- 1Department of Dermatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
- 2Department of Immunology and Molecular Genetics, Kawasaki Medical School, Kurashiki, Japan
- 3Department of Molecular Oncology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
Considering the role of epidermal keratinocytes, they occupy more than 90% of the epidermis, form a physical barrier, and also function as innate immune barrier. For example, epidermal keratinocytes are capable of recognizing various cytokines and pathogen-associated molecular pattern, and producing a wide variety of inflammatory cytokines, chemokines, and antimicrobial peptides. Previous basic studies have shown that the immune response of epidermal keratinocytes has a significant impact on inflammatory skin diseases. The purpose of this review is to provide foundation of knowledge on the cytokines which are recognized or produced by epidermal keratinocytes. Since a number of biologics for skin diseases have appeared, it is necessary to fully understand the relationship between epidermal keratinocytes and the cytokines. In this review, the cytokines recognized by epidermal keratinocytes are specifically introduced as “input cytokines”, and the produced cytokines as “output cytokines”. Furthermore, we also refer to the existence of biologics against those input and output cytokines, and the target skin diseases. These use results demonstrate how important targeted cytokines are in real skin diseases, and enhance our understanding of the cytokines.
1 Introduction
In recent years, many biologics targeting cytokines have been clinically used for inflammatory skin diseases. Therefore, we must understand the importance of cytokines in the pathogenesis of the diseases. It is widely known that cytokines mainly function among immunocytes such as lymphocytes, but in fact, epidermal keratinocytes, which are resident cells, also recognize and produce various cytokines.
Epidermal keratinocytes occupy 90% or more of the epidermis, form a physical barrier (1). On the other hand, epidermal keratinocytes also form an innate immunological barrier with the potential to mount an innate immune response. For example, epidermal keratinocytes also express a variety of cytokine receptors, and microbial sensors such as Toll-like receptor (TLR) 1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR9, MDA5 (melanoma differentiation-associated gene 5) and RIG-I (retinoic acid-inducible gene-I) (2–6). Also, epidermal keratinocytes are capable of producing inflammatory cytokines and chemokines (5, 6). In addition, the cells show antibacterial activity by expressing antibacterial peptides such as defensins, cathelicidin, and S100 proteins (7). Through these immunological functions, epidermal keratinocytes play an important role in the pathogenesis of inflammatory skin diseases including atopic dermatitis (AD), psoriasis, several pustular dermatoses and so on (8–10).
The purpose of this review is to provide foundation of knowledge on the cytokines which are recognized or produced by epidermal keratinocytes. Since a number of biologics for skin diseases have appeared, it is necessary to fully understand the relationship between epidermal keratinocytes and the cytokines. We here focus on pro- or anti-inflammatory cytokines except growth factors in epidermal keratinocytes. The cytokines directly recognized by epidermal keratinocytes are specifically introduced as “input cytokines”, and the produced cytokines as “output cytokines”. Furthermore, we also refer to the existence of biologics against those input and output cytokines and the target skin diseases. Some of these biologics have already been approved and are in use, while others have not been shown to be effective. Recognizing these findings will enhance our understanding of the cytokines.
2 “Input cytokines” in epidermal keratinocytes
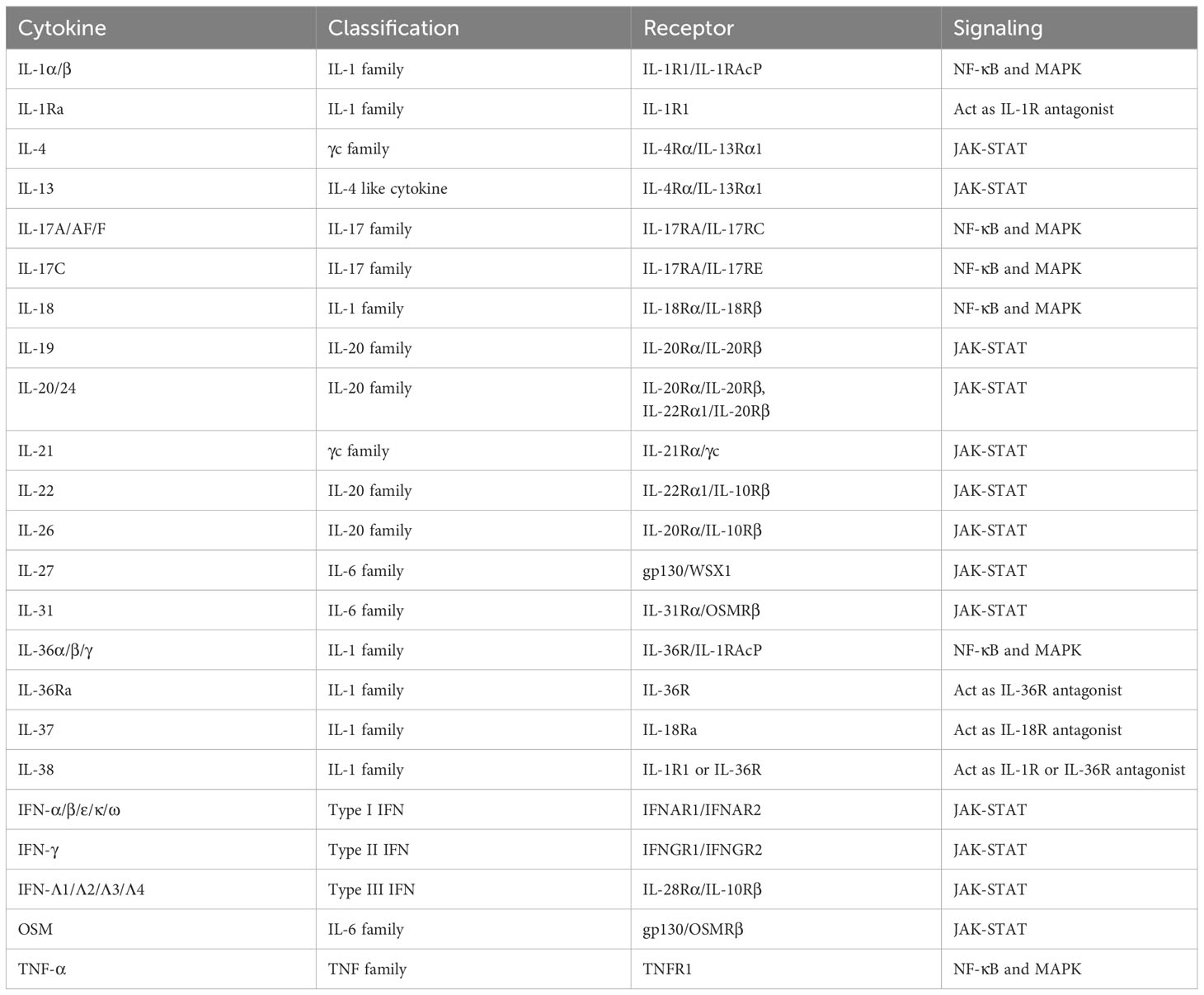
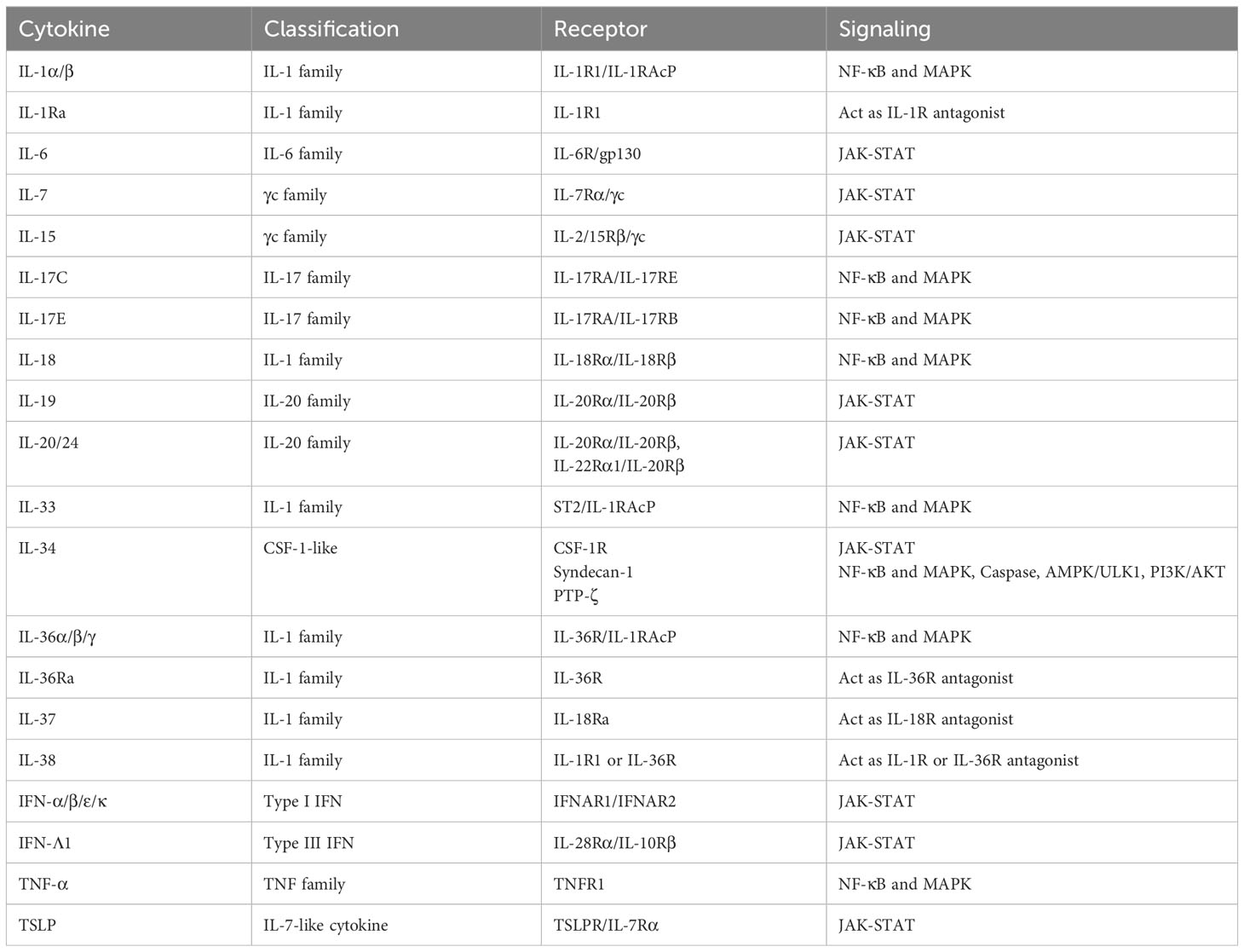
“Input cytokines” in epidermal keratinocytes include IL-1α/β/Ra, IL-4, IL-13, IL-17A/AF/C/F, IL-18, IL-19, IL-20, IL-21, IL-22, IL-24, IL-26, IL-27, IL-31, IL-36α/β/γ/Ra, IL-37, IL-38, IFN-α/β/ε/γ/κ/λ1/λ2/λ3/λ4/ω, oncostatin M (OSM) and TNF-α (Figures 1–3, Table 1).
