- 1Comparative Immunogenetics Laboratory Department of Veterinary Pathobiology, Texas A&M University, College Station, TX, United States
- 2Amino Acid Laboratory, Department of Animal Science, Texas A&M University, College Station, TX, United States
- 3Department of Microbial Pathogenesis and Immunology, College of Medicine, Texas A&M Health Science Center, Texas A&M University, Bryan, TX, United States
Comparative animal models generate fundamental scientific knowledge of immune responses. However, these studies typically are conducted in mammals because of their biochemical and physiological similarity to humans. Presently, there has been an interest in using teleost fish models to study intestinal immunology, particularly intestinal mucosa immune response. Instead of targeting the pathogen itself, a preferred approach for managing fish health is through nutrient supplementation, as it is noninvasive and less labor intensive than vaccine administrations while still modulating immune properties. Amino acids (AAs) regulate metabolic processes, oxidant-antioxidant balance, and physiological requirements to improve immune response. Thus, nutritionists can develop sustainable aquafeeds through AA supplementation to promote specific immune responses, including the intestinal mucosa immune system. We propose the use of dietary supplementation with functional AAs to improve immune response by discussing teleost fish immunology within the intestine and explore how oxidative burst is used as an immune defense mechanism. We evaluate immune components and immune responses in the intestine that use oxidant-antioxidant balance through potential selection of AAs and their metabolites to improve mucosal immune capacity and gut integrity. AAs are effective modulators of teleost gut immunity through oxidant-antioxidant balance. To incorporate nutrition as an immunoregulatory means in teleost, we must obtain more tools including genomic, proteomic, nutrition, immunology, and macrobiotic and metabonomic analyses, so that future studies can provide a more holistic understanding of the mucosal immune system in fish.
1 Introduction
Animals have benefited comparative immunology for over a millennium by providing a scientific platform to better understand biological processes, with human and veterinary medicine only significantly diverging in the early 20th century (1, 2). Comparative animal models generate fundamental scientific knowledge on immune responses, and mammals have been favored for those studies for their similarities with human biochemical mechanisms and physiology. To further expand our understanding of immunity, research in lower vertebrates such as zebrafish (Danio rerio) has rapidly gained popularity in immunology since 1997 (3), and has led to the use of other teleost species as models to investigate immune responses, particularly within the intestine. One component of interest is mucus, which is composed of antimicrobial peptides (AMPs), bactericidal enzymes, immunoglobulins, mucins, glycoproteins, and immune cells (4). Although fish may experience different pathogens compared with humans and other mammals, they are valuable models for intestinal inflammatory responses with many immune genes and mechanisms conserved from fish to mammals (3, 5, 6). Teleost fish dominate the species raised on aquaculture farms and when using these species as immunology models, we can further improve fish health via both animal science and veterinary biomedical research. Ultimately, research in multiple teleost species is crucial to fully investigate the natural history of biochemical mechanisms that can be applied to human biomedical research as well.
Farmed fish is a popular protein source for human consumption, and the Food and Agriculture Organization (FAO) reported that production in 2020 reached 87.5 million tonnes. This is higher than previous years and reduces the need for capture fisheries (7). The COVID-19 pandemic drove aquaculture production in the last few years due to restriction in fishing activities, with FAO projecting that production will reach 106 million tonnes by 2030 (7). To meet market demands and environment changes, sustainable farming techniques are used to improve the growth performance of fish within the industry (8, 9). Sustainable techniques for aquaculture are evaluated according to societal standards of impact on the environment, animal welfare, as well as safety for both the animals and workers (8, 10). Water quality and habitat are a sector of sustainable management to limit the spread of disease, especially in highly stocked farms (8, 9, 11).
Mucosal surfaces are in direct contact with the water environment to provide the first line of defense against pathogens. Currently, vaccines provide the best method to prevent infectious disease, as they can be efficient and cost-effective (12). However, the protection is limited to specific known pathogens and research is still identifying the most effective administration routes for existing vaccines in fish (13–15). Mucosal vaccines are available and have been explored for they are non-invasive and decrease the risks of exposure to pathogens; however, there are challenges with vaccine efficacy from administration (16–18). For long-term B and T memory responses to be strong at mucosal surfaces, the immunization needs to adhere to the surface and penetrate the mucus; however, mucosal surfaces dilute the immunization and, despite increasing the dosage, vaccines can be broken down by proteases present on these surfaces (18, 19). In the gut, one challenge is that the antigen often needs to break intestinal tolerance through stimulation of lymphocytes within the lamina propria of mucosal tissues (17, 20). The use of antibiotics in the aquaculture industry is another approach to control infections but can be expensive (21) and if misused, can lead to selection of bacteria with antibiotic resistance in humans (15).
Instead of targeting the pathogen itself, nutrition has become a preferred approach for managing fish health for it is less invasive or labor intensive than administering vaccines while still providing immune modulation properties to fight infections and limit losses from diseases (13, 22). Using nutrition to modulate fish immune responses can enhance immunological functions by targeting biochemical mechanisms for immunostimulation (13). Nutrient immunostimulants include vitamins, minerals, fatty acids, and amino acids (AAs), which all provide a non-toxic, non-invasive, and environmentally friendly method to support immune responses (23). These feed additives as immunostimulants can enhance the expression of genes related to humoral and cellular immunities (23, 24). Additionally, nutrient immunostimulants can regulate and shape immune responses by activating cytokine secretion, receptor expression and other components within the innate and adaptive immune systems (23, 25). AAs as nutrient immunostimulants are a niche of immunonutrition that depends on cellular requirements for an immune response. Cells require more metabolic fuels and nutrients during an infection, particularly AAs for protein synthesis until the pathogen is cleared (13). The availability of nutrients can impact oxidative-antioxidant balance by promoting the production of metabolites from AAs such as glutathione (GSH) and nitric oxide (NO). Amino acids are the building blocks of proteins including antioxidant enzyme systems such as superoxide dismutase (SOD) and are essential for physiological development of teleost fish (26). All AAs are required to produce immune cells and antibodies, implying that their deficiency can weaken the immune system. Supplemental AAs in diets can act as an immunostimulant to modulate immune responses partly via antioxidant mechanisms (27). In immunonutrition, morphological and transcriptional analyses have been excellent tools to understand the health status of the gut, which can be examined after feed supplementation to determine optimal immune capacity to prevent infections (Abdul 28). By understanding AA impact on gut immune health, diets can be modified to favor specific immune responses that will not lead to oxidative stress but instead promote homeostasis once the pathogen has been cleared.
In this review, we will evaluate select AAs and their metabolites in oxidant-antioxidant balance and their use to improve mucosal immune capacity as well as gut integrity. We will have a brief overview of teleost fish immunology, including both innate and adaptive responses within the intestine. We will then expand on a particular immune defense mechanism called oxidative burst, which produces reactive oxygen species (ROS) to destroy bacterial pathogens, and discuss how this mechanism is activated through stress that fish encounter within aquaculture. This will lead to a discussion of the relationship between oxidative stress and gut mucosal immune responses under various environmental stressors, and current studies utilizing specific AAs to modulate mucosal immune responses in teleost intestine. Lastly, we will provide our perspectives on overcoming the experimental challenges in AA immunonutrition for more effective modulation of teleost gut immunity through oxidant-antioxidant balance.
2 Teleost fish immunology
2.1 Overview of mucosal and intestinal immune response
Although fish lack organized lymphoid structures in their gut, the gut wall contains a range of immune cells such as plasma cells, other lymphocytes, and antigen-presenting cells (APCs) (17, 29). The mucosal barrier maintains homeostasis in the organism through local immune responses to sustain tolerance with mutual and commensal bacteria (30). Differentiated epithelial cells called goblet cells within the mucosa prevent pathogen entry by secreting mucins, high molecular weight glycoproteins that can contain antimicrobial properties. Goblet cells are a typical morphological marker to identify active mucosal immune responses, for example in brown trout (Salmo trutta) during parasitic infections, the secreted mucus contains acidic glycoconjugates that increase mucus viscosity for intestinal protection (31). Intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) are lymphocytes that reside in the epithelial and lamina propria layers respectively, and recognize antigens to induce an adaptive immune response to maintain gut homeostasis and kill the pathogen (32).
The gastrointestinal tract of teleost fish has evolved to their aquatic lifestyle and feeding habits, but it is important to note that not all teleost fish have a stomach. For instance, zebrafish and common carp (Cyprinus carpio) lack stomachs and instead have an anterior intestine called the intestinal bulb, whereas fish like Nile tilapia (Oreochromis niloticus) and Atlantic cod (Gadus morhua) have a true stomach (3). The intestine is divided into three main regions: the pyloric caeca, midgut, and hindgut, which all aid in food digestion, nutrient absorption, and mucosal immunity. From their investigation in rainbow trout (Onchorhynchus mykiss) intestine, Verdile et al. (32) reported that gut segments should be accounted for when determining the relationship between metabolism and immune response. Typically, histological parameters used to determine gut health in response to dietary supplementation are based on classical morphological changes in the fish fed experimental and control diets, including villus length, lamina propria height, quantity of IELs, and qualitative changes in mucus secretion (32, 33). Differences in segment morphology could result from commensal bacteria populations within specific segments affecting the morphology of the brush border. Ultimately, these morphological changes can impact the expression of genes encoding immune signaling factors like chemokines and cytokines, which could lead to alterations in cellular signaling for immune defense. This is because enterocytes undergo rapid turnover to function properly and an imbalance between cell proliferation and apoptosis can lead to tissue dysfunction plus damage to the intestinal morphology (34).
Besides morphological analyses of the gut, other biomarkers present valuable information to better understand mechanisms impacted from supplementation. For example, intestinal alkaline phosphatase (ALP) activity is a biomarker for mature enterocytes and can be used in conjunction with the immunohistochemical localization of proliferating cell nuclear antigen (PCNA), where PCNA signal confirms the presence of stem cells (33). These signals tend to be within intestinal folds of the villi, and when identified with ALP activity, mature differentiated epithelial cells can be recognized (33). Alternatively, a lack of ALP leads to increased sensitivity to microbes and high levels of neutrophils, which modulate the composition and function of the gut microbiota (3). If pathogens have the ability to affect morphology as well as microbiota populations throughout the length of the gut, experiments should be cognizant of their impact on immune-relevant gene expression that alter mucosal defense (35). Published articles often either sample one gut segment or multiple segments, so validation is needed for past work utilizing only a single segment or less-defined ones to fully understand mucosal immunity throughout the gut length.
Production of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) depends on oxygen to fuel the mitochondria to meet the cell’s energy needs in an immune response (36). Circulating oxygen diffuses into tissues through the oxygen cascade gradient and functions as a regulator of ROS generation in the mitochondria (36). The mucosal barrier maintains homeostasis through the selective transport of nutrients including electrolytes and water from the intestinal lumen into the bloodstream while disposing of harmful substances to prevent them from entering the internal environment (34, 37, 38). Tight junctions, for example, can regulate the mucosal barrier by directly preventing toxins from entering the lumen. Development of the disease is closely associated with tight junction structure damage of intestinal epithelial cells that are triggered by pro-inflammatory cytokine released by the activation of the intestinal mucosal immune system (39–41). Tumor necrosis factor α (TNF-α) is a pro-inflammatory cytokine that can disrupt the stability of tight junction, thereby introducing oxidative stress (41). Wu et al. (42), concluded from their work on Songpu mirror carp (Cyprinus carpio Songpu) that during Aeromonas hydrophila exposure, mRNA expression of tight junction structural protein genes was upregulated in intestinal mucosal cells to reduce intestinal permeability (42). This suggests that the regulation of mucosal tight junctions is to protect the integrity of the gut by controlling its environment and reduce oxidative damage. Intestinal epithelial cells communicate with the mucosa to facilitate an effective immune response, and this process starts with cellular signaling within the innate immune system.
2.2 Innate immune response
Innate immune cells and related proteins play crucial roles to neutralize pathogens. The process begins with the recognition of pathogen-associated molecular patterns in fish gut (PAMPs) by pattern recognition receptors (PRRs) expressed on gut epithelial cells (13). This interaction detects and recognizes pathogens that disrupt homeostasis and initiates chemical, biological, and/or mechanical responses to prevent further infection (43). Toll-like receptors (TLRs) are a type of PRR that have been evolutionarily conserved to mediate innate immune defense, recognizing PAMPs such as lipopolysaccharides (LPS), a component of bacterial cell walls. In fish, LPS activates the expression of inflammatory cytokine genes, such as the interleukins, IL-1β, IL-8, IL-17A/F, and TNF-α, likely mediated by TLR4 (44). TLR-4 is an important receptor for inflammation because it activates the nuclear factor kappa B (NF-κB) pathway, leading to the release of inflammatory mediators, including chemokines and cytokines (42, 45, 46). Conversely, some fish do not present TLR-4 or an ortholog to recognize LPS (47, 48). A dual-luciferase reporter assay was used to compare NF-κB activation in gilthead seabream (Sparus aurata), spotted green pufferfish (Tetraodon nigroviridis), and zebrafish showed that LPS signaled a TLR4 independently from the MyD88 pathway (47). The MyD88 is a signaling protein for TLRs induced by NF-κB for immune signaling and interestingly the zebrafish TLR4 orthologs negatively regulated the MyD88-dependent pathway, suggesting that TLR4 is a negative regulator to provide resistance to bacterial endotoxins (47, 48). Other TLRs have been identified in teleost species such as rock cod (Eleginops maclovinus) (49). Different portions of the intestine were investigated following exposure to the bacterium Francisella noatunensis and modulated the transcription of innate PRRs TLR1, TLR5 and TLR8 were observed. The transcriptional profiles of the evaluated TLRs were likely based on the binding capabilities of TLR1 to bacterial lipopeptides and LPS, TLR5 to flagellin, and TLR8 to single-stranded bacterial RNA (49, 50). These findings showed that TLR1, TLR5, and TLR8 recognized F. noatunensis in different parts of the gut at different levels, further supporting that the receptors on the gut following infection modulate transcription differently amongst the length of the intestine (49). Activation of specific TLRs can cause downstream immune effects based on the cytokines and chemokines being produced.
Notable cytokines that can attract phagocytic cells to the site of infection include IL-1β, a proinflammatory cytokine produced by monocytes/macrophages that signals the proliferation of leukocytes and downstream production of additional chemokines and other cytokines (51). IL-2 is an example of a downstream cytokine that can further promote inflammation through increased signaling of IL- 1β and NO production to recruit neutrophils, while stimulating T cells of adaptive immunity (51, 52). IL-8 is a pro-inflammatory chemokine that induces neutrophil and macrophage migration during oxidative stress or when infected by a pathogen to clean cellular debris and generate new tissue (51, 53). IL-17A/F are cytotoxic cytokines that CD4+ helper T cells use to communicate inflammation through AMPs. IL-17A is produced by adaptive Th17 cells, inducing the expression of cytokines such as IL-1β, TNF-α, and IL-6 in yellow catfish (Pelteobagrus fulvidraco) (54). IL-17F is responsible for protecting epithelial cells against bacterial invasion, upregulating other proinflammatory cytokines such as TNF-α, a multifunctional proinflammatory cytokine that drives the proliferation of immunocytes and phagocytic activity. Hoseinifar et al. (51) reported that TNF-α could enhance the phagocytic ability, ROS activity, and apoptosis of granulocytes/monocytes, suggesting that the leukocyte phagocytosis mechanism could be regulated by TNF-α in rainbow trout (Oncorhynchus mykiss). Research in isolated leukocytes of largemouth bass (Micropterus salmoides) confirmed TNF-α’s role in phagocytosis, leading to an enhancement in phagocytic ability, ROS activity, and apoptosis of granulocytes (55).
