- 1Hepatobiliary Pancreatic Center, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 2School of Medicine, Tsinghua University, Beijing, China
- 3School of Clinical Medicine, Tsinghua University, Beijing, China
- 4Thorgene Co., Ltd., Beijing, China
Background: New treatment strategies are needed to improve outcomes for patients with advanced cholangiocarcinoma (CCA) due to the limited efficacy of current first-line chemotherapy regimens. Although the combination of hepatic arterial infusion chemotherapy (HAIC), lenvatinib, and programmed cell death protein-1 (PD-1) inhibitors has been extensively evaluated in the treatment of advanced hepatocellular carcinoma, their roles in advanced CCA remain poorly understood. The purpose of this study is to compare the efficacy and safety of HAIC plus lenvatinib with or without PD-1 inhibitors in patients with advanced CCA.
Methods: Between March 2019 to June 2022, patients diagnosed with advanced CAA who received HAIC plus lenvatinib with or without PD-1 inhibitors treatment were reviewed for eligibility. Efficacy was evaluated according to survival and tumor response, and safety was evaluated according to the incidence of adverse events (AEs).
Results: Fifty-five patients with advanced CCA were included in the study, and they were divided into the HAIC+lenvatinib (LEN)+PD-1 inhibitors (PD-1i) group (n = 35) and HAIC+LEN group (n = 20). The median follow-up time was 14.0 (5–42) months. Patients in the HAIC+LEN+PD-1i group had significantly better PFS (HR = 0.390; 95% CI 0.189-0.806; p = 0.001) and OS (HR = 0.461; 95% CI 0.229-0.927; p = 0.01) than those in the HAIC+LEN group. The HAIC+LEN+PD-1i group showed a higher objective response rate and disease control rate than the HAIC+LEN group but did not find a significant difference. The incidence of grade 1-2 and grade 3-4 AEs was not significantly higher in the HAIC+LEN+PD-1i group compared to the HAIC+LEN group, whereas two patients (5.7%) in the HAIC+LEN+PD-1i group experienced grade 5 immune-mediated pneumonia.
Conclusion: HAIC plus lenvatinib with PD-1 inhibitors is safe and well-tolerated, and has the potential to prolong the survival of patients with advanced CCA. The addition of PD-1 inhibitors may enhance the efficacy of HAIC and lenvatinib. Therefore, the combined therapy has the potential to become a treatment option for advanced CCA.
Introduction
Cholangiocarcinoma (CCA), a malignant tumor originating from the intrahepatic, perihilar, or distal (extrahepatic) bile duct, accounts for about 3% of all digestive system malignancies and 2% of all cancer-related deaths worldwide (1, 2). Surgical treatment is the only curative method for CCA. However, as most patients are diagnosed with advanced CCA accompanied by multiple lesions and lymph node metastasis, although surgical treatment has made progress in recent years, the prognosis of CCA is still poor, with a high recurrence rate of up to 60% within two years, a reoperation rate of only 9%, and a 5-year survival rate of less than 5%. The median survival time for unresectable hilar CCA is about 10 months, while for intrahepatic CCA, it is as short as 6 months (2–4). The gemcitabine plus cisplatin regimen is considered the first-line chemotherapy standard for advanced or metastatic CCA (5). However, the efficacy of current first-line chemotherapy regimens is very limited (3, 5, 6). Therefore, new treatment strategies are needed to improve outcomes for patients with advanced CCA.
Hepatic arterial infusion chemotherapy (HAIC) of oxaliplatin, fluorouracil, and leucovorin (FOLFOX) is a regional chemotherapy that delivers high concentrations of chemotherapy drugs directly to the tumor site via the hepatic artery. Arterial administration of FOLFOX not only reduces toxicity and side effects, but also achieves higher drug concentrations and stronger anti-tumor effects than systemic delivery (7–9). FOLFOX-HAIC has demonstrated clinical benefits for patients with advanced CCA, including improved response rates and survival outcomes (8, 10).
Lenvatinib is a multi-target anti-angiogenic drug, showing favorable anti-tumor angiogenesis effect in a variety of solid tumors and improving T-cell infiltration in the tumor microenvironment (11–14). Lenvatinib has shown promising activity in the treatment of advanced CCA (15). Furthermore, many studies on hepatobiliary malignancies have confirmed the synergistic effect of lenvatinib and HAIC (14–17). Programmed cell death protein-1 (PD-1) inhibitors are a type of immunotherapy that blocks the PD-1 pathway, which is responsible for inhibiting the immune response against tumors. PD-1 inhibitors have also shown durable responses and improved survival outcomes in advanced CCA (18). However, the response rates of CCA are still unsatisfactory, whether treated with PD-1 inhibitors alone or in combination with targeted therapy.
The combination of HAIC, lenvatinib, and PD-1 inhibitors may result in synergistic anti-tumor effects, as each therapeutic approach targets different pathways involved in tumor growth and survival, and concurrent use of multiple treatments can overcome drug resistance which often limits clinical efficacy of single-agent therapies (19–21). Although the combination of HAIC, lenvatinib and PD-1 inhibitors has been extensively evaluated in the treatment of advanced hepatocellular carcinoma, their roles in advanced CCA remain poorly understood. Thus, we hypothesize that this is a promising combinatorial approach for advanced CCA. The objective of this study is to evaluate the safety and efficacy of HAIC plus lenvatinib with or without PD-1 inhibitors in patients with advanced CCA, and to compare the therapeutic effect of these two treatment regimens. The findings from this study may provide new insights into the treatment of advanced CCA and guide the development of future therapeutic strategies.
Materials and methods
After the institutional review board (IRB) of Beijing Tsinghua Changgung Hospital reviewed and approved the patient data analysis, medical records of patients with advanced CCA from our center who were received HAIC plus lenvatinib with or without PD-1 inhibitors at our center from March 2019 to June 2022 were reviewed. The study was conducted ethically, following the guidelines of the Declaration of Helsinki, and all participants provided informed consent for treatment.
Patient selection
Patient inclusion criteria for this study were as follows (1): Patients aged from 18 to 80 years (2); Patients diagnosed with advanced CCA through enhanced computed Tomography (CT), magnetic resonance imaging (MRI), and histopathology at our center (3); Patients with advanced CCA with multiple lesions, vascular invasion, local lymph node metastasis, and distant metastasis (4); Patients who opted out of systemic chemotherapy (5); Patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) within the range of 0-2 prior to HAIC treatment (6); Patients who underwent at least one cycle of HAIC plus lenvatinib with or without two cycles of PD-1 inhibitor treatment (7); Patients with complete follow-up records.
In contrast, exclusion criteria were (1): Patients with co-existing malignant tumors (2); Patients with histopathologically confirmed mixed tumors of hepatocellular carcinoma and CCA (3); Patients with histopathologically confirmed gallbladder carcinoma (3); Patients who were allergic to chemotherapy drugs, lenvatinib, or PD-1 inhibitors (4); Patients without enhanced CT or MRI images before treatment (5); Patients with uncontrolled hypertension and severe cardiovascular disease (6); Patients classified as Child-Pugh class C (7); Patients with a history of active autoimmune diseases (8); Patients with a history of idiopathic pneumonia and drug-induced pneumonia (9); Patients with severe active infections excluding hepatitis B virus (HBV) and hepatitis C virus (HCV) (10); Patients with severe gastrointestinal bleeding within 4 weeks prior to treatment (11); Patients with severe thrombosis within 6 months prior to treatment (12); Patients who received other treatments, including but not limited to transarterial chemoembolization (TACE), radiotherapy, or systemic chemotherapy (13); Patients who previously participated in clinical trials (14); Patients missing clinical data or follow-up.
Prior to initial treatment, laboratory data and enhanced CT or MRI images were collected and documented within 2 weeks. The inclusion and exclusion process of this study was depicted in Figure 1, leading to the final inclusion of 55 patients.
