- 1Department of Endocrinology, Chengdu Shuangliu Hospital of Traditional Chinese Medicine, Chengdu, China
- 2Department of Ultrasound, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Human Cell Biology and Genetics, School of Medicine, Southern University of Science and Technology, Shenzhen, China
- 4Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Immune checkpoint inhibitors (ICIs) are increasingly being used in the treatment of advanced human malignancies. ICIs-related adverse events, including pancreatitis and diabetes, have been individually characterized in the literature. The co-occurrence of ICIs-related pancreatitis with diabetes is rare and easily overlooked, but it is often severe or fatal. We present a patient with renal tumor resection who was treated with injection of the PD-L1 inhibitor toripalimab and eventually developed acute pancreatitis and fulminant type 1 diabetes mellitus. In addition, we conducted a literature review of ICIs-related pancreatitis with diabetes. The case in our report presented with paroxysmal abdominal pain and loss of appetite. Intravenous fluids and insulin infusion improved the patient’s pancreatitis and explosive hyperglycemia. This article suggests that ICIs can affect endocrine and exocrine functions of the pancreas, while providing information and new perspectives for the diagnosis and treatment of this challenging rare disease, helping inspire clinicians for the early identification and effective management of similar cases.
1 Introduction
Immune checkpoint inhibitors (ICIs) include anticytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), antiprogrammed cell death 1 receptor and its ligand (anti-PD-1/anti-PD-L1) (1). These novel drugs can enhance the immune system’s ability to eliminate cancer cells by triggering the reactivation and expansion of T lymphocytes (1, 2). Therefore, ICIs are widely used to treat malignant melanoma, non-small cell lung carcinoma (NSCLC), head and neck squamous cell carcinoma, and malignancies of the genitourinary and hematological systems (3). During the past decade, the life expectancy of end-stage cancer patients has increased significantly because of using ICIs (4).
However, the widespread use of ICIs has been followed by a series of immune-related adverse events (irAEs) associated with their mechanism of action. These irAEs can influence almost all organ systems, from minor self-limiting symptoms to severe life-threatening incidents, including the dermatologic, gastrointestinal, hepatic, pulmonary, and endocrine systems (5). ICIs-associated pancreatic injury (ICIs-PI) is rare but can cause metabolic and nutritional disorders, and even death (6, 7). Timely recognition of the condition and initiation of treatment can hugely impact the patient’s health and quality of life. In this paper, we described a patient with acute pancreatitis (AP) and fulminant type 1 diabetes mellitus (FT1DM) induced by toripalimab (anti-PD-1), conducted a systematic review of relevant case reports and attempted to raise awareness about ICIs-PI.
2 Case descriptions
A 58-year-old Chinese man (70.0 kg, body mass index: 21.1 kg/m2) diagnosed with clear cell renal cell carcinoma (WHO/ISUP nuclear grade was 4) underwent left nephrectomy in August 2021. In December 2021, the patient began immunotherapy with toripalimab (240 mg/dose), a humanized antiprogrammed cell death 1 receptor. Eleven weeks after initiating toripalimab treatment, he visited the hospital with a seven-day history of paroxysmal abdominal pain and the resulting loss of appetite. He had a 30-year history of smoking but had quit smoking for a year and had no history of alcohol consumption. Furthermore, he had no personal or family history of endocrine or autoimmune diseases.
2.1 Disease progression
Figure 1A shows the overall changes during the disease course of the patient. A day before admission, the patient’s mid-upper abdominal pain worsened. After admission, the patient’s blood amylase was 313 U/L (28.0-100.0 U/L), blood lipase was 670 U/L (23.0-300.0 U/L), and random blood glucose was 4.3 mmol/L. Total cholesterol and triglycerides were slightly elevated, while liver and kidney function, electrolytes, blood gas analysis and blood ketone bodies were not significantly abnormal. Glycosylated hemoglobin was 7.3%. Abdominal ultrasound revealed an “enlarged pancreas with uneven parenchymal echogenicity” (Figures 1B, C), and abdominal computed tomography (CT) showed a “full pancreas with slightly blurred peripheral space” (Figure 1D). On the third day after hospitalization, the patient developed dry mouth and excessive fluid intake, increased urination, and fatigue. Laboratory tests showed the patient’s blood glucose was 43.6 mmol/L, and urine glucose was positive (+++), while other indicators were not notable. After the patient’s condition stabilized, we performed oral glucose tolerance tests (OGTT), insulin, and C-peptide release experiments along with islet autoantibodies. Data showed the patient had a complete loss of islet function (Figure 1E) and was negative for islet autoantibodies (Supplementary Table 1). Subclinical hyperthyroidism (TSH 0.01 mIU/L, FT4 21.85 pmol/L) was also detected, and thyroid autoantibodies (TPOAb, TSI) were negative. The thyroid ultrasound did not show hypervascularization. Detailed laboratory test values for the patient are shown in Supplementary Table 2. We continuously tested the patient’s lipase and glucose levels during treatment. The patient’s symptoms subsided after two weeks of treatment, and his lipase levels returned to 231.0 U/L, amylase levels to 83.0 U/L, and fasting glucose to 6.5 mmol/L; Subsequently, the patient was discharged from the hospital.

