
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 29 September 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1265797
 Nisa Vorasoot1,2
Nisa Vorasoot1,2 Madeleine Scharf3
Madeleine Scharf3 Ramona Miske3
Ramona Miske3 Smathorn Thakolwiboon4
Smathorn Thakolwiboon4 Divyanshu Dubey1,4
Divyanshu Dubey1,4 John R. Mills1
John R. Mills1 Sean J. Pittock1,4
Sean J. Pittock1,4 Anastasia Zekeridou1,4
Anastasia Zekeridou1,4 Anthonina Ott3
Anthonina Ott3 Andrew McKeon1,4*
Andrew McKeon1,4*Background: Purkinje cytoplasmic autoantibody type 1 (PCA-1)/anti-Yo autoimmunity is a common high-risk paraneoplastic neurological disorder, traditionally attributed antigenically to cerebellar degeneration–related protein 2 (CDR2), predominantly affecting women with gynecologic or breast adenocarcinoma. Single-modality CDR2 testing may produce false-positive results. We assessed the performance characteristics of the more recently purported major PCA-1/Yo antigen, CDR2-like (CDR2L), side by side with CDR2, in a line blot format.
Methods: CDR2 and CDR2L were tested in six specimen groups (serum and cerebrospinal fluid (CSF)). Group 1, PCA-1/Yo mouse brain indirect immunofluorescence assay (IFA) positives; Group 2, PCA-1/Yo IFA mimics; Group 3, suspected CDR2 line blot false positives; Group 4, consecutive patient samples tested for neural antibodies over 1 year; Group 5, healthy subject serums; and Group 6, polyclonal (non-specific) immunoglobulin G (IgG)-positive serums.
Results: Group 1: Of 64 samples tested, all but two were CDR2 positive (both CSF samples) and all were CDR2L positive. In individual patients, CDR2L values were always higher than CDR2. The two “CDR2L-only” positives were CSF samples with low titer PCA-1/Yo by IFA with serum negativity but with typical clinical phenotype. Group 2: All 51 PCA-1/Yo mimics were CDR2/CDR2L negative. Group 3: Nine samples [six of 1289 (0.47%) serums and three of 700 CSF samples (0.43%) were PCA-1/Yo IFA negative/CDR2 positive; two of the six available (serums from the same patient) were also CDR2L positive; the other four CDR2L negative had low CDR2 values (17–22). Group 4: Twenty-two patients had unexpected CDR2 or CDR2L positivity; none had tissue IFA positivity. Eleven of the 2,132 serum (0.5%) and three of the 677 CSF (0.4%) samples were CDR2 positive; median value was 19 (range, 11–48). Seven of the 2,132 serum (0.3%) and three of the 677 CSF (0.4%) samples were CDR2L positive; median value was 18 (range, 11–96). Group 5: All 151 healthy serum samples were negative. Group 6: One of the 46 polyclonal serum samples was CDR2L positive. Optimum overall performance was accomplished by requiring both CDR2 and CDR2L positivity in serum (sensitivity, 100%; and specificity, 99.9%) and positivity for CDR2L in CSF (sensitivity, 100%; and specificity, 99.6%).
Conclusion: CDR2L provides additional PCA-1/anti-Yo sensitivity in CSF, and dual positivity with CDR2 provides additional specificity assurance in serum. Combining antigen-specific and tissue-based assays optimizes PCA-1/anti-Yo testing.
Purkinje cytoplasmic autoantibody type 1 (PCA-1, also known as anti-Yo) is a biomarker of paraneoplastic neurological autoimmunity, usually manifesting as cerebellar ataxia (paraneoplastic cerebellar degeneration) in women with gynecologic or breast adenocarcinoma (1, 2). The diagnosis is typically accomplished in serum or CSF by screening for a criterion-based pattern of patient antibody staining of neuronal cytoplasmic elements of rodent brain tissue by immunohistochemical assay [either indirect immunofluorescence assay (IFA) or immunoperoxidase-based] and confirmed by Yo antigen [cerebellar degeneration–related protein 2 (CDR2)]–specific immunoblot (3–5). CDR2 testing, when used in isolation or as a screening test, has association with significant numbers of false-positive results (6, 7). CDR2-like (CDR2L) is now recognized as another major antigen in PCA-1/Yo autoimmunity and, perhaps, the main antigen (8, 9). Testing utilizing CDR2L line blot or cell-based assay has been reported to have improved performance characteristics over CDR2 (6, 10). Here, we compared the performance of CDR2 and CDR2L antigens in a line blot format among six patient and control groups (among over 3,000 tested).
This retrospective study was approved by the Mayo Clinic Institutional Review Board (IRB, 21-001297). Medical records of patients who consented to research review were included.