Monocytes, macrophages, and neutrophils are granulocytes that mediate non-specific immunity by promoting inflammation and phagocytosis. Within macrophages, there are two major subpopulations: M1 and M2 macrophages. M1 has pro-inflammatory properties and expresses TNFα, whereas M2 has anti-inflammatory properties from increased expression of TGF-β, an anti-inflammatory cytokine (30, 56, 57). Monocytes and macrophages predominantly produce ROS and NO intracellularly, while neutrophils produce these molecules both intracellularly and extracellularly (4, 58–60). NO is a cytotoxic molecule of macrophages that mediates inflammation (26), where inflammation can be categorized as acute or chronic. Acute inflammation involves a series of events resulting in increases in blood flow as well as monocyte and neutrophil trafficking (61, 62). Acute inflammation can be shut down by inhibiting proinflammatory pathways and activating anti-inflammatory cytokines, like TGF-β. If the pathogen persists, the inflammation can become chronic by increasing blood flow through an increase in capillary permeability resulting from high amounts of antinutritional factors due to inadequate feed formulation (62–64). Chronic inflammation can lead to cellular resource depletion, tissue damage, and fibrosis unless it is optimally regulated by anti-inflammatory responses to neutralize ROS (63, 65). Acute inflammation can be prevented by inhibiting proinflammatory pathways and activating anti-inflammatory cytokines, such as TGF-β. If the pathogen persists, the inflammation can become chronic by increasing blood flow through an increase in capillary permeability resulting from either high amounts of antinutritional factors or inadequate feed intakes of nutrients (62, 63, 66). Chronic inflammation can lead to cellular resource depletion, tissue damage, and fibrosis unless it is optimally regulated by anti-inflammatory responses to neutralize ROS (63, 65). The cellular signaling in response to changes in an infection from acute to chronic depends on the cytokine and chemokines secreted from these immune cells. Inflammasomes provide surveillance, monitoring the infection site to promote the recruitment of immune cells causing inflammation, followed by expression of genes for anti-inflammatory signals to restore homeostasis and destroy damaged cells through an intracellular death process defined as apoptosis (67, 68).
In both fish and mammals, cytokines and chemokines are secreted through the bloodstream interacting with hepatocytes (68). This initiates crosstalk with various plasma proteins to signal for immune pathways like the complement system to be upregulated (69–71, 71, 72). The complement system in mammals includes three pathways with series of cascades of the cleaving until a terminal complex is lysed (69, 71, 73). Most subunits of the complement system have been identified in teleost fish and share structural similarities at the protein level with mammalian orthologs (69). Interestingly, teleost fish have variable isoforms of some components, notably C3 (74); C4 (75), C7 (76), C8 (77), C2/CFB (78), CFH (79), and CR3 (77). The various isoforms suggest different specificities for cleavage activation; however, the complement components are understudied, including the mechanisms involved to activate systems such as kallikrein (69, 80). Within the blood coagulation pathway in mammals, there is an important cascade with serine proteases called the kinin-kallikrein cascade that participates in blood clotting, blood pressure regulation and inflammation (81). Wong and Takei (82) found that the teleost fishes Nile tilapia and rainbow trout shared very little similarity in homologous structures of kininogens with mammals and had some components missing at the genomic level (82). A particular enzyme responsible for the creation of large structures with a high binding affinity had been lost through evolution within the teleost group (82). Instead, teleost fish have analogous structures within the kallikrein cascade and have a unique lectin activity known as the kalliklectin cascade (83). Andreeva et al. (81), investigated Atlantic cod serum for intracellular proteins to understand this cascade further, and observed no mammal-like osmotically active albumin but found osmotically active proteins that functionally replace the albumin independently from osmoregulation (81). The results from this experiment suggest that teleost albumin function was lost in fish during evolution, but other intracellular proteins are needed to maintain osmotic homeostasis (83). Their results demonstrated that these other plasma proteins are involved with transport in binding lectin to pathogens through the complement system and in immune regulation (83). To further explore the roles of neutralization of pathogens, we will discuss a crucial defense mechanism of teleost fish against invading pathogens: oxidative burst.
2.2.1 Oxidative (respiratory) burst and ROS
In teleost fish, ROS and NO (a reactive nitrogen species) defend against pathogens by causing oxidative damage to their DNA, proteins, and lipids (26). In erythrocytes and immunocytes of these animals, the mitochondria function as an oxygen regulator by generating ROS and using energy to synthesize antioxidants. In fish, molecules of ROS are formed via many biochemical reactions (including NADPH oxidase and SOD), whereas NO is produced from arginine (84). At metabolic rest, ROS yield is typically low until a stressor induces a fight or flight response via activating the protein kinase C signaling pathway (36, 85). The primary electron donor for superoxide generation is NADPH, which is formed mainly via the hexose monophosphate shunt in endotoxin or phorbol myristate acetate (PMA)-stimulated macrophages to perform oxidative burst (86). Oxidative burst is primarily seen in phagocytic leukocytes where its receptors interact with the pathogen (59). When the pathogen is engulfed, a phagosome undergoes a series of steps to fuse with a lysosome, in which the lysosome activates the NADPH oxidase complex on the extracellular membrane of the phagolysosome to produce 90% of cell-derived ROS in the mitochondria (36, 59, 87). Organisms lacking this complex can suffer from chronic infections, leading to hyper-inflammation that can be fatal (86, 88). The NADPH oxidase complex consists of several subunits, including p22phox, gp91phox, p47phox, p67phox, and Rac (89, 90). The subunit gp91phox is first stimulated during the complex’s activation, and electrons are transferred from NADPH to O2 within the cytosol of the cell, resulting in the production of ROS, such as superoxide anion (O2-) with the enzyme SOD (89, 90). Superoxide anion is converted to hydrogen peroxide with catalase (CAT) and can be further converted to water with peroxidase (89, 90). ROS must be removed by either enzymatic antioxidants including SOD, CAT, glutathione peroxidase (GPx), and peroxiredoxin (Prx); or non-enzymatic antioxidants including vitamins C and E, and GSH (36). In teleost fish, antioxidant enzymes are similar among different vertebrate species, where the main variability is in distribution of these enzymes in different tissues (36). Hydrogen peroxide (H2O2) is produced by leukocytes as part of their oxidative burst response involved in host defense (62, 91, 92). H2O2 can modulate gene expression through transcriptional factors and signaling molecules to regulate the late phase of leukocyte recruitment for inducing the production of the chemokine CXCl8 (93). In addition, H2O2 may regulate epigenetic modifications of transcriptional factor accessibility to CXCl8 and other genes encoding inflammatory factors.
Throughout evolution, higher oxygen capacity has become favored in fish based on their high activity levels. Fish cells may favor antioxidant protection in order to continue other important metabolic functions (36). Feeding and starvation conditions display the mediation of oxidative-antioxidant balance through the promotion or inhibition of ROS. Schvezov et al. (94) reported that rainbow trout (Oncorhynchus mykiss) after one feeding had molecular changes in oxidative defenses over a 96-hour post-feeding period (94). The highest antioxidant enzyme activities were found in the liver, followed by the intestine and stomach, with the lowest activities observed in the gills (94, 95). Interestingly, CAT was found in all tissues except for the liver, in contrast to GPx, which had the highest activity in the liver (94), suggesting that there may be interorgan relationships in antioxidant defense in the liver to favor oxidant-antioxidant balance. During an immune response, the liver has special mechanisms to scavenge ROS as well as synthesizing proteins like lipoproteins, such as very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), regulating intracellular protein turnover, producing bile acid, and synthesizing GSH (96–98). In order to perform these biomechanisms, the liver needs to take up the AAs, including: glycine, methionine, and proline (97). The bile can be excreted into the duodenum through the bile duct in response to a digestion-induced increase in the concentration of bicarbonate from ductal epithelial cells (97). Bile acids have the ability to conjugate with glycine and taurine to form bile salts to facilitate intestinal absorption of dietary lipids, including lipid-soluble vitamins like the antioxidant vitamin E to mediate antioxidant balance (26, 99). Lowered CAT activity in fish liver has been observed during starvation while SOD and GPx activities have been reported to increase, leading to oxidative damage (94, 100–102). A low SOD/GPx ratio will give better protection against oxidative stress because the efficiency of SOD is relatively higher for short periods of stress due to the balance between free radical production and antioxidant enzyme activity (94). To modulate oxidant-antioxidant balance with an immunostimulant supplement, one should be aware of the synergistic cooperation between immune response and the enzymes SOD, CAT, and GPx.
GPx is the main molecule in the response to reduce lipid hydroperoxides to their corresponding alcohols, thereby protecting the cell against lipid peroxidation (26). Stress has been shown to increase the activity of GPx in Atlantic salmon and rainbow trout 12 h post-exposure to 15 min of hypoxia stress from overcrowding (103). Interestingly, GPx activity may be affected by cortisol, a circulating glucocorticoid hormone that is a common biomarker for stress across various animal species. This result indicates that enzymes with antioxidant properties can be triggered in a stress response (103). Elevated concentrations of cortisol may induce the production of ROS, but cytokines produced from inflammation can act like regulatory molecules to facilitate immunosuppression (103, 104). Teleost fish are constantly exposed to various environmental stressors that can lead to oxidative stress, causing an imbalance of ROS, making it challenging to control the amount of free radicles through the ingestion of dietary antioxidants (103, 105).
2.2.2 Oxidative stress and immune response
Exposure to stress, especially throughout multiple stages of life, can lead to chronic oxidative stress, which may induce morphological changes that can affect fish metabolism, including digesting and absorption (103, 106). It was observed in Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) that high stocking densities had a decrease in the integrity of the intestinal mucosal barrier and decreased mRNA expression of interferon gamma (IFNγ), the type II interferon and regulator of the gut immune response (103, 107). Chronic oxidative stress can cause damage to cell proteins, leading to cell death. Cells can modify the pH of their surrounding environments based on nutrient availability for host defense (108). Most eukaryotic cells have a pH ranging from 7.2 to 7.4 at a resting metabolic state, then decreasing during an immune response to denature pathogenic proteins (108, 109). In phagocytosis, lysosomes have a pH5, although it can vary depending on the cell type and environmental stressors (108, 110). Vacuolar-type ATPase (V-ATPase) enzymes have been identified as the key mediators of organelle acidification, which use ATP to drive protons into the lumen and the rate can impact the generation of ROS (108, 111, 112). To achieve antigen presentation and activate the adaptive immune response, the acidic phagolysosomal system promotes the net export of protons outside of the cell through the inner membrane antiporter, GadC (108, 113). This transporter relies on intracellular glutamate for the production of γ-aminobutyric acid (GABA; an inhibitory neurotransmitter) and, under acidic conditions (e.g., < pH 6.5), exchanges intracellular GABA for extracellular glutamate. Animals also require basic AAs (e.g., arginine, ornithine, histidine, and lysine) to maintain gut homeostasis through biochemical pathways to maintain low pH in the gut (108, 113, 114). These exemplify the relationship between acidic intracellular compartments for activating components of adaptive immune response by presenting antigens to T cells and inducing their activation for downstream defenses.
Oxidative stress can alter the composition of the gut microbiota, resulting in unwanted inflammation that can lead to oxidative damage from ROS. Environmental stress like water pollution from antimony have been investigated in zebrafish (66). Toxic metals, like antimony, in high concentration can alter the microbiota and affect both immune response and nutrition metabolism (64, 115, 116). The enzymes SOD, CAT, and GPx are critical to detoxify organs after toxic metal exposure because the metals produce ROS (64, 117). Acetylcholinesterase (AChE) is an enzyme used as a biomarker to quantify environmental pollution because it degrades acetylcholine, a neurotransmitter (64). Under stress, ROS in excess can inhibit AChE, meaning nerve signals are excited and oxidative stress will not be alleviated due to the accumulation of acetylcholine (64). This suggests that the inhibitory effect on AChE activity when ROS production is high can decrease the total antioxidant capacity (T-AOC) of the cell (64). T-AOC assays can directly reflect the capacity of nonenzymatic and enzymatic systems in response to environmental stressors. Malondialdehyde (MDA) is a biomarker for oxidative stress that can be used with T-AOC assays because it is a product of lipid peroxidation, typically for polyunsaturated fatty acids (64, 117, 118). MDA can induce damage to the structure of biofilms of pathogens and affect their normal function leading to proapoptosis (42, 119). In zebrafish, MDA content in the intestine increased with exposure time of antimony due to the oxidative damage from ROS (64). With excessive ROS production, SOD may be highly active convert superoxide anion to hydrogen peroxide, and CAT activity may be enhanced for H2O2 removal to resist oxidative damage (66, 120, 121). As exposure time progresses, the excess H2O2 will all be removed, and CAT activity will decreases to equilibrium (64). Thus, antimony stress results in dysbiosis of the zebrafish antioxidant system, ultimately impacting immune response and potential gut commensal bacteria.
Although quantifying the three main antioxidant enzymes, SOD, CAT, and GPx is beneficial to examine the effect of T-AOC, a histological marker of interest is the melanomacrophages centers (MMCs). MMCs are phagocytes containing lipofuscin, melanin, and haemosiderin found in the liver, head kidney, spleen (122, 123), and skin (124). These are believed to function in both immune defenses and can potentially orchestrate immune responses as well as oxidative stress. Researchers can determine AA metabolism, nucleotide synthesis, glycolysis, tricarboxylic acid (TCA) cycle, and oxidative phosphorylation (OXPHOS) while quantifying expression for fatty acid oxidation (125). An example is an OXPHOS pathway called glutaminolysis, which sustains T cell response through important enzymes GLS1 (encoding the K-type mitochondrial glutaminase) and GLUD (encoding the mitochondrial glutamate dehydrogenase); and if glutamine is deprived, phosphorylation is reduced in the T cells. This results in spleen lacking T cell proliferation (125). T cell immunity can determine the outcome of the adaptive immune response through the need for B cell activation and the direction of other effector mechanisms.
