- 1Department of Endocrinology and Metabolic Diseases, Polish Mother’s Memorial Hospital—Research Institute, Lodz, Poland
- 2Institute of Information Technology, Lodz University of Technology, Lodz, Poland
- 3Department of Endocrinology and Metabolic Diseases, Medical University of Lodz, Lodz, Poland
Introduction: Graves’ disease (GD) and Graves’ orbitopathy (GO) development were suspected to be HLA-related in both Asian and Caucasian populations. However, most studies were performed with application of serological methods or low resolution genetic typing, which led to inconsistent results even among the same population. The present review is intended to summarize the state-of-art knowledge on the HLA significance in GD and GO in Asians and Caucasians, as well as to find the most significant alleles for each of the populations.
Methods: PubMed was searched for relevant articles using the following search terms: HLA plus thyroid-associated ophthalmopathy or Graves’ disease or Graves’ orbitopathy or thyroid eye disease or thyroid-associated orbitopathy.
Results: In Asian population GD was found to be associated mostly with B*46:01, DPB1*05:01, DRB1*08:02/03, DRB1*16:02, DRB1*14:03, DRB1*04:05, DQB1*05:02 and DQB1*03:03, while DRB1*07:01, DRB1*01:01, DRB1*13:02, DRB1*12:02 are potentially protective. HLA-B*38:02, DRB1*16:02, DQA1*01:02, DQB1*05:02 can be considered associated with increased risk of GO in Asians, while HLA-B*54:01 may play protective role. In Caucasians, C*07:01, DQA1*05:01, DRB1*03, DQB1*02:01 are associated with GD risk while DRB1*07:01, DQA1*02:01 may be protective. Significance of HLA in the course of GD and novel aspects of HLA amino acid variants and potential HLA-based treatment modalities were also discussed.
1 Introduction
Graves’ disease (GD) is an autoimmune thyroid disorder caused by production of antibodies against thyrotropin (TSH) receptor. TSH-receptor antibodies (TRAb) usually stimulate thyroid hormone production, but they can also block TSH-receptor (TSHR) or have ambivalent character with no impact on thyroid function (1). Additionally, insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) was demonstrated to play an important role in GD development (2). An activation of IGF-1R on orbital fibroblasts may result from the binding of stimulatory IGF-1R antibodies (IGF-1R-Ab) to IGF-1R. Synergistic crosstalk between TSHR and IGF-1R after binding of stimulatory TRAb to TSHR may also be a mechanism leading to IGF-1R activation (3). The prevalence of GD in general population is about 0.5-2.0% (1, 4). Like many other autoimmune diseases, GD is typically induced by environmental factors in genetically predisposed individuals (5, 6). Graves’ orbitopathy (GO) is the most frequent extrathyroidal manifestation of GD, with the estimated incidence of 2.67–3.3 cases/100 000/year in women and 0.54–0.9 cases/100 000/year in men (7). GO significantly deteriorates patients’ quality of life (QoL) and may even lead to a sight-threatening conditions (7).
Therefore, the knowledge on risk factors associated with GD and GO development seems to be crucial for the prevention and management of GO. Many environmental agents were demonstrated to be potential GD-triggering factors, including viral infections, vaccines (8), drugs (9), stress (5), reactive oxygen species (ROS) overproduction (10). Smoking, high serum TRAb levels, severe or unstable hyperthyroidism as well as hypercholesterolemia are already known risk factors of GO development and progression (7, 11).
However, as not all exposed individuals are affected and – on the other hand – GD and GO are more frequent among family members, it seems obvious that environmental factors always act on a genetic susceptibility which is crucial for the disease development. The strength of genetic susceptibility was proven in environmental, family and twin studies, which suggested that 70% of the risk of GD can be attributed to genetic factors (12). Among genes associated with the autoimmune response, human leukocyte antigen (HLA) genes seem to play a prominent role as a molecular background of GD and GO (13–15). Many different HLA alleles were postulated as GD and/or GO risk factors in Caucasian and Asian populations. The role of other genes such as TSHR, cytotoxic T lymphocyte-associated factor 4 (CTLA-4), protein tyrosine phosphatase non-receptor type 22 (PTPN22), Fc receptor like 3 (FCEL3), interleukin 2 receptor A (IL2RA), thyroglobulin (TG) or cluster of differentiation 40 (CD40) genes was also postulated in GD development (16–19). Similarly, the risk of GO occurrence in GD patients is associated with genetic susceptibility. Except for HLA, several other genes were suggested as GO-related, including TSHR, CTLA4, tumor necrosis factor (TNF), interferon γ (IFN γ), interleukin 1A (IL1A) and its receptor (IL1AR), protein tyrosine phosphatase non-receptor type 12 (PTPN12), peroxisome proliferator-activated receptor γ (PPAR γ) or intracellular adhesion molecule 1 (ICAM-1) genes (4, 20–24). There is evidence that the risk of GD and/or GO is not related to a single gene, but is a result of a complex interaction between genetic factors (4). Nevertheless, considering the importance of the major histocompatibility complex (MHC) for autoimmune responses and taking into account high polymorphism of HLA region, HLA seems to play a prominent role as a molecular background of GD and GO (4, 14, 15).
However, results of studies regarding either Caucasian or Asian population were not coherent. The major reasons of this inconsistency are: different sizes of study groups and different methods used by the researchers, including low resolution or serological methods (13). Serological methods detect antigens only, so their accuracy is very low and their application is currently not recommended. More precise results can be obtained with DNA typing methods for HLA analysis. These include i.a.: polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP), PCR sequence-specific oligonucleotide probe (PCR-SSOP), PCR sequence-specific primer (PCR-SSP), PCR single-strand conformation polymorphism (PCR-SSCP), and sequence-based typing (SBT). These methods, except for the SBT, require more probes and primers to maintain the acceptable (low or intermediate) resolution, and are unable to detect new polymorphism (25). On the other hand, SBT method can identify all sequence motifs and is capable of detecting new undefined alleles (25). Low resolution methods provide results for the entire allelic group, but not for a particular allele. Serological methods or low resolution DNA-based methods of HLA typing provide results only at antigen or allelic group level. The quality of the results was significantly improved by high-resolution DNA-based typing. In order to further improve this quality, next-generation sequencing (NGS) methods which are based on deep-sequencing of the entire HLA gene followed by bioinformatic processing, have been introduced to provide typing results at the allelic level (25, 26). The use of serological or low resolution methods may lead to erroneous conclusions and inconsistency in the study results depending on the applied method. More and more studies which demonstrated method-dependent error in HLA analysis are available. Among a strictly controlled cohort in whom HLA typing was performed for bone marrow transplantation, discrepancies exceeding 29% were reported between less sensitive methods and NGS method (27). Another important example of the risk related to low resolution methods is HLA-B*27 test, which is commonly used to confirm a diagnosis of ankylosing spondylitis. However, it has been recently demonstrated that alleles HLA-B*27:06 and HLA-B*27:09 are probably not associated with the disease, so the results based on less precise methods may lead to a wrong diagnosis (28). Therefore, the significance of applied HLA-typing method seems crucial to unequivocally determine HLA-related background of autoimmune diseases, including GD.
The purpose of this review is to summarize the current knowledge on the HLA significance in GD and GO in Asians and Caucasians, as well as to indicate the most reliable sets of high risk-related or protective alleles for each of the populations. Identification of an actual sets of such alleles can constitute a reliable tool for the individual risk assessment, and would play an important role in a development of personalized medicine. Additionally, other aspects of HLA significance in GD/GO were also summarized.
2 Methods
PubMed was searched for relevant articles using the following search terms: HLA plus one of the following: thyroid-associated ophthalmopathy or Graves’ disease or Graves’ orbitopathy or thyroid eye disease or thyroid-associated orbitopathy. Studies which analyzed HLA-associated susceptibility to GD were included only if comparison with healthy control group was performed. Results based exclusively on comparison with patients with other autoimmune diseases, without a control group, were excluded. In the case of HLA-related background of GO, only studies which provided comparison between GD patients with GO and without GO were included. A total number of 1197 studies were found through initial database searching. Subsequently, after thorough screening of titles and abstracts, 368 studies were selected after exclusion of duplicates, articles not available in English, or in full text, and irrelevant papers. These studies were reviewed in detail. Two hundred seventy two of these were excluded as they did not meet the inclusion criteria or due to data overlapping, insufficient data, or for other reasons. Study selection flowchart is available as a Supplementary Material. A total number of 96 papers were included and, on the basis of the studied population and analyzed factors, these papers were divided into the following groups of papers 1. Results on HLA-related GD risk in Asians, 2. Results on HLA-related GO risk in Asians, 3. Results on HLA-related GD risk in Caucasians, 4. Results on HLA-related GO risk in Caucasians, 5. Results on other correlations between HLA and GD/GO (recurrence risk, clinical course, amino acid variants significance etc.). For further analysis of Group 1 and Group 3, only studies performed with genetic (not serological) methods were included, and only alleles which were reported as related to increased or decreased risk in more than one study were presented as summary results.
3 Results
3.1 Population-dependent differences and comparison difficulties
An importance of significant ethnic differences was indicated in many studies on HLA-related susceptibility to GD (5, 6, 29–34). Furthermore, the results already published for either Asian or Caucasian populations are inconsistent (6, 29–34). Additionally, the symbols of alleles used for the previous methods differ from the ones currently used. Application of high-resolution methods changed the obtained results significantly, because antigens previously denoted by a given symbol have been separated into many different alleles (13). This fact obviously highly influenced the accuracy and coherence of the already published results.
