
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 06 October 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1242336
This article is part of the Research Topic Checkpoint Inhibition in Hematologic Malignancies View all 8 articles
Background: Immune checkpoint inhibitors (ICIs) has emerged as a popular cancer treatment approach. However, non-specific activation of T cells by ICIs can lead to immune-related adverse events (irAEs), including specific rheumatic manifestations. The study aimed to explore the current trend of ICIs associated rheumatic irAEs and summarize the knowledge structure through bibliometric methods.
Methods: The Web of Science Core Collection database (WoSCC) was selected for retrieving literature on ICIs associated rheumatic irAEs. To evaluate contributions from different countries/regions, institutions, journals, and authors, bibliometric analysis software, including VOSviewer and CiteSpace, as well as bibliometric online platforms, were utilized to construct and visualize bibliometric networks. Through the systematic review of this knowledge domain, future research directions were determined.
Results: In This study, a total of 803 publications on ICIs-associated rheumatic irAEs were included for analysis. The distribution of these publications revealed two distinct growth phases: a stable phase between 2007 to 2015 followed by rapid growth from 2016 to 2020. The United States emerged as the top contributor in terms of publications, citations, and h-index, with the majority of leading institutions and funding agencies located there. Apart from government funding, pharmaceutical companies such as Bristol Myers Squibb and Merck Company also play a significant role in drug development and research. Analysis of keywords and citation bursts indicated that the initial burst was related to “monoclonal antibody,” “anti-CLTA4 antibody,” and “melanoma”. This was followed by a rise in interest related to “sarcoidosis,” “safety,” “inflammatory arthritis,” and “preexisting autoimmune.”
Conclusion: This study summarized the global research trends concerning ICIs associated rheumatic irAEs. The findings can provide valuable insights into the current understanding of rheumatic irAEs, highlight the research trend and developments in the field. Future efforts should focus on developing classification criteria and guidelines, conducting prospective studies, investigating the mechanisms involved, and identifying biomarkers for prediction and monitoring of these events.
In recent years, immunotherapy has become increasingly common in cancer treatment, as they have shown remarkable success in inducing durable responses in some patients (1). Monoclonal antibodies targeting immunological checkpoints, or immune checkpoint inhibitors (ICIs), represent a rapidly growing class of these agents. By targeting the T-cell cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or the programmed cell death-ligand 1 (PD-1/PD-L1) coinhibitory receptors, ICIs, either as single agents or in combination, enhance antitumor T-cell activity, leading to unprecedented long-lasting tumor responses in patients with unresectable or advanced metastatic disease (2–6).
However, while these drugs non-specifically activate T cells, which can not only increase immune response against cancer cells, but also lead to immune-related adverse events (irAEs) that exhibit inflammatory or autoimmune-like side effects. These adverse events (AEs) are unique in comparison to conventional cancer therapies and can range from mild to severe, affecting any organ system in the body (7, 8). The incidence of irAEs depends on various factors, such as the type of ICIs used, the cancer type, and the individual patient’s characteristics. Common irAEs associated with ICIs include pneumonitis, colitis, hepatitis, endocrinopathies, and skin toxicities (9). Interestingly, specific rheumatic manifestations among irAEs have also been described, such as inflammatory arthritis, vasculitis, inflammatory myopathy, sicca syndrome, and scleroderma, etc (9). However, the prevalence and course of these rheumatic irAEs are not well-established, and detecting them during or after cancer treatment can be challenging, as they resemble conventional rheumatologic diseases (10). Clinical trials often overlook musculoskeletal/rheumatic events as a distinct organ system or report only high-grade and/or frequent adverse events occurring in at least 10% of the patients (11). Additionally, different researches might use varying terms to describe these irAEs due to their heterogeneous definitions, and real-life data suggest that they are underreported (12, 13). Also, evidence is lacking for the optimal diagnostic approach and management of these patients while allowing effective antitumor therapy to continue (9). Consequently, it is crucial to gain a comprehensive understanding of the current status, research trends and emerging topics.
Bibliometric analysis is a useful tool for achieving this goal, as it allows for the systematic and quantitative analysis of research publications in a particular field. No such studies have been published in the field of ICIs associated rheumatic irAEs. By using bibliometric analysis techniques, we aim to provide an overview of the studies and scholarly contributions, identify the most influential authors, institutions, and journals in this field and to map the most significant research topics and trends, which can contribute to the improvement of patient care and the development of safer immunotherapies. Furthermore, the results of this study can also inform policymakers and funding agencies about the most promising areas of research in the field of immunotherapy-related adverse events.
The bibliographic data were all obtained from the Science Citation Index Expanded (SCI-expanded, 1999-present) of the Web of Science Core Collection (WoSCC). Rheumatic irAEs were identified through literature review (9, 11, 14). To perform a comprehensive literature search of ICIs associated rheumatic irAEs, specific retrieval rules were designed as follows: #1: TS=(ipilimumab) or TS=(avelumab) or TS=(nivolumab) or TS=(atezolizumab) or TS=(pembrolizumab) or TS=(durvalumab) or TS=(cemiplimab) or TS=(relatimab) or TS=(“immune checkpoint inhibitor*”) or TS=(“immune checkpoint block*”) or TS=(“immune checkpoint therap*”). #2: TS=(vasculitis) or TS=(arteritis) or TS=(polyangiitis) or TS=(lupus) or TS=(“systemic sclerosis”) or TS=(scleroderma) or TS=(“skin thickening”) or TS=(raynaud*) or TS=(erythema nodosum) or TS=(sarcoidosis) or TS=(sarcoid-like) or TS=(“sicca symptoms”) or TS=(“dry mouth”) or TS=(parotitis) or TS=(“Sjögren’s syndrome”) or TS=(enthesitis) or TS=(dactylitis) or TS=(tenosynovitis) or TS=(synovitis) or TS=(“musculoskeletal pain”) or TS=(myalgia) or TS=(myositis) or TS=(dermatomyositis) or TS=(arthritis) or TS=(arthralgia) or TS=(*arthropathy) or TS=(“polymalgia rheumatica”) or TS=(rheumatic) or TS=(“connective tissue disease”). #3: #1 and #2. The time span of the search was from 1999 to 2022 (retrieved on November 24, 2022), and a total of 1187 records were collected from WoSCC. In the end, 803 ultimate documents were remained after excluding non-English literature and restricting document types to articles and reviews. The search details were shown in Figure 1.
