- 1Department of Laboratory Medicine, Hunan Provincial People’s Hospital (the First- Affiliated Hospital of Hunan Normal University), Changsha, China
- 2Department of Laboratory Medicine, the First- Affiliated Hospital of Hunan Normal University (Hunan Provincial People’s Hospital), Changsha, China
- 3Research Office of Clinical Laboratory, Clinical Translational Medicine Research Institute of Hunan Provincial People’s Hospital (the First-affiliated Hospital of Hunan Normal University), Changsha, China
Background: Primary biliary cholangitis (PBC) is a chronic intrahepatic cholestatic autoimmune liver disease characterized by inflammatory injury of small and medium-sized bile ducts in the liver. The pathogenesis of PBC has yet to be entirely understood. CD47/signal-regulatory protein alpha (SIRPα) is closely related to developing autoimmune diseases by promoting inflammatory response. However, the effect of CD47/SIRPα on inflammatory response in PBC patients is still unclear.
Objective: We investigated the expression of CD47/SIRPα and the effect of inflammatory cytokines on the CD47 expression, analyzed potential autoantibodies against CD47 and the effect of anti-CD47 antibody on the inflammatory response in PBC, provided laboratory basis for the study of the pathogenesis and targets for non-invasive diagnosis and treatment on PBC.
Methods: The expression levels of CD47 and SIRPα on peripheral blood mononuclear cells (PBMC) were measured in 14 patients with PBC (the PBC group) and 13 healthy subjects (the Control group) by flow cytometry (FCM). The PBMC derived from healthy subjects were stimulated with healthy subjects’ serum, PBC patients’ serum, IFN-α or TNF-α, and the CD47 expression level on CD14+ monocytes was detected by FCM. The level of serum anti-CD47 antibody or IFN-α in PBC patients and healthy subjects was analyzed by ELISA. FCM was used to examine the TNF-α expression level in CD14+ monocytes of healthy subjects stimulated with isotype control antibody, anti-CD47 antibody, LPS or LPS combined with CD47 antibody.
Results: The CD47 expression level on the CD14+ monocytes in PBC patients was statistically higher than that in the Control group (P<0.01). Compared with the Control group (PBMC+healthy serum), the CD47 expression on CD14+ monocyte stimulated with the PBC patients’ serum (PBMC+PBC patients’ serum) was increased (P<0.001); the CD47 expression on CD14+ monocyte stimulated with IFN-α (PBMC + IFN-α) increased gradually with the increased concentration of IFN-α (P<0.05). However, there was no similar trend on CD14+ monocyte stimulated with the TNF-α (PBMC+TNF-α) (P>0.05). The levels of serum anti-CD47 antibody and IFN-α in the PBC patients were higher than those in healthy subjects (P<0.05). The TNF-α expression level in CD14+ monocyte stimulated with the LPS (PBMC+LPS) or anti-CD47 antibody+LPS group (PBMC+LPS+anti-CD47 antibody) was significantly increased than that in the Control group (PBMC+isotype control antibody) (P<0.01 and P<0.001, respectively). The TNF-α expression level in CD14+ monocyte stimulated with the anti-CD47 antibody + LPS was higher than that with the LPS (P< 0.05).
Conclusion: The CD47 may be related to the pathogenesis of PBC by inflammatory response. The CD47/SIRPα signal were imbalanced in PBC patients. The presence of serum anti-CD47 antibodies in PBC patients provides a laboratory basis for clinical diagnosis and treatment.
1 Introduction
Primary biliary cholangitis (PBC) is a chronic intrahepatic cholestatic autoimmune liver disease characterized by inflammatory injury of small and medium-sized bile ducts in the liver. It occurs mainly in middle-aged and older women; the onset is mostly hidden. The early symptoms of the disease are not specific, and it is easy to miss or misdiagnose (1). The pathogenesis of PBC needs to be clarified. The interaction among environmental, genetic, and hormonal factors led to congenital and adaptive immune disorders and loss of self-tolerance (2), leading to antibody and T cell-mediated specific immune attacks against the liver, progressive inflammatory necrosis, and fibrosis of the liver (3, 4). The activation of inflammatory mediators such as type I interferon (IFN-I) and tumor necrosis factor (TNF), innate liver lymphocytes, and natural killer T cells played a crucial role in the pathogenesis of the disease (5). In addition, due to the existence of the ‘gut-liver axis’, when the intestinal mucosa was damaged, Lipopolysaccharides (LPS), the cell wall component of Gram-negative bacteria, entered the liver through the portal vein, which would cause activation of Toll-like receptors 4 (TLR4) and nuclear factor kappa-B (NF-κB) signaling pathway, increased the expression of inflammatory factors, and aggravated the liver from autoimmune damage (6).
CD47 is a transmembrane protein expressed in different cell types, such as thymocytes, T and B cells, monocytes, erythrocytes and nerve cells. It belongs to the immunoglobulin superfamily (7) and is a supramolecular complex composed of integrin, G protein, and cholesterol (8). It interacts with corresponding ligands and mediates cell proliferation, migration, phagocytosis, apoptosis, immune homeostasis, and inhibition of nitric oxide signaling (9, 10). signal-regulatory protein alpha (SIRPα) binds to CD47 to initiate an inhibitory signaling pathway, resulting in weakened phagocytosis of macrophages to malignant cells (11).
The binding of CD47 on erythrocyte to the SIRPα on macrophages prevented erythrocyte phagocytosis by suppressing phagocytic activity. The polycythemia phenotype was restored by intercepting CD47-SIRPα through either anti-CD47 treatment or loss of the inhibitory SIRPα-signal in a PV mouse model (12). Both solid and hematologic malignancies expressed higher levels of CD47, which bound with SIRPα to protect the tumor cell against macrophage-mediated phagocytosis (13–15). The CD47 enabled cancer cells to escape innate and adaptive immune surveillance leading to metastatic spread, which could be restricted by the administration of anti-CD47 antibodies through affecting tumor growth and tumor microenvironment signaling (16, 17). Anti CD47 therapy promoted T cell secretion of pro-inflammatory cytokines in an undifferentiated pleiomorphic sarcoma which expressed highly CD47 (18).
CD47-SIRP α signaling pathway is related to the development of autoimmune diseases. CD47 deficiency improved ocular autoimmune inflammation (19) and furthered macrophage-mediated phagocytosis in type I diabetes (20). CD47 facilitated autoimmune valvular carditis through damaging macrophage efferocytosis and increasing cytokine production (21). T cell activation was regulated by macrophages through CD47/SIRPA in inflammatory bowel disease (22). Jin et al. (7) reported that the CD47 expression level on monocytes in SLE patients was significantly increased, promoting SLE patients’ inflammatory response. However, the effect of CD47/SIRPα on the inflammatory response in PBC patients has not been reported.
Therefore, the present research clarified the expression of CD47/SIRPα on mononuclear cells in PBC patients, investigated the effect of serum inflammatory cytokines on the CD47 expression in PBC patients, analyzed potential autoantibodies against CD47 in PBC patients, and explored the role of CD47 and anti-CD47 antibody in the inflammatory response of PBC, provided laboratory basis for the study of the pathogenesis and targets for non-invasive diagnosis and treatment on PBC.
2 Materials and methods
2.1 Research object
From December 2021 to February 2023, 14 PBC patients and 13 healthy subjects were selected from Hunan Provincial People’s Hospital (the First Affiliated Hospital of Hunan Normal University). Diagnosis of PBC was based on the published American Association for the Study of Liver Diseases (AASLD) criteria, APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis (2022) (23). Patients were excluded with one or more of the following conditions: (1) severe cardiovascular disease, kidney disease and other serious diseases; (2) acute and chronic infectious diseases and various non-PBC immune system diseases; (3) pregnant or lactating women; (4) liver cancer or other types of malignant tumor. The ethics committee of Hunan Provincial People’s Hospital approved the exemption of informed consent, because theresearch used the remaining samples after clinical testing, which did not cause additional harm to patients.