3.2 Asian population
3.2.1 HLA and GD development
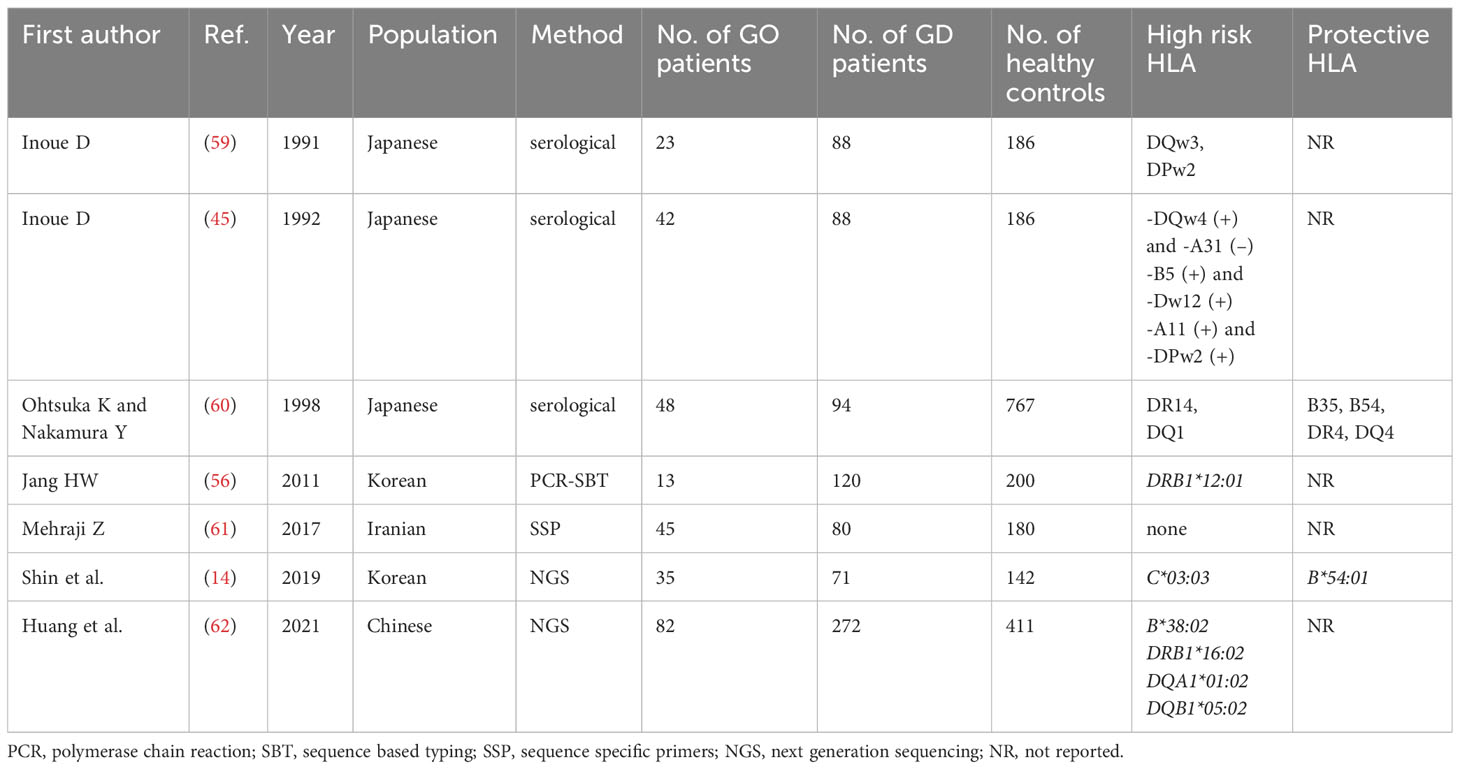
Many studies on the potential significance of HLA for GD in Asians were performed. Since 1978, several of them have postulated the impact of HLA-B*46 in the development of GD (31–33). However, with application of serological methods, in some studies, HLA*Bw46 significance was demonstrated in males only, with lack of correlation in females (34–36). Similar gender-limited relationship was observed between GD and HLA-DR9 and DQB1*03:03 (36). A metanalysis of 14 studies performed by Li et al. in 2013 demonstrated association between HLA-B*46 and GD in Asians (31). Although, most of the authors of the included studies applied serological methods, more recent reports confirmed this correlation (37–40). The overview of the published results on HLA significance in GD are demonstrated in Table 1. It is clearly visible that the obtained results are not consistent and potential high risk alleles or protective ones are different even in similar populations (i.e. in the Chinese, the Japanese, the Korean etc.). Several antigens other than Bw46 were postulated by authors who used serological methods, including, for example, HLA-DRw8, -DQw4, -B5, Dw12 and –A11 antigens (44–46). At the end of the 20th century, polymerase chain reaction (PCR) sequence specific oligonucleotide probe (SSOP) method was introduced and many authors applied it at least for MHC class II analysis. Similarly to the serology-based reports, the studies with combined serological and SSOP methods revealed inconsistent results. Chan et al. reported that the risk of GD was higher in patients with HLA-A*2, Cw1, DRB1*16:02, DRB1*03:01, DRB1*14:05, DRB5*02, DQB1*05:02, while the presence of HLA-DRB1*15:01 and -DQB1*03:01 played a protective role (35). On the other hand, Japanese authors presented that the most important factor was the presence of HLA-DPB1*05:01 and/or HLA-A*2, with the risk being the highest in carriers of both of them (46). In one Taiwanese study, HLA-A*02:07 was found a GD risk factor (37), while other Taiwanese authors showed correlation between GD and HLA-B*46:01, DPB1*05:01, DQB1*03:02, DRB1*15:01 and DRB1*16:02, with the strongest relationship existing with HLA-DPB1*05:01 (38).
There are discrepancies in the obtained results, not only between methods applied by the authors or between subpopulations based on patients’ nationality, but also between studies performed with the same method and among the same subpopulation (40–57). Additionally, many studies included MHC class II alleles only, or even one group mostly HLA-DRB1 (48–52). Therefore, direct comparison of the results, or any attempt to include the studies into metanalysis, would be subjected to high risk of error. The most reliable results obtained with NGS method are scarce. Katahira et al. did not compare their GD patients to a control group but only to patients with Hashimoto thyroiditis. Therefore, they are not suitable for comparison with others. Ueda et al. presented results as a list of alleles associated with high risk of GD and a list of 3 haplotypes which showed significant protective effects against the development of GD in Japanese population (HLA-A*24:02-C*12:02-B*52:01-DRB1*15:02-DQB1*06:01-DPB1*09:01 and HLA-A*24:02-C*07:02-B*07:02-DRB1*01:01-DQB1*05:01-DPB1*04:02, and HLA-A*33:03-C*14:03-B*44:03-DRB1*13:02-DQB1*06:04-DPB1*04:01). Taking into account the difficulties in direct comparison of the results, we decided to analyze a number of studies which revealed a given allele as the high risk one or the protective one. In order to obtain the most reliable results we included all studies which used methods which allowed to obtain allelic specificity. We did not include any of the results obtained by serological methods. As it was stated above, a metanalysis of those studies previously confirmed a significance of HLA-B46 only.
Our current analysis demonstrated that the following alleles were most commonly reported as related to high risk of GD in Asians: B*46:01 (7 studies), DPB1*05:01 (7 studies), DRB1*16:02 (4 studies), DRB1*08:02/03 (4 studies), DRB1*14:03 (3 studies), DRB1*04:05 (3 studies), DQB1*05:02 (3 studies), DQB1*03:03 (3 studies, including two studies with significance for children only and one study with significance for males only) (Table 2). On the other hand, the following alleles were reported as potentially protective: DRB1*07/DRB1*07:01 (4 studies), DRB1*01:01 (3 studies), DRB1*13:02 (3 studies), DRB1*12:02 (3 studies) (Table 2).
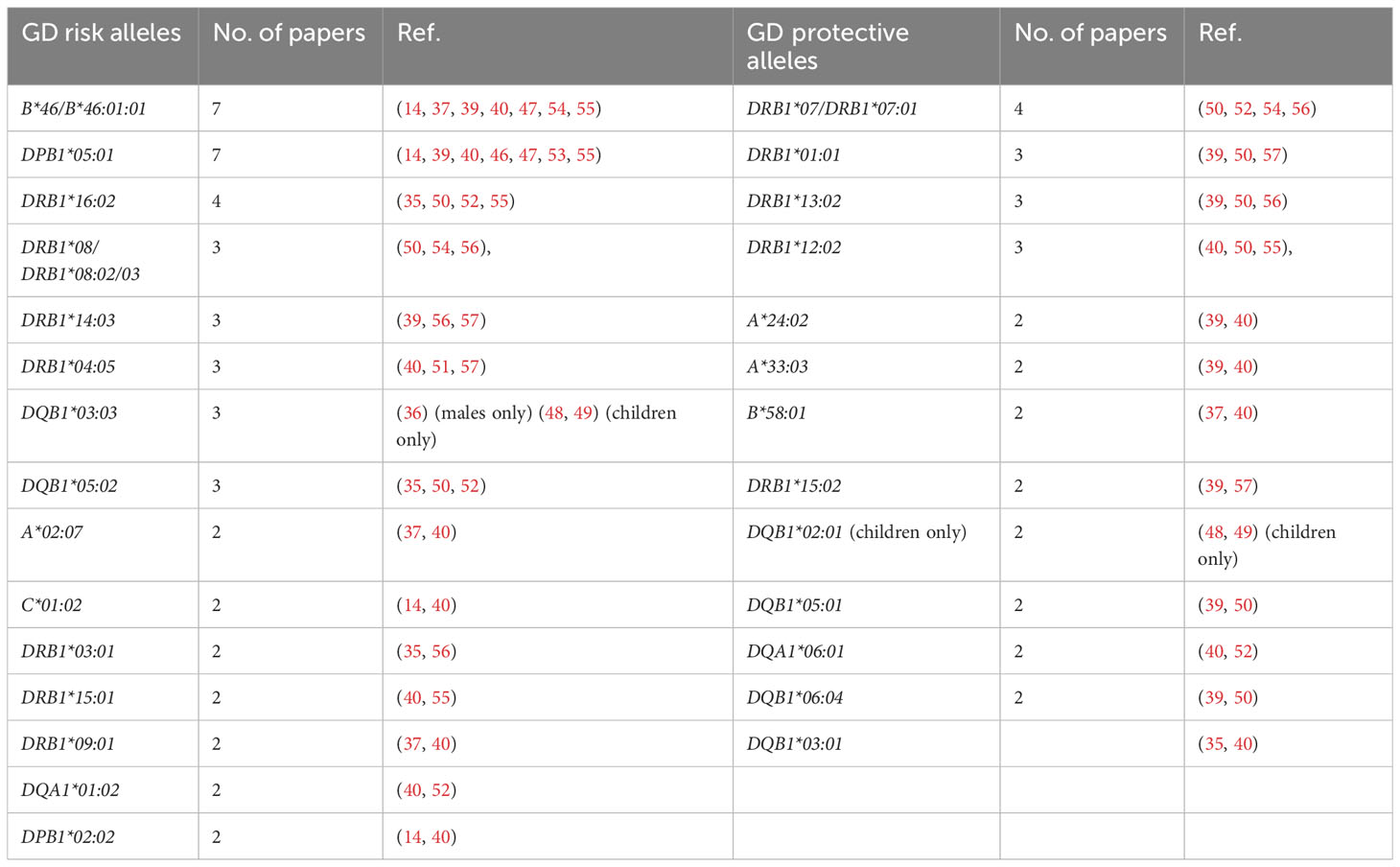
Table 2 Alleles reported as Graves’ disease (GD)-related in more than one study in Asian population.
It is worth indicating, that among the alleles found as related to high risk of GD on the basis of our present review, HLA-DRB1*16:02 is in linkage disequilibrium with DQB1*05:02 (58), so they cannot be considered independent risk predictors. Additionally, among alleles demonstrated as related to lower risk HLA-DRB1*01:01 is in linkage disequilibrium with DQB1*05:01, DRB1*12:02 is in linkage disequilibrium with DQA1*06:01 and DRB1*13:02 is in linkage disequilibrium with DQB1*06:04 (58). These correlations must be taken into account if independent associations are analyzed.
3.2.2 HLA and GO development
Similarly to GD, the results concerning HLA associations with GO development are inconsistent. The overview of the published results is presented in Table 3. Inoue et al. used serological method in Japanese patients and demonstrated that only DQw3 seemed to be a GO risk factor (59). However, in a study published a year later, Inoue et al. found that the risk of GO was associated with the following sets of HLA antigens: HLA-DQw4 without presence of -A31, HLA-A11 without presence of -DPw2, and with a co-presence of HLA-B5 and -Dw12 (45). Other authors applied serological methods, in another group of patients from Japan, and obtained entirely different results (60). They reported that predisposition to severe GO can be related to a presence of HLA-DR14 and DQ1 antigens, while HLA-B35, B54, DR4, and DQ4 were postulated to play a protective role (60).