We conducted data analysis and result visualization using multiple tools, including Citespace (Version 5.6.R2), VOSviewer (Version 1.6.18), and an online platform (https://bibliometric.com/), as well as two R packages, Bibliometrix and Biblioshiny (Version 4.1.2).
CiteSpace is a Java-based visualization tool that offers a range of visualization options, such as network maps, centrality and burst maps, which reveal clusters and dynamics related to a specific scientific topic (15). The centrality of nodes is calculated to reveal their importance within the network (16). The burst maps were applied to detect keywords and references, which recognizes sudden significant increases in a scientific activity over a certain period (17). VOSviewer is another Java-based bibliometric analysis software that allows for the exploration and visualization of research characteristics from various perspectives, providing three types of network maps: the network visualization map, the overlay visualization map, and the density visualization map (15). In the network visualization map, each node corresponds to parameters, such as countries/regions, institutions, journals, authors, or keywords. The node size represents its strength (e.g., the number of publications, citations, and occurrences) while the thickness of the link represents the strength of the network associations. The color-coded nodes and links represent clusters and connections, respectively, and clusters with the same color are automatically assigned to closer terms (18). The python pyechats was used to build the world map, while an online bibliometric analysis platform was applied to visualize the distribution and international cooperation among countries/regions. The different tools used in this study allowed for a comprehensive and nuanced analysis of the data and provided valuable insights into the current status, knowledge base, research trends, and emerging topics in the field of rheumatic irAEs associated with ICIs.
Performing a quantitative analysis of publications in a specific field can provide valuable insights into academic hotspots and trends. In this study, we identified 803 publications, including 516 articles and 287 reviews, through the screening process illustrated in Figure 1. The first literature, which was a review article, on ICIs associated rheumatic irAEs was published in the United States of America (USA) in 2007, following the approval of the CTLA4 blockade (Figure 2) (19). The number of publications per year has steadily increased over the past 16 years, with occasional dips, and peaked in 2020, with two distinct growth phases: a stationary phase from 2007 to 2015, followed by a rapid growth phase from 2016 to 2020 (Figure 2). Of the 803 documents, 82.44% were published within the last 5 years, with a total citation count of 35,506 times and an average of 44.22 citations per paper. As ICIs have become more popular in recent years, rheumatic irAEs have received greater attention.