2.3 Adaptive immune response
In teleost adaptive immunity, lymphocytes are activated by innate immunity or the antigen itself binding to the lymphocyte antigen receptor, producing a specified response to eliminate pathogens and affording memory protection (13). This activation begins at peripheral lymphoid tissues, including GALT, where naïve lymphocytes first encounter antigen to perform either cell-mediated immunity or humoral immunity (114). Cell-mediated immunity starts with naïve T cells, lymphocytes that have not been exposed to antigen that is specific for their antigen receptor. Naïve T cells will circulate through the blood and peripheral lymphoid tissues in search of the antigen of the pathogen, which will be presented by proteins of the major histocompatibility complex (MHC) on APCs such as dendritic cells (29, 114, 126). This specific antigen and co-stimulation will then activate the naïve T cells and spark proliferation and differentiation into effector cells such as cytotoxic T cells (CD8+) or helper T cells (CD4+), which will clonally expand and secrete cytokines and chemokines (29). Cytotoxic T-cells can target and kill infected cells via antigen presentation from MHC class I on the cell surface, where MHC class I high allelic polymorphism provides broad pathogen recognition at the host population level (114, 127). T-helper cells have diverse functions based on the cytokines or chemokines produced (114). For example, T-helper 1 cells (Th1) activate infected macrophage microbicidal functions via phagocytosis by antigen presentation by MHC class II. Th1 cells during an inflammatory response can secrete proinflammatory cytokines including IL-1β, TNFα and IFN-γ, that will activate M1 macrophages that will kill the pathogen (128). In contrast, during an anti-inflammatory processes, Th2 cells activate M2 macrophages that secrete cytokines IL-4 and IL-10 and synthesize polyamines to promote wound healing and defense against helminths (128, 129). Another noteworthy Th cell are Th17 cells that stimulate neutrophils for inflammatory responses, as well as maintaining homeostasis at the epithelial barriers (29, 114).
Teleost fish have B lymphocytes that produce immunoglobulins of three isotypes: IgM, IgD, and IgT/IgZ. Antibodies are immunoglobulins that are synthesized and secreted by plasma cells (30, 130–132). IgM are the primary immunoglobulin present and can also be found in the serum, while IgT/Z are mucosal-specific immunoglobulin analogous to mammalian IgA and is primarily found in the intestine and skin mucosal tissue (13, 114). Nomenclature was standardized to IgT from IgZ recently (133) although some teleost’s clades such as catfish lack the isotype signifying other potential mucosal immune defense mechanisms (134).
Lymphocytes proliferate with intense protein synthesis and, therefore, a sufficient bioavailability of AAs is important metabolic processes (including ATP production, oxidation, antioxidant defense, and the production of antibodies and cytokines) and immune responses (26). Protein synthesis requires a large amount of energy, with the minimum energetic cost estimated to be 40 to 50 mmol ATP equivalents per gram of protein synthesized (26, 135–137). In growing fish, about 20 to 30% of dietary AAs are used for protein accretion (138, 139). By combining knowledge from nutrition and immunology, we can develop immunostimulants through AA supplementation to prevent the damage caused by ROS, while still promoting effective immune responses when fish are infected. There has been extensive research done on mechanisms that regulate the antioxidant defense system in mammals but not with teleost fish. Some of the reasons are experimental limitations from the amount of serum or plasma in juvenile fish, as well as challenges to isolate the leukocytes of fish to measure ROS and NO production. It is critical to find solutions to using minimal amounts of samples to take these quantitative measurements to evaluate the health and welfare of these fish within aquaculture.
3 Immunonutrition
3.1 Protein metabolism and cellular AA requirements
The nutritional requirements for AAs depend on numerous factors including the species, developmental stage, physiological status, gut microbiota, environment, and diseases (27, 140–143). Both immune response and antioxidant activity can be modulated through dietary supplementation as a non-invasive approach for controlling infectious diseases and minimize stress (21, 98, 144, 145). The teleost intestine has a plethora of digestive enzymes that facilitate the breakdown of complex nutrients into simple molecules that are absorbed into the intestinal mucosal cells by transporters. This process can regulate the downstream activation of the immune response through direct or indirect metabolic pathways. Understanding of their regulatory properties in the immune system in relation to AA requirements is imperative because protein feedstuffs contain different composition of AAs (138). For example, plant protein as the sole protein source in fish diets increases the risk of deficiency in important AAs like taurine, a sulfur-containing AA with antioxidant and osmoregulatory properties (138).
When using an AA as a supplement to aid immunity, it is important to understand its metabolic processes, including degradation in the gut, that will in turn affect the concentration of the AA in blood circulation, thus impacting dietary requirements (84, 98). To better understand this, we must evaluate digestibility and bioavailability of AAs prior to establishing an effective nutritional program for teleost fish. Digestibility of dietary protein is a percentage of AAs released from protein in the diet by the small intestine due to the actions of proteases and peptidases (84). This process is crucial because dietary proteins have no nutritional value unless hydrolyzed to free AAs and small peptides in the gut (84, 146, 147).
For fish, the dietary requirement for protein ranges from 30-60% of dietary dry matter, which is much greater than that for terrestrial mammals and birds (98). However the whole-body AA composition is largely similar among animals including fish, pigs, and chickens (138, 148, 149). For omnivorous teleost fish, their protein requirement is lower compared to carnivorous fish, and therefore the digestibility of plant-sourced feedstuffs is also important to consider because they generally have anti-nutritional factors including protease inhibitors (138, 150, 151). As mentioned previously, sustainable farming techniques are used in the aquaculture industry, including the use of plant-sourced feedstuff (such as soybean meal) instead of fish meal (84). In many regions of the world, the main plant-sourced ingredient is soybean meal; however, some teleost fish can have a limited capacity to use its protein because of a reduced activity of proteases in the digestive tract (53, 152). Important anti-nutrients present in soybean meal include not only protease inhibitors but also lectins, phytic acid, saponins, phytoestrogens, antivitamins, and allergens (150). There are two groups of protease inhibitors in soybean meal (trypsin inhibitors): Kunitz-type (153) and Bowman-Birk type 1 that reduces the digestibility of dietary protein (138, 154). These protease inhibitors can affect protein utilization by reducing trypsin activity in the small intestine by 30% (150, 155). To combat the effects of antinutritional factors, dietary supplementation with proteases can improve the digestibility of soybean meal in rainbow trout (138, 156).
The use of fishmeal has rapidly declined due to the serious environmental damages from high phosphorus content in fish fecal matter, creating poor water quality (157). Soybean meal is considered the most economic and environmentally friendly; however, compared to fishmeal, soybean meal is deficient in some AAs (e.g., glycine and methionine) and lacks taurine (158). Song et al. (157) performed a feeding trial in juvenile starry flounder (Platichthys stellatus) investigating differences in growth performance between diets with the protein component being fish meal or soybean hydrolysates (an improved soy protein with enzymatic modifications). They found that the soybean hydrolysates could replace part of fish meal in the diet without affecting the growth performance of the fish (157). This led to new initiatives of nutritionists within aquaculture to further understand protein metabolism in the context of fish health.
Intracellular protein undergo various cellular processes that regulates cellular integrity through protein synthesis and degradation (110). Proteases are separated by the membrane to control the rate of degradation during an immune response, like phagocytosis. Under stress, the lysosomal-vacuolar pathway is the dominant process for protein turnover in the liver, but other cells may rely on non-lysosomal proteolytic pathways (110, 159). Secretory factors from the duodenum, such as secretin produced by the S-cells, regulate secretions from the stomach, pancreas and liver, and cholecystokinin (CCK) by I-cells to stimulate the secretion of pancreatic digestive enzymes (84, 98, 147, 160). CCK aids in lipid digestion by first stimulating the gallbladder to contract and release the stored bile salts into the intestine, then signaling for enhancing the secretion of pancreatic digestive enzymes (84, 147). It is important to note that there are upper limits to dietary AA intake and that excessive AAs (e.g., > 2% glutamine and > 3% arginine in corn- and soybean meal-based diets for growing pigs) can become toxic (158). For example, supplementation with excessive methionine (e.g., 0.17% of diet) can reduce the life span in rats (158, 161). In addition, dietary supplementation with 563 ppm canavanine (a plant metabolite analogous to L-arginine) can reduce weight gain and feed intake in chicks (162). By contrast, dietary supplementation with 4% glutamate is safe for growing pigs (158).
3.2 AA Immunostimulants and intestinal immunity
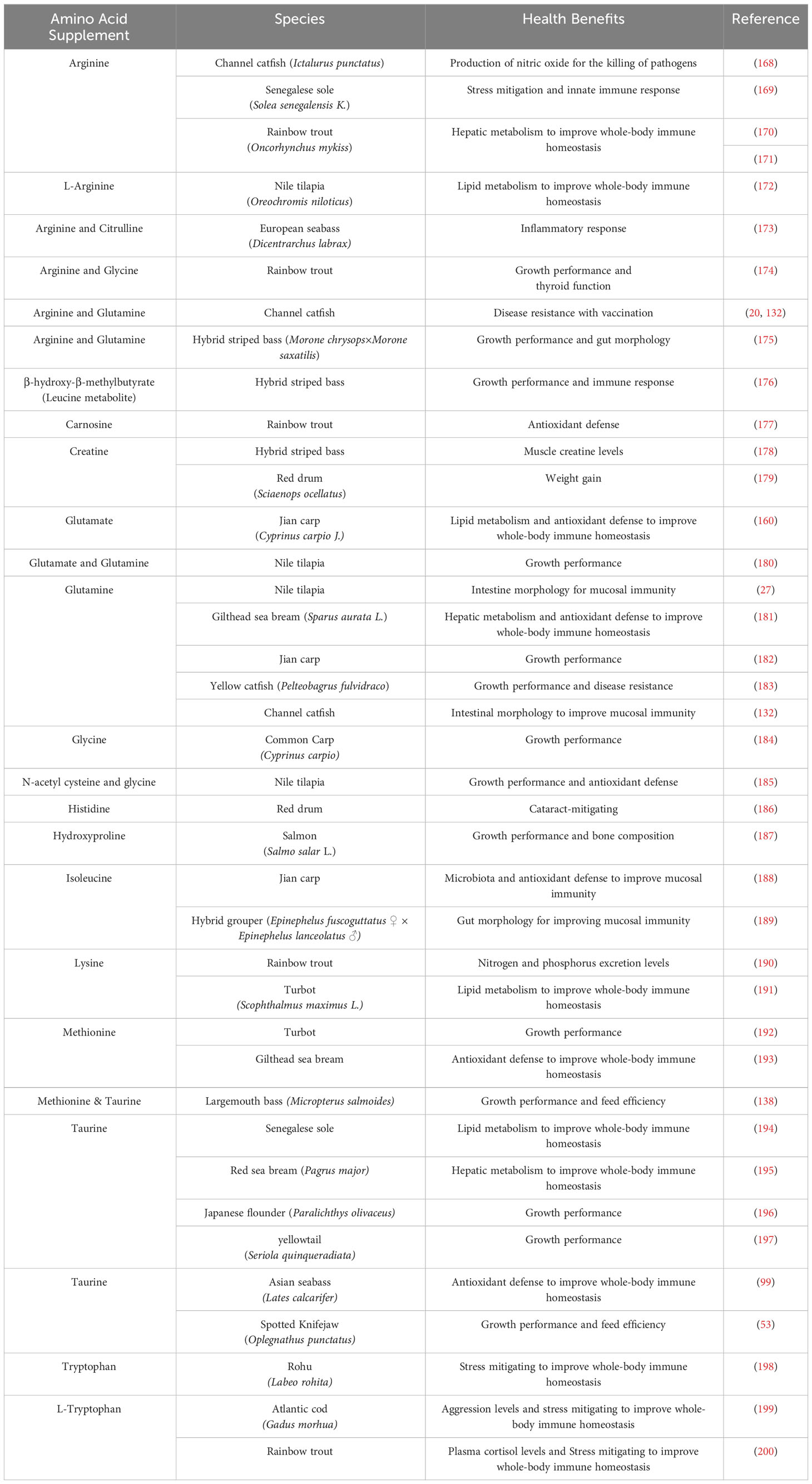
Historically, AA requirements and functions in fish species have been determined primarily based on dose-response trials utilizing different protein sources. However, different feedstuffs have very different compositions of AAs (84, 147, 163, 164). Fatty acids and glucose can be stored in the body as triacylglycerols and glycogen, respectively, and when not utilized, they can accumulate and eventually impair the function of cells and tissues (138). Compared to mammals, fish liver and kidneys have high AA oxidation, but fish metabolism is more energetically efficient because they can excrete ammonia directly into their environment without needing ATP (26, 138, 141, 147). There are nine AAs that are not synthesized from any dietary AAs and have traditionally been regarded as nutritionally essential in all fish: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine (84). It remains unknown whether fish can synthesize arginine de novo (158). These AAs have a direct relationship with protein synthesis, so if one of the 9 nutritionally essential AAs is limited, it can impact intracellular protein synthesis and possibly proteolysis (26). Recently there have been suggestions to reevaluate the parameters for AAs because the term “non-essential” is a misnomer due to the critical role these AAs have in protein synthesis as well as immunity (84, 141, 147, 165). A widely accepted term, functional AAs, refer to AAs that not only participate in but also regulate key metabolic pathways. These AAs include arginine, glutamate, glutamine, glycine, and proline (138, 166, 167). Understanding optimal AA requirement in teleost fish cells is important for developing ideal diets and improving health through disease resistance, but only a handful of AAs have been extensively researched to investigates its health benefits. Table 1 summarize the current AA supplementation studies performed in teleost fish. We will discuss arginine as well as glutamate and glutamine in this review for they may directly influence antioxidant balance.
3.2.1 Glutamine and glutamate
Glutamine is endogenously synthesized from glutamate and ammonia by glutamine synthetase, then deaminated into glutamate and ammonia by phosphate-activated glutaminase, meaning that both glutamine and glutamate play a valuable role in ammonia detoxification (138, 201, 202). Both glutamine and glutamate are required for ammonia detoxification in fish through the enzymes glutamine synthetase and glutaminase (GLS), demonstrating that there is a glutamine–glutamate cycle to regulate the intracellular concentrations of glutamine (138, 202). Both of these AAs are major sources of energy in the intestinal mucosa and glutamine can activate the mTOR pathway to initiate polypeptide synthesis (84, 147). Carvalho et al. (27) showed that 1.33% (dry matter basis) is the most optimal amount of glutamine in the diet for the immune function of juvenile Nile tilapia (27). Through histological analysis, the proximal intestine, villus height was higher and the ratio of villus:crypt was higher (27). GSH is an antioxidant molecule that participates in the teleost intestinal mucosal immune response by protecting the epithelium from oxidative damage caused by pathogens. GSH also improves the functionality of enterocytes to repair, proliferate, and recruit immune cells (84). Carvalho et al. (27) found that dietary supplementation with up to 1.71% of glutamine increases GSH concentration in the intestine, which is essential to prevent oxidative stress and adverse health consequences (27). This suggests that oxidative damage can be associated with inflammatory molecules, including changes in the expression and/or activities of antioxidative enzymes in reflection of oxidative stress. Glutamine supplementation may also be required to support immune responses in tilapia by serving as a precursor for glutamate, thereby mediating GSH synthesis and scavenging ROS to maintain cellular redox balance (27, 185).
