasono Bio, Shanghai). The sequences were aligned in GenBank and the results showed that the strains were all Lactobacillus crispatus, which was named L.crispatus FSCDJY67L3. The strain was deposited on 17 October 2022 at the China General Microbial Strain Deposit and Management Center (CGMCCNo.25925). In this research paper, L. crispatus FSCDJY67L3 and Lactobacillus strains (strains from Jiangnan University, Wuxi, China) were grown in MRS medium at 37°C. Moreover, H. pylori SS1 (from the National Centre for Type Culture Collection) was grown in Brain-Heart Infusion broth with 5% fetal bovine serum at 37°C in a micro-oxygenated atmosphere (85% N2, 10% CO2, 5% O2). The Lactobacillus strains and H. pylori bacteria were harvested by centrifugation at 8000 g for 10 min at 4 °C. They were subsequently washed twice with PBS and suspended in PBS and pH 4 artificial gastric juice (containing 0.9% NaCl and 0.3% pepsinogen), respectively, to achieve a concentration of 1×109 CFU/mL for each sample.
2.2 Strains co-aggregation capacity
The co-aggregation ability analysis was performed according to Collado et al. (16). Briefly, lactic acid bacteria suspension (2 mL) and H. pylori SS1 suspension (2 mL) were mixed as well as vortexed for 10 s to determine co-aggregation capacity. The mixture was incubated at 37°C for 2 h, and the OD600 value of the bacterial solution was measured. The co-aggregation ability was calculated by the equation as follows:
Where OD Lactobacillus, OD H. pylori, and OD mixture represent, respectively, the absorbance at 600 nm of lactobacillus, H. pylori, and their mixture after incubating at 37°C for 2 h.
2.3 Scanning electron microscopy morphological observation of strains co-aggregation
The sample preparation method was referred to by Holz et al. (15). L. crispatus FSCDJY67L3 and H. pylori SS1 suspensions were mixed in equal volumes to induce co-aggregation. After incubating for 2 h at room temperature, bacterial co-aggregates were obtained by centrifugation. Subsequently, the co-aggregates were suspended in a glutaraldehyde fixation solution (4%) and kept in a cold room (4°C) overnight. The fixed co-aggregates were then centrifuged, and a gradient dehydration process was carried out using ethanol solutions (70%, 80%, 90%, 95%, and 100%) for 10 min each. The dehydrated co-aggregates were allowed to air-dry at room temperature to ensure complete evaporation of organic reagents. Afterward, they were encapsulated in plastic wrap to prevent sample splatter and subjected to vacuum freeze-drying for 2 days. Following freeze-drying, the samples underwent palladium sputter coating and were observed using scanning electron microscopy (SEM) (HITACHI SU8100, Tokyo, Japan) at high magnification of 5000× and 10000×.
2.4 Participants and ethics
From November 2021 to December 2021, the study enrolled patients aged 18 to 65 years at the Sixth People’s Hospital of Yancheng City (Jiangsu Province, China). The subjects enrolled met the following inclusion criteria: (i) men and women aged from 18 to 65 years old, half from each group; (ii) confirmed diagnosis of H. pylori infection after study entry (≤3 months) by 14C urea breath test, rapid urease test, or histology; (iii) without symptoms of discomfort, except for H. pylori infection; (iv) prior anti- H. pylori treatment was not received; and (v) understood the details of the study and provided written informed consent. Patients were excluded if they met any of the following criteria: a history of gastrointestinal surgery (except for appendectomy), pregnancy or lactation, a history of any severe disease or condition (e.g., severe cardiovascular, endocrine, hepatic, or renal dysfunction), a mental illness that could potentially hinder collaboration, lack of self-cognitive judgment, substance abuse (alcohol or drugs), or failure to meet the study requirements. Additionally, patients who had taken antibiotics or probiotics in the month prior to inclusion were also excluded. The following criteria were used to determine patient exclusion: (a) if the subject was unwell or had special physiological changes in their body not appropriate to continue participating in the study, (b) if the subject elected to opt out or terminate their participation in the study, and (c) if the subject failed to undergo laboratory procedures, take antibiotics or other probiotic products, undergo a medical examination, and take stool or blood-related samples during the study. If the subject failed to fill in the relevant scale on time, the subject was considered lost to follow-up.
The experimental scheme was approved and implemented by the Yan Cheng People’s Hospital Research Ethics Board (approval no. of the ethics committee: ET2021085) and registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR2100053710). The Declaration of Helsinki and pertinent local laws were followed during the planning and execution of this study. All study participants signed an informed consent form.
Gastrointestinal Symptom Rating Scale (GSRS) (17): All patients attended an interview for the recall of gastrointestinal symptoms. The 15-item GSRS to assess the severity and frequency of symptoms was reported. According to the degree of score statistics (0~3 points), the score is proportional to the severity of symptoms. The following symptoms were specifically investigated: abdominal pain, heartburn, acid regurgitation, sucking sensations in the epigastrium, nausea and vomiting, borborygmus, abdominal distension, eructation, increased flatus, decreased/increased passage of stools, loose/hard stools, sense of urgency of evacuation, and feeling of incomplete evacuation.
2.5 Study design
In this study, a double-blind, randomized, and placebo-controlled trial was used to evaluate the eradication rate and safety of L. crispatus FSCDJY67L3 in patients with confirmed H. pylori infection. Patients randomly received 2 g probiotics (5 × 109 CFU/package L. crispatus FSCDJY67L3) or matching placebo twice a day for one month. In general, probiotic doses ranging from 1.0 × 109 to 1.0 × 1010 colony-forming units (CFU) per day have been shown to be well tolerated in the general population (18). Blood samples were collected prior to the experiment and after the intervention for further analysis, including blood routine and blood biochemical indexes related to liver and kidney function. In addition, we compared the changes in the intestinal flora of subjects after the L. crispatus FSCDJY67L3 intervention using 16S rDNA amplicon sequencing. Chao1 and Shannon indexes were used to characterize the α-diversity of the intestinal flora in subjects after the L. crispatus FSCDJY67L3 intervention. PCoA analysis was used to characterize the β-diversity.
2.6 Intestinal microbial composition
Fecal samples were collected before and after the experiment and were used to analyze changes in the composition of the gut microbiota. The resolved bacteria were extracted using the feces genome extraction kit from USA MP Company. After extraction, PCR was performed for the V3~V4 region of bacterial 16S rDNA. The PCR amplification system was as follows: 25 µL 2 × Premix Taq, 1 µL upstream primer 341F, 1 µL downstream primer 806R, 1 µL genomic DNA template, and 22 µL ddH2O. Amplification conditions were as follows: 95°C for 8 min; 95°C for 35 s, 52°C for 35 s, and 72°C for 40 s, 30 cycles; 72°C for 8 min. The gel was recovered using the ultra-thin agarose-gel purification and recovery kit from Beijing Tian Gen Biochemical Technology Co., LTD., and the purified fecal DNA was mixed according to the equal quality library samples and then sequenced by machine. QIIME1 software was used for data analysis after the microflora was disembarked.
2.7 Statistical analysis
SPSS22.0 software was used for data analysis, and GraphPad Prism 9 was used to make various statistical graphs. All data are expressed as mean ± standard deviation, and differences between groups were compared by one-way analysis of variance (ANOVA) with Duncan post-hoc analysis to correct for multiple comparisons. Differences were considered statistically significant when the p< 0.05.
