- 1Breast Tumor Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 2Department of Ultrasonography, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Department of Thoracic Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
Background: The use of immune checkpoint inhibitors (ICIs) in cancer treatment has led to an increase in immune-related adverse events (irAEs), which can cause treatment discontinuation and even fatal reactions. The purpose of this study was to evaluate the usefulness of the peripheral biomarker neutrophil to lymphocyte ratio (NLR) in predicting irAEs.
Methods: A systematic search of databases was conducted to identify studies on the predictive value of NLR for irAEs. The standardized mean difference (SMD) was used to compare continuous NLR, while crude odds ratios (ORs) were calculated for categorized NLR if adjusted ORs and 95% confidence intervals (CIs) were not provided in the original study.
Results: The meta-analysis included 47 studies with a total of 11,491 cancer patients treated with ICIs. The baseline continuous NLR was significantly lower in patients with irAEs compared to those without (SMD=-1.55, 95%CI=-2.64 to -0.46, P=0.006). Similarly, categorized NLR showed that lower baseline NLR was associated with increased irAEs (OR=0.55, 95%CI=0.41-0.73, P<0.001). Subgroup analysis revealed that the OR for predicting irAEs with NLR cut-off values of 3 and 5 was 0.4 and 0.59, respectively. Interestingly, increased baseline NLR was associated with a higher incidence of immune-related liver injury (OR=2.44, 95%CI=1.23-4.84, I2 = 0%, P=0.010).
Conclusion: Our study suggests that lower baseline NLR is associated with a higher risk of overall irAEs. However, further studies are needed to determine the best cut-off value and explore the efficacy of NLR in predicting specific types of irAEs.
Introduction
Over the past few decades, immunotherapies have emerged as a milestone in the treatment of cancer, resulting in durable responses for several types of cancer and extended overall survival (1). Immune checkpoint inhibitors (ICIs) are increasingly being used for certain cancers and have shown remarkable efficacy in promoting long-term survival in patients with metastatic disease. Also, they are gradually becoming a therapeutic option for earlier-stage cancers (2, 3). Currently, the most commonly used ICIs for tumors are anti-programmed death-1 (PD-1) and its ligand (PD-L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) inhibitors (4). When tumors occur, PD-L1 expressed on tumor cells binds with PD-1 to down-regulate the response of T cells, allowing tumor cells to escape immune recognition and destruction, thereby promoting tumor growth (5). CTLA-4 is another receptor expressed on T cells that binds to CD80 and CD86 ligands on antigen-presenting cells. This interaction results in weakened activation of effector T cells and participates in tumor immune escape (6).
However, the increased use of ICIs has led to an increase in the occurrence of immune-related adverse events (irAEs) (7). Unlike conventional radiotherapy and chemotherapy, the anticancer immune responses may also result in adverse side effects due to self-tolerance impairment caused by autoreactive lymphocytes and autoantibodies, disruption of normal tissue immune homeostasis, and subsequent off-target immune and inflammatory responses (8). IrAEs can affect any organ or system in the body, most commonly in the skin, gastrointestinal tract, lungs, musculoskeletal system, and endocrine organs such as the thyroid, adrenal gland, and pituitary gland (9). Although most irAEs are mild and manageable when promptly recognized and appropriately treated, some severe irAEs may necessitate the termination of immunotherapy or the addition of immunosuppressants. Moreover, severe or fatal toxic reactions can occur, posing significant challenges to immunotherapy (10, 11). For example, the reported case fatality rate of immune-related myocarditis is approximately 20%-50% (10). Furthermore, once patients are diagnosed with neurotoxicity following ICIs, almost 80% of them are judged to have grade 3-4 neurotoxicity, and approximately one-third of them die due to irAEs (12).
Exploring biomarkers for predicting the efficacy of ICIs has been one of the focuses of immunotherapy (13). Peripheral blood biomarkers are economical, convenient, and easily obtainable in clinical practice, making them a commonly adopted option. Some studies have demonstrated their prognostic value in both therapeutic efficacy and survival outcomes (14). However, due to the possibilities of drug discontinuation or even death caused by irAEs, recent studies have begun to seek predictive indicators for irAEs to prevent the occurrence of side effects earlier (15). The neutrophil-to-lymphocyte ratio (NLR) is an indicator that reflects systematic inflammation (16). Previous studies have demonstrated that an elevated NLR is a significant risk factor associated with poorer survival outcomes in oncological patients, including those diagnosed with lung cancer, breast cancer and hepatocellular carcinoma (17–19). However, the role of NLR in predicting irAEs remains controversial (14, 20). Therefore, the aim of this review and meta-analysis is to evaluate the overall predictive value of NLR in irAEs in patients undergoing immunotherapy and to explore a suitable NLR cutoff for clinical use.
Materials and methods
This study was designed based on the preferred reporting items for systematic review and meta-analysis (PRISMA) 2020 guidelines (21). The aim was to evaluate the predictive value of peripheral NLR for irAEs in oncological patients treated with ICIs.
Search strategy
The authors conducted a systematic search of PubMed, Ovid Medline, Embase, and Cochrane Database of Systematic Reviews up to March 25th, 2023. Additionally, grey literature was searched using Google Scholar and related conference websites such as the European Society of Medical Oncology and American Society of Clinical Oncology. The search terms used were “immune checkpoint inhibitor,” “immune-related adverse event,” and “neutrophil to lymphocyte ratio.” The detailed search strategy is provided in Supplementary Table 1. All the studies containing titles and abstracts were imported into Endnote X9 to find duplicate studies and then for literature screening.
Selection criteria
Studies were included if they met the following criteria: 1) included cancer patients treated with ICIs, 2) reported the incidence of irAEs, and 3) evaluated NLR as a predictive value for irAE. Exclusion criteria were: 1) in vitro or in vivo studies, 2) no available data on continuous NLR, categorized number of NLR by cut-off, or odds ratio associated with irAE, and 3) case reports or case series with a sample size of less than 10. There was no restriction on study design, but studies were limited to the English language. Conference could be included if the data could be extracted from the abstracts and other review and meta-analysis were screened for further including studies. Data from the same project or center will be selected as one for further meta-analysis.
Literature screening, data extraction and quality assessment
Two researchers (W Zhang and YF Tan) independently screened the titles and abstracts according to the inclusion and exclusion criteria. The full text was further evaluated if the abstracts could not be determined or data could not be extracted. Disagreements were resolved by discussion with a third investigator (J Liu). Data from eligible studies were extracted into a standard form that included study characteristics, NLR-related items, patient characteristics, and the incidence of irAE in all or different subtypes. NLR could be recorded in baseline or post-treatment of ICIs. The continuous or categorized number of NLR were collected in terms of adverse event (AE) group and non-AE group. Odd ratios (ORs) with corresponding 95% confidence intervals (CIs) were also collected when available. Multivariate or adjusted ORs were preferentially included, otherwise univariate ORs was included or calculated based on the original data of the article. The quality of included studies was assessed using the Newcastle-Ottawa Scale (NOS) tool (22). Studies with NOS scores higher than 6 were considered of high quality, while studies with NOS scores of 5 or less were defined as moderate quality.
Statistical analysis
The main outcome of the meta-analysis was the predictive value of NLR for irAE in cancer patients. If the continuous NLR provided as medians and ranges instead of means and standard deviations (SD), the authors converted them into means and SD using the formula provided by Hozo et al. (23). The standardized mean difference (SMD) was used to evaluate the difference in continuous NLR between irAE and non-irAE groups. If categorized NLR was provided based on the NLR cutoff provided by articles, the authors calculated the ORs and 95% CIs. The authors summarized crude ORs or adjusted ORs for reporting pooled ORs and 95% CIs. Subgroup analysis was performed based on different NLR cutoffs provided by each study and other variables such as ethnicity, ICI type, cancer type, and irAE type were also analyzed. The χ2 test combined with the I2 statistics were used for evaluating statistical heterogeneity (a P value of lower than 0.05 with I2≥50% indicated the presence of heterogeneity). If heterogeneity was absent, a fixed-effects model was applied; otherwise, a random-effects model was used. Sensitivity analysis was performed by omitting individual studies one by one to check the influence of each study. Publication bias was evaluated using funnel plots and the Egger test. The statistical analysis was performed by Stata software (version 15.0, Stata Corporation, College Station, TX, USA). P value < 0.05 was set as significant difference.