In Iranian cohort, no differences in allele distribution between patients with and without GO was postulated (61).On the contrary, in a Korean study, HLA-C*03:03 allele had higher frequency of occurrence in GO as compared to non-GO patients (14). In a Chinese cohort described by Huang et al., the HLA-B*38:02, DRB1*16:02, DQA1*01:02 and DQB1*05:02 were postulated as GO high risk alleles (62). No consistent results were found (Table 3), therefore, no allele can be unequivocally selected as actually GO related. Taking into account the significance of the applied method and the size of groups, the results presented by Huang et al. in 2021 can be considered the most reliable, as they were performed by NGS method in 82 GO patients, 272 GD patients and 411 healthy controls (Table 3) (62). Therefore the alleles HLA-B*38:02, DRB1*16:02, DQA1*01:02 and DQB1*05:02 can be considered associated with increased risk of GO in Asians, although such relationship was demonstrated in one study only and requires further confirmation. Moreover, HLA-DRB1*16:02 is in linkage disequilibrium with DQA1*01:02 and DQB1*05:02 (58), so their associations with GO cannot be considered independent. On the other hand, the significance of HLA-B*38:02 seems to be entirely independent. In this study, no potentially protective alleles were selected, however there was a concordance in two previous studies in regard to HLA-B*54/HLA-B*54:01 which was demonstrated to play a protective role (14, 60). Therefore HLA-B*54:01 can be considered as associated with lower GO risk in Asian population.
3.3 Caucasian population
3.3.1 HLA and GD development
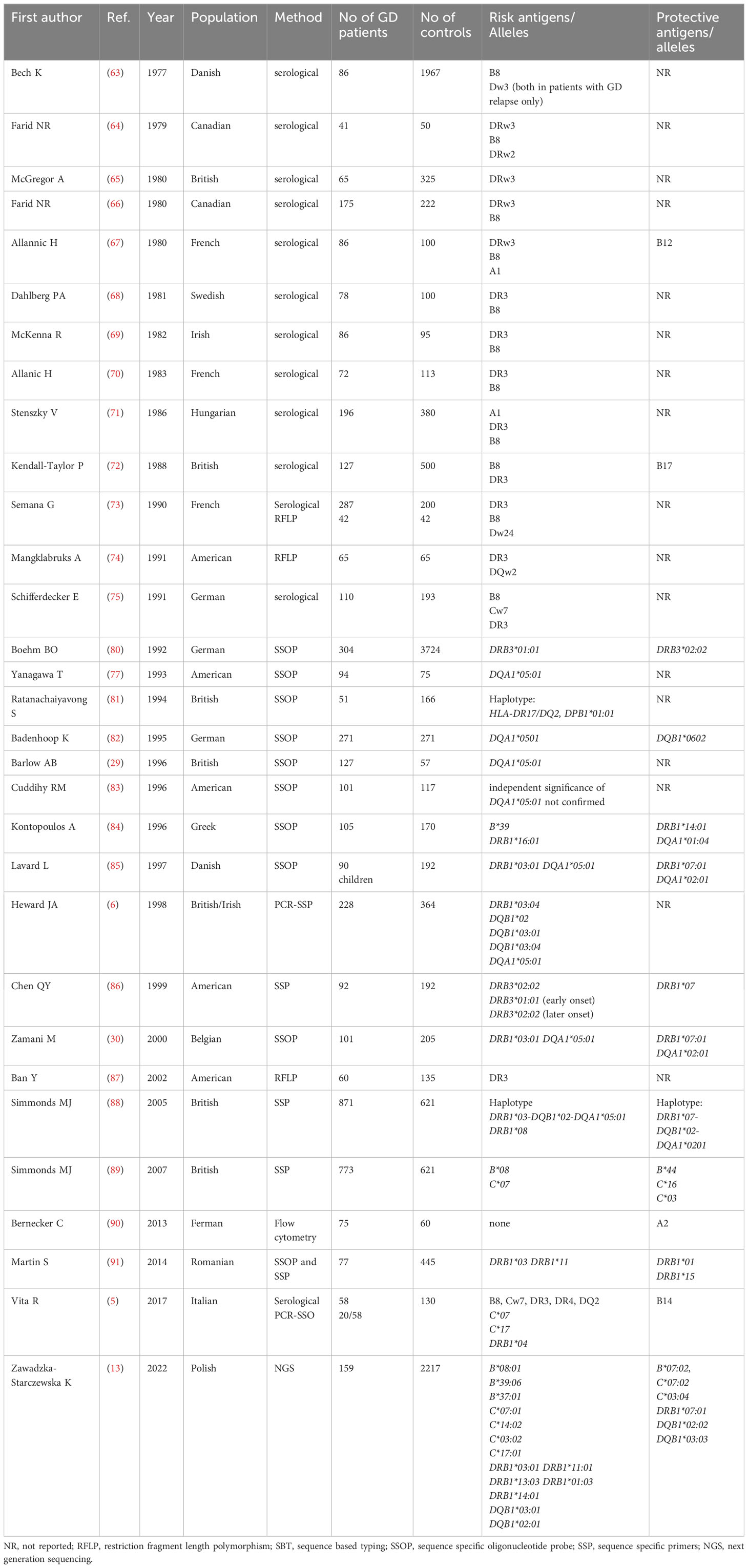
In Caucasians, GD was initially reported to be associated with allelic group DRB1*03, i.e. with B8 and Dw3antigens (63). Many serological studies performed between 1978 and 1991 confirmed the significance of B8and DRw3 (63–75). In different Caucasian populations, the frequency of DRB1*03 in GD patients was demonstrated to range between 40% and 55%, while in the general population it ranged between 15% and 30%. The relative risk (RR) of GD in DRB1*03 carriers was assessed as approximately 4 (76). Since 1993, HLA-DQA1*05 has been reported as associated with GD (77, 78) and a few years later, HLA-DQB1*02 was also demonstrated to be related to GD in Caucasians (6). For many years, the results of studies were concordant mainly with regard to the increased risk of GD in carriers of HLA-DRB1*03 and of the alleles DQA1*05:01, DQB1*02:01 which are in linkage disequilibrium with HLA-DRB1*03 in Caucasians (6, 74, 77). Both case-control and family studies, demonstrated that a haplotype DRB1*03- DQA1*05-DQB1*02 can be considered a predictor of the development of GD (78).
However, HLA-DRB1*03 is well known to be associated with an increased risk of many autoimmune diseases, not exclusively with GD. Increased risks of Hashimoto’s thyroiditis, myasthenia gravis, Addison’s disease, diabetes mellitus type 1, systemic lupus erythematosus (SLE) were reported (79).
Many other alleles were postulated, but hardly any consistency was found between the results. Vita et al. found that frequencies of HLA-C*07, -C*17 and -DRB1*04 are significantly higher in patients with GD than in controls (5), while Heward et al. suggested that HLA-DQB1*03:01/04 and -DQB1*02 can play a role in GD occurrence (6).
Recently, the significance of B*08:01, B*39:06, B*37:01, C*07:01, C*14:02, C*03:02, C*17:01, DRB1*03:01, DRB1*11:01, DRB1*13:03, DRB1*01:03, DRB1*14:01, DQB1*03:01, DQB1*02:01 was demonstrated by our research team with application of NGS method (13). Unfortunately, it was the first study which applied NGS in Caucasian patients, therefore, no comparison with other NGS-based studies is possible. The overview of studies focused on associations between HLA and GD in Caucasian population is presented in Table 4.
Similarly to Asian population, there were discrepancies in the applied methods, group size and analyzed MHC classes (13, 63–91). Most of the studies included only one MCH class, mainly class 2, while some studies included even only one gene, predominantly HLA-DRB1 (Table 4). Therefore, due to difficulties in direct comparison of the results, we analyzed numbers of studies which revealed a given allele as a high risk one or a protective one. In order to obtain the most reliable results we included studies which used methods which allowed to obtain allelic specificity, or studies which reported allele group concordant with results in which a specific allele was provided (e.g. DRB1*03 and DRB1*03:01/04). We did not include any of the results obtained by serological methods.
Our present analysis demonstrated that the following alleles were most commonly reported as related to high risk of GD in Caucasians: DQA1*05:01 (7 studies), DRB1*03, including DRB1*03:01/04 (6 studies), C*07/C*07:01 (3 studies), DQB1*02/DQB1*02:01 (3 studies) (Table 5). On the other hand, the following alleles were reported as potentially protective: DRB1*07/DRB1*07:01 (5 studies), DQA1*02:01 (3 studies), C*03/C*03:04 (2 studies), DQB1*02/DQB1*02:02 (2 studies) (Table 5).
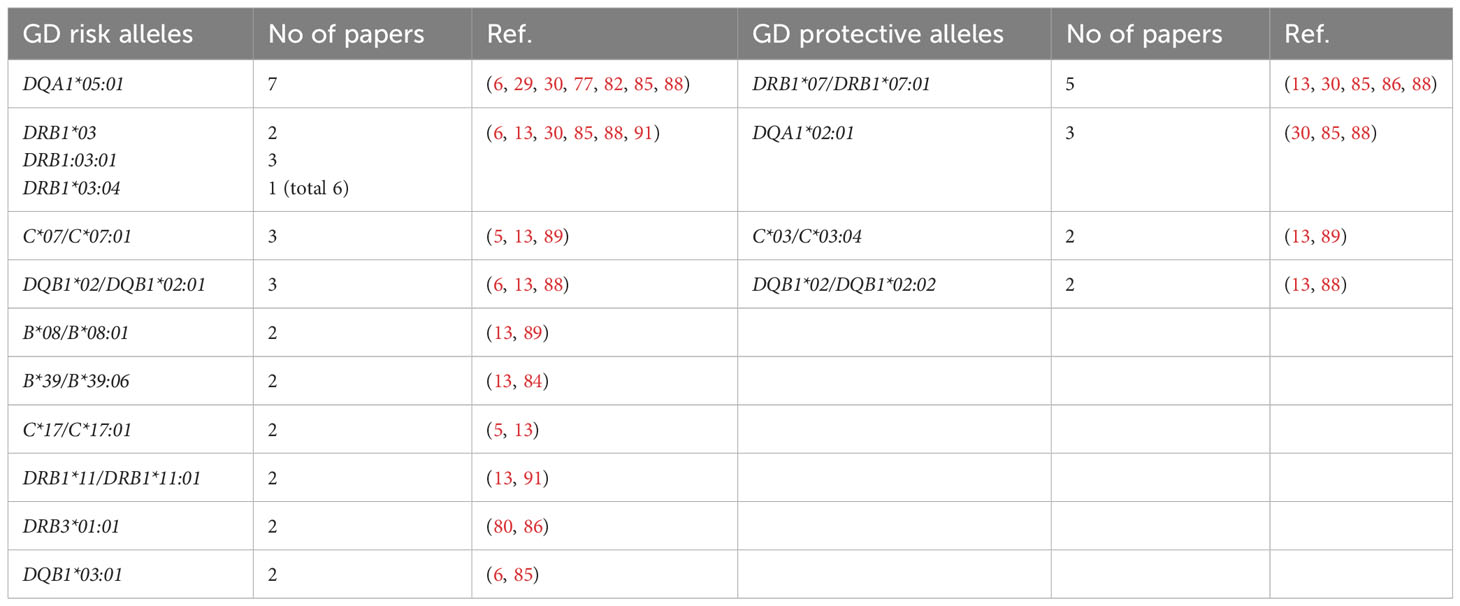
Table 5 Alleles reported as Graves’ disease (GD)-related in more than one study in Caucasian population.
