- Chengdu Seventh People’s Hospital & Chengdu Tumor Hospital, Chengdu, Sichuan, China
Background: Radiofrequency ablation (RFA) and chemotherapy are used to treat lung cancer or pulmonary metastases, but no direct comparison of overall survival (OS) has been published. The present study aimed to assess the OS of RFA and/or chemotherapy in patients with lung cancer or pulmonary metastases who were not candidates for surgical resection.
Methods: To identify relevant studies, the following databases were electronically searched from their inception to 31 March 2023: PubMed, Embase, Web of Science, Cochrane Library, Scopus, Ovid, ScienceDirect, SinoMed, China National Knowledge Infrastructure Database, Chongqing VIP Chinese Science and Technology Periodical Database, Wanfang Database, LILACS, ClinicalTrials.gov, and Chictr.org. Manual retrieval was also conducted. We used published hazard ratios (HRs) if available or estimates from other survival data.
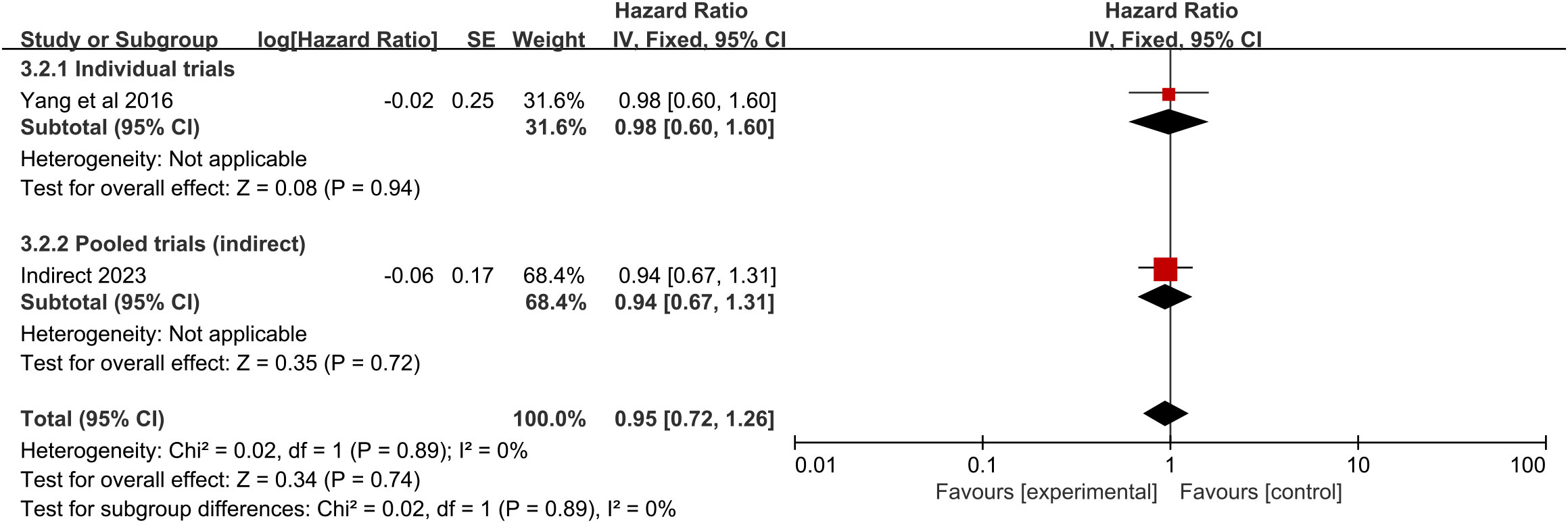
Results: A total of 1,387 participants from 14 trials were included in the final analysis. Patients treated with RFA combined with chemotherapy significantly improved OS compared with those treated with chemotherapy alone [HR 0.50, 95% confidence interval (CI) 0.41–0.61; p < 0.00001], with an absolute difference at 12 months of 29.6% (95% CI 23.7–35.5), at 24 months of 19.2% (95% CI 10.1–28.2), and at 36 months of 22.9% (95% CI 12.0–33.7). No statistically significant difference was observed in the subgroups of case type, cancer type, chemotherapy drugs, and tumor size. The HR for OS with RFA plus chemotherapy vs. RFA alone was 0.53 (95% CI 0.41–0.70; p < 0.00001), corresponding to a 27.1% (95% CI 18.3–35.8), 31.0% (95% CI 19.9–41.9), and 24.9% (95% CI 15.0–34.7) absolute difference in survival at 12 months, 24 months, and 36 months, respectively. Subgroup analysis by geographic region and TNM stage showed that RFA combined with chemotherapy still significantly improved OS compared to RFA. The HR of RFA vs. chemotherapy was 0.98 (95% CI 0.60–1.60; p = 0.94), with an absolute difference at 12 months of 1.4% (95% CI -19.2 to 22.1), at 24 months of 7.8% (95% CI -11.3 to 26.8), and at 36 months of 0.3% (95% CI -13.2 to 13.8). The overall indirect comparison of OS for RFA vs. chemotherapy was 0.95 (95% CI 0.72–1.26; p = 0.74). Data on progression-free survival were not sufficiently reported.
Conclusion: RFA combined with chemotherapy might be a better treatment option for patients with lung cancer or pulmonary metastases than chemotherapy alone or RFA alone. The comparison between RFA and/or chemotherapy remains to be specifically tested.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=335032, identifier CRD42022335032.
Introduction
In 2020, the estimated number of new cases of lung cancer diagnosed worldwide was 2,206,771 (11.4%) and lung cancer-related deaths were 1,796,144 (18.0%) (1). Lung cancer is the second most commonly diagnosed cancer and the top cause of cancer deaths globally (1, 2). Because the prophase of clinical symptoms is not obvious, over 70% of patients already have advanced disease at the time of presentation (3–5). The survival of lung cancer in advanced stages is still very poor, with an overall 5-year survival rate of 9.5%–18% (5, 6). Above 54%, patients with cancer may develop pulmonary metastases, for many tumors involve the lung for distant spread (5). In colorectal cancer, patients who present with lung metastasis have a 5-year survival rate of less than 10% compared with 91% of those without metastasis (7).
Surgical resection is preferred in the treatment of lung tumors and pulmonary metastases. However, a significant number of patients who are not candidates for surgical resection receive multidisciplinary synthetic treatment (8). Chemotherapy, as the main adjuvant method in the treatment of cancer, has been widely applied in advanced lung cancer treatment. Platinum-based chemotherapy is an essential part of the treatment of locally advanced lung cancer. Their effects on the survival of patients are still far from satisfactory because the median overall survival (OS) was only 9 months and the over 1-year survival rate was 30% (9). Radiofrequency ablation (RFA), a minimally invasive technique, has been gradually introduced for pulmonary tumor treatment in recent years. In a large prospective trial of RFA for lung cancer (10), there was no difference in response between primary and metastasized lung tumors. The OS of 1 year is 70% in patients with non-small cell lung cancer (NSCLC) and 89% at 1 year in patients with colorectal metastases. Combining systemic therapy with local therapy is always the focus of clinical inquiry. The combination of RFA with chemotherapy improves survival, offers higher treatment efficacy, and delays disease progression (11–13).
Three reviews and meta-analyses have been published on this subject (14–16). They focused on lung tumors in nonsurgical patients, short-term clinical effects, survival rate, local tumor progression, quality of life, recrudescence, and drug toxicity (14–16). However, all of the trials that were included in these meta-analyses were conducted before 2014. Furthermore, they did not evaluate survival by hazard ratio (HR). The limited scope of previous reviews and the recent publication of a number of studies assessing OS for patients treated with RFA and/or chemotherapy require a new comprehensive meta-analysis.
Our objective was to conduct a systematic review and meta-analysis to compare the survival of RFA and/or chemotherapy on lung cancer and pulmonary metastases in patients who are nonsurgical candidates and try to provide evidence in support of clinical work in choosing appropriate treatment options.
Methods
We conducted and reported this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (17). The protocol was registered with PROSPERO (registration number: CRD42022335032) at the start of our investigation.
Research question
We aimed to evaluate the survival of RFA, chemotherapy, and RFA plus chemotherapy for patients with lung cancer and pulmonary metastases.
