
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 31 July 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1229558
This article is part of the Research TopicNon-cellular Immunotherapies in Pediatric MalignanciesView all 7 articles
 Patrick Greve1,2†
Patrick Greve1,2† Auke Beishuizen2†
Auke Beishuizen2† Melanie Hagleitner2
Melanie Hagleitner2 Jan Loeffen2
Jan Loeffen2 Margreet Veening2
Margreet Veening2 Marianne Boes1,3
Marianne Boes1,3 Victor Peperzak1
Victor Peperzak1 Claudius Diez2
Claudius Diez2 Friederike Meyer-Wentrup2*
Friederike Meyer-Wentrup2*Introduction: Classical Hodgkin lymphoma (cHL) is the most common pediatric lymphoma. Approximately 10% of patients develop refractory or recurrent disease. These patients are treated with intensive chemotherapy followed by consolidation with radiotherapy or high-dose chemotherapy and autologous stem cell reinfusion. Although this treatment is effective, it comes at the cost of severe long-term adverse events, such as reduced fertility and an increased risk of secondary cancers. Recently, promising results of inducing remission with the immune checkpoint inhibitor nivolumab (targeting PD-1) and the anti-CD30 antibody-drug conjugate Brentuximab vedotin (BV) +/- bendamustine were published.
Methods: Here we describe a cohort of 10 relapsed and refractory pediatric cHL patients treated with nivolumab + BV +/- bendamustine to induce remission prior to consolidation with standard treatment.
Results and discussion: All patients achieved complete remission prior to consolidation treatment and are in ongoing complete remission with a median follow-up of 25 months (range: 12 to 42 months) after end-of-treatment. Only one adverse event of CTCAE grade 3 or higher due to nivolumab + BV was identified. Based on these results we conclude that immunotherapy with nivolumab + BV +/- bendamustine is an effective and safe treatment to induce remission in pediatric R/R cHL patients prior to standard consolidation treatment. We propose to evaluate this treatment further to study putative long-term toxicity and the possibility to reduce the intensity of consolidation treatment.
Classical Hodgkin lymphoma (cHL) (1) is the most frequent lymphoma in children. Chemo- and radiotherapy results in a 5-year event-free survival (EFS) of 89% (2, 3). However, approximately 10% of patients present with recurrent or refractory (R/R) disease. Currently, there is no uniform standard of care for treatment of pediatric R/R cHL patients. In general, salvage chemotherapy is designed to induce remission with as limited long- and short-term toxicity as possible (4–6). Remission may be induced by IGEV chemotherapy (ifosfamide, gemcitabine, vinorelbine) followed by radiotherapy or HD-BEAM (bendamustine, etoposide, cytarabine and melphalan) chemotherapy with autologous stem cell reinfusion depending on stage of disease at recurrence (4). Achieving a complete metabolic remission (CMR) prior to consolidation therapy predicts outcome of pediatric R/R cHL treatment (7). EFS in adults is better in patients in CMR prior to HD chemotherapy and autologous stem cell reinfusion (8). When choosing the best treatment for remission induction in children with R/R cHL special attention must be paid to efficacy as well as long-term toxicity: Although advances in therapy have reduced the long-term toxicity in cHL survivors, follow-up studies still show increased mortality rates (5.1-fold higher risk of death due to causes other than HL) (9) and late toxicity in this population up to decades after their cHL diagnosis (10, 11). Specifically, premature ovarian failure has been reported in 6-34% of female long-term survivors and elevated FSH levels with low inhibin B in a median of 51.5% and 45% respectively in male survivors (12). In addition, 12.7% of childhood HL survivors develop a novel second cancer within 30 years of the initial diagnosis, which is 11 times higher than the expected incidence in the general population (13). Finally, a 4- to 6-fold increased standardized incidence ratio of coronary heart disease or heart failure compared with the general population has been reported (14).
The malignant HL cells express CD30 and are characterized by upregulated expression of the immune checkpoint molecule programmed death-ligand 1 (PD-L1) that binds to the receptor programmed cell death protein 1 (PD-1) as part of an immune evasive phenotype (15). Consequently, clinical efficacy of anti-PD1 checkpoint inhibition by the anti-PD-1 antibody nivolumab and targeting of malignant cHL cells with the anti-CD30 antibody-drug conjugate Brentuximab vedotin (BV) alone and in combination has been demonstrated in adult (16–19) and partly in pediatric R/R cHL patients (20, 21). Recently, encouraging results of the Checkmate 744 trial were published for children, adolescents and young adults (age 5 to 30 years) with R/R cHL after treatment with Nivolumab + BV +/- bendamustine (22). In this study 31 patients < 18 years were treated. The rate of complete metabolic response (CMR) (assessed according to the Lugano classification) (23) was 59% after induction with nivolumab + BV and 94% before consolidation. The one-year progression free survival rate was reported to be 91%. Nivolumab + BV treatment induced grade 3/4 treatment-related adverse events in 18% of patients. In addition, first results of the treatment for low risk patients consisting of nivolumab + BV followed by radiotherapy have been published recently (24). CMR before radiotherapy was reached in 88.9% of treated children with a 3-y EFS rate of 78.3%.
