
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 05 September 2023
Sec. Alloimmunity and Transplantation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1229266
 Qi Ji1†
Qi Ji1† Yongping Zhang1†
Yongping Zhang1† Yixin Hu1†
Yixin Hu1† Lixia Liu2†
Lixia Liu2† Shanbo Cao2
Shanbo Cao2 Li Gao1
Li Gao1 Bohan Li1
Bohan Li1 Yuanyuan Tian1
Yuanyuan Tian1 Lingjun Kong1
Lingjun Kong1 Shuiyan Wu3
Shuiyan Wu3 Jing Ling1
Jing Ling1 Peifang Xiao1
Peifang Xiao1 Jun Lu1
Jun Lu1 Jie Li1
Jie Li1 Yanhua Yao1
Yanhua Yao1 Jiayue Qin2*
Jiayue Qin2* Shaoyan Hu1*
Shaoyan Hu1*Background: Methotrexate (MTX), utilized as a graft-versus-host disease (GvHD) prophylactic agent in allogeneic hematopoietic stem cell transplantation (allo-HSCT), has been proven to effectively decrease the occurrence of the peri-engraftment syndrome (Peri-ES) and acute GvHD (aGvHD). Changes in the pharmacodynamics of MTX are closely associated with gene polymorphisms in genes encoding drug-metabolizing enzymes and transporters. Nevertheless, the current studies mainly concentrate on leukemia or autoimmune diseases, and limited studies on allo-HSCT were reported.
Methods: Here, we retrospectively assessed the relationship between MTX-related transporter and metabolizing enzyme gene polymorphisms, clinical characteristics, and outcomes in 57 pediatric patients who received haploid HSCT (haplo-HSCT) with malignant tumors at a single center.
Results: We discovered all gene polymorphisms were in the Hardy–Weinberg equilibrium in our cohort. We discovered a significant correlation between platelet recovery time and ABCB1 (1236C>T) (p = 0.042). Compared with patients with SLCO1B1 (1865+4846T>C) TT, patients with SLCO1B1 (1865+4846T>C) TC/CC had an increased incidence of Peri-ES (p = 0.030). Based on the multivariate Cox analysis, we discovered that SLCO1B1 (1865+4846T>C) TT genotype was an independent protective factor for Peri-ES morbidity (hazard ratio (HR) = 0.464, p = 0.031), and the dose of mononuclear cells reinfused was significantly correlated with II–IV aGvHD (HR = 2.604, p = 0.039).
Conclusion: In summary, our findings prove that the host’s genotypes might modify the risk of developing Peri-ES, contribute to a better understanding of the inter-individual difference in efficacy, and facilitate the development of individualized approaches to GvHD prophylaxis.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective method to treat hematological malignancies. However, patients still face various complications after transplantation, including peri-engraftment syndrome (Peri-ES), graft-versus-host disease (GvHD), and infection, which adversely affect the survival and prognosis of patients (1–3). Methotrexate (MTX) is one of the main drugs used to prevent GvHD after HSCT, which effectively reduces the incidence of Peri-ES and acute GvHD (aGvHD) (4). However, MTX may also cause various adverse reactions, including severe mucositis, bone marrow suppression, hepatotoxicity, etc. (4, 5). Changes in the pharmacodynamics of MTX are closely associated with gene polymorphisms in genes encoding drug-metabolizing enzymes and transporters, as demonstrated by the therapy of acute lymphocytic leukemia with high-dose MTX (HD-MTX), which were rarely reported in populations undergoing allo-HSCT. Limited reports had focused on the association between gene polymorphisms and Peri-ES.
This study aims to verify the effects of MTX-related transporter and metabolizing enzyme gene polymorphisms on Peri-ES and aGvHD after haploid HSCT (haplo-HSCT) in children.
This retrospective longitudinal cohort study was conducted in a single center at the Children’s Hospital of Soochow University (Suzhou, China). Patients who received allo-HSCT were enrolled between January 1, 2019, and June 30, 2021. The study inclusion criteria consisted of 1) first-time transplant, 2) haplo-HSCT, 3) patients aged less than 18 years, 4) three or four intravenous doses of MTX (three doses: day 1 at 15 mg/m2 and days 3 and 6 at 10 mg/m2; four doses: day 1 at 15 mg/m2 and days 3, 6, and 11 at 10 mg/m2) and cyclosporine A or tacrolimus (po q12h starting on the day 5 before transplantation) for GvHD prophylaxis, and 5) presence of informed consent to methotrexate genotyping. Patients who had undergone a second or subsequent transplantation or received GvHD prophylaxis without MTX were excluded from the study. All available follow-up data until December 31, 2022, were analyzed.