3 Results
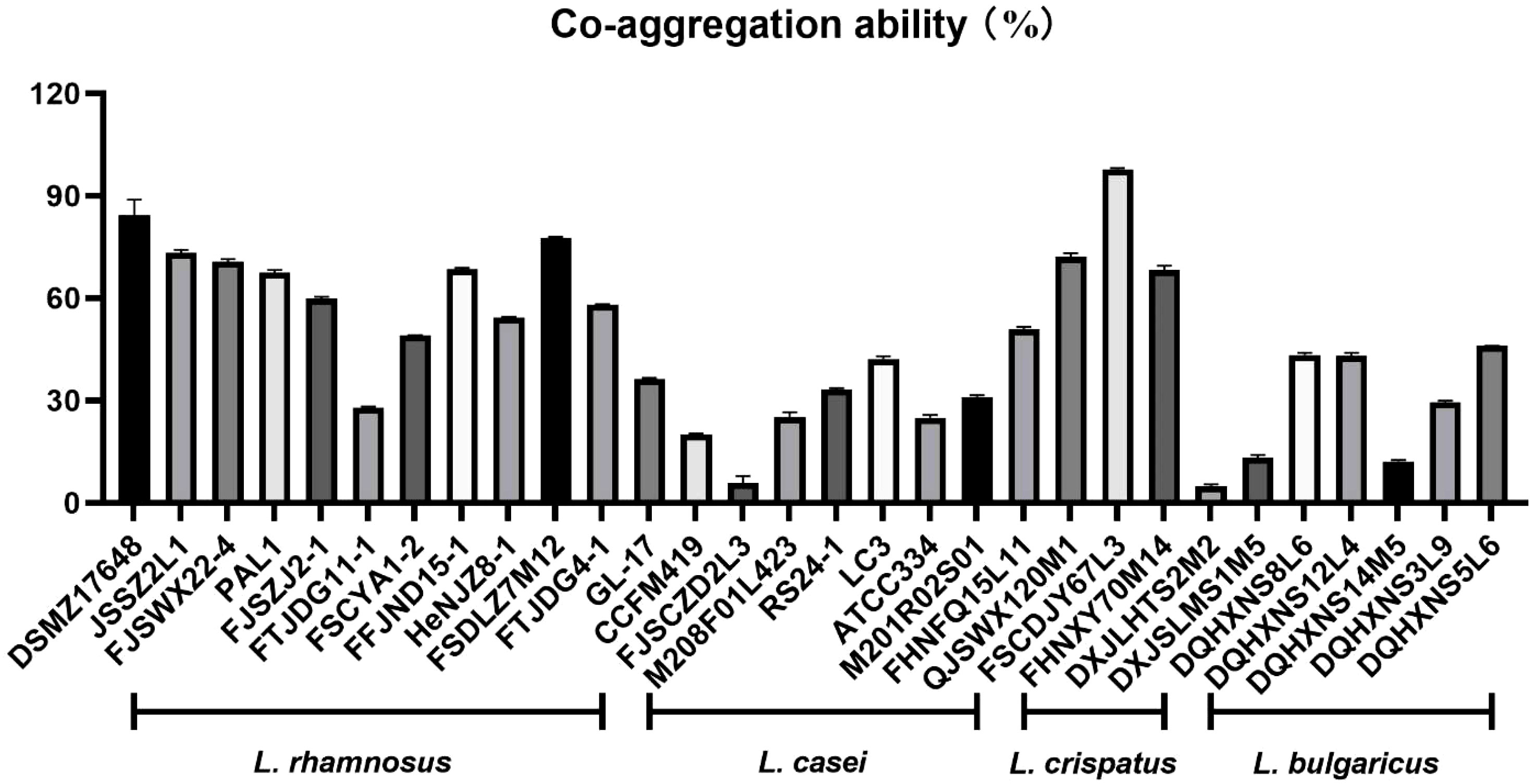
3.1 The analyses of co-aggregation ability
In this study, Lactobacilli with excellent ability to co-aggregate H. pylori were selected from a large amount of Lactobacillus species (Figure 1). Interestingly, L. crispatus FSCDJY67L3 exhibited the strongest co-aggregation with H. pylori among all Lactobacillus strains. The co-aggregation rate between L. crispatus FSCDJY67L3 and H. pylori was above 97.78%.
3.2 SEM micro-morphological analysis of co-aggregates
Cold field emission scanning electron microscopy was used to observe the morphology of strain co-aggregation. In previous studies, H. pylori morphology was generally S-shaped or spiraled. However, it may age into a cocoon or globular shape as the environment changes. L. crispatus FSCDJY67L3 was long and cylindrical and co-aggregated with H. pylori as shown in Figure 2. It interacted with the surface of H. pylori in a strongly binding interaction. In addition, the binding site for H. pylori seemed to not be present on the flagellar structure of L. crispatus FSCDJY67L3, which is in agreement with the results reported by Holz et al. (15). Notably, L. crispatus FSCDJY67L3 also exhibited self-aggregation properties, suggesting that the formation of larger co-aggregates was facilitated.
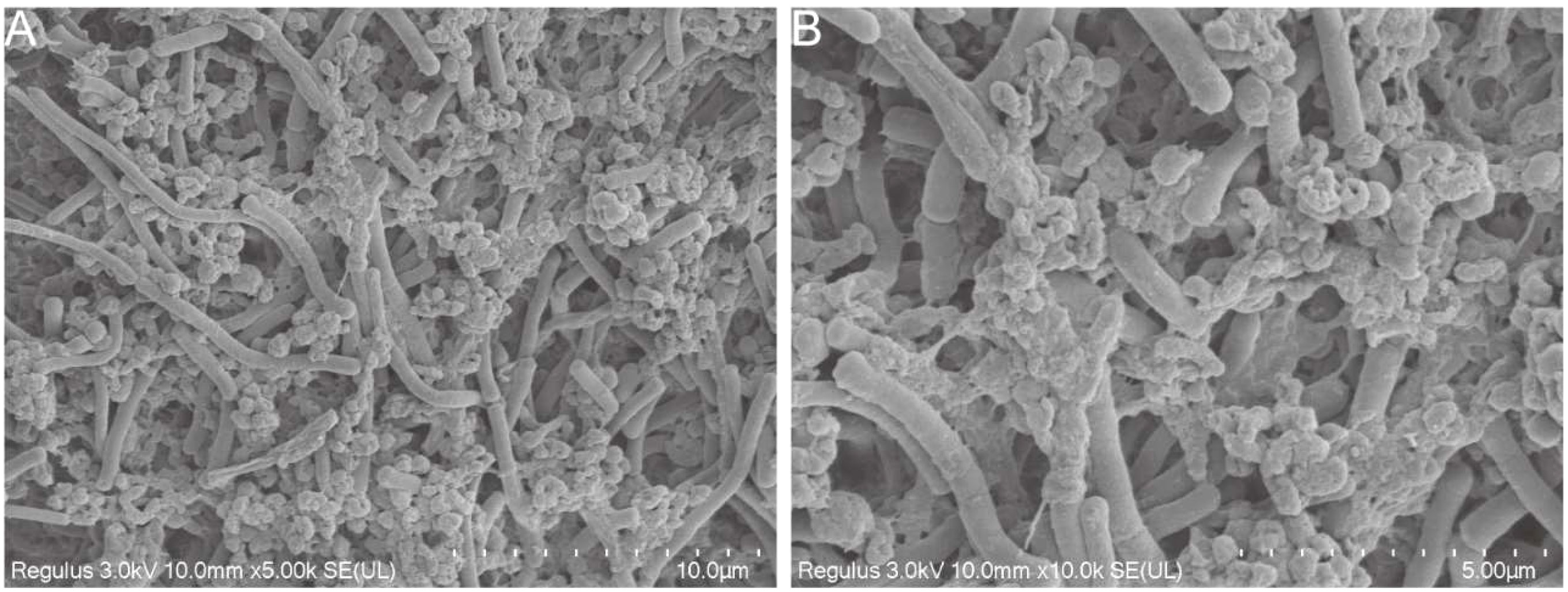
Figure 2 SEM morphological observation of strains co-aggregation. (A) 5000×magnification; (B) 10000×magnification.