In the current study we present the real-world experience of treating 10 pediatric R/R cHL patients with nivolumab + BV +/- bendamustine as an effective and safe therapy to induce remission prior to consolidation with HD-BEAM chemotherapy and autologous stem cell reinfusion or radiotherapy.
We present data on 10 children (<18 years) with R/R cHL treated at the Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands. The primary manifestation of cHL was initially treated according to the Euronet PHL-interim or -C2 treatment protocols: 2 OEPA cycles (vincristine, etoposide, prednisolone and doxorubicine) followed by 1 to 4 COPDAC-28 cycles (28 days; cyclophosphamide, vincristine, prednisolone and dacarbazine) or 2 to 4 DECOPDAC-21 cycles (21 days; doxorubicin, etoposide, cyclophosphamide, vincristine, prednisolone and dacarbazine) +/- radiotherapy (25). R/R cHL was confirmed by pathology, when clinically possible, and staging performed by total body MRI scan, FDG-PET-CT scan and abdominal ultrasound. Patients were stratified into 2 groups (standard and low risk) according the EuroNet-PHL guidelines (4) and as published (22, 24). Disease was considered refractory if remission had not been achieved or if progression occurred < 3 months after end-of-treatment. All patients and/or parents or guardians were informed about standard chemotherapy (4) and offered to choose treatment with nivolumab + BV. They provided written informed consent according to national laws and in agreement with the declaration of Helsinki (2013). This study is IRB-approved and registered under a national trial registry number 7744.
All patients were treated as published for the Checkmate 744 trial (22, 24): Patients received 4-6 cycles of nivolumab (3 mg/kg on day 8 of cycle 1 and on day 1 thereafter) + BV (1.8 mg/kg on day 1 of every cycle). If CMR [Deauville score < 4, according to Lugano criteria (23)] was reached after cycle 4, patients from the low risk group received consolidation treatment by involved-field radiotherapy. After achieving CMR patients from the standard risk group were treated with HD-BEAM chemotherapy followed by autologous stem cell reinfusion. Autologous peripheral CD34+ blood stem cells were harvested in standard risk patients after the 3rd or 4th cycle of nivolumab + BV according to standard procedures. In patients not in CMR after the 4th nivolumab + BV cycle treatment was intensified by administering 2 cycles of BV + bendamustine (90 mg/m2 on day 1 and 2). One patient was treated with DHAP (dexamethasone, high-dose cytarabine, cisplatinum) + BV (26) because of progressive disease after BV + bendamustine. Treatment toxicity was assessed according to the National Cancer Institute Common Terminology Criteria (CTC) to evaluate adverse events.
Response to treatment was evaluated by total body MRI scan and FDG-PET-CT after 2 and 4 cycles of nivolumab + BV or after the second cycle of bendamustine + BV according to the Lugano criteria (23). A Deauville score <4 was considered a CMR.
Laboratory tests were performed according to standardized procedures at the laboratories for clinical chemistry at the University Medical Center Utrecht and the Princess Máxima Center. Serum TARC levels were measured by ELISA at the laboratory of the UMC Utrecht.
Data were descriptively analyzed using Stata 18 SE for Windows (StataCorp., College Station, TX, USA). Continuous data were shown as median and range or as mean values and range when useful. Categorical data were shown as frequencies and percentages.
We here report the results of 10 patients treated for R/R cHL at the Princess Máxima Center for Pediatric Oncology (Table 1). The median age at primary cHL diagnosis was 13 years (range: 9-16). Nine children had received first-line cHL therapy according to the EuroNet PHL-C2 treatment protocol and one according to the EuroNet PHL-interim treatment recommendation (25) at our center (Table 1). Two patients had received radiotherapy after completion of chemotherapy. Notably, 5 patients were in complete metabolic remission (CMR) at the end of first line treatment.
Recurrences occurred on average 236 days (range 8-804, median 176 days) after end-of-treatment and were diagnosed during routine follow-up visits in 90% of cases. One case came to attention due to a visit scheduled at the patients’ request after feeling a swelling the neck. In 9/10 patients R/R disease was detected in the initially involved lymph node region. 3/10 patients showed progressive disease during treatment (1 at early and 2 at late response assessment). In 20% of cases the relapse occurred at a site previously treated by radiotherapy. One patient presented with a second recurrence. Nine R/R cHL cases were confirmed by pathology. In 1 patient a biopsy was considered too dangerous due to the bleeding risk because of the anatomical localization. The diagnosis of this specific cHL recurrence was made based on detection of enlarging FDG-PET-positive (Deauville score 5) lymph nodes in the previously afflicted lymph node region, an increase of the erythrocyte sedimentation rate (ESR) from normal to 63 mm/1st h and serum TARC-elevation to 971 pg/ml.
In all patients staging was performed with total body MRI and FDG-PET-CT scans. It demonstrated Ann-Arbor stage I/II disease extension in 50% and stage III/IV in 50% of patients. Characteristic “B-symptoms” for cHL such as fever, drenching night sweats or weight loss >10% body weight were absent in 90% of patients treated for R/R disease. One patient with refractory disease had B-symptoms at first presentation. Three patients presented with an ESR >30 mm/1st hour. Recently, the cytokine TARC (CCL-17) has been characterized as a biomarker for cHL in children (27–29). At diagnosis of R/R cHL serum TARC levels were elevated (>880 pg/ml) in 8/10 patients and hemoglobin levels reduced (<7.4 mmol/L) in 4/10 patients.
