
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 08 November 2023
Sec. Autoimmune and Autoinflammatory Disorders: Autoinflammatory Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1240811
Background: There is increasing evidence pointing to a close relationship between sarcopenia and inflammatory bowel disease. However, it remains unclear whether or in which direction causal relationships exist, because these associations could be confounded.
Methods: We conducted a two-sample bidirectional mendelian randomization analysis using data from European genome-wide association studies of the appendicular lean mass(n = 450,243), walking pace(n = 459,915), grip strength (left hand, n = 461,026; right hand, n = 461,089), inflammatory bowel disease (25,042 patients and 34,915 controls), ulcerative colitis (12,366 patients and 33,609 controls), and Crohn’s disease (12,194 patients and 28,072 controls) to investigate the causal relationship between sarcopenia-related traits and inflammatory bowel disease and its subtypes on each other. The inverse-variance weighted method was used as the primary analysis method to assess the causality, and a comprehensive sensitivity test was conducted.
Results: Genetically predicted appendicular lean mass was significantly associated with inflammatory bowel disease (OR = 0.916, 95%CI: 0.853–0.984, P = 0.017), ulcerative colitis (OR =0.888, 95%CI: 0.813–0.971, P = 0.009), and Crohn’s disease (OR = 0.905, 95%CI: 0.820–0.999, P = 0.049). Similar results also revealed that the usual walking pace was causally associated with Crohn’s disease (OR = 0.467, 95%CI: 0.239–0.914, P = 0.026). Reverse mendelian randomization analysis results found that genetic susceptibility to inflammatory bowel disease, and Crohn’s disease were associated with lower appendicular lean mass. A series of sensitivity analyses ensured the reliability of the present research results.
Conclusion: The mendelian randomization study supports a bidirectional causality between inflammatory bowel disease, Crohn’s disease and appendicular lean mass, but no such bidirectional causal relationship was found in ulcerative colitis. In addition, genetically predicted usual walking pace may reduce the risk of Crohn’s disease. These findings have clinical implications for sarcopenia and inflammatory bowel disease management.
Sarcopenia is a gradual and systemic skeletal muscle disease characterized by a loss of skeletal muscle mass or function that happens regularly with aging and is associated with an increased risk of poor outcomes such as falls, disability, impaired function, frailty, hospitalization, and mortality (1–4). Sarcopenia was classified as a disease by the World Health Organization in 2016 and given an International Classification of Diseases-10 code (5). Sarcopenia can be primary (age-related) or accompany a variety of chronic illnesses in middle age appendiceal skeletal muscle mass, such as inflammation, malnutrition, neoplastic disease, or organ failure (1, 6, 7). According to estimates, sarcopenia affects 10% to 16% of aged people worldwide (8). Along with older persons, those who are underweight, women, and those who have other chronic ailments are more susceptible to developing sarcopenia and its detrimental effects on their health (9). Sarcopenia incidence is rising quickly as the world’s population ages (10, 11), and by 2050, 500 million people are expected to have the condition (12).
Inflammatory bowel disease (IBD) is an idiopathic inflammatory illness of the gastrointestinal tract that comprises Crohn’s disease (CD) and ulcerative colitis (UC) (13). Over the last decade, the prevalence of IBD has increased worldwide (14). Muscle loss is a typical pathophysiological aspect of many chronic gastrointestinal illnesses, including IBD (15). When compared to healthy individuals, patients with IBD have a lower lean body mass composition and reduced muscle mass by up to 60% (14, 16). Even at a young age, patients with IBD might develop sarcopenia (17). Sarcopenia may exist even in IBD patients in remission (18). When IBD patients are divided into those who are UC or CD, those who are CD have a higher frequency of sarcopenia than those who are UC (52% vs. 37%, respectively) (17). Sarcopenia affects up to 50% of IBD patients and is related to adverse clinical outcomes (19), such as longer hospitalization, intestinal resection, and an increased risk of post-operative complications (15, 20, 21).
Several previous studies have shown that patients with sarcopenia have a higher risk of developing IBD and vice versa (8, 15, 17, 18, 22). However, Pedersen et al. found no difference in the incidence of sarcopenia between people with IBD and those without IBD (23). The relationship between sarcopenia and IBD is unclear and controversial, and their genetically predicted causal effect remains unexplored. Here, we aim to evaluate the causality between Sarcopenia-related traits and IBD and its subtypes (UC and CD) using a two-sample Mendelian randomization (MR) design. MR is a genetic epidemiological research method that greatly improves the ability to investigate the causal relationship between traits and diseases because it addresses some of the common drawbacks of traditional epidemiological studies (such as reverse causation) and ensures that results are unaffected by confounding factors (24, 25). This study was conducted in both directions (i.e. bidirectional MR), as this is also an advantageous strategy to orient the direction of causal effects between two variables (26).
Using a two-sample MR analytic approach, which is an extension of MR where the effects of genetic instruments on exposure and outcome are extracted from two different datasets, we evaluated the causality between sarcopenia-related characteristics and IBD and its subtypes (CD and UC) (27).The three core MR assumptions for assuming causal relationship are as follows (1): the genetic variants have a strong connection to the exposure (2); there don’t exist unmeasured confounders of the associations between outcome and genetic variants; and (3) the genetic variants effect the outcome just via the exposure (28).
Due to the fact that the current study is a secondary analysis of data that is available to the public, no separate ethical approval was necessary. The GWAS summary statistics are accessible at https://gwas.mrcieu.ac.uk/. The details of GWAS used to get the summary statistics for each trait are listed in Supplementary Table 1. The flowchart of the bidirectional MR design is shown in Figure 1A.