3.3 Subjects
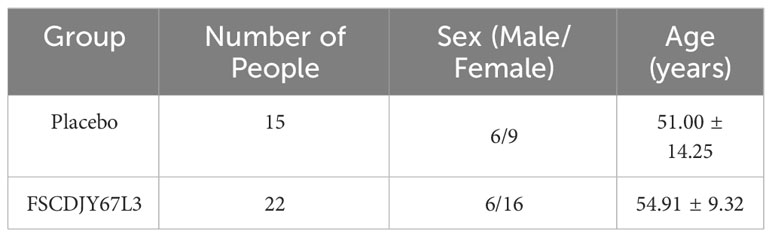
In the present research, patients with H. pylori infection (total of 44) were recruited and randomized to complete the treatment protocol with a placebo (n=20) or with L. crispatus FSCDJY67L3 (n=24). Unfortunately, seven patients dropped out during the process of the study, including two in the L. crispatus FSCDJY67L3 group and five in the placebo group. There were no patients discontinued from treatment and no loss to follow-up. The basic information on the enrolled population is shown in Table 1. There was no significant difference in age or sex among the groups of subjects.
3.4 Effect of L. crispatus FSCDJY67L3 on H. pylori eradication rates
A clinical trial in patients with a family history of gastric cancer demonstrated that eradication of H.pylori significantly reduced the risk of gastric cancer compared to persistent infection (19). Therefore, it is crucial to decrease the load of H. pylori in the human organism. The expiratory value of 14C is a widely used clinical indicator for the diagnosis of H. pylori infection, which reflects the H. pylori load in patients. Importantly, the sensitivity and specificity of 14C expiratory testing usually exceed 95% (20). As shown in Figure 3, the reduction rate of 14C expiratory value in H. pylori-positive patients was significantly increased (67.19%) after the intervention of L. crispatus FSCDJY67L3. However, patients with increased 14C expiratory values showed a negative rate of reduction in expiratory values in the placebo group. This result indicated that L. crispatus FSCDJY67L3 could effectively reduce the H. pylori load in patients.
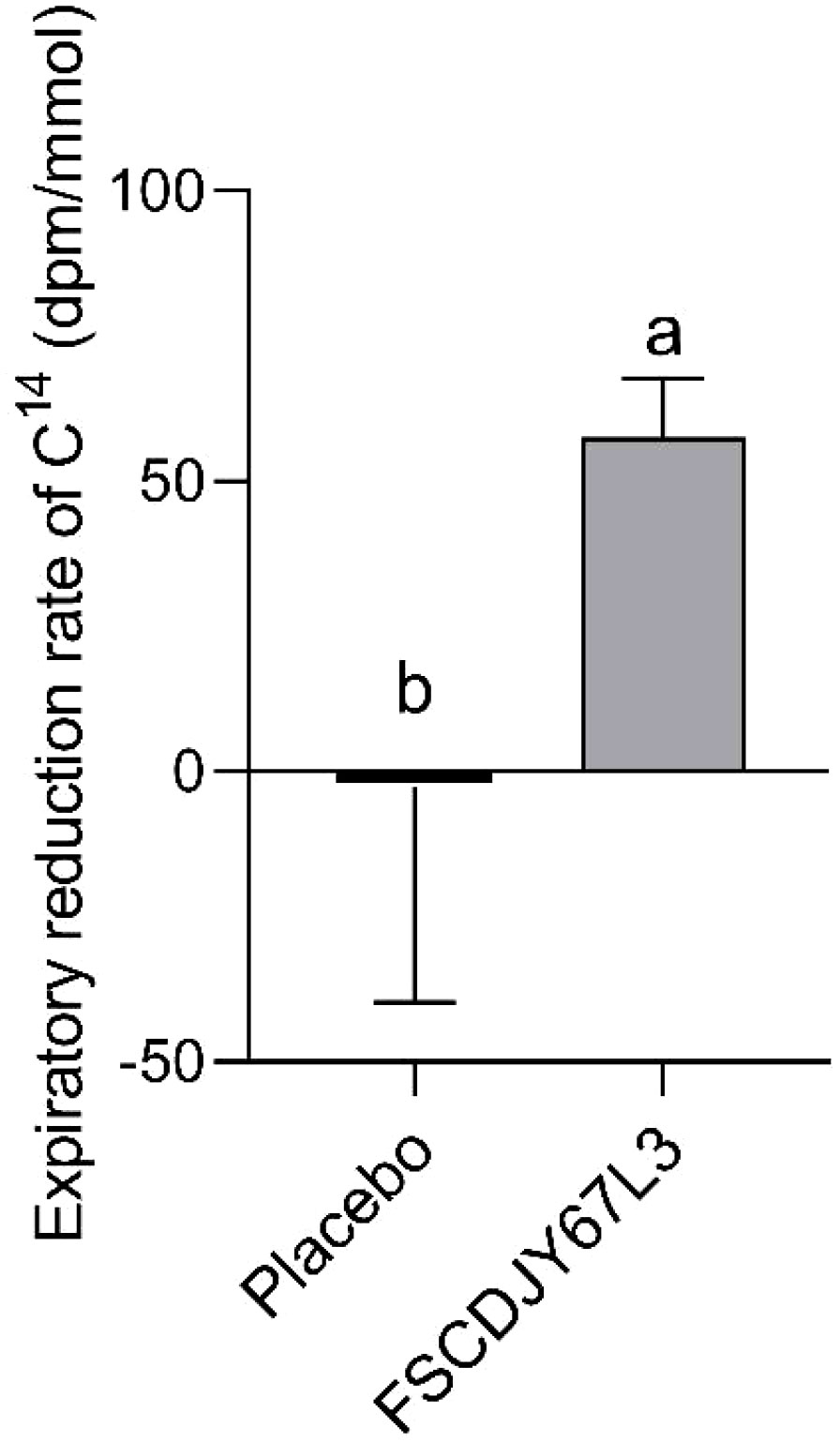
Figure 3 Effect of FSCDJY67L3 intervention on 14C expiratory value in Helicobacter pylori-positive patients. Different lowercase letters indicate significant differences between the two groups.
3.5 Effect of L. crispatus FSCDJY67L3 on gastrointestinal symptom in H. pylori-positive patients
In this study, we evaluated the gastrointestinal symptoms presented by all patients, which were quantified with the GSRS score (21). There was a significant difference in gastrointestinal symptoms in H. pylori-positive patients before and after L. crispatus FSCDJY67L3 intervention. As seen in Figure 4, the GSRS score of the placebo group changed from 3.47 ± 2.00 to 4.59 ± 2.01 (p>0.05) after the intervention. In addition, the GSRS score of the L. crispatus FSCDJY67L3 group decreased from 3.2 ± 1.01 to 2.5 ± 1.19 (p<0.05), indicating that the symptoms of gastrointestinal discomfort caused by H. pylori were significantly improved.