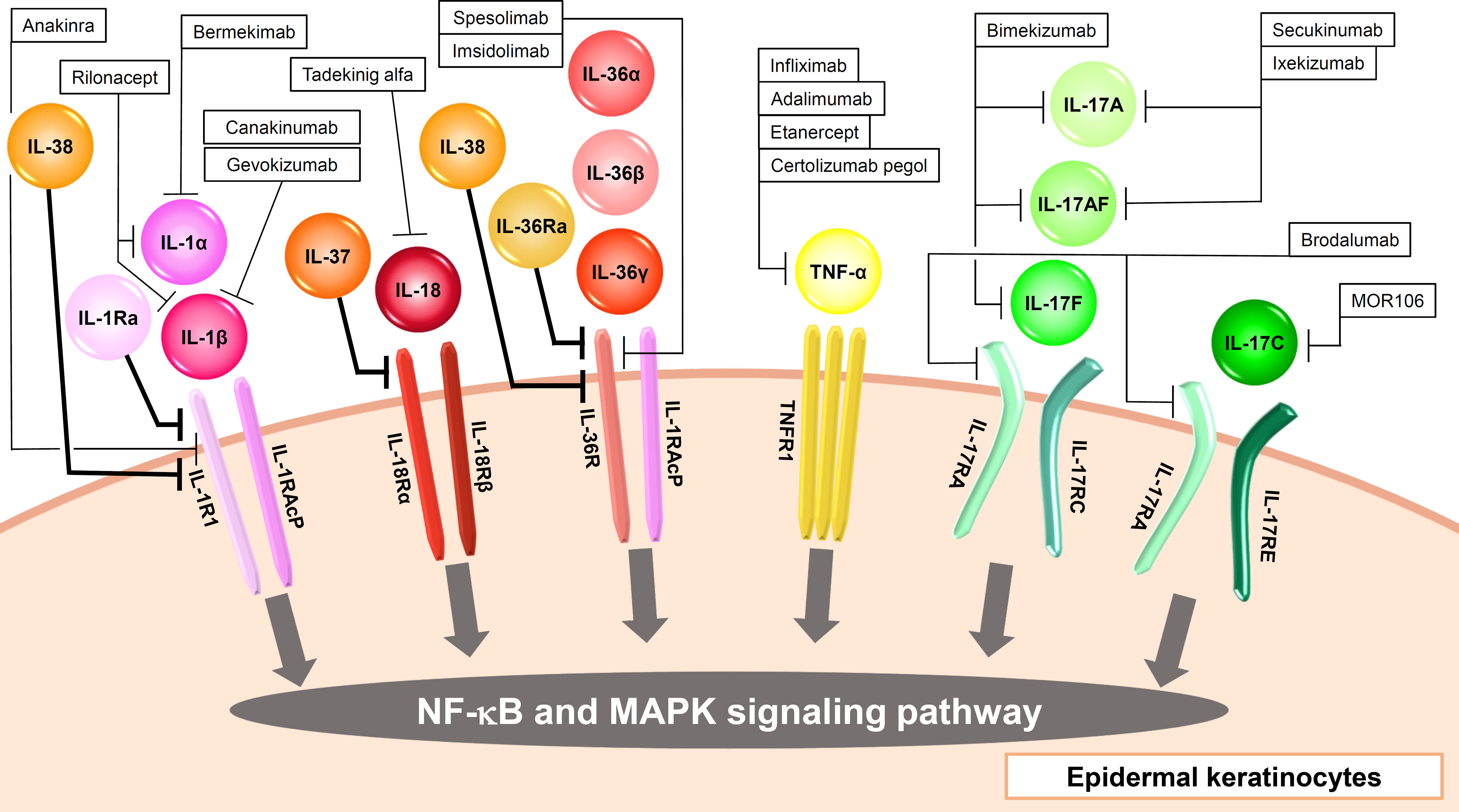
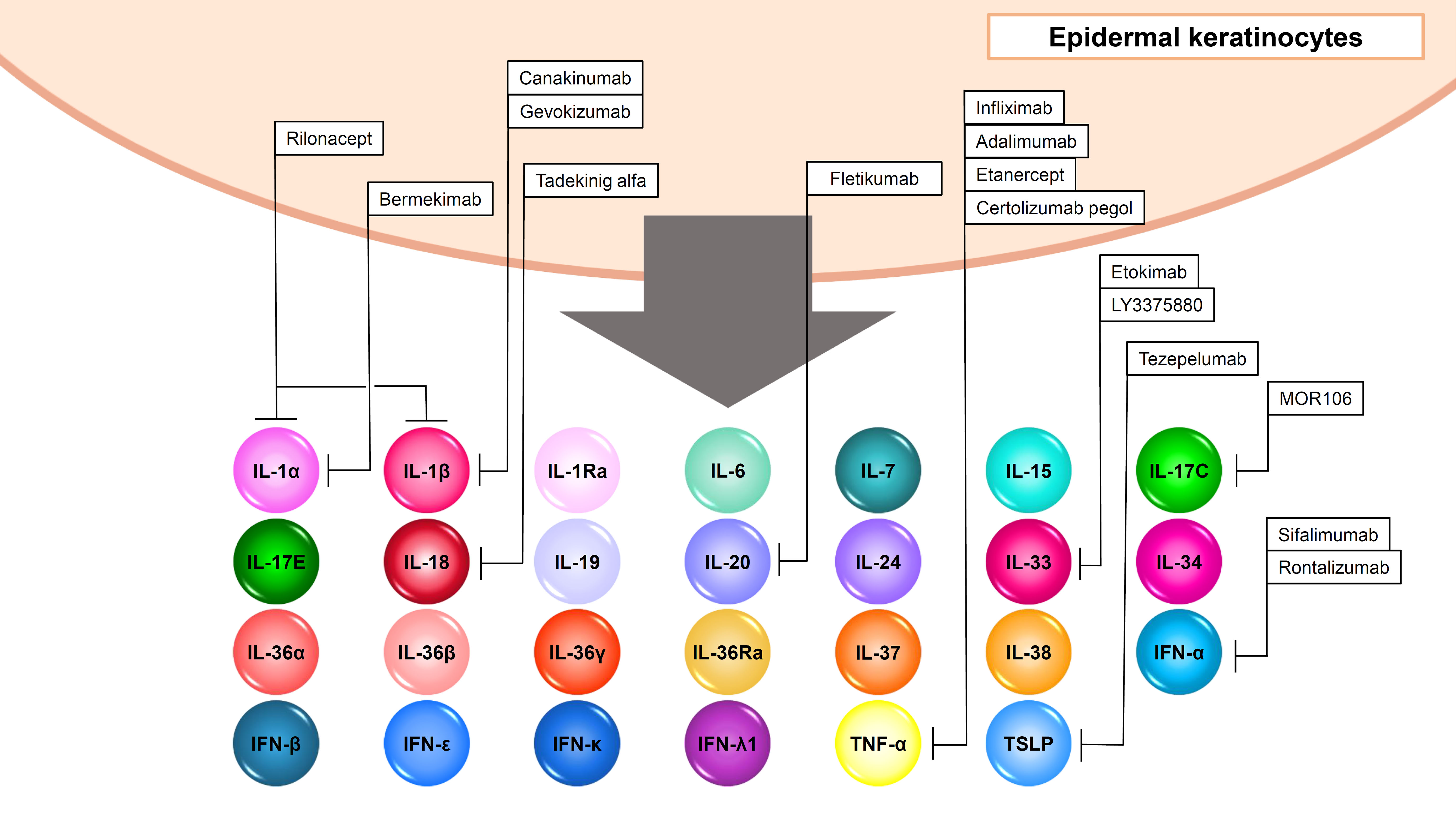
Figure 1 Input cytokines which activate NF-κB and MAPK signaling pathway in epidermal keratinocytes.
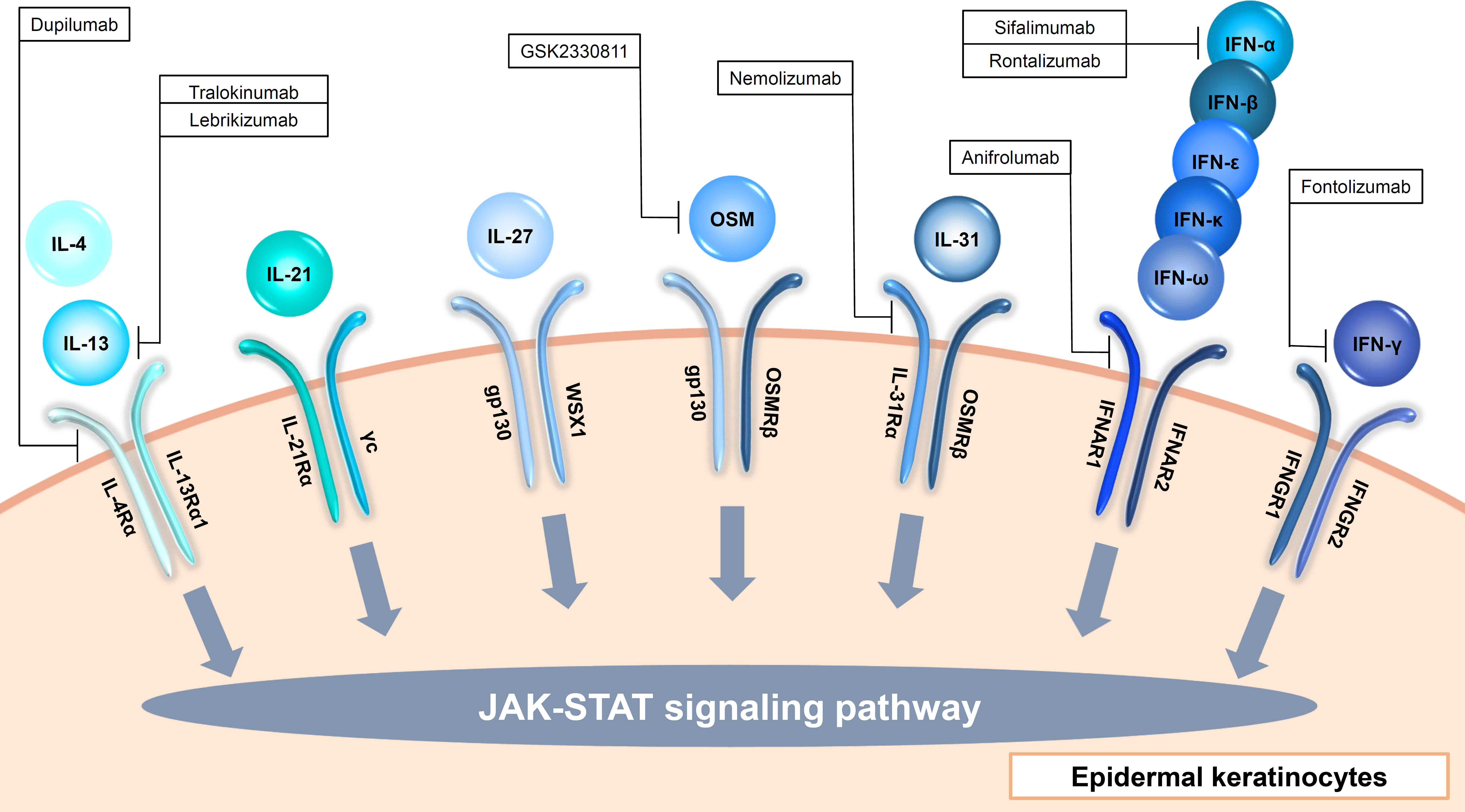
Figure 2 Input cytokines which activate JAK-STAT signaling pathway in epidermal keratinocytes (γc cytokines, IL-6 family cytokines, type I IFNs and type II IFN).