Results
Study selection
A total of 5,418 studies were identified from the four databases using the search strategy. After deleting the duplicated studies, 3,405 studies were screened by titles and abstracts, and 242 studies were eligibility for full-text review. Grey literature was also searched but no additional studies or information were included. Other related reviews and meta-analyses were screened for further inclusion of the studies. Finally, 47 studies (3, 10, 13, 14, 16, 20, 24–64) were included in our review (Figure 1).
Characteristics of included studies
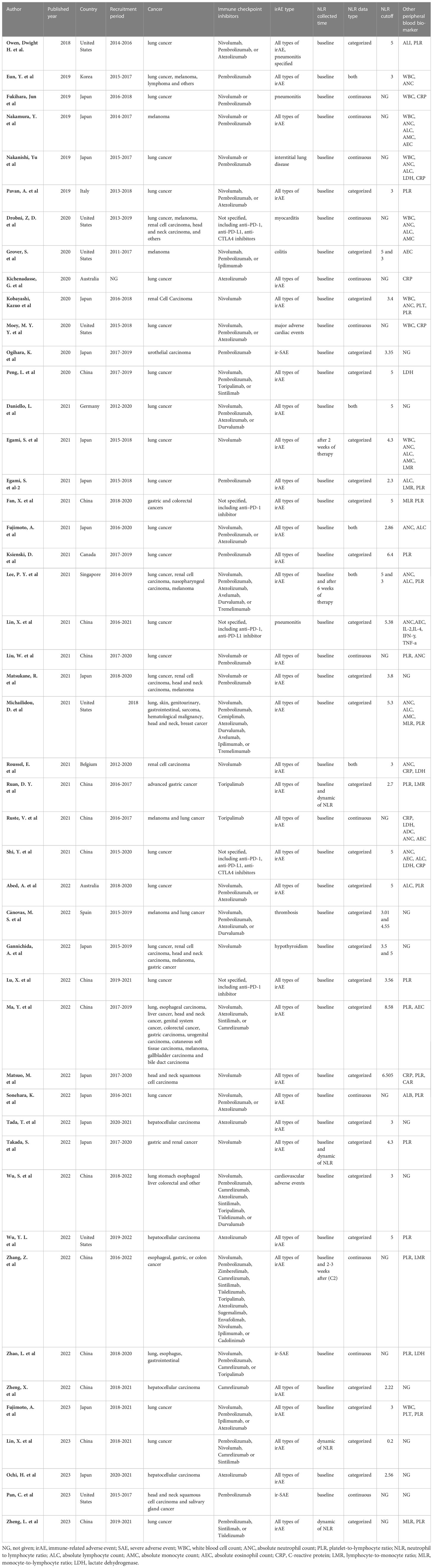
Table 1 shows the characteristics of the 47 included studies. All studies were published between 2018 to 2023, with recruitment periods between 2014 to 2021. Thirty-three studies were reported from Asia, with 16 from Japan, 15 from China, and the remaining two from Korea and Singapore, respectively. The other 14 studies were from the United States (n=7), Australia (n=2), Italy, Germany, Canada, Belgium, and Spain (each reporting one study). Regarding cancer type, 21 studies only included lung cancer patients, four studies only included hepatocellular carcinoma, three studies only included renal cell or urothelial carcinoma, two studies only included melanoma, one study only included head and neck squamous cell carcinoma, one study only included gastric cancer, and the remaining 15 studies contained mixed cancers. In terms of the types of ICIs, 25 studies only evaluated the irAEs of PD-1 inhibitors, four studies only evaluated the irAEs of Atezolizumab (a PD-L1 inhibitor), and the remaining 18 studies contained PD-1/PD-L1 and CTLA-4 inhibitors. Of the 47 studies, 35 evaluated all types of irAEs, three studies only reported ir-SAE, three studies only reported immune-related pneumonitis and interstitial lung disease, three studies reported cardiovascular adverse events, and the remaining three studies reported colitis, thrombosis, and hypothyroidism, respectively. Regarding peripheral NLR, 44 studies evaluated the predictive value of baseline NLR, three studies assessed the value of NLR 2-6 weeks after ICI treatment, and four studies evaluated the predictive value of dynamic NLR in irAEs. Other peripheral blood biomarkers were also evaluated, of which 21 studies discussed the predictive value of platelet to lymphocyte ratio.
Meta-analysis
A total of 11, 491 patients were included in our meta-analysis, with a median number of 115 (range 45 to 1548) enrolled in each study (Supplementary Table 2). Sixty-seven percent of patients were male, with a median age ranging from 16 to 89 years. A total of 2,836 irAEs were reported, with a median incidence of 24.5% ranging from 3% to 70%. Twenty-two studies reported the incidence of different subtypes of irAEs (Supplementary Table 3). The most common irAE was dermatologic disorders, with an incidence ranging from 6.1% to 77.7%. The incidence of pneumonitis, endocrinopathy, gastrointestinal disorders, and liver injury was 1.7%-33.3%, 4.4%-36.4%, 1.3%-29.2%, and 0.4%-27.5%, respectively.
Predictive value of continuous NLR for irAE
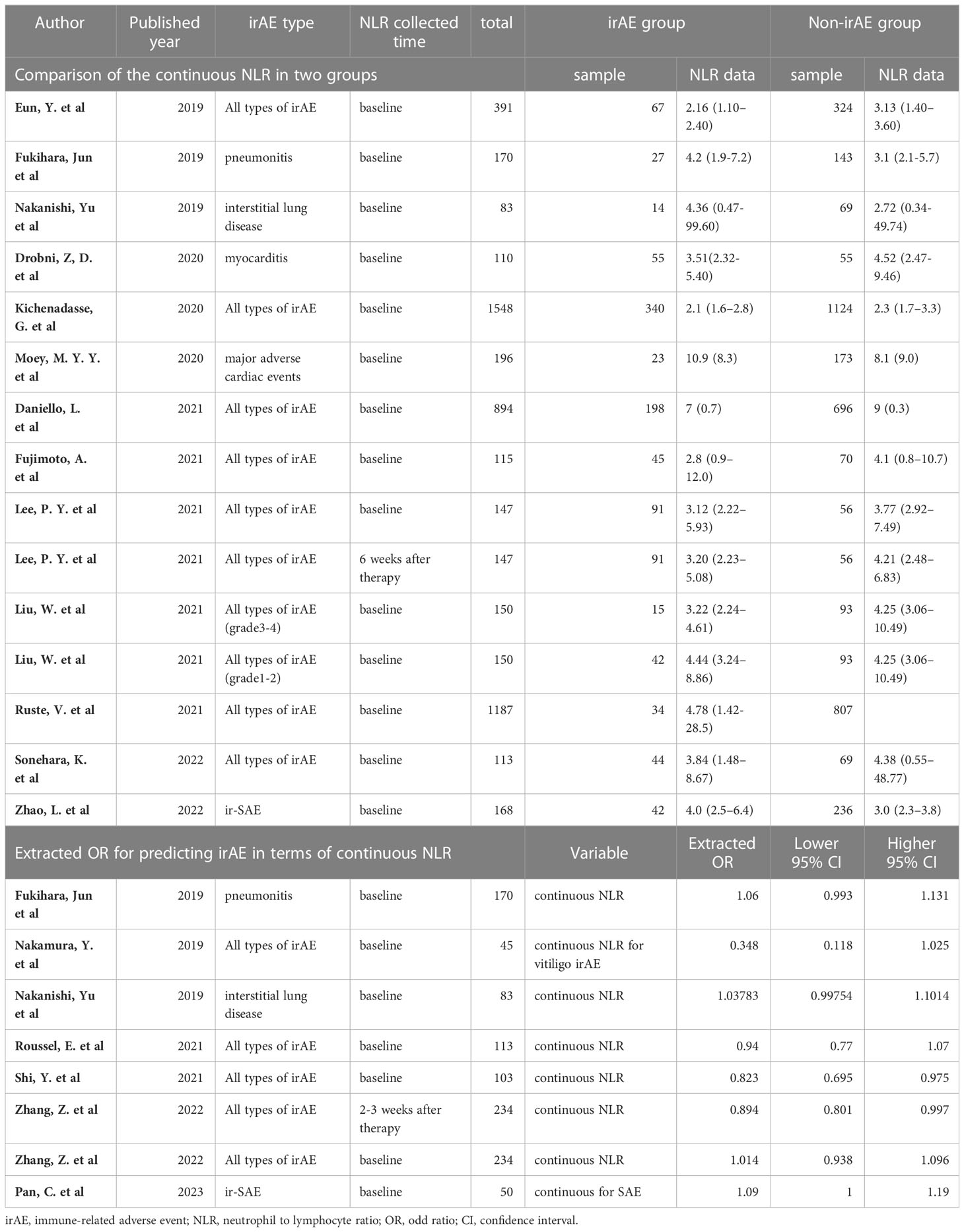
Among the identified studies, thirteen listed continuous NLR in both irAE and non-irAE groups. Of these, eight analyzed all types of irAEs (Table 2). Median baseline NLR ranged from 2.1 to 10.9 in irAE group, compared with 2.3 to 9 in non-irAE group. Seven studies compared baseline peripheral NLR in all types of irAEs, and were thereby included in the meta-analysis. As shown in Figure 2A, the baseline NLR was significantly lower in the irAE group compared with the non-irAE group (SMD=-1.55, 95%CI=-2.64 to -0.46, P=0.006, I2 = 99.1%, random effect model).