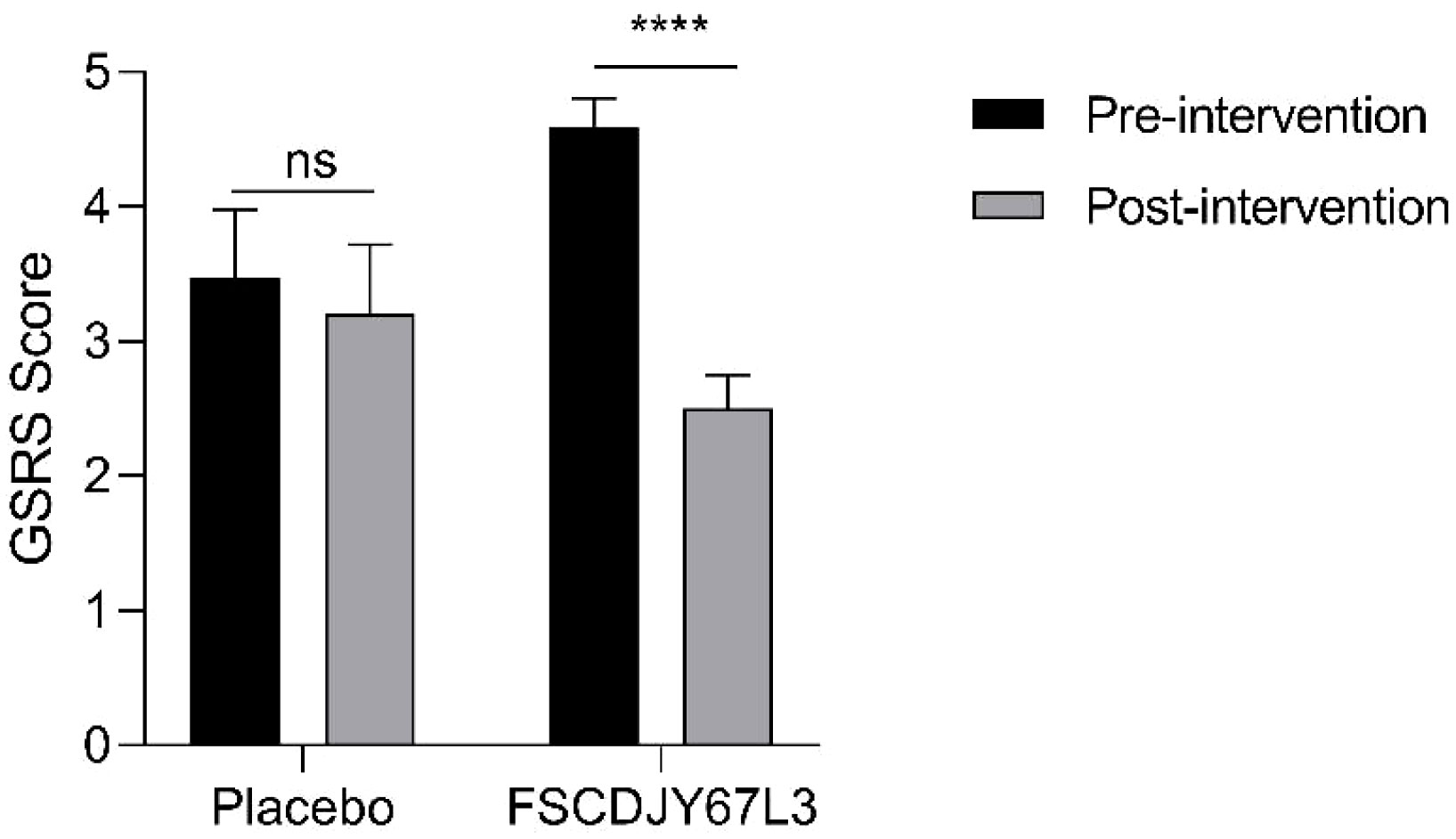
Figure 4 Effect of FSCDJY67L3 intervention on gastrointestinal discomfort in Helicobacter pylori-positive patients. ****p<0.0001. ns, no significance.
3.6 Effect of L. crispatus FSCDJY67L3 on blood index in H. pylori-positive patients
The changes in the number of white blood cells, red blood cells, hemoglobin concentration, platelet count, the content of basic phospholipase, alanine aminotransferase, aspartate aminotransferase, and total bilirubin in patients with H. pylori infection in the L. crispatus FSCDJY67L3 and placebo groups are shown in Figures 5, 6. In the placebo and L. crispatus FSCDJY67L3 groups, there were no significant differences between the immune mediators in the serum of the patients before and after the intervention.
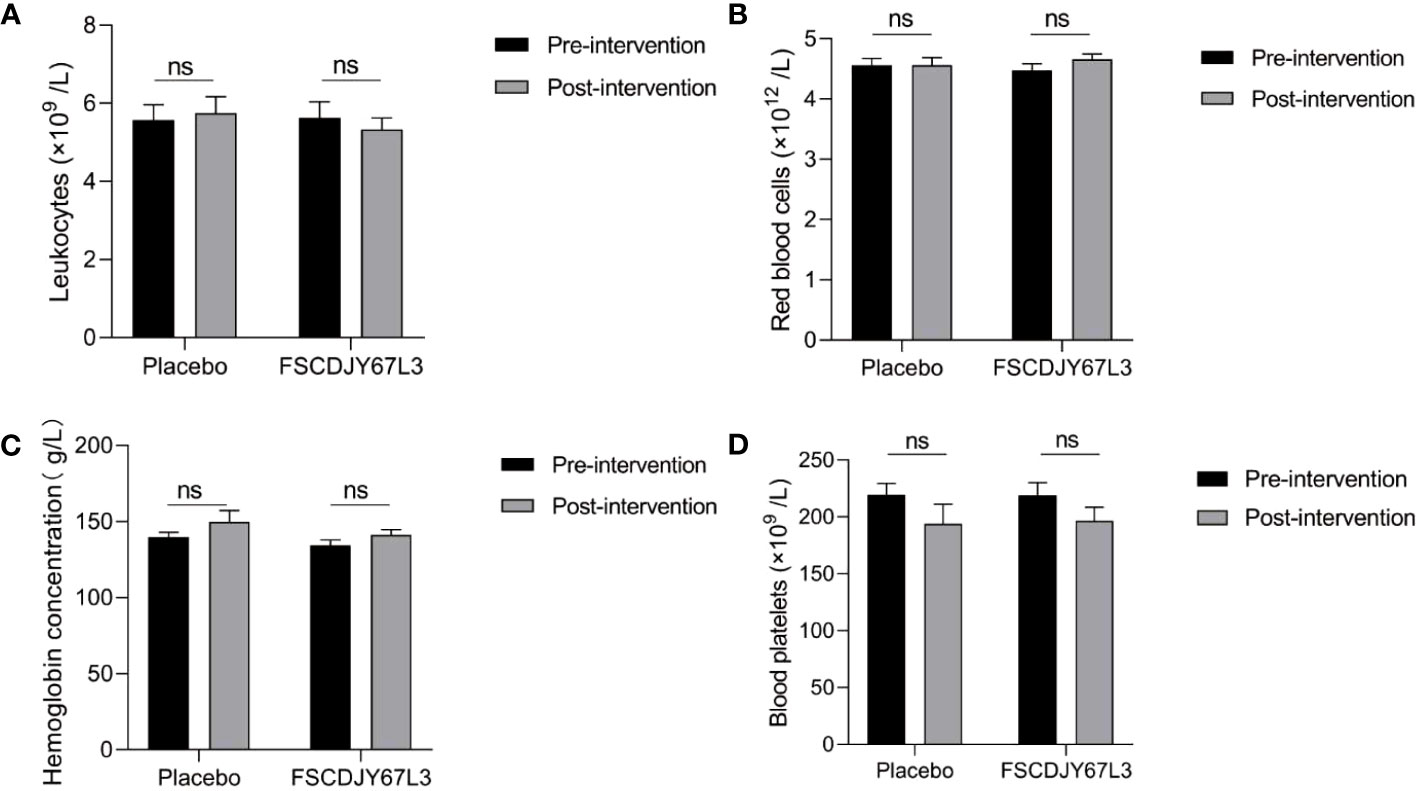
Figure 5 Effect of FSCDJY67L3 intervention on blood routine indexes in Helicobacter pylori-positive patients pre- and post-intervention. (A) Leukocyte count; (B) Red blood cells; (C) Hemoglobin concentration; (D) Blood platelets. ns, no significance.