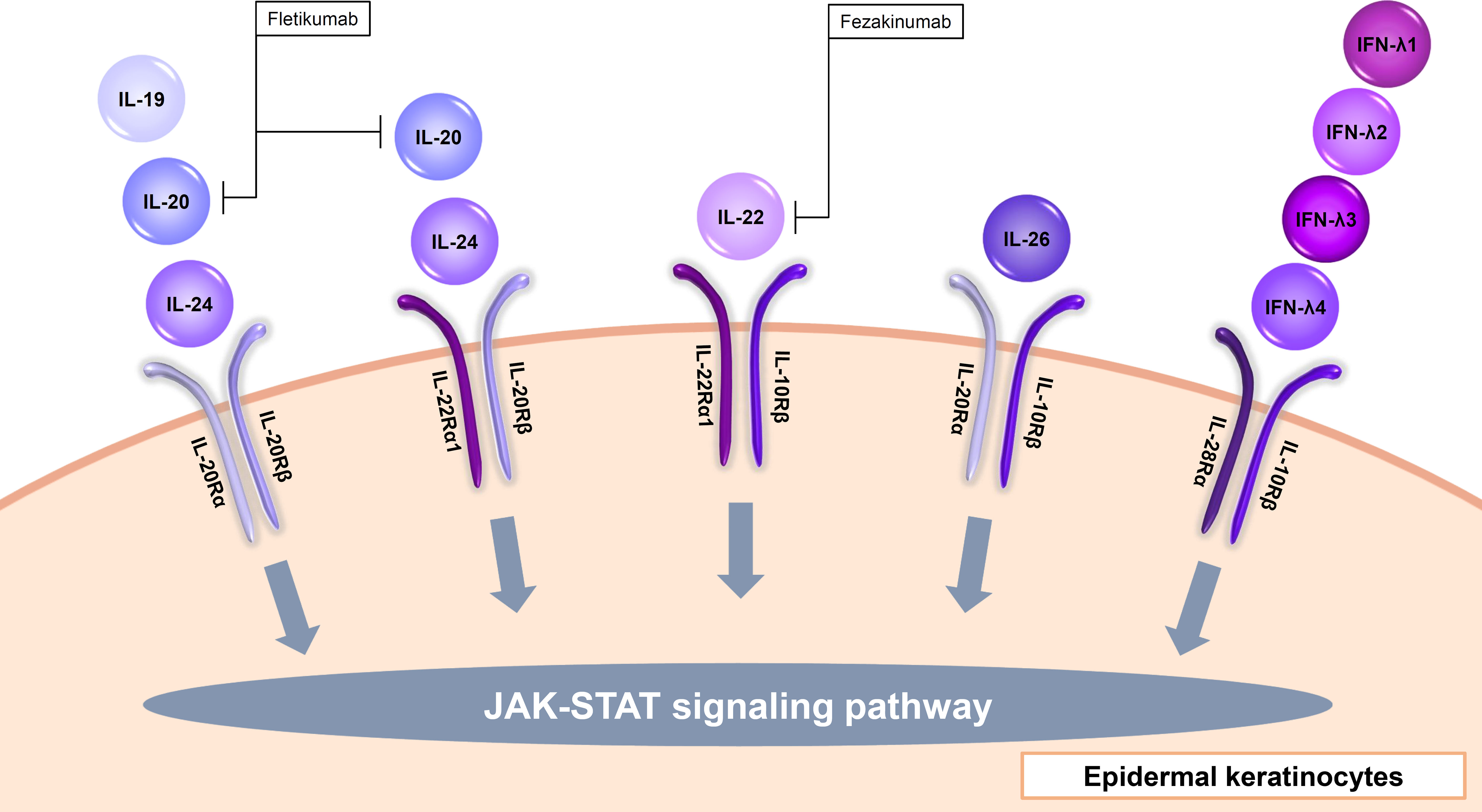
Figure 3 Input cytokines which activate JAK-STAT signaling pathway in epidermal keratinocytes (IL-20 family cytokines and type III IFNs).
2.1 IL-1 family cytokines
The IL-1 family consists of 11 cytokines which are further divided into inflammatory cytokines with agonistic activity (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, IL-36γ) and anti-inflammatory cytokines with antagonistic activity (IL-1Ra, IL-36Ra, IL-37, IL-38) (11). They are also classified into three subfamilies (IL-1, IL-18, IL-36 subfamily) according to their structures and receptors (11). Most of the human IL-1 family cytokine genes are located on chromosome 2, and the IL-18 and IL-33 genes are located on chromosomes 11 and 9, respectively (12). Among them, epidermal keratinocytes recognize IL-1α, IL-1β, IL-18, IL-36α, IL-36β, IL-36γ, IL-1Ra, IL-36Ra, IL-37, and IL-38 (Figure 1, Table 1).
IL-1α and IL-1β bind to IL-1R1, and this binding signals via TIR-MyD88, leading to NF-κB and MAPK activation (Figure 1, Table 1) (13). IL-1RAcP is the co-receptor for IL-1R1 (Figure 1, Table 1). IL-1-bound IL-1RI associates with IL-1RAcP to form a heterodimer. Signal transduction requires the presence of IL-1RI and IL-1RAcP molecules. IL-1α is produced as precursors and activated by calpain (10). IL-1β is also produced as precursors and activated by casepase-1. IL-1α and IL-1β induce inflammatory cytokines including TNF-α and IL-6, and chemokines including IL-8 in epidermal keratinocytes (14). IL-1Ra binds to IL-1R1, and this cytokine work as the inhibitor of IL-1α and IL-1β (Figure 1, Table 1) (13).
IL-1 signaling is thought to play an important role in not only autoinflammatory diseases but also various inflammatory skin diseases. Therefore, it has attracted attention as a therapeutic target. Anakinra, a recombinant IL-1Ra which blocks the activities of the proinflammatory cytokines IL-1α and IL-1β, is clinically used for rheumatoid arthritis (RA), neonatal-onset multisystem inflammatory disease (NOMID), cryopyrin-associated periodic syndromes (CAPS), systemic juvenile idiopathic arthritis (sJIA), adult-onset Still disease (AOSD), Schnitzler’s Syndrome (SS), and deficiency of IL-1RA (DIRA) (15). However, it was not significantly effective in a phase II randomized, double-blind clinical trial for palmoplantar pustulosis (PPP) (16). On the other hand, another group reported that anakinra up to 300mg daily showed positive responses with localized and generalized pustular psoriasis (GPP) in a phase II open-label trial (17). Anakinra has also been used in hidradenitis suppurativa (HS) by several groups with controversial results (10). Rilonacept is an IL-1 receptor fusion protein consisting of the Fc portion of human IgG1 and the human IL-1 receptor which traps both IL-1α and IL-1β, and clinically used for familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS), and recurrent pericarditis (15). A clinical trial for cold contact urticaria (CCU) is currently ongoing with this agent (18). Canakinumab is a human anti-IL-1β monoclonal antibody, and clinically used for FCAS, MWS, CAPS, familial Mediterranean fever (FMF), mevalonate kinase deficiency (MKD), tumor necrosis factor receptor-associated periodic syndrome (TRAPS), and AOSD (15). Canakinumab has also shown contradictory efficacy results in HS (10). In an open-label prospective study, this agent was effective for pyoderma gangrenosum (PG) (10). Bermekimab, a human anti-IL-1α monoclonal antibody, showed efficacy in phase II open-label studies in HS patients (10). Gevokizumab is a humanized anti-IL-1β monoclonal antibody, and clinical trials for PG are currently ongoing with this agent (18).
IL-18 binds to IL-18Rα, and this binding signals via TIR-MyD88, leading to NF-κB and MAPK activation (Figure 1, Table 1) (13). IL-18Rβ is the co-receptor for IL-18Rα (Figure 1, Table 1). Like IL-1 signaling, the signal transduction requires the heterodimerization of IL-18Rα and IL-18Rβ (Figure 1, Table 1) . IL-18 is produced as precursors and activated by casepase-1 (10). Epidermal keratinocytes express IL-18Rα and IL-18Rβ. When IL-18 binds to these receptors on the surface of keratinocytes, it triggers a signaling cascade within the cells, leading to various cellular responses such as the induction of CXCL9, CXCL10, CXCL11, major histocompatibility complex (MHC) class I, and MHC class II expression (19, 20). IL-18 is considered to be involved in the pathogenesis of psoriasis, AD, and AOSD, and tadekinig alfa, a human recombinant IL-18-binding protein, is currently investigated in a phase II open-label clinical trial on patients with AOSD (18).
IL-36α, IL-36β, and IL-36γ bind to IL-36R, and these binding signal via TIR-MyD88, leading to NF-κB and MAPK activation (Figure 1, Table 1) (13). IL-1RAcP is the co-receptor for IL-36R (Figure 1, Table 1). Like IL-1 and IL-18 signaling, the signal transduction requires the heterodimerization of IL-36R and IL-1RAcP (Figure 1, Table 1). IL-36 cytokines are produced as precursors and activated by neutrophil-derived proteases (10). Similar to IL-1α and IL-1β, IL-36α, IL-36β, and IL-36γ induce TNF-α, IL-6, IL-8, G-CSF, GM-CSF, CXCL1, CXCL10, CCL20, and RANTES in epidermal keratinocytes (21, 22).