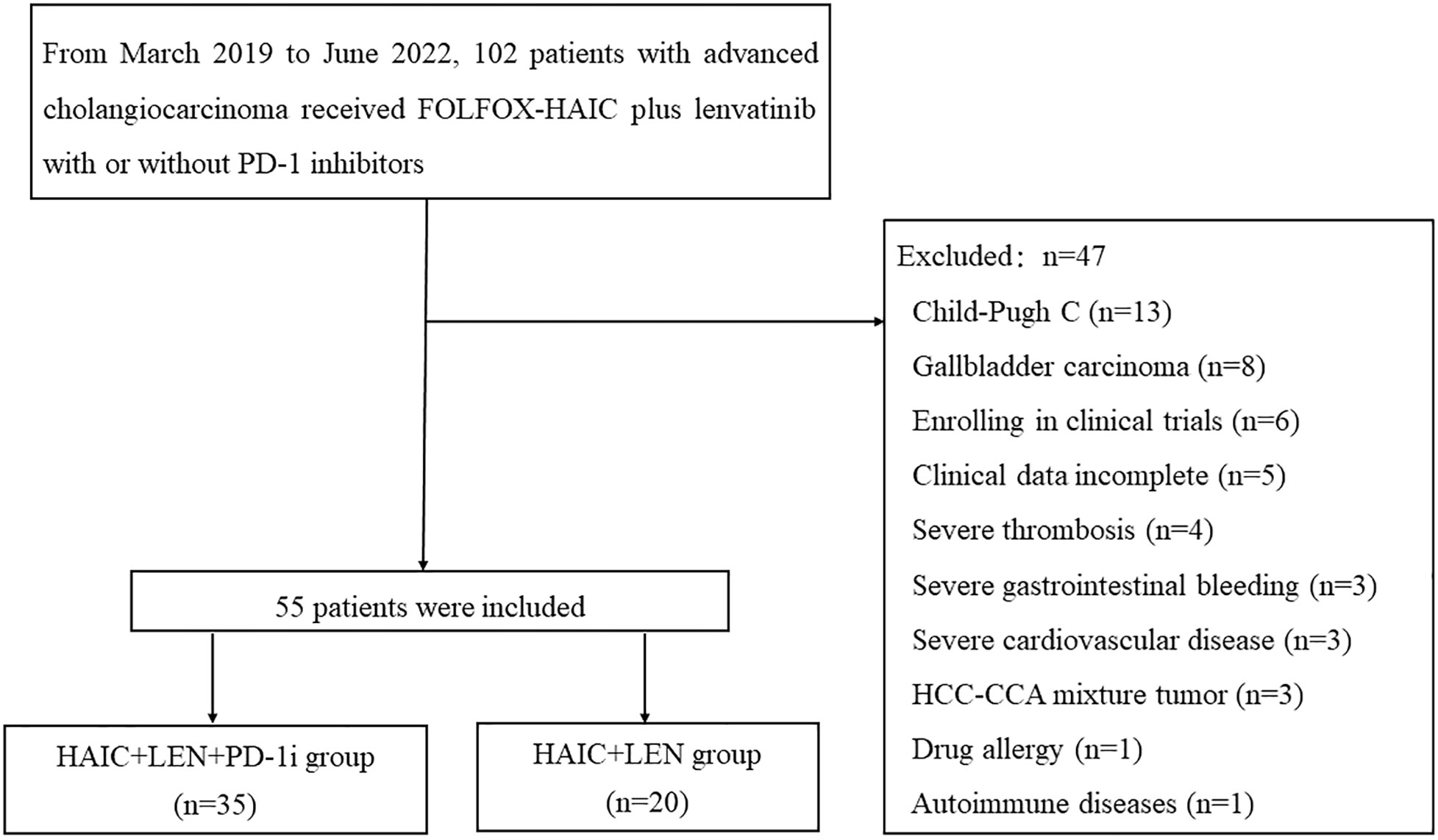
Figure 1 Flow diagram of study design. FOLFOX-HAIC, hepatic arterial infusion chemotherapy of oxaliplatin, fluorouracil, and leucovorin; HCC, hepatocellular carcinoma; CCA, cholangiocarcinoma; LEN, lenvatinib; PD-1i, PD-1 inhibitors.
Data collection
We sourced clinical data from the electronic medical record system of Beijing Tsinghua Changgung Hospital. The following parameters were collected and analyzed for the study: Age, gender, number of HAIC treatment cycles, comorbidities, HBV status, ECOG-PS score, number of tumors, white blood cell count (WBC), neutrophil count (NEUT), lymphocyte count (LY), platelet count (PLT), serum albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin count (TBIL), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), cholinesterase (CHE), liver function classification (Child-Pugh score), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), protein induced by vitamin K absence II (PIVKA-II), tumor stage (TNM staging based on the American Joint Committee on Cancer 8th edition), presence of portal vein tumor thrombus, vascular invasion, distant metastases, and treatment with pre-emptive percutaneous transhepatic cholangial drainage (PTCD).
Treatment protocol
Patients received liver protection, antiemetic, pain relief and other symptomatic treatments prior to HAIC. For patients with hyperbilirubinemia, PTCD drainage was performed before surgery and HAIC was administered after bilirubin levels had decreased to less than three times the normal range. Each HAIC treatment cycle lasts between 1 to 3 days, during which femoral artery puncture and catheter insertion are performed with the aid of digital subtraction angiography (DSA) to accurately select tumor feeding arteries. If necessary, the gastroduodenal artery or right gastric artery can be embolized with a spring coil. 5-fluorouracil is continuously administered for up to 12 hours per day at a total dose of 1500mg, while patients receive 50mg of oxaliplatin and 300mg of calcium folinate nightly for two hours. The interval between two HAIC treatment cycles is anywhere from 2 to 5 weeks, and patients receive 1 to 9 cycles of HAIC treatment.
Prior to HAIC treatment or one week after treatment, depending on bilirubin levels, patients were given lenvatinib (Japan Eisai Co, Ltd) orally at a dosage of either 8 mg (≤60 kg) or 12 mg (>60 kg) depending on their body weight. If patients were unable to tolerate HAIC, they continued taking lenvatinib; if patients were unable to tolerate lenvatinib, they reduced or stopped taking lenvatinib and continued with HAIC treatment.
PD-1 inhibitors were administered via intravenous drip within 1 to 2 weeks after HAIC treatment, with a dose of 200 mg every 3 weeks. Patients must receive at least 2 cycles of PD-1 inhibitor treatment. For patients with hyperbilirubinemia, the treatment can be initiated once the bilirubin level returns to within 2 times the normal range after PTCD drainage. The PD-1 inhibitors used in this study mainly include camrelizumab (28/35, 80.0%), sintilimab (4/35, 11.4%), and tislelizumab (3/35, 8.6%).
Treatment was discontinued in the event of disease progression (PD), the patient being unable to tolerate toxic or adverse reactions, patient refusal of treatment or change of treatment regimen. Enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was performed every 2 to 3 months, while follow-up visits were scheduled every 3 months.
Outcomes and assessments
The primary endpoints were overall survival (OS) and progression-free survival (PFS). OS was defined as the duration from the commencement of the initial therapy till the occurrence of death owing to any cause or the last follow-up. PFS was the duration from the beginning of the primary therapy till either the progression of the disease or the administration of bridging therapy and transplantation, or the last follow-up. Modified Response Evaluation Criteria in Solid Tumors (mRECIST) and RECIST version 1.1 were the standard methods employed by radiologists and hepatobiliary surgeons to assess the tumor response. The response criteria involved the determination of complete response (CR), partial response (PR), stable disease (SD), and PD. Objective response rate (ORR) was defined as the sum of CR and PR, whereas disease control rate (DCR) was determined from the sum of CR, PR, and SD. The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was utilized to evaluate treatment-related adverse events (AEs).