Neutrophil engraftment was defined as a neutrophil count >0.5 × 109/L for three consecutive days. Platelet engraftment was defined as a platelet count exceeding 20 × 109/L for seven consecutive days without transfusion. Primary graft failure (GF) was defined as the failure of neutrophil engraftment at day +28 after HSCT, while secondary GF was defined as the development of neutrophil count <0.5 × 109/L occurring after the initial engraftment. Peri-ES was defined according to the previously described criteria (6). Moreover, diagnosis and grading of aGvHD were performed based on the standard criteria (7). Patients who survived >30 days post-HSCT with successful engraftment were evaluated for aGvHD analysis.
Genomic DNA was isolated from bone marrow at diagnosis. A targeted 27-gene sequencing panel was used for next-generation sequencing (NGS) at Acornmed Biotechnology Co., Ltd (Tianjin, China). Multiplex libraries were sequenced with the NovaSeq instrument (Illumina). The following criteria were performed to filter raw variants: average sequencing depth on target per sample ≥1,000×, mapping quality ≥30, and base quality ≥30. Alignment of the trimmed reads was performed with the Burrows–Wheeler alignment (BWA; version 0.7.12). PCR duplicates were marked with the MarkDuplicates tool from Picard. BaseRecalibrator from Genome Analysis Toolkit (GATK; version 3.8) was used to realign and recalibrate the BWA data. Variant calling was used in Mutect2. ANNOVAR software was performed to annotate all the variants, including dbSNP. The MTX-related transporter and metabolizing enzyme gene polymorphisms include SLCO1B1 (1865+4846T>C), SLCO1B1 (521T>C), MTRR (66A>G), MTHFR (665C>T), ABCB1 (1236C>T), ABCB1 (3435C>T), ABCB1 (1000-44C>T), ABCB1 (1554 + 24A>G), and ABCB1 (1725 + 38C>T).
This primary purpose of interest was the development of Peri-ES and aGvHD after haplo-HSCT with different MTX-related transporter and metabolizing enzyme gene polymorphism status. The t-test and Mann–Whitney U test were routinely used for the comparison of the quantitative variables between two groups with parametric and non-parametric distribution, respectively. Categorized variables were compared using chi-square or Fisher’s exact test. Event time distributions for the clinical outcomes were estimated using a cumulative incidence curve with a log-rank test. Univariate and multivariate analyses were used to compare the relative risk by Cox regression. Variables were selected by univariate Cox regression analysis, and those with p < 0.2 were subsequently enrolled in multivariate Cox regression analysis based on the likelihood ratio test. All data were analyzed using SPSS software (version 22.0) or R (version 3.5.2). p < 0.05 was considered statistically significant.
The baseline characteristics of 57 Chinese pediatric patients are described in Table 1. All 57 patients were treated with the myeloablative conditioning regimen based on busulfan (Bu) and cyclophosphamide (Cy) and received at least three doses of MTX as GvHD prophylaxis. The mean age at HSCT was 5.1 years, and more men (80.8%) were enrolled in our cohort. Acute leukemias, including mixed-phenotype acute leukemia (MAPL), was the most common etiology, which constituted 94.7% (54/57) of the cohort. All patients achieved hematopoietic recovery from haploidentical donors. The median time to neutrophil and platelet engraftment was 12 days (range, 10–23) and 12 days (range, 5–39), respectively.
MTX-related transporter and metabolizing enzyme gene polymorphism frequencies for the patients are shown in Figure 1 and Table 2. A total of nine kinds of gene polymorphisms were detected, and the most common variant type was ABCB1 (1236C>T), followed by MTHFR (665C>T) and ABCB1 (3435C>T) (Figure 1). All MTX-related transporter and metabolizing enzyme gene polymorphisms were discovered to be in the Hardy–Weinberg equilibrium in our cohort (all p > 0.05, Table 2). In addition, ABCB1 (1236C>T) was found to be related to platelet recovery day (p = 0.042, Supplementary Table 1).