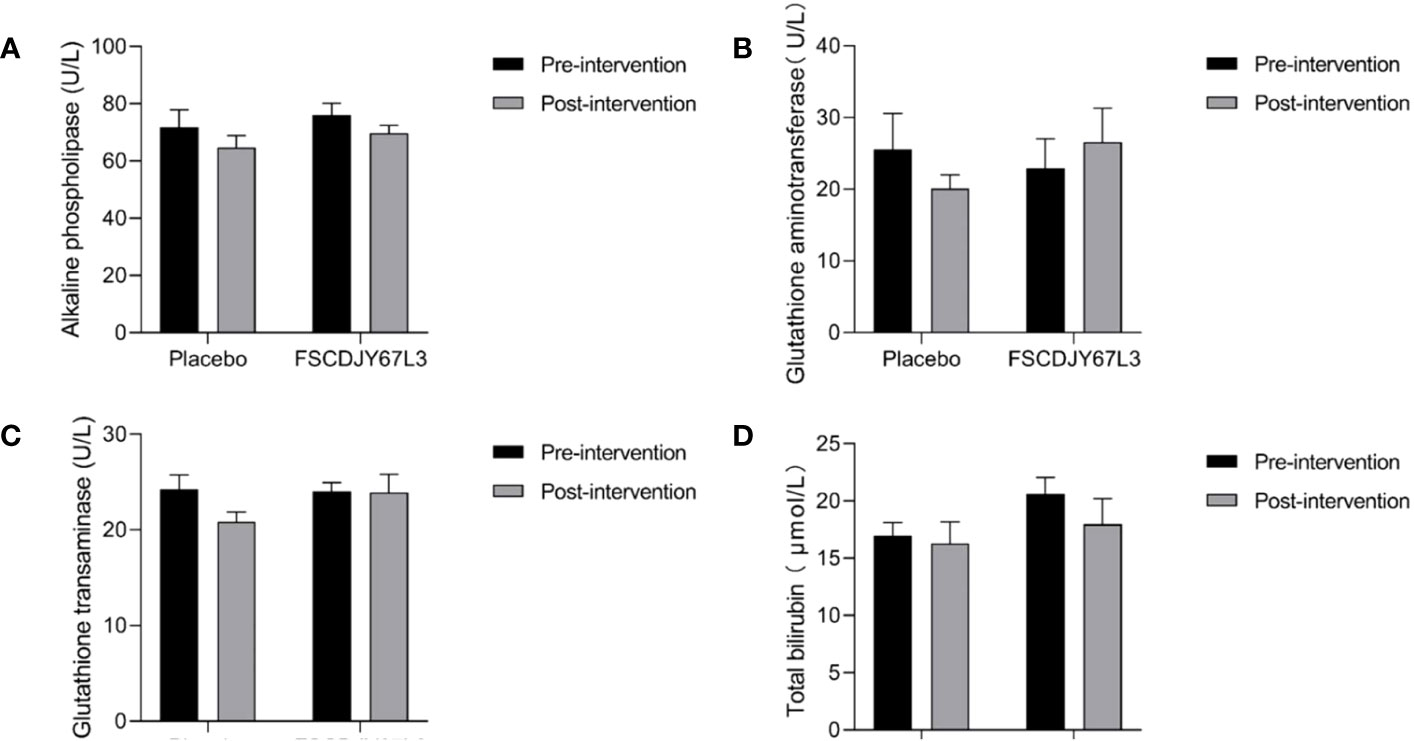
Figure 6 Effect of FSCDJY67L3 intervention on blood biochemical indexes refers to liver function in Helicobacter pylori-positive patients pre- and post-intervention. (A) Alkaline phospholipase content; (B) Glutathione aminotransferase content; (C) Glutathione transaminase content; (D) Total bilirubin.
As shown in Figure 7, the content of urea and uric acid refers to blood biochemical indicators of renal function in H. pylori-positive patients and showed no significant changes before and after the placebo and L. crispatus FSCDJY67L3 intervention. However, in the placebo group, the creatinine content of H. pylori-positive patients changed from 56.62 ± 17.94 before intervention to 74.05 ± 14.51 (p<0.01), and that of H. pylori-positive patients changed from 54.46 ± 7.82 before intervention to 70.30 ± 15.07 after intervention (p<0.005).
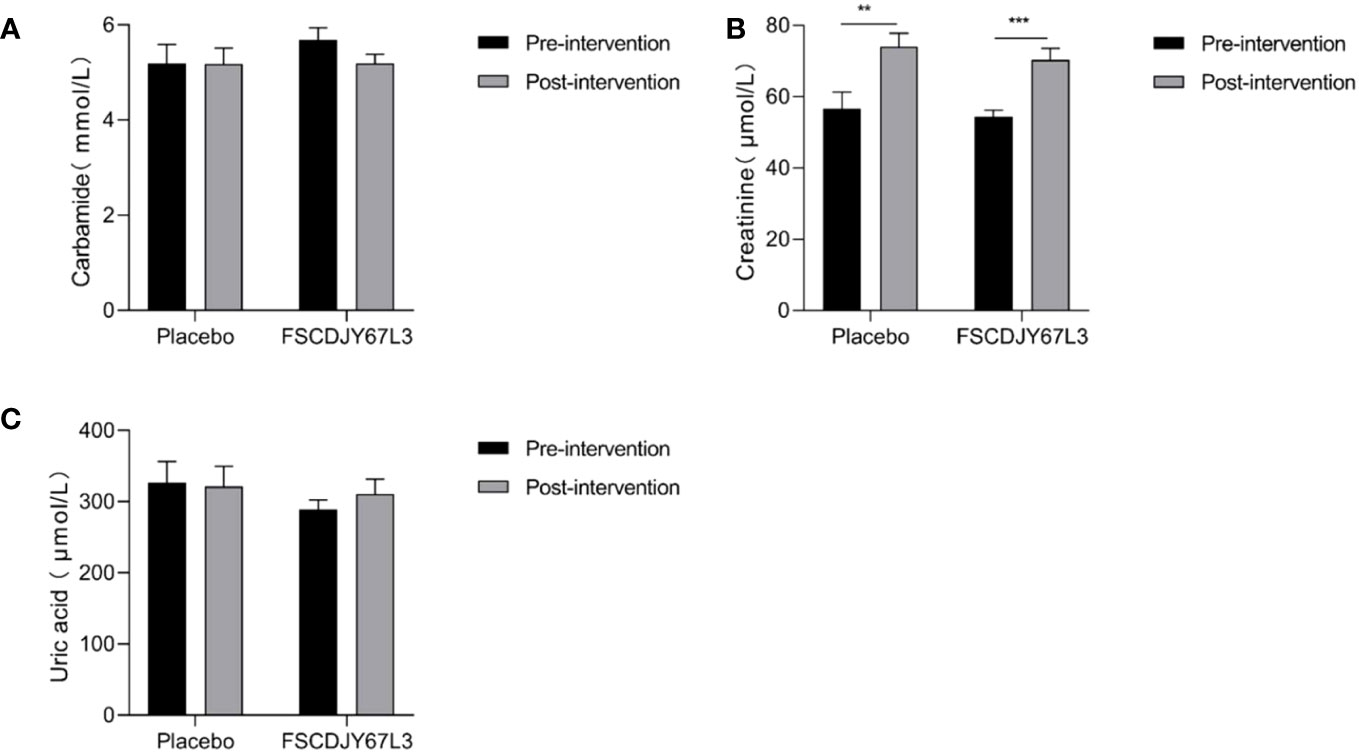
Figure 7 Effect of FSCDJY67L3 intervention on blood biochemical indexes refers to renal function in Helicobacter pylori-positive patients pre- and post-intervention. (A) Carbamide content; (B) Creatinine content; (C) Uric acid content. **p<0.01, ***p<0.005.