The significance of HLA-DRB1*03:01 as GD high risk allele is the only one which is common for both Asian and Caucasian populations. The alleles HLA-DRB1*03:01, DQA1*05:01 and DQB1*02:01 are in strong linkage disequilibrium in Caucasian population (58). Therefore, their significance cannot be considered entirely independent, however, the single presence of any of them can be correlated with the risk of GD. Moreover, some studies demonstrated a common association of HLA-B*08:01, which was also reported as GD high risk allele, with DRB1*03:01 and DQB1*02:01 (6, 13, 29, 30, 77). In our recent study, the combination of these three alleles occurred in 22% of patients with GD, while it was found only in 5.87% of the healthy control group (13). Interestingly, in patients in whom HLA-B*08:01 was present, it occurred with alleles other than the ones described above only in 1.9% of GD patients (13). HLA-B*08:01 is in linkage disequilibrium with HLA-C*07:01 – another high risk allele, whose significance in GD was also demonstrated (92, 93).
Therefore, it can be concluded that HLA-B*08:01, C*07:01, DRB1*03:01, DQA1*05:01, DQB1*02:01 and can be considered the most important predictors of GD development in Caucasians.
On the other hand, HLA-DRB1*07:01 was the allele most commonly demonstrated to be a protective one. Interestingly, this observation is the only one consistent with results of studies in Asians in regard to the protective alleles. It should be indicated that HLA-DRB1*07:01 is in linkage disequilibrium with two other potentially protective alleles – HLA-DQB1*02:02 and DQA1*02:01 (58). HLA-C*03:04, which is in no linkage disequilibrium with other discussed alleles, seems to be the only entirely independent protective allele (34, 92). Therefore, the alleles HLA-C*03:04, DRB1*07:01, DQB1*02:02 and DQA1*02:01 can be considered a group of GD protective alleles.
Some differences in the results between Caucasian and Asian populations seem unexpected. Among the alleles, which are in linkage disequilibrium with HLA-DQA1*05:01, only DRB1*03:01 was proved to be GD-related in both Asian and Caucasian populations (13, 30, 35, 54, 91). However, these linkage disequilibrium-based correlations are not exclusive for the Caucasian population, but are common in all analyzed populations, including Asians (58). Therefore, it remains unexplained why these correlations found in Caucasians are absent in Asians, in whom completely different alleles were reported as high risk of GD development. Furthermore, on the basis of the present review, a phenomenon of opposite roles of HLA-DQB1*02:01 can be observed. This allele was demonstrated as high risk in Caucasians (6, 13), but it was also reported as protective in Asian pediatric studies (48, 49). This population-dependent opposite correlation indicates the potential significance of other factors influencing GD risk in either population. Further studies with application of high resolution methods are required to solve this puzzle.
3.3.2 HLA and GO
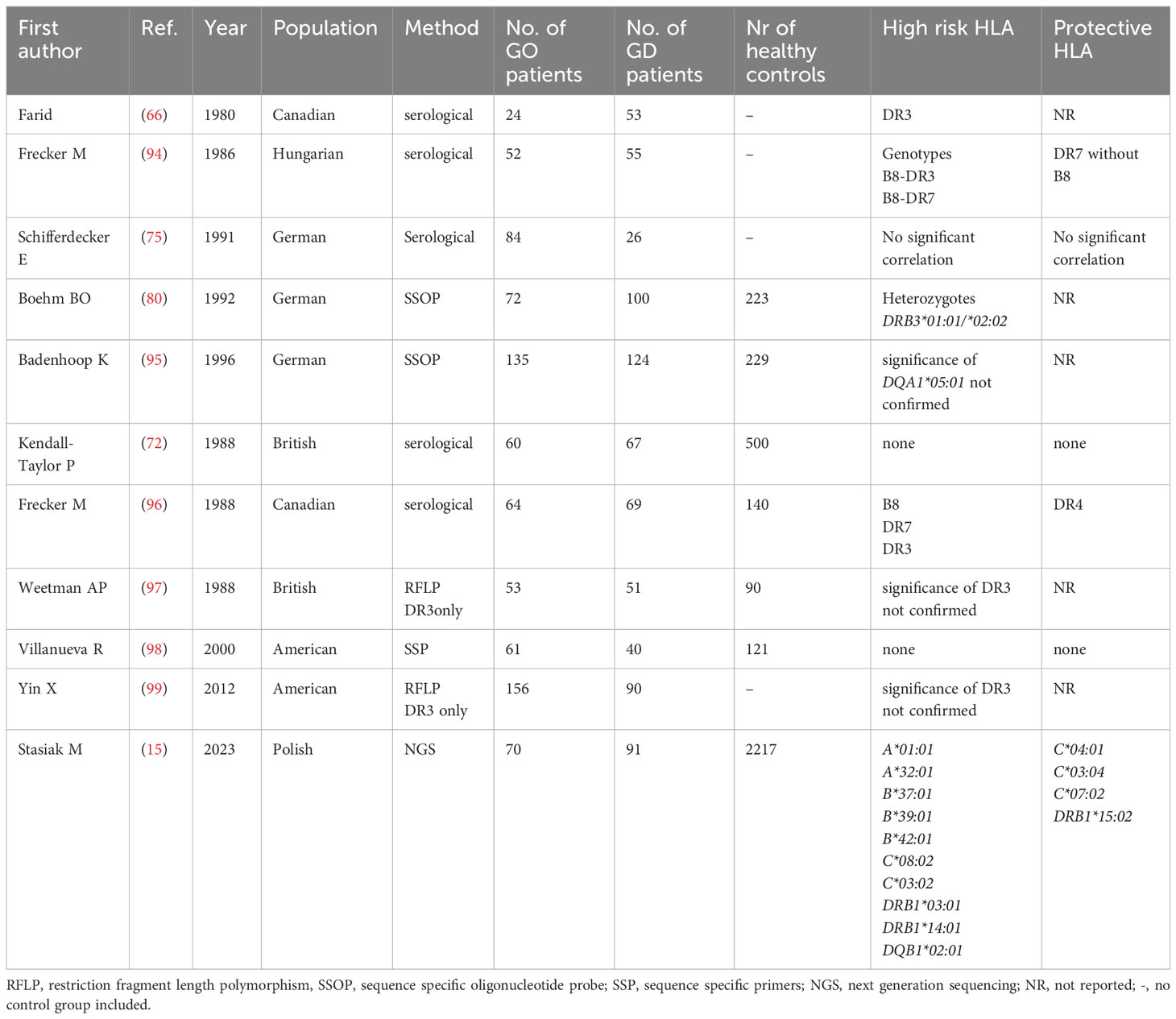
Similarly to the current state of art in Asians, the results concerning HLA associations with GO development in Caucasians are inconsistent (Table 6). From the first study in 1980, DR3 antigen was being demonstrated in serological studies to be not only associated with GD high risk, but also with GO development (66). However, several studies, using different methods, both serological and genetic, did not confirmed such association (36, 72, 97) and the results were highly divergent (80, 94–99). Even a quite recent study performed by Yin et al. did not confirm the existence of HLA-related susceptibility to GO in the group of patients with GD and postulated the importance of environmental or epigenetic factors only (99). However, the authors of that study, similarly to some other earlier ones, focused only on the frequency of HLA-DR3, without assessing the frequencies of other alleles, and applied low resolution method. Recently, the significance of HLA-DRB1*03:01 was demonstrated by our research team with application of NGS method (15). We performed genotyping of both MCH classes and found that the frequency of several alleles is significantly higher in GO group as compared to either patients with GD without GO or healthy controls. Except for the previously mentioned HLA-DRB1*03:01, this group of alleles includes HLA-B*08:01, B*39:06, B*37:01, C*07:01, C*14:02, C*03:02, C*17:01, DRB1*11:01, DRB1*13:03, DRB1*01:03, DRB1*14:01, DQB1*03:01, DQB1*02:01. On the other hand, alleles HLA-C*04:01, C*03:04, C*07:02 and DRB1*15:02 were significantly less frequent in GO patients as compared to GD without GO or controls (15). Unfortunately, it was the first study which applied NGS in Caucasian patients, and therefore – similarly to the studies on GD described above – no comparison with other NGS-based studies is possible. The overview of studies focused on associations between HLA and GO in Caucasian is presented in Table 6.
In our study, based on NGS method, HLA-DQB1*02:01 was also found to be a high risk allele (15). Such phenomenon could be expected as this allele is in linkage disequilibrium with HLA-DRB1*03:01 (58). Interestingly, another allele demonstrated to be GO-related in our study – i.e. HLA-DRB1*14:01, was previously observed to be GO-related in Japanese patients (60) (Table 1). This similarity is the only one between our results in Caucasian population and the already published data for Asian population in regard to GO. Nevertheless, such a lack of coherence between Asians and Caucasians could be expected, because HLA-related susceptibility for many autoimmune diseases differs between these two populations. In our NGS-based study, the highest risk of GO was associated with the presence of HLA-C*08:02 (OR 6.9) and -B*37:01 (OR 4.5) as well as DRB1*14:01 (OR 6.2) (15). There is no linkage disequilibrium between these three alleles (58, 92, 93), so the presence of any of them constitutes independent high risk factor.
Interestingly, in the same study, we demonstrated a protective effect of HLA-C*04:01 allele, which was previously reported as related to increased risk of subacute thyroiditis (SAT) (100, 101). This coincidence can be considered an explanation of the fact that SAT and GO actually occur together extremally rarely in clinical practice. It is worth noting that HLA alleles had already been suggested to be related with the course of SAT and GD in patients with simultaneous presence of both these diseases (102).
3.4 Other associations
3.4.1 Asian population
3.4.1.1 HLA and GD course, comorbidities and relapse
In a large study performed in Taiwanese patients with GD, significant associations between HLA and comorbidities were found (40). In this group, genotypes HLA-A*11:01-A*33:03 and A*02:07-A*11:01, as well as HLA-B*40:01-B*58:01 were significantly correlated with heart disease. Additionally, genotypes HLA-A*24:02-A*24:02 and HLA-B*46:01-B*46:01 were significantly associated with stroke, while genotypes HLA-B*46:01-*46:01 and DPA1*01:03-DPA1*01:03 as well as DPB1*02:01-DPB1*05:01 were correlated with hypertension. On the other hand, genotypes HLA-DPA1*01:03-DPA1*01:03 and DPA1*01:03-DPA1*02:01 were significantly more frequent among the subjects with GD and diabetes than in those with GD without diabetes (40).
Some studies suggested that the age of GD onset is HLA-dependent. As it was indicated above, it was postulated that HLA-Bw46 is associated with younger age of GD onset (32, 34), while B5 was suggested as related to older age of onset (32). Few years later, Onuma et al. published entirely opposite results and stated that HLA-B46 is related to late onset of GD, while DPB1*05:01 is associated with early onset (47). In Japanese and Chinese studies, which included children only, DQB1*03:03 was demonstrated to be GD-related (48, 49). However, in a recent study performed in Korean children, previously postulated significance of HLA-B*46:01 was confirmed, and – additionally – HLA-C*01:02, DPB1*02:02, DPB1*05:01, occurred to be significantly associated with GD (14).