When T cells are activated to combat an infection, the transporters ASCT2 and SNAT2 are upregulated to increase glutamine availability for the formation of glutamate in the mitochondria by GLS (125, 203). It will then be converted to alpha-ketoglutarate (AKG) by glutamate dehydrogenase (GLUD) through a metabolic process called glutaminolysis to promote cell proliferation, protein synthesis, and maintain redox balance (125, 204, 205). AKG is a precursor to glutamine and an intermediate of the TCA cycle that may play a role in antioxidant capacity during an immune response (42). The TCA cycle can facilitate NADPH production and provide extra ATP to the intestinal mucosal cells by promoting electron transport in the respiratory chain and maintaining the activity of the ion transporters (125, 206–208). This reduces the electron leakage from intracellular sodium/calcium ion loading and slows the formation of free radical damage (125, 209). Exogenous AKG promotes the absorption of nutrients through modulating intestinal AA metabolism by activating NF-κB to favor upregulation of expression for pro-inflammatory cytokines (46, 125, 210–213). NF-κB signaling in epithelial cells maintains immune homeostasis in mucosal tissues, and when in a resting metabolic state, NF-κB is complexed with an inactive inhibitory protein IκB-α (125). During oxidative stress, inflammatory signals can activate the enzyme IκB kinase (IKK) to phosphorylate the IκBα protein, which causes ubiquitination (125, 203). Ubiquitination is an ATP-dependent process that is highly regulated for homeostasis, and the dissociation of IκB-α from NF-κB can cause degradation of IκB-α which will activate NF-κB to be translocated into the nucleus (125, 214). It has been reported that commensal bacteria may have the ability to activate NF-κB in epithelial cells by stimulating pattern recognition receptors including TLR and NLRs (125, 215). For example, TLR4 recognizes inflammation to then activate the myeloid differentiation factor 88 (MyD88)-dependent pathway in Th cells that will release inflammatory cytokines (42, 216, 217).
Wu et al. (42) investigated effects of dietary supplementation with 1% AKG in Songpu mirror carp as an alternative to AAs to modulate immune response. They challenged the mirror carp with Aeromonas hydrophila and witnessed abnormal swimming behavior where the carp sought more oxygen on the water surface (42). Increasing oxygen needs was consistent with the data mRNA expression indicating a systemic immune response due to stress. Within intestinal tissues, they observed TLR4b intracellular signal transduction through a MyD88-independent pathway that upregulated expression of inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8 in addition to down regulating claudins and ZO-1 (42). Claudin-1, claudin-3, claudin-7, claudin-11 and zonula occludens-1 (ZO-1) were upregulated exhibiting that AKG supplementation could increase activities of tight junction structural protein genes in intestinal mucosal cells to promote integrity and reduce intestinal permeability. Oxidative stress in the intestine was quantified through the enzyme CAT while total antioxidant capacity used the MDA biomarker, and both demonstrated a decrease in content during AKG supplementation (42). AKG could reduce oxidative damage caused by A. hydrophila infection by increasing antioxidant capacity promoting scavenging radical properties. By inhibiting the generation of oxygen free radicals, it prevents lipid peroxidation as well as tissue damage (42, 218). AKG is capable of promoting expression of claudins under stress and can alleviate the stress response by reshaping the cytoskeletal microfilaments through the myosin light chain kinase (MLCK) signaling pathway (42, 218–220). MLCK functions in tight junction permeability by regulating the protein abundances of claudins, occludins, and zonula occludens (ZOs) (42). AKG supplementation in Songpu mirror carp can improve the contraction of the actin ring before the tight junctions, stabilizing the tight junction proteins and their surroundings cytoskeletal protein distributions to reduce intestinal permeability (41, 42, 221, 222). Protection of tight junction structure of intestinal epithelial cells during an infection increases intestinal mucosal permeability, therefore activating intestinal mucosal immune response. There are other AAs and associated metabolites that can enter immune cells through various junctions (158). However, these junctions and transporters need to be extensively studied for each AA.
3.2.2 Arginine
Another AA of interest for modulating immune responses is arginine, a nutritionally essential AA required by teleost fish for growth and immunity (26, 223). Arginine-derived NO is a cytotoxic molecule of macrophages that mediates inflammation (84, 147), where inflammation can be categorized as acute or chronic. Arginine is abundant in tissues and through the production of NO by macrophages, it can facilitate an immune response (26, 84, 147). NO is a cytotoxic molecule of macrophages that mediates inflammation through the production of cytokines that can directly or indirectly affect gene expression (84, 141, 147, 167, 224). In teleost fish, there is likely limited endogenous arginine synthesis due to the low activity of the enzyme carbamoyl phosphate synthase III and pyrroline-5-carboxylate synthase (84, 147, 225, 226). In contrast to most mammals, teleost fish may not synthesize citrulline from glutamate, glutamine, and proline but can produce citrulline from arginine via NO synthase at a very low rate (26, 84, 147). Hoseini et al. (227) investigated the effects of dietary arginine supplementation on systemic immune responses in common carp that were challenged with ammonia stress (227). They observed that arginine supplementation reduced ammonia levels in the plasma, elevated plasma glutamate and declined glutamine is due to lower available ammonia in the arginine-treated fish (227). After ammonia exposure, plasma arginine decreased in fish fed both the control and the arginine supplemented diets, but paradoxically the decrease in plasma arginine was greater in the arginine-supplemented group, possibly due to enhanced protein synthesis in the whole body. Plasma glutamate and glutamine decreased after exposure to ammonia, which could be due to the reaction of glutamate with ammonia to form glutamine and the excretion of glutamine from the body to help the fish cope with ammonia toxicity (227, 228).
From these observations, Hoseini et al. (227) concluded that arginine supplementation may be beneficial for ammonia tolerance in common carp to facilitate detoxification through AA metabolisms (227). Hoseini et al. (229) also investigated effects of dietary arginine supplementation on the common carp that were exposed to density stress. They observed that both arginine-deficient or excessive diets had detrimental effects on fish health, causing cortisol elevation, innate immunity weakness, and down-regulation of cytokine gene expression. In addition, they observed that optimum arginine levels inhibited stress-induced cortisol secretion and lysozyme activity through the downregulation of expression of IL-1β and IL-8 genes. The interaction of dietary arginine levels and stocking density on plasma cortisol suggest that different stress types might affect arginine requirement in common carp potentially through different metabolic pathways (229). Optimum dietary arginine levels boosted fish health and suppressed stress signals by stimulating the expression of inducible NO synthase (iNOS) to produce NO (229, 230). iNOS also regulates the secretion of inflammatory cytokines through activation of guanylate cyclase, as well as the release of inflammatory cytokines by binding all subtypes of glutamate receptor (229, 231, 232). At low cortisol levels in the plasma, there may be higher cytokine gene expression for regulatory cytokines and polyamine production for arginine-dependent cell proliferation (229). This is consistent with the report that arginine may increase the resistance of channel catfish to Edwardsiela ictaluri by producing NO (138, 167, 233).
M1 macrophages are believed to metabolize arginine into NO through the action of iNOS resulting in a macrophage population with increased microbicidal activity (57, 128). M2 macrophages metabolize arginine by converting it to ornithine and further converting ornithine into polyamines through ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (SAMdc) (128, 234, 235). Between the two subtypes of macrophages, competition for arginine is a balance between inflammatory response and cellular repair, but the competition between the two macrophage subtypes can deplete the arginine pool, therefore making the organism more susceptible to disease (128, 236, 237). M1 macrophages depend on extracellular arginine levels and when there is a sufficient supply the cells exports citrulline; in contrast under depleted supplies the cells import citrulline (128, 238). This is done through the formation of polyamines, thereby regulating the inflammatory response through inhibiting inflammatory mediators, antioxidant properties, as well as cell proliferation (128, 239, 240). M2 macrophages directly convert arginine to ornithine for polyamine synthesis for tissue repair as a result of increases in the enzymatic activities of ODC1, ODC2, SAMdc1, and SAMdc2 in the liver and head kidney (128). In addition, polyamines are produced by exfoliated enterocytes to also scavenge ROS and protect cells from oxidative stress (240, 241). Intracellular polyamine oxidation leads to an increase in ROS that causes DNA damage in activated immune cells (240, 242, 243). More studies are needed to further understand how polyamines affect both protein synthesis and gene expression for immune proteins. Li et al. (223) reported effects of dietary 1% and 2% L-arginine supplementation for 8 weeks on intestinal homeostasis in tilapia. They observed altered composition, diversity, and functional profiles of gut microbiota with increasing α-diversity and altering β-diversity Li et al. (223). Through evaluating the enhanced mRNA expression for Pparα, acox1, and Pgc-1α in tilapia intestinal tissues, Li et al. (223) found that arginine may regulate lipid metabolism in the intestine (223). Li et al. (223) verified the activity by culturing IPEC-1 cells (small- intestinal epithelial cells) to determine lipid metabolism through starving the cells and using oleic acid to induce triglyceride accumulation (223). These authors found that L-arginine mediated the AMP-activated protein kinase (AMPK) signaling pathway and mRNA levels were measured for Acox 1 and Acox 2 (223). When extracellular arginine availability was limited, triglycerides accumulated in the cells. Through examining the sequence of the 16S ribosomal RNA gene, Li et al. (223) and Ren et al. (244) found that dietary L-arginine supplementation affected the composition of the intestinal microbiota and therefore the intestinal immune response (223, 244). Since the intestine catabolizes about 40% of dietary arginine, its supplementation inhibited lipid accumulation in the intestine as confirmed through BODIPY staining to reveal reduced triglycerides content (223, 245, 246). Elevated mRNA levels for Pparα, Acox1, and Pgc-1α were associated with increased fatty acid oxidation through the activation of the in the enterocytes (223). Thus, there may be a trade-off between growth and the immune response in which growth is hindered until the infection is resolved (128). Dietary arginine supplementation could improve the physiological processes of digestion and the absorption of nutrients from the diet, thus enhancing growth performance and immune responses (particularly intestinal mucosal immunity).
4 Discussion
Phagocytosis involves pathogen enclosure in a phagosome and its subsequent destruction inside this structure via the fusion with the lysosome or the action of ROS. Current knowledge of teleost mucosal immunity is based on studies performed on fish species in addition to other species belonging to different genera, families, and orders. The inherent differences make it challenging to consolidate these findings to identify a comprehensive landscape on gut mucosal response facilitated through AA supplementation by modulating oxidant-antioxidant balance. Systematic studies are necessary for a comprehensive understanding of immunity in all fish, but a lack of specific antibodies against these cell markers is a limiting factor for the study of immune cell subsets in most teleost’s (20). Whole genome sequencing information from phylogenetically diverse organisms can offer other tools by comparing immune receptors (43). Proteomic analysis can validate individual proteins in the intestine compared to systemic efficiency from phagocytosis (247). Primary cells lines also provide a valuable model, but to our knowledge no one has created a gut mucosa primary cell line. We hope that researchers will be successful in developing a gut mucosa primary cell line as well as mono- and poly-clonal antibodies for some teleost fish species to phenotype leukocytes and quantify cellular regulation of certain immune cell populations. An another powerful tool that can be used to analyze distinct gene expression datasets from the same experiment in samples of interest is called non-metric multidimensional scaling (NMDS) (128). This method has not been utilized to a large extent, but should be incorporated in future studies because the plots can display a clear separation between uninfected and infected groups, as well as enzymatic activities (128). Another appealing perspective would be to look further into the effects of both pathogen and AA supplementation on epigenetics, which is defined as the heritable alterations of gene expression through covalent modifications of DNA in addition to core histones without alterations in the DNA sequence (248). This process is initiated by S-adenosylmethionine-dependent DNA and histone methylation, histone acetylation by acetyl-CoA, histone phosphorylation, and histone ubiquitination (249).
In recent years, there has been growing interest in the role of dietary AAs in the immune responses of fish, especially their mucosal immunity. Adding crystalline AAs in diets for fish can effectively improve intestinal mucosal integrity, enhance growth performance, modulate intestinal microbiota, and improve resistance to infections (250, 251). To further understand teleost, gut mucosal immunity in response to dietary AA supplementation, future studies should involve a collaboration of genomics, proteomics, nutrition, immunology, macrobiotic and metabonomic analyses. The collaboration of different specialties in a single feeding trial, even for one teleost species can provide a more holistic understanding of the mucosal immunity in fish.
Amino acid supplementation has a promising future for its ability to modulate immune response through metabolic processes, oxidant-antioxidant balance, and physiological requirements (158, 164). While the immunonutrition field has made advances, only a few research teams have investigated cell- and tissue-specific energy requirements in gut mucosal immune responses. By bridging the knowledge of protein metabolism during oxidative burst or oxidative stress, nutritionists can develop sustainable aquafeeds through modifying AA composition to promote specific immune response, including gut mucosal immunity. More nutritional experiments are warranted to investigate the dosage effect and chronic impact of using AA as a dietary immunostimulant in fish immunity. An exploration of the response of fish at different stages to dietary AA supplementation in terms of their immune system development will also lay a solid physiological basis for the broad application of using AA to modulate fish immune systems. Rapid advances in the field of teleost gut mucosal immunity require more sophisticated tools, especially when utilizing the zebrafish model in the biomedical science field to understand biochemical mechanisms to find cure for inflammatory diseases. Overall, we propose further investigation on dietary supplementation with functional AAs to improve immune response. Availability of well-developed tools in aquaculture models will have major impacts on the provision of high-quality fish protein for global food security by understanding the molecular impacts on immune components in the gut as well as the cellular mechanisms for oxidant-antioxidant balance. Through research, we can compare various AAs and characterize their antioxidant benefits to make the aquaculture more sustainable by improving plant-based diets with amino acid supplementation to help fish within aquaculture resist pathogenic microbes to improve the growth and health of the aquatic animals.
Author contributions
KH drafted the work for this review, contributing to majority of the interpretation of data for the work. MC provided approval for publication of the immunology content, while contributing and revising it critically for important intellectual content. GW also provided approval for publication of both the immunology and nutrition content. Both WH and GW also contributed to the nutrition and amino acid concepts, and all authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Agriculture and Food Research Initiative Competitive Grant (2021-67015-34534) from the USDA National Institute of Food and Agriculture.
Conflict of interest
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From ‘One medicine’ to ‘One health’ and systemic approaches to health and well-being. Prev Veterinary Med (2011) 101(3–4):148–56. doi: 10.1016/j.prevetmed.2010.07.003
2. Mwangi W, de Figueiredo P, Criscitiello MF. One health: addressing global challenges at the nexus of human, animal, and environmental health. PloS Pathog (2016) 12(9):1–8. doi: 10.1371/journal.ppat.1005731
3. Brugman S. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol (2016) 64:82–92. doi: 10.1016/j.dci.2016.02.020
4. Dezfuli BS, Bosi G, DePasquale JA, Manera M, Giari L. Fish innate immunity against intestinal helminths. Fish Shellfish Immunol (2016) 50:274–87. doi: 10.1016/j.fsi.2016.02.002
5. Cheesman SE, Guillemin K. We know you are in there: conversing with the indigenous gut microbiota. Res Microbiol (2007) 158(1):2–9. doi: 10.1016/j.resmic.2006.10.005
6. Yang Y, Tomkovich S, Jobin C. Could a swimming creature inform us on intestinal diseases? Lessons from zebrafish. Inflammatory Bowel Dis (2014) 20(5):956–66. doi: 10.1097/01.MIB.0000442923.85569.68
7. Food and Agriculture Organization of the United Nations. The state of world fisheries and aquaculture 2022. Towards blue transformation. (Rome: FAO) (2022). doi: 10.4060/cc0461en (Accessed January 20, 2023).