3.7 Analysis of intestinal microbial composition in H. pylori-positive patients
Previous research presented the theory that changes in intestinal flora are associated with a range of gastrointestinal diseases and systemic diseases (22). In addition, Luyi et al. demonstrated that stool samples from H. pylori-infected individuals showed reduced abundance of Clostridium perfringens and total anaerobic bacteria as compared to H. pylori-negative individuals (23). Therefore, the modification of the intestinal flora could be considered as a criterion to reflect the pathological status of the organism.
After intervention with the L. crispatus FSCDJY67L3, no significant changes in intestinal flora Chao1 and Shannon index were observed in H. pylori-positive infected patients (Figure 8), indicating that L. crispatus FSCDJY67L3 intervention could not alter community richness and microbial diversity in H. pylori-positive infected individuals. The further the distance between the different groups in the PCoA analysis indicated the greater the difference in their intestinal flora. As shown in Figure 9, there was no significant distance between the two groups before and after the intervention, suggesting L. crispatus FSCDJY67L3 could not contribute to the difference in intestinal flora in H. pylori-positive individuals.
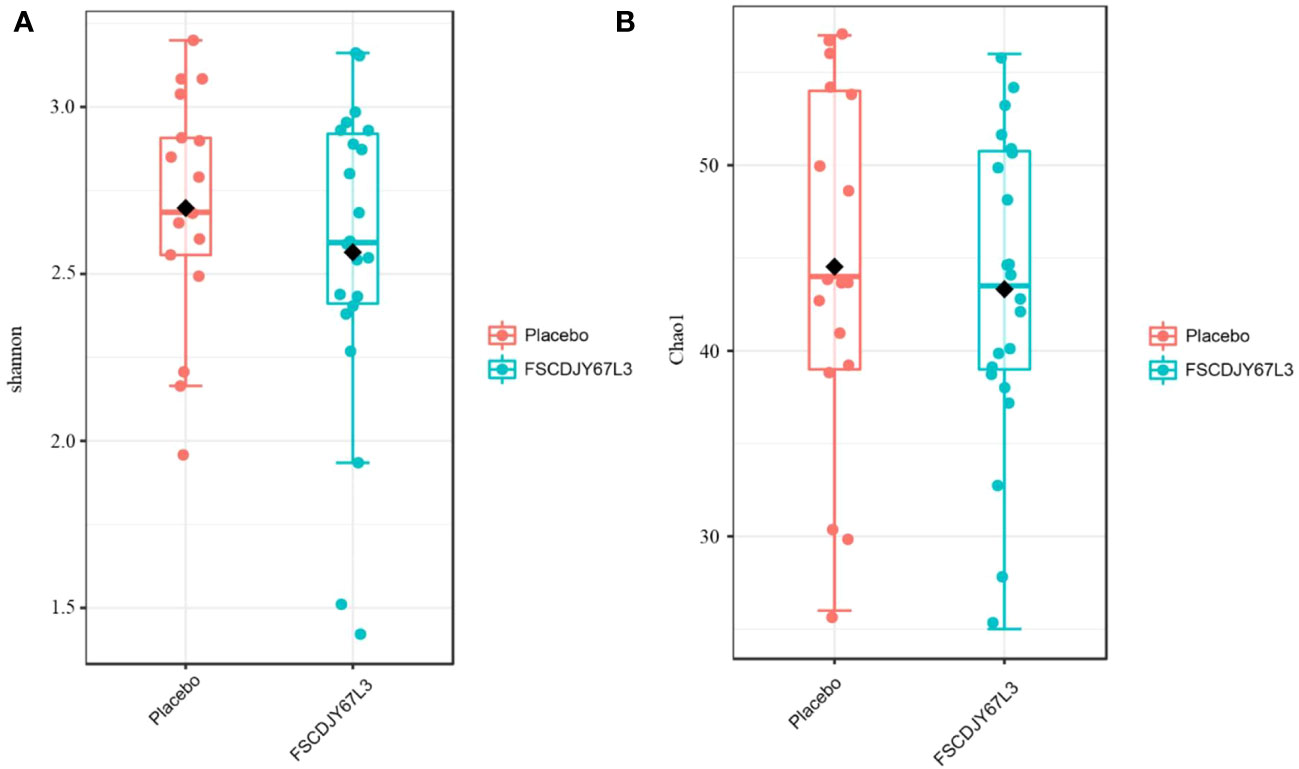
Figure 8 Changes of intestinal flora diversity in Helicobacter pylori-positive patients after intervention. (A) Shannon index; (B) Chao1 index.
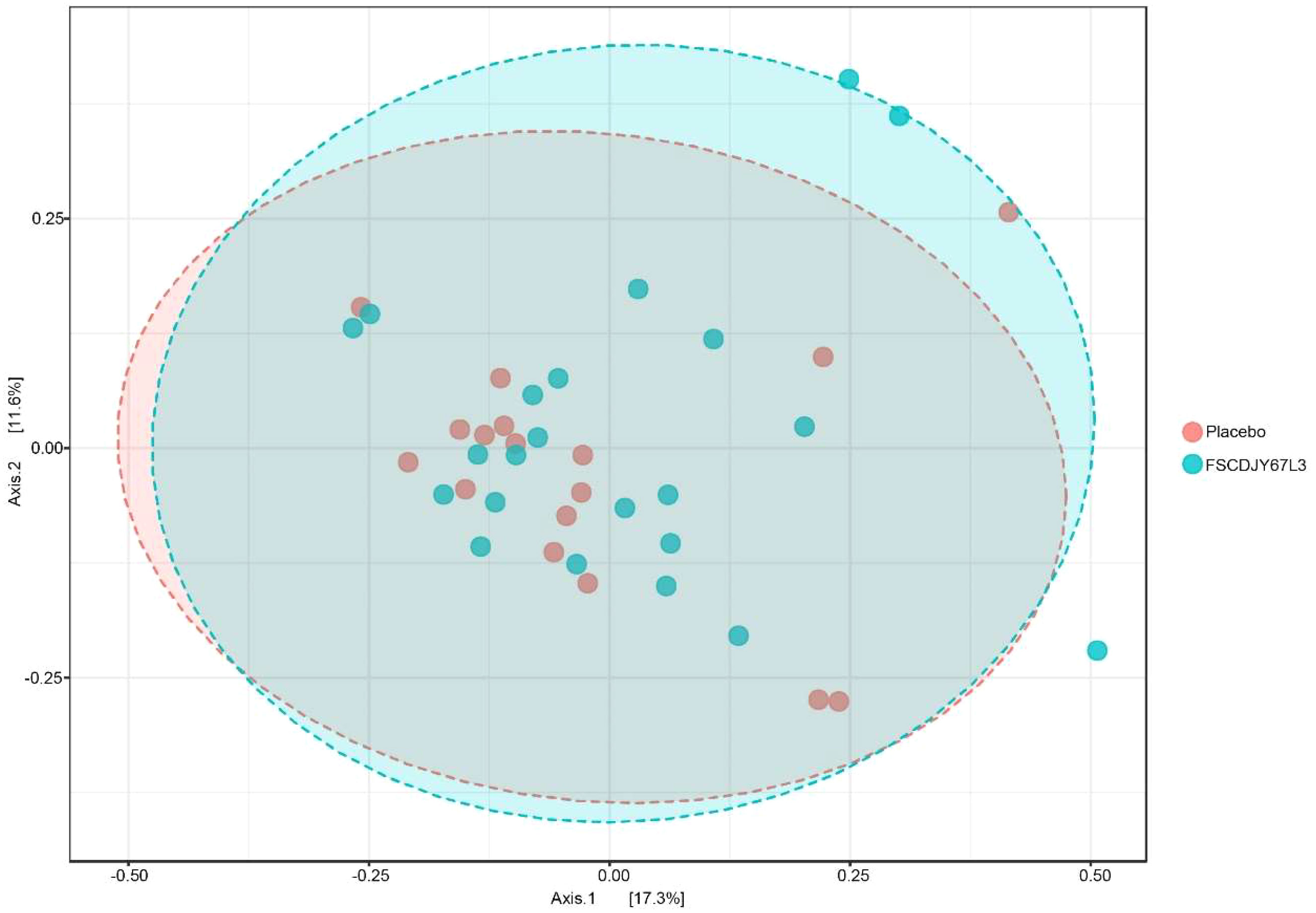
Figure 9 Principal coordinates analysis of intestinal flora composition in Helicobacter pylori-positive patients after intervention.