2.2 Peripheral blood experiments
2.2.1 Extraction of peripheral blood mononuclear cells
EDTA anticoagulant venous blood of PBC patients or healthy subjects diluted 2-4 times with PBS was mixed with Ficoll lymphatic separation solution at 1:1, centrifuged at 400 g, 18°C-20°C for 40 min. The PBMC layer was taken and centrifuged twice to leave the PBMC for use.
2.2.2 Detection of CD47/SIRPα expression on the mononuclear cells
After treated with Fixable Viability Dye eF780(#65-0865, eBioscience, USA) and FC receptor blockers (#564765, BD Biosciences, Germany), PBMCs (105 cells/mL) were incubated for 30 min at 4°C in the dark with 5ul of fluorescent dyes CD3-Percp-Cy5.5 (#560835, BD Biosciences), CD19-BV421 (#562440, BD Biosciences), CD14-BV510 (#563079, BD Biosciences), CD56-BV650(#564057, BD Biosciences), SIRPα-APC (#17-1729-42, eBioscience), 20ul of CD16-PE (#555407, BD Biosciences) or CD47-FITC (#556045, BD Biosciences). After washing, the cells were detected by CytoFlex V5-B5-R3 Flow cytometer (No: 38385, Beckman USA). The expressions of CD47 and SIRPα in CD3+ T cells, CD19+ B cells, CD56+ NK cells or CD14+ monocytes in PBMC were analysed using flowjo 6.2 software. The gate-drawing strategies for CD3+ T cells, CD19+ B cells, CD56+ NK cells and CD14+ monocytes were shown in Figure 1.
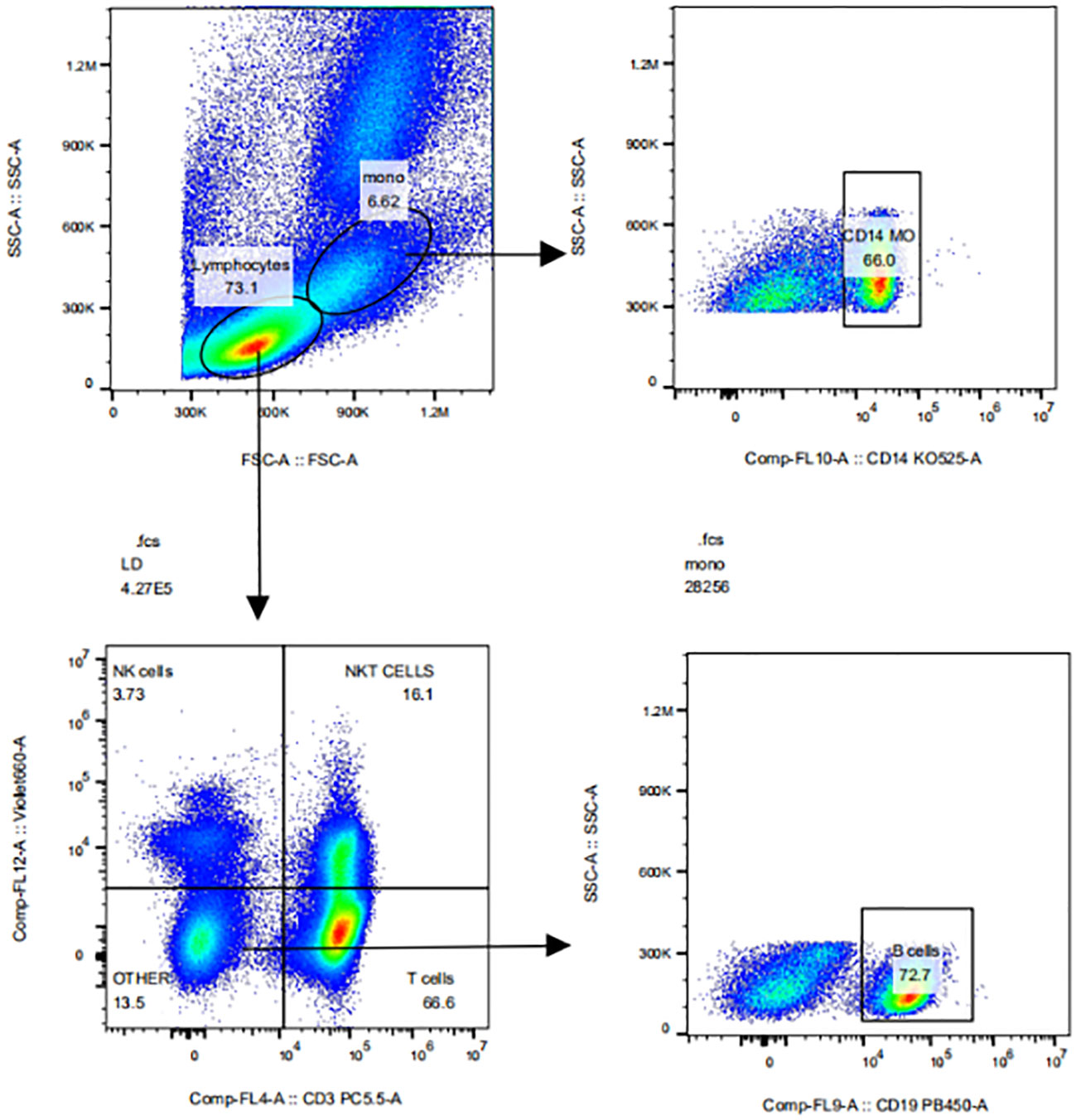
Figure 1 The gate-drawing strategies of CD3+T cells, CD19+B cells, CD56+NK cells and CD14+ monocytes.
2.2.3 Detection of anti-CD47 antibody level and IFN-α level
According to the instructions, the serum anti-CD47 level and the IFN-α level in the PBC patients and healthy subjects were detected by ELISA kits (#F-111548-A, FANKEW, China) (#F10665-A, FANKEW, China).
2.2.4 Detection of ANA and AMA
Antinuclear antibody (ANA) and antimitochondrial antibody (AMA) were detected by indirect immunofluorescence method [EUROIMMUN Medical Diagnostics (China) Co., Ltd.], autoimmune liver disease antibodies were detected by membrane strip immunoblotting (Suzhou Haobo Co., Ltd.), and serum immunoglobulins by rate scattering turbidimetric assay (Siemens, USA), liver function was detected by the automatic biochemical analyzer (Hitachi Medical Instrument Co., Ltd.).
2.3 In vitro experiments
2.3.1 Detection of CD47 expression on the CD14+ monocytes
PBMCs were extracted from healthy subjects. PBMC samples were divided into the Control, PBC, IFN-α, and TNF-α groups. Cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS). According to the grouping, 100 uL of healthy human serum, 100 uL of PBC patient serum, IFN-α (#ab200262, absin, China) (0/10/50) ng/mL, or TNF-α (#ab259410, absin) (0/10/50) ng/mL was added to PBMC (105 cells/mL), mixed and incubated at 37°C, 5% CO2 incubator overnight (12 h). Cells were collected and treated with Fixable Viability Dye eF780(#65-0865, eBioscience, USA) and FC receptor blockers (#564765, BD Biosciences, Germany). After washing, 5 μL of fluorescent dye CD14-BV510 (#563079, BD Biosciences) and 20ul of CD47-FITC (#556045, BD Biosciences) were added and incubated at 4°C in the dark for 30 min. After washing, the cells were detected by CytoFlex V5-B5-R3 Flow cytometer (No: 38385, Beckman USA) Flow cytometer.