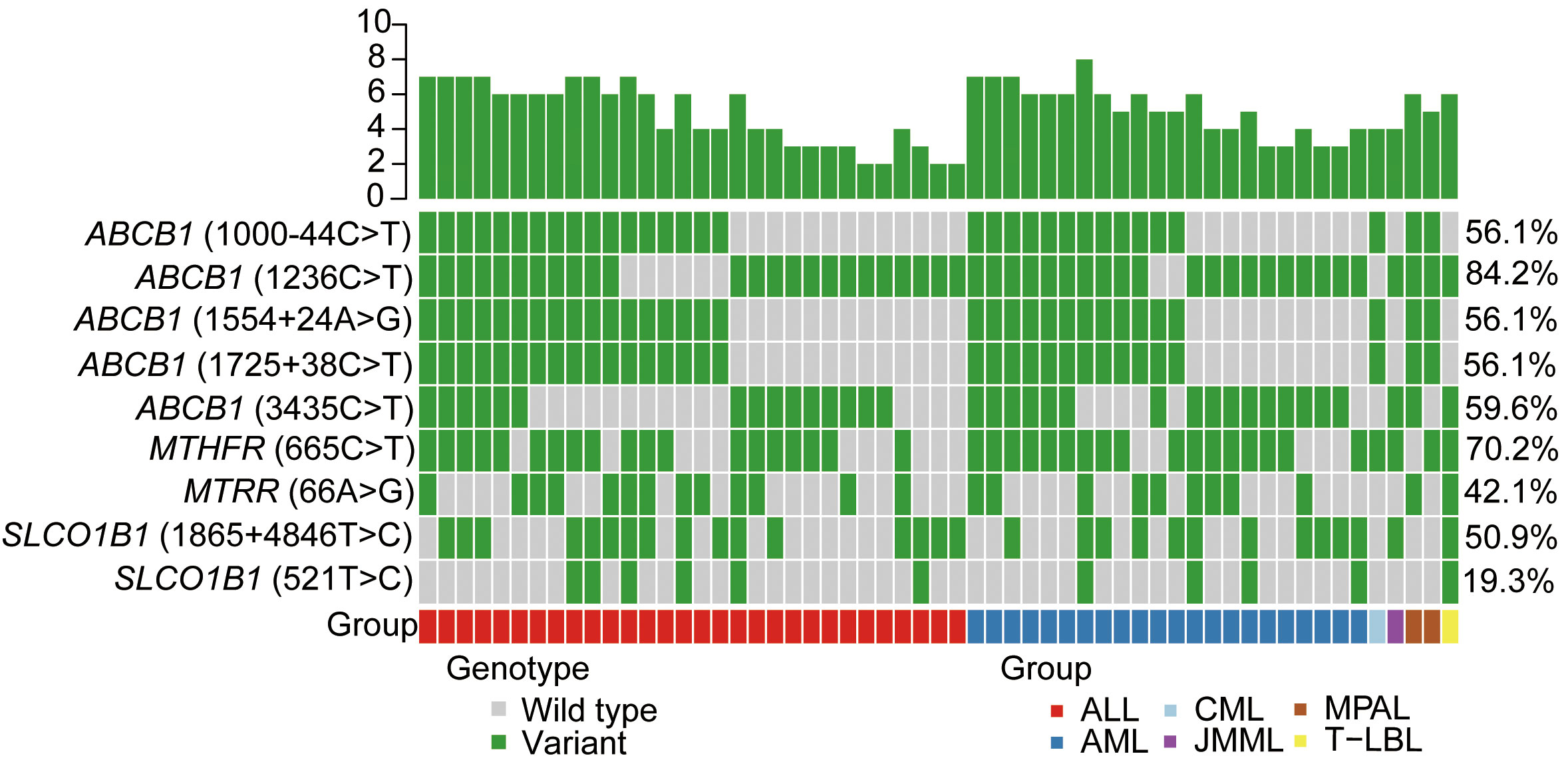
Figure 1 MTX-related transporter and metabolizing enzyme gene polymorphisms detected in our cohort. Heatmap shows the specific variants in each patient based on different genotypes, according to different subtypes of malignant hematological diseases. MTX, methotrexate; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; JMML, juvenile myelomonocytic leukemia; MPAL, mixed-phenotype acute leukemia; T-LBL, T-cell lymphoblastic lymphoma.
In this study, 61.4% of patients (35/57) developed Peri-ES; 94% of patients (33/35) with Peri-ES received methylprednisolone therapy and improved. There was an increased morbidity rate of Peri-ES in the patients with SLCO1B1 (1865+4846T>C) TC/CC (p = 0.030, Figure 2A). Based on multivariate analysis, SLCO1B1 (1865+4846T>C) TT genotype (hazard ratio (HR) = 0.464, p = 0.031) was an independent protective factor for Peri-ES morbidity. However, no association was found between other gene polymorphisms with Peri-ES, such as ABCB1, MTHFR, and MTRR polymorphisms (Table 3).