There were no emergency presentations due to a large mediastinal mass. All patients had a Karnofsky/Lansky score above 80 and presented without relevant comorbidities. There were 8 patients in the standard and 2 patients in the low-risk treatment group (Table 2).
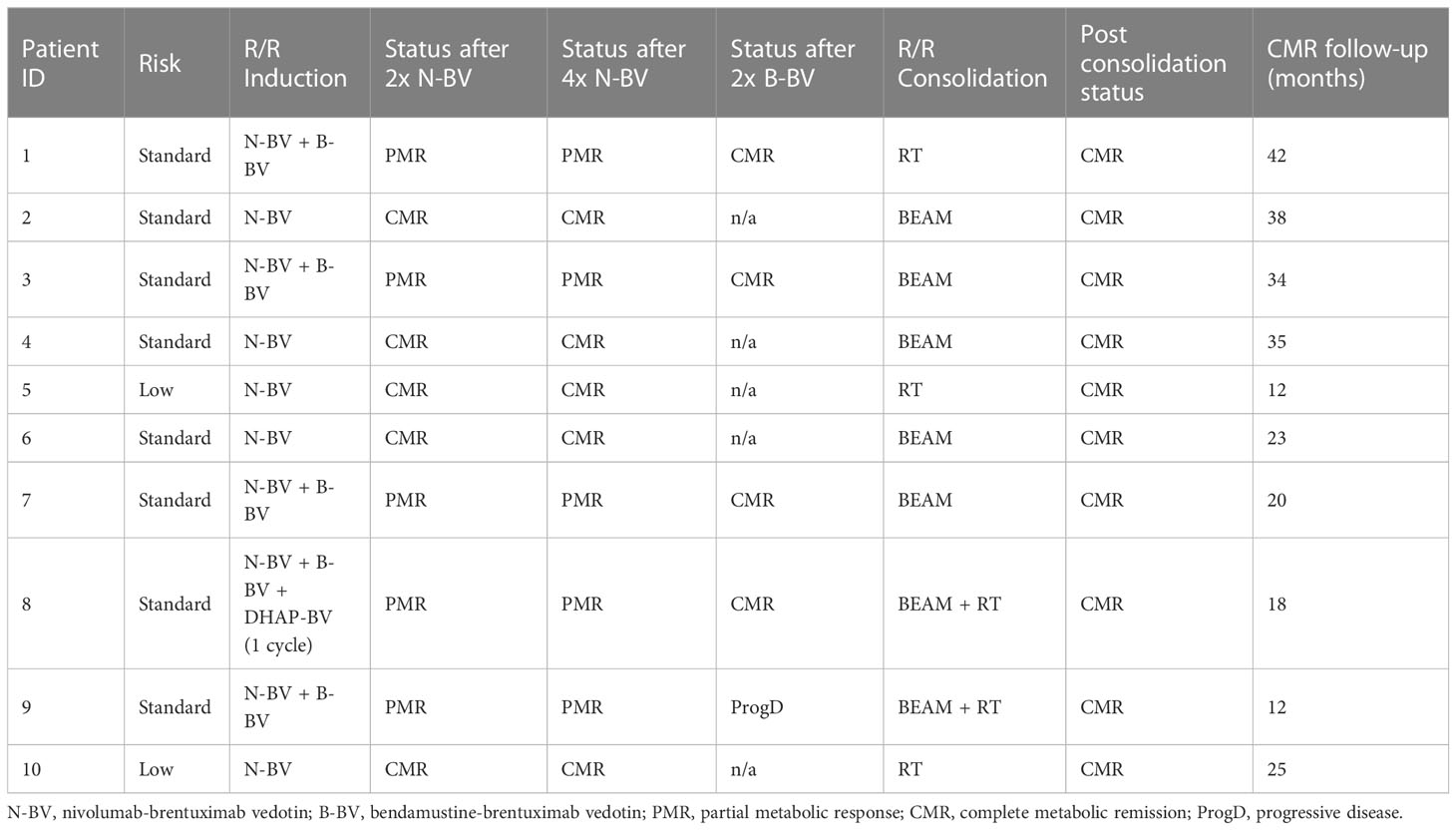
Table 2 Clinical characteristics of R/R HL patients and response to nivolumab + BV +/- bendamustine.
Treatment of R/R disease was on average initiated 16 days (range: 8-26) after diagnosis. Treatment was given according to the Checkmate 744 trial protocol: The first cycle of immunotherapy was started with the administration of BV followed by infusion of nivolumab 7 days later. Afterwards nivolumab + BV was administered on the same day every 3 weeks in our outpatient clinic (22). Immunotherapy induced a CMR (Deauville score <4) in 5 patients after 2 administrations of nivolumab + BV. Four patients did not achieve CMR after 4 nivolumab + BV cycles. Three patients achieved CMR after 2 and 1 patient after 3 cycles of BV in combination with bendamustine. In 1 patient with a FDG-PET-CT scan suspicious for only a partial metabolic response a CT-guided biopsy was performed that did not detect viable cHL tissue. A second patient showed progressive disease after 2 cycles of BV + bendamustine. Treatment was intensified by 1 course of DHAP + BV (26), which induced CMR (Figure 1), followed by consolidation with HD-BEAM with autologous stem cell reinfusion. Also this patients is still in ongoing remission for 16 months. TARC levels normalized concomitantly with CMR induction (Figure 2). Peripheral stem cell harvest after 2 to 4 cycles of nivolumab + BV was successful in all standard risk patients. On average 5.2 x106 CD34+ cells/kg body weight were harvested.