Statistical analysis
To compare the baseline characteristics of patients receiving HAIC plus lenvatinib with PD-1 inhibitors and HAIC plus lenvatinib without PD-1 inhibitors for advanced CCA, statistical tests including Pearson’s chi-square test, Fisher’s exact test, or Wilcoxon rank sum test were conducted. Normally distributed variables were described using mean ± standard error (SE), while median was used for non-normally distributed variables. Survival analysis was performed using the Kaplan-Meier method, and differences in survival curves were assessed using the log-rank test. Baseline variables that were deemed clinically relevant or demonstrated a univariate association with outcome were included in a multivariate Cox proportional-hazards regression model. The variables were selected judiciously, taking into account the number of available events, to ensure that the final model was parsimonious. This regression analysis comprised patients’ basic information, treatment method, tumor status, and more to calculate hazard ratios (HR) and confidence intervals (CI). R4.2.2 software was used for all descriptive and multivariate analyses. Statistical significance was considered when p-values <0.05 using a two-tailed test. All data analysis was conducted using R language and Statistical Package for the Social Sciences (SPSS, version 24.0, IBM, USA).
Results
Patient and tumor characteristics
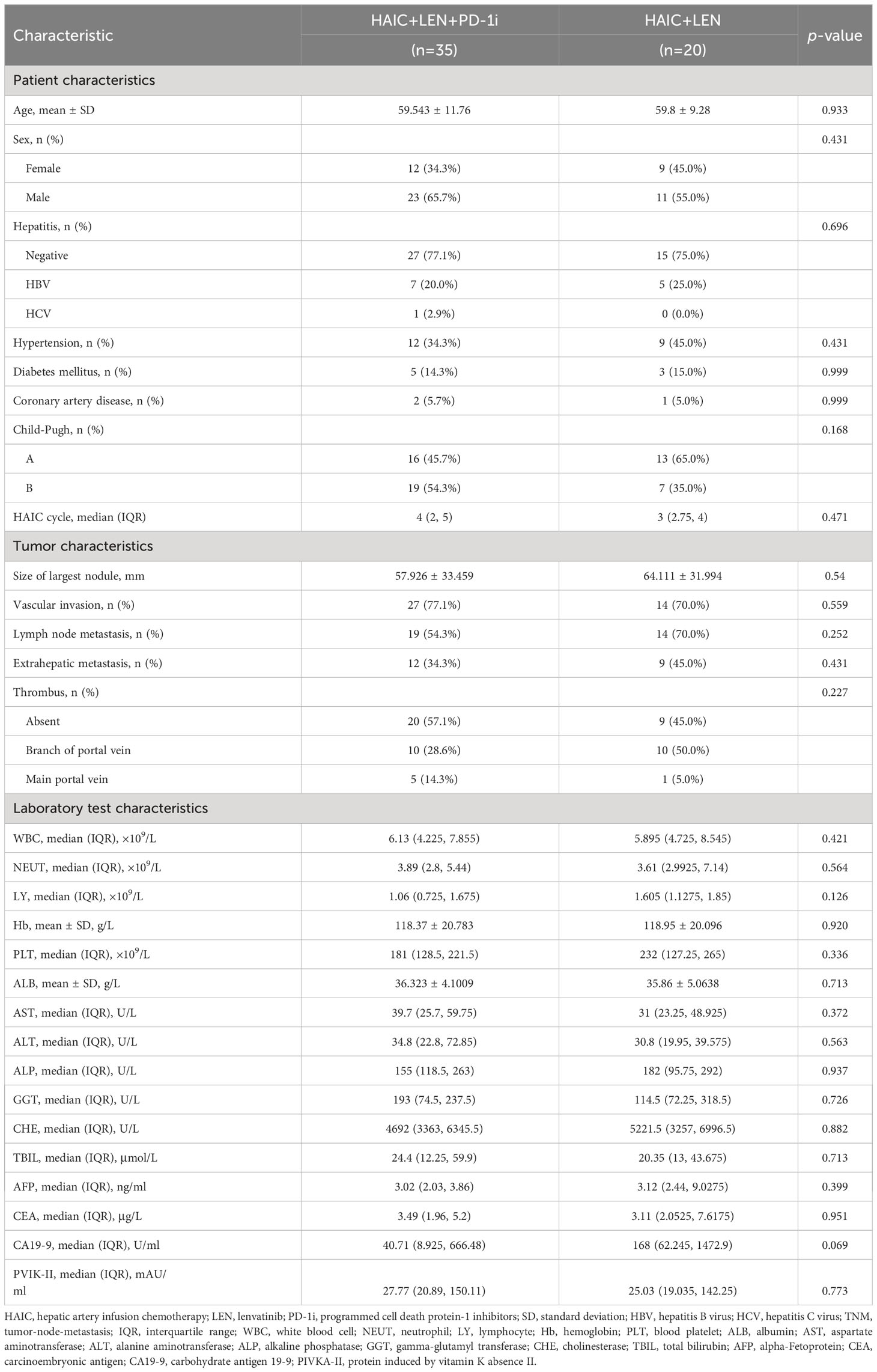
From March 2019 to June 2022, a total of 55 advanced CCA patients were enrolled in this study. 35 patients received HAIC plus lenvatinib with PD-1 inhibitors, while 20 patients received only HAIC plus lenvatinib treatment. The HAIC+LEN+PD-1i group received 1 to 6 cycles of HAIC, with a median of 4 cycles, while the HAIC+LEN group received 1 to 9 cycles of HAIC, with a median of 3 cycles. Table 1 displayed the demographic data and baseline characteristics of the two groups, which did not differ significantly in clinical variables.
Survival
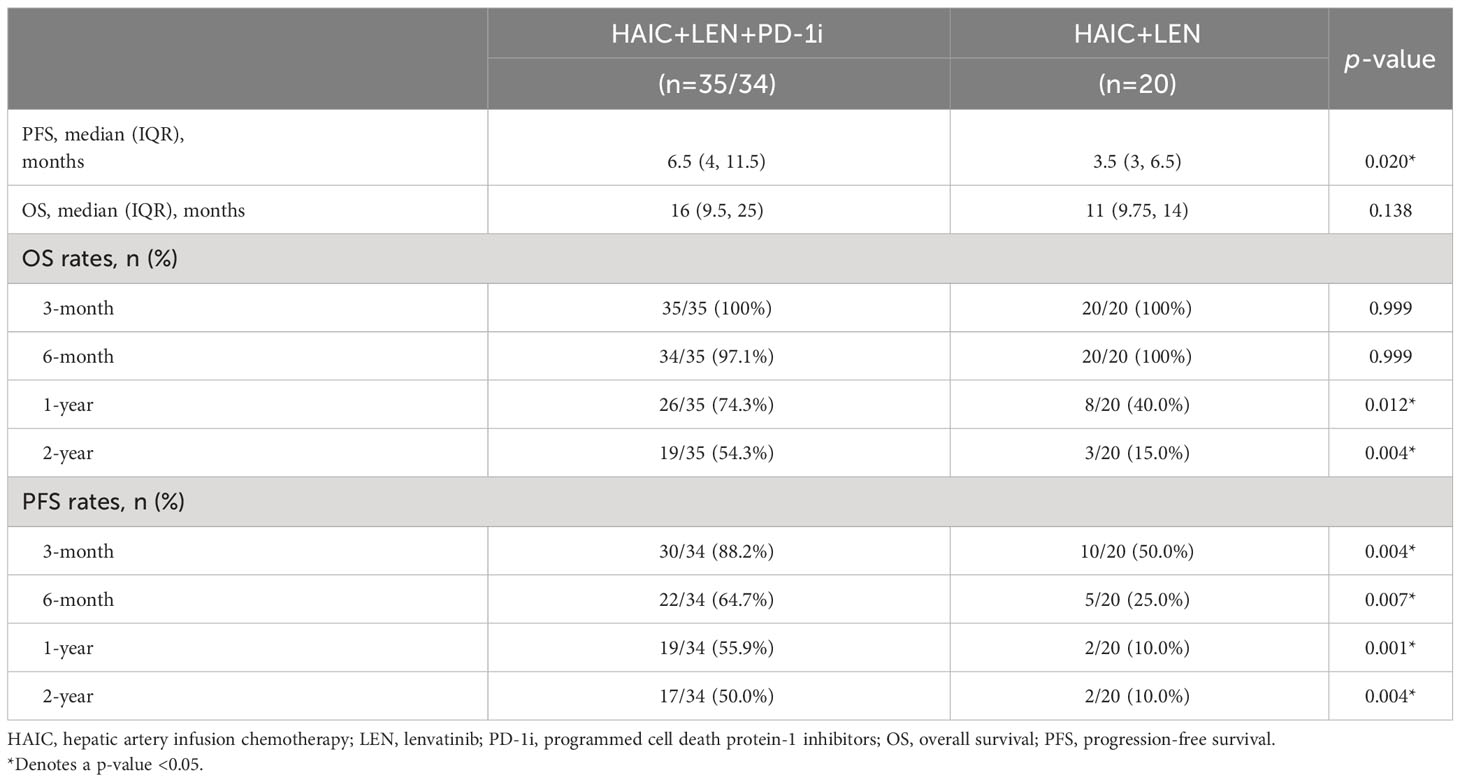