IL-36Ra binds to IL-36R, and this cytokine works as the inhibitor of IL-36α, IL-36β, and IL-36γ (Figure 1, Table 1) (13). Deficiency of IL-36Ra develop GPP, which suggests the importance of IL-36 signaling in the disease (23). In fact, spesolimab, a humanized anti-interleukin-36 receptor monoclonal antibody which blocks human IL-36α-, IL-36β-, and IL-36γ-induced IL-36R activation, show significant clinical improvement in GPP (24). Additional studies of spesolimab are currently being performed in patients with PPP and HS (10). Imsidolimab is also a humanized anti-interleukin-36 receptor monoclonal antibody which blocks human IL-36α-, IL-36β-, and IL-36γ-induced IL-36R activation, and clinical trials for HS and GPP are currently ongoing with this agent (10).
IL-37 is an anti-inflammatory cytokine, and reported to suppress the production of CXCL8, IL-6, and S100A7 which are induced by the mixture of five proinflammatory cytokines in human keratinocyte cell line HaCaT cells (25). Extracellularly, IL-37 binds to IL-18Ra and recruits IL-1R8 to form the IL-37/IL-1R8/IL-18Ra complex, inhibiting IL-18R-dependent inflammation (10).
IL-38 is also anti-inflammatory cytokine, and reported to inhibit IL-36γ-induced inflammatory molecules in epidermal keratinocytes (26). IL-38 binds to IL-1RAcP or IL-36R, and works as the inhibitor of IL-1α/β or IL-36α/β/γ, respectively (10).
2.2 Gamma chain cytokines
The γc cytokines family consists of IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, and among them, IL-4 and IL-21 are input cytokines in epidermal keratinocytes (Figure 2, Table 1) (27). IL-13, another type 2 cytokine which shares IL-4Rα and IL-13Rα1 with IL-4, is also an input cytokine in the cells (Figure 2, Table 1) (28). Th2 cells release IL-4 and IL-13, and type 2 innate lymphoid cells produce IL-13 (29). The IL-4 and IL-13 signaling in the cells decrease the expression of filaggrin, loricrin, an involucrin via JAK-STAT pathway (30, 31). These cytokines also suppress ceramide synthesis and inhibit the expression of elongases which lengthen fatty acid chain in the cells (32–34). In addition, antimicrobial peptides expression is also suppressed by IL-4 and IL-13 in the cells, which enhances the susceptibility to infection (35). Furthermore, IL-4 and IL-13 increase serine protease KLK7 expression and function in the cells (36). Recently, these cytokines were also reported to impair TLRs-mediated barrier functions in the early phases of AD (37). These findings suggest that IL-4 and IL-13 contribute to not only allergic inflammation but also barrier dysfunction. The importance of IL-4 and IL-13 in skin diseases is found in recent biologics. Anti-IL-4Rα antibody dupilumab which blocks both IL-4 and IL-13 signaling and anti-IL-13 antibody including tralokinumab and lebrikizumab show clinical efficacy in AD (38–41). In addition, dupilumab represents significant improvement in prurigo nodularis (PN) (42).
IL-21 is produced by NKT and CD4(+) T cells, and signals via JAK-STAT pathway (Figure 2, Table 1) (43). IL-21R is up-regulated in patients with systemic sclerosis (SSc) and might be involved in the pathogenesis of SSc via induction of VEGF (44). IL-21 is also highly expressed in the skin of individuals with psoriasis, and stimulates epidermal keratinocytes to proliferate and causes epidermal hyperplasia (45).
2.3 IL-6 family cytokines
The IL-6 family consists of 11 cytokines and shares 130-kDa signal-transducing β-receptor subunit gp130, except IL-31 (46–48). All the cytokines activate JAK-STAT signaling pathway. Among them, IL-27 induces CXCL9, CXCL10, CCL2, CCL5, and enhance anti-viral activity in epidermal keratinocytes (49–51). Another IL-6 family member, OSM, is also recognized via gp130 and OSM receptor beta (OSMRβ) by epidermal keratinocytes (Figure 2, Table 1) (52). OSM is produced by T cells, monocytes, macrophages, hepatocytes and endothelial cells (52). OSM is involved with innate immunity, angiogenesis, adhesion, motility, tissue remodeling, cell cycle and transcription in epidermal keratinocytes (52, 53). Since this cytokine show synergy with TNF-α, IL-1α, IL-17A, and IL-22 in production of antimicrobial peptides, it is considered to be involved in pathogenesis of psoriasis (53). OSM is also implicated in the pathogenesis of SSc, and a randomized phase 2 study ofan anti-OSM monoclonal antibody GSK2330811 in SSc was conducted. However, its effects were not different from placebo (54). IL-31 is also an input cytokine which signals through heterodimeric receptors composed of the OSMRβ and the interleukin 31 receptor alpha (IL-31Rα) (Figure 2, Table 1) (48). IL-31 is mainly produced by Th2 cells, and suppresses the skin barrier protein expression such as filaggrin and involucrin and induces the expression of several chemokines in epidermal keratinocytes (55, 56). IL-31Rα is also expressed in sensory nerves and IL-31 promotes nerve fiber extension, suggesting that IL-31 is involved in pruritus in AD (57). Actually, nemolizumab, a humanized monoclonal antibody against IL-31Rα which blocks signaling from IL-31, provides improvement of pruritis in patients with AD in a 16-week, double-blind, phase 3 trial (58).
2.4 IL-17 family cytokines
The IL-17 family consists of 6 homodimers IL-17A to IL-17F and 1 heterodimer IL-17AF (59). On the other hand, the IL-17 receptor family consists of 5 molecules, IL-17RA-RE (59). IL-17RA is a common receptor and forms heterodimeric complexes with IL-17RB, IL-17RC and IL-17RE. Epidermal keratinocytes recognize IL-17A, IL-17C, IL-17F, and IL-17AF and then strongly produce inflammatory cytokines, chemokines, and antimicrobial peptides (Figure 1, Table 1) (60). IL-17A, IL-17AF, and IL-17F are mainly produced by Th17 cells, and share the heterodimeric receptor of IL-17RA and IL-17RC (Figure 1, Table 1) (59). Binding of these cytokines to their receptors recruits Act1 to which TRAF6 binds. TAK1-NF-κB and MAPK-AP1 axes are activated downstream of TRAF6. IL-17C are produced by epithelial cells rather than immune cells, and binds to the heterodimeric receptor of IL-17RA and IL-17RE, and shows similar activation to IL-17A (Figure 1, Table 1). However, the ability to induce inflammation in epidermal keratinocytes is reported to be stronger in the order of IL-17A, IL-17AF, IL-17F, and IL-17C (60). These cytokines, especially IL-17A, are considered to play a critical role in the pathogenesis of psoriasis, and anti-IL-17A antibody including secukinumab and ixekizumab, anti-IL-17A/IL-17F antibody bimekizumab, and anti-IL-17RA antibody brodalumab which blocks the signaling of IL-17A, IL-17A/F, IL-17F, IL-17C, and IL-17E, show high clinical efficacy in psoriasis (61–64). Secukinumab and bimekizumab are also reported to be clinically effective in HS (65, 66).
2.5 IL-20 family cytokines
The IL-20 family consists of IL-19, IL-20, IL-22, IL-24, IL-26, and type III IFNs. IL-19, IL-20, and IL-24 signal through the IL-20Rα/IL-20Rβ heterodimer. Furthermore, IL-20 and IL-24 also signal through the IL-22Rα1/IL-20Rβ heterodimer (Figure 3, Table 1) (67). IL-19, IL-20, and IL-24 is mainly produced by myeloid cells but can also be produced by epidermal keratinocytes (68). TNF-α and IFN-γ enhance IL-20Rα expression in the cells (69). These cytokines all induce epidermal keratinocytes to proliferate and to express inflammatory and immunomodulatory mediators through activation of STAT3 (67). IL-20 was considered to be involved in the pathogenesis of psoriasis, and a phase I study with an anti-IL-20 monoclonal antibody fletikumab for psoriasis was conducted, however the study was terminated due to lack of efficacy (70).
IL-22 is known to exert protective functions in barrier defense, tissue repair, and homeostasis depending on the context, in various organs including the skin (71). Epidermal keratinocytes recognize IL-22 through the IL-22Rα1 and IL-10Rβ heterodimer (Figure 3, Table 1) (72). IL-22 is mainly produced by Th1, Th17, and Th22 cells and also type 3 innate lymphoid cells (72–74). IL-22 up-regulates, in a dose-dependent manner, the expression of S100A7, S100A8, S100A9, a group of proinflammatory molecules belonging to the S100 family of calcium-binding proteins, as well as the matrix metalloproteinase 3, the platelet-derived growth factor A, and the CXCL5 chemokine (75). IL-22 also down-regulates the expression of genes associated with keratinocyte differentiation such as filaggrin (75). In addition, IL-22 strongly induces hyperplasia of reconstituted human epidermis (75). Therefore, IL-22 is considered to contribute to the acanthosis in psoriasis and lichenification in AD. However, the inhibitors of and IL-22 (fezakinumab) did not show sufficient improvement in psoriasis (70). On the other hand, fezakinumab, anti-IL-22 antibody, showed clinical efficacy in moderate-to-severe AD (76).
IL-26 is an input cytokine in epidermal keratinocytes. IL-26 is produced mainly by Th1, Th17, or natural killer cells (77, 78). IL-26R is a heterodimer composed of two receptor proteins: IL-20Rα and IL-10Rβ (Figure 3, Table 1) (79). IL-26 enhances the production of FGF1, FGF2, and FGF7 from epidermal keratinocytes and vascular endothelial cells (80). These may promote angiogenesis in patients with T cell-mediated skin inflammation, including psoriasis (80). IL-26 enhanced IL-8, IL-1β, CCL20, IL-33, and β-defensin 2 expression via JAK1, JAK2, and TYK2 in normal human epidermal keratinocytes (81). These may be involved in the pathogenesis of AD (81).
2.6 type I interferons
Type I interferon (IFN) members consist of IFN-α, IFN-β, IFN-ϵ, IFN-κ and IFN-ω, and bind to the heterodimeric receptor of IFN-α/β receptor 1(IFNAR1) and 2(IFNAR2), resulting in the activation of JAK1 and non-receptor tyrosine kinase 2 (TYK2) and the formation of STAT1-STAT2-IRF9 complex which is called ISGF3 (82). Almost all cell types produce type I IFNs (82). Since epidermal keratinocytes express both IFNAR1 and IFNAR2, the cells recognize type I IFNs (Figure 2, Table 1) (83). For example, IFN-κ induces IFN-κ expression itself and enhances the anti-viral activity against HSV-1 in epidermal keratinocytes (84). In addition, IFN-α and IFN-κ increase IL-6 production in the cells, which is considered to be associated with the pathogenesis of cutaneous lupus erythematosus (85).