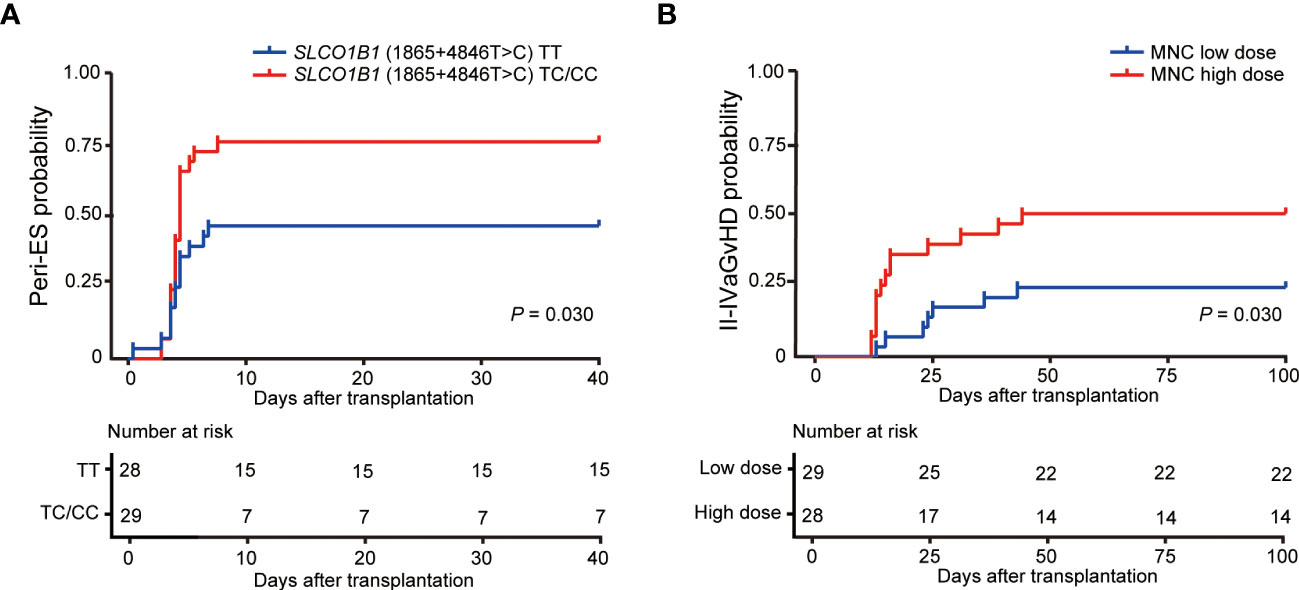
Figure 2 Effects of SLCO1B1 (1865+4846T>C) gene polymorphisms and MNC doses on the morbidity of Peri-ES (A) and II–IV aGvHD (B), respectively. Peri-ES, Peri-engraftment syndrome; aGvHD, acute graft-versus-host disease; MNC, mononuclear cells.
In the study, 36.8% of patients (21/57) developed grade II–IV aGvHD, including eight (14.0%) with grade II, eight (19.3%) with grade III, and five (8.8%) with grade IV. With respect to organ severity stage, 20 patients developed stage 2–4 cutaneous aGvHD, and eight patients developed stage 2–4 GI aGvHD. Only one child developed stage 4 liver aGvHD. Compared with mononuclear cell (MNC) low dose according to the median dose value in our cohort, patients with MNC high dose had an increased incidence of II–IV aGvHD (p = 0.030, Figure 2B). Also, we conducted univariate and multivariate Cox regression analyses for II–IV aGvHD and III–IV aGvHD. Our findings suggested that SLCO1B1 (521T>C) genotype tended to be associated with II–IV aGvHD in univariate analysis (p = 0.175), and only the dose of MNC reinfused was significantly related to II–IV aGvHD as an independent risk factor in multivariate analysis (HR = 2.604, p = 0.039, Table 4). Unfortunately, no significant association between III–IV aGvHD after haplo-HSCT and MTX-related gene polymorphisms was discovered (Supplementary Table 2).
In this study, we analyzed the correlation between MTX-related transporter and metabolizing enzyme gene polymorphisms, Peri-ES, and aGvHD after haplo-HSCT in 57 pediatric patients with malignant hematological diseases. Basic characteristics and transplant outcomes between different MTX-related transporter and metabolizing enzyme gene polymorphisms were compared, in which we primarily found that patients with ABCB1 (1236C>T) were associated with platelet recovery day. When considering the time of disease occurrence, we found that patients with SLCO1B1 (1865+4846T>C) TC/CC genotype had a higher incidence of Peri-ES. Furthermore, our Cox regression analysis revealed that SLCO1B1 (1865+4846T>C) TT genotype was an independent protective factor against the overall incidence of Peri-ES.