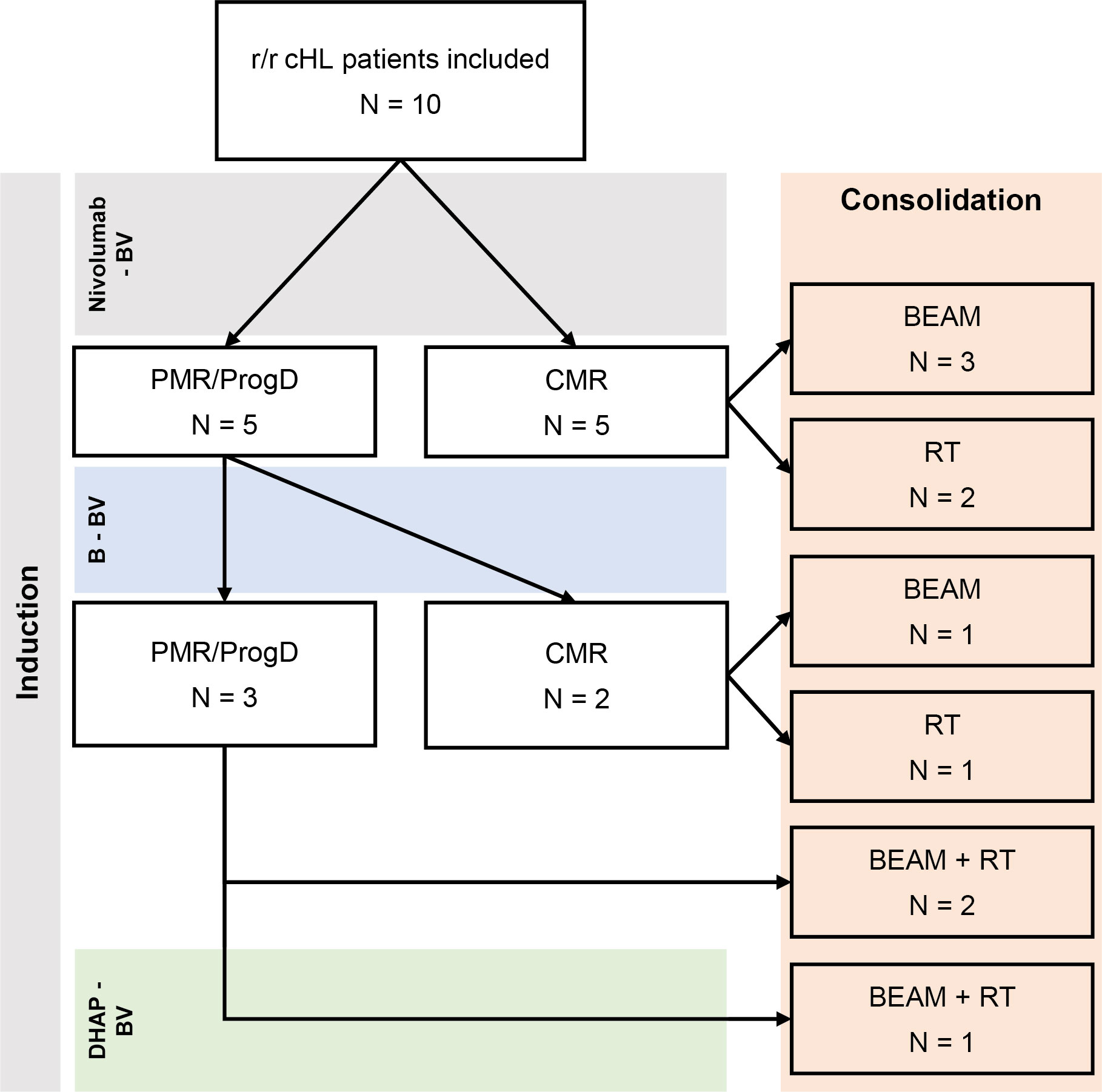
Figure 1 Treatment with nivolumab + BV +/- bendamustine. Flowchart of included patients and their treatment. Five patients had CMR after 4 cycles of N-BV treatment and were consolidated with HD-BEAM (n = 3) or RT (n = 2). Those with PMR continued with 2 cycles of bendamustine + BV, leading to CMR in 4 more patients, consolidated with HD-BEAM (n = 1), RT (n = 1) or HD-BEAM + RT (n = 2). The last patient was treated with a single cycle of DHAP + BV, leading to CMR, and had consolidation therapy with HD-BEAM + RT.