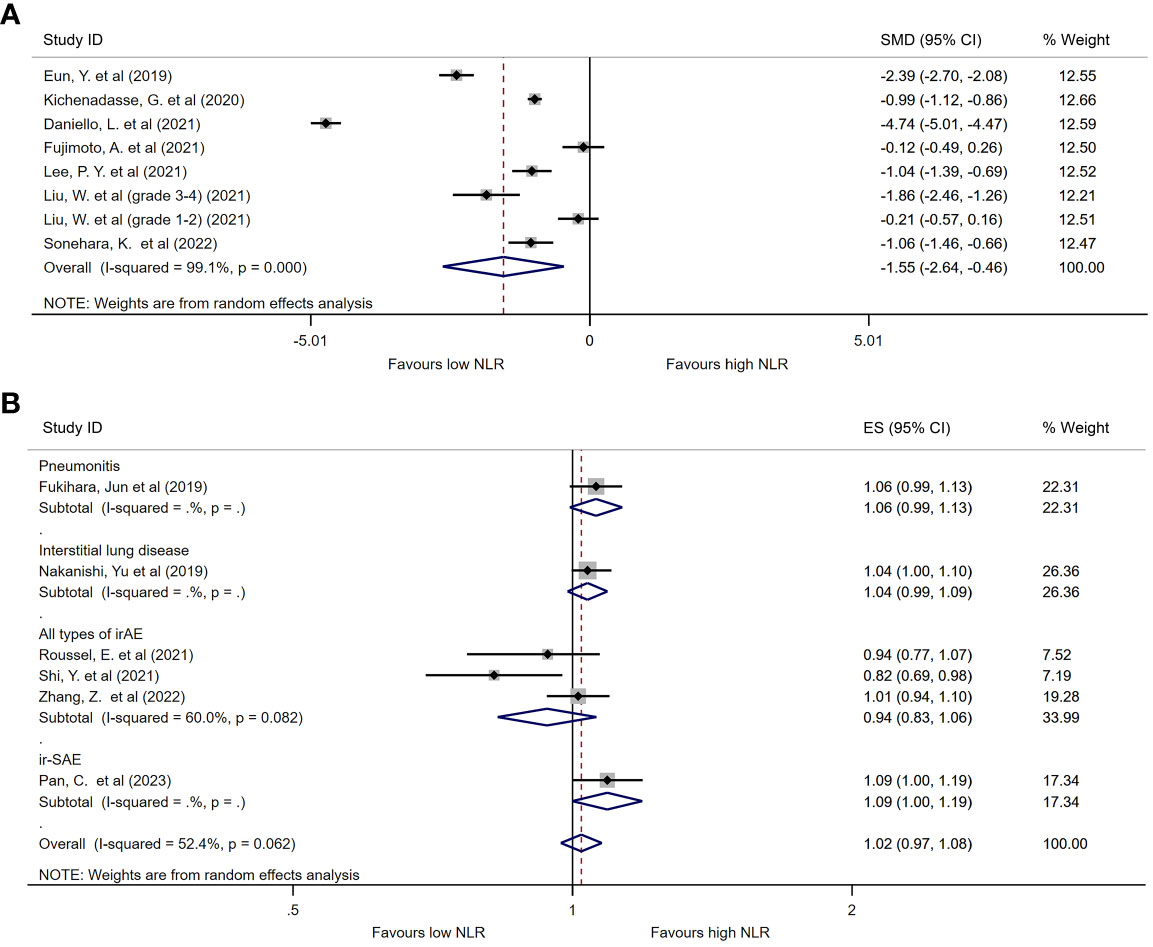
Figure 2 Forest plot comparing continuous NLR between patients who experienced irAEs and those who did not. (A) Comparison of mean NLR values between groups. (B) Pooled ORs based on continuous NLR data.
Seven studies evaluated the ORs of continuous NLR in predicting irAEs, of which three reported baseline continuous NLR in all types of irAEs (Table 2). Although a trend was found suggesting that the incidence of irAEs could be lower with an increase in continuous NLR, there was no statistical difference observed (OR=0.94, 95%CI=0.83 to 1.06, P=0.311, Figure 2B).