The following groups of patient samples were evaluated. Groups 1–4 were samples derived from clinical laboratory service, Neuroimmunology Laboratory, Mayo Clinic. Groups 5 and 6 were additional control samples tested. Neurological and cancer histories were obtained where possible for samples with unexpected results. Group 1 (PCA-1/Yo IFA positives): Samples (64) were serum (39) or CSF (25) from 48 patients identified in our clinical service laboratory (three were evaluated neurologically at Mayo Clinic, 34 elsewhere). All samples yielded PCA-1/Yo by tissue IFA (January 2019 to December 2022). Fifteen patients had serum/CSF pairs available for testing. Group 2 (PCA-1/Yo IFA mimics): There were 53 samples (30 serum and 23 CSF samples) with immunohistochemical staining mimicking but not fulfilling criteria for PCA-1/Yo (January 2019 to December 2022). Group 3 (suspected CDR2 line blot false positives): There were nine samples [six of the 1,289 serum (0.47%) and three of the 700 CSF (0.43%) samples] reflexed from IFA (27 February 2022 to 14 March 2023) without PCA-1/Yo by IFA, in which CDR2 was incidentally detected during confirmation testing for another antibody (e.g., anti-Hu). Six (five serum and one CSF samples) were available for CDR2L testing. Group 4 (consecutive Mayo Clinic referred for neural antibody testing): There were 2,809 consecutive available specimens (2,132 serum and 677 CSF samples; including 410 serum/CSF pairs), from patients all neurologically evaluated at Mayo Clinic, and referred for neural antibody testing in the Mayo Clinic Neuroimmunology Laboratory (19 September 2016 to 25 October 2017). Group 5 (healthy subject serums): There were 151 serum samples from healthy adult donors (EUROIMMUN). Group 6 (a potential source of false positives through non-specific IgG interference): There were 46 serum samples from patients without neurological disease but known to have polyclonal hypergammaglobulinemia (a potential interfering substance in IgG-binding assays).
IFA utilizing a composite of murine brain, gut, and kidney was performed as previously described (11). Criteria for PCA-1/Yo by indirect murine tissue–based IFA include neuronally restricted cytoplasmic staining throughout the cerebrum, myenteric plexus, and cerebellum. The distinctive features of the cerebellar staining (Figure 1A) include Purkinje (P) neuron cytoplasm with a “chunky” appearance, scattered molecular (M) layer neurons, and sparing of the granular (G) layer, save for isolated Golgi cells (GCs). In addition, there should be an absence of staining of control tissues (such as gastrointestinal mucosa and kidney parenchyma). Examples of PCA-1 mimics are shown in Figures 1B–E. For CDR2 and CDR2L line blot, each patient specimen was incubated with multi-antigen–coated test strips (EUROIMMUN) and was tested as previously described (12). CDR2 and CDR2L purification had been achieved by immobilized metal affinity chromatography. Bound IgG was detected by application of enzyme-conjugated anti-human IgG catalyzing a color reaction, and densitometric data were acquired by EUROBlotOne/EUROLINEScan software (performed at Mayo Clinic in Groups 1–3 and 6; cutoff for positive was ≥15) or by flatbed scanner/EUROLINEScan software (performed at EUROIMMUN in Groups 4 and 5; cutoff for positive was ≥11).