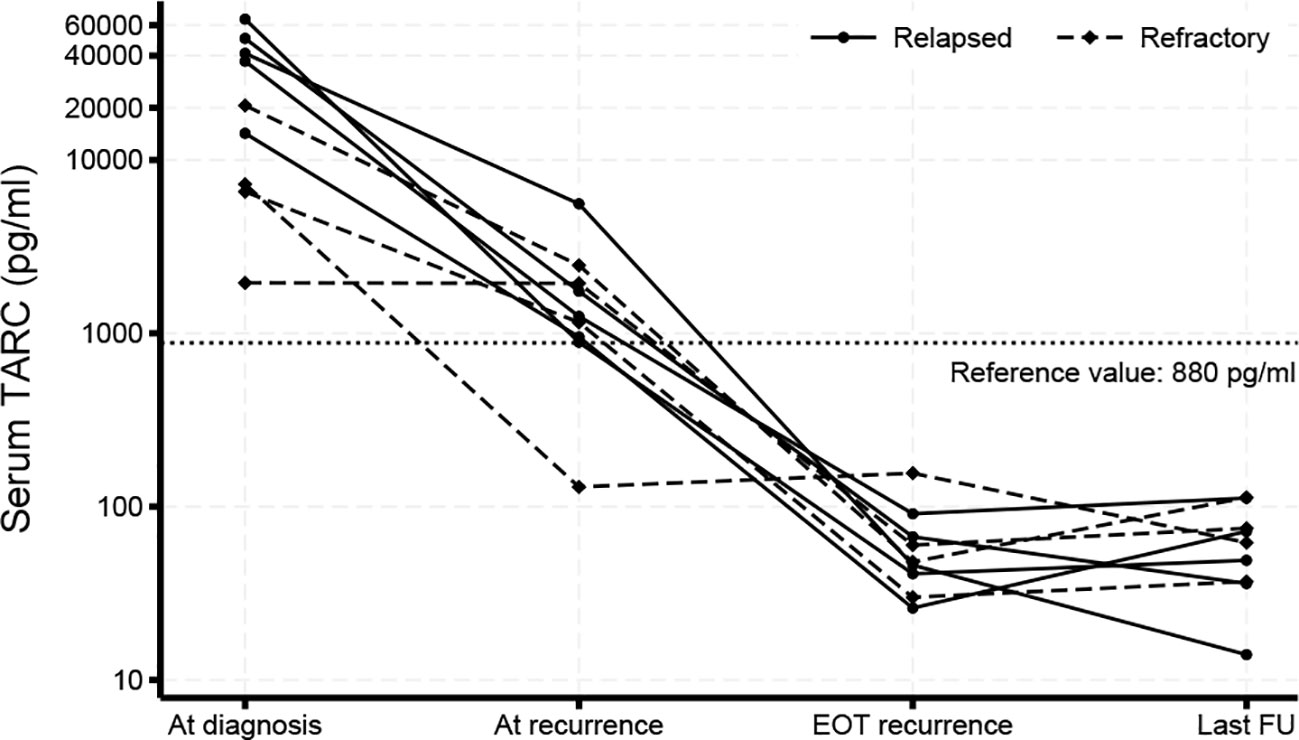
Figure 2 Serum TARC levels. Serum TARC levels were measured by ELISA prior to initiation of treatment of primary disease and at diagnosis of R/R cHL, at end-of-treatment (EOT) of R/R disease and during follow-up (FU). Values above 880 pg/ml were considered elevated based on previous findings (28). TARC levels decreased during treatment and were still normal at the last follow-up moment.
The treatment was well tolerated and administered in the outpatient clinic (Table 3). We observed a single CTC grade 3 or higher adverse event: an increase of ALT and AST more than five times the upper limit of normal. Both ALT and AST recovered spontaneously and there were no signs of impaired liver function and no suspicion of drug-induced liver injury. Bilirubin levels stayed within the reference range. However, the next nivolumab cycle was delayed, while the possible causes were evaluated. Mild side effects that were reported more than once included fever (n = 3), rash (n = 2), and fatigue (n = 2). One patient developed hypothyroidism attributed to nivolumab treatment, which recovered during follow-up.
Depending on the initial risk group stratification 7/10 patients received standard consolidation with HD-BEAM chemotherapy plus autologous stem cell reinfusion and 3/10 involved-field RT (30,6 to 36 Gy). One patient with 2nd recurrence had received prior HD-BEAM chemotherapy with autologous stem cell reinfusion as treatment for first recurrence and therefore received radiotherapy as consolation after reaching CMR. In 2/10 patients radiotherapy was administered after completing HD-BEAM chemotherapy with autologous stem cell reinfusion. The indications for additional consolidation radiotherapy in these 2 patients were detection of viable lymphoma after the 4th course of nivolumab + BV with CMR after bendamustine + BV; and progression after treatment with bendamustine + BV with CMR after one cycle of DHAP + BV. Most importantly, these 2 patients achieved CMR before starting consolidation. The toxicity observed after HD-BEAM was mild. No CTCAE adverse events above grade 3 were reported. Grade 3 CTCAE adverse events included febrile neutropenia (n = 5), weight loss (n = 1), nausea (n = 1), vomiting (n = 1), oral mucositis (n = 1) and isolated ALT increase (n = 1). On average 3.8 x106 CD34+ stem cells/kg body weight were infused. Leukocyte numbers had recovered to >5 x10e6/L after a mean of 11 days (range 11-15) after stem cell reinfusion with neutrophils reaching counts >4 x10e6/L after a mean of 13 days (range 10-16). At present all patients are in ongoing remission with a median follow-up time of 25 months (range: 12-42) since the end-of-treatment.