2.3.2 Detection of TNF-α level in the CD14+ monocytes
PBMCs were extracted from healthy subjects. PBMC samples were divided into the CD47 isotype control group, anti-CD47 antibody group, LPS group, and anti-CD47 antibody + LPS group. The cells were cultured in RPMI-1640 medium containing 10% FBS. According to the grouping, 1 μg/mL of isotype control (#14-4321-85, eBioscience), 1 μg/mL of anti-CD47 antibody (#16-0471-81, eBioscience), 3 μg/mL of LPS (#abs47014848, absin), 1 μg/mL of anti-CD47 antibody (#16-0471-81, eBioscience) + 3 μg/mL of LPS (#abs47014848, absin) was added to PBMC (105 cells/mL), respectively. After mixing, they were cultured in 37°C, 5% CO2 incubator for 5 h. Cells were collected and treated with Fixable Viability Dye eF780(#65-0865, eBioscience, USA) and FC receptor blockers (#564765, BD Biosciences, Germany). After washing, 5 μL of fluorescent dye CD14-BV510 (#563079, BD Biosciences) was added and incubated at 4°C for 30 min in the dark. Washing, an intracellular staining antibody (TNF-α-PE-c) (#557647, BD Biosciences) was added. Washing again, the cells were detected by CytoFlex V5-B5-R3 Flow cytometer (No: 38385, Beckman USA) Flow cytometer.
2.4 Statistical analysis
Statistical analysis was performed using Graph Prism 8.3.0 and SPSS Statistics 25 software. Flow data were analysed using the flowjo 6.2 software. The measurement data of normal distribution were expressed as mean ± standard deviation. One-way ANOVA was used to compare the differences among multiple groups, and Post Hoc post hoc test was used for pairwise comparison. The difference between the two groups was analyzed by independent sample t-test or paired t-test. Non-normal distribution data were expressed as median (quartile). Mann-Whitney U test was used for comparison between two groups and multiple groups. P<0.05 indicated a statistically significant difference.
3 Results
3.1 Clinical indicators of patients
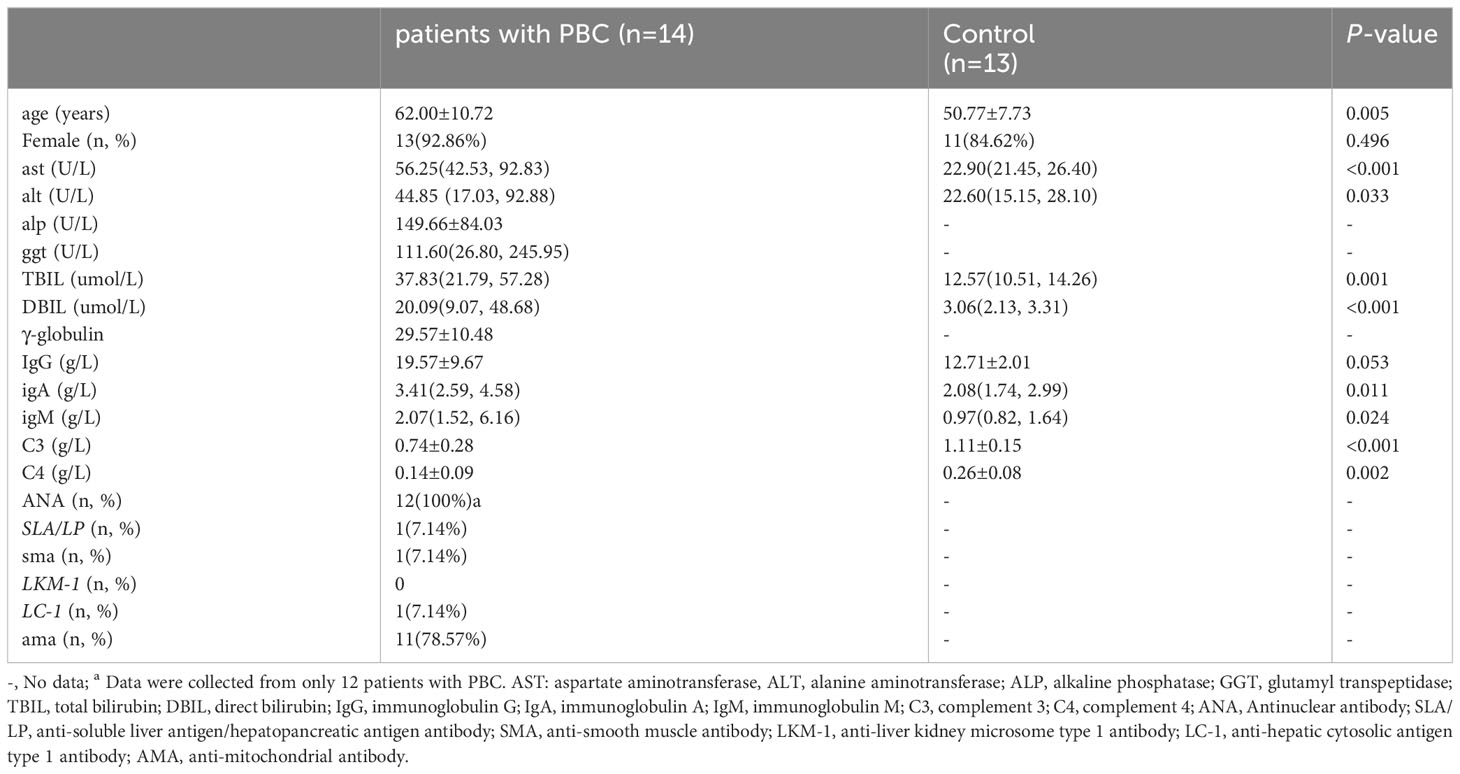
A total of 14 patients with PBC and 13 healthy subjects were collected. Among the enrolled 14 PBC patients, 12 cases were positive for ANA, 11 cases were positive for AMA, 1 case was positive for anti-soluble liver antigen/hepatopancreatic antigen antibody (SLA/LP), 1 case for anti-smooth muscle antibody (SMA) and 1 case for anti-hepatocyte cytoplasmic type 1 antibody (LC-1). There was no significant difference in gender and immunoglobulin G between the two groups (P>0.05). The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), immunoglobulin M (IgM) and immunoglobulin A (IgA) in PBC patients were higher than those in the control group (P<0.05). In contrast, complement 3 (C3) and complement 4 (C4) were opposite (P<0.05). The clinical indicators of PBC patients and healthy subjects enrolled in this research were shown in Table 1.
3.2 The expression levels of CD47 and SIRPα on mononuclear cells in PBC patients
The mean fluorescence intensity (MFI) of CD47 and SIRPα expression on the PBMC surface of PBC patients was shown in Figure 2. The expression levels of CD47 on CD14+ monocytes (81484 ± 31179), CD56+ NK cells (28982 ± 10467), CD3+ T cells (25124 ± 7565) and CD19+ B cells (22639 ± 5596) in PBC patients were statistically different (F=24.705, P<0.001). The CD47 expression level on CD14+ monocytes was higher than that on CD56+ NK cells, CD3+ T cells or CD19+ B cells in PBC patients (t=5.812, 6.573, 6.951, P<0.001, respectively), there were no significant difference in the CD47 expression levels among the CD3+ T cells, CD19+ B cells and CD56+ NK cells in PBC patients (P>0.05) (Figure 2A).