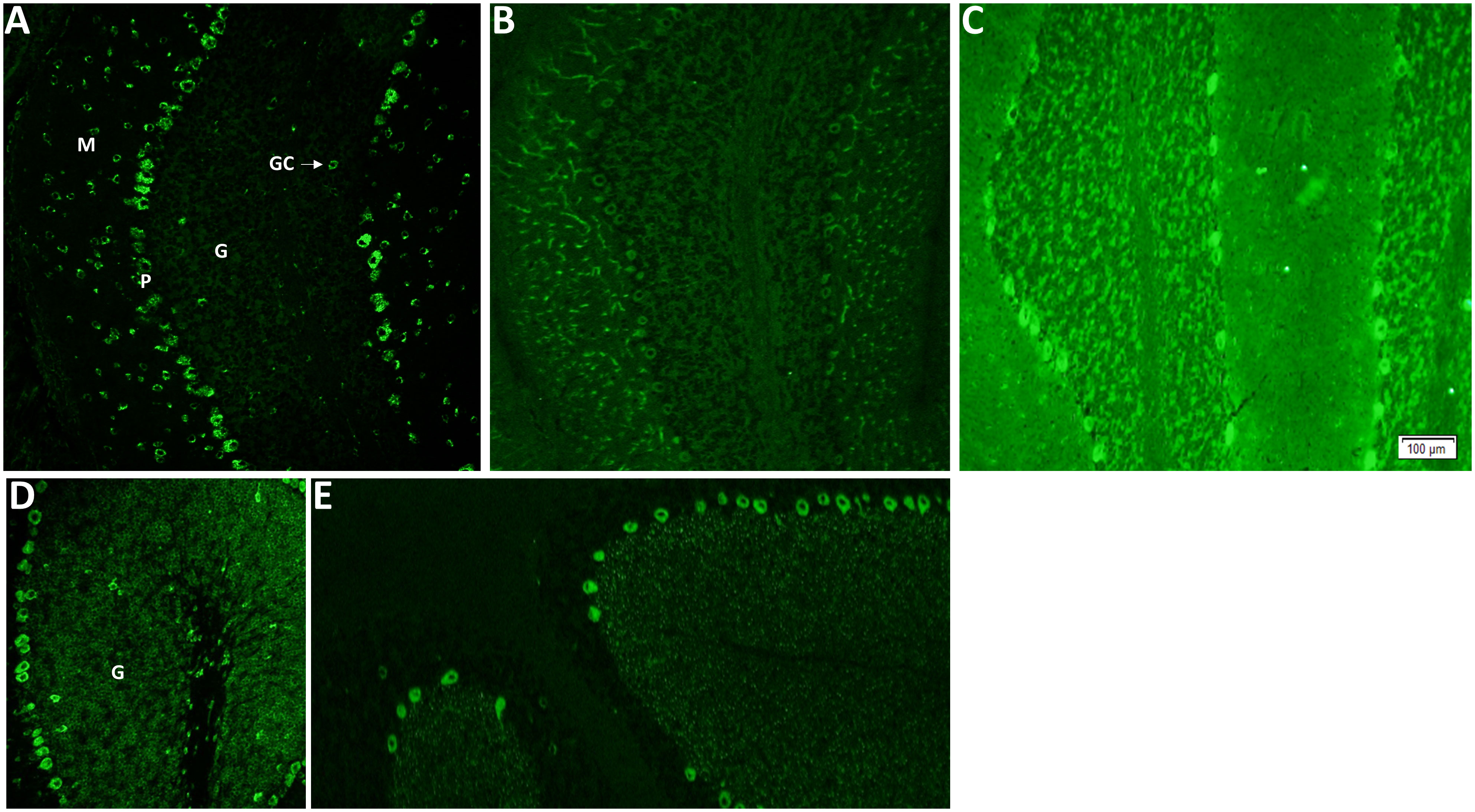
Figure 1 Indirect immunofluorescence assay utilizing mouse brain as substrate demonstrates Purkinje cytoplasmic antibody type 1 (PCA-1/Yo), other classified PCA antibodies, and mimics of PCA-1/Yo. (A) PCA-1-IgG/anti-Yo staining of cerebellum. Purkinje (P) neuron cytoplasm with a “chunky” appearance, scattered molecular (M) layer neurons and sparing of the granular (G) layer, save for isolated Golgi cells (GCs). Other classified PCA antibodies include PCA-2 (B; also known as MAP1B) and PCA-Tr (E; also known as DNER antibody). Unclassified mimics of PCA-1/Yo (protein target unknown) are diverse and exemplified in (C, D); a potentially treacherous PCA-1/Yo mimic is signal recognition particle (SRP) antibody (a biomarker of necrotizing myopathy). Although it produces diffuse cytoplasmic staining, it is distinguishable by the presence of granular (G) layer staining, which is largely absent for PCA-1/Yo, and staining of non-neural elements (such as proximal gastrointestinal mucosa, not shown).
Correlation of CDR2 and CDR2L values was evaluated using logistic regression analyses. Serum and CSF values were compared using Mann–Whitney U-test.
Anonymized data used for this study are available upon request.
All 48 patients were positive for PCA-1/Yo by tissue IFA (Table 1; Figure 1A). Median IFA titers were as follows: serum, 1:30,720 (range, 960–122,880; normal, ≤240); and CSF, 1:256 (range, 4–1,024; normal, ≤2). Typical of a PCA-1/Yo cohort, median age at testing was 64 years (range, 46–88 years), and all were women. The three patients neurologically assessed at Mayo Clinic had histories available. All had paraneoplastic cerebellar degeneration in the setting of adenocarcinoma (ovarian, 2; and breast, 1). Of 64 samples tested, all but two (96%) were CDR2 positive (median value of all positives, 132; range, 39–156), and all (100%) were CDR2L positive (median value, 186; range, 47–203). In serum, median value for CDR2 was 134 (range, 70–156) and that for CDR2L was 188 (range, 114–203). In CSF, median value for CDR2 was 127 (range, 5–148) and that for CDR2L was 178 (range, 47–195). Both CDR2L-only–positive samples were CSF (values, 47 and 115) and were from female patients, aged 50 and 64 years. Both were IFA serum negative (no CDR2/CDR2L testing possible as both serum samples had been discarded). Both had tissue IFA titers at the lower end of the positive range (end-point dilutions, 1:4 and 1:16). History, available in one of these two non-Mayo patients, revealed paraneoplastic cerebellar degeneration in the setting of breast adenocarcinoma.
All 53 samples were CDR2 and CDR2L negative (Table 1).
Overall, for data for Groups 1 and 2, there was a good correlation between CDR2 and CDR2L values (R2 = 0.970) (Figure 2). CDR2L values were always higher than CDR2 values in individual patients. CDR2L values (but not CDR2 values) were significantly higher in serum than that in CSF (p = 0.037).