Figure 1 (A) The clinical course of irAEs. (B, C) Ultrasound showed a diffusely enlarged and hypoechoic pancreas. (D) The abdominal CT scan showed diffuse enlargement of the pancreas with mild surrounded by inflammatory stranding [yellow arrow]. (E) Experimental results of the release of the insulin and C-peptides.
2.2 Diagnostic assessment
After completing four immunotherapy cycles, the patient experienced abdominal pain and elevated levels of pancreatic enzymes. The patient had no gallstones, normal triglycerides, no personal history of autoimmune disorders, and no alcohol abuse or overeating. An abdominal CT examination revealed diffuse pancreatic enlargement with no signs of tumor metastasis, leading to the diagnosis of ICIs-related pancreatitis (ICIs-P) (8, 9). After three days of hospitalization, the patient developed symptoms of dry mouth and thirst, with a sudden increase in blood sugar to 43.6 mmol/L and complete loss of pancreatic islet function (C-Peptide <0.003). The patient had no history of diabetes (3 weeks before admission, FBG 5.1 mmol/L, HbA1c 6.1%) and only a slight increase in glycated hemoglobin (HbA1c 7.3%) on admission. Therefore, FT1DM was diagnosed (10). According to the Naranjo Adverse Drug Reaction Probability Scale, the total score was 6; therefore, we considered that pancreatic damage was associated with toripalimab therapy in this case (11). Patients with elevated blood sugar levels after acute pancreatitis must be differentiated from those with postacute pancreatitis diabetes mellitus (PPDM-A) and stress diabetes mellitus. Glucose metabolism in PPDM-A is like that in T2DM with surviving islet function. Stress hyperglycemia manifests itself mainly as insulin resistance leading to increased blood sugar, which can return to normal after the stress factor has been eliminated (12, 13). Therefore, PPDM-A and stress hyperglycemia were not considered in this patient, but a diagnosis of ICIs-FT1DM was considered. The patient had no previous episodes of thyrotoxicosis, while subclinical hyperthyroidism (TSH 0.001 mIU/L, FT4 21.85 pmol/L) and negative thyroid autoantibodies (TPOAb, TSI) were measured at admission. This clinical situation was indicative of ICIs-related thyroiditis (14, 15).
2.3 Therapeutic intervention
According to the Common Terminology Criteria for Adverse Events (CTCAE 5.0) and the National Comprehensive Cancer Network (NCCN) guidelines (16), the patient’s elevation in blood glucose was classified as life-threatening (grade 4); amylase or lipase levels of > 2.0-5.0 × ULN were rated grade 3; therefore, toripalimab needed to be discontinued. After diagnosing pancreatitis induced by toripalimab, the patient’s vital signs were monitored, and fluids were replaced. The patient’s symptoms were relieved after treatment. However, on the third day following hospitalization, the patient abruptly developed FT1DM. So he was administered appropriate intravenous fluids and an insulin infusion. Subsequently, he still required insulin therapy. Therefore, blood glucose was controlled by subcutaneous insulin injections. Due to delays in thyroid autoantibody detection results and limited experience in this area, a small dose of methimazole was also used to reduce thyrotoxic symptoms in patients.When the diagnosis was confirmed, methimazole was immediately discontinued and clinical observations were performed in accordance with treatment guidelines (17, 18). The patient was discharged when his symptoms improved after two weeks of treatment. We revisited the patient three months later, who was doing well with stable glycemic control,thyroid function back to normal and no abdominal pain, signs of pancreatic exocrine insufficiency, or tumor aggravation.