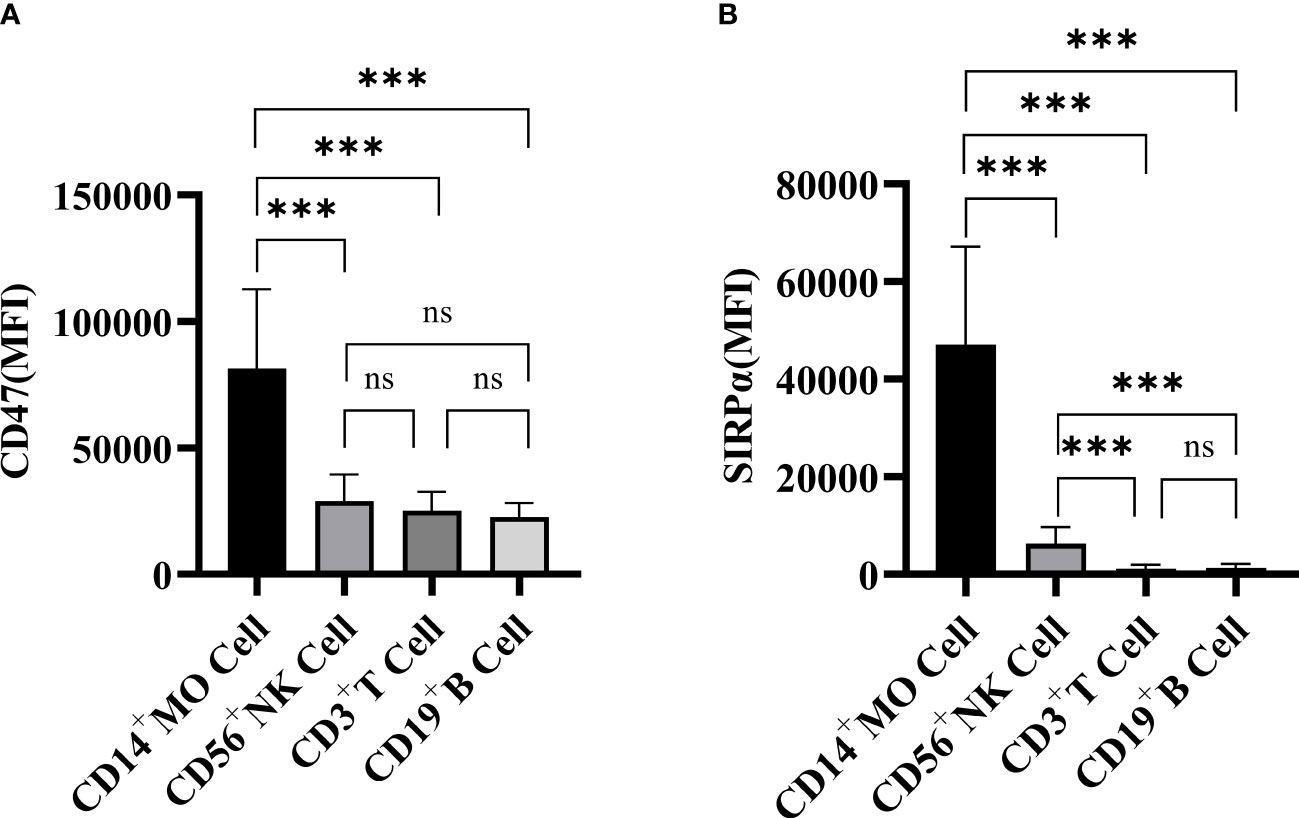
Figure 2 The expression of CD47 (A) and SIRPα (B) on CD3+ T cells, CD19+ B cells, CD56+ NK cells and CD14+ monocytes in PBC patients. ns: no statistical significance; ***P< 0.001, **P< 0.01, *P< 0.05.
The SIRPα expression levels on CD14+ monocytes (47112 ± 20060), CD56+ NK cells (6332 ± 3348), CD3+ T cells (1166 ± 785) and CD19+ B cells (1308 ± 813) in PBC patients were statistically different (F=33.367, P<0.001). The SIRPα expression level on CD14+ monocytes was higher than that on CD56+ NK cells, CD3+ T cells or CD19+ B cells in PBC patients (Figure 2B).
The CD47 expression level (Figures 3A–D) on CD14+ monocytes in PBC patients was higher than that in the Control group (81484 ± 31179, 39537 ± 11773; t=3.628, P<0.05) (Figure 3A). There was no significant difference in the SIRPα expression levels on CD3+ T cells, CD19+ B cells, CD56+ NK cells or CD14+ monocytes between PBC patients and the Control group (P>0.05) (Figures 3E–H).
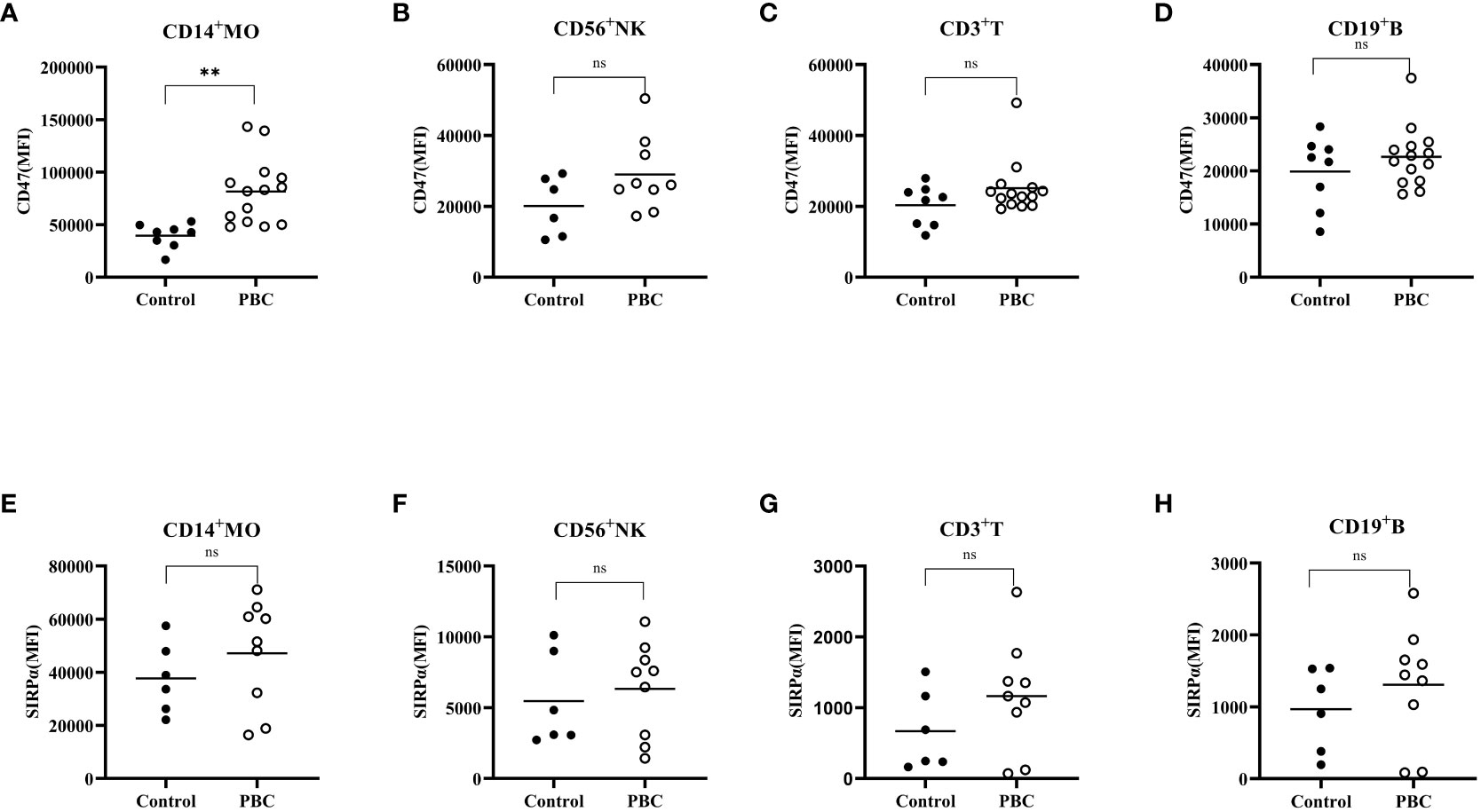
Figure 3 The expression of CD47 (A–D) and SIRPα (E–H) on CD3+ T cells, CD19+ B cells, CD56+ NK cells, and CD14+ monocytes in the Control group and PBC group. ns: There was no statistical significance between the two groups; ***P< 0.001, **P< 0.01, *P< 0.05.