The median follow-up time was 14.0 months (range, 5-42 months), with the last follow-up date being April 10, 2023. As shown in Figure 2, patients in the HAIC+LEN+PD-1i group had significantly better PFS (HR = 0.390; 95% CI 0.189-0.806; p = 0.001; Figure 2A) and OS (HR = 0.461; 95% CI 0.229-0.927; p = 0.01; Figure 2B) than those in the HAIC+LEN group. The median OS of the HAIC+LEN+PD-1i and HAIC+LEN groups were 16 months and 11 months, respectively (p = 0.138), while the median PFS of the two groups were 6.5 months and 3.5 months, respectively (p = 0.020). Notably, the 1-year and 2-year OS rates of the HAIC+LEN+PD-1i group were significantly higher than those of the HAIC+LEN group (p = 0.012 and p= 0.004, respectively), and the 3-month, 6-month, 1-year, and 2-year PFS rates of the HAIC+LEN+PD-1i group were significantly higher than those of the HAIC+LEN group (p = 0.004, p = 0.007, p = 0.001, and p = 0.004, respectively) (Table 2).
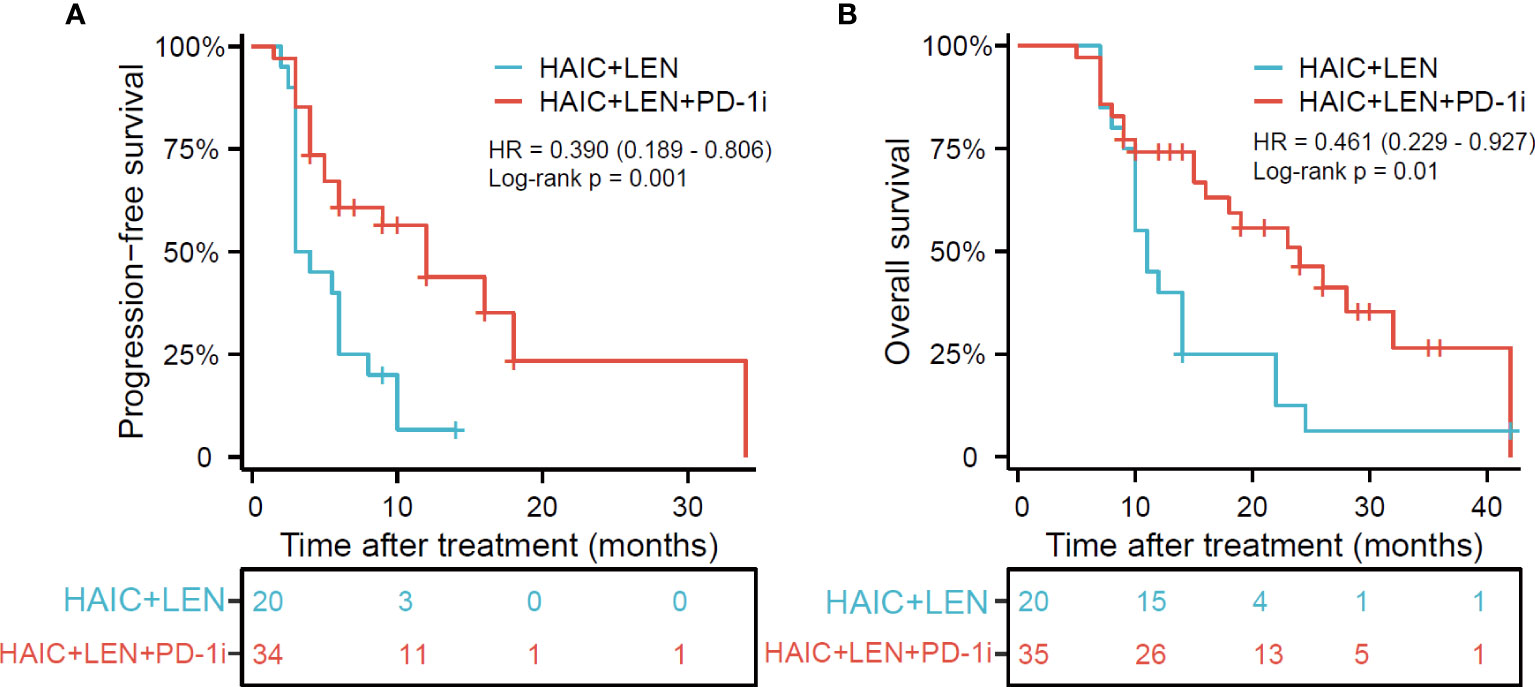
Figure 2 Kaplan-Meier plot for progression free survival (A) and overall survival (B) based on the HAIC+LEN+PD-1i group compared with the HAIC+LEN group. HAIC, hepatic arterial infusion chemotherapy; LEN, lenvatinib; PD-1i, PD-1 inhibitors; OS, overall survival; PFS, progression free survival. * One patient in the HAIC+LEN+PD-1i group was unable to accurately assess the time of disease progression, therefore only 34 patients were included in the evaluation of progression-free survival.
As shown in Figure 3, HAIC+LEN+PD-1i provided a clinical benefit for OS in patients with lymph metastasis (HR = 0.335; 95% CI 0.137-0.818; p = 0.016), extrahepatic metastasis (HR = 0.285; 95% CI 0.086-0.938; p = 0.039), and high CA199 level (HR = 0.172; 95% CI 0.055-0.531; p = 0.002), and provided a clinical benefit for PFS in patients with large tumor (HR = 0.277; 95% CI 0.083-0.924; p = 0.037), vascular invasion (HR = 0.402; 95% CI 0.182-0.889; p = 0.024), lymph metastasis (HR = 0.378; 95% CI 0.153-0.935; p = 0.035), extrahepatic metastasis (HR = 0.312; 95% CI 0.109-0.892; p = 0.030), portal vein tumor thrombus (HR = 0.26; 95% CI 0.087-0.783; p = 0.017), and high CA199 level (HR = 0.236; 95% CI 0.093-0.601; p = 0.002).