Types of studies
We included randomized controlled trials (RCTs), cohort studies, and case-control studies. Case series lacking comparator groups or follow-up less than 12 months were excluded. Published and unpublished studies, full articles, and abstracts satisfying the criteria listed below were included without any language restriction. For publications and unpublished works not subject to peer review (such as theses or reports), we would contract the authors to get the key data. We also hand-searched the reference lists of the included studies and topical reviews for potentially relevant articles.
Type of participant
We reviewed studies reporting on patients with lung cancer and/or pulmonary metastases who were not eligible for surgical resection and were receiving the treatment of RFA, chemotherapy, and RFA plus chemotherapy.
Type of intervention
The arms of the studies were only chemotherapy, RFA, and RFA plus chemotherapy. Other adjunctive therapies (e.g., microwave ablation and radiotherapy) and targeted treatments were excluded.
Type of outcome measure
The primary outcome was OS, defined as the time from randomization to death from any cause. The secondary outcome was progression-free survival (PFS), defined as the time from randomization to disease progression or death.
Search strategy
We systematically searched the following databases from their date of inception to 31 March 2023: PubMed, Embase, Web of Science, Cochrane Library, Scopus, Ovid, ScienceDirect, SinoMed, China National Knowledge Infrastructure Database (CNKI), Chongqing VIP Chinese Science and Technology Periodical Database (VIP), Wanfang Database, LILACS, ClinicalTrials.gov, and Chictr.org. There were no language restrictions, and they carried out translations if necessary. The search strategy included four core components: 1) lung cancer; 2) pulmonary metastases; 3) chemotherapy; and 4) RFA. The retrieval model was ((a) OR (b)) AND (c) AND (d). Medical Subject Headings (MeSH) terms, free-text terms, keywords, and subject words were identified for each of the above core components. MeSH terms were retrieved from PubMed. The search was based on PubMed and then adapted for other English databases. For other databases, the subject-word retrieval method was used. The searching strategy is presented in Supplementary Table S1. References from previous reviews and key articles retrieved were also reviewed and cross-referenced for relevant studies.
Selection of studies
We downloaded all titles and abstracts obtained by electronic searches to a reference management database (Microsoft Excel) and removed duplicate articles. The remaining titles and abstracts were independently reviewed by two authors. They excluded studies that clearly did not meet the inclusion criteria. We obtained full-text articles of the remaining articles, and two independent reviewers determined the eligibility of the retrieved papers. We resolved disagreements by consensus or by consulting the senior author if necessary. We documented reasons for exclusion during this process.
Data extraction and management
Two review authors independently extracted the data using the extraction template, which include study characteristics (authors, journal, year of publication, location, and funding), study questions (participants, comparison, aims, design, follow-up time, type of study, and size), results (outcomes, key findings), and conclusions. When a consensus on the data extraction cannot be obtained through consultations, the senior author will make a decision.
Assessment of risk of bias and reporting of study quality
Two reviewers independently evaluated the methodological quality of the included studies. For RCTs, we assessed the risk of bias and created applicability concerns graph using the Cochrane Risk of Bias Tool, which is structured into seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. The outcomes include low risk of bias, unclear risk of bias, and high risk of bias (18, 19).
For cohort and case-control studies, the Newcastle–Ottawa Scale (NOS) was used to assess the risk of bias (19). It assigns up to a maximum of nine points for the least risk of bias in three domains: 1) selection of the study groups (4 points); 2) comparability of groups (2 points); and 3) ascertainment of exposure and outcomes (3 points) (20). The maximum score of each study was 9. Studies with scores of 7 were considered to have a low risk of bias, scores of 4–6 were considered to have a moderate risk of bias, and scores <4 were considered to have a high risk of bias. We assessed that follow-up was adequate if the follow-up was in excess of 12 months. Any disputes will be settled via consensus or with the involvement of the senior author.
Measures of treatment effect
We used the HR for the comparison in each trial to assess the treatment effects.
Management of missing data
If there are missing data for the primary results, we will contact the corresponding authors to request the missing data. If the missing data cannot be obtained, the analysis will rely on the available data. HRs and 95% confidence intervals (CIs) not reported or supported were calculated by the survival curves (21).
Statistical synthesis
The meta-analysis was performed using Review Manager (RevMan V.5.4.1 for Windows; the Nordic Cochrane Centre, The Cochrane Collaboration, 2020) and R version 4.2.1. The chi-square heterogeneity test and I2 statistic were used to investigate the overall heterogeneity between trials. p < 0.10 or I2 > 50% indicated significant heterogeneity. If considerable heterogeneity was observed, a random-effects model was used to analyze the pooled effect estimate; otherwise, the fixed-effects model was used. If more than 10 trials were included, funnel plots and the Egger test were used to assess publication biases. To estimate the 12-month, 24-month, and 36-month absolute differences, survival rates were computed on all patients and the HR at the corresponding time period was used to compute survival in each group (22). We used indirect comparison to obtain estimates of the benefit of RFA compared with chemotherapy.
Subgroup analysis
When significant heterogeneity was found, subgroup and sensitivity analyses were performed to explore possible reasons for the heterogeneity. However, given that the main purpose of subgroup analyses was to assess differences between subgroups rather than to explore reasons for heterogeneity, we performed subgroup analyses regardless of the presence or absence of statistically significant heterogeneity. Subgroup analyses were conducted assessing the impact of case type, geographic region, cancer type, TNM stage, age, tumor size, chemotherapy drugs, and time of RFA and follow-up.
Results
Results of the search
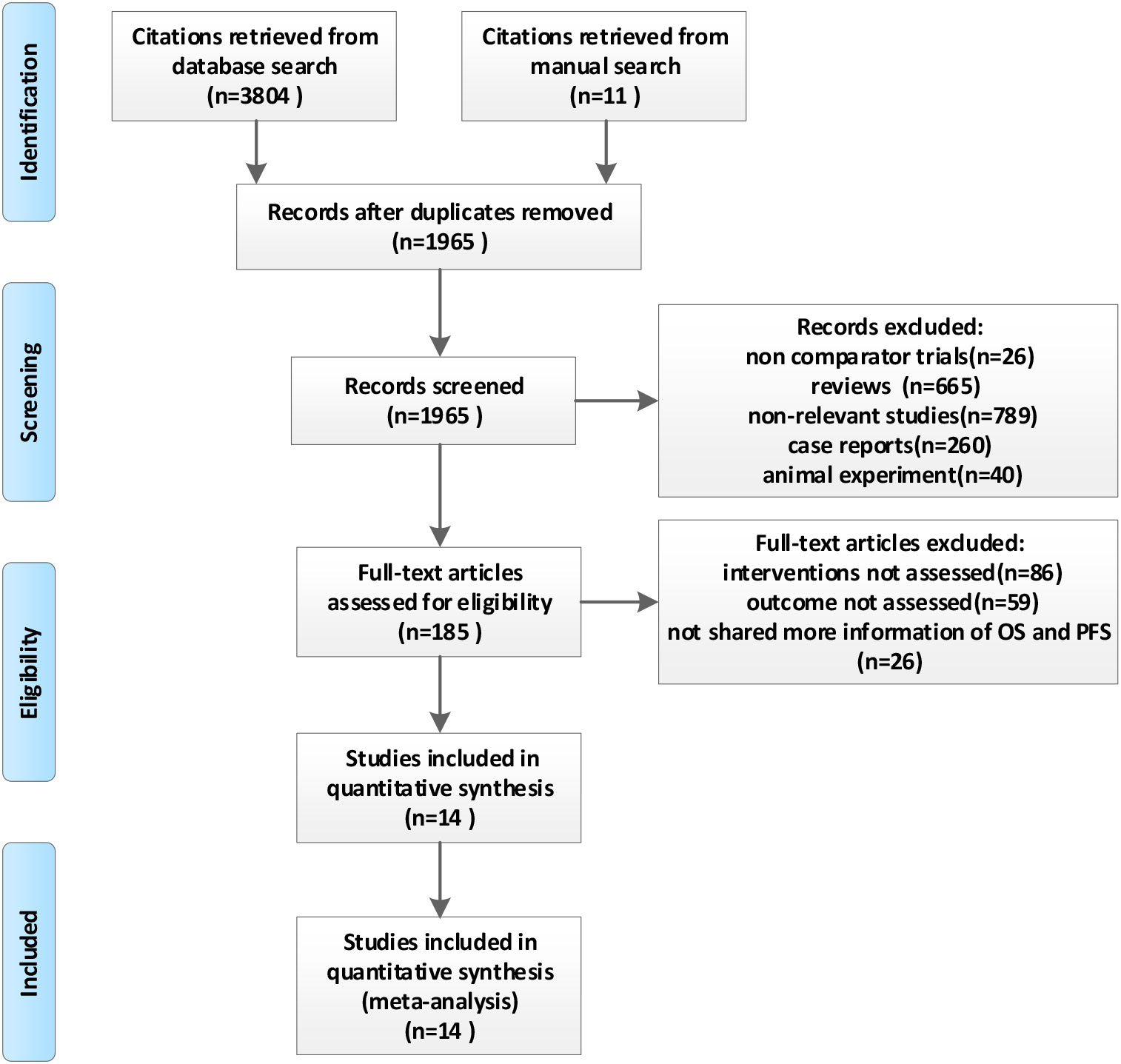
Figure 1 is a flowchart of the literature retrieval. Our literature identified 3,804 records from the database search results, and 11 additional articles were identified from manual searches. After removing the duplicate publications, 1,965 unique references were screened for eligibility by titles and abstracts. The remaining 185 publications were retrieved as full text or abstracts for detailed evaluation. Another 171 articles were excluded for the following reasons: interventions not assessed (n = 86), outcomes not assessed (n = 59), and not sharing more information about HR, OS, and PFS (n = 26). Finally, 14 trials representing 1,387 patients were included in this meta-analysis (11, 12, 23–34).