Predictive value of categorized NLR for irAE
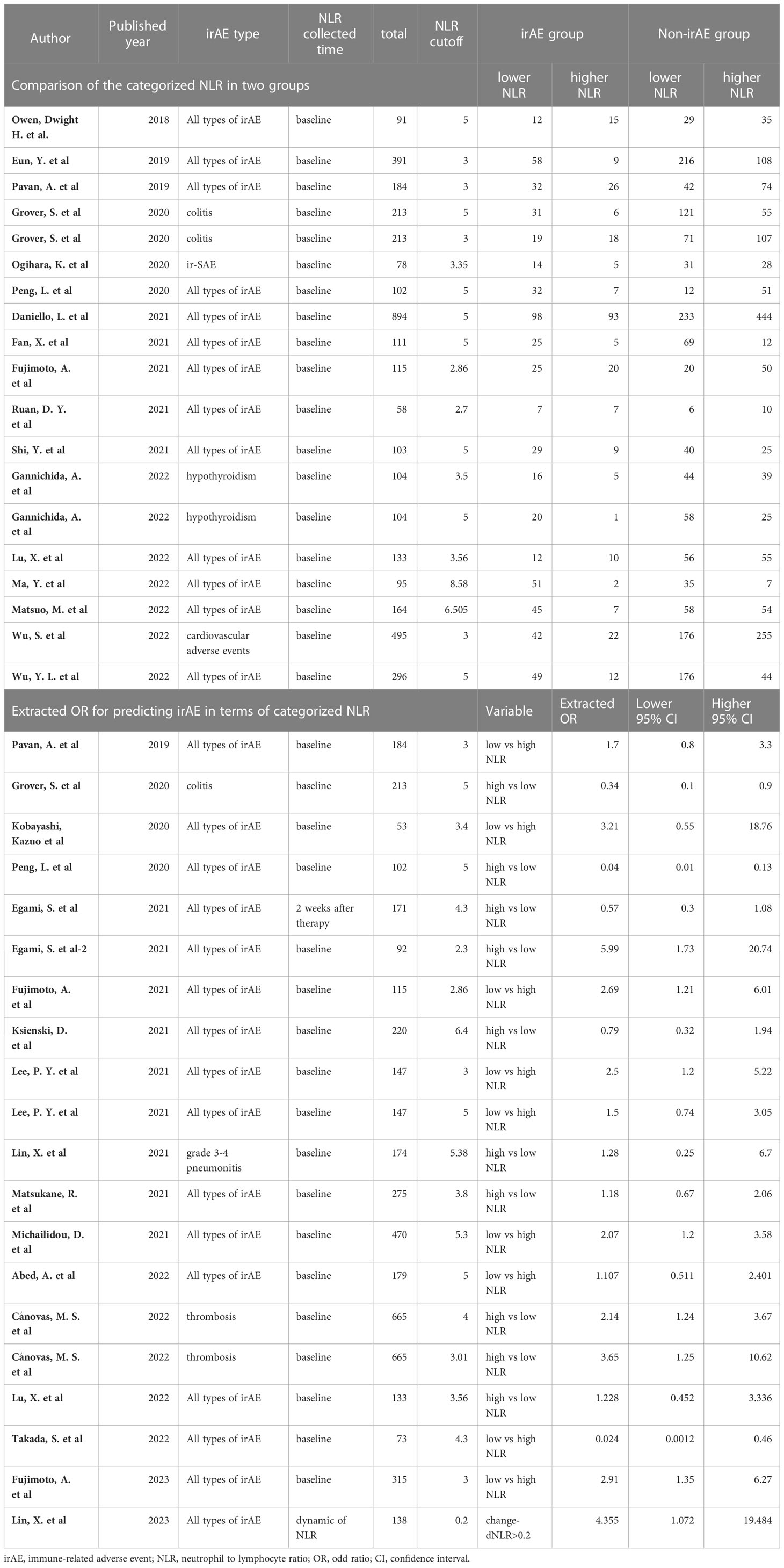
Twenty-nine studies categorized NLR to predict irAEs by different cut-offs (Table 3). The cut-offs of baseline NLR ranged from 2.3 to 8.58, with seven studies using 3 as the cut-off and twelve studies using 5 as the cut-off. Seventeen studies provided data on the number of lower NLR patients and higher NLR patients in both irAE and non-irAE groups. The pooled ORs for these studies were 0.424 (95%CI=0.308 to 0.584, P<0.001, Supplementary Figure 1). Additionally, 17 studies reported calculated ORs of categorized NLR in predicting irAEs, either in univariate or adjusted methods. The pooled ORs for these studies were 0.61 (95%CI=0.39 to 0.94, P=0.027, Supplementary Figure 2). Combining all studies reporting categorized NLR to predict irAEs, we found that lower NLR was associated with a higher incidence of irAEs (OR=0.55, 95%CI=0.41-0.73, I2 = 71.1%, P<0.001, Supplementary Figure 3).
Subgroup analysis
Subgroup analysis was performed to explore the potential sources of heterogeneity among studies. To avoid bias caused by studies reporting only one type of irAE (36, 42, 43, 47, 61), we included 24 studies that analyzed all types of irAE in the subgroup analysis based on different NLR cut-off values. Figure 3 presents the pooled ORs for all or individual NLR cut-offs. The overall pooled OR was consistent with the previous result (OR=0.55, 95%CI=0.41-0.73, I2 = 65.9%, P<0.001). Among the different NLR cut-offs, an NLR of 3 or less was associated with a significantly lower incidence of irAEs (OR=0.40, 95%CI=0.28-0.58, I2 = 0%, P<0.001), while an NLR of 5 or less also correlated with a lower incidence of irAEs (OR=0.59, 95%CI=0.36-0.97, I2 = 70.9%, P=0.036).
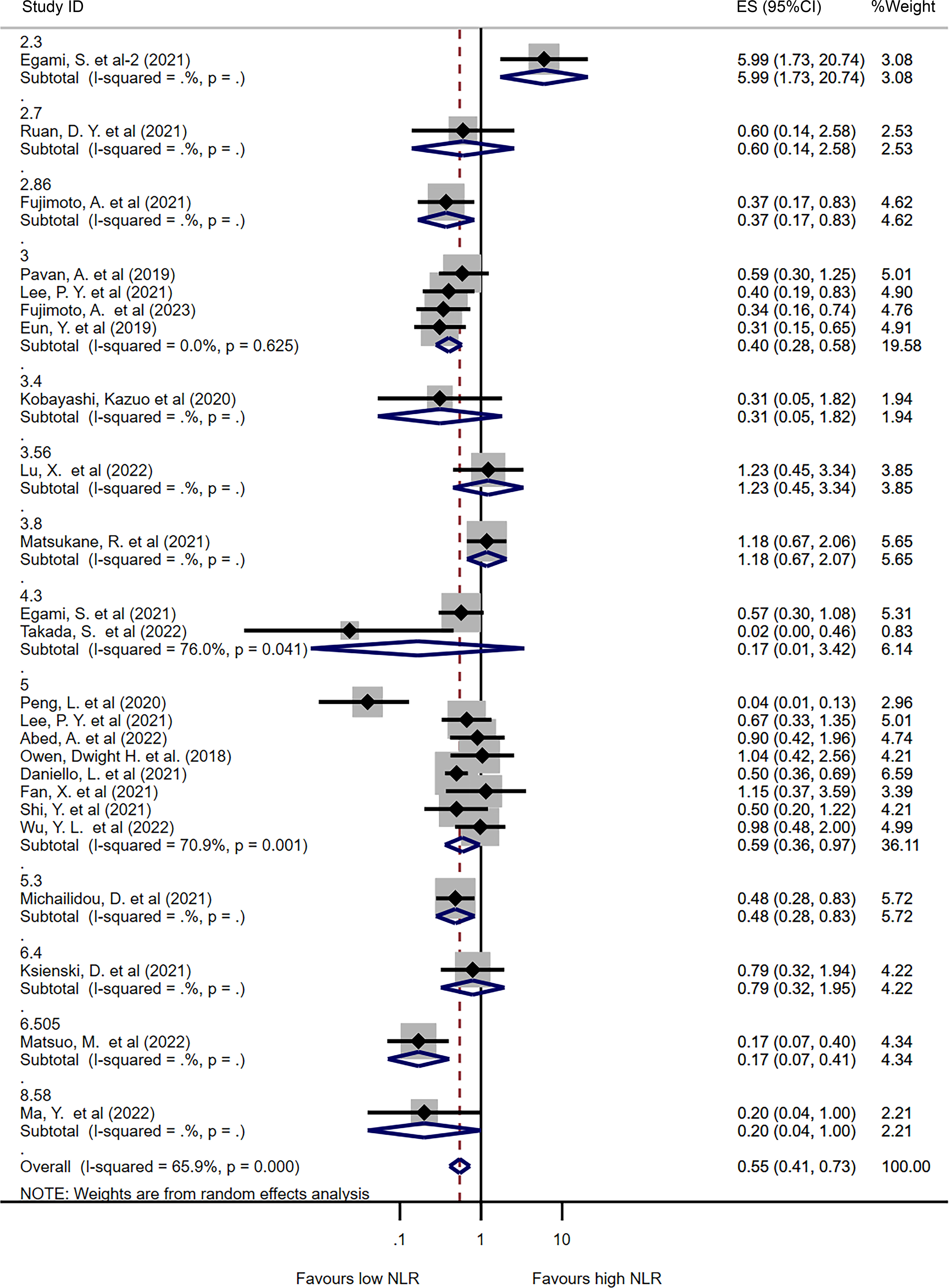
Figure 3 Forest plot comparing categorized NLR for overall irAEs using different cut-off values. Pooled ORs are shown for each cut-off.