In a recent study by Azizi et al. with application of SSP method, DQB1*05 HLA polymorphism was demonstrated to be related to GD relapse (103). The authors analyzed relapse rates in patient during 48 months period after methimazole withdrawal (103). Unfortunately, no other similar study in Asians is available for comparison.
3.4.2 Caucasian population
3.4.2.1 HLA and GD recurrence and course
In Caucasian population, HLA-DQA1*05 variant was demonstrated to have ability to predict relapse. Surprisingly, combinations with other HLA risk genes forming the risk haplotype DRB1*03-DQA1*05-DQB1*02 did not improve the predictive value (104). Several years earlier, no significance of HLA DQA1*05:01 in GD recurrence was reported by Badenhoop et al. (95) However, a study in which patients were observed for two years after antithyroid drug withdrawal revealed that HLA-DRB1*03, DQA1*05, and DQB1*02 polymorphisms are strong predictors for recurrence after antithyroid drug therapy (105). There is a strong linkage disequilibrium between the them and their significance in GD relapse in Caucasians should be considered.
Most of the studies focused on GD recurrence used serological methods. In a study performed by Shifferdecker et al. in German population, HLA DR5 was associated with relapse of GD, whereas HLA DR7 and B12 were negatively correlated with relapse (75). A role of B8 was postulated by Irvine et al, who observed significantly more common relapses in B8 positive patients (106). Another study demonstrated significance of HLA-DR3 haplotype and lack of association with HLA-B8 haplotype and GD relapse (107). Some studies underlined the role of HLA-DR3 (65, 108), while other did not confirmed this association (109). Similarly, de Bruin et al. found no significance of HLA-DR3, but demonstrated that HLA-Cw7 was associated with relapse, while HLA-DR4 was protective against recurrence (110). In a study by Young et al., neither HLA-B8 nor HLA-DR3 conferred increased likelihood of relapse (111). Significant association of GD relapse and DQA2 genotype was found with application of RFLP method (112). Most of the authors did not find any correlation between HLA and GD recurrence (113–117).
Few studies analyzed the severity of GD in regard to HLA. Preus et al. demonstrated that patients with severe course were characterized by a high frequency of HLA-A1 andHLA-B8, while mild course patients showed a higher frequency of HLA-B12 (118). Some studies focused directly on the HLA associations with GD onset. In a study by Lavard et al. DRB1*03 and DRB3*01:01 were associated with juvenile GD onset (<20 years of age), whereas DRB3*02:02 was associated with adult onset of the disease (85). Other clinical course-related associations demonstrated that HLA-DR3 positive GD patients were also found to be more resistant to radioiodine therapy than patients negative for these antigens (66). Additionally, patients with GD and haplotype HLA-DRB1*03/DRB1*11 were found to have higher FT4/TT3 ratio and anti-thyroglobulin antibody levels (91).
Unfortunately, most of studies which analyzed GD recurrence or the disease course were performed decades ago and applied serological methods. Therefore, the described discrepancies in the obtained results were not unexpected, and currently, no clear correlation in regard to HLA and GD severity or age of the disease onset can be confirmed. Further studies using high resolution methods may provide conclusive results.
3.4.2.2 HLA and non-genetic risk factors of GO
Increased level of total cholesterol (TC) and/or – of low-density lipoprotein cholesterol (LDL) is generally known to increase the risk of GO. Very recently, our research team demonstrated a significant correlation between the higher TC/LDL levels (11) and the occurrence of GO-related high-risk alleles (HLA-B*37:01 and C*03:02). Moreover, the presence of alleles associated with GD without GO (HLA-C*17:01 and B*08:01), as well as alleles which are in linkage disequilibrium with B*08:01 (i.e., HLA-DRB1*03:01 and DQB1*02:01), was shown to be correlated with lower TC levels (11). These results seem important as they provide evidence that correlations between TC/LDL and GO can be HLA-dependent and confirm the relevance of TC/LDL lowering therapy in the cases where the risk of the development of GO is significant.
3.4.3 Significance of HLA amino acid variants in Asians and Caucasians
The significance of amino acid variants of HLA molecules were also postulated in the context of risk of GD development. In a study by Shin et al., both Leu35 (OR = 23.38, P = 0.0002) and Glu55 (OR = 23.38, P = 0.0002) of HLA-DPB1 were strongly associated with GD (14). These authors concluded that amino-acid signatures of the HLA-DP β chain, might contribute to the molecular pathogenesis of GD (14). However, in Japanese population, amino acid variants in HLA-DRB1 allotypes were examined, and critical significance of position 9 amino acid variants for GD development was found (57). Glu-9 variant, which was reported as related to GD high risk, is encoded by HLA-DRB1*03:01, DRB1*04:05 and -DRB1*14:03 alleles, which confer susceptibility to GD in several studies. On the other hand, Cys-9 variant, which was reported as protective against GD, is encoded by HLA-DRB1*07:01, DRB1*01:01 and DRB1*15:02 alleles, which were demonstrated to be protective against GD (57). It is speculated that changes in the charge and polarity of amino acids at these positions modify the three-dimensional structure of the DR peptide-binding pocket, which leads to susceptibility to or protection against GD (57, 119). Negatively charged Glu-9 was demonstrated to confer susceptibility to GD, while uncharged Cys-9 conferred protection against GD (57).
It was postulated that HLA-DR3 (HLA-DRB1*03) Arg-74 is the critical amino acid for the development of GD (88, 120). The group HLA-DR3 includes more than 30 alleles. A HLA-DRβ (beta chain of HLA-DR3) pocket variant with arginine at position 74 (HLA-DRβ-Arg74) is associated with an increased risk of GD, while glutamine (HLA-DRβ-Gln74) variant was protective (88, 119, 120). Three-dimensional computer modeling of the HLA-DR pocket allowed proving that electrostatic potential of HLA-DRβ-Arg74 generates a more positively charged P4 HLA-DR pocket than HLA-DRβ-Gln74. Therefore, the susceptibility to GD may be related to electrostatic potential of HLA-DR pocket and positive charge can be a high risk factor. The significance of this phenomenon can be confirmed by the finding that, among DRB1 molecules, DRB1*03 contains a positively charged Arg at β74, and DRB1*07 contains a non-charged Gln at β74 position (88). This positive charge difference is believed to facilitate auto-antigen presentation and T-cell activation because of its increased binding affinity of pathogenic self-peptides to the HLA-DR pocket (121). Candidate pathogenic peptides in GD include peptides derived from TSHR and Tg. This interaction between peptides of thyroidal origin and the HLA-DRβ-Arg74 pocket was considered as potential therapeutic target (121). Such an antigen-specific immunotherapy may constitute a crucial progress in the GD therapy, as current treatment modalities do not reverse the autoimmune process. It is well known that TSHR is the major autoantigen in GD. A TSHR peptide, designated TSHR.132, was found to be a dominant TSHR peptide in GD (122–124). TSHR.132 was demonstrated to bind with high affinity to recombinant HLA-DRβ-Arg74 and to cells that express HLA-DRβ-Arg74 (121). Cepharanthine is a small molecule compound which was demonstrated to inhibit T-cell activation by TSHR.132 ex vivo in splenocytes isolated from humanized mice induced with EAGD (experimental autoimmune Graves’ disease) (123, 124). Cepharantine directly interacts with Arg74 and blocks the HLA-DR3 pocket variant associated with AITD including GD. Due to the significant role of HLA-DR3 in many autoimmune diseases, cepharantine may be a potentially efficient causative therapy for GD and other autoimmune disorders such as Addison’s disease, Hashimoto’s thyroiditis, myasthenia gravis or SLE (121, 123, 124).
Additional various HLA polymorphisms were also described as associated with GD. In two independent Caucasian populations, an intronic variant (rs3094228) in HLA complex P5 (HCP5) was demonstrated to be associated with GD susceptibility and age of onset, which indicates a potential role of long non-coding ribonucleic acids, including HCP5, in GD pathogenesis (125, 126). HCP5 gene polymorphism (rs3094228) was associated with an earlier age of GD onset and patients with higher number of the HCP5 risk alleles tend to have a significantly earlier onset of GD (126).