Study description
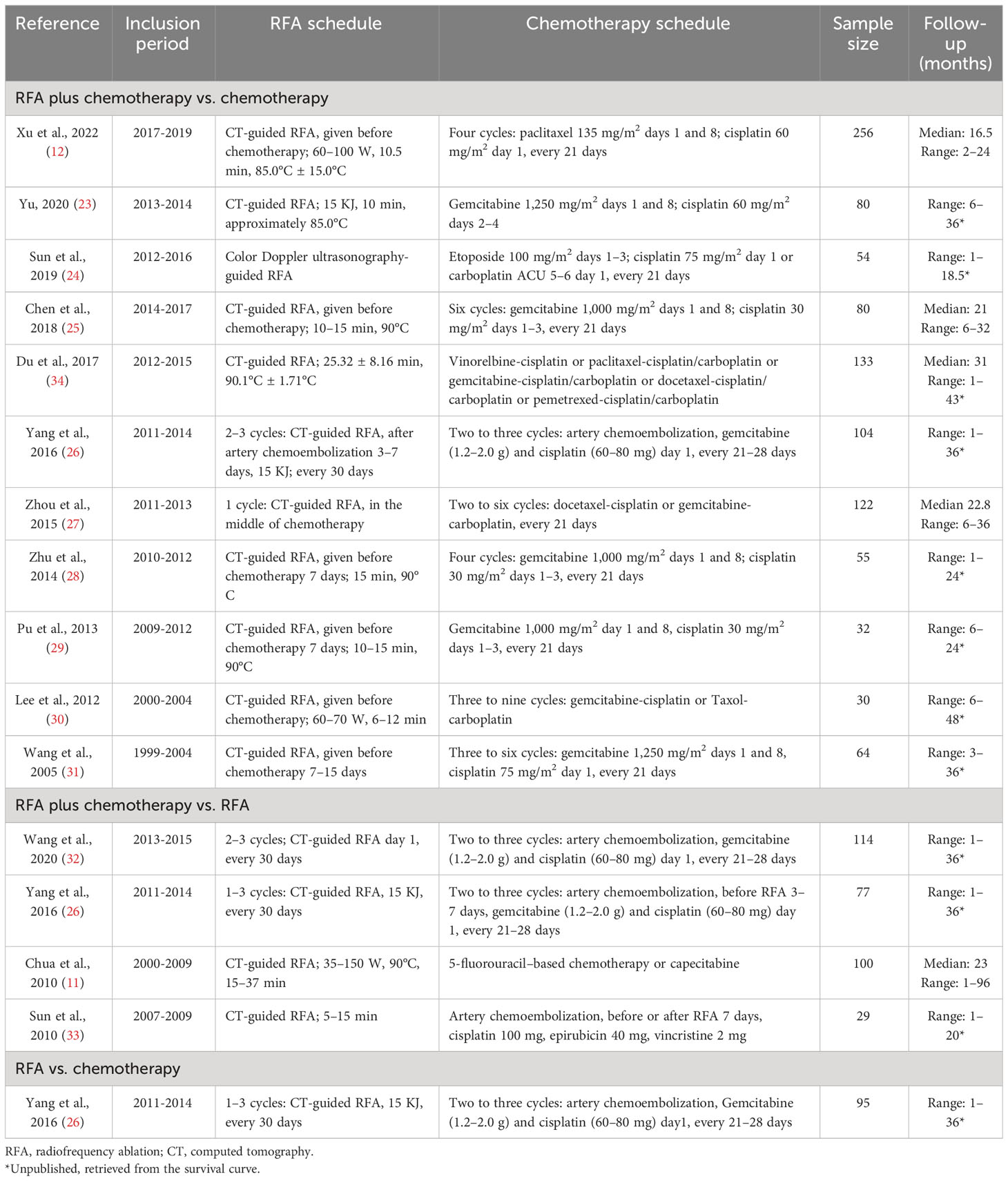
The characteristics of the studies in the evidence synthesis were summarized in Tables 1, 2. All of the studies were non-randomized comparisons. Nine studies were published from 2014 to 2022, and five were published before 2014. Most studies (11/14, 78.6%) were based in China. The sample size ranged from 29 to 256 patients. For NSCLC, the main lung cancer type, nine (n = 768) trials were only focused on NSCLC (23, 26–32, 34), and only one (n = 100) included patients with pulmonary metastases (11). Most patients in the trials were diagnosed with stage III or IV. Eleven studies were included in the comparison of RFA plus chemotherapy vs. chemotherapy alone (n = 1,010) (12, 23–31, 34). Four trials were included in the comparisons of RFA plus chemotherapy vs. RFA alone (n = 320) (11, 26, 32, 33). Only one study compared RFA with chemotherapy (n = 95) (26). One study included those three comparisons with RFA plus chemotherapy (n = 43), chemotherapy (n = 61), and RFA (n = 34) (26). Three studies (11, 31, 33) supplied HR in the articles; others were calculated by OS. Only one trial supplied the PFS curve (12).
Risk of bias assessment
Supplementary Figures S1, S2 provide the Cochrane risk of bias. The NOS results are listed in Supplementary Table S2. The majority of the included studies were felt to have a low risk of bias. The adequacy of follow-up was often not described in the included studies, which raises the question of bias.