We then analyzed the geographical distribution of the 803 retrieved articles and identified 52 countries/regions as the origins. The spatial distribution heatmap showed higher publication density in darker color and lower publication density in lighter color (Figure 3A). The USA ranked first among the top ten countries with 350 publications, followed by France (89), Japan (85), Germany (65), and China (62) (Table 1 and Figure 3B). A detailed analysis of citations and H-index across all countries/regions revealed that the USA had the most citations (24,786) and the highest H-index (71). Notably, Japan and China had relatively high numbers of publications, but their citation counts (1,218 and 328, respectively) and H-indices (20 and 9, respectively) were lower, indicating a need for improvement in the quality of their publications. By contrast, Germany, the United Kingdom (UK), Canada, and Australia have relatively lower publication output but higher citation rates (all exceeds 100 citations per article) compared to other countries, suggesting that the research they do publish is of high quality and has a significant impact on the field (Table 1 and Figure 3B). The USA still ranked first in the number of publications per year compared to other countries (Figure 3C). An analysis of international collaboration between countries showed that the USA had the most collaborations (mainly with France, Australia, and Italy), followed by France (Supplementary Figure 1A). We also performed a network visualization analysis using VOSviewer, which included countries/regions with at least five publications. The resulting network map consisted of 25 nodes, 7 clusters, and 273 links, with the USA located at the center of the node, indicating its significant contributions to research in this field (Supplementary Figure 1B).
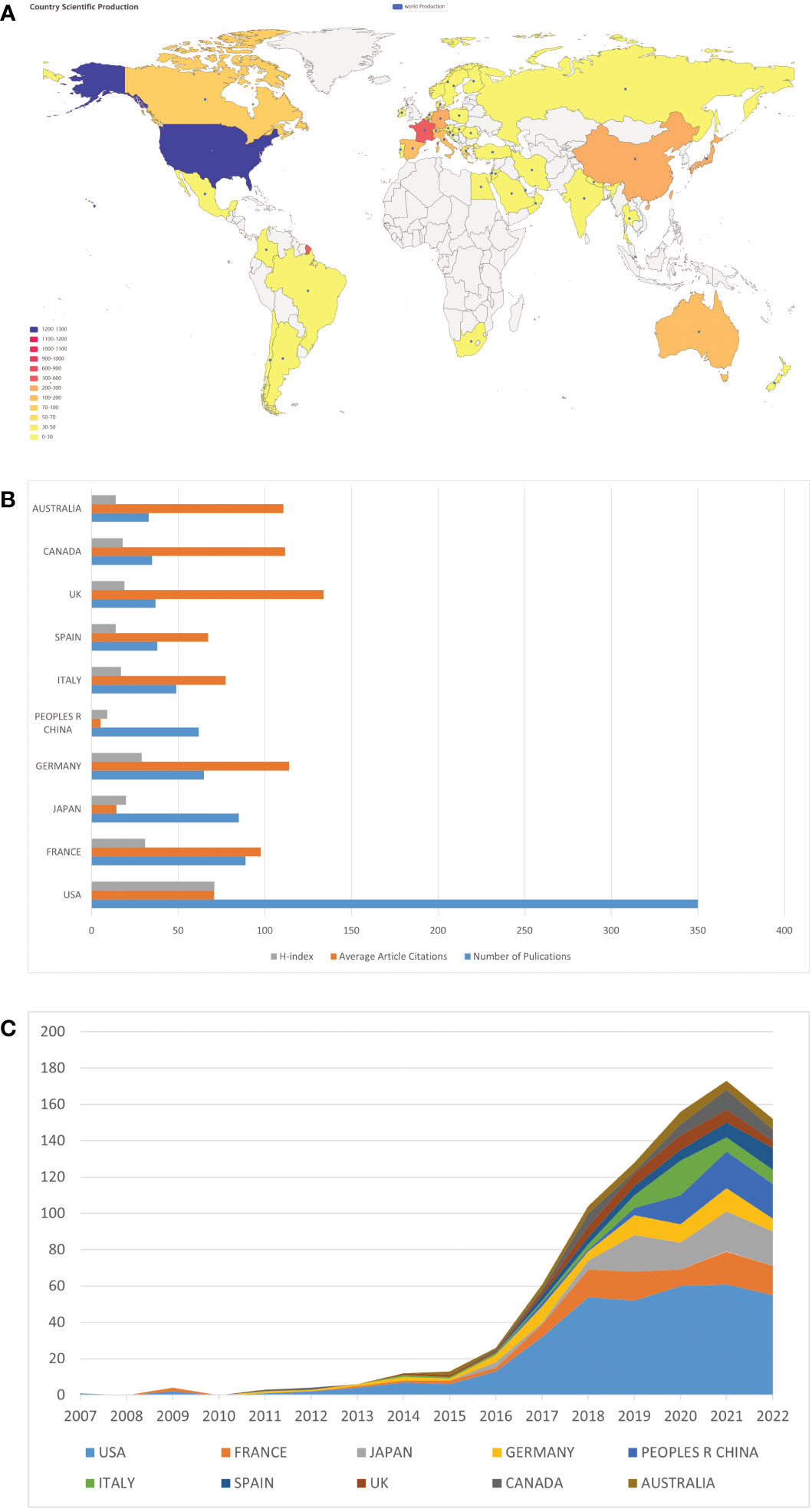
Figure 3 Country/region specific analyses of publications on ICIs associated rheumatic irAEs. (A) Geographic distribution of global publications. The color gradient in the visualization represents the number of publications, where the darker color indicates a higher number of publications while the lighter color represents a lower number of publications. (B) Number of publications, average citations per article, and H-index of the top 10 countries/regions. (C) Comparison of the number of publications by the top 10 countries/regions between 2007 and 2022.
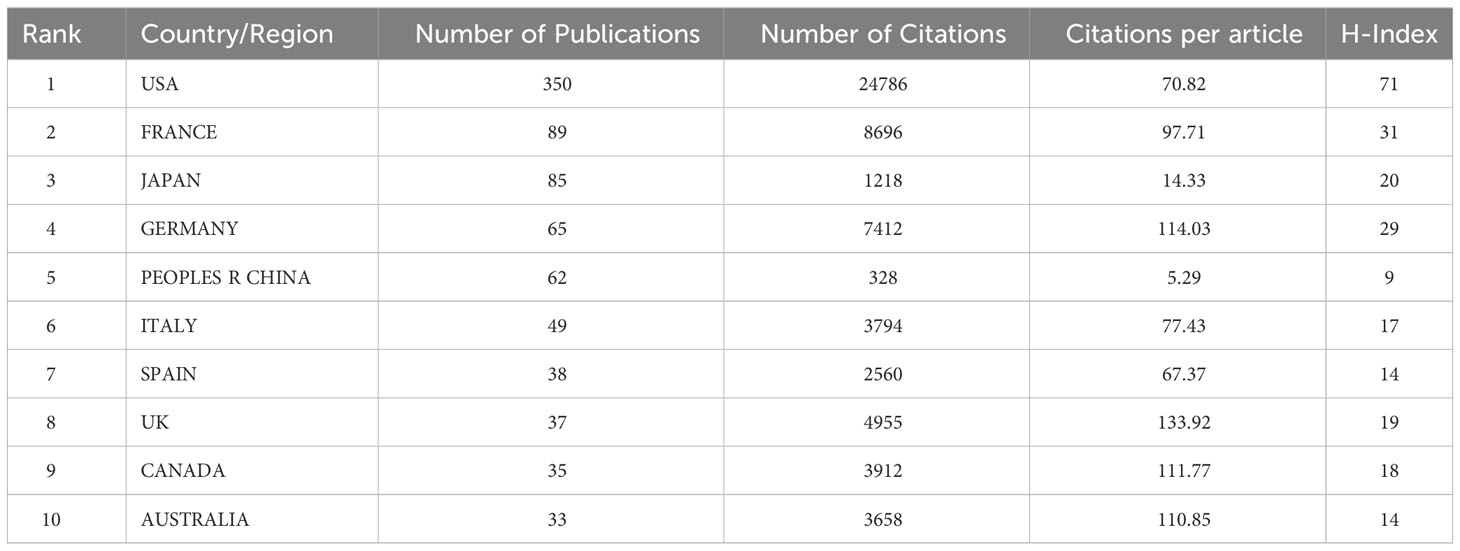
Table 1 The top 10 most productive countries and regions for ICI-related rheumatological side effects.
After removing duplicates and irrelevant organizations, we found 1354 institutions with publications related to ICIs associated rheumatic irAEs. The top 10 most productive institutions accounted for 63.14% (507 publications) of the total output, with Udice France Research University (63 publications), University of Texas System (57 publications), and Harvard University (56 publications) being the top three (Table 2). Six of the top 10 institutions came from the USA, and four came from France. Additionally, we visualized the institution citation analysis using VOSviewer, which included institutions with at least five publications (Figure 4). The resulting network map contained 73 nodes, 6 clusters, and 2027 links, with the University of Texas MD Anderson Cancer Center being the central node. This indicated that these institutions are key players in advancing research on ICIs associated rheumatic irAEs.