4 Conclusions
In Asian population, GD was found to be associated mostly with HLA-B*46:01, DPB1*05:01, DRB1*08:02/03, DRB1*16:02, DRB1*14:03, DRB1*04:05, DQB1*03:03 and DQB1*05:02, while DRB1*07:01, DRB1*01:01, DRB1*13:02, DRB1*12:02 are potentially protective. HLA-B*38:02, DRB1*16:02, DQA1*01:02, DQB1*05:02 can be considered associated with increased risk of GO in Asians, while HLA-B*54:01 may play protective role. In Caucasians, C*07:01, DQA1*05:01, DRB1*03, DQB1*02:01, are associated with GD risk while DRB1*07:01, DQA1*02:01 may be protective. Data are scarce in regard to GO in Caucasians, but HLA-B*08:01, B*39:06, B*37:01, C*07:01, C*14:02, C*03:02, C*17:01, DRB1*03:01, DRB1*11:01, DRB1*13:03, DRB1*01:03, DRB1*14:01, DQB1*03:01, DQB1*02:01 were found more frequently while alleles HLA-C*04:01, C*03:04, C*07:02 and DRB1*15:02 were significantly less frequent in GO patients as compared to GD without GO, or to controls. HLA polymorphisms potentially influence the course of GD, its recurrence risk, comorbidities and a presence of GO-related non-genetic risk factors. Further studies based on NGS methods are required to clearly demonstrate or confirm the correlations, as identification of an actual set of high risk and protective alleles in a given population can constitute a reliable tool for the individual risk assessment. Additionally, it may contribute to potential development of HLA-based treatment modalities.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
MS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. BS: Formal Analysis, Investigation, Methodology, Resources, Software, Writing – original draft. KZ-S: Investigation, Resources, Writing – review & editing. AL: Methodology, Project administration, Resources, Validation, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This review was financially supported by the Polish Mother’s Memorial Hospital-Research Institute, Lodz, Poland.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid (2016) 26:1343–421. doi: 10.1089/thy.2016.0229
2. Bartalena L, Tanda ML. Current concepts regarding Graves’ orbitopathy. J Intern Med (2022) 292:692–716. doi: 10.1111/joim.13524
3. Krieger CC, Morgan SJ, Neumann S, Gershengorn MC. Thyroid stimulating hormone (TSH)/insulin-like growth factor 1 (IGF1) receptor cross-talk in human cells. Curr Opin Endocr. Metab Res (2018) 2:29–33. doi: 10.1016/j.coemr.2018.01.007
4. Radziszewski M, Kuś A, Bednarczuk T. Genotype-phenotype correlations in Graves' disease. Best Pract Res Clin Endocrinol Metab (2023) 37:101745. doi: 10.1016/j.beem.2023.101745
5. Vita R, Lapa D, Trimarchi F, Vita G, Fallahi P, Antonelli A, et al. Certain HLA alleles are associated with stress-triggered Graves' disease and influence its course. Endocrine (2017) 55:93–100. doi: 10.1007/s12020-016-0909-6
6. Heward JM, Allahabadia A, Daykin J, Carr-Smith J, Daly A, Armitage M, et al. Linkage disequilibrium between the human leukocyte antigen class II region of the major histocompatibility complex and Graves’ disease: replication using a population case control and family-based study. J Clin Endocrinol Metab (1998) 83:3394–7. doi: 10.1210/jcem.83.10.5137
7. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol (2021) 185:G43–67. doi: 10.1530/EJE-21-0479
8. Chaudhary S, Dogra V, Walia R. Four cases of Graves' disease following viral vector severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) vaccine. Endocr J (2022) 69:1431–5. doi: 10.1507/endocrj.EJ22-0208
9. Rodríguez de Vera Gómez P, García-González JJ, Ravé-García R, López Ruiz R, Torres-Cuadro A, Eichau-Madueño S, et al. Graves' disease induced by Alemtuzumab in relapsing-remitting multiple sclerosis patients: an observational study in a reference center. J Endocrinol Invest. (2022) 45:1977–90. doi: 10.1007/s40618-022-01832-4
10. Morshed S, Latif R, Davies TF. Signal responses to neutral TSH receptor antibody - A cycle of damage in the pathophysiology of Graves' disease. J Autoimmun (2023) 136:103012. doi: 10.1016/j.jaut.2023.103012
11. Stasiak M, Zawadzka-Starczewska K, Tymoniuk B, Stasiak B, Lewiński A. Associations between lipid profiles and graves' Orbitopathy can be HLA-dependent. Genes (Basel). (2023) 14:1209. doi: 10.3390/genes14061209
12. Brix TH, Kyvik KO, Christensen K, Heged�s L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab (2001) 86:930–4. doi: 10.1046/j.1365-2265.1998.00450.x
13. Zawadzka-Starczewska K, Tymoniuk B, Stasiak B, Lewiński A, Stasiak M. Actual associations between HLA haplotype and graves' Disease development. J Clin Med (2022) 11:2492. doi: 10.3390/jcm11092492
14. Shin DH, Baek IC, Kim HJ, Choi EJ, Ahn M, Jung MH, et al. HLA alleles, especially amino-acid signatures of HLA-DPB1, might contribute to the molecular pathogenesis of early-onset autoimmune thyroid disease. PloS One (2019) 14:e0216941. doi: 10.1371/journal.pone.0216941
15. Stasiak M, Zawadzka-Starczewska K, Tymoniuk B, Stasiak B, Lewiński A. Significance of HLA in the development of Graves' orbitopathy. Genes Immun (2023) 24:32–8. doi: 10.1038/s41435-023-00193-z
16. Gu LQ, Zhu W, Zhao SX, Zhao L, Zhang MJ, Cui B, et al. Clinical associations of the genetic variants of CTLA-4, Tg, TSHR, PTPN22, PTPN12 and FCRL3 in patients with Graves' disease. Clin Endocrinol (Oxf) (2010) 72:248–55. doi: 10.1111/j.1365-2265.2009.03617.x
17. Wang PW, Chen IY, Juo SH, Hsi E, Liu RT, Hsieh CJ. Genotype and phenotype predictors of relapse of graves' disease after antithyroid drug withdrawal. Eur Thyroid J (2013) 1:251–8. doi: 10.1159/000342621
18. Pujol-Borrell R, Giménez-Barcons M, Marín-Sánchez A, Colobran R. Genetics of graves' Disease: special focus on the role of TSHR gene. Horm Metab Res (2015) 47:753–66. doi: 10.1055/s-0035-1559646
19. Razmara E, Salehi M, Aslani S, Bitaraf A, Yousefi H, Colón JR, et al. Graves' disease: introducing new genetic and epigenetic contributors. J Mol Endocrinol (2021) 66:R33–55. doi: 10.1530/JME-20-0078
20. Kuś A, Szymański K, Peeters RP, Miśkiewicz P, Porcu E, Pistis G, et al. The association of thyroid peroxidase antibody risk loci with susceptibility to and phenotype of Graves' disease. Clin Endocrinol (Oxf) (2015) 83:556–62. doi: 10.1111/cen.12640
21. Kuś A, Szymański K, Jurecka-Lubieniecka B, Pawlak-Adamska E, Kula D, Miśkiewicz P, et al. Gender-dependent and age-of-onset-specific association of the rs11675434 single-nucleotide polymorphism near TPO with susceptibility to Graves' ophthalmopathy. J Hum Genet (2017) 62:373–7. doi: 10.1038/jhg.2016.135
22. Khong JJ, Burdon KP, Lu Y, Leonardos L, Laurie KJ, Walsh JP, et al. Association of polymorphisms in MACRO domain containing 2 with thyroid-associated orbitopathy. Invest Ophthalmol Vis Sci (2016) 57:3129–37. doi: 10.1167/iovs.15-18797
23. Maciejewski A, Kowalczyk MJ, Gasińska T, Szeliga A, Prendecki M, Dorszewska J, et al. The role of vitamin D receptor gene polymorphisms in thyroid-associated orbitopathy. Ocul Immunol Inflamm (2020) 28:354–61. doi: 10.1080/09273948.2019
24. Pawlak-Adamska E, Frydecka I, Bolanowski M, Tomkiewicz A, Jonkisz A, Karabon L, et al. CD28/CTLA-4/ICOS haplotypes confers susceptibility to Graves' disease and modulates clinical phenotype of disease. Endocrine (2017) 55:186–99. doi: 10.1007/s12020-016-1096-1
25. Kishore A, Petrek M. Next-generation sequencing based HLA typing: Deciphering immunogenetic aspects of sarcoidosis. Front Genet (2018) 9:503. doi: 10.3389/fgene.2018.00503
26. Profaizer T, Kumánovics A. Human leukocyte antigen typing by next-generation sequencing. Clin Lab Med (2018) 38:565–78. doi: 10.1016/j.cll.2018.07.006
27. Mayor NP, Hayhurst JD, Turner TR, Szydlo RM, Shaw BE, Bultitude WP, et al. Recipients receiving better HLA-matched hematopoietic cell transplantation grafts, uncovered by a novel HLA typing method, have superior survival: A retrospective study. Biol Blood Marrow Transplant. (2019) 25:443–50. doi: 10.1016/j.bbmt.2018.12.768
28. Khan MA. Polymorphism of HLA-B27: 105 subtypes currently known. Curr Rheumatol Rep (2013) 15:362. doi: 10.1007/s11926-013-0362-y
29. Barlow AB, Wheatcroft N, Watson P, Weetman AP. Association of HLA-DQA1*0501 with Graves’ disease in English Caucasian men and women. Clin Endocrinol (1996) 44:73–7. doi: 10.1046/j.1365-2265.1996.634454.x
30. Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves disease. Am J Med Genet (2000) 95:432–7. doi: 10.1002/1096-8628(20001218)95:5<432::aid-ajmg5>3.0.co;2-7
31. Li Y, Yao Y, Yang M, Shi L, Li X, Yang Y, et al. Association between HLA-B*46 allele and Graves disease in Asian populations: a meta-analysis. Int J Med Sci (2013) 10:164–70. doi: 10.7150/ijms.5158
32. Hawkins BR, Ma JT, Lam KS, Wang CC, Yeung RT. Association of HLA antigens with thyrotoxic Graves' disease and periodic paralysis in Hong Kong Chinese. Clin Endocrinol (Oxf) (1985) 23:245–52. doi: 10.1507/endocrj1954.34.685
33. Naito S, Sasaki H, Arakawa K. Japanese Graves' disease: association with HLA-Bw46. Endocrinol Jpn (1987) 34:685–8. doi: 10.1507/endocrj1954.34.685
34. Yeo PP, Chan SH, Thai AC, Ng WY, Lui KF, Wee GB, et al. HLA Bw46 and DR9 associations in Graves' disease of Chinese patients are age- and sex-related. Tissue Antigens (1989) 34:179–84. doi: 10.1111/j.1399-0039.1989.tb01734.x
35. Chan SH, Lin YN, Wee GB, Ren EC, Lui KF, Cheah JS. Human leucocyte antigen DNA typing in Singaporean Chinese patients with Graves' disease. Ann Acad Med Singapore (1993) 22:576–9.
36. Cavan DA, Penny MA, Jacobs KH, Kelly MA, Jenkins D, Mijovic C, et al. The HLA association with Graves' disease is sex-specific in Hong Kong Chinese subjects. Clin Endocrinol (Oxf) (1994) 40:63–6. doi: 10.1111/j.1365-2265.1994.tb02444.x
37. Huang SM, Wu TJ, Lee TD, Yang EK, Shaw CK, Yeh CC. The association of HLA -A, -B, and -DRB1 genotypes with Graves' disease in Taiwanese people. Tissue Antigens (2003) 61:154–8. doi: 10.1034/j.1399-0039.2003.00016.x
38. Chen PL, Fann CS, Chu CC, Chang CC, Chang SW, Hsieh HY, et al. Comprehensive genotyping in two homogeneous Graves' disease samples reveals major and novel HLA association alleles. PloS One (2011) 6:e16635. doi: 10.1371/journal.pone.0016635
39. Ueda S, Oryoji D, Yamamoto K, Noh JY, Okamura K, Noda M, et al. Identification of independent susceptible and protective HLA alleles in Japanese autoimmune thyroid disease and their epistasis. J Clin Endocrinol Metab (2014) 99:E379–83. doi: 10.1210/jc.2013-2841
40. Liao WL, Liu TY, Cheng CF, Chou YP, Wang TY, Chang YW, et al. Analysis of HLA variants and graves' Disease and its comorbidities using a high resolution imputation system to examine electronic medical health records. Front Endocrinol (Lausanne) (2022) 13:842673. doi: 10.3389/fendo.2022.842673
41. Chan SH, Yeo PP, Lui KF, Wee GB, Woo KT, Lim P, et al. HLA and thyrotoxicosis (Graves' disease) in Chinese. Tissue Antigens (1978) 12:109–14. doi: 10.1111/j.1399-0039.1978.tb01306.x
42. Cho BY, Rhee BD, Lee DS, Lee MS, Kim GY, Lee HK, et al. HLA and graves' disease in Koreans. Tissue Antigens (1987) 30:119–21. doi: 10.1111/j.1399-0039.1987.tb01607.x
43. Tamai H, Tanaka K, Komaki G, Matsubayashi S, Hirota Y, Mori K, et al. HLA and thyrotoxic periodic paralysis in Japanese patients. J Clin Endocrinol Metab (1987) 64:1075–8. doi: 10.1210/jcem-64-5-1075
44. Tsai KS, Hsieh RP, Chang CC, Chen FW, Lee SC. Association of HLA-DR tissue types with Graves' disease in Taiwan. Taiwan Yi Xue Hui Za Zhi (1989) 88:336–41.