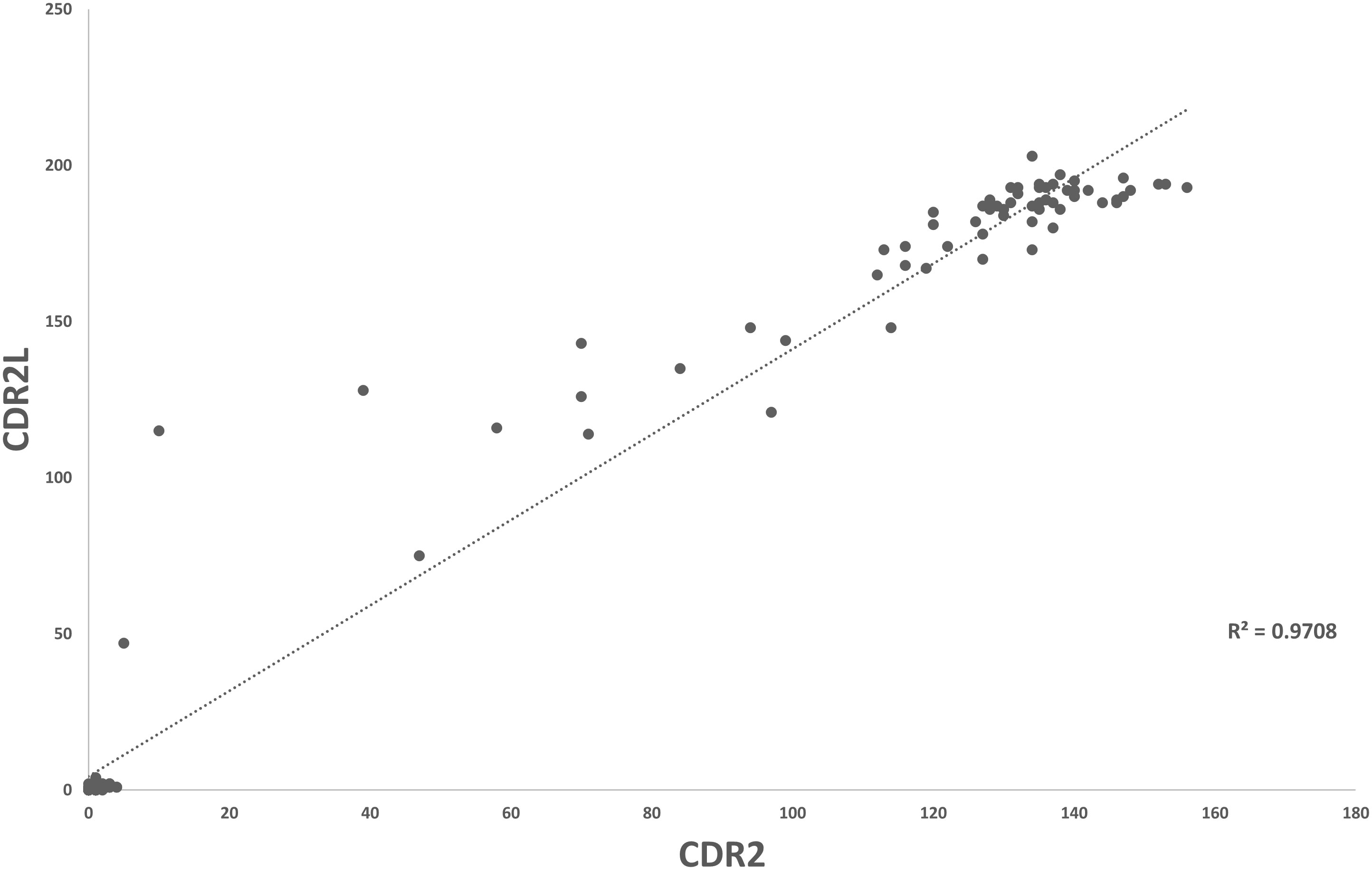
Figure 2 Scatter plot demonstrating correlation of CDR2- and CDR2L-positive values in patients with true-positive PCA-1/Yo (Group 1) and mimics (Group 2).
The historic CDR2 values for the six positive serum samples ranged from 17 to 59 (median, 22) and the three CSF samples from 19 to 30 (median value, 21). Six of the samples were available for CDR2L testing (five serum and one CSF samples). The solitary CDR2-positive CSF specimen tested was CDR2L negative. Two of the five PCA-1 IFA-negative/CDR2-positive serums were also CDR2L positive (value, 148 and 155, both from the same patient) (Table 1). This patient had antineuronal nuclear antibody type 1 (ANNA-1) detected by IFA and was also confirmed ANNA-1 (anti-Hu) antibody positive by line blot. Other small cell carcinoma paraneoplastic antibodies [SRY-box transcription factor 1 (SOX-1) (119) and Zic family member 4 (ZIC4) (145)] had also been detected by line blot only during clinical evaluation. The patient was an 80-year-old woman who presented with cerebellar ataxia and had small cell lung carcinoma (no history of breast or gynecologic adenocarcinoma). IFA for the three remaining specimens from two women and one man was reflexed from IFA for initially suspected non–PCA-1/Yo paraneoplastic antibodies (amphiphysin, 2; and ANNA-1, 1), and all ultimately were determined to be negative for those other antibodies. CDR2 values in those three samples were close to the cutoff for positive (≥15; range, 17–22), and CDR2L was negative in all. None of the three had CSF available for testing.