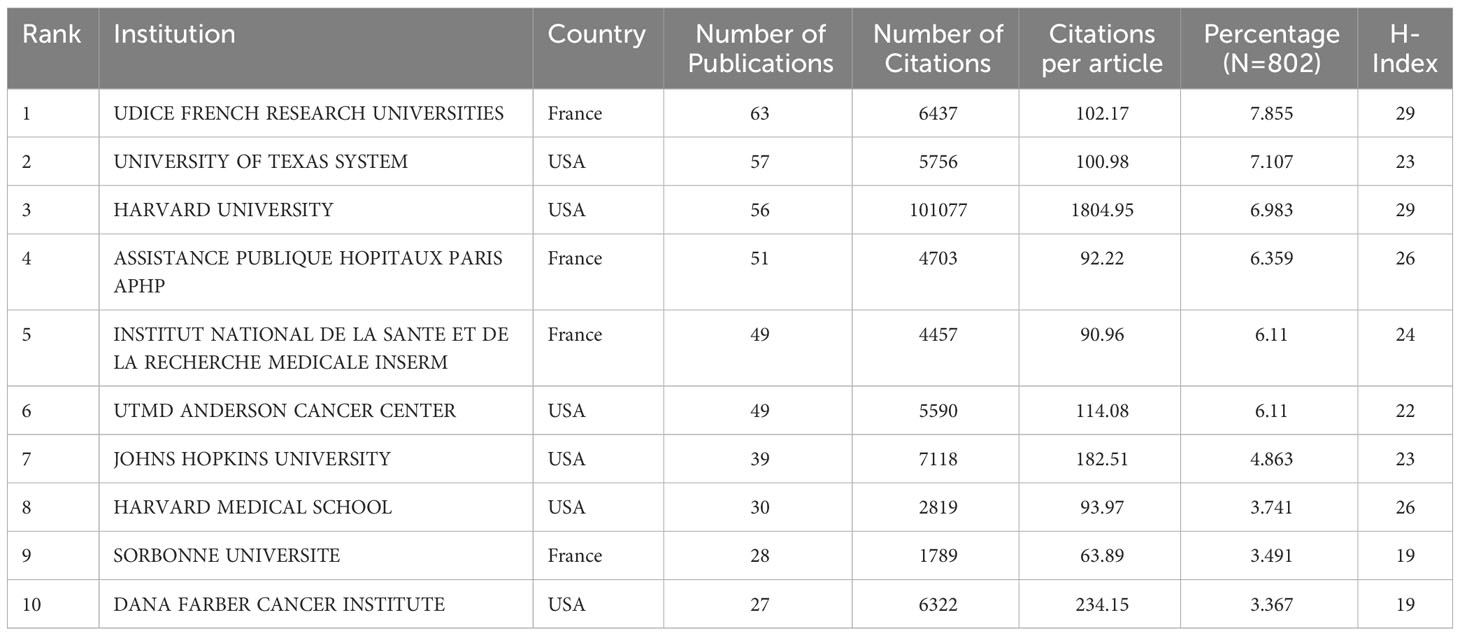
Table 2 A list of the top ten institutions with the most publications on ICI-related side effects in rheumatology.
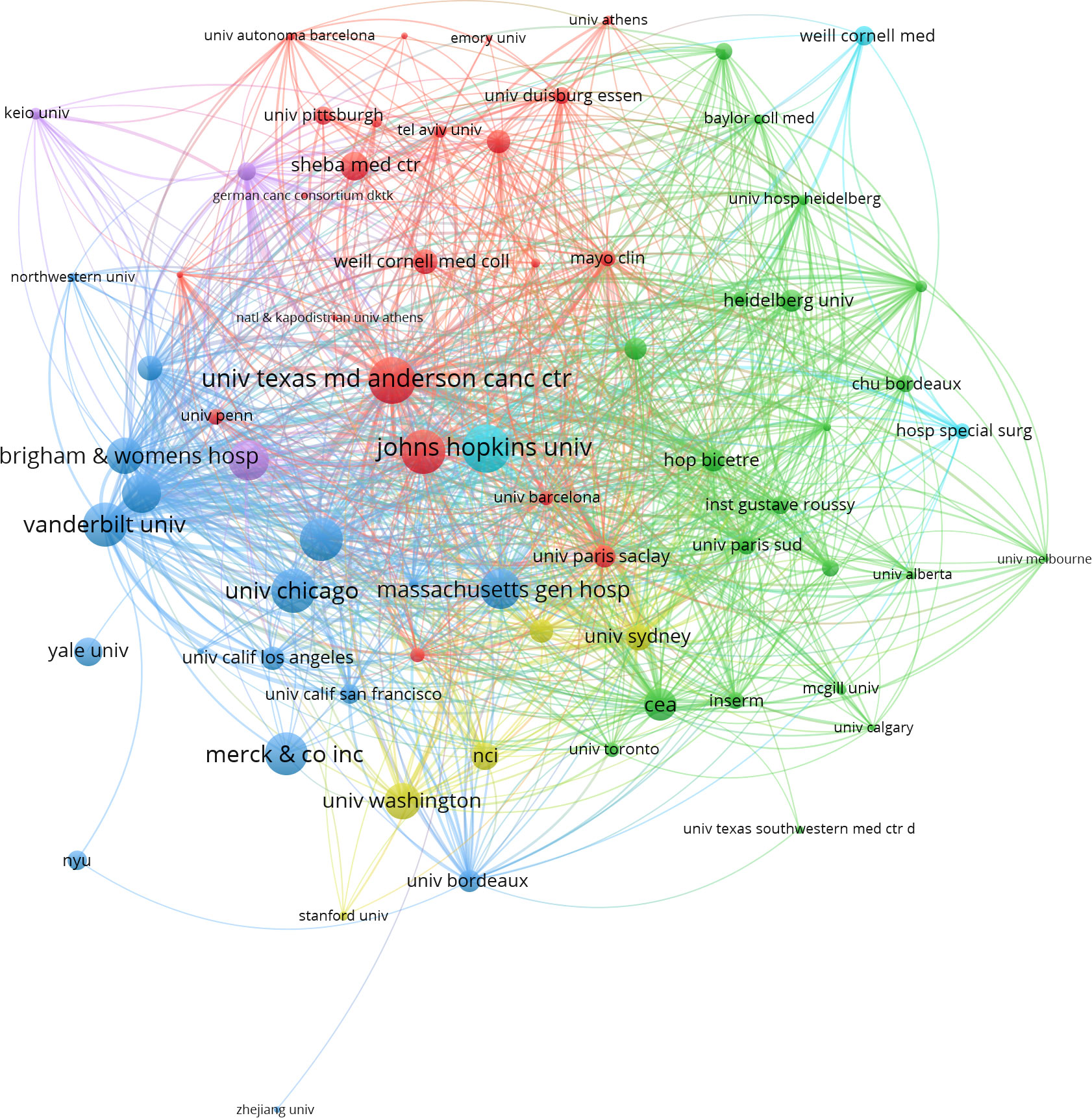
Figure 4 Co-citation analysis of institution using VOSviewer. In the network visualization, nodes represent institutions, and their size reflects the number of publications. Lines between nodes indicate co-citation relationship.