3 Discussion
The incidence of ICIs-related pancreatic irAEs (pancreatitis, hyperglycemia, elevated amylase/lipase, exocrine pancreatic insufficiency) is relatively low, with an incidence of 0.5%-4%, but can lead to poor quality of life and even be life-threatening (19). ICIs-P or ICIs-DM as the most important pancreatic exocrine or endocrine toxicity of irAEs has been reported separately in some studies (2, 20). However, their incidence and clinical characteristics are unclear. To our knowledge, literature reports are scarce on the incidence of ICIs-P combined with ICIs-DM. We reviewed literature on ICIs-P combined with ICIs-DM but found only three studies on ICIs-P and DM (21–23). Table 1 summarizes our case and the three cases reported to develop ICIs-P and ICIs-DM during treatment with ICIs. All patients were male with a mean age of 60.5 (range 49-72) years and had no history of diabetes. All three patients had a history of nivolumab treatment, and here we report the first case of ICIs-P combined with ICIs-FT1DM in a patient with renal cancer during treatment with toripalimab. Male sex and PD-1/PD-L1 blockers, particularly nivolumab, may be risk factors for immune-related pancreatitis and diabetes.

Table 1 Our case and summary of reported immune checkpoint inhibitors associated with pancreatitis and diabetes mellitus.
Of the three cases collected, one patient had pancreatitis and diabetes mellitus simultaneously, similar to our case (23), a case had acute pancreatitis 2 years after FT1DM (22), and a case had elevated blood glucose 3 months after the onset of autoimmune pancreatitis (type II) (21). This suggests that endocrine and exocrine damage to the pancreas can occur simultaneously or sequentially without a strict order of onset. Regarding the time of onset, ICIs-P and ICIs-FT1DM occurred in our case 11 weeks after starting ICIs treatment. The literature reports that early toxic pancreatic toxicity of ICIs may occur on day 1 of ICIs treatment (6) or late toxicity may occur after >1 year of therapy with ICIs (24) or even after the end of treatment (25). Delayed toxicity has been reported in other studies, which occurred 1 to 2 years following monotherapy (22). Therefore, ICIs-PI can appear at any time of treatment, and it is critical to consider the possibility of immune-associated pancreatitis/diabetes if there are any new symptoms of pancreatic injury (abdominal pain, elevated pancreatic enzymes, blood glucose, etc.) during ICIs treatment.
The mechanism by which ICIs are associated with pancreatic injury remains unclear, although several factors may be involved. However, excessive activation of T-lymphocytes, leading to the destruction of pancreatic cells, may be an important part of the pathogenesis (5).PD-1 negatively regulates immunity; PD-1 on the surface of T cells binds to PD-L1 on the surface of tumor cells, and T cells receive inhibitory signals, inactivation, and apoptosis occurs. Anti-PD-1/PD-L1 and anti-CTLA-4 monoclonal antibodies bind and block inhibitory signals. Therefore, the inhibitory signal is lifted, and T cells regain their tumor-killing function (26). PD-L1 is expressed not only in T cells, but also in pancreatic cells. When the PD-1 pathway is blocked, cancer-targeted T cells are activated, increasing the ratio of CD8+/CD4+ T lymphocytes in the peritoneal area. Increased CD8 + T cells may cause damage to pancreatic cells, including islet and acinar cells, leading to decreased endocrine and exocrine pancreatic function (2). This explains why ICI-related pancreatic injury can present with endocrine and exocrine dysfunction of the pancreas (Figure 2). However, this does not explain the low incidence, diverse clinical episodes (hyperglycemia, pancreatitis, pancreatic atrophy, and pancreatic exocrine insufficiency), and significant heterogeneity (9, 25).The exact mechanism of immune-related pancreatitis/diabetes requires further research.