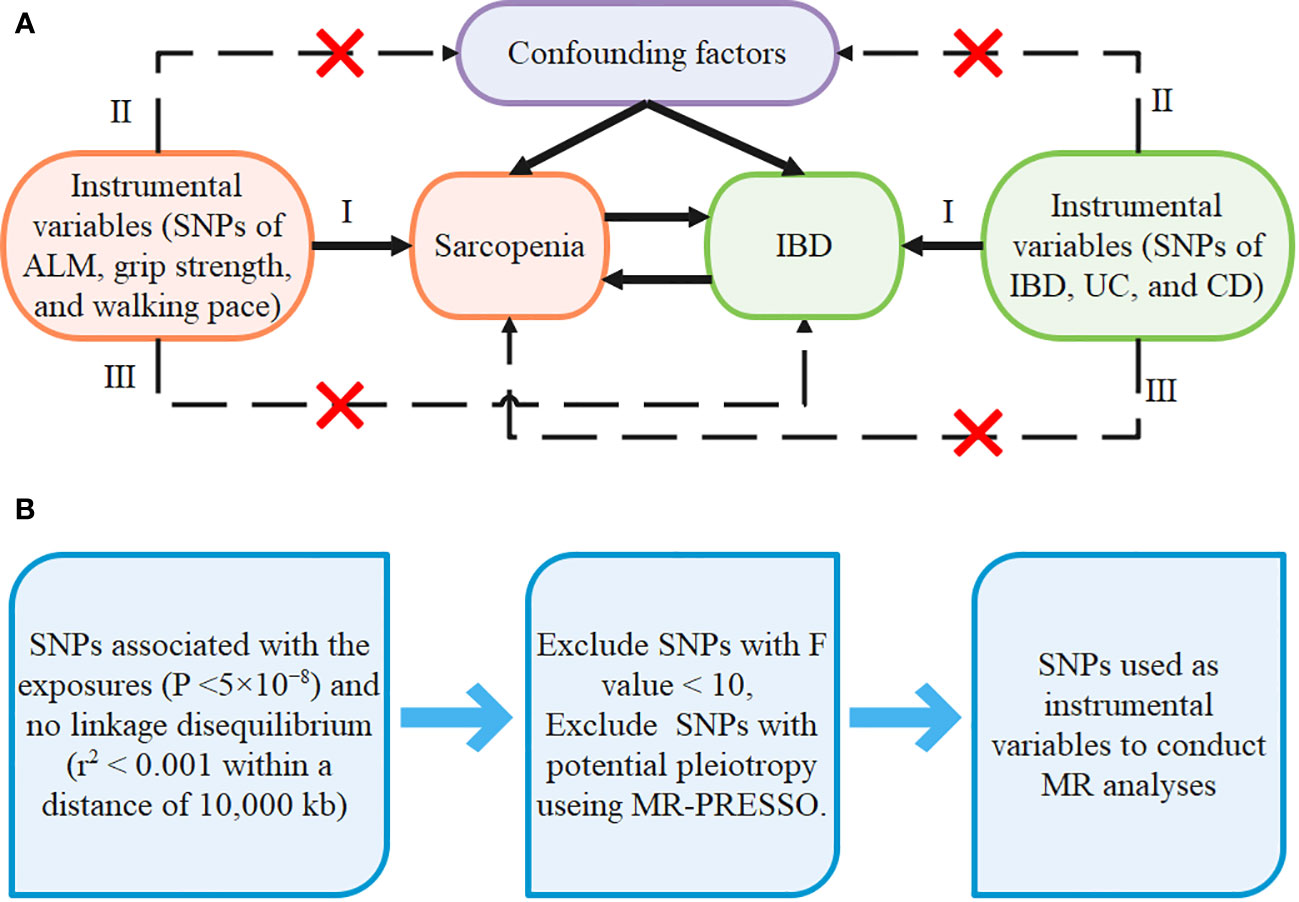
Figure 1 The design of our MR framework (A) Flowchart of the bidirectional Mendelian randomization study. (B) Flow diagram of SNPs screening.
We collected three functional parameters as sarcopenia-related traits, including appendicular lean mass (ALM), usual walking pace, and handgrip strength (left & right), since they are reliable predictors of sarcopenia (7).
As the most regularly used approximate index of muscle mass in sarcopenia research, ALM is regarded a reliable measure of muscle mass and is frequently used in the Asian Working Group for Sarcopenia (AWGS) and European Working Group on Sarcopenia in Older People (EWGSOP) diagnostic criterion for sarcopenia (29–31). The GWAS data of ALM was acquired from a meta-analysis of 450,243 UK Biobank participants (32), and ALM was measured using bioelectrical impedance analysis (33).
Since low physical performance is one of the characteristics of sarcopenia, gait speed measurement is also an essential diagnostic criteria for sarcopenia (34). In clinical practice, the walking pace is frequently employed as a quick, secure, and highly reliable test for sarcopenia (7). The summary statistics used as genetic predictors of walking pace were obtained from the UK Biobank, which comprises 459,915 individuals (35).
Grip strength is a widely used proxy of muscle fitness that can serve as a trustworthy alternative for overall muscle strength (7, 36, 37). Because the correlation between absolute handgrip strength and muscle strength may be higher than that of relative grip strength (38), we used absolute handgrip strength as a proxy for muscle strength. The UK Biobank provided the GWAS summary statistics for handgrip strength (left hand, n = 461,026; right hand, n = 461,089) (35). Handgrip strength was measured by adjusting hand dimensions with a calibrated hydraulic hand dynamometer (39).
The IEU Open GWAS database was used to acquire summary-level data for IBD and its subtypes (CD and UC). In the IBD data, there were 25,042 patients and 34,915 controls, and in the UC and CD data, there were 12,366 and 12,194 patients, respectively (40). The IBD data have included UC and CD subsets, the detailed table for the summary of UC, CD and IBD original data samples can be seen in the Supplementary Table 2, which was downloaded from the original literature (40). These diagnoses are based on radiographic, endoscopic, and histological criteria that are widely accepted (41). All study participants were of European ancestry to avoid pleiotropic deviation of cross-lineage cases (42).
For each MR analysis, we selected all single nucleotide polymorphisms (SNPs) associated with the exposure as instrumental variables. First, we selected SNPs at a genome-wide significance threshold (p<5×10-8) and there is no linkage disequilibrium at the selected SNPs (r2< 0.001 within a distance of 10,000 kb) (42, 43). Second, SNPs related to confounders and outcomes were excluded. We calculated the F-statistic to ensure the intensity of exposure, where a value greater than 10 is considered sufficiently robust to offset weak instrument bias (44), therefore the SNPs with F-statistic less than 10 were removed. The formula for calculating the SNPs’ R2 and F-statistics are as follows (45). Third, outliers were detected using MR-PRESSO models, and if any were found, they were deleted and re-analyzed using the remaining SNPs. Details of the SNPs used as instrumental variables in each MR analysis are available in Supplementary Tables 3, 4. The flow diagram of SNPs screening is shown in Figure 1B.
We selected inverse-variance weighted (IVW) MR with multiplicative random-effects as the main analysis method because it provides the most efficient combination of variant specificity ratio estimates and takes into account the heterogeneity of causal estimates obtained from individual variants (42, 45, 46). The results of IVW were visualized using forest plots. We also used four other additional MR analytical methods, including weighted median, MR-Egger, weighted mode, and simple mode, which allowed horizontal pleiotropy with a lower statistical capability than IVW.
In addition, a series of additional sensitivity analysis methods were performed to ensure the robustness of the results. We assessed the presence of heterogeneity amongst the variant-specific causal estimates using Cochran’s Q statistic and generated funnel plots (47, 48). p<0.05 values indicate significant heterogeneity and the funnel plot is expected to be symmetrical (49). MR-egger regression was used to test the pleiotropy and leave-one-out analysis was used to see if there was any bias due to individual SNP influencing the findings independently (50). Additionally, the MR-PRESSO method was used to detect outliers and identify horizontal pleiotropy, and if abnormal SNPs were detected, they were removed to eliminate pleiotropy effects (51).
All statistical analyses and results visualization were performed in R software (Version 4.2.3, https://www.r-project.org/) with the help of “two-sample MR” (v.0.5.1), “MR-PRESSO” (v.1.0) and “Forest plot” packages.
In the forward MR analysis, the results of estimating the causal effects of sarcopenia-related traits on IBD and its subtypes using the IVW method were shown in Figure 2. Scatter plots were provided in Figure 3. Genetically predicted ALM was significantly associated with IBD (OR = 0.916, 95%CI: 0.853–0.984, P = 0.017), UC (OR =0.888, 95%CI: 0.813–0.971, P = 0.009), and CD (OR = 0.905, 95%CI: 0.820–0.999, P = 0.049). We also found that the usual walking pace was causally associated with CD (OR = 0.467, 95%CI: 0.239–0.914, P = 0.026). There was no evidence to support the association between handgrip strength (right & left) with IBD and its subtypes (UC and CD). We discovered evidence of heterogeneity using Cochran’s Q, indicating that the IVW with random effects approach was acceptable (47). The MR-Egger intercept test did not provide any indications of directional pleiotropy (Table 1), and the funnel plot did not reveal any signs of symmetry deviation either [Supplementary File 1(A-L)]. In leave-one-out sensitivity analyses, the overall IVW estimates did not change on the exclusion of any variant [Supplementary File2(A-L)]. Overall, the higher appendicular lean mass is a protective factor for IBD and its subtypes, and a genetically elevated usual walking pace may reduce the risk of CD.