Survival analysis of RFA plus chemotherapy vs. chemotherapy
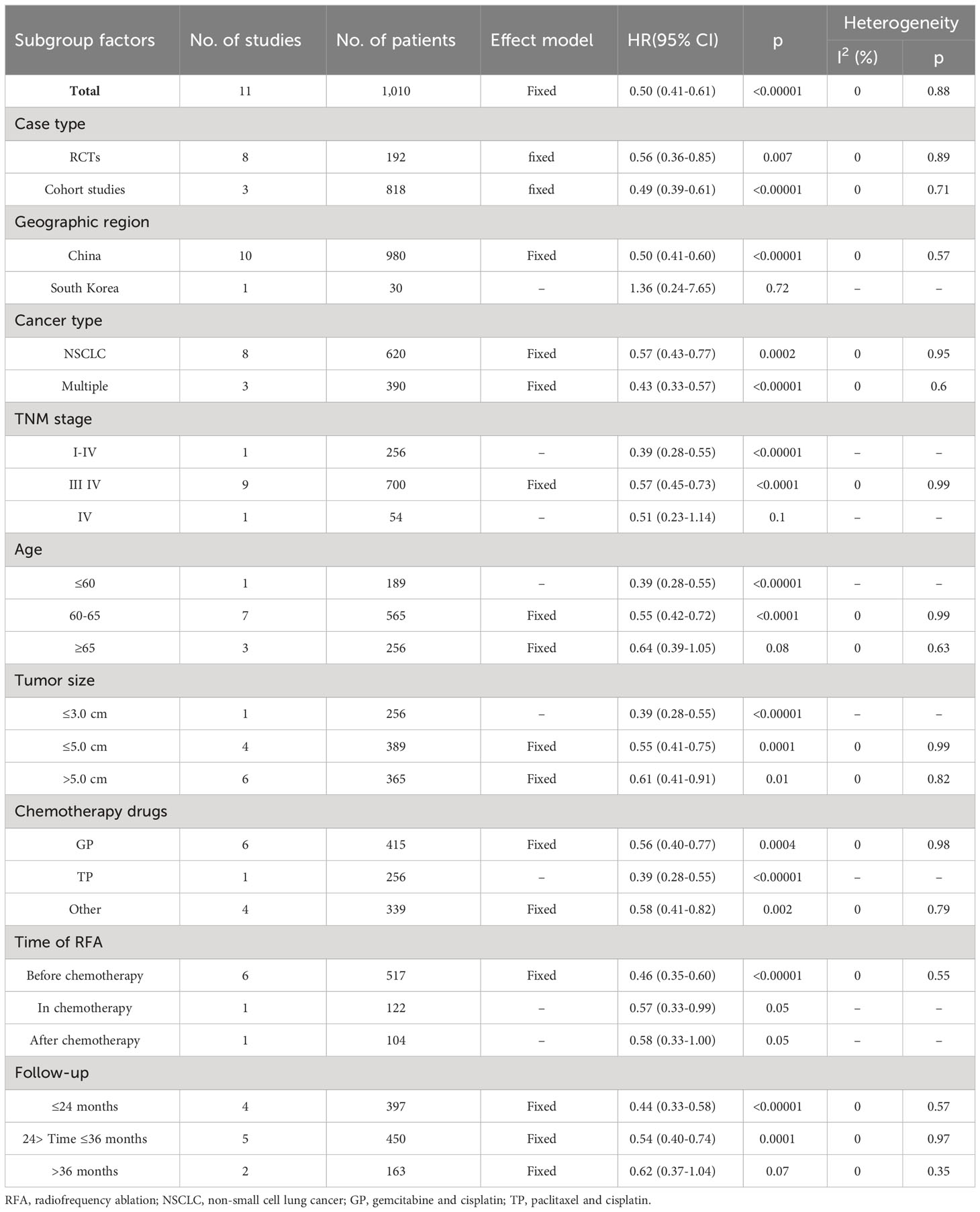
A significant benefit of OS was observed in favor of RFA plus chemotherapy vs. chemotherapy (HR 0.50, 95% CI 0.41–0.61; p < 0.00001) (Figure 2). This benefit corresponded to a 50% reduction in the risk of dying and an absolute benefit of 29.6% (95% CI 23.7–35.5), 19.2% (95% CI 10.1–28.2), and 22.9% (95% CI 12.0–33.7) at 12 months, 24 months, and 36 months, respectively (Figure 3A). Heterogeneity between trials was not significant (χ2 = 5.13, p = 0.88, I2 = 0%). We further performed the subgroup analysis, as shown in Table 3, and the primary result was independent of case type, cancer type, chemotherapy drugs, and tumor size. However, no statistically significant correlation was found in the Korean population (p = 0.72), TNM stage IV (p = 0.10), age ≥65 years (p = 0.08), RFA in or after the chemotherapy (p = 0.05), and follow-up periods longer than 36 months (p = 0.07) (Table 3).
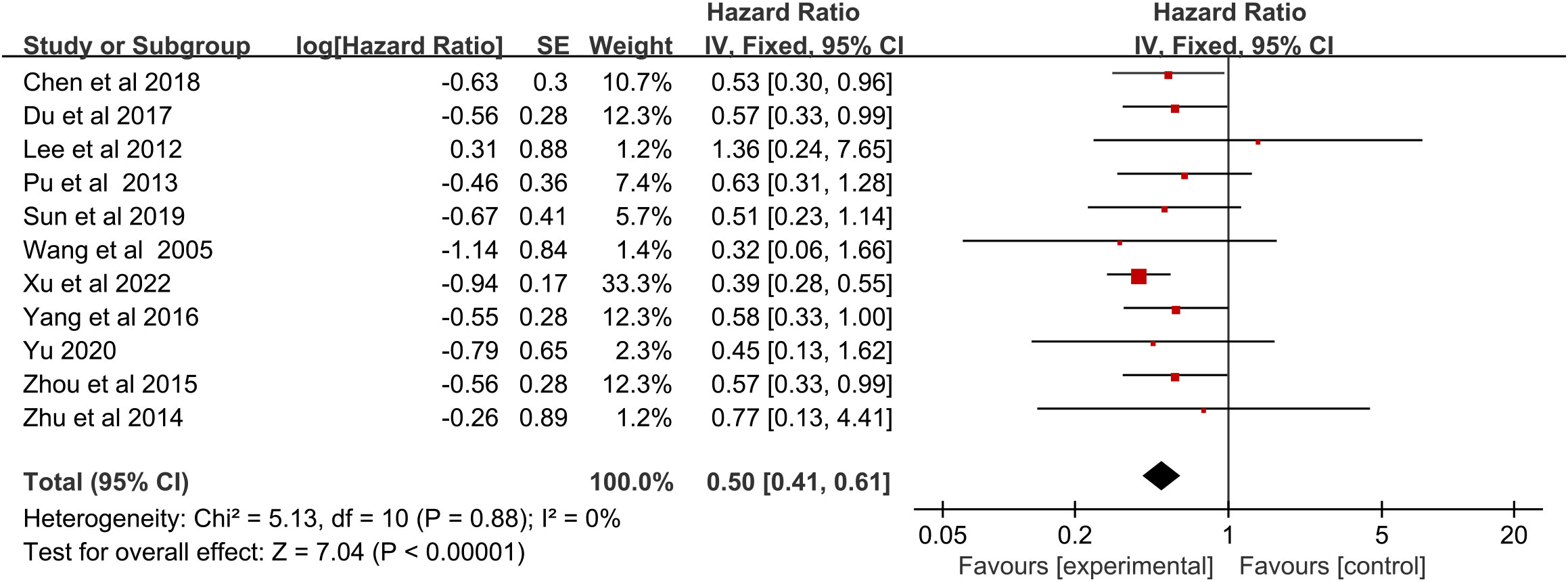
Figure 2 Overall survival for radiofrequency ablation plus chemotherapy compared with chemotherapy alone.
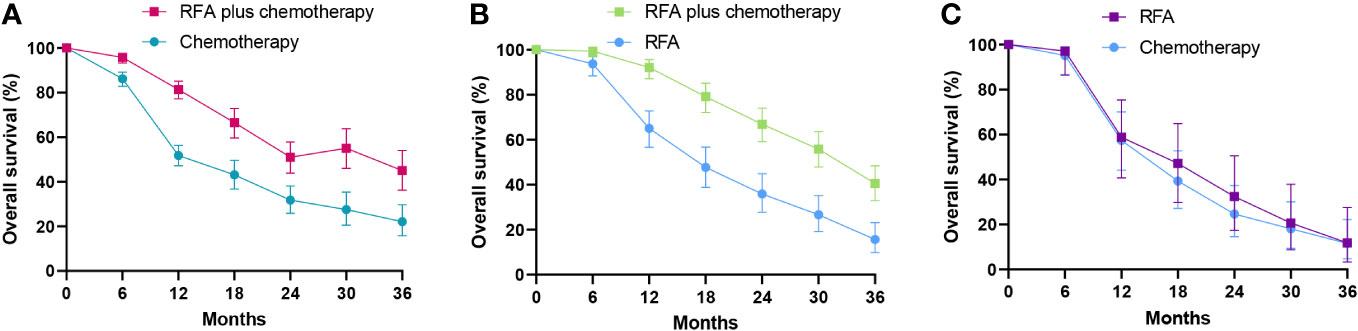
Figure 3 Comparison of overall survival curves for radiofrequency ablation and/or chemotherapy. (A) Overall survival curves of radiofrequency ablation plus chemotherapy compared with chemotherapy alone. (B) Overall survival curves of radiofrequency ablation plus chemotherapy compared with radiofrequency ablation alone. (C) Overall survival curves of radiofrequency ablation compared with chemotherapy. RFA, radiofrequency ablation.