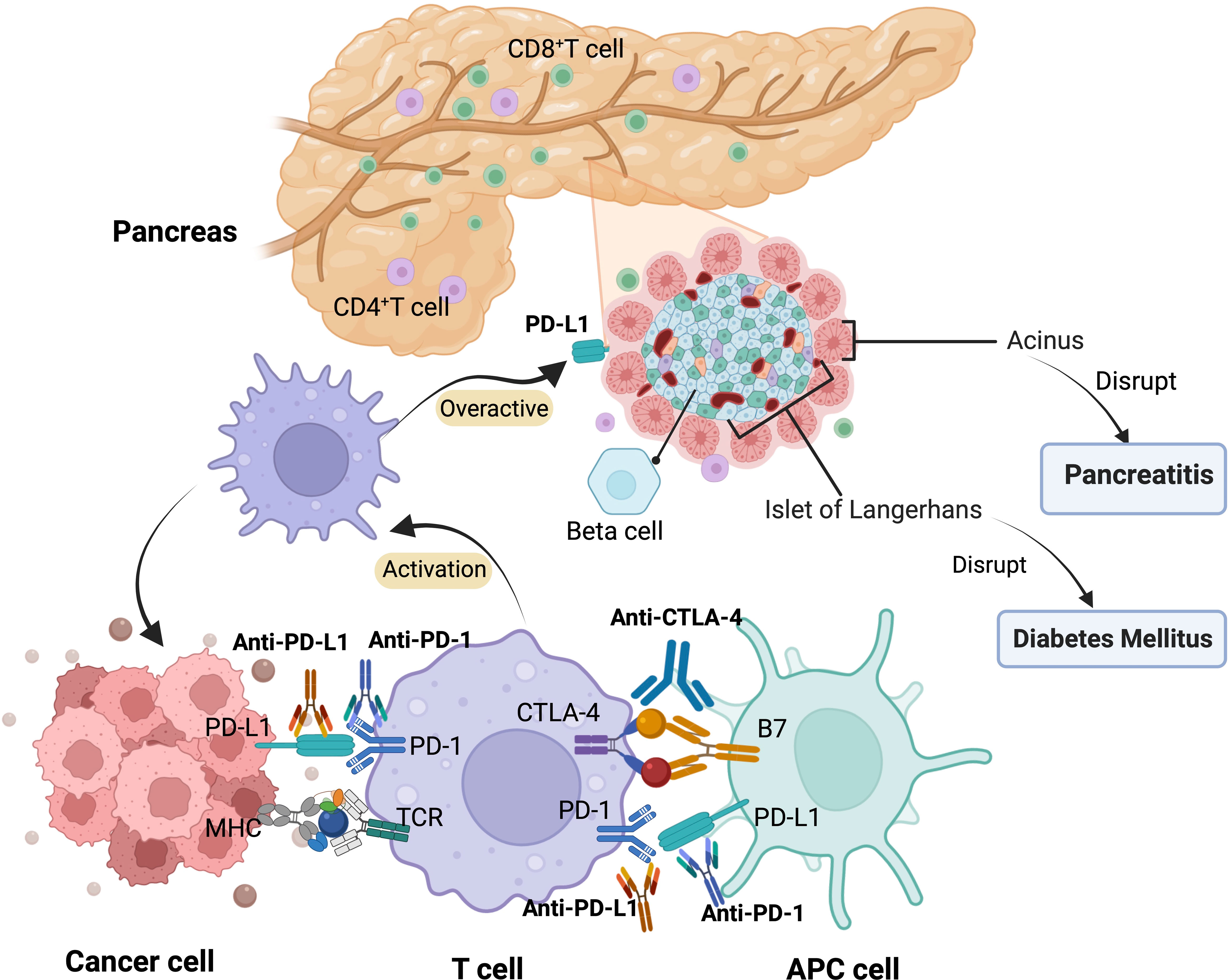
Figure 2 Mechanism of pancreatic adverse events related to ICIs. APC, antigen-presenting cell; MHC, major histocompatibility complex; TCR, T cell receptor.
NCCN guidelines for the treatment of ICIs-P state that for grade 2 pancreatitis (moderate), ICI treatment should be discontinued and 0.5-1 mg/kg/day prednisone/methylprednisolone should be administered until symptoms improve to grade ≤ 1 (16). For pancreatitis (severe and life-threatening), it is recommended to permanently discontinue immunotherapy and start therapy with 1-2 mg/kg/day of glucocorticoids. When symptoms of ICIs-P have improved to grade 1, steroids can be gradually reduced over 4-6 weeks. However, the NCCN guidelines are based on traditional acute pancreatitis and limited evidence on ICI-P, so current treatment options may not be effective for managing ICI-P combined with new-onset diabetes. A review study showed that corticosteroids have not been shown to have a significant benefit in shortening the acute phase of ICIs-P, preventing long-term adverse outcomes of ICIs-P, or improving overall survival, while early intravenous fluids may reduce the risk of long-term adverse outcomes of ICIs-P (27). AP combined with FT1DM in this patient, using corticosteroids, may cause difficulty controlling blood glucose, which may lead to acute ketoacidosis. No corticosteroids were administered to our patient. However, he was managed with active rehydration and insulin hypoglycemic therapy. The patient’s pancreatic enzymes decreased, blood glucose control and symptoms improved, and no signs of exocrine damage to the pancreas were found during follow-up. This suggests the importance of early identification and intervention, even if basic therapy is effective (rehydration, hypoglycemic reduction, etc.). Based on published literature and our case experience, the use of glucocorticoids can be evaluated according to the following criteria when pancreatitis co-occurs with ICIs-related diabetes: 1. the severity grade of ICIs-P (Grade 3-4 pancreatitis necessitates consideration of steroid therapy); 2. whether pancreatitis and diabetes manifested simultaneously; 3. whether it was complicated by irAEs in other organs.”