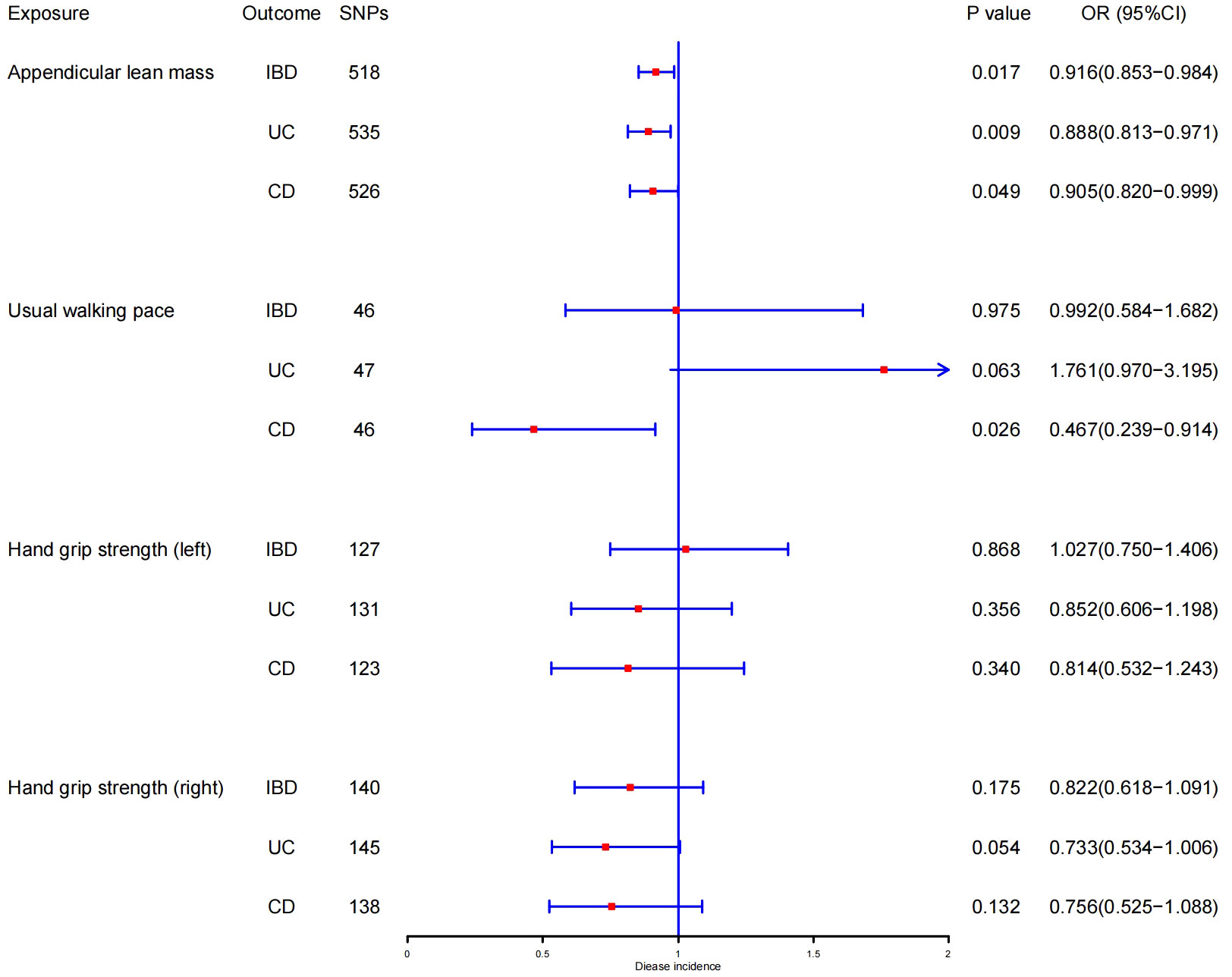
Figure 2 MR estimates using the IVW method of ALM, hand grip strength (right & left), and usual walking pace on IBD, UC, and CD. SNPs, single-nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
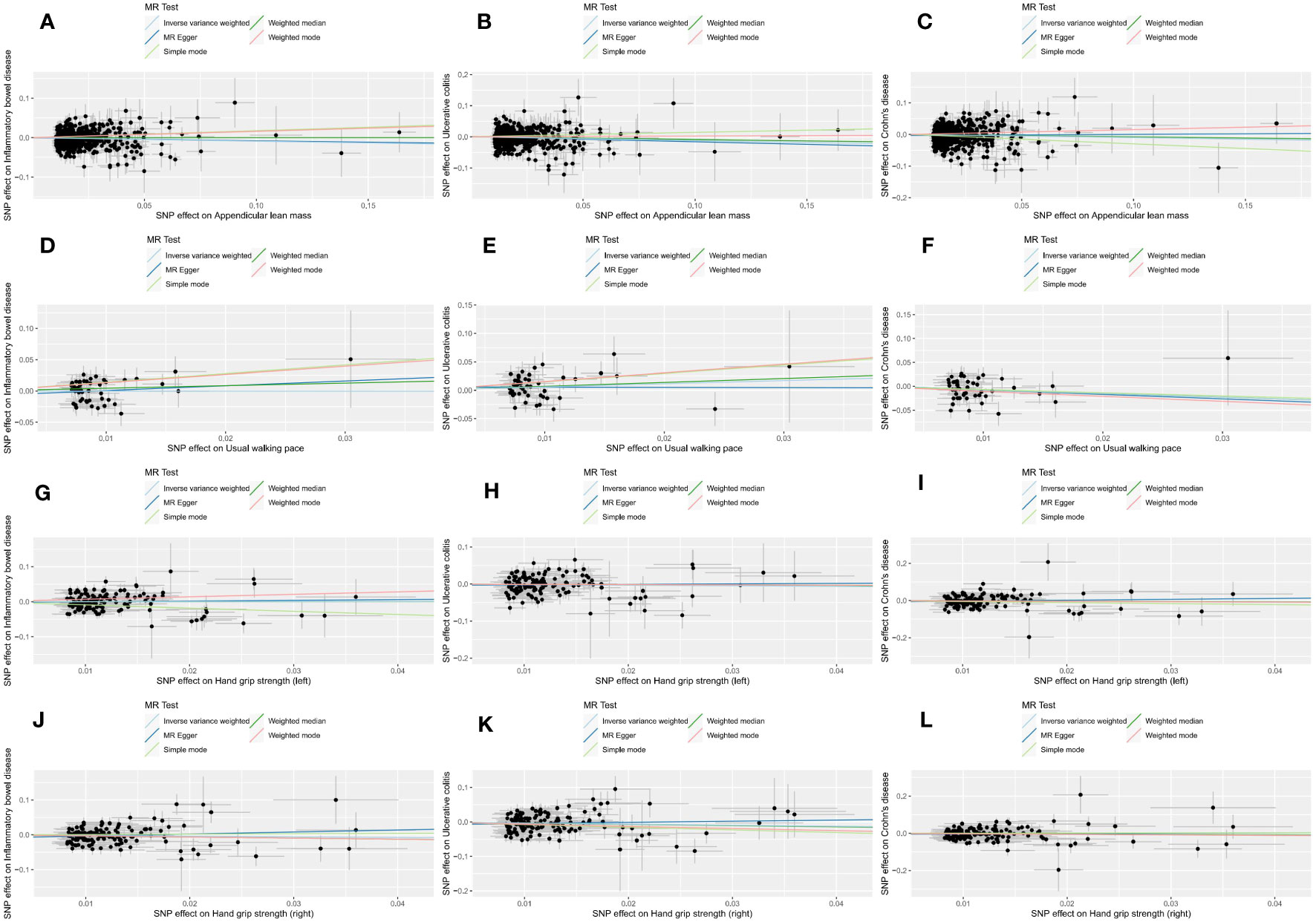
Figure 3 Scatter plots of forward MR analysis. The slope of each line represents the expected MR impact in various models. (A) ALM on IBD; (B) ALM on UC; (C) ALM on CD; (D) usual walking pace on IBD; (E) usual walking pace on UC; (F) usual walking pace on CD;(G) hand grip strength (left) on IBD; (H) hand grip strength (left) on UC; (I) hand grip strength (left) on CD; (J) hand grip strength (right) on IBD; (K) hand grip strength (right) on UC; (L) hand grip strength (right) on CD.