3.3 Serum IFN-α expression in PBC patients
The serum IFN-α level in PBC patients and healthy examiners were measured by ELISA. PBC patients had higher serum IFN-α levels than that in healthy examiners [(112.57(92.23, 174.78) pg/mL, 77.20(36.51, 116.70) pg/mL; Z=-2.10, P<0.05, Figure 4)].
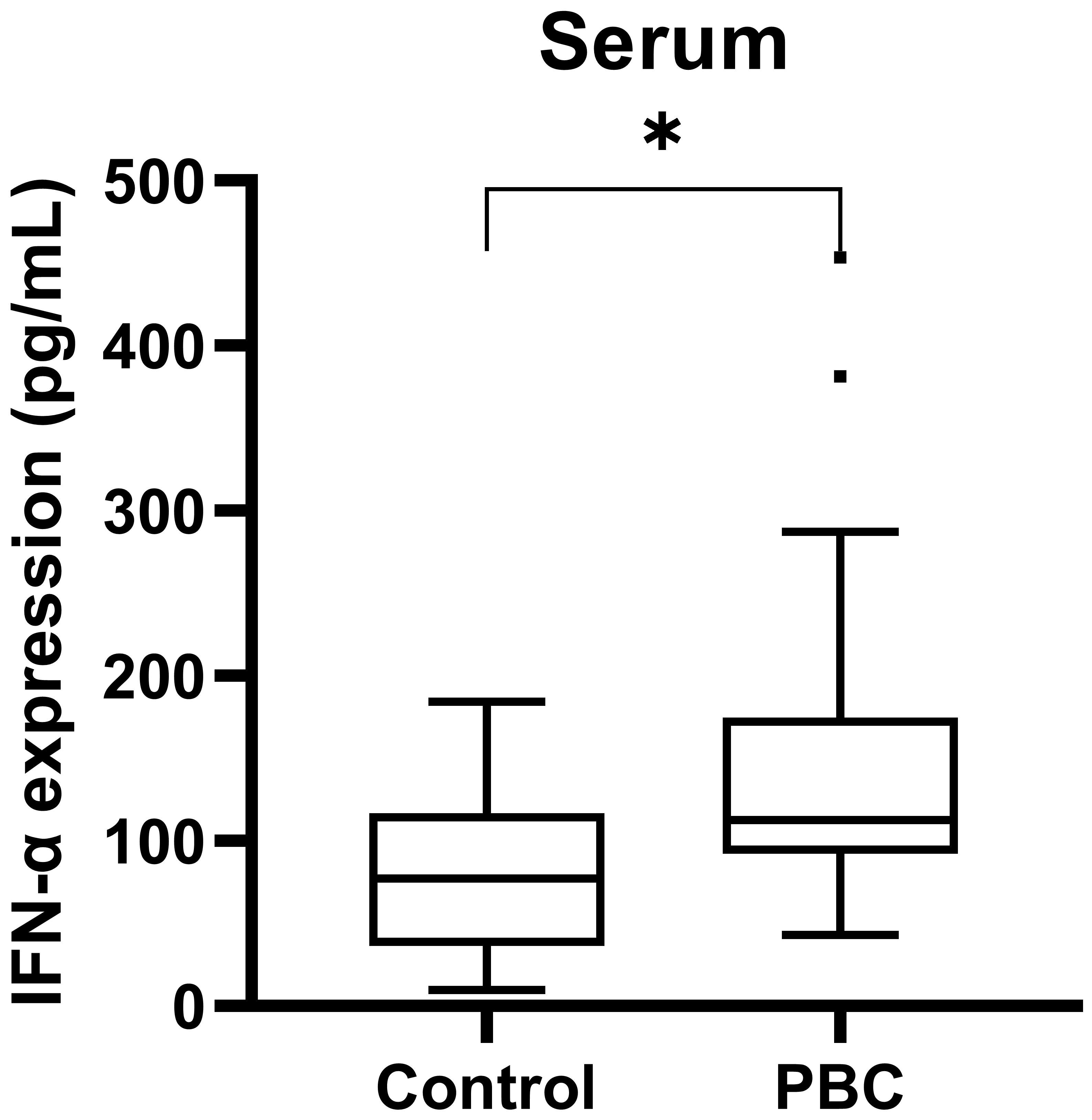
Figure 4 The serum IFN-α level in PBC patients and healthy examiners. ***P < 0.001, **P < 0.01, *P < 0.05.
3.4 The CD47 expression level on CD14+ monocytes incubated with PBC patients serum or recombinant IFN-α
PBMCs of healthy subjects were incubated with healthy subjects serum (n=5), PBC patients serum (n=9), different concentrations of recombinant IFN-α (n=4) or recombinant TNF-α (n=4), and the CD47 expression levels on cultured cells were detected. The CD47 expression level on CD14+ monocytes in the PBC group was significantly higher than that in the Control group (86.06% ± 8.98%, 30.09% ± 16.75%; t=6.94, P=0.001, Figure 5).
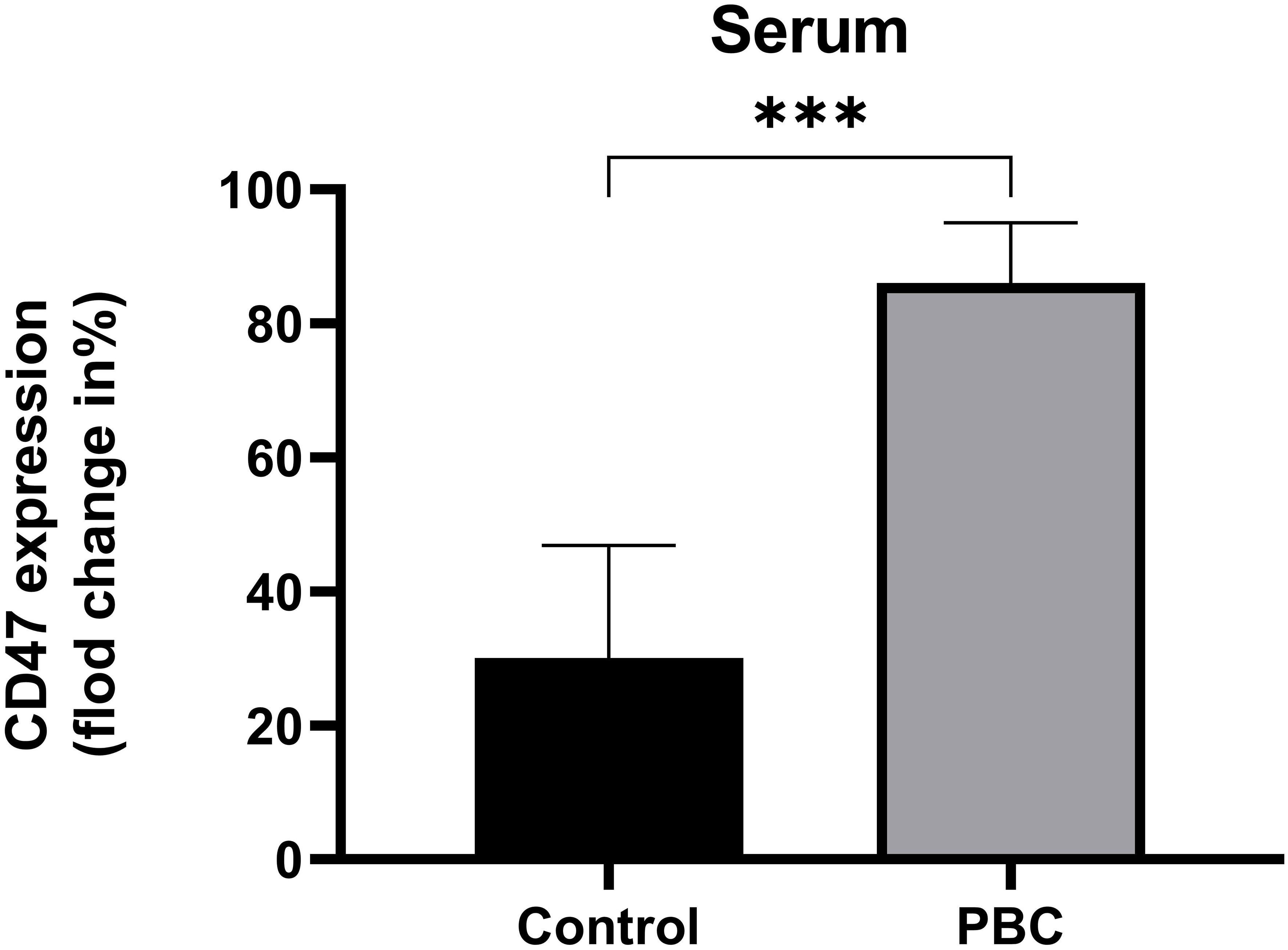
Figure 5 The CD47 expression levels on CD14+ monocytes in the Control group and PBC group. ***P ≤ 0.001, **P< 0.01, *P< 0.05.