45. Inoue D, Sato K, Enomoto T, Sugawa H, Maeda M, Inoko H, et al. Correlation of HLA types and clinical findings in Japanese patients with hyperthyroid Graves' disease: evidence indicating the existence of four subpopulations. Clin Endocrinol (Oxf) (1992) 36:75–82. doi: 10.1111/j.1365-2265.1992.tb02905.x
46. Dong RP, Kimura A, Okubo R, Shinagawa H, Tamai H, Nishimura Y, et al. HLA-A and DPB1 loci confer susceptibility to Graves' disease. Hum Immunol (1992) 35:165–72. doi: 10.1016/0198-8859(92)90101-r
47. Onuma H, Ota M, Sugenoya A, Inoko H. Association of HLA-DPB1*0501 with early-onset Graves' disease in Japanese. Hum Immunol (1994) 39:195–201. doi: 10.1016/0198-8859(94)90260-7
48. Ohtsuka K, Nakamura Y. Am Human leukocyte antigens associated with hyperthyroid Graves ophthalmology in Japanese patients. J Ophthalmol (1998) 126:805–10. doi: 10.1016/s0002-9394(98)00248-7
49. Wong GW, Cheng SH, Dorman JS. The HLA-DQ associations with Graves' disease in Chinese children. Clin Endocrinol (Oxf) (1999) 50:493–5. doi: 10.1046/j.1365-2265.1999.00661.x
50. Park MH, Park YJ, Song EY, Park H, Kim TY, Park DJ, et al. Association of HLA-DR and -DQ genes with Graves disease in Koreans. Hum Immunol (2005) 66:741–7. doi: 10.1016/j.humimm.2005.03.001
51. Iwama S, Ikezaki A, Kikuoka N, Kim HS, Matsuoka H, Yanagawa T, et al. Association of HLA-DR, -DQ genotype and CTLA-4 gene polymorphism with Graves' disease in Japanese children. Horm Res (2005) 63:55–60. doi: 10.1159/000083137
52. Wongsurawat T, Nakkuntod J, Charoenwongse P, Snabboon T, Sridama V, Hirankarn N. The association between HLA class II haplotype with Graves' disease in Thai population. Tissue Antigens (2006) 67:79–83. doi: 10.1111/j.1399-0039.2005.00498.x
53. Takahashi M, Yasunami M, Kubota S, Tamai H, Kimura A. HLA-DPB1*0202 is associated with a predictor of good prognosis of Graves' disease in the Japanese. Hum Immunol (2006) 67:47–52. doi: 10.1016/j.humimm.2006.02.023
54. Cho WK, Jung MH, Choi EJ, Choi HB, Kim TG, Suh BK. Association of HLA alleles with autoimmune thyroid disease in Korean children. Horm Res Paediatr (2011) 76:328–34. doi: 10.1159/000331134
55. Chen PL, Fann CS, Chu CC, Chang CC, Chang SW, Hsieh HY, et al. Comprehensive genotyping in two homogeneous Graves' disease samples reveals major and novel HLA association alleles. PloS One (2011) 28:e16635. doi: 10.1371/journal.pone.0016635
56. Jang HW, Shin HW, Cho HJ, Kim HK, Lee JI, Kim SW, et al. Identification of HLA-DRB1 alleles associated with Graves' disease in Koreans by sequence-based typing. Immunol Invest. (2011) 40:172–82. doi: 10.3109/08820139.2010.525571
57. Katahira M, Ogata H, Takashima H, Ito T, Hodai Y, Miwata T, et al. Critical amino acid variants in HLA-DRB1 allotypes in the development of Graves' disease and Hashimoto's thyroiditis in the Japanese population. Hum Immunol (2021) 82:226–31. doi: 10.1016/j.humimm.2020.12.007
58. Available at: www.ctht.info/Table%2013%20DRB1%20DQA1%20DQB1%20associations%20in%20various%20populations.pdf (Accessed 02, 2023).
59. Inoue D, Sato K, Maeda M, Inoko H, Tsuji K, Mori T, et al. Genetic differences shown by HLA typing among Japanese patients with euthyroid Graves' ophthalmopathy, Graves' disease and Hashimoto's thyroiditis: genetic characteristics of euthyroid Graves' ophthalmopathy. Clin Endocrinol (Oxf) (1991) 34:57–62. doi: 10.1111/j.1365-2265.1991.tb01736.x
60. Ohtsuka K, Nakamura Y. Human leukocyte antigens associated with hyperthyroid Graves ophthalmology in Japanese patients. Am J Ophthalmol (1998) 126:805–10. doi: 10.1016/s0002-9394(98)00248-7
61. Mehraji Z, Farazmand A, Esteghamati A, Noshad S, Sadr M, Amirzargar S, et al. Association of human leukocyte antigens class I and II with graves’ Disease in Iranian population. Iran. J Immunol (2017) 14:223–30.
62. Huang X, Liu G, Mei S, Cai J, Rao J, Tang M, et al. Human leucocyte antigen alleles confer susceptibility and progression to Graves' ophthalmopathy in a Southern Chinese population. Br J Ophthalmol (2021) 105:1462–8. doi: 10.1136/bjophthalmol-2020-317091
63. Bech K, Lumholtz B, Nerup J, Thomson M, Platz P, Ryser LF, et al. HLA antigens in Graves' disease. Acta Endocrinol Copenh (1977) 86:510. doi: 10.1530/acta.0.0860510
64. Farid NR, Sampson L, Noel EP, Barnard JM, Mandeville R, Larsen B, et al. A study of human leukocyte D locus related antigens in Graves' disease. J Clin Invest. (1979) 63:108–13. doi: 10.1172/JCI109263
65. McGregor AM, Smith BR, Hall R, Petersen MM, Miller M, Dewar PJ. Prediction of relapse in hyperthyroid Graves' disease. Lancet (1980) 1:1101–3. doi: 10.1016/s0140-6736(80)91551-2
66. Farid NR, Stone E, Johnson G. Graves' disease and HLA: clinical and epidemiologic associations. Clin Endocrinol (Oxf) (1980) 13:535–44. doi: 10.1111/j.1365-2265.1980.tb03421.x
67. Allanic H, Fauchet R, Lorcy Y, Heim J, Gueguen M, Leguerrier AM, et al. HLA and Graves' disease: An association with HLA-DRw3. J Clin Endocrinol Metab (1980) 56:863. doi: 10.1111/j.1365-2265.1980.tb03421.x
68. Dahlberg PA, Holmlund G, Karlsson FA, Säfwenberg J. HLA-A, -B, -C and -DR antigens in patients with Graves' disease and their correlation with signs and clinical course. Acta Endocrinol (Copenh) (1981) 97:42–7. doi: 10.1530/acta.0.0970042
69. McKenna R, Kearns M, Sugrue D, Drury MI, McCarthy CF. HLA and hyperthyroidism in Ireland. Tissue Antigens (1982) 19:97–9. doi: 10.1111/j.1399-0039.1982.tb01423.x
70. Allannic H, Fauchet R, Lorcy Y, Gueguen M, Le Guerrier AM, Genetet B. A prospective study of the relationship between relapse of hyperthyroid Graves' disease after antithyroid drugs and HLA haplotype. J Clin Endocrinol Metab (1983) 57:719–22. doi: 10.1210/jcem-57-4-719
71. Stenszky V, Kozma L, Balazś C, Bear JC, Farid NR. The role of HLA antigens in the manifestation and course of Graves' disease. Mol Biol Med (1986) 3:53–62.
72. Kendall-Taylor P, Stephenson A, Stratton A, Papiha SS, Perros P, Roberts DF. Differentiation of autoimmune ophthalmopathy from Graves' hyperthyroidism by analysis of genetic markers. Clin Endocrinol (Oxf) (1988) 28:601–10. doi: 10.1111/j.1365-2265.1988.tb03851.x
73. Semana G, Allanic H, Quillivic F, Vallejo MT, Simon JP, Genetet B, et al. Implication of the HLA-DRB3 gene in Graves' disease: predominance of allele Dw24. Hum Immunol (1990) 29:143–9. doi: 10.1016/0198-8859(90)90077-3
74. Mangklabruks A, Cox N, DeGroot LJ. Genetic factors in autoimmune thyroid disease analyzed by restriction fragment length polymorphisms of candidate genes. J Clin Endocrinol Metab (1991) 73:236–44. doi: 10.1210/jcem-73-2-236
75. Schifferdecker E, Kühnl P, Schöffling K, Manfras B, Holzberger G, Spielmann W, et al. Immunogenetic markers in patients with Graves' disease. Klin Wochenschr (1991) 69:256–60. doi: 10.1007/BF01666851
76. Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocr Rev (2003) 24:694–717. doi: 10.1210/er.2002-0030
77. Yanagawa T, Mangklabruks A, Chang YB, Okamoto Y, Fisfalen ME, Curran PG, et al. Human histocompatibility leukocyte antigen-DQA1*0501 allele associated with genetic susceptibility to Graves’ disease in a Caucasian population. J Clin Endocrinol Metab (1993) 76:1569–74. doi: 10.1210/jcem.76.6.8501164
78. Gough SC. The genetics of Graves' disease. Endocrinol Metab Clin North Am (2000) 29:255–66. doi: 10.1016/s0889-8529(05)70130-4
79. Gough SC, Simmonds MJ. The HLA region and autoimmune disease: Associations and mechanisms of action. Curr Genomics (2007) 8:453–65. doi: 10.2174/138920207783591690
80. Boehm BO, Kühnl P, Manfras BJ, Chen M, Lee JC, Holzberger G, et al. HLA-DRB3 gene alleles in Caucasian patients with Graves' disease. Clin Investig (1992) 70:956–60. doi: 10.1007/BF00180447
81. Ratanachaiyavong S, McGregor AM. HLA-DPB1 polymorphisms on the MHC-extended haplotypes of families of patients with Graves' disease: two distinct HLA-DR17 haplotypes. Eur J Clin Invest. (1994) 24:309–15. doi: 10.1111/j.1365-2362.1994.tb01090.x
82. Badenhoop K, Walfish PG, Rau H, Fischer S, Nicolay A, Bogner U, et al. Susceptibility and resistance alleles of human leukocyte antigen (HLA) DQA1 and HLA DQB1 are shared in endocrine autoimmune disease. J Clin Endocrinol Metab (1995) 80:2112–7. doi: 10.1210/jcem.80.7.7608264
83. Cuddihy RM, Bahn RS. Lack of an independent association between the human leukocyte antigen allele DQA1*0501 and Graves' disease. J Clin Endocrinol Metab (1996) 81:847–9. doi: 10.1210/jcem.81.2.8636314
84. Kontopoulos A, Harsoulis P, Adam K, Papadopoulos G, Polymenidis Z, Boudoulas H. Frequency of HLA antigens in Graves' hyperthyroidism and mitral valve prolapse. J Heart Valve Dis (1996) 5:543–7.