In addition, subgroup analysis was performed based on cancer type, with most studies including only lung cancer patients (n=12). The results showed that lower NLR values were associated with a higher incidence of irAEs in lung cancer patients (OR=0.60, 95%CI=0.39-0.92, I2 = 72.2%, P=0.018, Supplementary Figure 4). Subgroup analysis was also performed based on ICI type, with 12 studies including only PD-1 inhibitors, 6 studies including PD-1 and PD-L1 inhibitors, and 5 studies including PD-1, PD-L1, and CTLA-4 inhibitors. In patients treated with PD-1 inhibitors, lower NLR values were associated with a lower incidence of irAEs (OR=0.52, 95%CI=0.27-0.99, I2 = 80.2%, P=0.046, Supplementary Figure 5). Finally, subgroup analysis was performed based on publication area, with studies divided into Asian and non-Asian countries. Similar results were observed in both Asian and non-Asian publications (OR=0.50 for Asian, I2 = 74.3%, P=0.002; OR=0.56 for non-Asian, I2 = 0%, P<0.001, Supplementary Figure 6).
Predictive value of categorized NLR for specified irAE
We analyzed the pooled ORs of categorized NLR for specified irAE if two or more studies reported the predictive value. No significant difference was found in terms of pneumonitis, colitis or immune related endocrine dysfunction between irAE and non-irAE group. But interestingly, increased baseline NLR might associate with the increasing incidence of liver injury (OR=2.44, 95%CI=1.23-4.84, I2 = 0%, P=0.010, Figure 4).
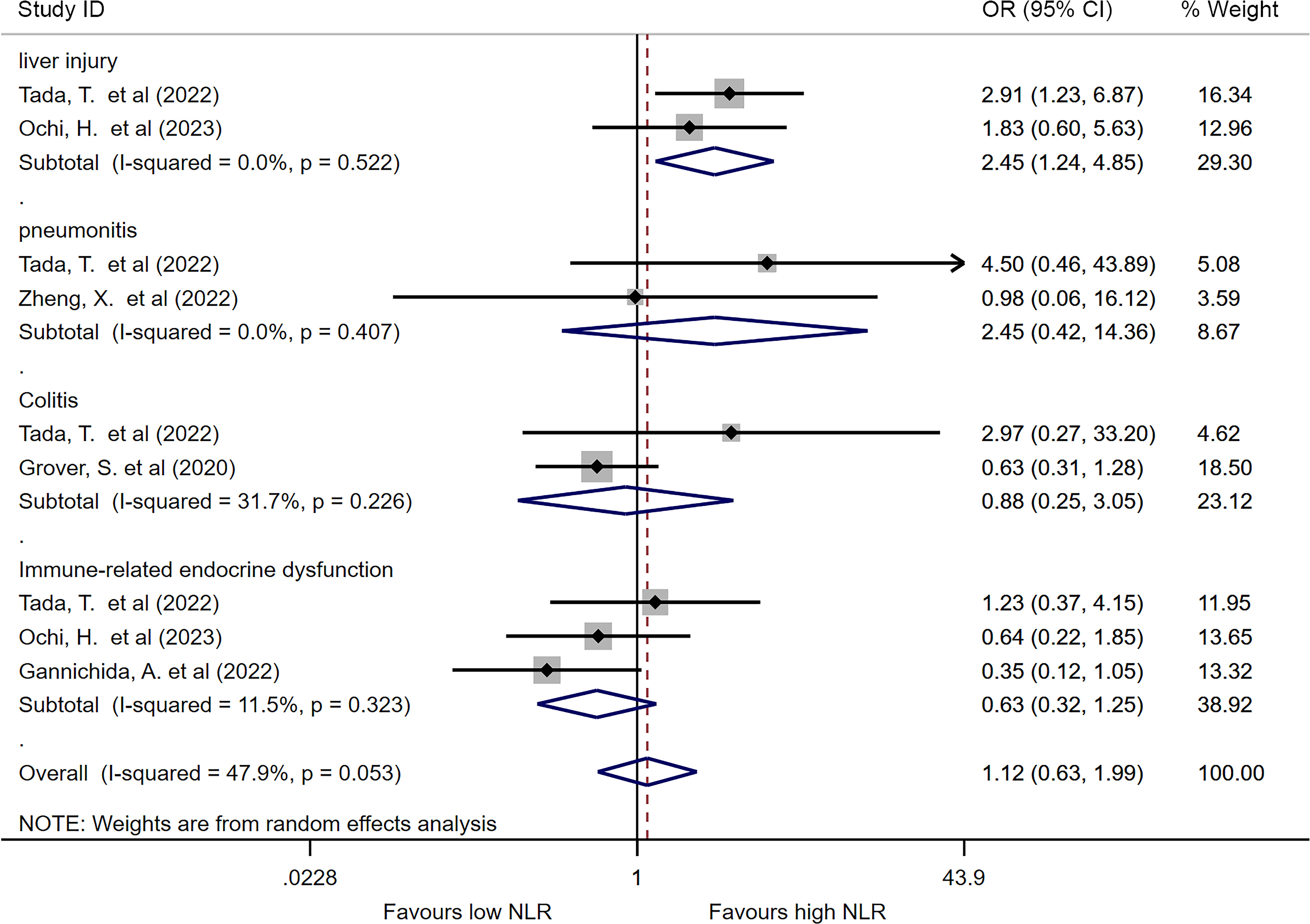
Figure 4 Forest plot comparing categorized NLR for specific irAEs. Pooled ORs are shown for each irAE.
Sensitivity analysis
We performed a sensitivity analysis to determine the potential source of heterogeneity. In continuous NLR for predicting irAE, the heterogeneity was influenced remarkably by each study due to the small number of involved studies (Supplementary Figure 7). However, in categorized NLR for predicting irAE, only a minority of studies were identified as contributing to the heterogeneity of pooled OR outcomes (Supplementary Figure 8). The overall estimate of the pooled ORs would not be significantly influenced when removing any study in turn.
Quality assessment and publication bias assessment
We considered 40 studies as high quality, while the other 7 studies had NOS scores of 5-6. The funnel plot of the included studies was symmetrical (Supplementary Figure 9). For studies comparing continuous NLR between irAE and non-irAE, the Egger’s test suggested no potential publication bias (P=0.744). Similarly, for studies calculating the pooled ORs in continuous or categorized NLR, no potential publication bias was identified by the Egger’s test (P=0.125 and P=0.377, respectively).
Discussion
In this meta-analysis, our objective was to examine the predictive ability of NLR for irAEs in cancer patients undergoing ICIs treatment. By utilizing a thorough and systematic meta-analysis approach, we evaluated 47 studies comprising 11,491 cancer patients and observed that NLR can serve as a predictor for adverse reactions.
The cancer immunoediting theory describes three stages of interaction between cancer and the immune system during tumorigenesis: elimination, equilibrium, and escape (65). During the elimination stage, both innate and adaptive immunity work together to induce chemokines or recognize tumor antigens to eliminate tumor cells. However, as the tumor progresses, tumor cells survive and reach a balance with the immune system. Eventually, tumor cells evade immune surveillance and gain the upper hand in the tumor microenvironment (TME) (65). The CTLA-4 and PD-1/PD-L1 pathways play a significant role in the escape process of the TME. Some tumors overexpress PD-L1, which increases suppressive co-stimulatory signal production, inhibiting T cell activation and proliferation. In addition, some tumors prompt regulatory T cells (Tregs) to express CTLA-4, leading to downregulation of CD80/CD86 expression in antigen-presenting cells, resulting in reduced production of cytokines such as interleukin 2, which affects the body’s anti-tumor capacity (66). While the mechanism of irAEs is still unclear, some studies have found that T cells are heavily infiltrated in tumor tissues of patients with irAEs (67). According to current findings, the mechanism of irAEs may include the over-activation of effector T cells caused by the inhibition of CTLA-4, PD-1 or PD-L1, reduced function of regulatory T cells, massive release of tumor necrosis factor and gamma interferon, toxic effects of neutrophils and macrophages, and production of antibodies by B cells (68).