4 Conclusions
Although the simultaneous occurrence of ICI-induced endocrine and exocrine pancreatic injuries is rare, it still requires more attention due to its life-threatening nature. Any new symptoms of pancreatic injury should be considered clinically significant for immunotherapy patients. Therefore, careful recognition and accurate diagnosis are extremely important. Despite the effectiveness of fluid therapy and insulin supplementation, the underlying mechanisms of immune pancreatic injury remain unclear and require further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Chengdu Shuangliu Hospital of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
WF, YG, and XS contributed equally to this work and share first authorship. YG and HW conceived and designed the study. WF were involved in clinical management. XZ, SZ, HZ, WY, and HW contributed to the literature search, data acquisition, and data analysis for the review. YG, WF, and XS drafted and revised the paper. YG contributed to all the figures. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Youth Science and Technology Innovation Project of the Department of Science and Technology of Sichuan Province (grant No.2021049 and No.2022082) and Sichuan Traditional Chinese Medicine Culture Collaborative Development Research Center Project (2022XT101).
Acknowledgments
We are grateful to the patient for allowing this paper to be published and for forgiving the flaws in the treatment.We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1243773/full#supplementary-material
References
1. Barnes Rogers CMAA, Cuddahy RMOT, Zawislak MPC. Management of acute pancreatitis associated with checkpoint inhibitors. J Advanced Practitioner Oncol (2020) 11:49–62. doi: 10.6004/jadpro.2020.11.1.3
2. Liu Y, Zhang H, Zhou L, Li W, Yang L, Li W, et al. Immunotherapy-associated pancreatic adverse events: current understanding of their mechanism, diagnosis, and management. Front Oncol (2021) 11:627612. doi: 10.3389/fonc.2021.627612
3. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) (2020) 12:738. doi: 10.3390/cancers12030738
4. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol (2018) 19:737–46. doi: 10.1016/S1470-2045(18)30261-4
5. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
6. Jiang R, Xu L, Huang Y, Fang C, Guo H, Li S, et al. Anti–PD-1 drug (Nivolumab) may induce acute and life-threatening pancreatitis in lung cancer patient. Pancreas (2018) 47:e53–54. doi: 10.1097/MPA.0000000000001107
7. Bai X, Jiang S, Zhou Y, Zhen H, Ji J, Li Y, et al. Common immune-related adverse events of immune checkpoint inhibitors in the gastrointestinal system: A study based on the US food and drug administration adverse event reporting system. Front Pharmacol (2021) 12:720776. doi: 10.3389/fphar.2021.720776
8. Alessandrino F, Sahu S, Nishino M, Adeni AE, Tirumani SH, Shinagare AB, et al. Frequency and imaging features of abdominal immune-related adverse events in metastatic lung cancer patients treated with PD-1 inhibitor. Abdom Radiol (Ny) (2019) 44:1917–27. doi: 10.1007/s00261-019-01935-2
9. Porcu M, Solinas C, Migali C, Battaglia A, Schena M, Mannelli L, et al. Immune checkpoint inhibitor-induced pancreatic injury: imaging findings and literature review. Target Oncol (2020) 15:25–35. doi: 10.1007/s11523-019-00694-w
10. Song SO, Yun JS, Ko SH, Ahn YB, Kim BY, Kim CH, et al. Prevalence and clinical characteristics of fulminant type 1 diabetes mellitus in Korean adults: A multi-institutional joint research. J Diabetes Investig (2022) 13:47–53. doi: 10.1111/jdi.13638
11. Zaki S. Adverse drug reaction and causality assessment scales. Lung India (2011) 28:152. doi: 10.4103/0970-2113.80343