2.7 type II interferon
Epidermal keratinocytes also recognize Type II IFN, IFN-γ which is produced by T cells, B cells, NK cells, NKT cells, and dendritic cells (86). IFN-γ binds to the heterodimeric IFN-γ receptor (IFNGR) complex comprising IFNGR1 and IFNGR2 (Figure 2, Table 1) (86). The signal phosphorylates and activates JAK1, JAK2, and STAT1, which leads to the homodimerization of STAT1 (86). Stimulation with IFN-γ in epidermal keratinocytes increases terminal differentiation of cells, inhibits proliferation, and enhance anti-viral activities (87, 88). Furthermore, IFN-γ cooperates with TNF-α and IL-17A to induce the production of cytokines, chemokines, and antimicrobial peptides (89–91). IFN-γ is increased in the skin lesions of psoriasis, and the disease was previously considered to be a Th1 disease. Therefore, a clinical trial with humanized anti–IFN-γ antibody (Fontolizumab) for moderate-severe plaque psoriasis was performed, however, no significant clinical changes were observed (92).
2.8 type III interferons
Type III IFNs, including IFN-λ1 (IL-29), IFN-λ2 (IL-28A), IFN-λ3 (IL-28B), and IFN-λ4, are involved in inhibiting viral infection similar to type I IFNs (93, 94). Type III IFNs act via the heterodimer of IL-28Rα and IL-10Rβ (Figure 3, Table 1) (93). These cytokines are input cytokines in epidermal keratinocytes, for example, IFN-λ1 is shown to enhance anti-viral activity through an increase in TLR3 in the cells (95).
2.9 tumor necrosis factor
Epidermal keratinocytes recognize TNF-α. TNF-α was discovered as a necrotic cytokine in solid tumors and later turned out to be a major cytokine involved in inflammation (96). TNF-α is produced from almost all cells, and are thought to exist both upstream and downstream of the pathological cascade of various inflammatory diseases. Since epidermal keratinocytes express TNFR1 receptors and produce TNF-α, autocrine phenomena are observed and an inflammatory loop is formed (Figure 1, Table 1) (97). The importance of TNF-α in various skin diseases is easily found in the clinical use of anti-TNF-α antibodies against the diseases. TNF-α inhibitors including infliximab, adalimumab, etanercept, and certolizumab pegol are clinically effective in psoriasis (8). Infliximab and adalimumab also show clinical efficacy in HS, and PG (98–101).
3 “Output cytokines” in epidermal keratinocytes
“Output cytokines” in epidermal keratinocytes include IL-1α/β/Ra, IL-6, IL-7, IL-15, IL-17C, IL-17E (IL-25), IL-18, IL-19, IL-20, IL-24, IL-33, IL-34, IL-36α/β/γ/Ra, IL-37, IL-38, IFN-α/β/ε/κ/λ1, thymic stromal lymphopoietin (TSLP), and TNF-α (Figure 4, Table 2).
3.1 IL-1 family cytokines
Epidermal keratinocytes produce inflammatory IL-1 family cytokines with agonistic activity including IL-1α, IL-β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ and anti-inflammatory IL-1 family cytokines with antagonistic activity including IL-1Ra, IL-36Ra, IL-37, and IL-38 (Figure 4, Table 2). Since IL-1α, IL-β, IL-18, IL-36α, IL-36β, and IL-36γ are also input cytokines and are capable of inducing themselves, inflammation loops are formed in the cells (Figures 1, 4, Tables 1, 2) (14, 102).
IL-33 is an IL-1-family cytokine that is over-expressed in the keratinocytes of patients with AD (103, 104). IL-33 is also in an activated state in the precursor and is rather inactivated when cleaved by caspase-1 or caspase-3 (105). IL-33 activates type 2 innate lymphoid cells which induce type 2 inflammation by producing IL-5 and IL-13 (103, 106). Therefore, IL-33 is thought to be involved in the pathogenesis of AD. However, anti-IL-33 antibody LY3375880 and etokimab or anti-IL-33 receptor ST2 antibody astegolimab did not show significant clinical improvement in AD (107–109).
3.2 Gamma chain cytokines
Among γc cytokines, epidermal keratinocytes produce IL-7 and IL-15 (Figure 4, Table 2) (27). IL-7 is produced under the stimuli with IFN-γ (110). IL-15 expression is increased in vitiligo epidermis, and is induced by oxidative stress via NF-κB (111). IL-7 and IL-15 derived from hair follicle keratinocytes regulate skin-resident memory T cell homeostasis (112). In a mouse model of alopecia areata, blockade of IL-7 signaling with anti-mouse IL-7Rα antibody suppressed inflammatory responses and reversed alopecia areata (113). Also, in a mouse model of vitiligo, blocking IL-15 signaling with an antibody reversed the disease symptoms (114).
Epidermal keratinocytes express TSLP which is an epithelial-derived IL7-like cytokine and initiate or perpetuate the Th2-type allergic inflammation via dendritic cells or group 2 innate lymphoid cells (Figure 4, Table 2) (115, 116). TSLP mediates STAT5 phosphorylation via kinases JAK1 and JAK2 (Table 2) (117). The levels of TSLP is significantly increased in the lesional skin of AD, indicating that TSLP is important for initiating the systemic Th2 immunity favorable for the development of allergic inflammation (115). Against this background, a randomized phase 2a clinical trial of the anti-TSLP monoclonal antibody tezepelumab in the treatment of moderate-to-severe AD patients was conducted but did not reach the targeted level of efficacy (118).
3.3 IL-6 family cytokines
IL-6, the first cytokine discovered in the IL-6 family, activates the JAK-STAT pathway and induces inflammation (46, 47). Epidermal keratinocytes also produce IL-6 under the stimuli with some TLR ligands, UVB, TNF-α, IL-17, IFN-γ, and so on (Figure 4, Table 2) (119–122). Anti-IL-6 receptor antibody such as tocilizumab shows clinical efficacy in rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), giant cell arteritis (GCA), and Castleman’s disease (CD) (123). The efficacy of tocilizumab in morphea, SSc, psoriasis, AD, vitiligo or PG has been also reported in case series, however, higher-level evidences have not been shown in these skin diseases (123).
3.4 IL-17 family cytokines
Epidermal keratinocytes produce IL-17C and IL-17E (IL-25) (Figure 4, Table 2). IL-17C controls the innate immune activity of epithelial cells in an autocrine manner (124). IL-17C is induced by TNF-α, IL-17A, and IFN-γ in epidermal keratinocytes (91). Anti-IL-17C antibody MOR106 showed no significant clinical improvement in AD although it was reported to be effective in mouse experiments (125, 126). IL-17E is produced by various cell types and induces Th2 responses (59). In AD, IL-17E derived from epidermal keratinocytes activates type 2 innate lymphoid cells, which drive IL-13 production (127). Therefore, IL-17E is considered to play an important role in the pathogenesis in AD. IL-17E is shown to be induced by IL-17A and IL-22 in epidermal keratinocytes (Figure 4, Table 2) (128).
3.5 IL-20 family cytokines
As described above, IL-19, IL-20, and IL-24 are input and output cytokines in epidermal keratinocytes (Figures 3, 4, Tables 1, 2). TNF-α, IL-17A, and IL-22 induces IL-19, IL-20, and IL-24 production in the cells (68, 129–131). These cytokines are considered to enhance psoriatic inflammation (130, 131).
3.6 type I interferons
Type I IFNs including IFN-α, IFN-β, IFN-ϵ, and IFN-κ are also output cytokines in epidermal keratinocytes (Figure 4, Table 2) (84). These cytokines are induced by TLR3 and TLR9 signaling, or Type I IFNs themselves in the cells (2, 84, 132). Since type I IFNs are considered to stimulate myeloid dendritic cells which produce IL-23 and contribute to the pathogenesis of psoriasis, randomized, double-blind, placebo-controlled, phase I study of MEDI-545 (Sifalimumab), an anti-IFN-α monoclonal antibody for plaque psoriasis was performed, however, it showed no significant clinical improvement (133). Sifalimumab was also expected to be a treatment for systemic lupus erythematosus (SLE), but the clinical trial was discontinued in favor of anifrolumab (134). Anifrolumab, a monoclonal antibody that binds to IFNAR1, therefore blocking the activity of all type I IFNs, are demonstrated to improve skin and joint disease activity in patients with SLE (134). Rontalizumab is also a monoclonal antibody, and did not show clinical efficacy including a phase 2 trial in SLE patients (134).
3.7 type III interferons
Among type III IFNs, IFN-λ1 is shown produced by epidermal keratinocytes stimulated with TLR3 ligand poly (I:C) or vesicular stomatitis virus (Figure 4, Table 2) (135). IFNλ and the IFNλ receptor are strongly expressed in the epidermis of cutaneous lupus erythematosus (CLE), SLE, lichen planus (LP) and dermatomyositis (135).
3.8 tumor necrosis factor
TNF-α is also an input and output cytokine as described above (Figures 1, 4, Tables 1, 2). TNF-α is induced by TNF-α itself, IL-1β, IL-17A, TLR ligands including poly (I:C), LPS, flagellin, CpG, ultraviolet light, anisomysin, palmitic acid and so on (4, 131, 136, 137).
3.9 Others
IL-32 is a proinflammatory cytokine which is produced by a variety of cells, including NK cells, T cells, monocytes, and epithelial cells (138, 139). IL-32 expression is increased in the epidermis of AD lesions, and the expression is induced by TNF−α and/or IFN-γ in cultured epidermal keratinocytes (139). However, IL-32 is not secreted by the cells and remains in the cells; therefore, this cytokine cannot be called an output cytokine in epidermal keratinocytes (139). This cytokine is considered to modulate keratinocyte apoptosis and contribute to the pathogenesis of AD (139).
IL-34 is an output cytokine in epidermal keratinocytes (Figure 4, Table 2). It exists as a homodimer consisting of 39 kDa monomers (140). IL-34 has no evident sequence homology with other cytokines (141). Likewise, IL-34 has only a 26% sequence homology with colony-stimulating factor 1 (CSF-1), yet they share a common receptor known as CSF-1R (Table 2) (141, 142). Furthermore, IL-34 has exhibits interactions with two distinct receptors: protein-tyrosine phosphatase (PTP)-ζ, and syndecan-1 (Table 2) (141). Through the investigation of IL-34-deficient (Il34LacZ/LacZ) reporter mice, it was found that keratinocytes and neurons were the main sources of IL-34 (143). Especially, IL-34 is highly expressed in the epidermis during murine embryogenesis (144). CSF-1R is expressed by dendritic cells (DCs) and macrophages, excluding CD11c+ precursors of DCs, whereas PTP-ζ is expressed by neural progenitors, glia, glioblastoma, B cells, and kidney tubular cells (141). Syndecan-1 is expressed by many cancers, such as myeloma, melanoma (141). IL-34 is considered to regulate major cellular functions, including cell adhesion, motility, proliferation, differentiation, survival, metabolism, and cytokine/chemokine expression (141).