Funding support is crucial for the advancement of any research field, as it attracts more researchers and institutions to dedicate their work to the area. Therefore, we identified the top 10 funding agencies that have provided crucial support to the field of ICIs associated rheumatic irAEs research (Table 3). Notably, the top five agencies are all from the USA, comprising of three research institutions, namely the USA Department of Health Human Services (HHS), National Institutes of Health (NIH), and NIH National Cancer Institute (NCI), as well as two companies, Bristol Myers Squibb and Merck Company. This finding highlights the significant financial support that the USA provides to this research field, thereby maintaining its leading position. The remaining funding agencies are from China, Japan, and Switzerland (Table 3).

Table 3 A list of the top 10 funding agencies contributed to rheumatological side effects related to ICIs.
Our bibliometric analysis identified 316 journals that have published articles on ICIs associated rheumatic irAEs, with the top 10 journals publishing 190 publications (23.66%) (Table 4). The Journal for ImmunoTherapy of Cancer had the highest number of publications (38), with 2259 citations and the highest h-index (22) (Table 4). Notably, the European Journal of Cancer had only 14 publications but the highest number of citations (2355), resulting in the highest citations per article (168.21). Both journals are classified as Q1 in the JCR partition. Frontiers in Immunology and Frontiers in Oncology had a relatively high number of publications (25 and 20, respectively), but their total citations were relatively low, and they were classified as Q1 and Q2 categories in the JCR partition (Table 4). We used Citespace software to analyze connections between journals cited in other journals and found a co-citation network map with 578 nodes and 4913 links (Figure 5). Centrality measurements revealed that no particular journal had high centrality or was highly cited in other journals (Supplementary Figure 2). Additionally, we conducted a dual map overlay of journals related to ICIs associated rheumatic irAEsand found that published studies focused on two field: molecular biology and immunology, and medicine, medical, and clinical (Supplementary Figure 3). The most cited publications originated from journals in the fields of molecular biology and genetics, and health, nursing, and medicine.

Figure 5 Journal co-citation analysis in the field of ICIs associated rheumatic irAEs using CiteSpace.
The bibliometric analysis of the 803 publications identified 5106 authors as contributors. Table 5 presents the top 10 most productive authors in the field, led by Suarez-Almazor ME with 21 papers and closely followed by Johnson DB with 18 publications. Notably, Robert C achieved the highest total citations with 4287, despite only having 11 publications in the field, averaging 389.73 citations per article. This highlights their valuable and impactful research contributions to the field.
To identify research frontiers and emerging trends in this field, we analyzed the top 10 most cited publications and citation burst (Table 6, Figure 6). Table 6 summarized the top 10 most cited publications in the field of ICIs associated rheumatic irAEs, covering the period from 2012 to 2018. The publication by Brahmer JR, published in 2018, received the highest number of citations, with 1893 citations, followed by Nanda R, with 1334 citations. Michot, JM published the third co-cited paper with 1304 citations. Subsequently, we applied burst detection in Citespace to identify research hotspots over time for references. Figure 6 displays the top 25 references with the most robust citation bursts, with the red line indicating the duration of the reference burst occurrence. The first burst of the co-cited reference was a case report that initially reported the induction of sarcoidosis during the treatment of metastatic melanoma with anti-CTLA4 monoclonal antibody, beginning in 2009 and ending in 2014 (20). Currently, two references are still in the burst, focusing mainly on ICIs associated fatal toxic effects and myocarditis. Thus, ICIs associated side effects will be further explored in the future (21, 22).
In addition to references, keywords can provide valuable insights into the research hotspots and directions of a specific research field. With the help of VOSviewer, we conducted a visual analysis of keywords to generate the visualization map based on keywords with a co-occurrence value above 20. Supplementary Figure 4A highlights closely related keywords grouped into three clusters, with 149 nodes, 9328 links, and a total link strength of 44142. “ICIs” was at the center of node in the red cluster, with 148 links and a link strength of 1958. “Nivolumab,” the center of node for the green cluster, has 147 connections and a total link strength of 1503. “Effect,” the center of node for the blue cluster, has 148 connections and a total link strength of 2328. Supplementary Figure 4B presents the density visualization map of keywords, with “effect,” “ICIs” and “nivolumab” as the top three keywords, appearing 228, 179 and 136 times, respectively. Supplementary Figure 4C demonstrates a time-lapse visualization map, with purple representing earlier dates and yellow representing more recent dates. “ipilimumab” was the top keyword in 2017, appearing 127 times. “Effect” was the top keyword in 2019, appearing 228 times, and “ICIs” in 2020, appearing 179 times. The pattern is consistent with drug research norms, in which researchers examine the effects of a drug before evaluating its long-term side effects. Finally, we applied burst detection in Citespace to identify research hotspots over time for keywords. Supplementary Figure 4D shows that the first burst of keywords included “monoclonal antibody,” “anti-CLTA4 antibody,” and “melanoma”. Subsequently, induced sarcoidosis (2012-2018, strength of 3.51), safety (2015-2017, strength of 3.48), inflammatory arthritis (2018-2019, strength of 3.43), and preexisting autoimmune (2019-2020, strength of 3.51) garnered increasing attention from researchers.