During tumorigenesis, neutrophils can produce cytokines and growth factors that lead to immune escape of tumors, and therefore promote tumor growth, invasion, and metastasis (69). On the other hand, lymphocytes, such as T cells, play a crucial role in anti-tumor immune response, suppressing tumor growth (70). Besides, elevated neutrophils can inhibit the anti-tumor function of lymphocytes, leading to weakened attack on mutated cells (71). The NLR imbalance can directly decrease the anti-tumor immune response, accelerating tumor invasion and metastasis, resulting in poor prognosis (32). However, the role of NLR in predicting irAEs is not fully understood. Previous studies have suggested that immune-related toxicities are a group of heterogeneous manifestations, with distinct immunopathogenic mechanisms and different histopathological phenotypes in each involved organ (63, 64). In our study, we found that a lower NLR indicated a higher incidence of all kinds of irAEs. Specifically, we observed that a higher NLR was associated with an increased incidence of immune-related liver injury, although only two studies were included for analysis (28, 32). These studies focused on ICI treatment in hepatocellular carcinoma patients, who may have underlying liver disease leading to distinct results. Wu et al. (62) suggested that a higher NLR could be associated with severe disease burden and liver dysfunction, with patients having an NLR >5 exhibiting higher incidence of elevated alpha-fetoprotein and neoplastic portal vein hypertension. However, more studies should be conducted to investigate the relationship between NLR and distinct types of irAEs.
The optimal cut-off value for NLR varied among the studies included in our analysis. As our studies presented, most studies used a cut-off of 5 to categorize high and low NLR, followed by 3. The criteria used to identify the best cut-off for NLR differed across studies, with some using median values or diagnostic experiments. Additionally, the cut-off of NLR was determined by various factors, including the study participants, tumor type, ICI agents, and risk factors considered, leading to heterogeneity in the analysis of the impact of NLR. Nonetheless, most studies suggested that lower NLR was associated with a higher incidence of irAEs, with this trend or significant difference being observed in the majority of studies included in the meta-analysis.
While our meta-analysis only considered baseline NLR as an indicator for predicting irAEs, some other studies have also investigated the predictive value of dynamic or post-treatment NLR (13, 33, 38, 46, 57, 60, 63). However, these studies have not found dynamic or post-treatment NLR to be a better predictor than baseline NLR, although more recent studies have focused on studying dynamic NLR as an independent predictor. Despite the heterogeneity resulting from potential risk factors, our conclusion was still consistent with the observed trend across studies.
Our study has several strengths. Firstly, to the best of our knowledge, this is the most comprehensive meta-analysis that includes 47 studies investigating the predictive value of peripheral NLR for irAEs. Secondly, our analysis not only compared NLR in categorized conditions based on cut-off values but also examined the impact of baseline continuous NLR in predicting irAEs, thereby strengthening our conclusions. Thirdly, we performed sufficient subgroup analyses based on different NLR cut-off values, cancer types, ICI agents, and ethnicities, and demonstrated that there were differences in the predictive value of NLR between overall irAEs and specific irAEs, such as immune-related liver injury, which has not been reported in previous studies. Additionally, all the included studies were of moderate to high quality, and sensitivity analyses showed robust results.
There are some limitations to our study that need to be considered. Firstly, as our meta-analysis is based on retrospective studies, there is a possibility of heterogeneity and publication bias among the studies. Future studies based on prospective design or individual patient data may provide more robust results. Secondly, due to the variability of cut-offs of NLR used in different studies, we could not determine a consensus on the best cut-off value based on our analysis, which may limit clinical guidance. Thirdly, although our study included a relatively comprehensive set of studies, negative results from non-publication studies could lead to selection bias. Finally, despite the differences in predictive value for different subtypes of irAEs, more studies are needed to investigate specific irAEs as there are currently limited reports available.
Conclusion
In summary, our meta-analysis revealed a significant association between lower baseline NLR and increased risk of irAEs. However, the predictive value of NLR varied among different types of irAEs, indicating a need for further subgroup analysis in evaluating the efficacy of peripheral biomarkers. The most frequently used cut-offs for NLR were 3 and 5, but a consensus on the best “cut-off” is required for future clinical guidance. Overall, our findings suggest that NLR can serve as a valuable tool in predicting irAEs, and further studies are necessary to explore its potential role in personalized immunotherapy management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
Design of the meta-analysis: WZ, YT, YL and JL. Literature screening: WZ, and YT. Quality assessment: WZ and YL. Statistics analysis: WZ. Write and revise: WZ, YT, YL, and JL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Guangdong Basic and Applied Basic Research Foundation (2020A1515110047) and Basic and applied basic research fund of Guangzhou basic research program (202201011562).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1234142/full#supplementary-material
Supplementary Figure 1 | Forest plot comparing pooled ORs based on raw data reported in the original studies.
Supplementary Figure 2 | Forest plot comparing pooled ORs based on adjusted ORs reported in the original studies.
Supplementary Figure 3 | Forest plot comparing pooled ORs based on both adjusted and crude ORs reported in the original studies.
Supplementary Figure 4 | Subgroup analysis of the pooled ORs in terms of cancer type.
Supplementary Figure 5 | Subgroup analysis of the pooled ORs in terms of ICI type.
Supplementary Figure 6 | Subgroup analysis of the pooled ORs in terms of publication area.
Supplementary Figure 7 | Sensitivity analysis of included studies for continuous NLR data.
Supplementary Figure 8 | Sensitivity analysis of included studies for categorized NLR data.
Supplementary Figure 9 | Funnel plot showing the publication bias assessment of the included studies.
Abbreviations
ICI, Immune checkpoint inhibitor; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; IrAE, immune-related adverse event; AE, adverse event; SAE, severe adverse event; NLR, neutrophil-to-lymphocyte ratio; PRISMA, the preferred reporting items for systematic review and meta-analysis; OR, Odd ratio; CI, confidence interval; NOS, Newcastle-Ottawa Scale; SD, standard deviations; SMD, standardized mean difference; TME, tumor microenvironment.