For context, two Mayo patients had expected PCA-1/Yo autoimmunity during the 1-year epoch (both had serum tested and one had CSF tested, and all samples positive). Both had paraneoplastic disorders in the setting of adenocarcinoma (of the breast and fallopian tube, respectively). Neither had samples residual for CDR2L testing. An additional 22 patients had unexpected CDR2 or CDR2L positivity, where tissue IFA was PCA-1/Yo negative. Thirteen patients were CDR2-only positive, eight patients were CDR2L-only positiv, and one patient was positive for both CDR2 and CDR2L (Table 2). For CDR2, 11 of the 2,132 serum (0.5%) and three of the 677 CSF (0.4%) samples were positive; median value was 19 (range, 11–48). For CDR2L, seven of the 2,132 serum (0.3%) and three of the 677 CSF (0.4%) samples were positive; median value was 18 (range, 11–96). Just one serum/CSF pair was weakly positive (Table 2, Patient 20). Eighteen of the 22 patients (82%) were men, and none had typical anti-Yo neurological or oncological phenotypes. For 21 of the 22 patients, positivity was for one analyte only: 13 were CDR2-only positive (11 were positive in serum and two in CSF) or eight were CDR2L-only positive (six were positive in serum, one in CSF, and one weakly positive in serum and CSF); all but one (Patient 13) had non-autoimmune neurological diagnoses (encephalitis), and just one (Patient 14) had active cancer (melanoma). The remaining patients (12) who had dual-CDR2/CDR2L positivity in CSF (serum negative) had a steroid-responsive presumed autoimmune meningoencephalitis.
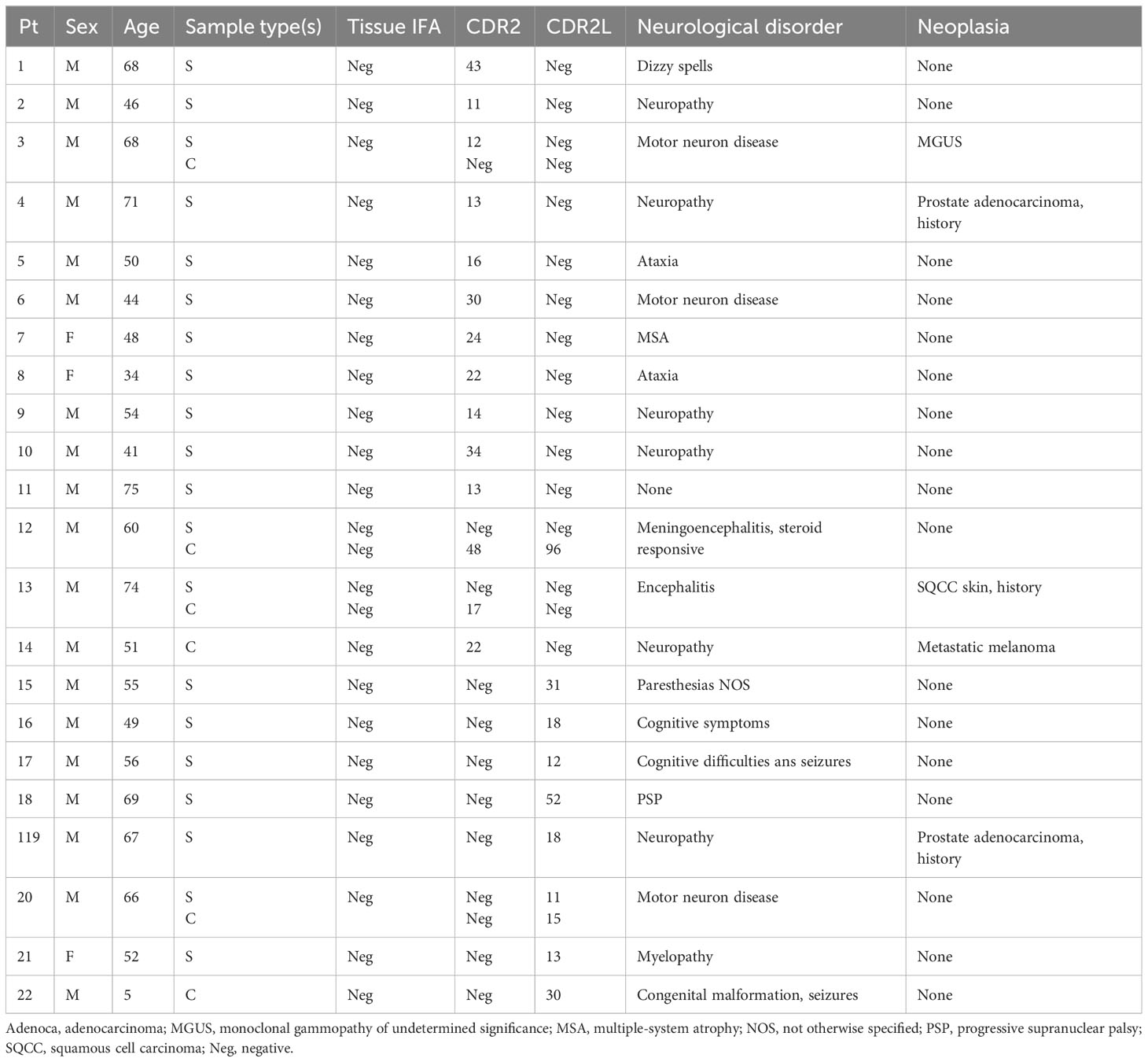
Table 2 Demographic, clinical, and serological data for Group 4 patients positive for CDR2 or CD2L by line blot.