In this study, we conducted a bibliometric analysis of publications related to ICI-induced rheumatic irAEs for the first time to gain insights into the global research trends in this field. In contrast to scoping reviews and systematic reviews, bibliometric analysis provides a powerful tool for summarizing current knowledge and identifying emerging trends. After excluding 384 publications that didn’t meet our inclusion criteria, we identified 803 publications in 316 journals from 1354 institutions in 52 countries/regions. The first article was published in 2007, describing the effectiveness of CTLA-4 inhibitors in Phase I/II clinical trials. It was also observed that some patients developed arthritis, and showed improvement after treatment with Cox-2 inhibitors. A significant increase in publications related to this topic over the years was revealed, particularly since 2015, indicating the growing importance of this field of research. One possible explanation for this trend is the increasing clinical use of ICIs since the first US Food and Drug Administration (FDA) approval of ipilimumab for late-stage (metastatic) melanoma in 2011 (23). The approval of several ICIs in 2015 for various types of cancer (24) led to a surge in their use and a subsequent increase in the reports of rheumatic irAEs. As more patients were treated and more AEs were reported, the interest and awareness of this topic increased among the research community.
The distribution of contributing countries/regions and institutions showed some characteristics. The USA was the most productive country in terms of publications, followed by France and Japan. The top 10 institutions accounted for 55.9% of the papers in this field, highlighting the significant contributions made by researchers from these countries. The USA’ dominant position can be attributed to its well-established research infrastructure, long history of medical research, top-ranked institutions, and funding agencies such as the National Institutes of Health (NIH), which is one of the most important research funding agencies in the world for basic and clinical research. Most of the top 10 institutions and funding agencies are based in the USA. In addition to government fundings, pharmaceutical companies such as Bristol Myers Squibb and Merck Company also play a significant role in drug development and research. Regarding the top 10 co-cited references, six of them are from the USA, and one is a collaborative effort between the USA and the UK. USA scholars primarily publish clinical research and clinical guidelines, which enables their work to be widely disseminated and cited. China produces a considerable amount of research papers, but the citation rate is relatively low. The reasons behind this include language barriers, inadequate funding and support from the government or pharmaceutical industries, limiting large-scale clinical trials in some areas. As a result, researchers may prioritize conducting meta-analyses and case reports that have less impact on the scientific community compared to original research articles and clinical trials, thereby leading to a decreased citation rate. To address this, Chinese authorities should allocate more resources towards conducting original research articles and large-scale clinical trials to potentially increase the impact of research findings and contribute to the advancement of this research areas.
We also found that international collaboration has been increasing over time, with collaboration between the USA and France being particularly strong. This is an encouraging trend as international collaboration can lead to the sharing of knowledge and expertise, resulting in a better understanding of scientific research. China’s high publication output in the field of immunotherapy and irAEs indicates a growing interest and investment in research, which presents valuable opportunities for collaboration with researchers around the world. However, there may be limitations to China’s collaboration with other countries due to language barriers and a lack of established relationships with researchers in other countries. Additionally, China faces funding and pharmaceutical development challenges, with many local companies focusing on generic drugs rather than original products. Nonetheless, China has a large population of cancer patients, making it an attractive market for the development of new therapies. By collaborating more closely with other countries, we can effectively facilitate knowledge exchange and sharing, while simultaneously driving innovation in the healthcare industry. This can lead to a better understanding of diseases, drugs, and treatment options, ultimately resulting in improved patient care.
Our analysis further identified the top journals and institutions that have contributed to research on ICIs associated rheumatic irAEs. The top ten most central journals in this field were identified, and the institutions with the highest number of publications were also analyzed. Our findings can help researchers identify the most relevant journals and institutions to publish their research and collaborate with other researchers in this field. Additionally, researchers can subscribe to these journals to stay up-to-date with the latest developments in the field.
We also analyzed the keywords used in these publications. Analyzing the keywords used in publications can offer information about the research topics and methodologies of the publications, and analysis of keywords’ co-occurrence can often detect research hotspots and directions in the research field. By generating a network visualization map for keywords with the co-occurrence value greater than 20 times, we found that the most frequently used keywords can be broadly categorized into three sections: ICIs and their tumor indications, the mechanisms and efficacy of ICIs, and the adverse reactions caused by ICIs. As time progressed, researchers gained a deeper understanding of ICIs beyond their development as cancer treatments, with emphasis on their mechanisms, efficacy, and adverse reactions. The research hotspots included autoimmune hypophysitis, induced sarcoidosis, safety, inflammatory arthritis, and preexisting autoimmune conditions. These hotspots provide insights into the current research focus and highlight areas that require further investigation.
However, it is also important to acknowledge potential limitations and biases of our study. Firstly, this study solely relied on literature from the WoSCC database, which may have resulted in the exclusion of publications from other sources. Secondly, the study overlooked non-English articles, as it is focused only on English-language publications. These factors should be considered while interpreting the results. Another limitation of this study could be the lack of attention paid to the quality of the publications. While bibliometric methods can provide a comprehensive analysis of the literature, they do not take into account the quality of the research. Future studies could consider incorporating quality assessment tools to better understand the impact and significance of the research conducted on ICIs-associated rheumatic irAEs. Upon reviewing the literatures, we found that majority of them focused solely on describing the phenomenon of ICIs associated rheumatic irAEs, with a lack of comprehensive research on the underlying mechanisms, risk factors, and prevention strategies. We believe that delving deeper into these areas will significantly contribute to enhancing the prognosis of cancer patients. Despite these limitations, our study provides valuable insights that can significantly support researchers in their decision-making process when it comes to selecting suitable journals and institutions to publish their research or collaborate with. Additionally, this study sheds light on the current research hotspots and directions within the specific field of research being examined. These findings can contribute to enhancing the quality and impact of researchers’ work, as well as facilitating fruitful collaborations and identifying potential areas for future exploration.