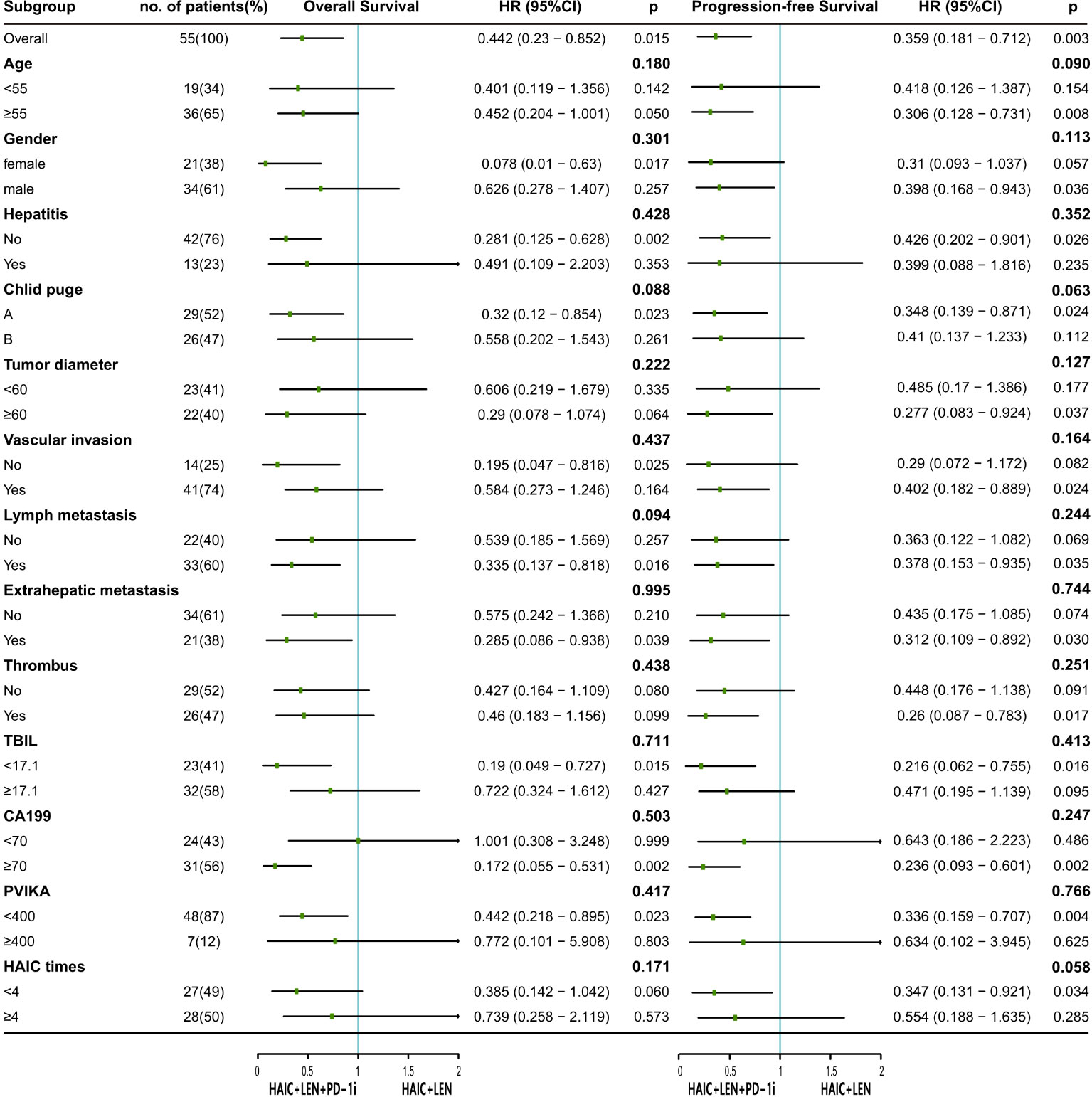
Figure 3 The forest plot analysis of factors associated with overall survival and progression free survival. HAIC, hepatic arterial infusion chemotherapy; LEN, lenvatinib; PD-1i, PD-1 inhibitors; HR, hazard ratio; 95% CI, 95% confidence interval; TBIL, total bilirubin; CA199, carbohydrate antigen 199; PVIK-II, protein induced by vitamin K absence II.
Tumor response
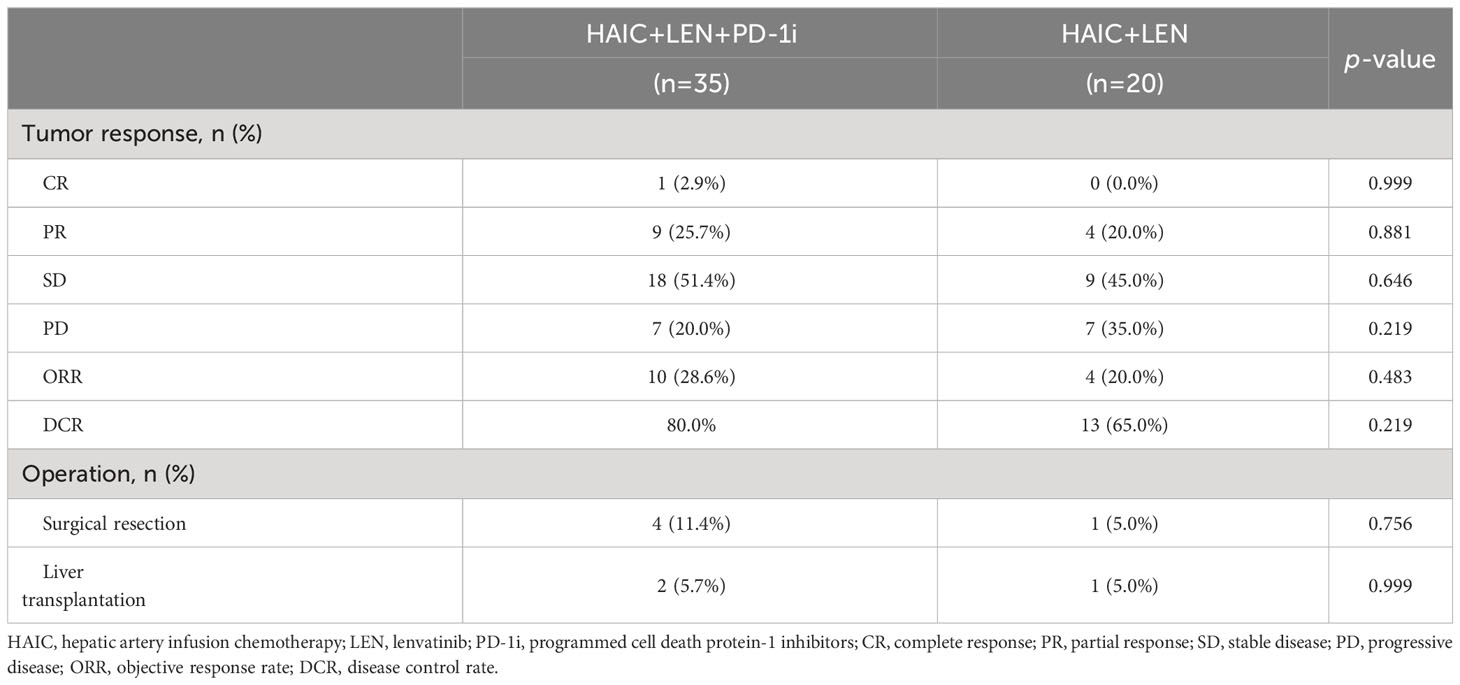
Radiographic evaluation by RECIST version 1.1, at the 3-month post-treatment imaging assessment, demonstrated that 7 (20.0%) patients in the HAIC+LEN+PD-1i group had PD, 18 (51.4%) patients showed SD, 9 (25.7%) patients achieved PR, and 1 (2.9%) patient achieved CR, resulting in an ORR of 28.6% and DCR of 80.0%. In the HAIC+LEN group, 7 (35.0%) patients had PD, 9 (45.0%) patients had SD, and 4 (20.0%) patients achieved PR, resulting in an ORR of 20.0% and DCR of 65.0%. The HAIC+LEN+PD-1i group showed a higher ORR and DCR than the HAIC+LEN group but did not find a significant difference (Table 3).