We report that combination immunotherapy with nivolumab + BV (+ bendamustine in 5/10 cases) induces complete remission in 10 pediatric R/R cHL patients and does not cause relevant toxicity during treatment and in the median follow-up period of 25 months (range: 12 to 42). CMR was achieved after only 2 cycles of immunotherapy in 50% of patients. 9/10 patients had achieved CMR after nivolumab +BV +/- bendamustine prior to consolidation treatment. One patient reached CMR after adding DHAP + BV to this treatment. While progressive disease was diagnosed after nivolumab +BV + bendamustine, DHAP + BV induced CMR. This may be due to cHL sensitization to DHAP by prior nivolumab administration (30). All patients are in ongoing remission with a median follow-up period of 25 months. Our findings add real-world evidence to the promising results of the CheckMate 744 study that were recently published (22, 24). Interestingly, nivolumab + BV has also shown promising results in the treatment of R/R primary mediastinal B cell lymphoma (31), a Non-Hodgkin lymphoma entity biologically closely related to cHL (32).
Even though we did not observe relevant toxicity > CTC grade 3, it is important to study possible long-term side effects, most importantly of PD-1 checkpoint inhibition by nivolumab. Children have a more active immune system in development, as characterized by increased T cell responses to infectious agents, when compared to adults, in whom immune senescence increases the presence of dysfunctional and exhausted T cells (33, 34), and could thus be more prone to long-term side effects of checkpoint inhibition. In adults, short- and long-term side effects of checkpoint inhibition are well-characterized and include most prominently different forms of autoimmunity (35). Only 1 possible adverse event related to immunotherapy, hypothyroidism induced by nivolumab, occurred during treatment but resolved spontaneously during follow-up. In the up to 42 months of follow-up we have observed no severe side effects attributable to nivolumab + BV + bendamustine treatment, but longer follow-up is needed to thoroughly compare morbidity and mortality of immunotherapy with current standard of care.
All patients still underwent standard consolidation treatment after reaching CMR. The question remains whether this is necessary. Recently, a case series of 4 children who did not receive HD chemotherapy consolidation after remission induction with BV plus gemcitabine reported ongoing complete response in all of them (36). If patients achieve durable CMR after nivolumab + BV +/- bendamustine without the need for intensive consolidation radio- or chemotherapy, their long-term quality of life may benefit significantly (37, 38).
In our cohort all patients tolerated HD-BEAM chemotherapy followed by autologous stem cell reinfusion very well. Immune reconstitution was fast and patients did not experience symptoms caused by > grade 3 CTC acute toxicity. This could be due to reduced acute toxicity of induction treatment with nivolumab + BV +/- bendamustine when compared to standard chemotherapy (4). This observation could be another benefit of remission induction with nivolumab + BV +/- bendamustine.
In conclusion, nivolumab + BV +/- bendamustine was effective and safe. All patients achieved complete remission before consolidation and are in ongoing complete remission with a median follow-up of 25 months after end-of-treatment. No relevant adverse events of CTCAE grade 3 or higher were reported and no side effects attributable to nivolumab or BV were identified. Our results support that immunotherapy with nivolumab + BV +/- bendamustine is an effective and safe treatment to induce remission in pediatric R/R cHL patients prior to standard consolidation treatment. We propose to evaluate this treatment further to study putative long-term toxicity and the possibility to reduce the intensity of consolidation treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
PG and AB collected the data and wrote the manuscript, MH, JL, MV, MB, and VP contributed to writing the manuscript, CD analyzed the data and wrote the manuscript and FM-W drafted the manuscript, analyzed data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was partially supported by a research grant from Lymph&Co (2018-LyCo-002), a Dutch non-profit research foundation that supports lymphoma research, and by the Ferenc foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Connors JM, Cozen W, Steidl C, Carbone A, Hoppe RT, Flechtner HH, et al. Hodgkin lymphoma. Nat Rev Dis Primers (2020) 6(1):61. doi: 10.1038/s41572-020-0189-6