Survival analysis of RFA plus chemotherapy vs. RFA
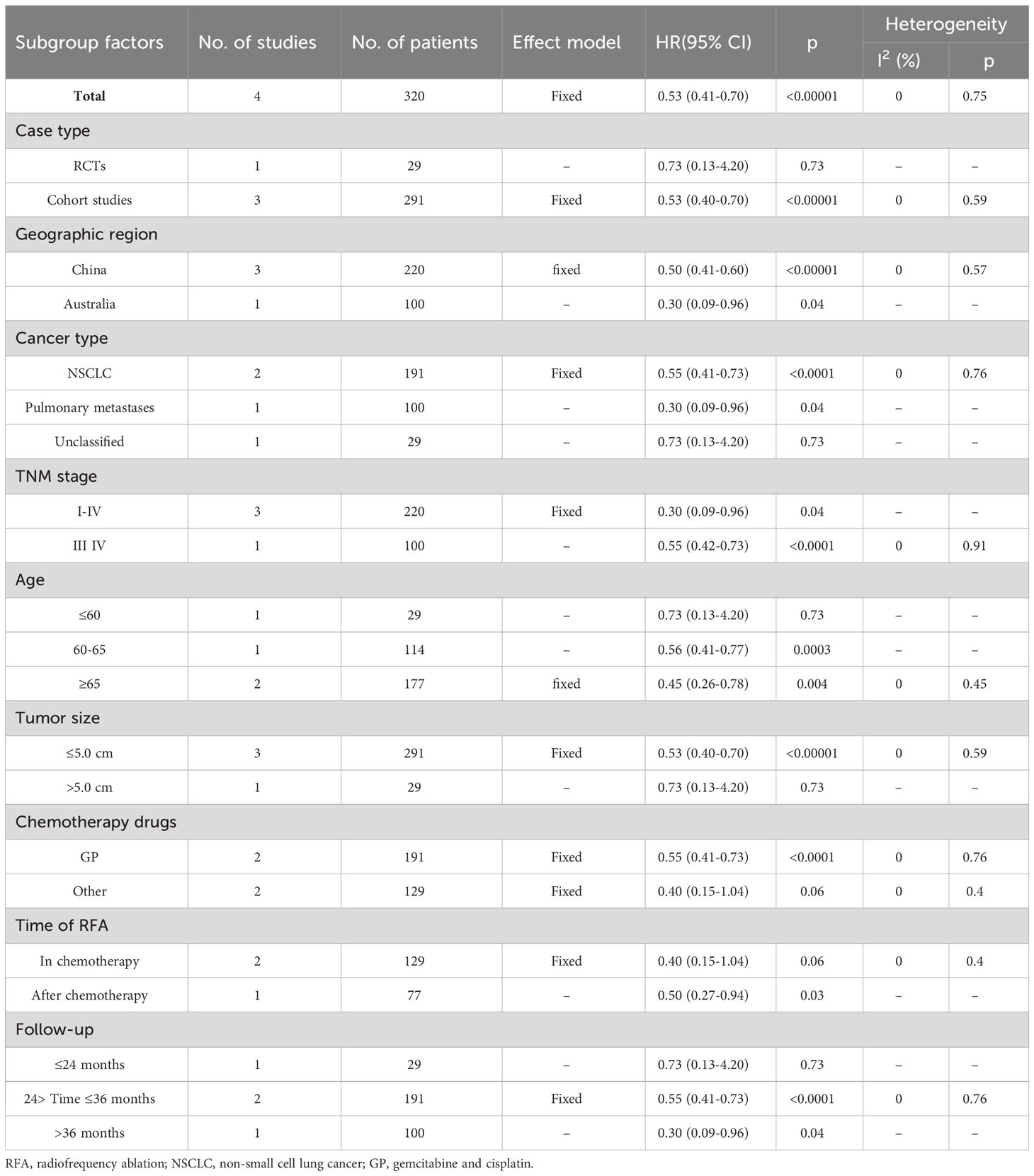
A significant benefit of OS was also observed in favor of RFA plus chemotherapy vs. RFA (HR 0.53, 95% CI 0.41–0.70; p < 0.00001) (Figure 4). This benefit corresponded to a 46% reduction in the risk of dying and an absolute benefit of 27.1% (95% CI 18.3–35.8), 31.0% (95% CI 19.9–41.9), and 24.9% (95% CI 15.0–34.7) at 12 months, 24 months, and 36 months, respectively (Figure 3B). Heterogeneity between trials was not significant (χ2 = 1.20, p = 0.75, I2 = 0%). As implied by the subgroup analysis, RFA combined with chemotherapy still significantly improved OS in various subgroups of geographic region and TNM stage (Table 4). No statistically significant correlation was detected in RCTs (p = 0.73), size larger than 5.0 cm (p = 0.73), age ≤60 years (p = 0.73), follow-up ≤24 months (p = 0.73), and RFA in the chemotherapy (p = 0.06) (Table 4).
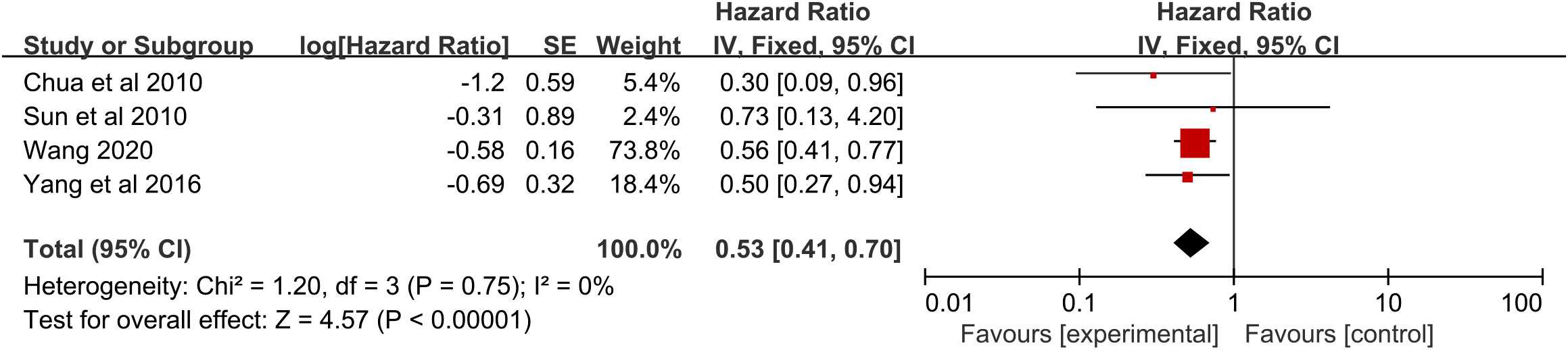
Figure 4 Overall survival for radiofrequency ablation plus chemotherapy compared with radiofrequency ablation alone.
Survival analysis of RFA vs. chemotherapy
The pooled analysis showed that, compared with chemotherapy alone, RFA did not significantly increase OS. There is only one trial evaluating RFA vs. chemotherapy. No significant difference in the survival rate was detected in this trial (HR = 0.98, 95% CI 0.60–1.60; p = 0.94) (Figure 5), with an absolute benefit of 1.4% (95% CI -19.2 to 22.1), 7.8% (95% CI -11.3 to 26.8), and 0.3% (95% CI -13.2 to 13.8) at 12 months, 24 months, and 36 months, respectively (Figure 3C). From the indirect comparison, the HR was 0.94 (95% CI 0.67–1.31; p = 0.72). Combining the indirect and direct comparisons yielded an overall HR of 0.95 (95% CI 0.72–1.26; p = 0.74) (Figure 5). The heterogeneity was not significant (χ2 = 0.02, p = 0.89, I2 = 0%).
Published bias analysis
Assessment of publication bias was performed using funnel plots (Supplementary Figure S3) and the Egger regression model. The Egger test showed that no publication bias was present for RFA plus chemotherapy vs. chemotherapy (p = 0.0986 > 0.05).
Discussion
This meta-analysis demonstrates a statistical survival advantage for patients treated with RFA plus chemotherapy compared with those treated with chemotherapy alone or RFA alone, while RFA seems not to significantly improve OS compared with chemotherapy. Our findings provide strong evidence that RFA plus chemotherapy can improve survival in the disease.