4 Discussion
H.pylori is a major human pathogen, listed by the World Health Organization as one of the 20 pathogens that pose the most serious threat to human health because of its drug resistance (24). As H. pylori drug resistance increases, some antibiotics (e.g., amoxicillin, clarithromycin, and metronidazole) have a diminished therapeutic effect on H. pylori. Notably, antibiotics were considered to be able to completely eradicate H. pylori at an earlier time. Therefore, we need new therapeutic strategies to tackle this global problem. Recently, a therapeutic strategy was proposed, which combines probiotics and antibiotics in a quadruple therapy to completely eradicate H. pylori. In this process, probiotics are used as a supplement to play a therapeutic role (25, 26).
In the human intestine, Lactobacillus is present in high quantities and exhibits adhesion properties, allowing it to co-aggregate with pathogenic bacteria. Current research on the co-aggregation ability of Lactobacillus to alleviate H. pylori infection has primarily focused on a strain of Lactobacillus reuteri DSMZ17648, which was screened by German researchers (15). However, it was not yet possible to alleviate H. pylori infection in China by co-aggregation of Lactobacillus strains. This study aims to identify a Lactobacillus strain with superior co-aggregation ability compared to DSMZ17648 through in vitro screening and evaluate its effectiveness in alleviating H. pylori infection through clinical trials.
We characterized the co-aggregation ability of H. pylori and probiotic strains in vitro and demonstrated that FSCDJY67L3 and H. pylori showed the highest co-aggregation ability (27). In the DSMZ17648 clinical trial research, there was a 20.00% decrease in exhaled breath values after ingestion in subjects (28). In comparison, after the intervention by L. crispatus FSCDJY67L3, the breath values of patients decreased by 67.19% in the 14C urease breath test. This indicates that L. crispatus FSCDJY67L3 not only has a strong co-aggregation effect but also exhibits better clinical efficacy in alleviating H. pylori infection.
In this study, we monitored gastric symptoms in patients to illustrate the positive modulating effect of FSCDJY67L3. It could significantly improve the gastrointestinal symptoms in H. pylori-positive patients, which may be related to the reduction of H. pylori load in patients after ingestion of L. crispatus FSCDJY67L3. Lactobacillus reuteri DSM17938 and ATCC PTA 6475 also showed similar effects (29).
We also evaluated the safety of L. crispatus FSCDJY67L3 intervention by the blood routine and blood biochemical indicators of the subjects with liver and kidney function markers. There was no significant difference in the abovementioned indexes of H. pylori-positive patients in the placebo and L. crispatus FSCDJY67L3 groups except for the creatinine content. There were a number of serum markers related to the renal function that were affected in subjects after placebo or FSCDJY67L3 intervention. However, the normal reference range for creatinine in adults is 44 to 133 μmol/L, indicating that the creatinine levels of the patients were within the normal range. The variation in creatinine levels in patients may be related to their own state and physiological habits. The clinical trial of Lb. reuteri DSMZ17648 also showed that the intervention of Lactobacillus did not affect the blood routine and blood biochemical indicators as safety parameters (15), which was consistent with the results of this study. In conclusion, L. crispatus FSCDJY67L3 is a safe probiotic whose intervention does not affect routine blood indicators, or liver and kidney function in H. pylori-positive patients.
After 30 days of intervention with L. crispatus FSCDJY67L3, there was no significant change in the diversity of the gut microbiota in the placebo and lactobacilli intervention groups. The results are consistent with previous studies, where a short period of Lactobacillus intervention did not significantly affect the intestinal flora of the organism (30–32). Several long-term factors, such as genetics, environmental factors, diet, disease, and stress, determine the structure of the intestinal flora of the host (33, 34). Short-term Lactobacillus interventions may not significantly influence the intestinal flora of the organism. Based on this, L. crispatus FSCDJY67L3 is safe for humans.
The present study has a number of limitations that need to be acknowledged. We did not enroll infants and adolescents under 18 years of age in clinical trials. A study published in The Lancet Child & Adolescent Health suggests that the global prevalence of H. pylori infection in children and adolescents aged 18 years and younger is 32.3%. The rate is significantly lower than the global average value of H. pylori infection (35). Therefore, populations under the age of 18 years were not enrolled in our study. On the other hand, a long-term intervention clinical trial is a challenge for patient recruitment. Therefore, the clinical trial intervention period was short in this research, which led to the inability to state whether the long-term intervention would have a negative impact on the strains themselves. Moreover, only one clinical trial with a small sample size was conducted in this study. Future clinical experimental studies with large sample sizes, multi-center, and more comprehensive and rigorous designs are needed. Subsequently, synergistic effects between these strains and conventional therapeutic drugs for H. pylori eradication or dietary components with antagonistic effects on H. pylori may also be explored.
5 Conclusion
In this study, we found that L. crispatus FSCDJY67L3 exhibited a strong ability to co-aggregate with H. pylori in artificial gastric fluid (pH=3). Hence, it could significantly reduce H. pylori load and improve gastrointestinal symptoms in H. pylori-positive patients. On the other hand, L. crispatus FSCDJY67L3 showed no influence on routine blood indices and blood biochemical indices related to liver and kidney function. In addition, it also exhibited no change in the composition and diversity of the intestinal flora of the subjects before and after the intervention. Based on this, L. crispatus FSCDJY67L3 shows great promise in the preparation of products, such as food or pharmaceutical products, for the prevention and/or treatment of H. pylori infection.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Yan cheng People’s Hospital (ET2021085). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QH: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. JW: Formal Analysis, Writing – review & editing. HZ: Data curation, Writing – review & editing. XL: Supervision, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Shanghai Municipal Government State-Owned Assets Supervision and Administration Commission Enterprise Innovation Development and Capacity Enhancement (No.2022013).