IL-39 is a cytokine composed of IL-23Ap19 and Epstein–Barr virus-induced (EBI) 3 heterodimer which was firstly reported in 2015 (145). This cytokine is shown to be produced by B cell lymphocytes and activate neutrophils (146, 147). Our group researched about the expression of IL-39 in human epidermal keratinocytes, however our ELISA experiment and LC-Ms/Ms analyses did not detect the heterodimeric cytokine IL-39 in epidermal keratinocytes (148). So far, this cytokine cannot be called an output cytokine in epidermal keratinocytes Table 3.
4 Conclusion
In this review, we introduced that epidermal keratinocytes recognize and produce a large number of cytokines and are deeply involved in the pathogenesis of these diseases. The number of output cytokines appears to be lower compared to that of input cytokines in the cells. This might suggest that epidermal keratinocytes are cells that are responsible for innate immunity rather than adaptive immunity, and that they are excellent at functioning as sensor cells rather than the control tower. The immunological functions of epidermal keratinocytes in innate immunity requires further investigation.
We also referred to the existence of biologics against those input and output cytokines and the target skin diseases. Current biologics have a significant impact on immune cells throughout the body, which can lead to side effects such as serious infections. If we could target only cytokines derived from epidermal keratinocytes through the development of drug delivery that specifically acts on cells, it will be possible to suppress only excessive immune reactions in the skin caused by pathological activation of epidermal keratinocytes, which should be a safer treatment.
Author contributions
SM, YK, and KS wrote the manuscript. TM, KT, and MO contributed to writing and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study received funding from a Grant-in-Aid for Scientific Research (C) (no. 20K08672). The authors declare that this study received funding from Sun Pharma Ltd., AbbVie GK, Maruho Co., Ltd., Eli Lilly Japan Inc., and LEO Pharma K.K. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank Hiroto Morizane for making the figures in this manuscript.
Conflict of interest
SM received research support from Sun Pharma Ltd., AbbVie GK, and Maruho Co., Ltd., and honoraria for lectures from Eli Lilly Japan K.K., AbbVie GK, Pfizer Japan Inc., Torii Pharmaceutical Co., Ltd, Sanofi K.K., and Maruho Co., Ltd. D. TM received scholarship donations from Eli Lilly Japan, Inc., LEO Pharma K.K., and AbbVie.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Scieglinska D, Krawczyk Z, Sojka DR, Gogler-Piglowska A. Heat shock proteins in the physiology and pathophysiology of epidermal keratinocytes. Cell Stress Chaperones (2019) 24(6):1027–44. doi: 10.1007/s12192-019-01044-5
2. Morizane S, Yamasaki K, Muhleisen B, Kotol PF, Murakami M, Aoyama Y, et al. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J Invest Dermatol (2012) 132(1):135–43. doi: 10.1038/jid.2011.259
3. Kalali BN, Kollisch G, Mages J, Muller T, Bauer S, Wagner H, et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J Immunol (2008) 181(4):2694–704. doi: 10.4049/jimmunol.181.4.2694
4. Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol (2007) 127(2):331–41. doi: 10.1038/sj.jid.5700530
5. Chieosilapatham P, Kiatsurayanon C, Umehara Y, Trujillo-Paez JV, Peng G, Yue H, et al. Keratinocytes: innate immune cells in atopic dermatitis. Clin Exp Immunol (2021) 204(3):296–309. doi: 10.1111/cei.13575
6. Jiang Y, Tsoi LC, Billi AC, Ward NL, Harms PW, Zeng C, et al. Cytokinocytes: the diverse contribution of keratinocytes to immune responses in skin. JCI Insight (2020) 5(20):e14206. doi: 10.1172/jci.insight.142067
7. Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol (2012) 39(3):225–30. doi: 10.1111/j.1346-8138.2011.01483.x
8. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
9. Beck LA, Cork MJ, Amagai M, De Benedetto A, Kabashima K, Hamilton JD, et al. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov (2022) 2(5):100131. doi: 10.1016/j.xjidi.2022.100131
10. Iznardo H, Puig L. IL-1 family cytokines in inflammatory dermatoses: pathogenetic role and potential therapeutic implications. Int J Mol Sci (2022) 23(16):9479. doi: 10.3390/ijms23169479
11. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev (2018) 281(1):8–27. doi: 10.1111/imr.12621
12. Malik A, Kanneganti TD. Function and regulation of IL-1alpha in inflammatory diseases and cancer. Immunol Rev (2018) 281(1):124–37. doi: 10.1111/imr.12615
13. Martin P, Goldstein JD, Mermoud L, Diaz-Barreiro A, Palmer G. IL-1 family antagonists in mouse and human skin inflammation. Front Immunol (2021) 12:652846. doi: 10.3389/fimmu.2021.652846
14. Komine M, Rao LS, Freedberg IM, Simon M, Milisavljevic V, Blumenberg M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J Invest Dermatol (2001) 116(2):330–8. doi: 10.1046/j.1523-1747.2001.01249.x
15. Broderick L, Hoffman HM. IL-1 and autoinflammatory disease: biology, pathogenesis and therapeutic targeting. Nat Rev Rheumatol (2022) 18(8):448–63. doi: 10.1038/s41584-022-00797-1
16. Cro S, Cornelius VR, Pink AE, Wilson R, Pushpa-Rajah A, Patel P, et al. Anakinra for palmoplantar pustulosis: results from a randomized, double-blind, multicentre, two-staged, adaptive placebo-controlled trial (APRICOT). Br J Dermatol (2021) 186(2):245–56. doi: 10.1111/bjd.20653
17. Naik HB, Pichard DC, Schwartz DM, O'Brien M, Masciocchi M, Thompson J, et al. Anakinra for refractory pustular psoriasis: A phase II, open-label, dose-escalation trial. J Am Acad Dermatol (2022) 87(6):1380–3. doi: 10.1016/j.jaad.2022.07.065
18. Calabrese L, Fiocco Z, Satoh TK, Peris K, French LE. Therapeutic potential of targeting interleukin-1 family cytokines in chronic inflammatory skin diseases. Br J Dermatol (2022) 186(6):925–41. doi: 10.1111/bjd.20975
19. Kanda N, Shimizu T, Tada Y, Watanabe S. IL-18 enhances IFN-gamma-induced production of CXCL9, CXCL10, and CXCL11 in human keratinocytes. Eur J Immunol (2007) 37(2):338–50. doi: 10.1002/eji.200636420
20. Wittmann M, Purwar R, Hartmann C, Gutzmer R, Werfel T. Human keratinocytes respond to interleukin-18: implication for the course of chronic inflammatory skin diseases. J Invest Dermatol (2005) 124(6):1225–33. doi: 10.1111/j.0022-202X.2005.23715.x
21. Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M, et al. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36gamma induction in human epidermal keratinocytes. J Immunol (2014) 193(10):5140–8. doi: 10.4049/jimmunol.1302574
22. Carrier Y, Ma HL, Ramon HE, Napierata L, Small C, O'Toole M, et al. Inter regulation of Th17 cytokines and the IL 36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J Invest Dermatol (2011) 131(12):2428–37. doi: 10.1038/jid.2011.234
23. Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med (2011) 365(7):620–8. doi: 10.1056/NEJMoa1013068
24. Bachelez H, Choon SE, Marrakchi S, Burden AD, Tsai TF, Morita A, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med (2021) 385(26):2431–40. doi: 10.1056/NEJMoa2111563
25. Teng X, Hu Z, Wei X, Wang Z, Guan T, Liu N, et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol (2014) 192(4):1815–23. doi: 10.4049/jimmunol.1300047
26. Mercurio L, Morelli M, Scarponi C, Eisenmesser EZ, Doti N, Pagnanelli G, et al. IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment. Cell Death Dis (2018) 9(11):1104. doi: 10.1038/s41419-018-1143-3
27. Leonard WJ, Lin JX, O'Shea JJ. The gamma(c) family of cytokines: basic biology to therapeutic ramifications. Immunity (2019) 50(4):832–50. doi: 10.1016/j.immuni.2019.03.028
28. Akaiwa M, Yu B, Umeshita-Suyama R, Terada N, Suto H, Koga T, et al. Localization of human interleukin 13 receptor in non-haematopoietic cells. Cytokine (2001) 13(2):75–84. doi: 10.1006/cyto.2000.0814
29. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet (2020) 396(10247):345–60. doi: 10.1016/S0140-6736(20)31286-1
30. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol (2007) 120(1):150–5. doi: 10.1016/j.jaci.2007.04.031
31. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol (2008) 126(3):332–7. doi: 10.1016/j.clim.2007.11.006
32. Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight (2018) 3(4):e98006. doi: 10.1172/jci.insight.98006
33. Danso M, Boiten W, van Drongelen V, Gmelig Meijling K, Gooris G, El Ghalbzouri A, et al. Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J Dermatol Sci (2017) 88(1):57–66. doi: 10.1016/j.jdermsci.2017.05.005
34. Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol (2005) 124(4):786–92. doi: 10.1111/j.0022-202X.2005.23651.x
35. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med (2002) 347(15):1151–60. doi: 10.1056/NEJMoa021481
36. Morizane S, Yamasaki K, Kajita A, Ikeda K, Zhan M, Aoyama Y, et al. TH2 cytokines increase kallikrein 7 expression and function in patients with atopic dermatitis. J Allergy Clin Immunol (2012) 130(1):259–61 e1. doi: 10.1016/j.jaci.2012.03.006