12. Olesen SS, Svane HML, Nicolaisen SK, Kristensen JK, Drewes AM, Brandslund I, et al. Clinical and biochemical characteristics of postpancreatitis diabetes mellitus: A cross-sectional study from theDanish nationwideDD2 cohort. J Diabetes (2021) 13:960–74. doi: 10.1111/1753-0407.13210
13. Petrov MS. DIAGNOSIS OF ENDOCRINE DISEASE: Post-pancreatitis diabetes mellitus: prime time for secondary disease. Eur J Endocrinol (2021) 184:R137–49. doi: 10.1530/EJE-20-0468
14. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab (2016) 101:4431–39. doi: 10.1210/jc.2016-2300
15. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, KottsChade L, Withers S, et al. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab (2017) 102:2770–80. doi: 10.1210/jc.2017-00448
16. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw (2019) 17:255–89. doi: 10.6004/jnccn.2019.0013
17. Husebye ES, Castinetti F, Criseno S, Curigliano G, Decallonne B, Fleseriu M, et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur J Endocrinol (2022) 187:G1–21. doi: 10.1530/EJE-22-0689
18. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001
19. Zhang HC, Wang LS, Miller E. Hepatobiliary and pancreatic adverse events. Adv Exp Med Biol (2021) 1342:339–55. doi: 10.1007/978-3-030-79308-1_13
20. de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, van der Auwera BJ, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol (2019) 181:363–74. doi: 10.1530/EJE-19-0291
21. Dehghani L, Mikail N, Kramkimel N, Soyer P, Lebtahi R, Mallone R, et al. Autoimmune pancreatitis after nivolumab anti–programmed death receptor-1 treatment. Eur J Cancer (2018) 104:243–46. doi: 10.1016/j.ejca.2018.09.014
22. Yilmaz M, Baran A. Two different immune related adverse events occured at pancreas after nivolumab in an advanced RCC patient. J Oncol Pharm Pract (2022) 28:255–58. doi: 10.1177/10781552211028636
23. Munakata W, Ohashi K, Yamauchi N, Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol (2017) 105:383–86. doi: 10.1007/s12185-016-2101-4
24. Domínguez Bachiller M, Carrasco Piernavieja L, Toro Chico P, Perez Encinas M. 5PSQ-039 Pancreatitis induced by immunotherapy? Two case reports. Eur J Hosp pharmacy. Sci Pract (2020) 27:A167–68. doi: 10.1136/ejhpharm-2020-eahpconf.356
25. Capurso G, Archibugi L, Tessieri L, Petrone MC, Laghi A, Arcidiacono PG. Focal immune-related pancreatitis occurring after treatment with programmed cell death 1 inhibitors: a distinct form of autoimmune pancreatitis? Eur J Cancer (2018) 95:123–26. doi: 10.1016/j.ejca.2018.02.006
26. Eshet Y, Baruch EN, Shapira-Frommer R, Steinberg-Silman Y, Kuznetsov T, Ben-Betzalel G, et al. Clinical significance of pancreatic atrophy induced by immune-checkpoint inhibitors: A case–control study. Cancer Immunol Res (2018) 6:1453–58. doi: 10.1158/2326-6066.CIR-17-0659
Keywords: immune checkpoint inhibitors, immune-related adverse events, immunotherapy, case report, literature review
Citation: Fang W, Gao Y, Shi X, Zhang X, Zhou S, Zhu H, Yan W and Wang H (2023) Immune checkpoint inhibitors-related pancreatitis with fulminant type 1 diabetes mellitus: case report and literature review. Front. Immunol. 14:1243773. doi: 10.3389/fimmu.2023.1243773
Received: 21 June 2023; Accepted: 08 September 2023;
Published: 28 September 2023.
Edited by:
Nora Möhn, Hannover Medical School, GermanyReviewed by:
Jeehee Yoon, Chonnam National University Bitgoeul Hospital, Republic of KoreaDifei Lu, Peking University, China
Copyright © 2023 Fang, Gao, Shi, Zhang, Zhou, Zhu, Yan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Gao, gaoyangxueshu@163.com; Huanping Wang, fangw139@163.com
†These authors have contributed equally to this work and share first authorship
 Wei Fang1†
Wei Fang1†