To our knowledge, this paper represents the most comprehensive and up-to-date review of the treatment comparisons (RFA plus chemotherapy vs. chemotherapy, RFA plus chemotherapy vs. RFA, RFA vs. chemotherapy) for lung cancer and pulmonary metastases. Few previous meta-analyses have used HR to compare the OS of RFA and/or chemotherapy. Liu et al. (15) found that RFA combination with chemotherapy increased survival rate (response rate (RR) = 1.49, 95% CI 1.35–1.65) and reduced postoperative recrudescence (RR = 0.51, 95% CI 0.32–0.82) compared with chemotherapy for advanced NSCLC. In another meta-analysis (14), the authors also found that RFA plus chemotherapy improved the OS rate compared to chemotherapy alone for advanced NSCLC (1 year, RR = 2.01, 95% CI 1.41–2.86; 2 years, RR = 2.48, 95% CI 1.51–4.07; 3 years, RR = 2.29, 95% CI 1.24–4.22). Peter et al. (16) conducted a meta-analysis of the survival outcomes among lung tumors in nonsurgical patients treated with RFA plus post-ablation chemotherapy vs. RFA. They found that RFA plus post-ablation chemotherapy of lung tumors yielded improved outcomes in terms of local tumor progression, OS, and disease-free survival compared with RFA alone (16). A key limitation of those studies was that they used adjusted and unadjusted odds ratios that do not take into account time-to-over outcome measures.
Since most patients with lung cancer have advanced disease (stage III or IV), they miss the optimal therapeutic window for curative resection. For patients with inoperable cancer, chemotherapy is the mainstay of management. It is a systemic treatment because the chemicals or drugs travel throughout the body and kill cancer cells. The main international guidelines recommend platinum-based chemotherapy as the standard of care for first-line therapy of advanced lung cancer, while those compounds indiscriminately attack all rapidly dividing cells, leading to severe side effects and inducing drug resistance (35). Furthermore, residual tumor cells remain present within resolving lesions after chemotherapy. The posttreatment prognosis of these patients remains poor. The meta-analysis of SCLC suggests that the 6-month survival rate was 75.3% and 72.7% and the 1-year survival rate was 36.2% and 35.0% for cisplatin and carboplatin, respectively (36). Another meta-analysis of NSCLC, which included 38 randomized trials, showed that the 1-year survival rate for the platinum-containing regimens was 34% (37). Thus, more effective and less invasive strategies for advanced lung cancer remain a widespread necessity. As a precise localized and minimally invasive technique, RFA has good safety and effectiveness that can improve the clinical treatment effect and prolong the survival time of patients. It has been widely used in the clinical treatment of lung cancer (38). RFA is also an alternative to surgery for local treatment to eradicate the tumors and has been officially approved as a treatment for NSCLC. The principle of RFA uses high-frequency electromagnetic waves to make intracellular polar molecules agitate and friction to generate heat, leading to protein degeneration and the killing of tumor cells. RFA has an obvious advantage in lung cancer treatment, for a high amount of air in the lung can speed up the accumulation of heat, which causes a rapid temperature increase.
Only one study that met the inclusion criteria for this meta-analysis has directly compared RFA with chemotherapy for NSCLC (26). It seemed that RFA was not significantly beneficial compared with chemotherapy for OS (HR 0.98). Additionally, the absolute difference between 12 months and 36 months was very small in the trial. To help with future trial design and individual patient treatment decisions, we intended to measure the relative survival benefits when these two therapy modalities were directly compared. Weak evidence (5%) in favor of chemotherapy was found in the overall indirect comparison of RFA and chemotherapy (HR 0.95). Shi and Xu (39) compared the survival time and quality of life in patients with lung metastasis from a malignant tumor of the digestive tract between RFA via fiber-optic bronchoscopy and conservative chemotherapy. They reported that the 3-year survival rate with RFA (53.3%) was significantly higher than that of chemotherapy (31.1%) (p < 0.05). Lee et al. (30) reported that the 3-year lung survival rate of NSCLC patients with RFA was 33.3% and chemotherapy alone was 32.4%. The disadvantage may be caused by the fact that RFA effectively kills tumor cells directly. However, this indirect comparison might have been prone to selection bias, and more direct comparisons are needed to test it.
RFA plus chemotherapy provided a better OS. Compared with chemotherapy alone, the HR was 0.50. And compared with RFA alone, the HR was 0.53. We speculate that the improvement in OS of RFA plus chemotherapy compared with RFA alone or chemotherapy alone is due to the following reasons: Firstly, RFA can not only effectively kill tumor cells but also release tumor antigens that can provoke a systemic immune response (40). RFA induces massive necrotic cell death through frictional heating and inflammatory effects. Inflammatory infiltrates that include neutrophils, macrophages, dendritic cells, and natural killer cells are found in the transitional zone. B cells and T cells are specific to the ablated tissue. These immune cell subsets have also been observed in distant untreated tumors and the bloodstream in both patients and animals (41). These results suggest an overall immune activation by RFA. The levels of immunoglobulin A, immunoglobulin G, and immunoglobulin M were increased significantly after RFA (42). The immune response provoked by the localized RFA treatment may have a therapeutic effect on distant primary lesions. This may be the reason why tumor markers decreased after RFA. The combined use of RFA and chemotherapy could decrease further than chemotherapy alone (38). Secondly, chemotherapy resistance limits our ability to effectively treat lung cancer. Some lung tumors are intrinsically resistant to chemotherapy, and in virtually all cases, even the initial responders rapidly develop acquired resistance. RFA induced coagulation necrosis and cell death in the centrally located hypoxic tumor, which is typically less responsive to chemotherapy. RFA, which is a type of hyperthermia, inhibits DNA polymerase-mediated damage repair, increases functional multidrug resistance (MDR) proteins, and abrogates drug resistance (43). In the short-term effect study of middle- and late-period NSCLC, the effective rate of chemotherapy was 27.3%, RFA was 64.3%, and RFA combined with chemotherapy was 80.0%. A previous meta-analysis (15) also indicated that RFA combined with chemotherapy improved short-term effect than chemotherapy (RR = 0.93, 95% CI 0.72–1.20). Thirdly, the goal of combining chemotherapy with thermal ablation is often to enhance tumor cell death in the peripheral or transitional zone, which, at sublethal temperatures, is an area recovering from reversible injury. Apoptosis that is triggered by heat-induced cell injury is increased by the cytotoxic injury of chemotherapies. Chemotherapy is more sensitive to oxygen-enriched cells than to hypoxic cells, while RFA is more sensitive to hypoxic cells. RFA can cause “in situ thermal injury” to the large tumor mass, which can lead to a “chemotherapy-sensitizing effect” and make the chemotherapy more effective (44). The synergistic effect of the combination of RFA and chemotherapy has been proven (45). Finally, RFA targets tumors that can be seen in imaging but cannot treat subclinical or small lesions. Moreover, insufficient RFA can lead to the expression of tumor stem markers, promote the generation of tumor stem cells, and further lead to residual cancer recurrence. Chemotherapy is a systemic treatment that has a better effect on subclinical lesions, small lesions, and residual cancer. The benefit of combination survival was also proven in the rabbit VX2 lung tumor model (46). The combination has complementary advantages that increase the disease control rate, objective response rate, and survival (13, 47).
Furthermore, we performed subgroup analyses on case type, therapeutic approaches, and patient characteristics and tried to delve into their applicability. For the Chinese population, a statistically significant correlation was found between the combined application of RFA and chemotherapy vs. sole chemotherapy, whereas this difference was not significant in the Korean population (p = 0.72). Simultaneously, Chinese and Australian patients with lung cancer had comparable outcomes when comparing RFA combined with chemotherapy vs. RFA alone. The literature reports the results of RFA treatment for lung cancer in the United States (48) and Europe (49), but unfortunately, we did not find any trials of relevant comparisons. This may be due to a variety of factors, including the sample size, dissimilarities in populations and biological characteristics of tumors, and disparities in local clinical management (50). It indicated that variances in patient populations across different regions could impact the efficacy of the treatment. The combination of RFA with chemotherapy significantly improved survival compared to chemotherapy alone in different tumor sizes, drugs, and types of lung cancer including NSCLC, SCLC, and pulmonary metastases. Hiraki et al. (51) showed in their meta-analysis that tumor type did not impact local control by RFA in the lungs. Maybe the benefit is derived from the combined synergistic effects of both comprehensive treatment strategies and local therapies.