References
1. Esfahani K, Roudaia L, Del Rincon SV, Papneja N, Miller WH. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol (2020) 27:87–97. doi: 10.3747/co.27.5223
2. Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, et al. Immune checkpoint therapy-current perspectives and future directions. Cell (2023) 186:1652–69. doi: 10.1016/j.cell.2023.03.006
3. Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer (2018) 19:e893–900. doi: 10.1016/j.cllc.2018.08.008
4. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun (2020) 11:3801. doi: 10.1038/s41467-020-17670-y
5. Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discovery (2015) 5:915–9. doi: 10.1158/2159-8290.CD-15-0563
6. Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers (2021) 13(6):1440. doi: 10.3390/cancers13061440
7. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6:38. doi: 10.1038/s41572-020-0160-6
8. Bardoscia L, Pasinetti N, Triggiani L, Cozzi S, Sardaro A. Biological bases of immune-related adverse events and potential crosslinks with immunogenic effects of radiation. Front Pharmacol (2021) 12:746853. doi: 10.3389/fphar.2021.746853
9. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J immunother Cancer (2019) 7:1–11. doi: 10.1186/s40425-019-0805-8
10. Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, Lee C, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc (2020) 9:e018306. doi: 10.1161/JAHA.120.018306
11. Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol (2020) 6:865–71. doi: 10.1001/jamaoncol.2020.0726
12. Duong SL, Barbiero FJ, Nowak RJ, Baehring JM. Neurotoxicities associated with immune checkpoint inhibitor therapy. J neuro-oncol (2021) 152:265–77. doi: 10.1007/s11060-021-03695-w
13. Lee PY, Oen KQX, Lim GRS, Hartono JL, Muthiah M, Huang DQ, et al. Neutrophil-to-lymphocyte ratio predicts development of immune-related adverse events and outcomes from immune checkpoint blockade: A case-control study. Cancers (2021) 13(6):1308. doi: 10.3390/cancers13061308
14. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer immunol immunother CII (2020) 69:1813–22. doi: 10.1007/s00262-020-02585-w
15. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol Off J Am Soc Clin Oncol (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
16. Fujimoto A, Toyokawa G, Koutake Y, Kimura S, Kawamata Y, Fukuishi K, et al. Association between pretreatment neutrophil-to-lymphocyte ratio and immune-related adverse events due to immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer (2021) 12:2198–204. doi: 10.1111/1759-7714.14063
17. Zhang W, Liu C, Tan Y, Jiang L, Yan L, Yang J, et al. Role of liver resection in treating intermediate and advanced stage adolescent and young adult hepatocellular carcinoma patients: A propensity-matching cohort study. Int J Surg (2018) 54:259–64. doi: 10.1016/j.ijsu.2018.03.051
18. Huang L, Jiang S, Shi Y. Prognostic significance of baseline neutrophil-lymphocyte ratio in patients with non-small-cell lung cancer: a pooled analysis of open phase III clinical trial data. Future Oncol (London England) (2022) 18:1679–89. doi: 10.2217/fon-2021-1304
19. Rottier P, Emile G, Johnson A, Levy C, Allouache D, Hrab I, et al. Pretreatment neutrophil to lymphocyte ratio as prognostic factor in metastatic breast cancer treated with cyclin dependent kinase 4/6 inhibitors. Front Oncol (2022) 12:1105587. doi: 10.3389/fonc.2022.1105587
20. Egami S, Kawazoe H, Hashimoto H, Uozumi R, Arami T, Sakiyama N, et al. Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: a multicenter retrospective study. J Cancer (2021) 12:2105–12. doi: 10.7150/jca.53242
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.) (2021) 372:n71. doi: 10.1136/bmj.n71
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
24. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer (2019) 20(6):442–50.e4. doi: 10.1016/j.cllc.2019.07.006
25. Kobayashi K, Iikura Y, Hiraide M, Yokokawa T, Aoyama T, Shikibu S, et al. Association between immune-related adverse events and clinical outcome following nivolumab treatment in patients with metastatic renal cell carcinoma. In Vivo (Athens Greece) (2020) 34:2647–52. doi: 10.21873/invivo.12083
26. Nakanishi Y, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Nakashima T, et al. Pre-existing interstitial lung abnorMalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Invest (2019) 57:451–9. doi: 10.1016/j.resinv.2019.05.002
27. Shi Y, Liu J, Zhang D, Liu X, Yue Y, Zhou Q, et al. Correlations between peripheral blood biomarkers and clinical outcomes in advanced non-small cell lung cancer patients who received immunotherapy-based treatments. Trans Lung Cancer Res (2021) 10(12):4477–93. doi: 10.21037/tlcr-21-710
28. Ochi H, Kurosaki M, Joko K, Mashiba T, Tamaki N, Tsuchiya K, et al. Usefulness of neutrophil-to-lymphocyte ratio in predicting progression and survival outcomes after atezolizumab-bevacizumab treatment for hepatocellular carcinoma. Hepatol Res Off J Japan Soc Hepatol (2023) 53:61–71. doi: 10.1111/hepr.13836
29. Ogihara K, Kikuchi E, Shigeta K, Okabe T, Hattori S, Yamashita R, et al. The pretreatment neutrophil-to-lymphocyte ratio is a novel biomarker for predicting clinical responses to pembrolizumab in platinum-resistant metastatic urothelial carcinoma patients. Urologic Oncol (2020) 38:602.e601–602.e610. doi: 10.1016/j.urolonc.2020.02.005
30. Pan C, Wu QV, Voutsinas J, Houlton JJ, Barber B, Futran N, et al. Neutrophil to lymphocyte ratio and peripheral blood biomarkers correlate with survival outcomes but not response among head and neck and salivary cancer treated with pembrolizumab and vorinostat. Head Neck (2023) 45:391–7. doi: 10.1002/hed.27252
31. Roussel E, Kinget L, Verbiest A, Debruyne PR, Baldewijns M, Van Poppel H, et al. C-reactive protein and neutrophil-lymphocyte ratio are prognostic in metastatic clear-cell renal cell carcinoma patients treated with nivolumab. Urologic Oncol (2021) 39:239.e217–239.e225. doi: 10.1016/j.urolonc.2020.12.020
32. Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Neutrophil-lymphocyte ratio predicts early outcomes in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter analysis. Eur J Gastroenterol Hepatol (2022) 34:698–706. doi: 10.1097/MEG.0000000000002356
33. Zheng L, Xiong A, Wang S, Xu J, Shen Y, Zhong R, et al. Decreased monocyte-to-lymphocyte ratio was associated with satisfied outcomes of first-line PD-1 inhibitors plus chemotherapy in stage IIIB-IV non-small cell lung cancer. Front Immunol (2023) 14:1094378. doi: 10.3389/fimmu.2023.1094378
34. Zheng X, Qian K. Neutrophil-to-lymphocyte ratio predicts therapy outcomes of transarterial chemoembolization combined with tyrosine kinase inhibitors plus programmed cell death ligand 1 antibody for unresectable hepatocellular carcinoma. Anti-cancer Drugs (2022) 34(6):775–82. doi: 10.1097/CAD.0000000000001458
35. Abed A, Law N, Calapre L, Lo J, Bhat V, Bowyer S, et al. Human leucocyte antigen genotype association with the development of immune-related adverse events in patients with non-small cell lung cancer treated with single agent immunotherapy. Eur J Cancer (2022) 172:98–106. doi: 10.1016/j.ejca.2022.05.021
36. Cánovas MS, Garay DF, Moran LO, Pérez JR, Rubio CMG, de Mena ML, et al. Immune checkpoint inhibitors-associated thrombosis in patients with lung cancer and melanoma: a study of the Spanish society of medical oncology (SEOM) thrombosis and cancer group. Clin Trans Oncol (2022) 24:2010–20. doi: 10.1007/s12094-022-02860-5
37. Daniello L, Elshiaty M, Bozorgmehr F, Kuon J, Kazdal D, Schindler H, et al. Therapeutic and prognostic implications of immune-related adverse events in advanced non-small-cell lung cancer. Front Oncol (2021) 11:703893. doi: 10.3389/fonc.2021.703893
38. Egami S, Kawazoe H, Hashimoto H, Uozumi R, Arami T, Sakiyama N, et al. Absolute lymphocyte count predicts immune-related adverse events in patients with non-small-cell lung cancer treated with nivolumab monotherapy: A multicenter retrospective study. Front Oncol (2021) 11:618570. doi: 10.3389/fonc.2021.618570