37. Donetti E, Riva F, Indino S, Lombardo G, Baruffaldi Preis F, Rosi E, et al. Th2 cytokines affect the innate immune barrier without impairing the physical barrier in a 3D model of normal human skin. J Clin Med (2023) 12(5):1941. doi: 10.3390/jcm12051941
38. Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol (2021) 184(3):450–63. doi: 10.1111/bjd.19573
39. Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol (2021) 184(3):437–49. doi: 10.1111/bjd.19574
40. Silverberg JI, Guttman-Yassky E, Thaci D, Irvine AD, Stein Gold L, Blauvelt A, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med (2023) 388(12):1080–91. doi: 10.1056/NEJMoa2206714
41. Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet (2017) 389(10086):2287–303. doi: 10.1016/S0140-6736(17)31191-1
42. Yosipovitch G, Mollanazar N, Stander S, Kwatra SG, Kim BS, Laws E, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med (2023) 29(5):1180–90. doi: 10.1038/s41591-023-02320-9
43. Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol (2008) 26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316
44. Distler JH, Jungel A, Kowal-Bielecka O, Michel BA, Gay RE, Sprott H, et al. Expression of interleukin-21 receptor in epidermis from patients with systemic sclerosis. Arthritis Rheum (2005) 52(3):856–64. doi: 10.1002/art.20883
45. Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med (2009) 15(9):1013–5. doi: 10.1038/nm.1995
46. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol (2015) 16(5):448–57. doi: 10.1038/ni.3153
47. Murakami M, Kamimura D, Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity (2019) 50(4):812–31. doi: 10.1016/j.immuni.2019.03.027
48. Hermanns HM.Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiologyCytokine Growth Factor Rev (2015) 26(5):545–58doi: 10.1016/j.cytogfr.2015.07.006
49. Fujiwara S, Nagai H, Oniki S, Yoshimoto T, Nishigori C. Interleukin (IL)-17 versus IL-27: opposite effects on tumor necrosis factor-alpha-mediated chemokine production in human keratinocytes. Exp Dermatol (2012) 21(1):70–2. doi: 10.1111/j.1600-0625.2011.01384.x
50. Zeitvogel J, Dalpke A, Eiz-Vesper B, Kracht M, Dittrich-Breiholz O, Werfel T, et al. Human primary keratinocytes show restricted ability to up-regulate suppressor of cytokine signaling (SOCS)3 protein compared with autologous macrophages. J Biol Chem (2012) 287(13):9923–30. doi: 10.1074/jbc.M111.280370
51. Kwock JT, Handfield C, Suwanpradid J, Hoang P, McFadden MJ, Labagnara KF, et al. IL-27 signaling activates skin cells to induce innate antiviral proteins and protects against Zika virus infection. Sci Adv (2020) 6(14):eaay3245. doi: 10.1126/sciadv.aay3245
52. Finelt N, Gazel A, Gorelick S, Blumenberg M. Transcriptional responses of human epidermal keratinocytes to Oncostatin-M. Cytokine (2005) 31(4):305–13. doi: 10.1016/j.cyto.2005.05.005
53. Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, et al. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol (2007) 178(7):4615–22. doi: 10.4049/jimmunol.178.7.4615
54. Denton CP, Del Galdo F, Khanna D, Vonk MC, Chung L, Johnson SR, et al. Biological and clinical insights from a randomized phase 2 study of an anti-oncostatin M monoclonal antibody in systemic sclerosis. Rheumatol (Oxford) (2022) 62(1):234–42. doi: 10.1093/rheumatology/keac300
55. Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis. Exp Dermatol (2018) 27(4):327–31. doi: 10.1111/exd.13533
56. Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Luscher-Firzlaff J, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol (2012) 129(2):426–33, 33.e1-8. doi: 10.1016/j.jaci.2011.10.042
57. Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol (2016) 138(2):500–8.e24. doi: 10.1016/j.jaci.2016.02.020
58. Kabashima K, Matsumura T, Komazaki H, Kawashima M, Nemolizumab JPSG. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med (2020) 383(2):141–50. doi: 10.1056/NEJMoa1917006
59. McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity (2019) 50(4):892–906. doi: 10.1016/j.immuni.2019.03.021
60. Tollenaere MAX, Hebsgaard J, Ewald DA, Lovato P, Garcet S, Li X, et al. Signalling of multiple interleukin (IL)-17 family cytokines via IL-17 receptor A drives psoriasis-related inflammatory pathways. Br J Dermatol (2021) 185(3):585–94. doi: 10.1111/bjd.20090
61. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med (2014) 371(4):326–38. doi: 10.1056/NEJMoa1314258
62. Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med (2015) 373(14):1318–28. doi: 10.1056/NEJMoa1503824
63. Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med (2016) 375(4):345–56. doi: 10.1056/NEJMoa1512711
64. Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet (2021) 397(10273):475–86. doi: 10.1016/S0140-6736(21)00126-4
65. Kimball AB, Jemec GBE, Alavi A, Reguiai Z, Gottlieb AB, Bechara FG, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet (2023) 401(10378):747–61. doi: 10.1016/S0140-6736(23)00022-3
66. Glatt S, Jemec GBE, Forman S, Sayed C, Schmieder G, Weisman J, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, placebo-controlled randomized clinical trial. J JAMA Dermatol (2021) 157(11):1279–88. doi: 10.1001/jamadermatol.2021.2905
67. Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol (2007) 178(4):2229–40. doi: 10.4049/jimmunol.178.4.2229
68. Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol (2009) 39(12):3570–81. doi: 10.1002/eji.200939687
69. Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) (2009) 87(5):523–36. doi: 10.1007/s00109-009-0457-0
70. Kragstrup TW, Andersen T, Heftdal LD, Hvid M, Gerwien J, Sivakumar P, et al. The IL-20 cytokine family in rheumatoid arthritis and spondyloarthritis. Front Immunol (2018) 9:2226. doi: 10.3389/fimmu.2018.02226
71. Prignano F, Donetti E. Looking at interleukin-22 from a new dermatological perspective: from epidermal homeostasis to its role in chronic skin diseases. Dermatology (2022) 238(5):829–36. doi: 10.1159/000524119
72. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity (2004) 21(2):241–54. doi: 10.1016/j.immuni.2004.07.007
73. Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci U.S.A. (2009) 106(51):21795–800. doi: 10.1073/pnas.0911472106
74. Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science (2010) 330(6004):665–9. doi: 10.1126/science.1194597
75. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol (2005) 174(6):3695–702. doi: 10.4049/jimmunol.174.6.3695
76. Guttman-Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J Am Acad Dermatol (2018) 78(5):872–81.e6. doi: 10.1016/j.jaad.2018.01.016
77. Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol (2002) 168(11):5397–402. doi: 10.4049/jimmunol.168.11.5397
78. Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol (2008) 180(11):7423–30. doi: 10.4049/jimmunol.180.11.7423
79. Sheikh F, Baurin VV, Lewis-Antes A, Shah NK, Smirnov SV, Anantha S, et al. Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J Immunol (2004) 172(4):2006–10. doi: 10.4049/jimmunol.172.4.2006
80. Itoh T, Hatano R, Komiya E, Otsuka H, Narita Y, Aune TM, et al. Biological effects of IL-26 on T cell-mediated skin inflammation, including psoriasis. J Invest Dermatol (2019) 139(4):878–89. doi: 10.1016/j.jid.2018.09.037
81. Kamijo H, Miyagaki T, Hayashi Y, Akatsuka T, Watanabe-Otobe S, Oka T, et al. Increased IL-26 Expression Promotes T Helper Type 17- and T Helper Type 2-Associated Cytokine Production by Keratinocytes in Atopic Dermatitis. J Invest Dermatol (2020) 140(3):636–44.e2. doi: 10.1016/j.jid.2019.07.713
82. Hile GA, Gudjonsson JE, Kahlenberg JM. The influence of interferon on healthy and diseased skin. Cytokine (2020) 132:154605. doi: 10.1016/j.cyto.2018.11.022
83. Wang P, Gamero AM, Jensen LE. IL-36 promotes anti-viral immunity by boosting sensitivity to IFN-alpha/beta in IRF1 dependent and independent manners. Nat Commun (2019) 10(1):4700. doi: 10.1038/s41467-019-12318-y
84. Li Y, Song Y, Zhu L, Wang X, Richers B, Leung DYM, et al. Interferon kappa is important for keratinocyte host defense against herpes simplex virus-1. J Immunol Res (2020) 2020:5084682. doi: 10.1155/2020/5084682
85. Stannard JN, Reed TJ, Myers E, Lowe L, Sarkar MK, Xing X, et al. Lupus skin is primed for IL-6 inflammatory responses through a keratinocyte-mediated autocrine type I interferon loop. J Invest Dermatol (2017) 137(1):115–22. doi: 10.1016/j.jid.2016.09.008
86. Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol (2018) 9:847. doi: 10.3389/fimmu.2018.00847
87. Saunders NA, Jetten AM. Control of growth regulatory and differentiation-specific genes in human epidermal keratinocytes by interferon gamma. Antagonism by retinoic acid and transforming growth factor beta 1. J Biol Chem (1994) 269(3):2016–22.
88. Kajita AI, Morizane S, Takiguchi T, Yamamoto T, Yamada M, Iwatsuki K. Interferon-gamma enhances TLR3 expression and anti-viral activity in keratinocytes. J Invest Dermatol (2015) 135(8):2005–11. doi: 10.1038/jid.2015.125
89. Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol (1999) 162(1):494–502.