The subgroup analysis also showed the OS benefit for RFA prior to chemotherapy than for RFA during and after chemotherapy in the comparison of RFA plus chemotherapy and chemotherapy. These findings are comparable to a previously published research by Matsui et al. (52) who retrospectively studied 21 patients who underwent metastasectomy for pulmonary metastases from esophageal cancer. They estimated that 1-year, 3-year, and 5-year survival rates for RFA prior to chemotherapy were 93.3%, 63.8%, and 47.9%, respectively, while those for RFA after chemotherapy were 87.5%, 31.3%, and 31.3% respectively. This difference may be due to the fact that RFA effectively reduces the tumor size, alters the microenvironment of the tumor cells and triggers an immune response, and reduces drug resistance, thus helping to improve the efficacy of subsequent chemotherapy.
When conducting subgroup analyses comparing chemotherapy combined with RFA to RFA, we found tumor size >5.0 cm did differ from the overall analysis. The local efficacy of RFA for the treatment of lung cancer depends on tumor size and the type of electrode used (53). Dupuy et al. (48) reported the results of the American College of Surgeons Oncology Group Z4033 Trial that prospectively evaluated RFA for stage IA NSCLC in medically inoperable patients in 2015. The difference in local control between tumors 3 cm or less in size and tumors larger than 3 cm and therapeutic outcomes are better in smaller cancers. Kodama et al. (54) and Herrera et al. (55) reported a better treatment response in tumors smaller than 5 cm. RFA is strictly dependent on anatomical criteria, such as nodule size and location. Therefore, lesions larger than 5 cm should be excluded from RFA (56).
Our study has certain limitations that should be taken into account. Several studies included in the analysis were not RCTs. Only one retrospective study has been designed to compare chemotherapy directly with RFA for patients with NSCLC (26). Therefore, we conducted a cross-study analysis of data from the comparing of RFA plus chemotherapy with chemotherapy alone and RFA plus chemotherapy with RFA alone. The PFS of those comparisons was also low in the literature. The HR was not directly obtained by the included studies; therefore, it might have led to deviations in the calculated HR. In making treatment decisions, we need to consider toxicity or side effects. However, the adverse events were not analyzed in this paper because the definition, measurement, and reporting of adverse events in the trials were not standard-grade toxicities.
Conclusion
In conclusion, based on the present evidence, RFA plus chemotherapy improves OS compared with RFA alone or chemotherapy alone. Further research is still needed to compare the efficacy and safety of RFA plus chemotherapy and RFA alone or chemotherapy alone.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors were involved in the design of the trial, organization of the study, manuscript writing, and approval of final version of the manuscript. ZY, XL, HY, LH and RZ developed the protocol. ZY, XL, HY, BW, DX, LH and YH generated the search strategy, performed literature search and select. ZY, XL, HY, LH and RZ extracted and summarized the data. ZY did the data analyses, and XL and YH checked the data analysis and validity. ZY, XL, HY, BW, DX, YH and BD interpreted the data. ZY and XL were chief.
Funding
This work was supported by the Sichuan provincial administration of tradition Chinese medicine (2021MS157).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1240149/full#supplementary-material
References
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today (2020). Available at: https://gco.iarc.fr/today/.
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
3. Siddiqui F, Vaqar S, Siddiqui AH. Lung Cancer (2022). Available at: https://www.ncbi.nlm.nih.gov/books/NBK482357/.
4. Rossi A, Chiodini P, Sun JM, O'Brien ME, von Plessen C, Barata F, et al. Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol (2014) 15:1254–62. doi: 10.1016/S1470-2045(14)70402-4
5. Yuh D, Vricella L, Yang S, Doty J. Johns Hopkins Textbook of Cardiothoracic Surgery. 2nd ed. New York: McGraw-Hill Education Medical (2014) p. 119–55.
6. Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, et al. Lung cancer screening, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2018) 16:412–41. doi: 10.6004/jnccn.2018.0020
7. Jamil A, Kasi A. Lung Metastasis (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK553111/.
8. Raz DJ, Zell JA, Ou SH, Gandara DR, Anton-Culver H, Jablons DM. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest (2007) 132:193–9. doi: 10.1378/chest.06-3096
9. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med (2002) 346:92–8. doi: 10.1056/NEJMoa011954
10. Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol (2008) 9:621–8. doi: 10.1016/S1470-2045(08)70155-4
11. Chua TC, Thornbury K, Saxena A, Liauw W, Glenn D, Zhao J, et al. Radiofrequency ablation as an adjunct to systemic chemotherapy for colorectal pulmonary metastases. Cancer (2010) 116:2106–14. doi: 10.1002/cncr.24952
12. Xu F, Cai Z, Xu B, Song J, Liu H, Li X, et al. Clinical research on the combined use of systemic chemotherapy and CT-guided radiofrequency ablation in treatment of lung cancer. LASER Med Sci (2022) 37:233–9. doi: 10.1007/s10103-020-03222-9
13. Yu S, Wu ZZ, Si HT, Yang S, Liu GM, Zhao XD. Short-term effect analysis of radiofrequency ablation combined chemotherapy on middle and late period non-small cell lung cancer. Oncol Lett (2016) 12:4399–402. doi: 10.3892/ol.2016.5276
14. Wei Y, Li Z, Mi D, Wen Z, Li Z. A systematic review of radiofrequency ablation combined with radiotherapy or chemother-apy in the treatment of advanced non-small cell lung cancer. Modern Oncol (2015) 23:2609–14. doi: 10.3969/j.issn.1672-4992.2015.18.17
15. Liu W, Zeng X, Liu X, Li X, Guo Y. Radiofrequency ablation and chemotherapy combination versus chemotherapy monotherapy in non-small cell lung cancer:A systematic review of meta analysis. Chin Clin Oncol (2012) 17:530–8. doi: 10.3969/j.issn.1009-0460.2012.06.012
16. Peter A, Sandra J, Mario V, LYR M, Stephen O. Local tumor control and survival outcomes of percutaneous radiofrequency ablation plus post-ablation chemotherapy for lung tumors in nonsurgical patients: A meta-analysis. J Clin Oncol (2013) 31:9550. doi: 10.1200/jco.2013.31.15_suppl.9550
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.3736/jcim20090918
18. Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA(editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons. (2019).
20. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2011). Available at: https://www.ohri.ca/programs/clinic.
21. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. TRIALS (2007) 8:16. doi: 10.1186/1745-6215-8-16
22. Lacas B, Bourhis J, Overgaard J, Zhang Q, Grégoire V, Nankivell M, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol (2017) 18:1221–37. doi: 10.1016/S1470-2045(17)30458-8
23. Yu X. Clinical efficacy of chemotherapy combined with radiofrequency ablation in the treatment of locally advanced non small cell lung cancer. J Clin Res (2020) 37:136–8. doi: 10.3969/j.issn.1671-7171.2020.01.044
24. Sun J, Luo J, Xu L, Zhao L, Si T, Chen S, et al. Discussion on the treatment mode of liver metastasis in patients with extensive small cell lung cancer. J Tianjin Med Univ (2019) 25:577–80.
25. Chen D, Ye J, Xiong X, Wang J, Shi D, Peng G. Therapeutic efficacy of radiofrequency ablation combined with chemotherapy for advanced non-small cell lung cancer. Chin J Clin Oncol Rehabil (2018) 25:1073–6.