The CD47 expression levels in the IFN-α 0 ng/mL group, IFN-α 10 ng/mL group and IFN-α 50 ng/mL group were statistically different (F=6.41, P<0.05). The CD47 expression level in IFN-α 50 ng/mL group was higher than that in IFN-α 0 ng/mL group (69.03% ± 6.97%, 37.75% ± 20.21%; t=2.93, P=0.05, Figure 6A). The CD47 expression levels in the different concentrations of recombinant TNF-α groups were not statisticallydifferent (P>0.05), Figure 6B.
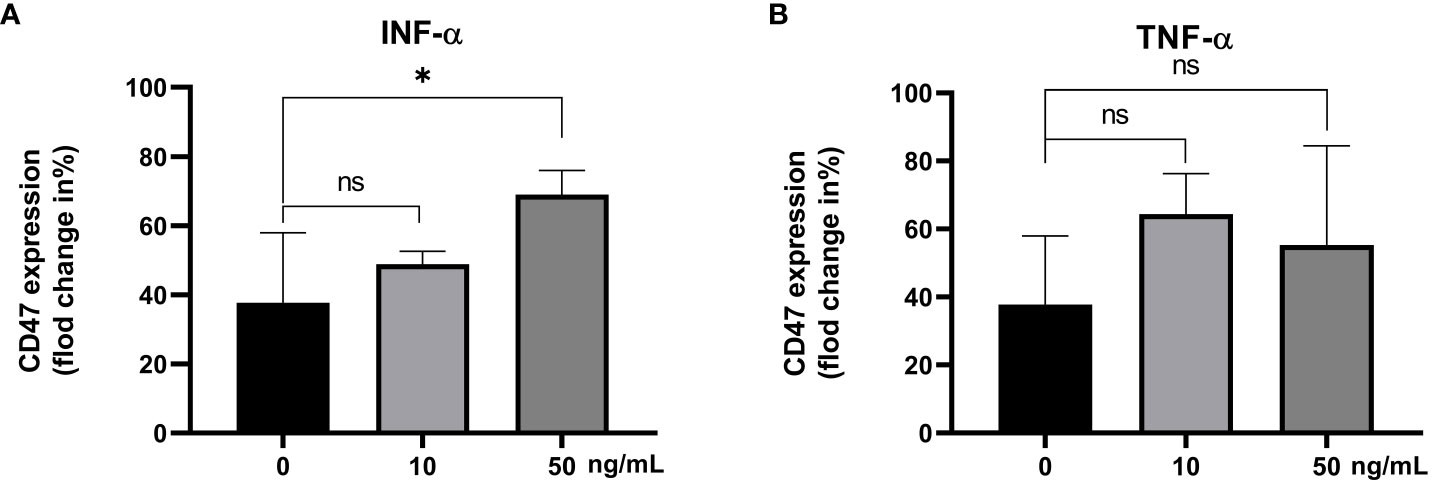
Figure 6 The CD47 expression levels on CD14+ monocytes incubated with different concentrations of the IFN-α (A) and TNF-α (B). ns: There was no statistical significance between the two groups; ***P< 0.001, **P< 0.01, *P< 0.05.
3.5 Serum anti-CD47 antibody level in PBC patients
The serum anti-CD47 antibody levels in PBC group (n=10) and the Control group (n=9) were detected by ELISA. The level of serum anti-CD47 antibody in PBC patients was significantly higher than that in the Control group [4437.50(3850.00, 10631.25) ng/L,3018.75(2443.75, 3475.00) ng/L; Z=-3.43, P=0.001, Figure 7)].

Figure 7 The serum anti-CD47 antibody level in the PBC group and the Control group. ***P≤ 0.001, **P< 0.01, *P< 0.05.
3.6 The TNF-α expression level in CD14+ monocytes incubated with the anti-CD47 antibody
PBMC in healthy subjects was incubated with an isotype control antibody, anti-CD47 antibody, LPS or anti-CD47 antibody+LPS. The TNF-α expression levels in CD14+ monocytes in cultured PBMC were detected. There were significant differences in the TNF-α expression in CD14+ monocytes among the isotype control group, anti-CD47 antibody group, LPS group and anti-CD47 antibody + LPS group (F=76.58, P<0.001). The TNF-α expression level in CD14+ monocytes in the anti-CD47 antibody group was not different from that in the isotype control group (3.76% ± 1.51%, 2.19% ± 0.51%; t=1.70, P>0.05), the TNF-α expression level in CD14+ monocytes in the LPS group was higher than that in the isotype control group (35.43% ± 9.85%, 2.19% ± 0.51%; t=5.84, P<0.01), the TNF-α expression level in CD14+ monocytes in the anti-CD47 antibody + LPS group was higher than that in the LPS group (57.80% ± 3.61%, 35.43% ± 9.85%; t=3.70, P<0.05) (Figure 8).