90. Simanski M, Rademacher F, Schroder L, Schumacher HM, Glaser R, Harder J. IL-17A and IFN-gamma synergistically induce RNase 7 expression via STAT3 in primary keratinocytes. PloS One (2013) 8(3):e59531. doi: 10.1371/journal.pone.0059531
91. Morizane S, Nomura H, Tachibana K, Nakagawa Y, Iwatsuki K. The synergistic activities of the combination of tumour necrosis factor-alpha, interleukin-17A and interferon-gamma in epidermal keratinocytes. Br J Dermatol (2018) 179(2):496–8. doi: 10.1111/bjd.16443
92. Harden JL, Johnson-Huang LM, Chamian MF, Lee E, Pearce T, Leonardi CL, et al. Humanized anti-IFN-gamma (HuZAF) in the treatment of psoriasis. J Allergy Clin Immunol (2015) 135(2):553–6. doi: 10.1016/j.jaci.2014.05.046
93. Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol (2003) 4(1):69–77. doi: 10.1038/ni875
94. Lazear HM, Nice TJ, Diamond MS. Interferon-lambda: immune functions at barrier surfaces and beyond. Immunity (2015) 43(1):15–28. doi: 10.1016/j.immuni.2015.07.001
95. Zhang SQ, Zhang Z, Luo X, Yang S, Chai Y, Huang HL, et al. Interleukin 29 enhances expression of Toll receptor 3 and mediates antiviral signals in human keratinocytes. Inflammation Res (2011) 60(11):1031–7. doi: 10.1007/s00011-011-0364-z
96. Sethi JK, Hotamisligil GS. Metabolic Messengers: tumour necrosis factor. Nat Metab (2021) 3(10):1302–12. doi: 10.1038/s42255-021-00470-z
97. Mizuno K, Morizane S, Takiguchi T, Iwatsuki K. Dexamethasone but not tacrolimus suppresses TNF-alpha-induced thymic stromal lymphopoietin expression in lesional keratinocytes of atopic dermatitis model. J Dermatol Sci (2015) 80(1):45–53. doi: 10.1016/j.jdermsci.2015.06.016
98. Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, Zouboulis CC, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med (2016) 375(5):422–34. doi: 10.1056/NEJMoa1504370
99. Yamasaki K, Yamanaka K, Zhao Y, Iwano S, Takei K, Suzuki K, et al. Adalimumab in Japanese patients with active ulcers of pyoderma gangrenosum: Final analysis of a 52-week phase 3 open-label study. J Dermatol (2022) 49(5):479–87. doi: 10.1111/1346-8138.16337
100. Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol (2010) 62(2):205–17. doi: 10.1016/j.jaad.2009.06.050
101. Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, Griffiths CE, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut (2006) 55(4):505–9. doi: 10.1136/gut.2005.074815
102. Boutet MA, Nerviani A, Pitzalis C. IL-36, IL-37, and IL-38 cytokines in skin and joint inflammation: A comprehensive review of their therapeutic potential. Int J Mol Sci (2019) 20(6):1257. doi: 10.3390/ijms20061257
103. Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci (2019) 96(1):2–7. doi: 10.1016/j.jdermsci.2019.08.006
104. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity (2005) 23(5):479–90. doi: 10.1016/j.immuni.2005.09.015
105. Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U.S.A. (2009) 106(22):9021–6. doi: 10.1073/pnas.0812690106
106. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med (2013) 210(13):2939–50. doi: 10.1084/jem.20130351
107. Laquer V, Parra V, Lacour JP, Takahashi H, Knorr J, Okragly AJ, et al. Interleukin-33 antibody failed to demonstrate benefit in a phase II, double-blind, randomized, placebo-controlled study in adult patients with moderate-to-severe atopic dermatitis. Br J Dermatol (2022) 187(4):599–602. doi: 10.1111/bjd.21631
108. Maurer M, Cheung DS, Theess W, Yang X, Dolton M, Guttman A, et al. Phase 2 randomized clinical trial of astegolimab in patients with moderate to severe atopic dermatitis. J Allergy Clin Immunol (2022) 150(6):1517–24. doi: 10.1016/j.jaci.2022.08.015
109. Yang N, Chen Z, Zhang X, Shi Y. Novel targeted biological agents for the treatment of atopic dermatitis. BioDrugs (2021) 35(4):401–15. doi: 10.1007/s40259-021-00490-x
110. Ariizumi K, Meng Y, Bergstresser PR, Takashima A. IFN-gamma-dependent IL-7 gene regulation in keratinocytes. J Immunol (1995) 154(11):6031–9.
111. Chen X, Guo W, Chang Y, Chen J, Kang P, Yi X, et al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8(+) T cells activation via JAK-STAT pathway in vitiligo. Free Radic Biol Med (2019) 139:80–91. doi: 10.1016/j.freeradbiomed.2019.05.011
112. Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, et al. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med (2015) 21(11):1272–9. doi: 10.1038/nm.3962
113. Dai Z, Wang EHC, Petukhova L, Chang Y, Lee EY, Christiano AM. Blockade of IL-7 signaling suppresses inflammatory responses and reverses alopecia areata in C3H/HeJ mice. Sci Adv (2021) 7(14):eabd1866. doi: 10.1126/sciadv.abd1866
114. Richmond JM, Strassner JP, Zapata L Jr., Garg M, Riding RL, Refat MA, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med (2018) 10(450):eaam7710. doi: 10.1126/scitranslmed.aam7710
115. Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol (2002) 3(7):673–80. doi: 10.1038/ni805
116. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med (2013) 5(170):170ra16. doi: 10.1126/scitranslmed.3005374
117. Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U.S.A. (2010) 107(45):19455–60. doi: 10.1073/pnas.1008271107
118. Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol (2019) 80(4):1013–21. doi: 10.1016/j.jaad.2018.11.059
119. Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med (2009) 15(12):1377–82. doi: 10.1038/nm.2062
120. de Vos S, Brach M, Budnik A, Grewe M, Herrmann F, Krutmann J. Post-transcriptional regulation of interleukin-6 gene expression in human keratinocytes by ultraviolet B radiation. J Invest Dermatol (1994) 103(1):92–6. doi: 10.1111/1523-1747.ep12391818
121. Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol (1998) 111(4):645–9. doi: 10.1046/j.1523-1747.1998.00347.x
122. Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol (2011) 131(3):677–87. doi: 10.1038/jid.2010.340
123. Choong DJ, Tan E. Does tocilizumab have a role in dermatology? A review of clinical applications, its adverse side effects and practical considerations. Dermatol Ther (2021) 34(4):e14990. doi: 10.1111/dth.14990
124. Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol (2011) 12(12):1159–66. doi: 10.1038/ni.2156
125. Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. Neutralization of IL-17C reduces skin inflammation in mouse models of psoriasis and atopic dermatitis. J Invest Dermatol (2018) 138(7):1555–63. doi: 10.1016/j.jid.2018.01.036
126. Thaci D, Singh D, Lee M, Timmis H, Jacobs D, Passier P, et al. Phase 1 and 2 randomized clinical studies determine lack of efficacy for anti-IL-17C antibody MOR106 in moderate-severe atopic dermatitis. J Clin Med (2022) 11(23):7244. doi: 10.3390/jcm11237244
127. Leyva-Castillo JM, Galand C, Mashiko S, Bissonnette R, McGurk A, Ziegler SF, et al. ILC2 activation by keratinocyte-derived IL-25 drives IL-13 production at sites of allergic skin inflammation. J Allergy Clin Immunol (2020) 145(6):1606–14.e4. doi: 10.1016/j.jaci.2020.02.026
128. Borowczyk J, Buerger C, Tadjrischi N, Drukala J, Wolnicki M, Wnuk D, et al. IL-17E (IL-25) and IL-17A differentially affect the functions of human keratinocytes. J Invest Dermatol (2020) 140(7):1379–89.e2. doi: 10.1016/j.jid.2019.12.013
129. Tohyama M, Hanakawa Y, Shirakata Y, Dai X, Yang L, Hirakawa S, et al. IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression. Eur J Immunol (2009) 39(10):2779–88. doi: 10.1002/eji.200939473
130. Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, Babel N, et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol (2014) 134(11):2757–67. doi: 10.1038/jid.2014.308
131. Ikeda K, Morizane S, Akagi T, Hiramatsu-Asano S, Tachibana K, Yahagi A, et al. Obesity and dyslipidemia synergistically exacerbate psoriatic skin inflammation. Int J Mol Sci (2022) 23(8):4312. doi: 10.3390/ijms23084312
132. Takiguchi T, Morizane S, Yamamoto T, Kajita A, Ikeda K, Iwatsuki K. Cathelicidin antimicrobial peptide LL-37 augments interferon-beta expression and antiviral activity induced by double-stranded RNA in keratinocytes. Br J Dermatol (2014) 171(3):492–8. doi: 10.1111/bjd.12942
133. Bissonnette R, Papp K, Maari C, Yao Y, Robbie G, White WI, et al. A randomized, double-blind, placebo-controlled, phase I study of MEDI-545, an anti-interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. J Am Acad Dermatol (2010) 62(3):427–36. doi: 10.1016/j.jaad.2009.05.042
134. Bruera S, Chavula T, Madan R, Agarwal SK. Targeting type I interferons in systemic lupus erythematous. Front Pharmacol (2022) 13:1046687. doi: 10.3389/fphar.2022.1046687
135. Zahn S, Rehkamper C, Kummerer BM, Ferring-Schmidt S, Bieber T, Tuting T, et al. Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNlambda) in cutaneous lupus erythematosus. J Invest Dermatol (2011) 131(1):133–40. doi: 10.1038/jid.2010.244
136. Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med (1990) 172(6):1609–14. doi: 10.1084/jem.172.6.1609
137. Johansen C, Funding AT, Otkjaer K, Kragballe K, Jensen UB, Madsen M, et al. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J Immunol (2006) 176(3):1431–8. doi: 10.4049/jimmunol.176.3.1431
138. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity (2005) 22(1):131–42. doi: 10.1016/j.immuni.2004.12.003
139. Meyer N, Zimmermann M, Burgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol (2010) 125(4):858–65.e10. doi: 10.1016/j.jaci.2010.01.016
140. Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science (2008) 320(5877):807–11. doi: 10.1126/science.1154370
141. Baghdadi M, Umeyama Y, Hama N, Kobayashi T, Han N, Wada H, et al. Interleukin-34, a comprehensive review. J Leukoc Biol (2018) 104(5):931–51. doi: 10.1002/JLB.MR1117-457R
142. Guillonneau C, Bezie S, Anegon I. Immunoregulatory properties of the cytokine IL-34. Cell Mol Life Sci (2017) 74(14):2569–86. doi: 10.1007/s00018-017-2482-4
143. Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol (2012) 13(8):753–60. doi: 10.1038/ni.2360
144. Wang Y, Bugatti M, Ulland TK, Vermi W, Gilfillan S, Colonna M. Nonredundant roles of keratinocyte-derived IL-34 and neutrophil-derived CSF1 in Langerhans cell renewal in the steady state and during inflammation. Eur J Immunol (2016) 46(3):552–9. doi: 10.1002/eji.201545917
145. Ramnath D, Tunny K, Hohenhaus DM, Pitts CM, Bergot AS, Hogarth PM, et al. TLR3 drives IRF6-dependent IL-23p19 expression and p19/EBI3 heterodimer formation in keratinocytes. Immunol Cell Biol (2015) 93(9):771–9. doi: 10.1038/icb.2015.77
146. Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, et al. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J Immunol (2016) 46(6):1343–50. doi: 10.1002/eji.201546095
147. Wang X, Liu X, Zhang Y, Wang Z, Zhu G, Han G, et al. Interleukin (IL)-39 [IL-23p19/Epstein-Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin Exp Immunol (2016) 186(2):144–56. doi: 10.1111/cei.12840
Keywords: epidermal keratinocytes, input cytokines, output cytokines, biologics, inflammatory skin diseases
Citation: Morizane S, Mukai T, Sunagawa K, Tachibana K, Kawakami Y and Ouchida M (2023) “Input/output cytokines” in epidermal keratinocytes and the involvement in inflammatory skin diseases. Front. Immunol. 14:1239598. doi: 10.3389/fimmu.2023.1239598
Received: 13 June 2023; Accepted: 20 September 2023;
Published: 10 October 2023.
Edited by:
Hyun Je Kim, Seoul National University, Republic of KoreaReviewed by:
Yong Woo Jung, Korea University, Republic of KoreaElena Donetti, University of Milan, Italy
Copyright © 2023 Morizane, Mukai, Sunagawa, Tachibana, Kawakami and Ouchida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin Morizane, emFuZW1vcmlAY2Mub2theWFtYS11LmFjLmpw
 Shin Morizane
Shin Morizane Tomoyuki Mukai
Tomoyuki Mukai Ko Sunagawa1
Ko Sunagawa1