8. Rector ME, Filgueira R, Bailey M, Walker TR, Grant J. Sustainability outcomes of aquaculture eco-certification: Challenges and opportunities. Rev Aquac (2023) 15(2):840–52. doi: 10.1111/raq.12763
9. Perry WB, Lindsay E, Payne CJ, Brodie C, Kazlauskaite R. The role of the gut microbiome in sustainable teleost aquaculture. Proc R Soc B: Biol Sci (2020) 287:(1926). doi: 10.1098/rspb.2020.0184
10. International Trade Centre. ITC standards map app. (2023). Available at: https://standardsmap.org/en/identify?products=Aquaculture. (Accessed June 7, 2023).
11. Weitzman J, Steeves L, Bradeford J, Filgueira R. Far-field and near-field effects of marine aquaculture. In: World seas: an environmental evaluation, 2nd ed. Cambridge, MA: Academic Press (2019), 197–220. doi: 10.1016/B978-0-12-805052-1.00011-5
12. Rombout JHWM, Abelli L, Picchietti S, Scapigliati G, Kiron V. Teleost intestinal immunology. Fish Shellfish Immunol (2011) 31(5):616–26. doi: 10.1016/j.fsi.2010.09.001
13. Pohlenz C, Gatlin DM. Interrelationships between fish nutrition and health. Aquaculture (2014) 431:111–17. doi: 10.1016/j.aquaculture.2014.02.008
14. Weir H, Chen PL, Deiss TC, Jacobs N, Nabity MB, Young M, et al. DNP-KLH yields changes in leukocyte populations and immunoglobulin isotype use with different immunization routes in zebrafish. Front Immunol (2015) 6:606. doi: 10.3389/fimmu.2015.00606
15. Yang DX, Yang H, Cao C, Jiang M, Zheng J, Peng B. Succinate promotes phagocytosis of monocytes/macrophages in teleost fish. Front Mol Biosci (2021) 8:644957. doi: 10.3389/fmolb.2021.644957
16. Munang’andu HM, Mutoloki S, Evensen Ø. An overview of challenges limiting the design of protective mucosal vaccines for finfish. Front Immunol (2015) 6:542. doi: 10.3389/fimmu.2015.00542
17. Docando F, Nuñez-Ortiz N, Serra CR, Arense P, Enes P, Oliva-Teles A, et al. Mucosal and systemic immune effects of bacillus subtilis in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol (2022) 124:142–55. doi: 10.1016/j.fsi.2022.03.040
18. Venu G, Dutta S, Panwar K, Sarkar R, Sinha E, Arun A, et al. Mucosal vaccines: Strategies and challenges: A brief overview. Pharma Innovation (2023) 12(6):4980–90.
19. Clark MA, Hirst BH. Expression of junction-associated proteins differentiates mouse intestinal M cells from enterocytes. Histochem Cell Biol (2002) 118:137–47. doi: 10.1007/s00418-002-0433-z
20. Pohlenz C, Buentello A, Criscitiello MF, Mwangi W, Smith R, Gatlin DM. Synergies between Vaccination and Dietary Arginine and Glutamine Supplementation Improve the Immune Response of Channel Catfish against Edwardsiella ictaluri. Fish Shellfish Immunol (2012b) 33(3):543–51. doi: 10.1016/j.fsi.2012.06.005
21. Naiel MAE, El-hameed SAAA, Arisha AH, Negm SS. Gum arabic-enriched diet modulates growth, antioxidant defenses, innate immune response, intestinal microbiota and immune related genes expression in tilapia fish. Aquaculture (2022) 556:738249. doi: 10.1016/j.aquaculture.2022.738249
22. Kiron V. Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci Technol (2012) 173(1–2):111–33. doi: 10.1016/j.anifeedsci.2011.12.015
23. Vijayaram S, Ringø E, Zuorro A, van Doan H, Sun Y. Beneficial roles of nutrients as immunostimulants in aquaculture: A review. Aquaculture Fisheries (2023). doi: 10.1016/j.aaf.2023.02.001
24. Mandujano-Tinoco EA, Sultan E, Ottolenghi A, Gershoni-Yahalom O, Rosental B. Evolution of cellular immunity effector cells; perspective on cytotoxic and phagocytic cellular lineages. Cells (2021) 10(8):1853. doi: 10.3390/cells10081853
25. Thépot V, Campbell AH, Rimmer MA, Paul NA. Meta-analysis of the use of seaweeds and their extracts as immunostimulants for fish: a systematic review. Rev Aquacult (2021) 13:907–33. doi: 10.1111/raq.12504
26. Li P, He W, Wu G. Composition of amino acids in foodstuffs for humans and animals. In: Wu G ed. Amino acids in nutrition and health: advances in experimental medicine and biology, vol. 1332. Cham: Springer (2021). doi: 10.1007/978-3-030-74180-8_11
27. Carvalho PLPF, Xavier WS, Guimarães MG, Rodrigues EJD, Furuya WM, Yamamoto FY, et al. Dietary Glutamine Improves Growth and Intestinal Morphology of Juvenile GIFT Tilapia (Oreochromis niloticus) but Has Limited Effects on Innate Immunity and Antioxidant Capacity. Aquaculture (2023) 563:738976. doi: 10.1016/j.aquaculture.2022.738976
28. Kari ZA, Kabir MA, Mat K, Rusli ND, Razab MKAA, Ariff NSNA, et al. The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquaculture Rep (2021) 21:100815. doi: 10.1016/j.aqrep.2021.100815
29. Mitchell CD, Criscitiello MF. Comparative study of cartilaginous fish divulges insights into the early evolution of primary, secondary and mucosal lymphoid tissue architecture. Fish Shellfish Immunol (2020) 107(PB):435–43. doi: 10.1016/j.fsi.2020.11.006
30. Stosik M, Tokarz-Deptuła B, Deptuła W. Immunity of the intestinal mucosa in teleost fish. Fish Shellfish Immunol (2023) 133:108572 doi: 10.1016/j.fsi.2023.108572
31. Díaz AO, García AM, Goldemberg AL. Glycoconjugates in the mucosa of the digestive tract of cynoscion guatucupa: A histochemical study. Acta Histochemica (2008) 110(1):76–85. doi: 10.1016/j.acthis.2007.08.002
32. Attaya A, Secombes CJ, Wang T. Effective Isolation of GALT Cells: Insights into the Intestine Immune Response of Rainbow Trout (Oncorhynchus mykiss) to Different Bacterin Vaccine Preparations. Fish Shellfish Immunol (2020) 105:378–92. doi: 10.1016/j.fsi.2020.06.051
33. Verdile N, Pasquariello R, Scolari M, Scirè G, Brevini TAL, Gandolfi F. A Detailed Study of Rainbow Trout (Onchorhynchus mykiss) Intestine Revealed That Digestive and Absorptive Functions Are Not Linearly Distributed along Its Length. Animals (2020) 10:(4). doi: 10.3390/ani10040745
34. Wang B, Wu G, Zhou Z, Dai Z, Sun Y, Ji Y, et al. Glutamine and intestinal barrier function. Amino Acids (2015) 47(10):2143–54. doi: 10.1007/s00726-014-1773-4
35. Firmino JP, Galindo-Villegas J, Reyes-López FE, Gisbert E. Phytogenic bioactive compounds shape fish mucosal immunity. Front Immunol (2021) 12:695973. doi: 10.3389/fimmu.2021.695973
36. Filho DW. Reactive oxygen species, antioxidants and fish mitochondria. Front Bioscience (2007) 12(4). doi: 10.2741/2141
37. Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr (2012) 3(5):687–96. doi: 10.3945/an.112.002683
38. Ruth M, Field C. The immune modifying effects of amino acids on gut-associated lymphoid tissue: discovery service for endeavour college of natural health library. J Anim Sci Biotechnol (2013) 4(27):1–10. doi: 10.1186/2049-1891-4-27
39. Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol - Gastrointestinal Liver Physiol (2012) 303:(7). doi: 10.1152/ajpgi.00155.2012
40. Oshima T, Miwa H. Gastrointestinal mucosal barrier function and diseases. J Gastroenterol (2016) 51(8):768–78. doi: 10.1007/s00535-016-1207-z
41. Zhou H, Zhu H, Yao XM, Qian J, Yang J, Pan X, et al. Metformin regulates tight junction of intestinal epithelial cells via MLCK-MLC signaling pathway. Eur Rev Med Pharmacol Sciencies (2017) 21:5239–46. doi: 10.26355/eurrev_201711_13847
42. Wu D, Fan Z, Li J, Zhang Y, Wang C, Xu Q, et al. Evaluation of Alpha-Ketoglutarate Supplementation on the Improvement of Intestinal Antioxidant Capacity and Immune Response in Songpu Mirror Carp (Cyprinus carpio) After Infection with Aeromonas hydrophila. Front Immunol (2021) 12:690234. doi: 10.3389/fimmu.2021.690234
43. Criscitiello MF, de Figueiredo P. Fifty shades of immune defense. PloS Pathog (2013) 9(2):1–6. doi: 10.1371/journal.ppat.1003110
44. Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol (2009) 21(4):317–37. doi: 10.1093/intimm/dxp017
45. Shi L, Feng L, Jiang WD, Liu Y, Jiang J, Wu P, et al. Immunity Decreases, Antioxidant System Damages and Tight Junction Changes in the Intestine of Grass Carp (Ctenopharyngodon idella) during Folic Acid Deficiency: Regulation of NF-KB, Nrf2 and MLCK mRNA Levels. Fish Shellfish Immunol (2016) 51:405–19. doi: 10.1016/j.fsi.2016.02.029
46. Xia HL, Wang ZW, Li Y, Chen WJ, Long M, Yu DP, et al. Characterization of tumor necrosis factor receptor associated factor 3 (TRAF3) in nile tilapia: expression profiles and functions in NF-κB pathway. Acta Hydrobiologica Sin (2023) 47(2):308–15. doi: 10.7541/2023.2020.269
47. Sepulcre MP, Alcaraz-Pérez F, López-Muñoz A, Roca FJ, Meseguer J, Cayuela ML, et al. Evolution of lipopolysaccharide (LPS) recognition and signaling: fish TLR4 does not recognize LPS and negatively regulates NF-KB activation. J Immunol (2009) 182(4):1836–45. doi: 10.4049/jimmunol.0801755
48. Martínez-López A, Tyrkalska SD, Alcaraz-Pérez F, Cabas I, Candel S, Morcillo FJM, et al. Evolution of LPS recognition and signaling: the bony fish perspective. Dev Comp Immunol (2023) 145:0–3. doi: 10.1016/j.dci.2023.104710
49. Díaz-Ibarrola D, Martínez D, Vargas-Lagos C, Saravia J, Vargas-Chacoff L. Transcriptional Modulation of Immune Genes in Gut of Sub-Antarctic Notothenioid Fish Eleginops maclovinus Challenged with Francisella noatunensis subsp. noatunensis. Fish Shellfish Immunol (2022) 124(January):56–65. doi: 10.1016/j.fsi.2022.03.044
50. Rauta PR, Samanta M, Dash HR, Nayak B, Das S. Toll-like receptors (TLRs) in aquatic animals: signaling pathways, expressions and immune responses. Immunol Lett (2014) 158(1–2):14–24. doi: 10.1016/j.imlet.2013.11.013
51. Hoseinifar SH, Shakouri M, Yousefi S, Doan HV, Shafiei S, Yousefi M, et al. Humoral and Skin Mucosal Immune Parameters, Intestinal Immune Related Genes Expression and Antioxidant Defense in Rainbow Trout (Oncorhynchus mykiss) Fed Olive (Olea europea L.) Waste. Fish Shellfish Immunol (2020) 100:171–78. doi: 10.1016/j.fsi.2020.02.067
52. Byadgi O, Chen CW, Wang PC, Tsai MA, Chen. SC. De Novo Transcriptome Analysis of Differential Functional Gene Expression in Largemouth Bass (Micropterus salmoides) after Challenge with Nocardia seriolae. Int J Mol Sci (2016) 17(8):1–17. doi: 10.3390/ijms17081315
53. Wu D, Xu H, Yang Y, Feng W, Han T, Wang J. Can taurine supplementation in a diet with soybean meal instead of fish meal improve the growth performance, feed utilization, and antioxidant capacity of spotted knifejaw (Oplegnathus punctatus)? Water (Switzerland) (2022) 14:(21). doi: 10.3390/w14213393
54. Zhou X, Zhang GR, Ji W, Shi ZC, Ma XF, Luo ZL, et al. Expression and function analysis of interleukin-17A/F1, 2, and 3 genes in yellow catfish (Pelteobagrus fulvidraco): distinct bioactivity of recombinant IL-17A/F1, 2, and 3. Front Immunol (2021) 12:626895. doi: 10.3389/fimmu.2021.626895
55. Yang S, Ma Y, Lou X, Zhou Z, Zhang H, Yi S, et al. The role of TNF-α in the phagocytosis of largemouth bass (Micropterus salmoides) leukocytes. Fish Shellfish Immunol (2023) 132:108488. doi: 10.1016/j.fsi.2022.108488
56. Hodgkinson JW, Grayfer L, Belosevic. M. Biology of bony fish macrophages. Biology (2015) 4(4):881–906. doi: 10.3390/biology4040881
57. Wiegertjes GF, Wentzel AS, Spaink HP, Elks PM, Fink IR. Polarization of immune responses in fish: the ‘Macrophages first’ Point of view. Mol Immunol (2016) 69:146–56. doi: 10.1016/j.molimm.2015.09.026
58. Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol (2007) 171(1):2–8. doi: 10.2353/ajpath.2007.070135
59. Rieger AM, Hall BE, Barreda DR. Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Dev Comp Immunol (2010) 34(11):1144–59. doi: 10.1016/j.dci.2010.06.006
60. Havixbeck JJ, Barreda DR. Neutrophil development, migration, and function in teleost fish. Biology (2015) 4(4):715–34. doi: 10.3390/biology4040715
61. Schmid-Schönbein GW. Analysis of inflammation. Annu Rev Biomed Eng (2006) 8:93–151. doi: 10.1146/annurev.bioeng.8.061505.095708
62. Martínez-Navarro FJ, Martínez-Morcillo FJ, de Oliveira S, Candel S, Cabas I, García-Ayala A, et al. Hydrogen peroxide in neutrophil inflammation: lesson from the zebrafish. Dev Comp Immunol (2020) 105:103583. doi: 10.1016/j.dci.2019.103583
63. Peixoto D, MaChado M, Azeredo R, Costas B. Chronic inflammation modulates opioid receptor gene expression and triggers respiratory burst in a teleost model. Biology (2022) 11:(5). doi: 10.3390/biology11050764
64. Wang M, Chen Z, Wang Y, Zou J, Li S, Guo X, et al. Largemouth Bass (Micropterus salmoides) Exhibited Better Growth Potential after Adaptation to Dietary Cottonseed Protein Concentrate Inclusion but Experienced Higher Inflammatory Risk during Bacterial Infection. Front Immunol (2022) 13:997985. doi: 10.3389/fimmu.2022.997985
65. Chadzinska M, Savelkoul HFJ, Kemenade BMLV. Morphine affects the inflammatory response in carp by impairment of leukocyte migration. Dev Comp Immunol (2009) 33(1):88–96. doi: 10.1016/j.dci.2008.07.004
66. Wang C, Yuan Z, Li J, Liu Y, Li R, Li S. Acute effects of antimony exposure on adult zebrafish (Danio rerio): from an oxidative stress and intestinal microbiota perspective. Fish Shellfish Immunol (2022) 123:1–9. doi: 10.1016/j.fsi.2022.02.050
67. Tang Y, Yang S, Yao M, Yang M, Wei L, Chen H, et al. Hemoglobin induces inflammation through NF-kB signaling pathway and causes cell oxidative damage in grass carp (Ctenopharyngodon idella). Front Immunol (2022) 13:1044299. doi: 10.3389/fimmu.2022.1044299