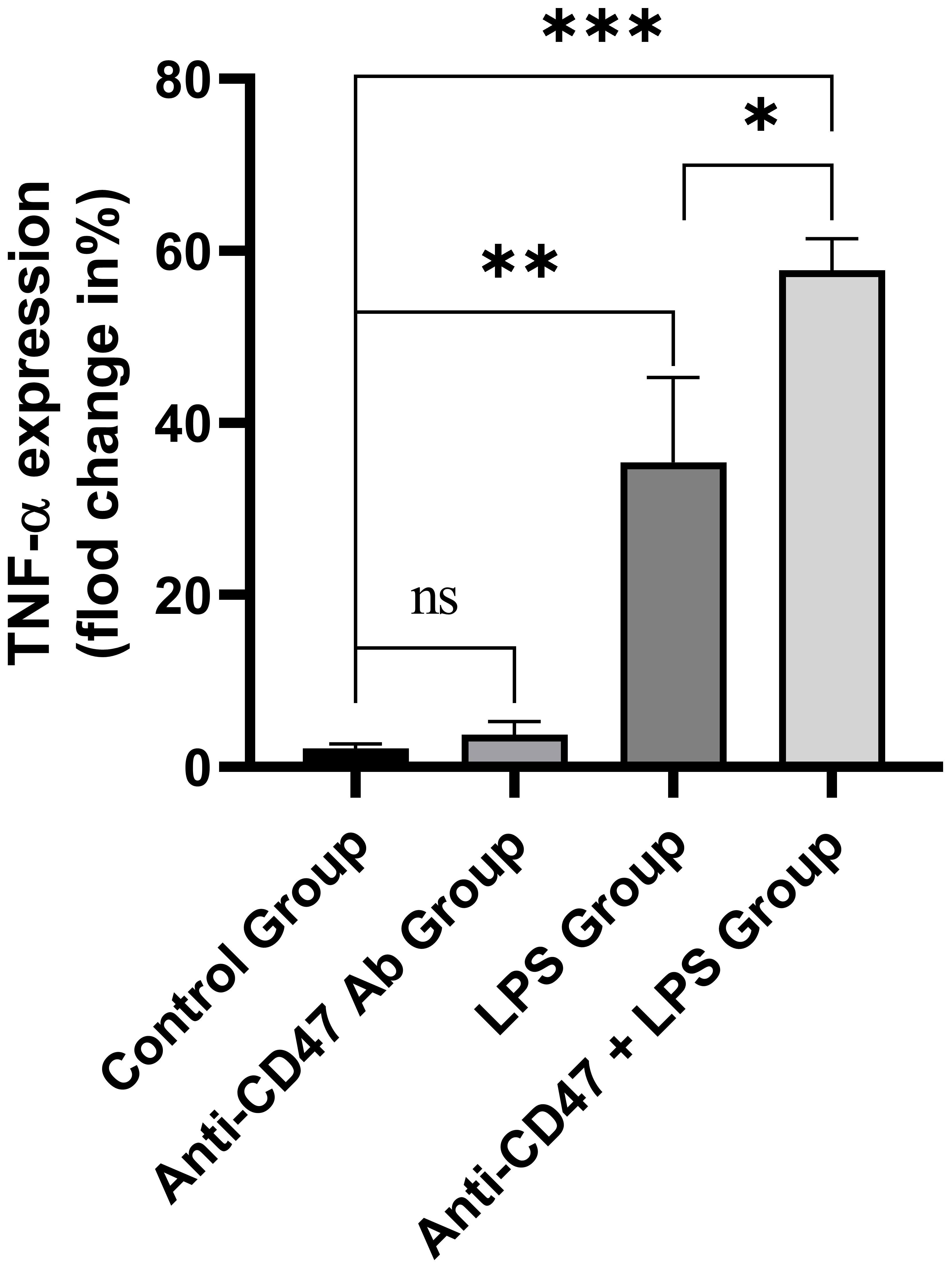
Figure 8 The TNF-α expression levels in CD14+ monocytes in the isotype control group, anti-CD47 antibody group, LPS group and anti-CD47 antibody+LPS group. ns: There was no statistical significance between the two groups; ***P< 0.001, **P< 0.01, *P< 0.05.
4 Discussion
Immunosurveillance among normal cells, defective cells and foreign pathogens is regulated by cell surface receptors, which mediate the interaction between immune cells and their targets. These ‘ self ‘ signal markers interact with proteins expressed on the surface of phagocytes to inhibit phagocytosis (24). CD47 is a protein expressed in almost all body cells, providing a ‘ do not eat me ’ signal to host phagocytes (including neutrophils and macrophages) (25). Cancer cells escaped the recognition and killing of host phagocytes by expressing high levels of CD47 (26, 27), indicating that pathogenic results will occur in the case of CD47 overexpression. The present research found increased CD47 expression level on CD14+ monocytes in PBC patients, consistent with the report (7).
When the CD47 and SIRPα are expressed on the monocytes at the same time, the sum of the two signals may determine the final effect of CD47 and SIRPα on cell response (28). Our research showed that the CD47 expression level was increased in PBC patients. In contrast, the expression of SIRPα was normal on CD14+ monocytes in PBC patients, suggesting that immune regulation of the CD47/SIRPα signals were imbalanced in PBC patients. The research also showed IFN-β and IFN-γ/TNF-α decreased erythrophagocytosis by human monocytes in vitro, which was independent from the increase in SIRP-α or SHP-1 expression (29).
Our research also showed that the PBC patients’ serum with increased IFN-α level could improve the CD47 expression on CD14+ monocytes from healthy subjects. Recombinant IFN-α also increased the CD47 expression on CD14+ monocytes in a dose-dependent manner. The data showed enhanced expression of IFN-I and toll-like receptor-3 in PBC (30) and IFN-I signaling as a necessary component of the sex bias in murine autoimmune cholangitis (31). The self-derived IFN inducers and a lack of negative feed-back signals downregulating the IFN response contributed to the continuous IFN production in SLE (32). Therefore, we speculated that increased serum IFN-α caused by self-derived IFN inducers or a lack of negative feed-back signals could promote CD47 expression in CD14+ monocytes in PBC patients.
PBC is an autoimmune disease, which can produce a variety of autoantibodies, which helps to diagnose autoimmune diseases. Our research found that the level of anti-CD47 autoantibodies in PBC patients was higher than that in healthy subjects, suggesting that there were autoantibodies against CD47 in PBC patients. Anti-CD47 antibody enhanced the phagocytosis of macrophages by binding to the Fc receptor on the macrophages, which might destroy the interaction of CD47/SIRPα, increase the phagocytic activity of macrophages, lead to an aggravation of experimental autoimmune encephalomyelitis and autoimmune nephritis (33, 34).
Studies have shown that the immune response of effector CD4+ T cells (Th1, Th17 and follicular helper T cells) and CD8+ T cells to autoantigens (expressed by hepatocytes and biliary epithelium) was related to PBC pathogenesis (35, 36). Activated Th1 and Th17 cells released various inflammatory factors to promote the occurrence and development of PBC (5). When PBC patients were exposed to LPS due to infection, LPS could bind to cell surface receptors (such as TLR 4/CD14) and induced the secretion of pro-inflammatory cytokines (such as TNF-α, IL-1, IL-6, IL-8), promoting inflammation. Our results showed that the anti-CD47 antibody could not promote TNF-α expression in CD14+ monocytes. However, LPS could, and TNF-α expression level was higher when stimulated with the combination of the anti-CD47 antibody and LPS. We assumed that anti-CD47 antibody can enhance the pro-inflammatory effect of LPS, aggravate liver lesions, and form a vicious circle.
In summary, increased inflammatory cytokines promote the expression of CD47 on the CD14+ monocytes in PBC patients. There was anti-CD47 antibody in PBC patients which could enhance the pro-inflammatory effect of LPS, aggravate liver lesions, and form a vicious circle. Blocking the imbalance of CD47/SIRPα signal may contribute to treatment for PBC patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Medical Ethics Committee of Hunan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because This research utilized samples obtained after clinical testing without causing harm to patients.
Author contributions
CL: Conceptualization, Supervision, Writing – review & editing. XS: Writing – original draft, Data curation, Software. WJ: Writing – original draft, Investigation, Methodology. LL: Writing – original draft, Formal Analysis. ZZ: Data curation, Investigation, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hunan Provincial Natural Science Foundation of China (No. 2022JJ50023); the Project of Hunan Provincial Department of Education of China (No. 22A0065); and the project of Hunan Provincial Health Commission of China (no. 202111001254).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sarcognato S, Sacchi D, Grillo F, Cazzagon N, Fabris L, Cadamuro M, et al. Autoimmune biliary diseases: primary biliary cholangitis and primary sclerosing cholangitis. Pathologica (2021) 113(3):170–84. doi: 10.32074/1591-951x-245
2. Tanaka A. Current understanding of primary biliary cholangitis. Clin Mol Hepatol (2021) 27(1):1–21. doi: 10.3350/cmh.2020.0028
3. Prieto J, Banales JM, Medina JF. Primary biliary cholangitis: pathogenic mechanisms. Curr Opin Gastroenterol (2021) 37(2):91–8. doi: 10.1097/mog.0000000000000703