In the HAIC+LEN+PD-1i group, 4 patients underwent surgical resection, of which 3 patients were pathologically confirmed complete necrosis of the tumor, 2 patients received liver transplantation, of which 1 patient was pathologically confirmed 90% necrosis, and 1 patient was pathologically confirmed 30% necrosis. In the HAIC group, 1 patient underwent surgical resection, and 1 patient underwent liver transplantation. All of them died of disease progression or recurrence. The surgical conversion rates of two groups were 17.1% and 10%, respectively (Table 3).
Safety and tolerability
As shown in Table 4, based on the CTCAE 5.0 standards, the incidence of AEs for the HAIC+LEN+PD-1i group and the HAIC+LEN group were 97.1% and 90%, respectively. In the HAIC+LEN+PD-1i group, nausea (77.1%), fatigue (71.4%), and vomiting (65.7%) were the most common grade 1-2 AEs, and the most common grade 3-4 AEs were hypertension (42.9%), hand-foot syndrome (8.6%), and immune-mediated pneumonia (8.6%). In the HAIC+LEN group, the most common grade 1-2 AEs were fatigue (70.0%), vomiting (65.0%), and elevated transaminases (65.0%), and the most common grade 3-4 AEs were hypertension (45.0%), and hand-foot syndrome (20.0%). There was no significant difference observed in the incidence of grade 1-2 and grade 3-4 AEs between the two groups. However, two patients (5.7%) in the HAIC+LEN+PD-1i group experienced grade 5 immune-mediated pneumonia, and both died due to the grade 5 AE induced by treatment.

Table 4 The adverse events in the two groups according to Common Terminology Criteria for Adverse Events version 5.0.
Prognostic factor analysis
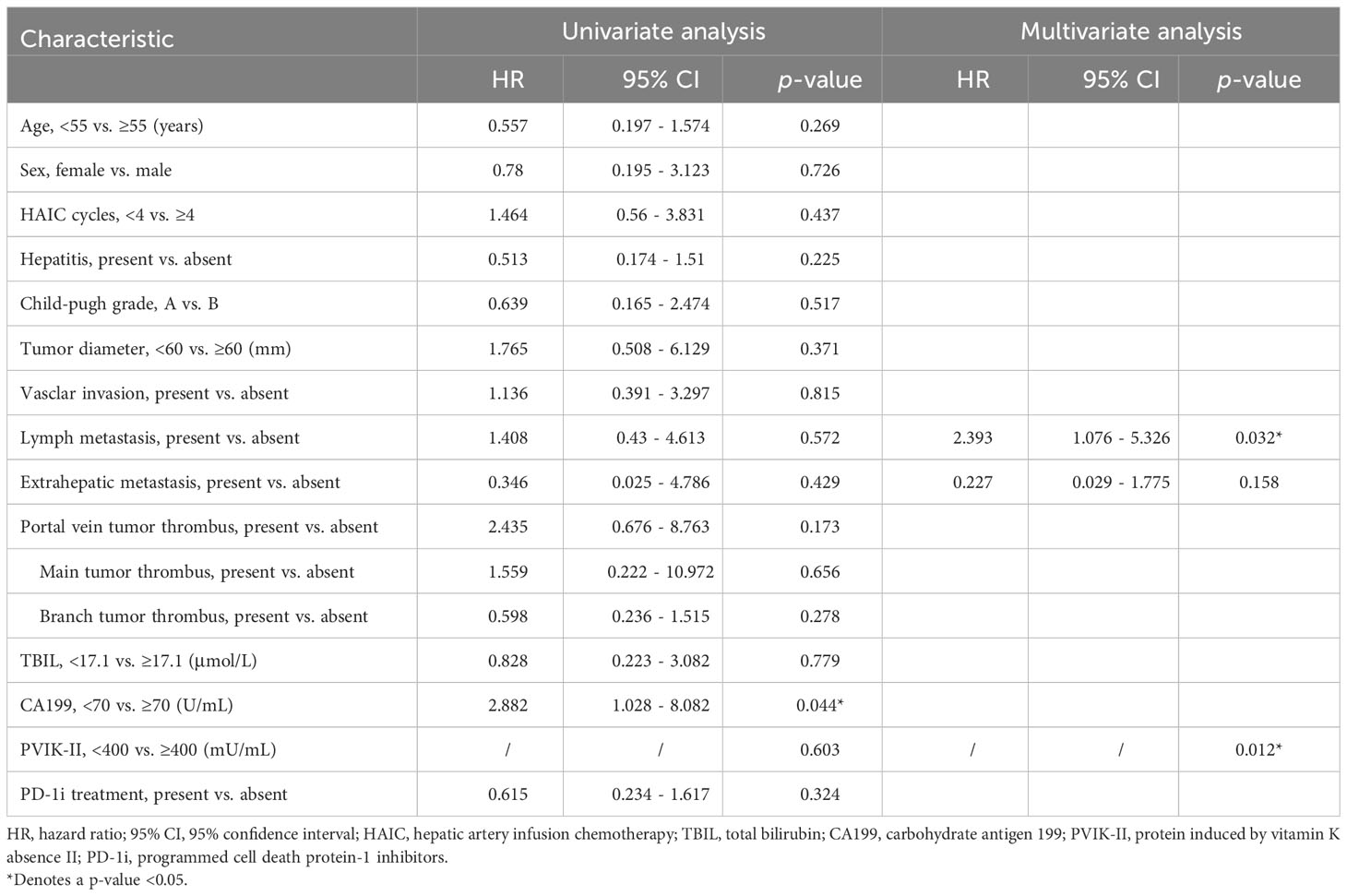
The prognostic factors for survival are shown in Tables 5, 6. The presence of PD-1 inhibitors was identified as an independent protective factor for PFS (HR = 0.368; 95% CI 0.168-0.807; p = 0.013). In addition, multivariate analyses identified that lymph metastasis was a risk factor for OS (HR = 2.393; 95% CI 1.076-5.326; p = 0.032), and extrahepatic metastasis was a risk factors for PFS (HR = 2.592; 95% CI 1.155-5.814; p = 0.021), while Child-pugh grade A was a protective factor for PFS (HR = 0.428; 95% CI 0.187-0.978; p = 0.044).
Discussion
This is the first clinical study to compare the efficacy and safety of HAIC plus levatinib with or without PD-1 inhibitors in the treatment of advanced CCA. It represented a potential shift in the approach to improving local chemotherapy response in advanced CCA patients. We found that the combination of HAIC plus lenvatinib with PD-1 inhibitors was promising, with significantly better OS and PFS compared to patients who received HAIC plus lenvatinib without PD-1 inhibitors. It is important to note that the incorporation of PD-1 inhibitors for advanced CCA patients did not result in a higher occurrence of AEs, and effective interventions were utilized to mitigate most of AEs observed in both groups. Furthermore, the study identified lymph metastasis, extrahepatic metastasis, and Child-pugh grade B as predictors of unfavorable prognosis.
For advanced CCA, there are several local treatment options available, such as radiofrequency ablation, radiotherapy, transarterial chemoembolization (TACE), and HAIC. Among these options, HAIC appears to have a unique advantage in treating CCA, as it can target diffuse and multiple lesions regardless of tumor size, location, or associated hyperbilirubinemia (8, 9, 16, 22–24). The ABC-06 clinical trial demonstrated that advanced CCA patients who had failed first-line chemotherapy could benefit from FOLFOX chemotherapy (25). FOLFOX-HAIC not only reduces systemic toxicity and side effects, but also achieves higher local drug concentrations and stronger anti-tumor effects than systemic delivery (7–9). A prospective phase II study reported a total effective rate of 67.6%, DCR of 89.2%, median PFS of 12.2 months, and median OS of 20.5 months with FOLFOX-HAIC treatment in advanced perihilar CCA (8). Ishii et al. (10) found that advanced intrahepatic CCA patients treated with FOLFOX-HAIC had significantly better OS than those receiving standard chemotherapy. Therefore, FOLFOX-HAIC may be an encouraging treatment option for advanced CCA due to its high tumor control, survival benefit, and low toxicity.