2. Mauz-Korholz C, Metzger ML, Kelly KM, Schwartz CL, Castellanos ME, Dieckmann K, et al. Pediatric Hodgkin lymphoma. J Clin Oncol (2015) 33(27):2975–85. doi: 10.1200/JCO.2014.59.4853
3. Belsky JA, Hochberg J, Giulino-Roth L. Diagnosis and management of Hodgkin lymphoma in children, adolescents, and young adults. Best Pract Res Clin Haematol (2023) 36(1):101445. doi: 10.1016/j.beha.2023.101445
4. Daw S, Hasenclever D, Mascarin M, Fernandez-Teijeiro A, Balwierz W, Beishuizen A, et al. Risk and response adapted treatment guidelines for managing first relapsed and refractory classical Hodgkin lymphoma in children and young people. Recommendations from the EuroNet pediatric Hodgkin lymphoma group. Hemasphere (2020) 4(1):e329. doi: 10.1097/HS9.0000000000000329
5. Harker-Murray PD, Drachtman RA, Hodgson DC, Chauvenet AR, Kelly KM, Cole PD. Stratification of treatment intensity in relapsed pediatric Hodgkin lymphoma. Pediatr Blood Cancer (2014) 61(4):579–86. doi: 10.1002/pbc.24851
6. Schellong G, Dorffel W, Claviez A, Korholz D, Mann G, Scheel-Walter HG, et al. Salvage therapy of progressive and recurrent Hodgkin’s disease: results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol (2005) 23(25):6181–9. doi: 10.1200/JCO.2005.07.930
7. Metzger ML, Hudson MM, Krasin MJ, Wu J, Kaste SC, Kun LE, et al. Initial response to salvage therapy determines prognosis in relapsed pediatric Hodgkin lymphoma patients. Cancer (2010) 116(18):4376–84. doi: 10.1002/cncr.25225
8. Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood (2010) 116(23):4934–7. doi: 10.1182/blood-2010-05-282756
9. de Vries S, Schaapveld M, Janus CPM, Daniels LA, Petersen EJ, van der Maazen RWM, et al. Long-term cause-specific mortality in Hodgkin lymphoma patients. J Natl Cancer Inst (2021) 113(6):760–9. doi: 10.1093/jnci/djaa194
10. Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van’t Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol (2003) 21(18):3431–9. doi: 10.1200/JCO.2003.07.131
11. Nunez-Garcia B, Clemente MB, Sanchez JC, Royuela A, Ibarguen BCS, Mendez M, et al. Long-term outcomes in Hodgkin lymphoma survivors. Temporary trends and comparison with general population. Hematol Oncol (2023). doi: 10.1002/hon.3131
12. Drechsel KCE, Pilon MCF, Stoutjesdijk F, Meivis S, Schoonmade LJ, Wallace WHB, et al. Reproductive ability in survivors of childhood, adolescent, and young adult Hodgkin lymphoma: a review. Hum Reprod Update (2023) 29(4):486–517. doi: 10.1093/humupd/dmad002
13. Maule M, Scelo G, Pastore G, Brennan P, Hemminki K, Tracey E, et al. Risk of second Malignant neoplasms after childhood leukemia and lymphoma: an international study. J Natl Cancer Inst (2007) 99(10):790–800. doi: 10.1093/jnci/djk180
14. van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med (2015) 175(6):1007–17. doi: 10.1001/jamainternmed.2015.1180
15. Ansell SM. Hodgkin lymphoma: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol (2022) 97(11):1478–88. doi: 10.1002/ajh.26717
16. Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood (2021) 138(6):427–38. doi: 10.1182/blood.2020009178
17. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med (2015) 372(4):311–9. doi: 10.1056/NEJMoa1411087
18. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II checkMate 205 trial. J Clin Oncol (2018) 36(14):1428–39. doi: 10.1200/JCO.2017.76.0793
19. Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood (2019) 134(14):1144–53. doi: 10.1182/blood.2019000324
20. Locatelli F, Mauz-Koerholz C, Neville K, Llort A, Beishuizen A, Daw S, et al. Brentuximab vedotin for paediatric relapsed or refractory Hodgkin’s lymphoma and anaplastic large-cell lymphoma: a multicentre, open-label, phase 1/2 study. Lancet Haematol (2018) 5(10):e450–e61. doi: 10.1016/S2352-3026(18)30153-4
21. Cole PD, McCarten KM, Pei Q, Spira M, Metzger ML, Drachtman RA, et al. Brentuximab vedotin with gemcitabine for paediatric and young adult patients with relapsed or refractory Hodgkin’s lymphoma (AHOD1221): a Children’s Oncology Group, multicentre single-arm, phase 1-2 trial. Lancet Oncol (2018) 19(9):1229–38. doi: 10.1016/S1470-2045(18)30426-1
22. Harker-Murray P, Mauz-Korholz C, Leblanc T, Mascarin M, Michel G, Cooper S, et al. Nivolumab and brentuximab vedotin with or without bendamustine for R/R Hodgkin lymphoma in children, adolescents, and young adults. Blood (2023) 141(17):2075–84. doi: 10.1182/blood.2022017118
23. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
24. Harker-Murray PD, Cole PD, Hoppe BS, Hodgson DC, Beishuizen A, Garnier N, et al. Response-adapted therapy (tx) with nivolumab plus brentuximab vedotin (nivo + BV) without autologous hematopoietic cell transplantation (auto-HCT) in children, adolescents, and young adults (CAYA) with low-risk relapsed/refractory (R/R) classic Hodgkin lymphoma (cHL): CheckMate 744. J Clin Oncol (2023) 41(16 supplement). doi: 10.1200/JCO.2023.41.16_suppl.7515