The outcomes of reverse MR analysis were displayed in Figure 4. The scatter plots were provided in Figure 5. Genetically predicted IBD and CD were significantly related with lower ALM, (OR = 0.988, 95%CI: 0.981–0.995, P = 0.001) and (OR = 0.991, 95%CI:0.986–0.997, P = 0.002), respectively. However, there was no correlation between UC and ALM (p > 0.05). The IVW results also indicated that IBD, UC, and CD had no causal effect on the usual walking pace and handgrip strength (left & right). Sensitivity analysis revealed no significant horizontal pleiotropy (Table 2). The funnel plot and leave-one-out sensitivity analysis can be found in the Supplementary Files 1(M-X), 2(M-X). In other words, UC will not increase the risk of ALM, and there is a positive correlation between genetic vulnerability to IBD, CD, and the risk of ALM.
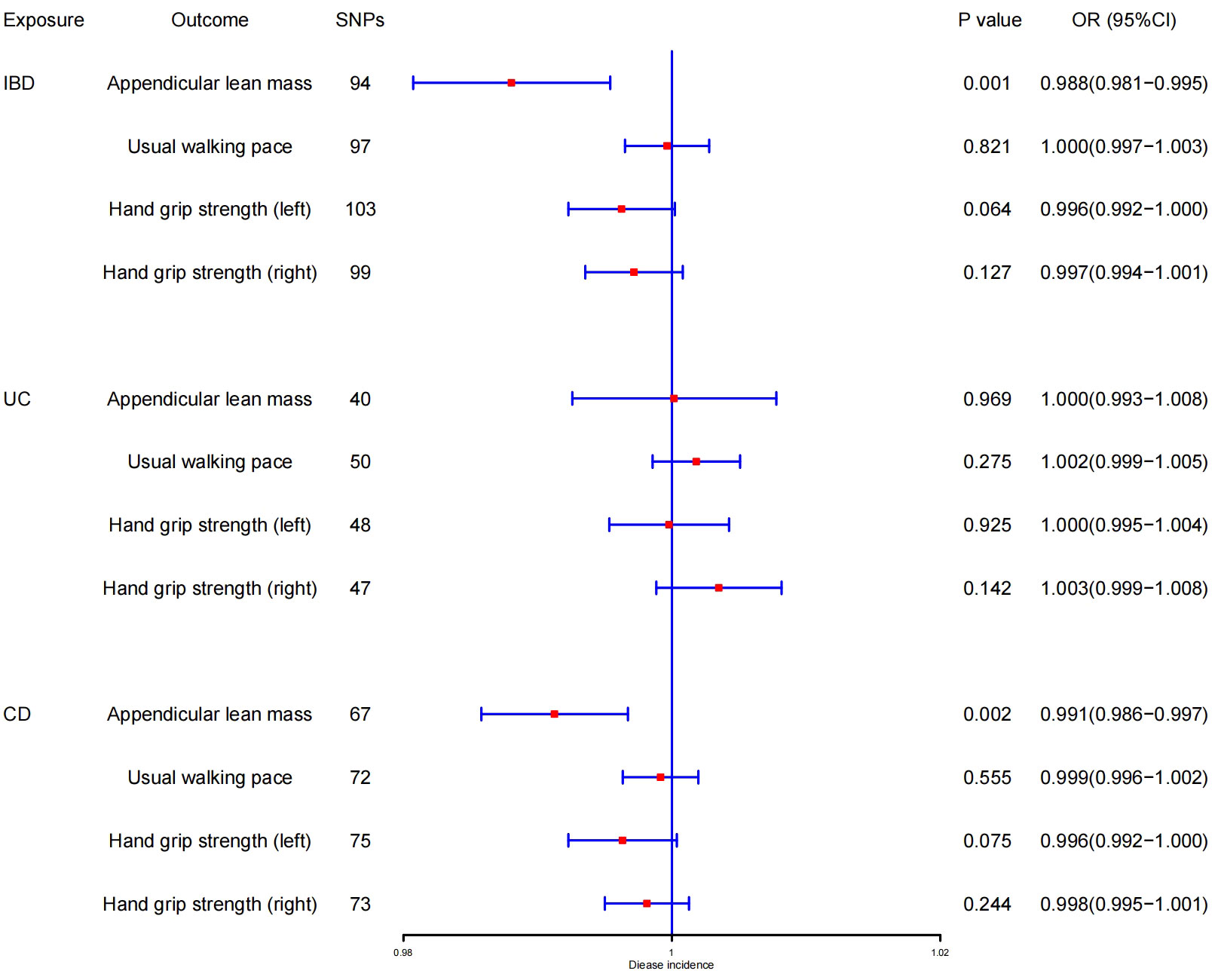
Figure 4 MR estimates using the IVW method of IBD, UC, and CD on ALM, hand grip strength (right & left), and usual walking pace. SNPs, single-nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
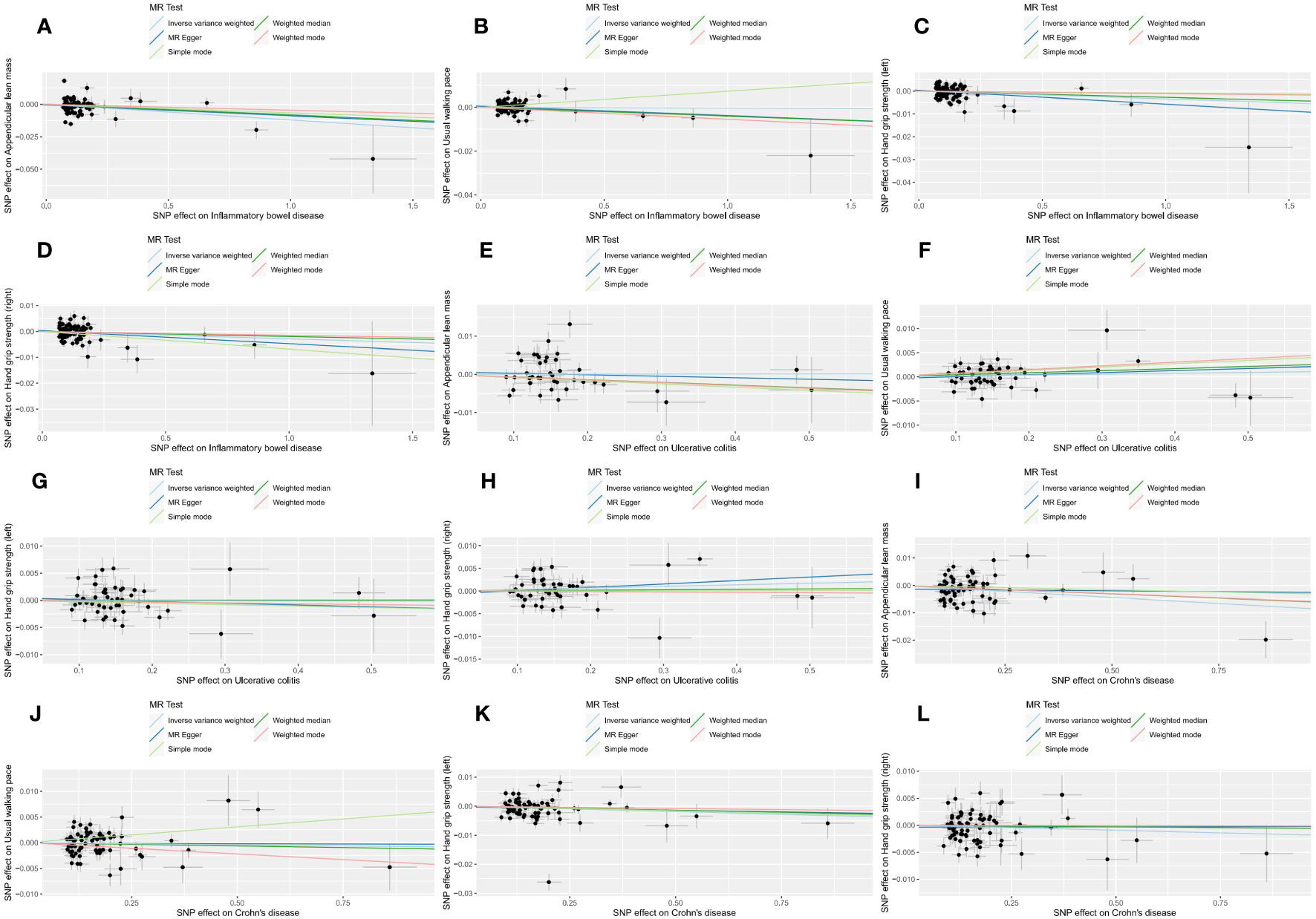
Figure 5 Scatter plots of reverse MR analysis. The slope of each line represents the expected MR impact in various models. (A) IBD on ALM; (B) IBD on usual walking pace; (C) IBD on hand grip strength (left); (D) IBD on hand grip strength (left); (E) UC on ALM; (F) UC on usual walking pace;(G) UC on hand grip strength (left); (H) UC on hand grip strength (right); (I) CD on ALM; (J) CD on usual walking pace; (K) CD on hand grip strength (left); (L) CD on hand grip strength (right).
In conclusion, we investigated the causality between sarcopenia and IBD on each other using large-scale GWAS summary data. MR analyses indicated a potential causal relationship between ALM and IBD and revealed that more ALM is a protective factor for IBD, UC, and CD. Furthermore, we found evidence of a potential causal role for the usual walking pace in CD. In the inverse MR analysis, genetically predicted IBD and CD were significantly associated with a lower ALM. No evidence supported the causal relationship between handgrip strength with IBD and its subtypes (UC and CD).
Previous research has demonstrated that a variety of factors, including chronic inflammation, malnutrition, vitamin deficiency, and an imbalance in the muscle-gut axis, might contribute to sarcopenia (52). IBD patients are at risk for weight loss, malnutrition, and long-term glucocorticoid usage, all of which have an adverse effect on muscular strength and mass. The premature and “accelerated” sarcopenia development has been found in other populations affected by factors such as malnutrition, chronic inflammation, and immobility, which are associated with IBD (36). Another study also found