Lenvatinib is a small molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) 1-3, fibroblast growth factor receptor (FGFR) 1-4, and platelet-derived growth factor receptor (PDGFR)-α, thereby inhibiting tumor angiogenesis, and has shown some efficacy against various solid tumors (13). These receptors are also highly expressed in CCA. Therefore, lenvatinib may be a potential systemic targeted therapy for CCA and has been used as second-line therapy for CCA (4, 26). Lenvatinib has demonstrated anti-tumor activity in CCA and has a tolerable safety profile. A multicenter phase II study reported by Ueno et al. showed that median PFS was 3.19 months, while median OS was 7.35 months in unresectable CCA patients who received lenvatinib treatment (15). While direct comparisons are not feasible when considering differences in background characteristics, the PFS and OS demonstrated in both groups of patients in our study were superior to those achieved with lenvatinib monotherapy for unresectable CCA. This indirectly confirms the safety and efficacy of the combination therapy.
Immunotherapy has shown promising results in various malignancies, including advanced biliary tract tumors treated with PD-1 inhibitors. Deng et al. conducted a retrospective analysis of 42 advanced intrahepatic CCA patients treated with PD-1 inhibitors and reported a median OS, PFS, and time to progression (TTP) of 19.3, 11.6, and 11.6 months, respectively (18). To improve the clinical efficacy of PD-1 inhibitors for advanced CCA, various combination therapies are being explored, such as PD-1 inhibitors combined with chemotherapy, targeted therapy, and local treatment (27, 28). The phase III TOPAZ-1 study reported by Oh et al. (29) demonstrated that Gemcitabine and cisplatin plus immunotherapy showed promising efficacy and acceptable safety in patients with biliary tract cancer. Recently, durvalumab combined with gemcitabine and cisplatin has been confirmed as a first-line treatment by the guidelines of Food and Drug Administration (FDA) and National Comprehensive Cancer Network (NCCN). It is worth noting that the PFS in our study was shorter compared to the PFS in the TOPAZ-1 study (3.5 months vs 5.7 months). We believe that this difference may be attributed to the retrospective nature of our study, where the inclusion criteria were more lenient compared to the TOPAZ-1 study, resulting in patients with poorer baseline characteristics, such as older age and more severe underlying disease. These factors could potentially impact the patients’ survival. Furthermore, this may reflect a reality that HAIC is a localized treatment method primarily targeting liver disease. In cases of extrahepatic disease, the effectiveness of HAIC in achieving systemic control may be suboptimal compared to conventional chemotherapy. Shi et al. (30) reported that lenvatinib plus PD-1 inhibitors showed an active trend towards improving survival in advanced CCA patients after failure with cisplatin-gemcitabine chemotherapy. Similar trends have been found in advanced intrahepatic CCA patients treated with PD-1 inhibitors and lenvatinib after failure of chemotherapy (31). In a retrospective analysis of advanced CCA patients receiving lenvatinib plus PD-1/PD-L1 inhibitors and combined with gemcitabine and oxaliplatin (Gemox) chemotherapy, Zhu et al. (32) reported a median OS of 13.4 months, median PFS of 9.27 months, and ORR, DCR, and clinical benefit rate of 43.9%, 91.2%, and 73.7%, respectively. Shi et al. (33) analyzed advanced intrahepatic CCA patients treated with the same treatment regimen and reported median OS, PFS, and duration of response (DoR) of 22.5, 10.2, and 11.0 months, respectively. Thus, the combination of lenvatinib, PD-1/PD-L1 inhibitors, and Gemox chemotherapy may be an effective and tolerable treatment option for advanced CCA. Moreover, a multicenter retrospective study confirmed that early HAIC combined with PD-1 inhibitors could effectively prolong the OS of advanced CCA patients (34).
Because each treatment targets different pathways involved in tumor growth and survival, combining HAIC, lenvatinib, and PD-1 inhibitors may produce a synergistic anti-tumor effect. Additionally, this combination can overcome the resistance mechanisms of single therapies and enhance anti-tumor immunity. Therefore, HAIC plus lenvatinib with PD-1 inhibitors is a promising combination approach for treating malignancies. This combination has been extensively studied in liver cancer (19–21). Mei et al. (19) found that additional HAIC treatment was significantly associated with better treatment response and survival benefits in advanced hepatocellular carcinoma (HCC) patients treated with lenvatinib plus PD-1 inhibitors. Lai et al. (20) demonstrated that FOLFOX-HAIC plus lenvatinib and PD-1 inhibitors showed encouraging anti-tumor activity in patients with high-risk advanced HCC. An et al. (21) compared the efficacy and safety of lenvatinib and PD-1 inhibitors with lenvatinib alone in advanced HCC refractory to HAIC and showed that this combination therapy of PD-1 inhibitors plus lenvatinib has promising survival benefits. In addition, a systematic review of 311 clinical trials demonstrated that HAIC plus lenvatinib and PD-1 inhibitors effectively delayed disease progression, prolonged survival, and improved quality of life in HCC patients with portal vein tumor thrombus (35).
This study is the first to compare the clinical outcomes of combined treatment of HAIC plus lenvatinib with or without PD-1 inhibitors in advanced CCA patients, and has demonstrated the promising safety and efficacy of the combination of the three treatments. Our efforts are aimed at guiding future research and improving the quality and safety of management for advanced CCA in clinical practice. For advanced CCA patients who refuse systemic chemotherapy or who have failed previous chemotherapy or symptomatic support, we recommend that they receive HAIC plus lenvatinib with PD-1 inhibitors. Therefore, the results of this study may play a crucial role in the current management of advanced CCA.
There were some limitations to this study. Firstly, its retrospective nature confined the analysis to previously aggregated data. The study was susceptible to extraneous factors that were beyond our control, such as variations in data collection and probable partiality. Secondly, due to the limited number of patients included in the study, some of the significant findings may have been observed merely by chance. Therefore, the findings of this study should be confirmed in larger, prospective, cohort studies before being definitively accepted. Thirdly, the study sample was only composed of elderly Chinese patients, limiting our generalizability to other races undergoing similar treatment. Therefore, further study is required to verify if the observed trends are applicable to other countries.
Conclusion
HAIC plus lenvatinib with PD-1 inhibitors is safe and well-tolerated in advanced CCA, and has the potential to prolong the survival of patients with advanced CCA. The addition of PD-1 inhibitors may enhance the efficacy of HAIC and lenvatinib. Therefore, the combined therapy has the potential to become a treatment option for advanced CCA. However, further study involving larger cohorts is necessary.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board and ethics committee at Beijing Tsinghua Changgung Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
This work was designed by ZW, YJW, and YZ. The data were collected by YJW, BW, YL, YQW, ZR, and XY. The data were analyzed by ZW, YJW, and BW. This manuscript was written by ZW and YJW. QC provided assistance in the revision of the manuscript. All authors approved the final version.
Funding
This study was funded by Beijing Medical Award Foundation (YXJL-2020-0972-1215).