4. Baier JL, Mattner J. Mechanisms of autoimmune liver disease. Discovery Med (2014) 18(100):255–63.
5. Blesl A, Stadlbauer V. The gut-liver axis in cholestatic liver diseases. Nutrients (2021) 13(3):1018. doi: 10.3390/nu13031018
6. Singla B, Lin HP, Ahn W, Xu J, Ma Q, Sghayyer M, et al. Loss of myeloid cell-specific SIRPα, but not CD47, attenuates inflammation and suppresses atherosclerosis. Cardiovasc Res (2022) 118(15):3097–111. doi: 10.1093/cvr/cvab369
7. Park JK, Lee YJ, Park JS, Lee EB, Song YW. CD47 potentiates inflammatory response in systemic lupus erythematosus. Cells (2021) 10(5):1151. doi: 10.3390/cells10051151
8. Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα Immune checkpoint. Immunity (2020) 52(5):742–52. doi: 10.1016/j.immuni.2020.04.011
9. Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol (2001) 11(3):130–5. doi: 10.1016/s0962-8924(00)01906-1
10. Oldenborg PA. CD47: A cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol (2013) 2013:614619. doi: 10.1155/2013/614619
11. Dutta S, Ganguly A, Chatterjee K, Spada S, Mukherjee S. Targets of immune escape mechanisms in cancer: basis for development and evolution of cancer immune checkpoint inhibitors. Biol (Basel) (2023) 12(2):218. doi: 10.3390/biology12020218
12. Lysenko V, Schürch PM, Tuzlak S, van Wijk NW, Kovtonyuk LV, Becher B, et al. Blocking the CD47-SIRPα interaction reverses the disease phenotype in a polycythemia vera mouse model. Leukemia (2023) 37(6):1277–86. doi: 10.1038/s41375-023-01903-2
13. van Duijn A, van der Burg SH, Scheeren FA. CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer (2022) 10(7):e004589. doi: 10.1136/jitc-2022-004589
14. Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα Axis. Front Immunol (2020) 11:18. doi: 10.3389/fimmu.2020.00018
15. Lecoultre M, Dutoit V, Walker PR. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J Immunother Cancer (2020) 8(2):e001408. doi: 10.1136/jitc-2020-001408
16. Mackert JD, Stirling ER, Wilson AS, Westwood B, Zhao D, Lo HW, et al. Anti-CD47 immunotherapy as a therapeutic strategy for the treatment of breast cancer brain metastasis. bioRxiv (2023) 25. doi: 10.1101/2023.07.25.550566
17. Liu Z, Chen H, Ta N, Shi Z, Zhan L, Han T, et al. Anti-CD47 antibody enhances the efficacy of chemotherapy in patients with gastric cancer liver metastasis. J Cancer (2023) 14(3):350–9. doi: 10.7150/jca.80725
18. Ozaniak A, Smetanova J, Bartolini R, Rataj M, Capkova L, Hacek J, et al. A novel anti-CD47-targeted blockade promotes immune activation in human soft tissue sarcoma but does not potentiate anti-PD-1 blockade. J Cancer Res Clin Oncol (2023) 149(7):3789–801. doi: 10.1007/s00432-022-04292-8
19. Okunuki Y, Tabor SJ, Lee MY, Connor KM. CD47 deficiency ameliorates ocular autoimmune inflammation. Front Immunol (2021) 12:680568. doi: 10.3389/fimmu.2021.680568
20. Zhang J, Tan SB, Guo ZG. CD47 decline in pancreatic islet cells promotes macrophage-mediated phagocytosis in type I diabetes. World J Diabetes (2020) 11(6):239–51. doi: 10.4239/wjd.v11.i6.239
21. Meier LA, Faragher JL, Osinski V, Auger JL, Voeller R, Marath A, et al. CD47 promotes autoimmune valvular carditis by impairing macrophage efferocytosis and enhancing cytokine production. J Immunol (2022) 208(12):2643–51. doi: 10.4049/jimmunol.2100903
22. Kim H, Kim HK, Hong D, Kim M, Jang S, Yang CS, et al. Identification of ulcerative colitis-specific immune cell signatures from public single-cell RNA-seq data. Genes Genomics (2023) 45(7):957–67. doi: 10.1007/s13258-023-01390-w
23. You H, Ma X, Efe C, Wang G, Jeong SH, Abe K, et al. APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis. Hepatol Int (2022) 16(1):1–23. doi: 10.1007/s12072-021-10276-6
24. Maute R, Xu J, Weissman IL. CD47-SIRPα-targeted therapeutics: status and prospects. Immunooncol Technol (2022) 13:100070. doi: 10.1016/j.iotech.2022.100070
25. Kelley SM, Ravichandran KS. Putting the brakes on phagocytosis: "don't-eat-me" signaling in physiology and disease. EMBO Rep (2021) 22(6):e52564. doi: 10.15252/embr.202152564
26. Jia X, Yan B, Tian X, Liu Q, Jin J, Shi J, et al. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci (2021) 17(13):3281–7. doi: 10.7150/ijbs.60782
27. Bian HT, Shen YW, Zhou YD, Nagle DG, Guan YY, Zhang WD, et al. CD47: Beyond an immune checkpoint in cancer treatment. Biochim Biophys Acta Rev Cancer (2022) 1877(5):188771. doi: 10.1016/j.bbcan.2022.188771
28. Bouwstra R, van Meerten T, Bremer E. CD47-SIRPα blocking-based immunotherapy: Current and prospective therapeutic strategies. Clin Transl Med (2022) 12(8):e943. doi: 10.1002/ctm2.943
29. de Almeida AC, Barbosa SM, de Lourdes Rios Barjas-Castro M, Olalla-Saad ST, Condino-Neto A. IFN-β, IFN-γ, and TNF-α decrease erythrophagocytosis by human monocytes independent of SIRP-α or SHP-1 expression. Immunopharmacol Immunotoxicol (2012) 34(6):1054–9. doi: 10.3109/08923973.2012.697470
30. Takii Y, Nakamura M, Ito M, Yokoyama T, Komori A, Shimizu-Yoshida Y, et al. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Invest (2005) 85(7):908–20. doi: 10.1038/labinvest.3700285
31. Bae HR, Hodge DL, Yang GX, Leung PSC, Chodisetti SB, Valencia JC, et al. The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity. Hepatology (2018) 67(4):1408–19. doi: 10.1002/hep.29524
32. Eloranta ML, Rönnblom L. Cause and consequences of the activated type I interferon system in SLE. J Mol Med (Berl) (2016) 94(10):1103–10. doi: 10.1007/s00109-016-1421-4
33. Han MH, Lundgren DH, Jaiswal S, Chao M, Graham KL, Garris CS, et al. Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. J Exp Med (2012) 209(7):1325–34. doi: 10.1084/jem.20101974
34. Shi L, Bian Z, Chen CX, Guo YN, Lv Z, Zeng C, et al. CD47 deficiency ameliorates autoimmune nephritis in Fas(lpr) mice by suppressing IgG autoantibody production. J Pathol (2015) 237(3):285–95. doi: 10.1002/path.4574
35. Li Y, Li B, You Z, Zhang J, Wei Y, Li Y, et al. Cytotoxic KLRG1 expressing lymphocytes invade portal tracts in primary biliary cholangitis. J Autoimmun (2019) 103:102293. doi: 10.1016/j.jaut.2019.06.004
Keywords: PBC, cd47, SIRPα, immune checkpoint, inflammatory response
Citation: Su X, Jin W, Liu L, Zhu Z and Li C (2023) Regulation of CD47 expression on CD14+ monocytes by interferon-α in PBC patients. Front. Immunol. 14:1256995. doi: 10.3389/fimmu.2023.1256995
Received: 11 July 2023; Accepted: 13 November 2023;
Published: 04 December 2023.
Edited by:
Jiabo Wang, Capital Medical University, ChinaReviewed by:
Robert Cody Sharp, University of Florida, United StatesZhigang Hu, Wuxi Children’s Hospital, China
Copyright © 2023 Su, Jin, Liu, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cunyan Li, empqbGN5XzEyM0AxNjMuY29t
 Xi Su1,2
Xi Su1,2 Zifei Zhu
Zifei Zhu Cunyan Li
Cunyan Li