that individuals with IBD had fewer skeletal muscle and bone masses, with more visceral and “creeping” fat (53). Saul et al. discovered that experimental IBD mice exhibited lower skeletal muscle weight and fiber size, as well as lower muscle protein content in the gastrocnemius and quadriceps (54). According to numerous investigations, sarcopenia correlates with the severity of IBD and assists in determining treatment decisions for patients with IBD (55–57). In a recent meta-analysis, 52% of CD patients and 37% of UC patients experienced sarcopenia (17). These findings are significant because sarcopenia is known to have a considerable influence on duration of hospital stay, surgical outcomes, life quality, and mortality (22, 41, 58).
In reality, sarcopenia may be a practical and accessible risk assessment tool for people with IBD, such as the necessity for surgical intervention and post-operative complications (6). Currently, there is no curative treatment for CD, whereas UC can be cured by a pan proctocolectomy (59). It can have even more negative effects when sarcopenia and IBD coexist, previous research revealed that patients with CD who were sarcopenic had a higher risk of adverse events and intestinal resection than those who weren’t sarcopenic (15, 60). In a study of 72 IBD patients, sarcopenia significantly increased the risk of intestinal resection (23). The chance of postoperative complications in IBD was shown to be more than six times higher in people with sarcopenia, according to a meta-analysis (61). As a result, sarcopenia is a crucial therapeutic and prognostic consideration in the management of IBD.
Although the exact cause of sarcopenia in IBD patients is still unknown, there is evidence that it may be brought on by drug interactions, malabsorption, poor protein intake, chronic inflammation, and reduced physical activity (62). Recent research has also demonstrated that the insulin-like growth factor 1, phosphoinositide 3 kinase, protein kinase B, and mammalian target of rapamycin (IGF1/PI3K/Akt/mTOR) axis dysregulation is associated with a decrease in IGF1-R in muscle tissues from IBD patients (63). Sarcopenia is frequently associated with malnutrition as a result of chronic persistent inflammation (64). IBD patients usually have poor nutritional status, and it is known that malnutrition is a significant factor in the loss of muscle mass and the ensuing loss of function (58). Although the direct causality between sarcopenia and inflammation has not been established, there is evidence of an association within the context of IBD (65). A study of 441 adults over the age of 60 found that sarcopenic older people had significantly higher levels of tumor necrosis factor (TNF) – α and circulating interleukin (IL)-6 (66).