39. Eun Y, Kim IY, Sun JM, Lee J, Cha HS, Koh EM, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep (2019) 9:14039. doi: 10.1038/s41598-019-50574-6
40. Fan X, Wang D, Zhang W, Liu J, Liu C, Li Q, et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving anti-PD-1 therapy. Front Cell Dev Biol (2021) 9:638312. doi: 10.3389/fcell.2021.638312
41. Fujimoto A, Koutake Y, Hisamatsu D, Ookubo N, Yabuuchi Y, Kamimura G, et al. Risk factors indicating immune-related adverse events with combination chemotherapy with immune checkpoint inhibitors and platinum agents in patients with non-small cell lung cancer: a multicenter retrospective study. Cancer immunol immunother CII (2023) 72(7):2169–78. doi: 10.1007/s00262-023-03408-4
42. Gannichida A, Nakazawa Y, Kageyama A, Utsumi H, Kuwano K, Kawakubo T. Necessity of neutrophil-to-lymphocyte ratio monitoring for hypothyroidism using nivolumab in patients with cancer. World J Clin Oncol (2022) 13:641–51. doi: 10.5306/wjco.v13.i7.641
43. Grover S, Dougan M, Tyan K, Giobbie-Hurder A, Blum SM, Ishizuka J, et al. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer (2020) 126:3758–67. doi: 10.1002/cncr.32966
44. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Multiorgan immune-related adverse events during treatment with atezolizumab. J Natl Compr Cancer Network JNCCN (2020) 18:1191–9. doi: 10.6004/jnccn.2020.7567
45. Ksienski D, Wai ES, Alex D, Croteau NS, Freeman AT, Chan A, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Trans Lung Cancer Res (2021) 10:355–67. doi: 10.21037/tlcr-20-541
46. Lin X, Chen X, Long X, Zeng C, Zhang Z, Fang W, et al. New biomarkers exploration and nomogram construction of prognostic and immune-related adverse events of advanced non-small cell lung cancer patients receiving immune checkpoint inhibitors. Respir Res (2023) 24(1):64. doi: 10.1186/s12931-023-02370-0
47. Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front Oncol (2021) 11:698832. doi: 10.3389/fonc.2021.698832
48. Liu W, Liu Y, Ma F, Sun B, Wang Y, Luo J, et al. Peripheral blood markers associated with immune-related adverse effects in patients who had advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Manage Res (2021) 13:765–71. doi: 10.2147/CMAR.S293200
49. Lu X, Wan J, Shi H. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios are associated with the efficacy of immunotherapy in stage III/IV non-small cell lung cancer. Oncol Lett (2022) 24:266. doi: 10.3892/ol.2022.13386
50. Ma Y, Ma X, Wang J, Wu S, Wang J, Cao B. Absolute eosinophil count may be an optimal peripheral blood marker to identify the risk of immune-related adverse events in advanced Malignant tumors treated with PD-1/PD-L1 inhibitors: a retrospective analysis. World J Surg Oncol (2022) 20:242. doi: 10.1186/s12957-022-02695-y
51. Matsukane R, Watanabe H, Minami H, Hata K, Suetsugu K, Tsuji T, et al. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci Rep (2021) 11:1324. doi: 10.1038/s41598-020-79397-6
52. Matsuo M, Yasumatsu R, Masuda M, Toh S, Wakasaki T, Hashimoto K, et al. Inflammation-based prognostic score as a prognostic biomarker in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with nivolumab therapy. In Vivo (Athens Greece) (2022) 36:907–17. doi: 10.21873/invivo.12780
53. Michailidou D, Khaki AR, Morelli MP, Diamantopoulos L, Singh N, Grivas P. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci Rep (2021) 11:9029. doi: 10.1038/s41598-021-88307-3
54. Moey MYY, Tomdio AN, McCallen JD, Vaughan LM, O'Brien K, Naqash AR, et al. Characterization of immune checkpoint inhibitor-related cardiotoxicity in lung cancer patients from a rural setting. JACC CardioOncol (2020) 2:491–502. doi: 10.1016/j.jaccao.2020.07.005
55. Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, Watanabe R, et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Japanese J Clin Oncol (2019) 49:431–7. doi: 10.1093/jjco/hyy201
56. Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. oncologist (2019) 24:1128–36. doi: 10.1634/theoncologist.2018-0563
57. Ruan DY, Chen YX, Wei XL, Wang YN, Wang ZX, Wu HX, et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol Rep (2021) 9:560–70. doi: 10.1093/gastro/goab032
58. Ruste V, Goldschmidt V, Laparra A, Messayke S, Danlos FX, ROmano-Martin P, et al. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: A prospective study of the French REISAMIC registry. Eur J Cancer (Oxford Engl 1990) (2021) 158:217–24. doi: 10.1016/j.ejca.2021.08.048
59. Sonehara K, Tateishi K, Araki T, Komatsu M, Akahane J, Yamamoto H, et al. Predictive factors correlated with the development of immune-related adverse events in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Cancer Manage Res (2022) 14:427–35. doi: 10.2147/CMAR.S347852
60. Takada S, Murooka H, Tahatsu K, Yanase M, Umehara K, Hashishita H, et al. Identifying early predictive markers for immune-related adverse events in nivolumab-treated patients with renal cell carcinoma and gastric cancer. Asian Pacific J Cancer Prev APJCP (2022) 23:695–701. doi: 10.31557/APJCP.2022.23.2.695
61. Wu S, Bai H, Zhang L, He J, Luo X, Wang S, et al. Cardiovascular adverse events induced by immune checkpoint inhibitors: A real world study from 2018 to 2022. Front Cardiovasc Med (2022) 9:969942. doi: 10.3389/fcvm.2022.969942
62. Wu YL, Fulgenzi CAM, D'Alessio A, Cheon J, Nishida N, Saeed A, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic biomarkers in unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancers (2022) 14(23):5834. doi: 10.3390/cancers14235834
63. Zhang Z, Xie T, Qi C, Zhang X, Shen L, Peng Z. Peripheral blood biomarkers predictive of efficacy outcome and immune-related adverse events in advanced gastrointestinal cancers treated with checkpoint inhibitors. Cancers (2022) 14(15):3736. doi: 10.3390/cancers14153736
64. Zhao L, Li Y, Jiang N, Song X, Xu J, Zhu X, et al. Association of blood biochemical indexes and antibiotic exposure with severe immune-related adverse events in patients with advanced cancers receiving PD-1 inhibitors. J immunother (Hagerstown Md. 1997) (2022) 45:210–6. doi: 10.1097/CJI.0000000000000415
65. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol (2002) 3:991–8. doi: 10.1038/ni1102-991
66. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Sci (New York N.Y.) (2008) 322:271–5. doi: 10.1126/science.1160062
67. Läubli H, Koelzer VH, Matter MS, Herzig P, Dolder Schlienger B, Wiese MN, et al. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology (2018) 7:e1386362. doi: 10.1080/2162402X.2017.1386362
68. Khunger A, Battel L, Wadhawan A, More A, Kapoor A, Agrawal N. New insights into mechanisms of immune checkpoint inhibitor-induced cardiovascular toxicity. Curr Oncol Rep (2020) 22:65. doi: 10.1007/s11912-020-00925-8
69. Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol (2019) 9:1146. doi: 10.3389/fonc.2019.01146
70. Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol (2019) 234:8509–21. doi: 10.1002/jcp.27782
Keywords: immune checkpoint inhibitors, immune-related adverse events, neutrophil to lymphocyte ratio, biomarker, cancer treatment
Citation: Zhang W, Tan Y, Li Y and Liu J (2023) Neutrophil to Lymphocyte ratio as a predictor for immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Immunol. 14:1234142. doi: 10.3389/fimmu.2023.1234142
Received: 03 June 2023; Accepted: 24 July 2023;
Published: 09 August 2023.
Edited by:
Fan Mo, University of British Columbia, CanadaReviewed by:
Jiaofang Shao, Nanjing Medical University, ChinaStefania Canova, San Gerardo Hospital, Italy
Copyright © 2023 Zhang, Tan, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, emhhbmd3Mjg3QG1haWwuc3lzdS5lZHUuY24=; Jiang Liu, bGl1ajY1OEBtYWlsLnN5c3UuZWR1LmNu; Yuquan Li, bGl5cTYyQG1haWwuc3lzdS5lZHUuY24=
 Wei Zhang
Wei Zhang Yifei Tan2
Yifei Tan2