Conflict of interest
Author QC was employed by the company Thorgene Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Benson AB III, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Hepatobiliary cancers, version 2.2019 featured updates to the NCCN guidelines. J Natl Compr Cancer Network (2019) 17(4):303–10. doi: 10.6004/jnccn.2019.0019
2. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol (2020) 17(9):557–88. doi: 10.1038/s41575-020-0310-z
3. Massani M, Bonariol L, Stecca T. Hepatic arterial infusion chemotherapy for unresectable intrahepatic cholangiocarcinoma, a comprehensive review. J Clin Med (2021) 10(12):2552. doi: 10.3390/jcm10122552
4. Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Soreide K, Wigmore SJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surgery (2021) 273(2):240–50. doi: 10.1097/SLA.0000000000003801
5. Hall FM. Cisplatin plus gemcitabine for biliary tract cancer. New Engl J Med (2010) 363(2):192–. doi: 10.1056/NEJMc1005317
6. Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27:v28–37. doi: 10.1093/annonc/mdw324
7. Hou Z, Liu J, Jin Z, Qiu G, Xie Q, Mi S, et al. Use of chemotherapy to treat hepatocellular carcinoma. Bioscience Trends (2022) 16(1):31–45. doi: 10.5582/bst.2022.01044
8. Wang X, Hu J, Cao G, Zhu X, Cui Y, Ji X, et al. Phase II study of hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced perihilar cholangiocarcinoma. Radiology (2017) 283(2):580–9. doi: 10.1148/radiol.2016160572
9. Cai Z, He C, Zhao C, Lin X. Survival comparisons of hepatic arterial infusion chemotherapy with mFOLFOX and transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.611118
10. Ishii M, Itano O, Morinaga J, Shirakawa H, Itano S. Potential efficacy of hepatic arterial infusion chemotherapy using gemcitabine, cisplatin, and 5-fluorouracil for intrahepatic cholangiocarcinoma. PloS One (2022) 17(4):e0266707. doi: 10.1371/journal.pone.0266707
11. Lyu N, Wang X, Li J-B, Lai J-F, Chen Q-F, Li S-L, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: A biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol (2022) 40(5):468–+. doi: 10.1200/JCO.21.01963
12. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion A randomized clinical trial. JAMA Oncol (2019) 5(7):953–60. doi: 10.1001/jamaoncol.2019.0250
13. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: A review in hepatocellular carcinoma. Drugs (2019) 79(6):665–74. doi: 10.1007/s40265-019-01116-x
14. Goyal L, Kongpetch S, Crolley VE, Bridgewater J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev (2021) 95:102170. doi: 10.1016/j.ctrv.2021.102170
15. Ueno M, Ikeda M, Sasaki T, Nagashima F, Mizuno N, Shimizu S, et al. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: primary analysis results. BMC Cancer (2020) 20(1):1105. doi: 10.1186/s12885-020-07365-4
16. Wang T, Dong J, Zhang Y, Ren Z, Liu Y, Yang X, et al. Efficacy and safety of hepatic artery infusion chemotherapy with mFOLFOX in primary liver cancer patients with hyperbilirubinemia and ineffective drainage: a retrospective cohort study. Ann Trans Med (2022) 10(7):411. doi: 10.21037/atm-22-978
17. Zhao Y, Zhang Y-N, Wang K-T, Chen L. Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochim Et Biophys Acta-Reviews Cancer (2020) 1874(1):188391. doi: 10.1016/j.bbcan.2020.188391
18. Deng M, Li S, Wang Q, Zhao R, Zou J, Lin W, et al. Real-world outcomes of patients with advanced intrahepatic cholangiocarcinoma treated with programmed cell death protein-1-targeted immunotherapy. Ann Med (2022) 54(1):803–11. doi: 10.1080/07853890.2022.2048416
19. Mei J, Tang Y-H, Wei W, Shi M, Zheng L, Li S-H, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.618206
20. Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer (2022) 174:68–77. doi: 10.1016/j.ejca.2022.07.005
21. An C, Fu Y, Li W, Zuo M, Wu P. Postprogression treatment of lenvatinib plus PD-1 inhibitor in advanced hepatocellular carcinoma refractory to HAIC. Cancer (2023) 129(14):2235–44. doi: 10.1002/cncr.34764
22. Akinwande O, Dendy M, Ludwig JM, Kim HS. Hepatic intra-arterial injection of irinotecan drug eluting beads (DEBIRI) for patients with unresectable colorectal liver metastases: A systematic review. Surg Oncology-Oxford (2017) 26(3):268–75. doi: 10.1016/j.suronc.2017.05.003
23. Elganainy D, Holliday EB, Taniguchi CM, Smith GL, Shroff R, Javle M, et al. Dose escalation of radiotherapy in unresectable extrahepatic cholangiocarcinoma. Cancer Med (2018) 7(10):4880–92. doi: 10.1002/cam4.1734
24. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol (2016) 34(5):460–+. doi: 10.1200/JCO.2015.64.2710
25. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol (2021) 22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9
26. Nagino M. Surgical treatment of perihilar cholangiocarcinoma resection or transplant? Ann Surg (2018) 267(5):806–7. doi: 10.1097/SLA.0000000000002624
27. Nehls O, Klump B, Arkenau HT, Hass HG, Greschniok A, Gregor M, et al. Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective phase II trial. Br J Cancer (2002) 87(7):702–4. doi: 10.1038/sj.bjc.6600543
28. Konstantinidis IT, Koerkamp BG, Do RKG, Goenen M, Fong Y, Allen PJ, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer (2016) 122(5):758–65. doi: 10.1002/cncr.29824
29. Oh D-Y, Lee K-H, Lee D-W, Yoon J, Kim T-Y, Bang J-H, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol (2022) 7(6):522–32. doi: 10.1016/S2468-1253(22)00043-7
30. Shi C, Li Y, Yang C, Qiao L, Tang L, Zheng Y, et al. Lenvatinib plus programmed cell death protein-1 inhibitor beyond first-line systemic therapy in refractory advanced biliary tract cancer: A real-world retrospective study in China. Front Immunol (2022) 13:946861. doi: 10.3389/fimmu.2022.946861
31. Xie L, Huang J, Wang L, Ren W, Tian H, Hu A, et al. Lenvatinib combined with a PD-1 inhibitor as effective therapy for advanced intrahepatic cholangiocarcinoma. Front Pharmacol (2022) 13. doi: 10.3389/fphar.2022.894407
32. Zhu C, Xue J, Wang Y, Wang S, Zhang N, Wang Y, et al. Efficacy and safety of lenvatinib combined with PD-1/PD-L1 inhibitors plus Gemox chemotherapy in advanced biliary tract cancer. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1109292
33. Shi G-M, Huang X-Y, Wu D, Sun H-C, Liang F, Ji Y, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. . Signal Transduction Targeted Ther (2023) 8(1):106. doi: 10.1038/s41392-023-01317-7
34. Zhang T, Yang X, Yang X, Zheng K, Wang Y, Wang Y, et al. Different interventional time of hepatic arterial infusion with PD-1 inhibitor for advanced biliary tract cancer: a multicenter retrospective study. Am J Cancer Res (2022) 12(7):3455–63.
35. Yuanren G, Yin L, Liu R, Cui Y. The treatment of transarterial chemoembolization/hepatic arterial infusion chemotherapy combined with lenvatinib and PD-1 inhibitor is effective against hepatocellular carcinoma with portal vein tumor thrombus: A systematic review. Front Oncol (2023) 13. doi: 10.3389/fonc.2023.1054072
Keywords: advanced cholangiocarcinoma, hepatic arterial infusion chemotherapy, lenvatinib, programmed cell death protein-1 inhibitor, efficacy, safety
Citation: Wei Z, Wang Y, Wu B, Liu Y, Wang Y, Ren Z, Yang X, Chen Q and Zhang Y (2023) Hepatic arterial infusion chemotherapy plus lenvatinib with or without programmed cell death protein-1 inhibitors for advanced cholangiocarcinoma. Front. Immunol. 14:1235724. doi: 10.3389/fimmu.2023.1235724
Received: 06 June 2023; Accepted: 15 August 2023;
Published: 30 August 2023.
Edited by:
Kazunari Sasaki, Stanford University, United StatesReviewed by:
Yutaka Endo, The Ohio State University, United StatesSahin Lacin, Koç University Hospital, Türkiye
Copyright © 2023 Wei, Wang, Wu, Liu, Wang, Ren, Yang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuewei Zhang, emhhbmd5dWV3ZWkxMTIxQHNpbmEuY29t
†These authors have contributed equally to this work
 Zhanqi Wei
Zhanqi Wei Yajing Wang1,3†
Yajing Wang1,3† Zhizhong Ren
Zhizhong Ren Yuewei Zhang
Yuewei Zhang