Sarcopenia involves muscle loss and dysfunction, causing systolic dysfunction, endocrine and metabolic abnormalities, and affecting metabolic and immune/inflammatory responses throughout the body (67). In our MR Results, genetically predicted ALM was strongly associated with IBD and its subtypes. ALM is primarily affected by skeletal muscle mass, which also has endocrine functions that affect inflammatory response and systemic metabolism (67, 68). Low muscle mass is common in IBD patients (69, 70). Studies have shown that levels of pro-inflammatory factors, including CRP, IL-6, and TNF-α, are inversely correlated with ALM (71, 72). Sarcopenia is associated with factors such as progressive increase in fibrosis, chronic inflammatory state, altered muscle metabolism, and degeneration of the neuromuscular junction (73). From a mechanistic perspective, chronic inflammation is most likely associated with sarcopenia leading to IBD. In addition, fatigue and reduced quality of life associated with muscle loss can also contribute to the progression of IBD (74, 75). Studies have also revealed that individuals with IBD had lower grip strength values, however these studies only analyzed the differences in grip strength values between individuals with IBD and controls, not the prevalence of low grip strength (76, 77). In our study, no relationship between IBD and grip strength was found. This may be because IBD is not linked to low grip strength but rather grip strength reduction. However, since recognition of sarcopenia is relatively recent, the mechanism by which sarcopenia leads to IBD remains to be further investigated.
Despite the high prevalence and clinical significance of sarcopenia in IBD patients, few patients receive routine muscle assessment. Furthermore, due to the screening tools are not accurate, most cases of sarcopenia go undetected (78). Given the aging population, an increase in sarcopenia management healthcare costs is to be expected (79). Currently, physical activity is advised as the primary treatment for sarcopenia, and numerous studies have also demonstrated the effectiveness and safety of physical exercise among patients with IBD (7, 80, 81). Additionally, nutritional intervention is also an essential component in treating IBD patients, which may reduce the inflammatory state and the risk of sarcopenia (82, 83).It has also been shown that infliximab therapy can reverse IBD-associated sarcopenia in individuals with active Crohn’s disease, with 6 months of treatment leading to significant improvements in skeletal muscle volume and maximum isokinetic strength (84). Muscle health should be evaluated regularly in all IBD patients, and effective management of sarcopenia in IBD will assist improve prognosis.
This is the first two-sample MR study to investigate the causal association between sarcopenia-related traits and IBD. The main strength of this study is the MR analysis design, which reduces some of the limitations in observational studies, such as confounding and reverse causality. Additionally, sensitivity studies were carried out to assess the robustness of this study’s findings and to assure the consistency of the causal estimations, while several MR analysis techniques were used to verify the accuracy and validity of the results. Notwithstanding the strengths of our study, there are several limitations. First, we employed ALM rather than appendiceal skeletal muscle mass to measure muscle mass because bias in other non-fat soft tissue components such the lung and kidney could affect the results. Second, we were unable to determine the causal link between genetically predicted sarcopenia and IBD in different genders and age groups due to the paucity of data stratified by sex and age. Given that the onset factors of sarcopenia and inflammatory bowel disease are related to age and gender (85–87). Third, the generalizability of our conclusions to other ethnicities may be limited because the results of our study were based on people of European ancestry. Fourth, even if confounding factors were removed in this study, it is difficult to completely remove mediation and pleiotropy, and a larger scale GWAS are needed to verify the results.
Our MR Results support a bidirectional causal relationship between IBD, CD, and ALM, but such a bidirectional causal relationship was not found in UC. Furthermore, the genetically predicted usual walking pace was causally associated with CD. Our novel insights may provide a better reference for the identification, evaluation, and management of sarcopenia in IBD patients. In order to enhance clinical prognosis, we emphasized that IBD patients should receive proper nutrition and strengthen exercise to combat sarcopenia.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
X-JZ, J-BL and S-FZ conceived and designed this research. XJ and W-YW performed data analyses, interpreted results, and drafted the manuscript. X-JZ reviewed the final manuscript. All authors contributed to the article and approved the submitted version.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Guangdong Traditional Chinese Medicine Research Project (NO.20222052) and National Natural Science Foundation of China (Grant no. 81973814).
We gratefully acknowledge all the studies and databases that made GWAS summary data available.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1240811/full#supplementary-material
Supplementary File 1 | (A–L) Funnel plots of forward MR analysis. (M–X) Funnel plots of reverse MR analysis.
Supplementary File 2 | (A–L) Leave-one-out sensitivity analysis of forward MR. (M–X) Leave-one-out sensitivity of reverse MR analysis. (A–L) stand for ALM→IBD, ALM→UC, ALM→CD, Usual walking pace →IBD, Usual walking pace →UC, Usual walking pace →CD, Hand grip strength (left)→IBD, Hand grip strength (left)→UC, Hand grip strength (left)→CD, Hand grip strength (right)→IBD, Hand grip strength (right)→UC, Hand grip strength (right)→CD, respectively. (M–X) stand for IBD→ALM, IBD →Usual walking pace, IBD →Hand grip strength (left), IBD →Hand grip strength (left),UC→ALM, UC →Usual walking pace, UC →Hand grip strength (left), UC →Hand grip strength (left), UC→ALM, CD →Usual walking pace, CD →Hand grip strength (left), CD →Hand grip strength (left), respectively.
1. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (London England) (2019) 393(10191):2636–46. doi: 10.1016/s0140-6736(19)31138-9
2. Bianchi L, Ferrucci L, Cherubini A, Maggio M, Bandinelli S, Savino E, et al. The predictive value of the ewgsop definition of sarcopenia: results from the inchianti study. journals gerontol Ser A Biol Sci Med Sci (2016) 71(2):259–64. doi: 10.1093/gerona/glv129
3. Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Joint Bone Spine (2019) 86(3):309–14. doi: 10.1016/j.jbspin.2018.08.001
4. Sayer AA, Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing (2022) 51(10). doi: 10.1093/ageing/afac220
5. Anker SD, Morley JE, von Haehling S. Welcome to the icd-10 code for sarcopenia. J cachexia sarcopenia Muscle (2016) 7(5):512–4. doi: 10.1002/jcsm.12147
6. Faye AS, Khan T, Cautha S, Kochar B. Sarcopenia in inflammatory bowel diseases: reviewing past work to pave the path for the future. Curr Treat options Gastroenterol (2022) 20(3):250–60. doi: 10.1007/s11938-022-00389-8
7. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised european consensus on definition and diagnosis. Age Ageing (2019) 48(4):601. doi: 10.1093/ageing/afz046
8. Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism: Clin Exp (2023), 155533. doi: 10.1016/j.metabol.2023.155533
9. Petermann-Rocha F, Chen M, Gray SR, Ho FK, Pell JP, Celis-Morales C. Factors associated with sarcopenia: A cross-sectional analysis using uk biobank. Maturitas (2020) 133:60–7. doi: 10.1016/j.maturitas.2020.01.004
10. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J cachexia sarcopenia Muscle (2022) 13(1):86–99. doi: 10.1002/jcsm.12783
11. Fernandes LV, Paiva AEG, Silva ACB, de Castro IC, Santiago AF, de Oliveira EP, et al. Prevalence of sarcopenia according to ewgsop1 and ewgsop2 in older adults and their associations with unfavorable health outcomes: A systematic review. Aging Clin Exp Res (2022) 34(3):505–14. doi: 10.1007/s40520-021-01951-7
12. Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatrics Soc (2020) 68(7):1410–8. doi: 10.1111/jgs.16372
13. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet (London England) (2017) 390(10114):2769–78. doi: 10.1016/s0140-6736(17)32448-0
14. Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol (2023) 8(6):579–90. doi: 10.1016/s2468-1253(23)00011-0
15. Bamba S, Sasaki M, Takaoka A, Takahashi K, Imaeda H, Nishida A, et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with crohn's disease. PloS One (2017) 12(6):e0180036. doi: 10.1371/journal.pone.0180036
16. Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Alimentary Pharmacol Ther (2013) 38(3):213–25. doi: 10.1111/apt.12372
17. Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and inflammatory bowel disease: A systematic review. Inflammatory bowel Dis (2019) 25(1):67–73. doi: 10.1093/ibd/izy212
18. Ünal NG, Oruç N, Tomey O, Ömer Özütemiz A. Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission. Eur J Gastroenterol Hepatol (2021) 33(11):1367–75. doi: 10.1097/meg.0000000000002044
19. Gold SL, Raman M, Sands BE, Ungaro R, Sabino J. Review article: putting some muscle into sarcopenia-the pathogenesis, assessment and clinical impact of muscle loss in patients with inflammatory bowel disease. Alimentary Pharmacol Ther (2023) 57(11):1216–30. doi: 10.1111/apt.17498
20. Campbell JP, Teigen L, Manski S, Blumhof B, Guglielmo FF, Shivashankar R, et al. Sarcopenia Is More Prevalent among Inflammatory Bowel Disease Patients Undergoing Surgery and Predicts Progression to Surgery among Medically Treated Patients. Inflammatory bowel Dis (2022) 28(12):1844–50. doi: 10.1093/ibd/izac013
21. Bamba S, Inatomi O, Takahashi K, Morita Y, Imai T, Ohno M, et al. Assessment of body composition from ct images at the level of the third lumbar vertebra in inflammatory bowel disease. Inflammatory bowel Dis (2021) 27(9):1435–42. doi: 10.1093/ibd/izaa306
22. Pizzoferrato M, de Sire R, Ingravalle F, Mentella MC, Petito V, Martone AM, et al. Characterization of sarcopenia in an ibd population attending an italian gastroenterology tertiary center. Nutrients (2019) 11(10). doi: 10.3390/nu11102281
23. Pedersen M, Cromwell J, Nau P. Sarcopenia is a predictor of surgical morbidity in inflammatory bowel disease. Inflammatory bowel Dis (2017) 23(10):1867–72. doi: 10.1097/mib.0000000000001166
24. Evans DM, Davey Smith G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet (2015) 16:327–50. doi: 10.1146/annurev-genom-090314-050016
25. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32(1):1–22. doi: 10.1093/ije/dyg070
26. Burgess S, Daniel RM, Butterworth AS, Thompson SG. Network mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol (2015) 44(2):484–95. doi: 10.1093/ije/dyu176
27. Lawlor DA. Commentary: two-sample mendelian randomization: opportunities and challenges. Int J Epidemiol (2016) 45(3):908–15. doi: 10.1093/ije/dyw127
28. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
29. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Directors Assoc (2020) 21(3):300–7.e2. doi: 10.1016/j.jamda.2019.12.012
30. Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin densitometry (2015) 18(4):467–71. doi: 10.1016/j.jocd.2015.05.063
31. Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, aging, and body composition study–dual-energy X-ray absorptiometry and body composition working group. J Appl Physiol (Bethesda Md 1985) (1999) 87(4):1513–20. doi: 10.1152/jappl.1999.87.4.1513
32. Pei YF, Liu YZ, Yang XL, Zhang H, Feng GJ, Wei XT, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the uk biobank study. Commun Biol (2020) 3(1):608. doi: 10.1038/s42003-020-01334-0
33. Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: A consideration of the pros and cons. Aging Clin Exp Res (2017) 29(4):591–7. doi: 10.1007/s40520-016-0622-6
34. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing (2010) 39(4):412–23. doi: 10.1093/ageing/afq034
35. Mitchell RE EB MR, Raistrick CA, Paternoster L, Hemani G, Gaunt TR. MRC IEU UK Biobank GWAS pipeline version 2. Univ Bristol (2019). doi: 110.5523/bris.pnoat8cxo0u52p6ynfaekeigi.
36. Beenakker KG, Ling CH, Meskers CG, de Craen AJ, Stijnen T, Westendorp RG, et al. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev (2010) 9(4):431–6. doi: 10.1016/j.arr.2010.05.005
37. Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care (2015) 18(5):465–70. doi: 10.1097/mco.0000000000000202
38. Wind AE, Takken T, Helders PJ, Engelbert RH. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr (2010) 169(3):281–7. doi: 10.1007/s00431-009-1010-4
39. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. Uk biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
40. de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet (2017) 49(2):256–61. doi: 10.1038/ng.3760
41. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut (2019) 68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484
42. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations. Wellcome Open Res (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.2
43. Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med (2020) 18(1):312. doi: 10.1186/s12916-020-01778-5
44. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol (2011) 40(3):740–52. doi: 10.1093/ije/dyq151
45. Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in mendelian randomization: comparison of allele score and summarized data methods. Stat Med (2016) 35(11):1880–906. doi: 10.1002/sim.6835
46. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Stat Med (2017) 36(11):1783–802. doi: 10.1002/sim.7221
47. Bowden J, Hemani G, Davey Smith G. Invited commentary: detecting individual and global horizontal pleiotropy in mendelian randomization-a job for the humble heterogeneity statistic? Am J Epidemiol (2018) 187(12):2681–5. doi: 10.1093/aje/kwy185
48. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in mendelian randomisation studies with summary data and a continuous outcome. Stat Med (2015) 34(21):2926–40. doi: 10.1002/sim.6522
49. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiol (Cambridge Mass) (2017) 28(1):30–42. doi: 10.1097/ede.0000000000000559
50. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
51. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
52. Nishikawa H, Nakamura S, Miyazaki T, Kakimoto K, Fukunishi S, Asai A, et al. Inflammatory bowel disease and sarcopenia: its mechanism and clinical importance. J Clin Med (2021) 10(18). doi: 10.3390/jcm10184214