26. Yang X, Wu G, Li Z, Wu H, Sun Y, Wen H, et al. Efficacy for artery chemoembolization combined with radiofrequency ablation in the treatment of advanced non-small cell lung cancer. Natl Med J China (2016) 96:539–43. doi: 10.3760/cma.j.issn.0376-2491.2016.07.010
27. Zhou J, Li H, Lv W, Wang S, Zheng X, Lin L. Curative effect of radiofrequency ablation combined with chemotherapy on middle-late stage non-small cell lung cancer. J Pract Med (2015) 31:1786–9. doi: 10.3969/j.issn.1006-5725.2015.11.019
28. Zhu Y, Zhou C, Lu B, Zhou Y, Zhao W, Wang Y, et al. The short-term efficacy of radiofrequency ablation combined with chemotherapy in the treatment of locally advanced non small cell lung cancer. Modern Pract Med (2014) 26:829–30. doi: 10.3969/j.issn.1671-0800.2014.07.024
29. Pu D, Liao J. Radiofrequency ablation combined with GP chemotherapy for stage III and IV peripheral non-small cell lung cancer: a clinical observation. J Interventional Radiol (2013) 22:129–32. doi: 10.3969/j.issn.1008-794X.2013.02.010
30. Lee H, Jin GY, Han YM, Chung GH, Lee YC, Kwon KS, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardio Vasc Interventional Radiol (2012) 35:343–50. doi: 10.1007/s00270-011-0194-y
31. Wang S, Chen J, Cao W, Chen Y, Cheng B. The observation of the clinical effect for combination therapy of RFA with GP on advanced stage lung cancer. Chin J Clin Oncol (2005) 32:628–30. doi: 10.3969/j.issn.1000-8179.2005.11.009
32. Wang Q, Yu L. Efficacy and prognosis of radiofrequency ablation combined with artery chemoembolization in the treatment of advanced non small cell lung cancer. J Clin Res (2020) 37:925–8. doi: 10.3969/j.issn.1671-7171.2020.06.040
33. Sun H, Liu R, Liu Y, Yang Y, Shen H, Wang H, et al. Radiofrequency ablation combined with arterial infusion chemotherapy for locally advanced lung cancer. J Pract Oncol (2010) 24:168–71. doi: 10.3969/j.issn.1002-3070.2010.02.018
34. Du S, Qin D, Pang R, Zhao S, Hu M, Zhi X. Long-term efficacy of radiofrequency ablation combined with chemotherapy in the treatment of patients with advanced non-small cell lung cancer-A retrospective study. Zhongguo Fei Ai Za Zhi (2017) 20:675–82. doi: 10.3779/j.issn.1009-3419.2017.10.03
35. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther (2016) 16:653–60. doi: 10.1586/14737140.2016.1170596
36. Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the cocis meta-analysis of individual patient data. J Clin Oncol (2012) 30:1692–8. doi: 10.1200/JCO.2011.40.4905
37. D'Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, Shepherd FA. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol (2005) 23:2926–36. doi: 10.1200/JCO.2005.03.045
38. Xu F, Song J, Lu Y, Wang J, Wang J, Xiao H, et al. Clinical efficacy of systemic chemotherapy combined with radiofrequency ablation and microwave ablation for lung cancer: a comparative study. Int J Hyperther (2021) 38:900–6. doi: 10.1080/02656736.2021.1936214
39. Shi Y, Xu X. Survival time and quality of life follow -up of fiberoptic bronchoscopy ablation and conservative chemotherapy for treating patients with lung metastasis of Malignant tumor of digestive tract. Modern Digestion Intervention (2016) 21:383–6. doi: 10.3969/j.issn.1672-2159.2016.03.003
40. Qi X, Yang M, Ma L, Sauer M, Avella D, Kaifi JT, et al. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J Immunother Cancer (2020) 8:e001038. doi: 10.1136/jitc-2020-001038
41. Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer (2014) 14:199–208. doi: 10.1038/nrc3672
42. Cheng R, Li Y, Su L, Wang L, Cao Y. Clinical effect of cold and heat ablation on patients with advanced lung cancer and its influence on immune function. Am J Transl Res (2023) 15:2939–48.
43. Stein U, Jürchott K, Walther W, Bergmann S, Schlag PM, Royer HD. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J Biol Chem (2001) 276:28562–9. doi: 10.1074/jbc.M100311200
44. Li X, Wang J, Li W, Huang Z, Fan W, Chen Y, et al. Percutaneous CT-guided radiofrequency ablation for unresectable hepatocellular carcinoma pulmonary metastases. Int J Hyperther (2012) 28:721–8. doi: 10.3109/02656736.2012.736669
45. Exner AA, Weinberg BD, Stowe NT, Gallacher A, Wilson DL, Haaga JR, et al. Quantitative computed tomography analysis of local chemotherapy in liver tissue after radiofrequency ablation. Acad Radiol (2004) 11:1326–36. doi: 10.1016/j.acra.2004.09.007
46. Ueki A, Okuma T, Hamamoto S, Miki Y. Therapeutic effects of CT-guided radiofrequency ablation with concurrent platinum-doublet chemotherapy in a rabbit VX2 lung tumor model. Radiology (2017) 283:391–8. doi: 10.1148/radiol.2016160414
47. Huang BY, Zhou JJ, Yu ZQ, Song XY, Lin Y, Li XM, et al. Long-term observation of ct-guided radiofrequency ablation of lung neoplasm in 476 consecutive patients by a thoracic surgical service: a single-institutional experience. Acad Radiol (2017) 24:1517–25. doi: 10.1016/j.acra.2017.06.018
48. Dupuy DE, Fernando HC, Hillman S, Ng T, Tan AD, Sharma A, et al. Radiofrequency ablation of stage ia non-small cell lung cancer in medically inoperable patients: results from the american college of surgeons oncology group z4033 (alliance) trial. Cancer-Am Cancer Soc (2015) 121:3491–8. doi: 10.1002/cncr.29507
49. De Baère T, Aupérin A, Deschamps F, Chevallier P, Gaubert Y, Boige V, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol (2015) 26:987–91. doi: 10.1093/annonc/mdv037
50. Møller H, Coupland VH, Tataru D, Peake MD, Mellemgaard A, Round T, et al. Geographical variations in the use of cancer treatments are associated with survival of lung cancer patients. Thorax (2018) 73:530–7. doi: 10.1136/thoraxjnl-2017-210710
51. Hiraki T, Gobara H, Mimura H, Sano Y, Tsuda T, Iguchi T, et al. Does tumor type affect local control by radiofrequency ablation in the lungs? Eur J Radiol (2010) 74:136–41. doi: 10.1016/j.ejrad.2009.01.026
52. Matsui Y, Hiraki T, Gobara H, Fujiwara H, Iguchi T, Shirakawa Y, et al. Percutaneous radiofrequency ablation for pulmonary metastases from esophageal cancer: retrospective evaluation of 21 patients. J Vasc Interv Radiol (2014) 25:1566–72. doi: 10.1016/j.jvir.2014.06.030
53. Hiraki T, Gobara H, Mimura H, Toyooka S, Fujiwara H, Yasui K, et al. Radiofrequency ablation of lung cancer at okayama university hospital: a review of 10 years of experience. Acta Med Okayama (2011) 65:287–97. doi: 10.18926/AMO/47010
54. Kodama H, Yamakado K, Hasegawa T, Fujimori M, Yamanaka T, Takaki H, et al. Radiofrequency ablation using a multiple-electrode switching system for lung tumors with 2.0-5.0-cm maximum diameter: phase ii clinical study. Radiology (2015) 277:895–902. doi: 10.1148/radiol.2015141153
55. Herrera LJ, Fernando HC, Perry Y, Gooding WE, Buenaventura PO, Christie NA, et al. Radiofrequency ablation of pulmonary Malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg (2003) 125:929–37. doi: 10.1067/mtc.2003.18
Keywords: lung cancer, pulmonary metastases, radiofrequency ablation, chemotherapy, systematic review, meta-analysis, RFA
Citation: Yang Z, Lyu X, Yang H, Wang B, Xu D, Huo L, Zhang R, Huang Y and Diao B (2023) Survival after radiofrequency ablation and/or chemotherapy for lung cancer and pulmonary metastases: a systematic review and meta-analysis. Front. Immunol. 14:1240149. doi: 10.3389/fimmu.2023.1240149
Received: 14 June 2023; Accepted: 20 September 2023;
Published: 06 October 2023.
Edited by:
Jie Mei, Wuxi People’s Hospital Affiliated to Nanjing Medical University, ChinaReviewed by:
Hashem Obaid Alsaab, Taif University, Saudi ArabiaGaia Piperno, European Institute of Oncology (IEO), Italy
Copyright © 2023 Yang, Lyu, Yang, Wang, Xu, Huo, Zhang, Huang and Diao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziyi Yang, MTg1MDgzMjMzMTBAMTYzLmNvbQ==
 Ziyi Yang
Ziyi Yang Xia Lyu
Xia Lyu