68. Weir A, Vince JE. No longer married to inflammasome signaling: the diverse interacting pathways leading to pyroptotic cell death. Biochem J (2022) 479(10):1083–102. doi: 10.1042/BCJ20210711
69. Li MF, Zhang HQ. An overview of complement systems in teleosts. Dev Comp Immunol (2022) 137):104520. doi: 10.1016/j.dci.2022.104520
70. Boshra H, Li J, Sunyer JO. Recent advances on the complement system of teleost fish. Fish shellfish Immunol (2006) 20(2):239–62. doi: 10.1016/j.fsi.2005.04.004
71. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol (2010) 11(9):785–97. doi: 10.1038/ni.1923
72. Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol (2010) 31(4):154–63. doi: 10.1016/j.it.2010.01.002
73. Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol (2004) 41(11):1089–98. doi: 10.1016/j.molimm.2004.06.011
74. Sunyer JO, Zarkadis IK, Sahu A, Lambris JD. Multiple forms of complement C3 in trout that differ in binding to complement activators. Proc Natl Acad Sci (1996) 93(16):8546–51. doi: 10.1073/pnas.93.16.8546
75. Wang T, Secombes CJ. Complete sequencing and expression of three complement components, C1r, C4 and C1 inhibitor, of the classical activation pathway of the complement system in rainbow trout Oncorhynchus mykiss. Immunogenetics (2003) 55:615–28. doi: 10.1007/s00251-003-0622-5
76. Papanastasiou AD, Zarkadis IK. Gene duplication of the seventh component of complement in rainbow trout. Immunogenetics (2005) 57:703–8. doi: 10.1007/s00251-005-0028-7
77. Tam VK, Tsujikura M, Somamoto T, Nakao M. Identification of cDNA sequences encoding the complement components of Zebrafish (Danio rerio). Journal of the Faculty of Agriculture, Kyushu University (2009) 54(2):373–87. doi: 10.5109/16119
78. Bayne CJ, Gerwick L, Fujiki K, Nakao M, Yano T. Immune-relevant (including acute phase) genes identified in the livers of rainbow trout, Oncorhynchus mykiss, by means of suppression subtractive hybridization. Dev Comp Immunol (2001) 25(3):205–17. doi: 10.1016/S0145-305X(00)00057-4
79. Sun G, Li H, Wang Y, Zhang B, Zhang S. Zebrafish complement factor H and its related genes: identification, evolution, and expression. Funct Integr Genomics (2010) 10:577–87. doi: 10.1007/s10142-010-0182-3
80. Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, et al. Interaction between the coagulation and complement system. Adv Exp Med Biol (2008) 632:71–9. doi: 10.1007/978-0-387-78952-1_6
81. Andreeva AM, Bazarova ZM, Toropygin IY, Vasiliev AS, Fedorov RA, Pavlova PA, et al. Serum osmotically active proteins in the atlantic cod Gadus morhua. J Evol Biochem Phys (2023) 59:325–36. doi: 10.1134/S0022093023020023
82. Wong MKS, Takei Y. Lack of plasma kallikrein-kinin system cascade in teleosts. PloS One (2013) 8(11):e81057. doi: 10.1371/journal.pone.0081057
83. Tsutsui S, Yoshimura A, Iwakuma Y, Nakamura O. Discovery of Teleost Plasma Kallikrein/Coagulation Factor XI-Like Gene from Channel Catfish (Ictalurus punctatus) and the Evidence that the Protein Encoded by it Acts as a Lectin. J Mol Evol (2023) 91:536–51. doi: 10.1007/s00239-023-10113-4
84. Li XY, Zheng SX, Cheng KM, Ma XK, Wu G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part II: effects of supplementation with methionine or taurine on growth, feed utilization, and health. Amino Acids (2021) 53:49–62. doi: 10.1007/s00726-020-02922-4
85. Filho DW, Torres MA, Marcon JL, Fraga C, Boveris A. Comparative antioxidant defences in vertebrates-emphasis on fish and mammals. Trends Comp Biochem Physiol (2000) 7:33–45.
86. Baier-Anderson C, Anderson RS. Suppression of superoxide production by chlorothalonil in striped bass (Morone saxatilus) macrophages: the role of cellular sulfhydryls and oxidative stress. Aquat Toxicol (2000) 50(1–2):85–96. doi: 10.1016/S0166-445X(99)00092-2
87. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J (1973) 134(3):707–16. doi: 10.1042/bj1340707
88. Thrasher AJ, Keep NH, Wientjes F, Segal AW. Chronic granulomatous disease. BBA - Biochim Biophys Acta (1994) 9:1–24. doi: 10.1016/0925-4439(94)90100-7
89. Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, et al. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radical Res (2009) 43(6):523–32. doi: 10.1080/10715760902918683
90. Abarike ED, Jia C, Jian J, Tang J, Lu Y, Yu H. Characterization and expression of NADPH oxidase genes in stimulated splenic neutrophils of tilapia, Oreochromis niloticus by a mix of traditional chinese medicine and bacillus species (TCMBS). Aquaculture Res (2019) 50(1):93–105. doi: 10.1111/are.13871
91. Yoo SK, Freisinger CM, LeBert DC, Huttenlocher A. Early redox, src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J Cell Biol (2012) 199(2):225–34. doi: 10.1083/jcb.201203154
92. Dahlgren C, Karlsson A, Bylund J. Intracellular neutrophil oxidants: from laboratory curiosity to clinical reality. J Immunol (2019) 202(11):3127–34. doi: 10.4049/jimmunol.1900235
93. Oliveira S, Boudinot P, Calado Â, Mulero V. Duox1-derived H2O2 modulates Cxcl8 expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J Immunol (2015) 194(4):1523–33. doi: 10.4049/jimmunol.1402386
94. Schvezov N, Wilson RW, Urbina MA. Oxidative damages and antioxidant defences after feeding a single meal in rainbow trout. J Comp Physiol B: Biochemical Systemic Environ Physiol (2022) 192(3–4):459–71. doi: 10.1007/s00360-022-01435-8
95. Eliason EJ, Higgs DA, Farrell AP. Postprandial gastrointestinal blood flow, oxygen consumption and heart rate in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol - A Mol Integr Physiol (2008) 149(4):380–88. doi: 10.1016/j.cbpa.2008.01.033
96. Hou Y, Wang L, Yi D, Wu G. N-acetylcysteine and intestinal health : A focus on mechanisms of its actions. Front Bioscience Landmark (2015) 20(10):872–91. doi: 10.2741/4342
97. Li X, Zheng S, Wu G. Amino acid metabolism in the liver: nutritional and physiological significance. Adv Exp Med Biol (2020) 1265:71–95. doi: 10.1007/978-3-030-45328-2_5
99. Morshedi V, Rainis S, Hamedi S, Ghasemi A, Bagheri D, Gamoori R. Effects of dietary taurine amino acid on growth performance, mucosal and immune response, gene expression and antioxidant defence of Asian seabass (Lates calcarifer). Mol Biol Rep (2022) 49(5):3503–10. doi: 10.1007/s11033-022-07187-6
100. Pascual P, Pedrajas JR, Toribio F, López-Barea J, Peinado J. Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata). Chemico-Biological Interact (2003) 145(2):191–99. doi: 10.1016/S0009-2797(03)00002-4
101. Li XF, Xu C, Tian HY, Jiang GZ, Zhang DD, Liu WB. Feeding rates affect stress and non-specific immune responses of juvenile blunt snout bream Megalobrama amblycephala subjected to hypoxia. Fish Shellfish Immunol (2016) 49:298–305. doi: 10.1016/j.fsi.2016.01.004
102. Yang S, He K, Yan T, Wu H, Zhou J, Zhao L, et al. Effect of starvation and refeeding on oxidative stress and antioxidant defenses in yangtze sturgeon (Acipenser dabryanus). Fish Physiol Biochem (2019) 45(3):987–95. doi: 10.1007/s10695-019-0609-2
103. Djordjevic B, Morales-Lange B, Press CM, Olson J, Lagos L, Mercado L, et al. Comparison of circulating markers and mucosal immune parameters from skin and distal intestine of Atlantic salmon in two models of acute stress. Int J Mol Sci (2021) 22(3):1–13. doi: 10.3390/ijms22031028
104. Zou J, Secombes CJ. The function of fish cytokines. Biology (2016) 5(2):23. doi: 10.3390/biology502002
105. Lazado CC, Voldvik V. Temporal control of responses to chemically induced oxidative stress in the gill mucosa of Atlantic salmon (Salmo salar). J Photochem Photobiol B: Biol (2020) 205:111851. doi: 10.1016/j.jphotobiol.2020.111851
106. Harper C, Wolf JC. Morphologic effects of the stress response in fish. ILAR J (2009) 50(4):387–96. doi: 10.1093/ilar.50.4.387
107. Sundh H, Finne-Fridell F, Ellis T, Taranger GL, Niklasson L, Pettersen EF, et al. Reduced water quality associated with higher stocking density disturbs the intestinal barrier functions of Atlantic salmon (Salmo salar L.). Aquaculture (2019) 512:734356. doi: 10.1016/j.aquaculture.2019.734356
108. Criscitiello MF, Dickman MB, Samuel JE, de Figueiredo P. Tripping on acid: trans-kingdom perspectives on biological acids in immunity and pathogenesis. PloS Pathog (2013) 9(7):e1003402. doi: 10.1371/journal.ppat.1003402
109. Roos A, Boron WF. Intracellular pH. Physiol Rev (1981) 61(2):296–434. doi: 10.1152/physrev.1981.61.2.296
110. Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Biochim Biophys Acta - Proteins Proteomics (2012) 1824(1):3–13. doi: 10.1016/j.bbapap.2011.03.007
111. Hirata T, Nakamura N, Omote H, Wada Y, Futai M. Regulation and reversibility of vacuolar H+-ATPase. J Biol Chem (2000) 275(1):386–89. doi: 10.1074/jbc.275.1.386
112. Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol (2007) 8(11):917–29. doi: 10.1038/nrm2272
113. Bearson S, Bearson B, Foster. JW. Acid stress responses in enterobacteria. FEMS Microbiol Lett (1997) 147(2):173–80. doi: 10.1016/S0378-1097(96)00503-4
114. Mashoof S, Goodroe A, Du CC, Eubanks JO, Jacobs N, Steiner JM, et al. Ancient T-independence of mucosal IgX/A: gut microbiota unaffected by larval thymectomy in Xenopus laevis. Mucosal Immunol (2013) 6(2):358–68. doi: 10.1038/mi.2012.78
115. Kakade A, Salama ES, Usman M, Arif M, Feng P, Li X. Dietary Application of Lactococcus lactis Alleviates Toxicity and Regulates Gut Microbiota in Cyprinus carpio on Exposure to Heavy Metals Mixture. Fish Shellfish Immunol (2022) 120:190–201. doi: 10.1016/j.fsi.2021.11.038
116. Fenta MM. Heavy metals concentration in effluents of textile industry, tikur wuha river and milk of cows watering on this water source, Hawassa, Southern Ethiopia. Res J Environ Sci (2014) 8(8):422–34. doi: 10.3923/rjes.2014.422.434
117. Prokić MD, Radovanović TB, Gavrić JP, Faggio C. Ecotoxicological effects of microplastics: examination of biomarkers, current state and future perspectives. TrAC - Trends Analytical Chem (2019) 111:37–46. doi: 10.1016/j.trac.2018.12.001
118. Liu Y, Wang J, Wei Y, Zhang H, Xu M, Dai J. Induction of time-dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebrafish liver. Aquat Toxicol (2008) 89(4):242–50. doi: 10.1016/j.aquatox.2008.07.009
119. Busch CJ, Binder CJ. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim Biophys Acta - Mol Cell Biol Lipids (2017) 1862(4):398–406. doi: 10.1016/j.bbalip.2016.06.016
120. Mittler R. ROS are good. Trends Plant Sci (2017) 22(1):11–9. doi: 10.1016/j.tplants.2016.08.002
121. Chen L, Kaneko G, Li Y, Xie J, Wang G, Li Z, et al. Reactive oxygen species (ROS)-mediated regulation of muscle texture in grass carp fed with dietary oxidants. Aquaculture (2021) 544:737150. doi: 10.1016/j.aquaculture.2021.737150
122. Steinel NC, Bolnick DI. Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front Immunol (2017) 8:827. doi: 10.3389/fimmu.2017.00827
123. Liao Z, Lin D, Jia J, Cai R, Yu Y, Li W. Innate immune response to fasting and refeeding in the zebrafish kidney. Biomolecules (2021) 11:(6). doi: 10.3390/biom11060825
124. Agius C, Roberts RJ. Melano-macrophage centres and their role in fish pathology. J Fish Dis (2003) 26:499–509. doi: 10.1046/j.1365-2761.2003.00485.x
125. Li K, Wei X, Jiao X, Deng W, Li J, Liang W, et al. Glutamine metabolism underlies the functional similarity of T cells between nile tilapia and tetrapod. Advanced Sci (2023) 10(12):2201164. doi: 10.1002/advs.202201164
126. Neely HR, Guo J, Flowers EM, Criscitiello MF, Flajnik MF. Double-duty conventional dendritic cells in the amphibian Xenopus as the prototype for antigen presentation to B cells. Eur J Immunol (2018) 48:430–40. doi: 10.1002/eji.201747260
127. Loh Z, Huan X, Awate S, Schrittwieser M, Renia L, Ren EC. Molecular characterization of MHC class I alpha 1 and 2 domains in Asian seabass (Lates calcarifer). Int J Mol Sci (2022) 23:(18). doi: 10.3390/ijms231810688
128. Clark TC, Tinsley J, Sigholt T, Macqueen DJ, Martin SAM. Arginine, ornithine and citrulline supplementation in rainbow trout: free amino acid dynamics and gene expression responses to bacterial infection. Fish Shellfish Immunol (2020) 98:374–90. doi: 10.1016/j.fsi.2020.01.026
129. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol (2011) 11(11):723–37. doi: 10.1038/nri3073
130. Bengtén E, Clem LW, Miller NW, Warr GW, Wilson M. Channel catfish immunoglobulins: repertoire and expression. Dev Comp Immunol (2006) 30(1–2):77–92. doi: 10.1016/j.dci.2005.06.016
131. Mashoof S, Pohlenz C, Chen PL, Deiss TC, Gatlin D, Buentello A, et al. Expressed IgH μ and τ Transcripts share diversity segment in ranched thunnus orientalis. Dev Comp Immunol (2014) 43(1):76–86. doi: 10.1016/j.dci.2013.10.015
132. Pohlenz C, Buentello A, Bakke AM, Gatlin DM III. Free dietary glutamine improves intestinal morphology and increases enterocyte migration rates, but has limited effects on plasma amino acid profile and growth performance of channel catfish Ictalurus punctatus. Aquaculture (2012a) 370:32–9. doi: 10.1016/j.aquaculture.2012.10.002
133. Dornburg A, Ota T, Criscitiello MF, Salinas I, Sunyer JO, Magadán S, et al. From IgZ to IgT: A call for a common nomenclature for immunoglobulin heavy chain genes of ray-finned fish. Zebrafish (2021) 18(6):343–5. doi: 10.1089/zeb.2021.0071