25. Mauz-Korholz C, Landman-Parker J, Fernandez-Teijeiro A, Attarbaschi A, Balwierz W, Bartelt JM, et al. Response-adapted omission of radiotherapy in children and adolescents with early-stage classical Hodgkin lymphoma and an adequate response to vincristine, etoposide, prednisone, and doxorubicin (EuroNet-PHL-C1): a titration study. Lancet Oncol (2023) 24(3):252–61. doi: 10.1016/S1470-2045(23)00019-0
26. Hagenbeek A, Mooij H, Zijlstra J, Lugtenburg P, van Imhoff G, Nijland M, et al. Phase I dose-escalation study of brentuximab-vedotin combined with dexamethasone, high-dose cytarabine and cisplatin, as salvage treatment in relapsed/refractory classical Hodgkin lymphoma: The HOVON/LLPC Transplant BRaVE study. Haematologica (2019) 104(4):e151–e3. doi: 10.3324/haematol.2018.196899
27. Zijtregtop EAM, Diez C, Zwaan CM, Veening MA, Beishuizen A, Meyer-Wentrup FAG. Thymus and activation-regulated chemokine (TARC) as treatment response marker for paediatric Hodgkin lymphoma: A pilot study. Br J Haematol (2023) 200(1):70–8. doi: 10.1111/bjh.18473
28. Zijtregtop EAM M-WF, Wong WC, Hoogendijk R, Lopez-Yurda M, Zwaan MC, Beishuizen A. Plasma thymus and activation-regulated chemokine (TARC) as diagnostic marker in pediatric Hodgkin lymphoma. eJHaem (2020) 1(1):152–60. doi: 10.1002/jha2.41
29. Zijtregtop EAM, van der Strate I, Beishuizen A, Zwaan CM, Scheijde-Vermeulen MA, Brandsma AM, et al. Biology and clinical applicability of plasma thymus and activation-regulated chemokine (TARC) in classical Hodgkin lymphoma. Cancers (Basel) (2021) 13(4):884. doi: 10.3390/cancers13040884
30. Carreau NA, Pail O, Armand P, Merryman R, Advani RH, Spinner MA, et al. Checkpoint blockade treatment may sensitize Hodgkin lymphoma to subsequent therapy. Oncologist (2020) 25(10):878–85. doi: 10.1634/theoncologist.2020-0167
31. Zinzani PL, Santoro A, Gritti G, Brice P, Barr PM, Kuruvilla J, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: efficacy and safety from the phase II checkMate 436 study. J Clin Oncol (2019) 37(33):3081–9. doi: 10.1200/JCO.19.01492
32. Chapuy B, Stewart C, Dunford AJ, Kim J, Wienand K, Kamburov A, et al. Genomic analyses of PMBL reveal new drivers and mechanisms of sensitivity to PD-1 blockade. Blood (2019) 134(26):2369–82. doi: 10.1182/blood.2019002067
33. Mogilenko DA, Shchukina I, Artyomov MN. Immune ageing at single-cell resolution. Nat Rev Immunol (2022) 22(8):484–98. doi: 10.1038/s41577-021-00646-4
34. Mogilenko DA, Shpynov O, Andhey PS, Arthur L, Swain A, Esaulova E, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK(+) CD8(+) T cells as conserved hallmark of inflammaging. Immunity (2021) 54(1):99–115 e12. doi: 10.1016/j.immuni.2020.11.005
35. Wang SJ, Dougan SK, Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer (2023) 9(7):543–53. doi: 10.1016/j.trecan.2023.04.002
36. Buhtoiarov IN, Mba NI, Santos CDL, McCarten KM, Metzger ML, Pei Q, et al. Durable remission for four pediatric patients with high-risk relapsed classical Hodgkin lymphoma treated with brentuximab vedotin plus gemcitabine but without autologous stem cell transplantation: A report from the Children’s Oncology Group. Pediatr Blood Cancer (2022) 69(6):e29649. doi: 10.1002/pbc.29649
37. Bhatia S, Sklar C. Second cancers in survivors of childhood cancer. Nat Rev Cancer (2002) 2(2):124–32. doi: 10.1038/nrc722
38. Bhuller KS, Zhang Y, Li D, Sehn LH, Goddard K, McBride ML, et al. Late mortality, secondary Malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br J Haematol (2016) 172(5):757–68. doi: 10.1111/bjh.13903
Keywords: oncology, recurrent/refractory classical Hodgkin lymphoma, checkpoint inhibition, combination immunotherapy, targeted therapy
Citation: Greve P, Beishuizen A, Hagleitner M, Loeffen J, Veening M, Boes M, Peperzak V, Diez C and Meyer-Wentrup F (2023) Nivolumab plus Brentuximab vedotin +/- bendamustine combination therapy: a safe and effective treatment in pediatric recurrent and refractory classical Hodgkin lymphoma. Front. Immunol. 14:1229558. doi: 10.3389/fimmu.2023.1229558
Received: 26 May 2023; Accepted: 17 July 2023;
Published: 31 July 2023.
Edited by:
Paul Harker-Murray, Medical College of Wisconsin, United StatesReviewed by:
Jennifer Belsky, Riley Hospital for Children, United StatesCopyright © 2023 Greve, Beishuizen, Hagleitner, Loeffen, Veening, Boes, Peperzak, Diez and Meyer-Wentrup. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Friederike Meyer-Wentrup, Zi5tZXllci13ZW50cnVwQHByaW5zZXNtYXhpbWFjZW50cnVtLm5s
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.