Overall, our study is the first to use bibliometric methods such as CiteSpace and VOSviewer to comprehensively evaluate the current state and progress, as well as research trends and contributions in the field of ICI-induced rheumatic irAEs, which represents a new area of rheumatology that calls for urgent attention, research, and collaboration. Given the rapidly increasing use of ICIs in cancer treatment, rheumatologists can play a central role in the management of irAEs in the future, and multidisciplinary collaboration is essential to improving their recognition and outcomes. Future efforts should focus on developing classification criteria and guidelines for the diagnosis and management of rheumatic irAEs; conducting prospective studies to better estimate the incidence and prevalence of these events and understand their risk factors and patterns of onset, resolution, and persistence; investigating the immunopathogenic mechanisms involved, including the roles of different immune checkpoints, autoantibodies, and cytokines in their development and persistence; and identifying biomarkers for prediction and monitoring of these events based on their immunological and clinical features. These future directions are crucial to promoting better care for cancer patients treated with ICIs and advancing the field of rheumatology.
ICIs have emerged as a promising treatment option for various types of cancers. However, their use has been associated with various irAEs, including rheumatic irAEs, which is a growing concern now. Compared to other immune AEs, rheumatic irAEs typically involve the joints, muscles, and other connective tissues. While arthralgia and myalgia are common rheumatic irAEs reported in clinical trials, other case series and reports have described a wider spectrum of de novo rheumatic and systemic manifestations that mimic classic rheumatic diseases and can occur with ICIs (25, 26). These include polymyalgia rheumatica (PMR)-like syndromes and inflammatory arthritis syndromes, which are often encountered clinical presentations (13, 27, 28). Systemic manifestations have also been reported, including sicca syndrome, sarcoidosis, and all vessel-sized vasculitis (29–35). Interestingly, autoantibodies are often absent or at low titre in these patients (11). While these rheumatic irAEs can occur across all classes of ICI, they are most frequently and severely associated with combination treatments and may be linked with other organ-specific irAEs. Treatment of rheumatic irAEs generally depends on the CTCAE grade of the events, and may involve discontinuing ICI therapy, administering steroids or immunosuppressive agents, including biologics, and referral to a rheumatologist for specialized care (11). Careful evaluation and monitoring of treatment response and potential side effects are critical as these patients may be at higher risk for infections and other complications.
Currently, the underlying immunopathogenic mechanisms of rheumatic irAEs are not clear and several possible mechanisms have been proposed. One might involve generalized immune activation, due to checkpoint neutralization, which might result in inflammatory reactions in multiple organ systems, including the musculoskeletal system (9). It is reported that several primary immunodeficiencies associated with prominent autoimmune manifestations are linked to dysfunction or underexpression of CTLA4 (36). T cell exhaustion, which correlates with a state of low disease activity in some autoimmune diseases, such as anti-nuclear cytoplasmic antibody (ANCA)-associated vasculitis, is also a possible explanation for this mechanism (37). A study found increased production of inflammatory cytokines such as IL-17 following treatment with ICIs, as well as the effectiveness of cytokine targeting (especially by TNF inhibitors) at treating immune-related side effects further supports it (38–40). Another proposed mechanism of rheumatic irAEs is direct off-target effects of checkpoint inhibitors (9). ICIs may have off-target effects on cells other than T cells, including natural killer cells, dendritic cells, and macrophages, leading to autoimmune inflammation and rheumatic irAEs. However, the precise mechanisms by which these effects occur are not yet clear. The third proposed mechanism of rheumatic irAEs is epitope spreading (41). The diversity of T-cell receptor repertoires and the heterogeneity of tumor-associated antigens make it challenging for the immune system to recognize and eliminate tumors. However, ICIs can induce epitope spreading, which is the diversification of the epitope-specificity of T-cells. The new targets acquired by the T cells can include new antigens on self-proteins, leading to autoimmune inflammation and rheumatic irAEs (41). In addition, molecular mimicry is also suggested as a possible mechanism of ICIs-associated rheumatic irAEs. Some viral and bacterial antigens share sequence or conformational similarity with host antigens. The immune system, in response to infections, may produce antibodies and T cells that cross-react with host antigens, leading to autoimmune inflammation and rheumatic irAEs. Pre-existing asymptomatic autoimmunity is another possible mechanism involving ICIs that mediate immune activation in asymptomatic or pauci-symptomatic autoimmunity (9). However, against this argument is the fact that most patients with rheumatic irAEs test negative for serum autoantibodies. To date, rheumatic irAEs have not been thoroughly investigated for signature autoantibodies, HLA associations, or biomarkers of idiopathic disease. However, as investigations are ongoing, there is a desperate need to study immunopathogenic mechanisms for most irAEs as such investigations are far from complete.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LZ and QZ participated in the research design. LZ and KC participated in the bibliometric analysis. GM, LZ and QZ drafted the manuscript, QZ revised and polished the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1242336/full#supplementary-material
1. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell (2018) 175(2):313–26. doi: 10.1016/j.cell.2018.09.035
2. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell (2015) 27(4):450–61. doi: 10.1016/j.ccell.2015.03.001
3. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
4. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
5. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
6. Yu L, Sun M, Zhang Q, Zhou Q, Wang Y. Harnessing the immune system by targeting immune checkpoints: Providing new hope for Oncotherapy. Front Immunol (2022) 13:982026. doi: 10.3389/fimmu.2022.982026
7. Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discovery (2016) 15(4):235–47. doi: 10.1038/nrd.2015.35
8. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
9. Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol (2018) 14(10):569–79. doi: 10.1038/s41584-018-0074-9
10. Cappelli LC, Shah AA, Bingham CO 3rd. Cancer immunotherapy-induced rheumatic diseases emerge as new clinical entities. RMD Open (2016) 2(2):e000321. doi: 10.1136/rmdopen-2016-000321
11. Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis (2021) 80(1):36–48. doi: 10.1136/annrheumdis-2020-217139
12. Le Burel S, Champiat S, Mateus C, Marabelle A, Michot JM, Robert C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer (2017) 82:34–44. doi: 10.1016/j.ejca.2017.05.032
13. Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis (2018) 77(3):393–8. doi: 10.1136/annrheumdis-2017-212257
14. Bobirca A, Bobirca F, Ancuta I, Florescu A, Padureanu V, Florescu DN, et al. Rheumatic immune-related adverse events-A consequence of immune checkpoint inhibitor therapy. Biol (Basel) (2021) 10(6). doi: 10.3390/biology10060561
15. Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc (2005) 2005:724–8.
16. Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technology (2006) 57(3):359–77. doi: 10.1002/asi.20317
17. Kleinberg J. Bursty and hierarchical structure in streams. Data Min Knowledge Discovery. (2003) 7(4):373–97. doi: 10.1023/A:1024940629314
18. Chen Y, Li Y, Guo L, Hong J, Zhao W, Hu X, et al. Bibliometric analysis of the inflammasome and pyroptosis in brain. Front Pharmacol (2020) 11:626502. doi: 10.3389/fphar.2020.626502
19. Cranmer LD, Hersh E. The role of the CTLA4 blockade in the treatment of Malignant melanoma. Cancer Invest (2007) 25(7):613–31. doi: 10.1080/07357900701522315
20. Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology (2009) 218(1):69–70. doi: 10.1159/000161122
21. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/jamaoncol.2018.3923
22. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med (2019) 380(24):2377–9. doi: 10.1056/NEJMc1901677
23. Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res (2011) 17(22):6958–62. doi: 10.1158/1078-0432.CCR-11-1595
24. Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J (2021) 23(2):39. doi: 10.1208/s12248-021-00574-0
25. Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: A systematic review of the literature. Arthritis Care Res (Hoboken) (2017) 69(11):1751–63. doi: 10.1002/acr.23177
26. Abdel-Rahman O, Eltobgy M, Oweira H, Giryes A, Tekbas A, Decker M. Immune-related musculoskeletal toxicities among cancer patients treated with immune checkpoint inhibitors: a systematic review. Immunotherapy (2017) 9(14):1175–83. doi: 10.2217/imt-2017-0108
27. Richter MD, Crowson C, KottsChade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic syndromes associated with immune checkpoint inhibitors: A single-center cohort of sixty-one patients. Arthritis Rheumatol (2019) 71(3):468–75. doi: 10.1002/art.40745
28. Calabrese C, Kirchner E, Kontzias A, Velcheti V, Calabrese LH. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open (2017) 3(1):e000412. doi: 10.1136/rmdopen-2016-000412
29. Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis (2017) 76(1):43–50. doi: 10.1136/annrheumdis-2016-209595
30. Ramos-Casals M, Maria A, Suarez-Almazor ME, Lambotte O, Fisher BA, Hernandez-Molina G, et al. Sicca/Sjogren's syndrome triggered by PD-1/PD-L1 checkpoint inhibitors. Data from the International ImmunoCancer Registry (ICIR). Clin Exp Rheumatol (2019) 37 Suppl 118(3):114–22.
31. Lomax AJ, McGuire HM, McNeil C, Choi CJ, Hersey P, Karikios D, et al. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: Case series and immunophenotypic analysis. Int J Rheum Dis (2017) 20(9):1277–85. doi: 10.1111/1756-185X.13076
32. Tomelleri A, Campochiaro C, De Luca G, Cavalli G, Dagna L. Anti-PD1 therapy-associated cutaneous leucocytoclastic vasculitis: A case series. Eur J Intern Med (2018) 57:e11–e2. doi: 10.1016/j.ejim.2018.07.023
33. Padda A, Schiopu E, Sovich J, Ma V, Alva A, Fecher L. Ipilimumab induced digital vasculitis. J Immunother Cancer (2018) 6(1):12. doi: 10.1186/s40425-018-0321-2
34. van den Brom RR, Abdulahad WH, Rutgers A, Kroesen BJ, Roozendaal C, de Groot DJ, et al. Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatol (Oxford) (2016) 55(6):1143–5. doi: 10.1093/rheumatology/kew063
35. Roger A, Groh M, Lorillon G, Le Pendu C, Maillet J, Arangalage D, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) induced by immune checkpoint inhibitors. Ann Rheum Dis (2019) 78(8):e82. doi: 10.1136/annrheumdis-2018-213857
36. Lo B, Fritz JM, Su HC, Uzel G, Jordan MB, Lenardo MJ. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood (2016) 128(8):1037–42. doi: 10.1182/blood-2016-04-712612
37. McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature (2015) 523(7562):612–6. doi: 10.1038/nature14468
38. Manson G, Norwood J, Marabelle A, Kohrt H, Houot R. Biomarkers associated with checkpoint inhibitors. Ann Oncol (2016) 27(7):1199–206. doi: 10.1093/annonc/mdw181
39. Esfahani K, Miller WH Jr. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med (2017) 376(20):1989–91. doi: 10.1056/NEJMc1703047
40. Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo). (2013) 2013:857519. doi: 10.1155/2013/857519
Keywords: immune checkpoint inhibitor, rheumatic immune-related adverse event, bibliometric, research trends, VOSviewer, CiteSpace
Citation: Zeng L, Ma G, Chen K and Zhou Q (2023) Bibliometric analysis of rheumatic immune related adverse events associated with immune checkpoint inhibitors. Front. Immunol. 14:1242336. doi: 10.3389/fimmu.2023.1242336
Received: 19 June 2023; Accepted: 26 September 2023;
Published: 06 October 2023.
Edited by:
Jeffrey J. Pu, Harvard Medical School, United StatesReviewed by:
Devis Benfaremo, Marche Polytechnic University, ItalyCopyright © 2023 Zeng, Ma, Chen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiao Zhou, MjI5MjI5MzRAcXEuY29t; Kai Chen, NzMxNjI2MTc3QHFxLmNvbQ==
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.