85. Lavard L, Madsen HO, Perrild H, Jacobsen BB, Svejgaard A. HLA class II associations in juvenile Graves' disease: indication of a strong protective role of the DRB1*0701,DQA1*0201 haplotype. Tissue Antigens (1997) 50:639–41. doi: 10.1111/j.1399-0039.1997.tb02922.x
86. Chen QY, Huang W, She JX, Baxter F, Volpe R, Maclaren NK. HLA-DRB1*08, DRB1*03/DRB3*0101, and DRB3*0202 are susceptibility genes for Graves' disease in North American Caucasians, whereas DRB1*07 is protective. J Clin Endocrinol Metab (1999) 84:3182–6. doi: 10.1210/jcem.84.9.5991
87. Ban Y, Davies TF, Greenberg DA, Concepcion ES, Tomer Y. The influence of human leucocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): results of studies in HLA-DR3 positive AITD families. Clin Endocrinol (Oxf) (2002) 57:81–8. doi: 10.1046/j.1365-2265.2002.01566.x
88. Simmonds MJ, Howson JM, Heward JM, Cordell HJ, Foxall H, Carr-Smith J, et al. Regression mapping of association between the human leukocyte antigen region and Graves disease. Am J Hum Genet (2005) 76:157–63. doi: 10.1086/426947
89. Simmonds MJ, Howson JM, Heward JM, Carr-Smith J, Franklyn JA, Todd JA, et al. A novel and major association of HLA-C in Graves' disease that eclipses the classical HLA-DRB1 effect. Hum Mol Genet (2007) 16:2149–53. doi: 10.1093/hmg/ddm165
90. Bernecker C, Ostapczuk M, Vordenbäumen S, Ehlers M, Thiel A, Schinner S, et al. HLA-A2 phenotype may be protective against Graves' disease but not against Hashimoto's thyroiditis in Caucasians. Horm Metab Res (2013) 45:74–7. doi: 10.1055/s-0032-1323704
91. Martin S, Dutescu MI, Sirbu A, Barbu C, Albu A, Florea S, et al. The clinical value of human leukocyte antigen HLA-DRB1 subtypes associated to Graves' disease in Romanian population. Immunol Invest. (2014) 43:479–90. doi: 10.3109/08820139.2014.886261
92. Available at: www.ctht.info/Table%209%20CB%20ASSOCIATIONS.pdf (Accessed 02, 2023).
93. Available at: www.ctht.info/Table%208%20BC%20ASSOCIATIONS.pdf (Accessed 02, 2023).
94. Frecker M, Stenszky V, Balazs C, Kozma L, Kraszits E, Farid NR. Genetic factors in Graves' ophthalmopathy. Clin Endocrinol (Oxf) (1986) 25:479–85. doi: 10.1111/j.1365-2265.1986.tb03599.x
95. Badenhoop K, Donner H, Braun J, Siegmund T, Rau H, Usadel KH. Genetic markers in diagnosis and prediction of relapse in Graves' disease. Exp Clin Endocrinol Diabetes. (1996) 104 Suppl 4:98–100. doi: 10.1055/s-0029-1211712
96. Frecker M, Mercer G, Skanes VM, Farid NR. Major histocompatibility complex (MHC) factors predisposing to and protecting against Graves' eye disease. Autoimmunity (1988) 1:307–15. doi: 10.3109/08916938809010684
97. Weetman AP, So AK, Warner CA, Foroni L, Fells P, Shine B. Immunogenetics of graves' ophthalmopathy. Clin Endocrinol (Oxf) (1988) 28:619–28. doi: 10.1111/j.1365-2265.1988.tb03853.x
98. Villanueva R, Inzerillo AM, Tomer Y, Barbesino G, Meltzer M, Concepcion ES, et al. Limited genetic susceptibility to severe Graves' ophthalmopathy: no role for CTLA-4 but evidence for an environmental etiology. Thyroid (2000) 10:791–8. doi: 10.1089/thy.2000.10.791
99. Yin X, Latif R, Bahn R, Davies TF. Genetic profiling in Graves' disease: further evidence for lack of a distinct genetic contribution to Graves' ophthalmopathy. Thyroid (2012) 22:730–6. doi: 10.1089/thy.2012.0007
100. Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewinski A. Subacute thyroiditis is associated with HLA-B*18:01, -DRB1*01 and -C*04:01-the significance of the new molecular background. J Clin Med (2020) 9:534. doi: 10.3390/jcm9020534
101. Stasiak M, Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord (2021) 22:1027–39. doi: 10.1007/s11154-021-09648-y
102. Stasiak M, Lewinski A. Strong correlation between HLA and clinical course of subacute thyroiditis-A report of the three siblings. Genes (2020) 11:1282. doi: 10.3390/genes11111282
103. Azizi F, Amouzegar A, Tohidi M, Hedayati M, Khalili D, Cheraghi L, et al. Increased remission rates after long-term methimazole therapy in patients with graves' Disease: results of a randomized clinical trial. Thyroid (2019) 29:1192–200. doi: 10.1089/thy.2019.0180
104. Vejrazkova D, Vcelak J, Vaclavikova E, Vankova M, Zajickova K, Vrbikova J, et al. Recurrence of graves' Disease: what genetics of HLA and PTPN22 can tell us. Front Endocrinol (Lausanne) (2021) 12:761077. doi: 10.3389/fendo.2021.761077
105. Vos XG, Endert E, Zwinderman AH, Tijssen JG, Wiersinga WM. Predicting the risk of recurrence before the start of antithyroid drug therapy in patients with graves' Hyperthyroidism. J Clin Endocrinol Metab (2016) 101:1381–9. doi: 10.1210/jc.2015-3644
106. Irvine WJ, Gray RS, Morris PJ, Ting A. Correlation of HLA and thyroid antibodies with clinical course of thyrotoxicosis treated with antithyroid drugs. Lancet (1977) 2:898–900. doi: 10.1016/s0140-6736(77)90833-9
107. Winsa B, Dahlberg A, Jansson R, Agren H, Karlsson FA. Factors influencing the outcome of thyrostatic drug therapy in Graves' disease. Acta Endocrinol (Copenh) (1990) 122:722–8. doi: 10.1530/acta.0.1220722
108. Teng CS, Yeung RT, Kawa A, Nakamura S, Nomoto K, Arima N, et al. Thyrotrophin-binding inhibitory immunoglobulins and HLA-DRW3-two prognostic factors in Graves' disease. Aust N Z J Med (1981) 11:383–5. doi: 10.1111/j.1445-5994.1981.tb03517.x
109. Schleusener H, Schwander J, Fischer C, Holle R, Holl G, Badenhoop K, et al. Prospective multicentre study on the prediction of relapse after antithyroid drug treatment in patients with Graves' disease. Acta Endocrinol (Copenh) (1989) 120:689–701. doi: 10.1530/acta.0.1200689
110. De Bruin TW, Bolk JH, Bussemaker JK, Stijnen T, Schreuder GM, de Vries RR, et al. Graves' disease: immunological and immunogenetic indicators of relapse. Br Med J (Clin Res Ed) (1988) 296:1292–5. doi: 10.1136/bmj.296.6632.1292
111. Young ET, Steel NR, Taylor JJ, Stephenson AM, Stratton A, Holcombe M, et al. Prediction of remission after antithyroid drug treatment in Graves' disease. Q J Med (1988) 66:175–89.
112. Ratanachaiyavong S, Gunn CA, Bidwell EA, Darke C, Hall R, McGregor AM. DQA2 U allele: a genetic marker for relapse of Graves' disease. Clin Endocrinol (Oxf) (1990) 32:241–51. doi: 10.1111/j.1365-2265.1990.tb00860.x
113. Di Mario U, Vitillo M, Perfetti R, Mancuso M, Morellini M, Cappellacci S, et al. Humoral and cellular immunological factors as possible markers of clinical relapse in HLA-typed Graves' patients followed with time. Horm Metab Res (1989) 21:267–71. doi: 10.1055/s-2007-1009209
114. Wilson R, McKillop JH, Henderson N, Pearson DW, Thomson JA. The ability of the serum thyrotrophin receptor antibody (TRAb) index and HLA status to predict long-term remission of thyrotoxicosis following medical therapy for Graves' disease. Clin Endocrinol (Oxf) (1986) 25:151–6. doi: 10.1111/j.1365-2265.1986.tb01676.x
115. Madec AM, Allannic H, Genetet N, Gueguen M, Genetet G, Fauchet R, et al. T lymphocyte subsets at various stages of hyperthyroid Graves' disease: effect of carbimazole treatment and relationship with thyroid-stimulating antibody levels or HLA status. J Clin Endocrinol Metab (1986) 62:117–21. doi: 10.1210/jcem-62-1-117
116. Allannic H, Fauchet R, Lorcy Y, Gueguen M, Le Guerrier AM, Genetet B. A prospective study of the relationship between relapse of hyperthyroid Graves' disease after antithyroid drugs and HLA haplotype. J Clin Endocrinol Metab (1983) 57:719–22. doi: 10.1210/jcem-57-4-719
117. Dahlberg PA, Holmlund G, Karlsson FA, Säfwenberg J. HLA-A, -B, -C and -DR antigens in patients with Graves' disease and their correlation with signs and clinical course. Acta Endocrinol (Copenh) (1981) 97:42–7. doi: 10.1530/acta.0.0970042
118. Preus M, Frecker MF, Stenszky V, Balaźs C, Farid NR. A prognostic score for Graves' disease. Clin Endocrinol (Oxf) (1985) 23:653–61. doi: 10.1111/j.1365-2265.1985.tb01126.x
119. Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, et al. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci U.S.A. (2008) 105:14034–9. doi: 10.1073/pnas.0806584105
120. Ban Y, Davies TF, Greenberg DA, Concepcion ES, Osman R, Oashi T, et al. Arginine at position 74 of the HLA-DR beta1 chain is associated with Graves' disease. Genes Immun (2004) 5:203–8. doi: 10.1038/sj.gene.6364059
121. Lee HJ, Stefan-Lifshitz M, Li CW, Tomer Y. Genetics and epigenetics of autoimmune thyroid diseases: Translational implications. Best Pract Res Clin Endocrinol Metab (2023) 37:101661. doi: 10.1016/j.beem.2022.101661
122. Inaba H, Martin W, De Groot AS, Qin S, De Groot LJ. Thyrotropin receptor epitopes and their relation to histocompatibility leukocyte antigen-DR molecules in Graves' disease. J Clin Endocrinol Metab (2006) 91:2286–94. doi: 10.1210/jc.2005-2537
123. Li CW, Osman R, Menconi F, Concepcion E, Tomer Y. Cepharanthine blocks TSH receptor peptide presentation by HLA-DR3: Therapeutic implications to Graves' disease. J Autoimmun (2020) 108:102402. doi: 10.1016/j.jaut.2020.102402
124. Li CW, Osman R, Menconi F, Faustino LC, Kim K, Clarke OB, et al. Cepharanthine blocks presentation of thyroid and islet peptides in a novel humanized autoimmune diabetes and thyroiditis mouse model. Front Immunol (2021) 12:796552. doi: 10.3389/fimmu.2021.796552
125. Lane LC, Kuś A, Bednarczuk T, Bossowski A, Daroszewski J, Jurecka-Lubieniecka B, et al. An intronic HCP5 variant is associated with age of onset and susceptibility to graves disease in UK and polish cohorts. J Clin Endocrinol Metab (2020) 105:e3277–84. doi: 10.1210/clinem/dgaa347
Keywords: Graves’ disease, Graves’ orbitopathy, human leukocyte antigen, HLA, Asian population, Caucasian population, genotyping
Citation: Stasiak M, Stasiak B, Zawadzka-Starczewska K and Lewiński A (2023) Significance of HLA in Graves’ disease and Graves’ orbitopathy in Asian and Caucasian populations – a systematic review. Front. Immunol. 14:1256922. doi: 10.3389/fimmu.2023.1256922
Received: 11 July 2023; Accepted: 04 September 2023;
Published: 28 September 2023.
Edited by:
Fernando Gabriel Chirdo, CONICET Instituto de Estudios Inmunológicos y Fisiopatalógicos (IIFP), ArgentinaReviewed by:
Ricardo Pujol Borrell, Autonomous University of Barcelona, SpainDaniele Sola, University of Eastern Piedmont, Italy
Copyright © 2023 Stasiak, Stasiak, Zawadzka-Starczewska and Lewiński. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magdalena Stasiak, bXN0YXNpYWszM0BnbWFpbC5jb20=
 Magdalena Stasiak
Magdalena Stasiak Bartłomiej Stasiak2
Bartłomiej Stasiak2 Andrzej Lewiński
Andrzej Lewiński