134. Salinas I, Fernández-Montero Á, Ding Y, Sunyer JO. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev Comp Immunol (2021) 121:104079. doi: 10.1016/j.dci.2021.104079
135. Silvestre MPC. Review of methods for the analysis of protein hydrolysates. Food Chem (1997) 60(2):263–71. doi: 10.1016/S0308-8146(96)00347-0
136. Thannhauser TW, Sherwood RW, Scheraga HA. Determination of the cysteine and cystine content of proteins by amino acid analysis: application to the characterization of disulfide-coupled folding intermediates. J Protein Chem (1998) 17(1):37–43. doi: 10.1023/A:1022586413862
137. Tsao M, Otter DE. Quantification of glutamine in proteins and peptides using enzymatic hydrolysis and reverse-phase high-performance liquid chromatography. Analytical Biochem (1999) 269(1):143–48. doi: 10.1006/abio.1998.3091
138. Jia S, Li X, He W, Wu G. Protein-sourced feedstuffs for aquatic animals in nutrition research and aquaculture. In: Advances in experimental medicine and biology, vol. 1354. Spring, Cham: Springer International Publishing (2022). doi: 10.1007/978-3-030-85686-1_12
139. National Research Council. Nutrient requirements of fish and shrimp. Washington, DC: The National Academies Press (2011). doi: 10.17226/13039
140. Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. Regulatory role for L-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids (2012) 43(1):233–44. doi: 10.1007/s00726-011-1067-z
141. Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, et al. Dietary requirements of ‘Nutritionally non-essential amino acids’ by animals and humans. Amino Acids (2013) 44(4):1107–13. doi: 10.1007/s00726-012-1444-2
142. Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. L-glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids (2013) 45(3):501–12. doi: 10.1007/s00726-012-1264-4
144. Abdelghany MF, El-Sawy HB, Abd El-hameed SAA, Khames MK, Abdel-Latif HMR, Naiel. MAE. Effects of Dietary Nannochloropsis Oculata on Growth Performance, Serum Biochemical Parameters, Immune Responses, and Resistance against Aeromonas Veronii Challenge in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol (2020) 107(PA):277–88. doi: 10.1016/j.fsi.2020.10.015
145. Naiel MAE, Ismael NEM, Negm SS, Ayyat MS, Al-Sagheer AA. Rosemary Leaf Powder–Supplemented Diet Enhances Performance, Antioxidant Properties, Immune Status, and Resistance against Bacterial Diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture (2020) 526:735370. doi: 10.1016/j.aquaculture.2020.735370
146. Hou Y, Wang L, Yi D, Ding B, Yang Z, Li J, et al. N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids (2013) 45(3):513–22. doi: 10.1007/s00726-012-1295-x
147. Li XY, Zheng SX, Ma XK, Cheng KM, Wu G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part I: effects of poultry by-product meal and soybean meal on growth, feed utilization, and health. Amino Acids (2021) 53:33–47. doi: 10.1007/s00726-020-02920-6
148. Latshaw JD, Bishop BL. Estimating body weight and body composition of chickens by using noninvasive measurements. Poultry Sci (2001) 80(7):868–73. doi: 10.1093/ps/80.7.868
149. Lobley GE, Milne V, Lovie JM, Reeds PJ, Pennie K. Whole body and tissue protein synthesis in cattle. Br J Nutr (1980) 43(3):491–502. doi: 10.1079/bjn19800116
150. Francis G, Makkar HPS, Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture (2001) 199:197–227. doi: 10.1016/S0044-8486(01)00526-9
151. Kuz’mina VV, Ushakova NV. The effect of temperature, PH, and heavy metals (Copper, zinc) upon proteinase activities in the potential preys for typical and facultative piscivores. J Ichthyology (2007) 47(9):791–801. doi: 10.1134/S0032945207090123
152. Pervin MA, Jahan H, Akter R, Omri A, Hossain Z. Appraisal of different levels of soybean meal in diets on growth, digestive enzyme activity, antioxidation, and gut histology of tilapia (Oreochromis niloticus). Fish Physiol Biochem (2020) 46(4):1397–407. doi: 10.1007/s10695-020-00798-5
153. Roychaudhuri R, Sarath G, Zeece M, Markwell J. Stability of the allergenic soybean kunitz trypsin inhibitor. Biochim Biophys Acta (BBA) - Proteins Proteomics (2004) 1699(1–2):207–12. doi: 10.1016/j.bbapap.2004.02.014
154. Lin S, Luo L. Effects of Different Levels of Soybean Meal Inclusion in Replacement for Fish Meal on Growth, Digestive Enzymes and Transaminase Activities in Practical Diets for Juvenile Tilapia, Oreochromis niloticus×O. aureus. Anim Feed Sci Technol (2011) 168(1–2):80–7. doi: 10.1016/j.anifeedsci.2011.03.012
155. Robaina L, Izquierdo MS, Moyano FJ, Socorro J, Vergara JM, Montero D, et al. Soybean and lupin seed meals as protein sources in diets for Gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture (1995) 130(2–3):219–33. doi: 10.1016/0044-8486(94)00225-D
156. Dalsgaard J, Verlhac V, Hjermitslev NH, Ekmann KS, Fischer M, Klausen M, et al. Effects of exogenous enzymes on apparent nutrient digestibility in rainbow trout (Oncorhynchus mykiss) fed diets with high inclusion of plant-based protein. Anim Feed Sci Technol (2012) 171(2–4):181–91. doi: 10.1016/j.anifeedsci.2011.10.005
157. Song Z, Li H, Wang J, Li P, Sun Y, Zhang L. Effects of fishmeal replacement with soy protein hydrolysates on growth performance, blood biochemistry, gastrointestinal digestion and muscle composition of juvenile starry flounder (Platichthys stellatus). Aquaculture (2014) 426–427:96–104. doi: 10.1016/j.aquaculture.2014.01.002
159. Mortimore GE, Pösö AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr (1987) 7:539–64. doi: 10.1146/annurev.nutr.7.1.539
160. Zhao Y, Li JY, Yin L, Feng L, Liu Y, Jiang WD, et al. Effects of dietary glutamate supplementation on flesh quality, antioxidant defense and gene expression related to lipid metabolism and myogenic regulation in jian carp (Cyprinus carpio var. Jian). Aquaculture (2019) 502:212–22. doi: 10.1016/j.aquaculture.2018.12.050
161. Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr (1993) 123(2):269–74. doi: 10.1093/jn/123.2.269
162. Shqueir AA, Brown DL, Klasing KC. Canavanine content and toxicity of sesbania leaf meal for growing chicks. Anim Feed Sci Technol (1989) 25(1-2):137–47. doi: 10.1016/0377-8401(89)90114-4
163. Hou Y, He W, Hu S, Wu G. Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids (2019) 51(8):1153–65. doi: 10.1007/s00726-019-02751-0
164. Li P, Wu G. Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids (2020) 52(4):523–42. doi: 10.1007/s00726-020-02833-4
165. Smedley MA, Migaud H, McStay EL, Clarkson M, Bozzolla P, Campbell P, et al. Impact of dietary phosphorous in diploid and triploid atlantic salmon (Salmo salar L.) with reference to early skeletal development in freshwater. Aquaculture (2018) 490:329–43. doi: 10.1016/j.aquaculture.2018.02.049
166. Li P, Wu. G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids (2018) 50(1):29–38. doi: 10.1007/s00726-017-2490-6
167. Andersen SM, Waagbø R, Espe M. Functional amino acids in fish health and welfare. Funct Amino Acids fish Nutr (2016) 1:143–69. doi: 10.2741/757
168. Buentello JA, Gatlin DM III. Nitric oxide production in activated macrophages from channel catfish (Ictalurus punctatus): influence of dietary arginine and culture media. Aquaculture (1999) 179(1-4):513–21. doi: 10.1016/S0044-8486(99)00184-2
169. Costas B, Aragão C, Dias J, Afonso A, Conceição LE. Interactive effects of a high-quality protein diet and high stocking density on the stress response and some innate immune parameters of Senegalese sole Solea Senegalensis. Fish Physiol Biochem (2013) 39:1141–51. doi: 10.1007/s10695-013-9770-1
170. Fauzi IA, Haga Y, Satoh S, Kondo H, Hirono I. Effects of arginine supplementation on growth performance and plasma arginine, ornithine and citrulline dynamics of rainbow trout, Oncorhynchus mykiss. Aquaculture Res (2019) 50(4):1277–90. doi: 10.1111/are.14004
171. Mommsen TP, Moon TW, Plisetskaya EM. Effects of arginine on pancreatic hormones and hepatic metabolism in rainbow trout. Physiol Biochem Zoology (2001) 74(5):668–78. doi: 10.1086/322924
172. Li S, Zhang Y, Liu N, Chen J, Guo L, Dai Z, et al. Dietary L-arginine supplementation reduces lipid accretion by regulating fatty acid metabolism in Nile tilapia (Oreochromis niloticus). J Anim Sci Biotechnol (2020) 11:82. doi: 10.1186/s40104-020-00486-7
173. Azeredo R, MaChado M, Fontinha F, Fernández-Boo S, Conceição LE, Dias J, et al. Dietary arginine and citrulline supplementation modulates the immune condition and inflammatory response of European seabass. Fish Shellfish Immunol (2020) 106:451–63. doi: 10.1016/j.fsi.2020.07.060
174. Riley WW Jr., Higgs DA, Dosanjh BS, Eales JG. Influence of dietary arginine and glycine content on thyroid function and growth of juvenile rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture Nutr (1996) 2(4):235–42. doi: 10.1111/j.1365-2095.1996.tb00065.x
175. Cheng Z, Gatlin DM, Buentello A. Dietary supplementation of arginine and/or glutamine influences growth performance, immune responses and intestinal morphology of hybrid striped bass (Morone chrysops×Morone saxatilis). Aquaculture (2012) 362–363:39–43. doi: 10.1016/j.aquaculture.2012.07.015
176. Li P, Gatlin DM III. Evaluation of dietary supplementation of graded levels of β-Hydroxy-β-Methylbutyrate for hybrid striped bass (Morone chrysops× M. saxatilis). J Appl Aquaculture (2007) 19(3):77–88. doi: 10.1300/J028v19n03_05
177. Snyder GS, Gaylord TG, Barrows FT, Hardy RW. Effects of carnosine supplementation to an all-plant protein diet for rainbow trout Oncorhynchus mykiss. Aquaculture (2012) 338–341:72–81. doi: 10.1016/j.aquaculture.2011.12.042
178. Burns AF, Gatlin DM III. Effects of dietary creatine on juvenile hybrid striped bass in low-salinity and brackish waters. J World Aquaculture Soc (2022) 53(1):122–32. doi: 10.1111/jwas.12843
179. Stites W, Wang L, Gatlin DM III. Evaluation of dietary creatine and guanidinoacetic acid supplementation in juvenile red drum Sciaenops ocellatus. Aquaculture Nutr (2020) 26(2):382–9. doi: 10.1111/anu.13000
180. Macêdo ÉSD, Franco TSG, Natali MRM, Panaczevicz PAP, Rudnik AR, MIranda JAG, et al. Dietary glutamine-glutamate supplementation enhances growth performance and intestinal villi development in cage-farmed Nile tilapia fingerlings. Rev Bras Zootecnia (2021) 50:e20200010. doi: 10.37496/rbz5020200010
181. Coutinho F, Castro C, Rufino-Palomares E, Ordóñez-Grande B, Gallardo MA, Oliva-Teles A, et al. Dietary glutamine supplementation effects on amino acid metabolism, intestinal nutrient absorption capacity and antioxidant response of gilthead sea bream (Sparus aurata) juveniles. Comp Biochem Physiol Part A: Mol Integr Physiol (2016) 191:9–17. doi: 10.1016/j.cbpa.2015.09.012
182. Hu K, Zhang JX, Feng L, Jiang WD, Wu P, Liu Y, et al. Effect of dietary glutamine on growth performance, non-specific immunity, expression of cytokine genes, phosphorylation of target of rapamycin (TOR), and anti-oxidative system in spleen and head kidney of Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem (2015) 41:635–49. doi: 10.1007/s10695-015-0034-0
183. Jiao C, Zou J, Chen Z, Zheng F, Xu Z, Lin YH, et al. Dietary glutamine inclusion regulates immune and antioxidant system, as well as programmed cell death in fish to protect against Flavobacterium columnare infection. Antioxidants (2021) 11(1):44. doi: 10.3390/antiox11010044
184. Abbasi M, Mirghaed AT, Hoseini SM, Rajabiesterabadi H, Hoseinifar SH, Doan HV. Effects of dietary glycine supplementation on growth performance, immunological, and erythrocyte antioxidant parameters in common carp, Cyprinus carpio. Animals (2023) 13(3):412. doi: 10.3390/ani13030412
185. Xie S, Zhou W, Tian L, Niu J, Liu Y. Effect of N-acetyl cysteine and glycine supplementation on growth performance, glutathione synthesis, anti-oxidative and immune ability of nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol (2016) 55:233–41. doi: 10.1016/j.fsi.2016.05.033
186. Peachey BL, Scott EM, Gatlin DM. Dietary histidine requirement and physiological effects of dietary histidine deficiency in juvenile red drum Sciaenop ocellatus. Aquaculture (2018) 483:244–51. doi: 10.1016/j.aquaculture.2017.10.032
187. Aksnes A, Mundheim H, Toppe J, Albrektsen S. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture (2008) 275(1-4):242–9. doi: 10.1016/j.aquaculture.2007.12.031
188. Zhao J, Feng L, Liu Y, Jiang W, Wu P, Jiang J, et al. Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol (2014) 41(2):663–73. doi: 10.1016/j.fsi.2014.10.002
189. Zhou Z, Wu X, Li X, Dong Y, Wang X, Mu W, et al. The optimum dietary isoleucine requirement of juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Aquaculture Nutr (2020) 26(4):1295–310. doi: 10.1111/anu.13085
190. Cheng ZJ, Hardy RW, Usry JL. Plant protein ingredients with lysine supplementation reduce dietary protein level in rainbow trout (Oncorhynchus mykiss) diets, and reduce ammonia nitrogen and soluble phosphorus excretion. Aquaculture (2003) 218(1-4):553–65. doi: 10.1016/S0044-8486(02)00502-1