53. Bilski J, Mazur-Bialy AI, Wierdak M, Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol (2013) 64(2):143–55.
54. Saul D, Kosinsky RL. Dextran sodium sulfate-induced colitis as a model for sarcopenia in mice. Inflammatory bowel Dis (2020) 26(1):56–65. doi: 10.1093/ibd/izz127
55. Zhang T, Ding C, Xie T, Yang J, Dai X, Lv T, et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr (Edinburgh Scotland) (2017) 36(6):1586–92. doi: 10.1016/j.clnu.2016.10.004
56. Cushing KC, Kordbacheh H, Gee MS, Kambadakone A, Ananthakrishnan AN. Sarcopenia is a novel predictor of the need for rescue therapy in hospitalized ulcerative colitis patients. J Crohn's colitis (2018) 12(9):1036–41. doi: 10.1093/ecco-jcc/jjy064
57. Atlan L, Cohen S, Shiran S, Sira LB, Pratt LT, Yerushalmy-Feler A. Sarcopenia is a predictor for adverse clinical outcome in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr (2021) 72(6):883–8. doi: 10.1097/mpg.0000000000003091
58. Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, et al. Espen guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr (Edinburgh Scotland) (2017) 36(2):321–47. doi: 10.1016/j.clnu.2016.12.027
59. Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut (2011) 60(5):571–607. doi: 10.1136/gut.2010.224154
60. Zhang C, Yu D, Hong L, Zhang T, Liu H, Fan R, et al. Prevalence of sarcopenia and its effect on postoperative complications in patients with crohn's disease. Gastroenterol Res Pract (2021) 2021:3267201. doi: 10.1155/2021/3267201
61. Erős A, Soós A, Hegyi P, Szakács Z, Benke M, Szűcs Á, et al. Sarcopenia as an independent predictor of the surgical outcomes of patients with inflammatory bowel disease: A meta-analysis. Surg Today (2020) 50(10):1138–50. doi: 10.1007/s00595-019-01893-8
62. Dhaliwal A, Quinlan JI, Overthrow K, Greig C, Lord JM, Armstrong MJ, et al. Sarcopenia in inflammatory bowel disease: A narrative overview. Nutrients (2021) 13(2). doi: 10.3390/nu13020656
63. de Sire R, Rizzatti G, Ingravalle F, Pizzoferrato M, Petito V, Lopetuso L, et al. Skeletal muscle-gut axis: emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva gastroenterologica e dietologica (2018) 64(4):351–62. doi: 10.23736/s1121-421x.18.02511-4
64. Nardone OM, de Sire R, Petito V, Testa A, Villani G, Scaldaferri F, et al. Inflammatory bowel diseases and sarcopenia: the role of inflammation and gut microbiota in the development of muscle failure. Front Immunol (2021) 12:694217. doi: 10.3389/fimmu.2021.694217
65. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol (2017) 8:1045. doi: 10.3389/fphys.2017.01045
66. Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors il-6 and tnf-A. Eur J Med Res (2017) 22(1):25. doi: 10.1186/s40001-017-0266-9
67. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr (Edinburgh Scotland) (2014) 33(5):737–48. doi: 10.1016/j.clnu.2014.03.007
68. Li CW, Yu K, Shyh-Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J cachexia sarcopenia Muscle (2019) 10(3):586–600. doi: 10.1002/jcsm.12417
69. Bryant RV, Ooi S, Schultz CG, Goess C, Grafton R, Hughes J, et al. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Alimentary Pharmacol Ther (2015) 41(9):895–906. doi: 10.1111/apt.13156
70. Bryant RV, Schultz CG, Ooi S, Goess C, Costello SP, Vincent AD, et al. Obesity in inflammatory bowel disease: gains in adiposity despite high prevalence of myopenia and osteopenia. Nutrients (2018) 10(9). doi: 10.3390/nu10091192
71. Bekkelund SI, Jorde R. Lean body mass and creatine kinase are associated with reduced inflammation in obesity. Eur J Clin Invest (2017) 47(11):803–11. doi: 10.1111/eci.12802
72. Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The role of tumor necrosis factor alpha (Tnf-A) in autoimmune disease and current tnf-A Inhibitors in therapeutics. Int J Mol Sci (2021) 22(5). doi: 10.3390/ijms22052719
73. Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology (2008) 9(4):213–28. doi: 10.1007/s10522-008-9131-0
74. Huppertz-Hauss G, Høivik ML, Langholz E, Odes S, Småstuen M, Stockbrugger R, et al. Health-related quality of life in inflammatory bowel disease in a european-wide population-based cohort 10 years after diagnosis. Inflammatory bowel Dis (2015) 21(2):337–44. doi: 10.1097/mib.0000000000000272
75. McGing JJ, Radford SJ, Francis ST, Serres S, Greenhaff PL, Moran GW. Review article: the aetiology of fatigue in inflammatory bowel disease and potential therapeutic management strategies. Alimentary Pharmacol Ther (2021) 54(4):368–87. doi: 10.1111/apt.16465
76. Cioffi I, Marra M, Imperatore N, Pagano MC, Santarpia L, Alfonsi L, et al. Assessment of bioelectrical phase angle as a predictor of nutritional status in patients with crohn's disease: A cross sectional study. Clin Nutr (Edinburgh Scotland) (2020) 39(5):1564–71. doi: 10.1016/j.clnu.2019.06.023
77. Bin CM, Flores C, Alvares-da-Silva MR, Francesconi CF. Comparison between handgrip strength, subjective global assessment, anthropometry, and biochemical markers in assessing nutritional status of patients with crohn's disease in clinical remission. Digestive Dis Sci (2010) 55(1):137–44. doi: 10.1007/s10620-008-0692-1
78. Ter Beek L, Vanhauwaert E, Slinde F, Orrevall Y, Henriksen C, Johansson M, et al. Unsatisfactory knowledge and use of terminology regarding malnutrition, starvation, cachexia and sarcopenia among dietitians. Clin Nutr (Edinburgh Scotland) (2016) 35(6):1450–6. doi: 10.1016/j.clnu.2016.03.023
79. Norman K, Otten L. Financial impact of sarcopenia or low muscle mass - a short review. Clin Nutr (Edinburgh Scotland) (2019) 38(4):1489–95. doi: 10.1016/j.clnu.2018.09.026
80. Klare P, Nigg J, Nold J, Haller B, Krug AB, Mair S, et al. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: A prospective randomized controlled trial. Digestion (2015) 91(3):239–47. doi: 10.1159/000371795
81. Ng V, Millard W, Lebrun C, Howard J. Low-intensity exercise improves quality of life in patients with crohn's disease. Clin J sport Med (2007) 17(5):384–8. doi: 10.1097/JSM.0b013e31802b4fda
82. Bian D, Liu X, Wang C, Jiang Y, Gu Y, Zhong J, et al. Association between dietary inflammatory index and sarcopenia in crohn's disease patients. Nutrients (2022) 14(4). doi: 10.3390/nu14040901
83. Churchward-Venne TA, Breen L, Phillips SM. Alterations in human muscle protein metabolism with aging: protein and exercise as countermeasures to offset sarcopenia. BioFactors (Oxford England) (2014) 40(2):199–205. doi: 10.1002/biof.1138
84. Subramaniam K, Fallon K, Ruut T, Lane D, McKay R, Shadbolt B, et al. Infliximab reverses inflammatory muscle wasting (Sarcopenia) in crohn's disease. Alimentary Pharmacol Ther (2015) 41(5):419–28. doi: 10.1111/apt.13058
85. Choe HJ, Cho BL, Park YS, Roh E, Kim HJ, Lee SG, et al. Gender differences in risk factors for the 2 year development of sarcopenia in community-dwelling older adults. J cachexia sarcopenia Muscle (2022) 13(3):1908–18. doi: 10.1002/jcsm.12993
86. Forss A, Ludvigsson JF, Olén O. The burden of ibd: comparing Denmark and Sweden. Gastroenterology (2023) 164(7):1343–4. doi: 10.1053/j.gastro.2022.10.015
Keywords: sarcopenia, inflammatory bowel disease, Crohn’s disease, ulcerative colitis, Mendelian randomization, causal relationship
Citation: Jiao X, Wu W-y, Zhan S-f, Liu J-b and Zhang X-j (2023) A bidirectional Mendelian randomization study of sarcopenia-related traits and inflammatory bowel diseases. Front. Immunol. 14:1240811. doi: 10.3389/fimmu.2023.1240811
Received: 18 August 2023; Accepted: 24 October 2023;
Published: 08 November 2023.
Edited by:
Giuseppe Murdaca, University of Genoa, ItalyReviewed by:
Zhipeng Zheng, Zhejiang Chinese Medical University, ChinaCopyright © 2023 Jiao, Wu, Zhan, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-feng Zhan, enNmc3RvbmVAMTI2LmNvbQ==; Jian-bo Liu, MTM1Mzg3NTkwNzFAMTYzLmNvbQ==; Xian-jin Zhang, c2FubG9uZy1zaGluZUAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.