Conflict of interest
Authors QH, JW, and ZL were employed by the company Bright Dairy & Food Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer (2002) 2(1):28–37. doi: 10.1038/nrc703
2. Go MF. Natural history and epidemiology of Helicobacter pylori infection. Alimentary Pharmacol Ther (2002) 16(s1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x
3. Zhang M, Zhang C, Zhao J, Zhang H, Zhai Q, Chen W. Meta-analysis of the efficacy of probiotic-supplemented therapy on the eradication of H. pylori and incidence of therapy-associated side effects. Microbial Pathogenesis (2020) 147:104403. doi: 10.1016/j.micpath.2020.104403
4. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology (2019) 157(1):44–53. doi: 10.1053/j.gastro.2019.04.011
5. Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou J-M, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut (2022) 71(9):1724–62. doi: 10.1136/gutjnl-2022-327745
6. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology (2018) 155(5):1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007
7. Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Alimentary Pharmacol Ther (2016) 43(4):514–33. doi: 10.1111/apt.13497
8. Lesbros-Pantoflickova D, Corthèsy-Theulaz I, Blum AL. Helicobacter pylori and probiotics1,2. J Nutr (2007) 137(3):S812–8. doi: 10.1093/jn/137.3.812S
9. Chen Y-H, Tsai W-H, Wu H-Y, Chen C-Y, Yeh W-L, Chen Y-H, et al. Probiotic Lactobacillus spp. Act Against Helicobacter pylori-induced Inflammation. J Clin Med (2019) 8(1). doi: 10.3390/jcm8010090
10. Thuy TTD, Kuo PY, Lin SM, Kao CY. Anti-Helicobacter pylori activity of potential probiotic Lactiplantibacillus pentosus SLC13. BMC Microbiol (2022) 22(1). doi: 10.1186/s12866-022-02701-z
11. Zuo FL, Appaswamy A, Gebremariam HG, Jonsson AB. Role of Sortase A in Lactobacillus gasseri Kx110A1 Adhesion to Gastric Epithelial Cells and Competitive Exclusion of Helicobacter pylori. Front Microbiol (2019) 10:2770. doi: 10.3389/fmicb.2019.02770
12. Chen ME, Su CH, Yang JS, Lu CC, Hou YC, Wu JB, et al. Baicalin, baicalein, and Lactobacillus rhamnosus JB3 alleviated Helicobacter pylori infections in vitro and in vivo. J Food Sci (2018) 83(12):3118–25. doi: 10.1111/1750-3841.14372
13. Lesbros-Pantoflickova D, Corthesy-Theulaz I, Blum AL. Helicobacter pylori and probiotics. J Nutr (2007) 137(3):812S–8S. doi: 10.1093/jn/137.3.812S
14. Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol (2005) 3(4):320–32. doi: 10.1038/nrmicro1095
15. Holz C, Busjahn A, Mehling H, Arya S, Boettner M, Habibi H, et al. Significant Reduction in Helicobacter pylori Load in Humans with Non-viable Lactobacillus reuteri DSM17648: A Pilot Study. Probiotics Antimicrobial Proteins (2015) 7(2):91–100. doi: 10.1007/s12602-014-9181-3
16. Collado MC, Surono I, Meriluoto J, Salminen S. Indigenous dadih lactic acid bacteria: Cell-surface properties and interactions with pathogens. J Food Sci (2007) 72(3):M89–93. doi: 10.1111/j.1750-3841.2007.00294.x
17. Svedlund J, Sjödin I, Dotevall G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Digestive Dis Sci (1988) 33(2):129–34. doi: 10.1007/BF01535722
18. Jansson P-A, Curiac D, Ahrén IL, Hansson F, Niskanen TM, Sjögren K, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol (2019) 1(3):e154–e. doi: 10.1016/S2665-9913(19)30068-2
19. Choi IJ, Kim CG, Lee JY, Kim YI, Kook MC, Park B, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med (2020) 382(5):427–36. doi: 10.1056/NEJMoa1909666
20. Crowe SE. Helicobacter pylori infection. New Engl J Med (2019) 380(12):1158–65. doi: 10.1056/NEJMcp1710945
21. Svedlund J, Sjodin I, Dotevall G. GSRS - a clinical rating-scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic-ulcer disease. Digestive Dis Sci (1988) 33(2):129–34. doi: 10.1007/bf01535722
22. Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease [Review]. Trends Microbiol (2011) 19(7):349–59. doi: 10.1016/j.tim.2011.05.006
23. Chen L, Xu W, Lee A, He J, Huang B, Zheng W, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine (2018) 35:87–96. doi: 10.1016/j.ebiom.2018.08.028
24. Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications [Review]. Nat Rev Gastroenterol Hepatology. (2021) 18(9):613–29. doi: 10.1038/s41575-021-00449-x
25. Lu M, Yu S, Deng J, Yan Q, Yang C, Xia G, et al. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: A meta-analysis of randomized controlled trials. PloS One (2016) 11(10):e0163743. doi: 10.1371/journal.pone.0163743
26. Keikha M, Karbalaei M. Probiotics as the live microscopic fighters against Helicobacter pylori gastric infections. BMC Gastroenterol (2021) 21(1):388. doi: 10.1186/s12876-021-01977-1
27. Juntarachot N, Sunpaweravong S, Kaewdech A, Wongsuwanlert M, Ruangsri P, Pahumunto N, et al. Characterization of adhesion, anti-adhesion, co-aggregation, and hydrophobicity of Helicobacter pylori and probiotic strains. J Taibah Univ Med Sci (2023) 18(5):1048–54. doi: 10.1016/j.jtumed.2023.02.017
28. Mehling H, Busjahn A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass) as a new approach to Helicobacter pylori control in humans. Nutrients (2013) 5(8):3062–73. doi: 10.3390/nu5083062
29. Dore MP, Bibbo S, Pes GM, Francavilla R, Graham DY. Role of Probiotics in Helicobacter pylori Eradication: Lessons from a Study of Lactobacillus reuteri Strains DSM 17938 and ATCC PTA 6475 (Gastrus(R)) and a Proton-Pump Inhibitor. Can J Infect Dis Med Microbiol (2019) 2019:3409820. doi: 10.1155/2019/3409820
30. Yap TW-C, Gan H-M, Lee Y-P, Leow AH-R, Azmi AN, Francois F, et al. Helicobacter pylori eradication causes perturbation of the human gut microbiome in young adults. PloS One (2016) 11(3). doi: 10.1371/journal.pone.0151893
31. Brahe LK, Le Chatelier E, Prifti E, Pons N, Kennedy S, Blaedel T, et al. Dietary modulation of the gut microbiota - a randomised controlled trial in obese postmenopausal women [Article]. Br J Nutr (2015) 114(3):406–17. doi: 10.1017/s0007114515001786
32. Taverniti V, Scabiosi C, Arioli S, Mora D, Guglielmetti S. Short-term daily intake of 6 billion live probiotic cells can be insufficient in healthy adults to modulate the intestinal bifidobacteria and lactobacilli. J Funct Foods. (2014) 6:482–91. doi: 10.1016/j.jff.2013.11.014
33. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature (2018) 555(7695):210. doi: 10.1038/nature25973
34. Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients (2019) 11(12). doi: 10.3390/nu11122862
35. Yuan C, Adeloye D, Luk TT, Huang L, He Y, Xu Y, et al. Global Hlth Epidemiology Res G. The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child Adolesc Health (2022) 6(3):185–94. doi: 10.1016/s2352-4642(21)00400-4
Keywords: Lactobacillus crispatus, Helicobacter pylori, eradication, co-aggregation, load
Citation: Hong Q, Wang J, Zhang H, Liu X and Liu Z (2023) Study of the effect of Lactobacillus crispatus FSCDJY67L3 on Helicobacter Pylori eradication: a double-blind randomized controlled clinical trial. Front. Immunol. 14:1265995. doi: 10.3389/fimmu.2023.1265995
Received: 26 July 2023; Accepted: 29 September 2023;
Published: 26 October 2023.
Edited by:
Aijaz Ahmad, University of the Witwatersrand, South AfricaReviewed by:
Ravinder Kumar, University of Tennessee Health Science Center (UTHSC), United StatesWajihul Hasan Khan, All India Institute of Medical Sciences, India
Srivastava Vartika, University of the Witwatersrand, South Africa
Amber Khan, Scintillon Institute, United States
Copyright © 2023 Hong, Wang, Zhang, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenmin Liu, bGl1emhlbm1pbkBicmlnaHRkYWlyeS5jb20=
 Qing Hong
Qing Hong Jidong Wang1
Jidong Wang1 Xiaoming Liu
Xiaoming Liu Zhenmin Liu
Zhenmin Liu