191. Huang X, Song X, Wang X, Zhou H, Liu C, Mai K, et al. Dietary lysine level affects digestive enzyme, amino acid transport and hepatic intermediary metabolism in turbot (Scophthalmus maximus). Fish Physiol Biochem (2022) 48(4):1091–103. doi: 10.1007/s10695-022-01098-w
192. Gao Z, Wang X, Tan C, Zhou H, Mai K, He G. Effect of dietary methionine levels on growth performance, amino acid metabolism and intestinal homeostasis in turbot (Scophthalmus maximus L.). Aquaculture (2019) 498:335–42. doi: 10.1016/j.aquaculture.2018.08.053
193. Kokou F, Sarropoulou E, Cotou E, Kentouri M, Alexis M, Rigos G. Effects of graded dietary levels of soy protein concentrate supplemented with methionine and phosphate on the immune and antioxidant responses of gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol (2017) 64:111–21. doi: 10.1016/j.fsi.2017.03.017
194. Aragão C, Teodósio R, Colen R, Richard N, Rønnestad I, Luís JD, et al. Taurine supplementation to plant-based diets improves lipid metabolism in Senegalese sole. Animals (2023) 13(9):1501. doi: 10.3390/ani13091501
195. Hano T, Ito K, Kono K, Ito M, Ohkubo N, Mochida K. Effect of taurine supplementation on hepatic metabolism and alleviation of cadmium toxicity and bioaccumulation in a marine teleost, red sea bream, Pagrus major. Fish Physiol Biochem (2017) 43(1):137–52. doi: 10.1007/s10695-016-0274-7
196. Kim SK, Takeuchi T, Yokoyama M, Murata Y. Effect of dietary supplementation with taurine, β-alanine and GABA on the growth of juvenile and fingerling Japanese flounder Paralichthys olivaceus. Fisheries Sci (2003) 69(2):242–8. doi: 10.1046/j.1444-2906.2003.00614.x
197. Matsunari H, Takeuchi T, Takahashi M, Mushiake K. Effect of dietary taurine supplementation on growth performance of yellowtail juveniles Seriola quinqueradiata. Fisheries Sci (2005) 71:1131–5. doi: 10.1111/j.1444-2906.2005.01072.x
198. Kumar P, Saurabh S, Pal AK, Sahu NP, Arasu ART. Stress mitigating and growth enhancing effect of dietary tryptophan in rohu (Labeo rohita, Hamilton, 1822) fingerlings. Fish Physiol Biochem (2014) 40:1325–38. doi: 10.1007/s10695-014-9927-6
199. Basic D, Schjolden J, Krogdahl Å, von Krogh K, Hillestad M, Winberg S, et al. Changes in regional brain monoaminergic activity and temporary down-regulation in stress response from dietary supplementation with L-tryptophan in Atlantic cod (Gadus morhua). Br J Nutr (2013) 109(12):2166–74. doi: 10.1017/S0007114512004345
200. Lepage O, Vílchez IM, Pottinger TG, Winberg S. Time-course of the effect of dietary L-tryptophan on plasma cortisol levels in rainbow trout Oncorhynchus mykiss. J Exp Biol (2003) 206(20):3589–99. doi: 10.1242/jeb.00614
201. Albrecht J, Norenberg MD. Glutamine: A trojan horse in ammonia neurotoxicity. Hepatology (2006) 44(4):788–94. doi: 10.1002/hep.21357
202. Li X, Zheng S, Wu G. Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids (2020) 52(5):671–91. doi: 10.1007/s00726-020-02851-2
203. Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and MTORC1 kinase activation. Immunity (2014) 40(5):692–705. doi: 10.1016/j.immuni.2014.04.007
204. Yang L, Venneti S, Nagrath D. Glutaminolysis: A hallmark of cancer metabolism. Annu Rev Biomed Eng (2017) 19:163–94. doi: 10.1146/annurev-bioeng-071516-044546
205. Lian G, Gnanaprakasam JNR, Wang T, Wu R, Chen X, Liu L, et al. Glutathione de Novo Synthesis but Not Recycling Process Coordinates with Glutamine Catabolism to Control Redox Homeostasis and Directs Murine T Cell Differentiation. ELife (2018) 7:1–28. doi: 10.7554/eLife.36158
206. Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity (2016) 44(5):973–88. doi: 10.1016/j.immuni.2016.04.020
207. Wei X, Ai K, Li H, Zhang Y, Li K, Yang J. Ancestral T cells in fish require mTORC1-coupled immune signals and metabolic programming for proper activation and function. J Immunol (2019) 203(5):1172–88. doi: 10.4049/jimmunol.1900008
208. Wang Z, Liu F, Fan N, Zhou C, Li D, Macvicar T, et al. Targeting glutaminolysis: new perspectives to understand cancer development and novel strategies for potential target therapies. Front Oncol (2020) 10:589508. doi: 10.3389/fonc.2020.589508
209. Kivisäkk P, Khoury SJ. Co-signaling molecules in neurological diseases. Adv Exp Med Biol (2019) 1189:233–65. doi: 10.1007/978-981-32-9717-3_9
210. Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell (2006) 124(4):815–22. doi: 10.1016/j.cell.2006.02.001
211. Hsu E. The invention of lymphocytes. Curr Opin Immunol (2011) 23(2):156–62. doi: 10.1016/j.coi.2010.12.007
212. Flajnik MF. A cold-blooded view of adaptive immunity. Nat Rev Immunol (2018) 18(7):438–53. doi: 10.1038/s41577-018-0003-9
213. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol (2020) 38:541–66. doi: 10.1146/annurev-immunol-042718-041717
214. Li Q, Verma IM. NF-KB regulation in the immune system. Nat Rev Immunol (2002) 2(10):725–34. doi: 10.1038/nri910
215. Abreu MT. Toll-like receptor signaling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol (2010) 10(2):131–43. doi: 10.1038/nri2707
216. Trinchieri G, Sher A. Cooperation of toll-like receptor signals in innate immune defence. Nat Rev Immunol (2007) 7(3):179–90. doi: 10.1038/nri2038
217. Sakai J, Cammarota E, Wright JA, Cicuta P, Gottschalk RA, Li N, et al. Lipopolysaccharide-induced NF-KB nuclear translocation is primarily dependent on MyD88, but TNFα Expression requires TRIF and MyD88. Sci Rep (2017) 7(1):1–9. doi: 10.1038/s41598-017-01600-y
218. Ai F, Wang L, Li J, Xu Q. Effects of A-ketoglutarate (AKG) supplementation in low phosphorous diets on the growth, phosphorus metabolism and skeletal development of juvenile mirror carp (Cyprinus carpio). Aquaculture (2019) 507:393–401. doi: 10.1016/j.aquaculture.2019.03.047
219. He L, Zhou X, Huang N, Li H, Cui Z, Tian J, et al. Administration of alpha-ketoglutarate improves epithelial restitution under stress injury in early-weaning piglets. Oncotarget (2017) 8(54):91965–78. doi: 10.18632/oncotarget.20555
220. Jiang Q, Liu G, Wang X, Hou Y, Duan Y, Wu G, et al. Mitochondrial pathway is involved in the protective effects of alpha-ketoglutarate on hydrogen peroxide induced damage to intestinal cells. Oncotarget (2017) 8(43):74820–35. doi: 10.18632/oncotarget.20426
221. Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke J, et al. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci (2004) 117(10):2097–107. doi: 10.1242/jcs.01071
222. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon- γ and tumor necrosis factor-α Synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol (2005) 166(2):409–19. doi: 10.1016/S0002-9440(10)62264-X
223. Li S, Wang C, Wu Z. Dietary L-arginine supplementation of tilapia (Oreochromis niloticus) alters the microbial population and activates intestinal fatty acid oxidation. Amino Acids (2022) 54(3):339–51. doi: 10.1007/s00726-021-03018-3
224. Li P, Mai K, Trushenski J, Wu G. New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids (2009) 37(1):43–53. doi: 10.1007/s00726-008-0171-1
225. Korte JJ, Salo WL, Cabrera VM, Wright PA, Felskie AK, Anderson PM. Expression of Carbamoyl-Phosphate Synthetase III mRNA during the Early Stages of Development and in Muscle of Adult Rainbow Trout (Oncorhynchus Mykiss). J Biol Chem (1997) 272(10):6270–77. doi: 10.1074/jbc.272.10.6270
226. Ortiz-Severín J, Tandberg JI, Winther-Larsen HC, Chávez FP, Cambiazo V. Comparative analysis of salmon cell lines and zebrafish primary cell cultures infection with the fish pathogen Piscirickettsia salmonis. Microorganisms (2021) 9:(12). doi: 10.3390/microorganisms9122516
227. Hoseini SM, Vatnikov YA, Kulikov EV, Petrov AK, Hoseinifar SH, Doan HV. Effects of dietary arginine supplementation on ureagenesis and amino acid metabolism in common carp (Cyprinus carpio) exposed to ambient ammonia. Aquaculture (2019a) 511:734209. doi: 10.1016/j.aquaculture.2019.734209
228. Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids (2009) 37):153–68. doi: 10.1007/s00726-008-0210-y
229. Hoseini SM, Yousefi M, Hoseinifar SH, Doan HV. Effects of dietary arginine supplementation on growth, biochemical, and immunological responses of common carp (Cyprinus carpio L.), stressed by stocking density. Aquaculture (2019) 503:452–59. doi: 10.1016/j.aquaculture.2019.01.031
230. Chen G, Liu Y, Jiang J, Jiang W, Kuang. S, Tang L, et al. Effect of dietary arginine on the immune response and gene expression in head kidney and spleen following infection of jian carp with Aeromonas hydrophila. Fish Shellfish Immunol (2015) 44(1):195–202. doi: 10.1016/j.fsi.2015.02.027
231. Boczkowski J, Philip I, Tedgui A, Bernard C, Merva R, Desmonts J, et al. Effects of inhibition of nitric oxide synthesis on TNFα Serum levels in E.coli. Life Sci (1995) 57:(13). doi: 10.1016/0024-3205(95)02077-V
232. Werry EL, Liu GJ, Lovelace MD, Nagarajah R, Hickie IB, Bennett MR. Lipopolysaccharide-stimulated interleukin-10 release from neonatal spinal cord microglia is potentiated by glutamate. Neuroscience (2011) 175:93–103. doi: 10.1016/j.neuroscience.2010.10.080
233. Costas B, Conceição LEC, Dias J, Novoa B, Figueras A, Afonso A. Dietary arginine and repeated handling increase disease resistance and modulate innate immune mechanisms of Senegalese sole (Solea Senegalensis kaup 1858). Fish Shellfish Immunol (2011) 31(6):838–47. doi: 10.1016/j.fsi.2011.07.024
234. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Publishing Group (2008) 8(12):958–69. doi: 10.1038/nri2448
235. Balaña-Fouce R, Calvo-álvarez E, Álvarez-velilla R, Prada CF, Pérez-pertejo Y, Reguera RM. Role of trypanosomatid’s arginase in polyamine biosynthesis and pathogenesis. Mol Biochem Parasitol (2012) 181:85–93. doi: 10.1016/j.molbiopara.2011.10.007
236. Badurdeen S, Mulongo M, Berkley JA. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatric Res (2015) 77(2):290–7. doi: 10.1038/pr.2014.177
237. Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. PNAS (2017) 114(5):751–60. doi: 10.1073/pnas.1614958114
238. Qualls JE, Subramanian C, Rafi W, Smith AM, Balouzian L, Defreitas AA, et al. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe (2012) 12(3):313–23. doi: 10.1016/j.chom.2012.07.012
239. Messina L, Arcidiacono A, Spampinato G, MalaGuamera L, Berton G, Kaczmarek L, et al. Accumulation of ornithine decarboxylase MRNA accompanies activation of human and mouse monocytes/macrophages. FEBS (1990) 268(1):32–4. doi: 10.1016/0014-5793(90)80965-L
240. Hussain T, Tan B, Ren W, Rahu N, Dad R, Kalhoro DH, et al. Polyamines: therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids (2017) 49(9):1457–68. doi: 10.1007/s00726-017-2447-9
241. Moinard C, Cynober L, de Bandt JP. Polyamines: metabolism and implications in human diseases. Clin Nutr (2005) 24(2):184–97. doi: 10.1016/j.clnu.2004.11.001
242. Shah N, Thomas T, Shirahata A, Sigal LH, Thomas TJ. Activation of nuclear factor Kb by polyamines in breast cancer cells. Biochemistry (1999) 38(45):14763–74. doi: 10.1021/bi991291v
243. Li L, Rao JN, Bass BL, Wang JY. NF-KB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am J Physiol - Gastrointestinal Liver Physiol (2001) 280(5):992–1004. doi: 10.1152/ajpgi.2001.280.5.g992
244. Ren W, Chen S, Yin J, Duan J, Li T, Liu G, et al. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr (2014) 144:988–95. doi: 10.3945/jn.114.192120
245. Spaeth E, Windmueller HG. Metabolism of absorbed small intestine in viva and arginine by rat. Arch Biochem Biophysics (1976) 175:670–76. doi: 10.1016/0003-9861(76)90558-0
246. Wu G. Intestinal mucosal amino acid catabolism. J Nutr (1998) 128(8):1249–52. doi: 10.1093/jn/128.8.1249
247. Li H, Limenitakis JP, Greiff V, Yilmaz B, Schären O, Urbaniak C, et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature (2020) 584:274–8. doi: 10.1038/s41586-020-2564-6
248. Jia S, Li X, He W, Wu G. Oxidation of energy substrates in tissues of fish: metabolic significance and implications for gene expression and carcinogenesis. In: Wu G eds. Advances in experimental medicine and biology. Cham: Springer (2021). p. 67–83. doi: 10.1007/978-3-030-74180-8_5
249. Wang X, Hinson ER, Cresswell P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. A Cell Press J (2012) 2(2):96–105. doi: 10.1016/j.chom.2007.06.009
250. Nunes AJP, Sá MVC, Browdy CL, Vazquez-anon M. Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture (2014) 431:20–7. doi: 10.1016/j.aquaculture.2014.04.003
Keywords: teleost, immunology, amino acid, antioxidant, reactive oxygen species
Citation: Hissen KL, He W, Wu G and Criscitiello MF (2023) Immunonutrition: facilitating mucosal immune response in teleost intestine with amino acids through oxidant-antioxidant balance. Front. Immunol. 14:1241615. doi: 10.3389/fimmu.2023.1241615
Received: 19 June 2023; Accepted: 24 August 2023;
Published: 29 September 2023.
Edited by:
Jorge Galindo-Villegas, Nord University, NorwayReviewed by:
Susana Magadan, University of Vigo, SpainRoberta Marcoli, James Cook University, Australia
Copyright © 2023 Hissen, He, Wu and Criscitiello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael F. Criscitiello, bWNyaXNjaXRpZWxsb0Bjdm0udGFtdS5lZHU=; Guoyao Wu, Zy13dUB0YW11LmVkdQ==
 Karina L. Hissen
Karina L. Hissen Wenliang He2
Wenliang He2 Michael F. Criscitiello
Michael F. Criscitiello