None of the 151 serum samples were CDR2 or CDR2L positive (Table 1).
One of the 46 polyclonal serum samples (2%) was CDR2L positive (value, 118) (Table 1). This sample was then tested on IFA and was negative. None of the 46 samples were CDR2 positive.
To assess sensitivity and specificity, we aggregated all the data from patient groups where both CDR2 and CDR2L had been evaluated equally (Groups 1, 2, 4, 5, and 6) and used the presence or absence of tissue IFA criteria as the gold standard for PCA-1 autoimmunity. We did not include Group 3 data because CDR2L testing was only performed on the available nine false-positive CDR2-positive cases from that cohort of 1,989 samples tested. Sensitivity data were as follows: CDR2 in serum, 100%; CDR2L in serum, 100%; CDR2 in CSF, 92%; and CDR2L in CSF, 100%. Specificity data were as follows: CDR2 in serum, 99.5%; CDR2L in serum, 99.7%; CDR2 in CSF 99.6%; and CDR2L in CSF, 99.6%. Combining CDR2 and CDR2L with a requirement for detection of both analytes above the cutoff to yield a positive result, sensitivity was 100% in serum and 92% in CSF, and specificity was 99.9% for both specimen types. Combining CDR2 and CDR2L with a requirement for one analyte above the cutoff to yield a positive result, sensitivity was 100% in both serum and CSF samples, and specificity was 99.2% for serum and 99.6% for CSF.
PCA-1 (anti-Yo) autoimmunity is the second most common high-risk paraneoplastic antibody after ANNA-1 (anti-Hu) detected in clinical laboratory practice (13). Although PCA-1/Yo can be readily identified by tissue-based assay, there are also mimics encountered in laboratory practice that closely resemble the PCA-1/Yo staining pattern, and, thus, a robust confirmatory test is critically important. CDR2-based molecular testing has been the main source of confirmatory testing. However, questions have been raised regarding the utility of CDR2 because of the potential for false positives and false negatives (6). In addition, CDR2L has been proposed as the main antigen in PCA-1/Yo autoimmunity, which prompted us to study these antigens across a number of patient groups encompassing known PCA-1/Yo autoimmunity and potential sources of false positives (8, 9).
We addressed sensitivity of the line blot by comparing CDR2 and CDR2L as reflex confirmatory tests among samples meeting IFA criteria for PCA-1/Yo. The available histories from patients assessed neurologically at the Mayo Clinic indicated that this group was representative of PCA-1/Yo autoimmunity (women, with cerebellar ataxia and breast or gynecologic cancer). Both antigens had equivalent sensitivity to confirm PCA-1/Yo IFA results in serum (100%). However, CDR2L values were universally higher than CDR2 in PCA-1/Yo IFA-positive cases. This finding is consistent with those from a study using a peptide-based phage-immunoprecipitation platform (14). In particular, we found that CDR2L was more sensitive than CDR2 in CSF, being positive in two (of 17) low-titer IFA-positive cases where CDR2 was negative. Serum testing was also negative in those two cases. Thus, sensitivity for PCA-1/Yo autoimmunity is optimized by testing in CSF where serum is negative and confirming by CDR2L.
We addressed the specificity of the line blot by testing for CDR2 and CDR2L among samples with IFA mimicking PCA-1/Yo, sequential Mayo Clinic patients in whom neurological autoimmune testing had been requested, and healthy control serums and serums with the potential to produce interference in an immunoassay (polyclonal IgGs). Although the non-detection of CDR2/CDR2L among samples producing IFA features mimicking PCA-1/Yo and healthy controls was reassuring, some false positives were encountered for both analytes among consecutive Mayo Clinic patient samples generally referred for neurological autoimmune testing. These false positives were more commonly detected in serum than in CSF, usually for one analyte only and occurred more frequently for CDR2 than CDR2L. Overall, requiring positivity for both CDR2 and CDR2L optimized specificity for both serum and CSF samples, but there was some loss of sensitivity for CSF. Thus, optimum overall performance might be accomplished by requiring positivity for both CDR2 and CDR2L in serum and positivity for either in CSF. In the Mayo Clinic experience to date, the values for CDR2 false-positive results (highest being 59 for serum and 19 for CSF) have been lower than that for true positives (lowest being 70 for serum and 39 for CSF). It should be borne in mind that some laboratories screen by line blot and reflex to tissue-based assay for confirmation, the opposite of the approach described here.
The two patients encountered with immune-mediated neurological disorders in whom both CDR2 and CDR2L were detected at intermediate or high values, but without PCA-1/Yo observed by tissue IFA, did not have PCA-1/Yo typical phenotypes. The immunological and clinical significance of these findings are unknown. One patient was a man with steroid-responsive meningoencephalitis without cancer with dual positivity in CSF but not in serum. The other was a woman with cerebellar degeneration but in the context of ANNA-1 (anti-Hu) by tissue IFA, with dual-CDR2/CDR2L positivity in serum (CSF not available) and had the ANNA-1–associated neoplasm (small cell carcinoma) rather than PCA-1/Yo–associated breast or gynecologic neoplasia. Consistent with the immune response against multiple neural antigens encountered in small cell carcinoma, she was also positive for Hu, SOX-1, and ZIC4 by line blot (15, 16). It is possible that the PCA-1/Yo staining pattern by IFA was masked by coexisting ANNA-1 staining. The coexistence of PCA-1/Yo and ANNA-1 antibodies in patients with dual cancers is extremely rarely reported (17).
Limitations of our study include missing clinical data for some subjects, and the focus on line blots currently used commonly in clinical practice. Future studies will need to examine promising CDR2L cell–based assays in a similarly high-throughput laboratory practice (10). In addition, our sensitivity assessment was undertaken using PCA-1/Yo IFA criteria as the gold standard rather than clinical cohorts. However, in our experience, classical sensitivity assessments of paraneoplastic antibodies are uninformative because of the rarity of the individual antibodies. Even among well-defined seropositive autoimmune ataxias PCA-1/Yo accounts for just 30% of patients (18). PCA-1/Yo was recently reported to account for 1.6% of all positives in an academic clinical service laboratory (19). Clinical specificity was addressed among the very large and clinically diverse Group 4 cohort (consecutive Mayo Clinic patients tested over 13 months).
We conclude that, for PCA-1/Yo autoimmunity, CDR2L in the line blot format improves sensitivity in CSF and is optimized for specificity in serum when combined with CDR2. Our observations give emphasis to the importance of pairing tissue-based and antigen-specific assays for the diagnosis of paraneoplastic neurological disorders (3). Although a specificity of >99% can seem reassuring, in high-throughput clinical laboratory practice, even a small loss of specificity can translate into a false positives “issue” (20). Ideally, testing laboratories should screen for PCA-1/Yo by tissue-based assay and confirm by antigen-specific assay, such as the line blots described here. Line blot data used in isolation should be interpreted with caution, particularly for low positive results in serum. Pairing CDR2 and CDR2L may help overcome some interpretative issues.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Mayo Clinic Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
NV: Data curation, Investigation, Methodology, Writing – review & editing. MS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. RM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. ST: Formal Analysis, Writing – review & editing. DD: Data curation, Formal Analysis, Writing – review & editing. JM: Data curation, Formal Analysis, Writing – review & editing. SP: Data curation, Formal Analysis, Writing – review & editing. AZ: Data curation, Formal Analysis, Writing – review & editing. AO: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. AM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Institutes of Health (RO1NS126227).
Supported by the Center for Multiple Sclerosis and Autoimmune Neurology, Mayo Clinic.
MS is employed by Euroimmun. RM is employed by Euroimmun. SP is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; has patents pending for KLHL11-IgG and Septin-5-IgG; has issued for MAP1B-IgG as markers of neurologic autoimmunity and paraneoplastic disorders; has consulted for Alexion and Medimmune; and has received research support from Genentech, Grifols, Medimmune, and Alexion. DD has research support from Department of Defense CA210208 and PR220430 and UCB; has consulted for UCB, Immunovant, Argenx, and Astellas pharmaceuticals compensation for consulting activities paid directly to Mayo Clinic; and has patent licensed for KLHL11-IgG and patents pending for LUZP4-IgG and cavin-4-IgG as markers of neurological autoimmunity. AZ has patent applications pending on PDE10A-IgG and DACH1-IgG as biomarkers of paraneoplastic neurological autoimmunity, has received research funding from Genentech not relevant to this study, and has consulted without personal compensation for Alexion Pharmaceuticals. AO is employed by Euroimmun. A. McKeon reports research funding from National Institutes of Health NIH: RO1NS126227, U01NS120901; has patents issued for GFAP and MAP1B-IgGs and patents pending for PDE10A, Septins-5 and -7, and KLCHL11-IgGs; and has consulted for Janssen and Roche pharmaceuticals, without personal compensation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol (1983) 14(6):609–13. doi: 10.1002/ana.410140603
2. McKeon A, Tracy JA, Pittock SJ, Parisi JE, Klein CJ, Lennon VA. Purkinje cell cytoplasmic autoantibody type 1 accompaniments: the cerebellum and beyond. Arch Neurol (2011) 68(10):1282–9. doi: 10.1001/archneurol.2011.128
3. Graus F, Vogrig A, Muniz-Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm (2021) 8(4). doi: 10.1212/NXI.0000000000001014
4. Fathallah-Shaykh H, Wolf S, Wong E, Posner JB, Furneaux HM. Cloning of a leucine-zipper protein recognized by the sera of patients with antibody-associated paraneoplastic cerebellar degeneration. Proc Natl Acad Sci U.S.A. (1991) 88(8):3451–4. doi: 10.1073/pnas.88.8.3451
5. Altermatt HJ, Rodriguez M, Scheithauer BW, Lennon VA. Paraneoplastic anti-Purkinje and type I anti-neuronal nuclear autoantibodies bind selectively to central, peripheral, and autonomic nervous system cells. Lab Invest (1991) 65(4):412–20.
6. Herdlevaer I, Haugen M, Mazengia K, Totland C, Vedeler C. Paraneoplastic cerebellar degeneration: the importance of including CDR2L as a diagnostic marker. Neurol Neuroimmunol Neuroinflamm (2021) 8(2). doi: 10.1212/NXI.0000000000000963
7. Dechelotte B, Muniz-Castrillo S, Joubert B, Vogrig A, Picard G, Rogemond V, et al. Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm (2020) 7(3). doi: 10.1212/NXI.0000000000000701
8. Eichler TW, Totland C, Haugen M, Qvale TH, Mazengia K, Storstein A, et al. CDR2L antibodies: A new player in paraneoplastic cerebellar degeneration. PloS One (2013) 8(6):e66002. doi: 10.1371/journal.pone.0066002
9. Krakenes T, Herdlevaer I, Raspotnig M, Haugen M, Schubert M, Vedeler CA. CDR2L is the major yo antibody target in paraneoplastic cerebellar degeneration. Ann Neurol (2019) 86(2):316–21. doi: 10.1002/ana.25511
10. Erikstad KI, Herdlevaer I, Peter E, Haugen M, Totland C, Vedeler C. A cerebellar degeneration-related protein 2-like cell-based assay for anti-Yo detection in patients with paraneoplastic cerebellar degeneration. Eur J Neurol (2023) 30(6):1727–33. doi: 10.1111/ene.15786
11. Flanagan EP, Hinson SR, Lennon VA, Fang B, Aksamit AJ, Morris PP, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann Neurol (2017) 81(2):298–309. doi: 10.1002/ana.24881
12. Miske R, Scharf M, Stark P, Dietzel H, Bien CI, Borchers C, et al. Autoantibodies against the purkinje cell protein RGS8 in paraneoplastic cerebellar syndrome. Neurol Neuroimmunol Neuroinflamm (2021) 8(3). doi: 10.1212/NXI.0000000000000987
13. Kunchok A, McKeon A, Zekeridou A, Flanagan EP, Dubey D, Lennon VA, et al. Autoimmune/paraneoplastic encephalitis antibody biomarkers: frequency, age, and sex associations. Mayo Clin Proc (2022) 97(3):547–59. doi: 10.1016/j.mayocp.2021.07.023
14. O'Donovan B, Mandel-Brehm C, Vazquez SE, Liu J, Parent AV, Anderson MS, et al. High-resolution epitope mapping of anti-Hu and anti-Yo autoimmunity by programmable phage display. Brain Commun (2020) 2(2):fcaa059. doi: 10.1093/braincomms/fcaa059
15. Sabater L, Titulaer M, Saiz A, Verschuuren J, Gure AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology (2008) 70(12):924–8. doi: 10.1212/01.wnl.0000281663.81079.24
16. Bataller L, Wade DF, Graus F, Stacey HD, Rosenfeld MR, Dalmau J. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology (2004) 62(5):778–82. doi: 10.1212/01.WNL.0000113749.77217.01
17. Li ZC, Cai HB, Fan ZZ, Zhai XB, Ge ZM. Paraneoplastic neurological syndrome with positive anti-Hu and anti-Yo antibodies: A case report. World J Clin cases (2022) 10(13):4190–5. doi: 10.12998/wjcc.v10.i13.4190
18. Jones AL, Flanagan EP, Pittock SJ, Mandrekar JN, Eggers SD, Ahlskog JE, et al. Responses to and outcomes of treatment of autoimmune cerebellar ataxia in adults. JAMA Neurol (2015) 72(11):1304–12. doi: 10.1001/jamaneurol.2015.2378
19. Arino H, Ruiz Garcia R, Rioseras B, Naranjo L, Martinez-Hernandez E, Saiz A, et al. Frequency and referral patterns of neural antibody studies during the COVID-19 pandemic: experience from an autoimmune neurology center. Neurol Neuroimmunol Neuroinflamm (2023) 10(4). doi: 10.1212/NXI.0000000000200129
Keywords: autoimmune, ataxia, paraneoplastic, breast cancer, ovarian cancer
Citation: Vorasoot N, Scharf M, Miske R, Thakolwiboon S, Dubey D, Mills JR, Pittock SJ, Zekeridou A, Ott A and McKeon A (2023) CDR2 and CDR2L line blot performance in PCA-1/anti-Yo paraneoplastic autoimmunity. Front. Immunol. 14:1265797. doi: 10.3389/fimmu.2023.1265797
Received: 23 July 2023; Accepted: 12 September 2023;
Published: 29 September 2023.
Edited by:
Lidia Sabater, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), SpainReviewed by:
Raquel Ruiz García, Hospital Clinic of Barcelona, SpainCopyright © 2023 Vorasoot, Scharf, Miske, Thakolwiboon, Dubey, Mills, Pittock, Zekeridou, Ott and McKeon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew McKeon, bWNrZW9uLmFuZHJld0BtYXlvLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.