Peri-ES is defined as a clinical syndrome encompassing both engraftment syndrome (ES) and pre-engraftment syndrome (pre-ES), which is closely correlated with the prognosis (6). In our cohort, 61.4% of patients (35/57) developed Peri-ES, which was higher than the reported rate (6). Our data showed that patients receiving haplo-HSCT combined with umbilical cord blood (haplo-cord HSCT) had a trend of increasing incidence of Peri-ES (21/31 vs. 14/26, p = 0.283). It is well known that the incidence of Peri-ES is closely associated with umbilical cord blood transplantation (UCBT). Previous studies showed that granulocyte-macrophage colony-stimulating factor (GM-CSF) produced by cord blood-derived inflammatory monocytes played a crucial role in driving the pathology of pre-ES in UCBT (8). In our study, approximately half of the patients underwent haplo-HSCT combined with UCBT. Thus, the increased vulnerability to Peri-ES in our cohort may be linked to the presence of highly potent monocytes that produce GM-CSF within the UCB grafts. It was noteworthy that an overwhelmingly high proportion of patients underwent methylprednisolone therapy and improved.
MTX is a cornerstone drug in preventing GvHD and has been shown to significantly reduce the incidence of Peri-ES after UCBT (9). The disposition of MTX is a complex process that involves various transporters and enzymes. Renal excretion is the primary route of elimination for MTX. Due to the significant contribution of enterohepatic circulation to its disposition, both transporters in the kidneys and liver are important determinants of clearance (10). SLCO1B1, also known as OATP-2 or LST1, is a bidirectional transporter with 12 transmembrane domains encoded by SLCO1B1 located on chromosome 12p12, which is mainly expressed in the hepatocyte basolateral membrane and plays a crucial role in the uptake of endogenous and exogenous anionic compounds, including MTX (11).
The rs11045879 single-nucleotide polymorphism (SNP), also known as SLCO1B1 (1865+4846T>C), is an intronic variant of the SLCO1B1 gene located at chr12:21229685. The functional implications of SLCO1B1 (1865+4846T>C) have not been fully elucidated; however, its clinical relevance in MTX treatment is noteworthy. SLCO1B1 (1865+4846T>C) was identified in a genome-wide association study as one of the most important factors influencing MTX clearance in patients with acute lymphoblastic leukemia (ALL) (12). Several studies have shown that patients carrying SLCO1B1 (1865+4846T>C) C allele had a tendency to demonstrate increased MTX plasma concentrations and decreased MTX clearance rates, which is associated with an increased risk for MTX-related toxicity, including gastrointestinal toxicity and anal mucositis (12–17). Katja Goričar conducted a prognostic analysis on osteosarcoma patients undergoing HD-MTX therapy and discovered that SLCO1B1 (1865+4846T>C) C allele was associated with a higher event-free survival (16). The studies regarding the relationship between low-dose MTX and SLCO1B1 gene polymorphisms were rare. İrem Eldem found that the SLCO1B1 (1865+4846T>C) variant was associated with lower tolerances to MTX in children with ALL who had completed or were receiving maintenance therapy (18). In summary, the SLCO1B1 (1865+4846T>C) variant is closely associated with the pharmacokinetics and pharmacodynamics of MTX and deserves further investigation.
Our study suggested that SLCO1B1 gene polymorphism was related to Peri-ES, but the mechanism was unclear. The mechanism of MTX entering cells mainly involves the carrier molecules on the cell membrane, such as members of the classic anti-cancer drug transporter family in vitro and in vivo, which recognize and transport MTX-containing complexes on the cell membrane (19). Additionally, high concentrations of extracellular MTX can allow for passive diffusion into the cell (20, 21). As the dose of MTX used for GvHD is low, MTX is more likely to enter cells via transporters in children undergoing allo-HSCT. Previous studies had shown that short-term MTX was related to a lower rate of ES and III–IV aGvHD (4, 22). Narimatsu indicated that MTX may alter interactions among donor lymphocytes, facilitating engraftment and residual recipient immune cells capable of graft rejection, thereby modulating engraftment (22). However, as a folic acid antagonist, MTX requires cellular entry to exert its anti-proliferative function. Most SLCO1B1 gene polymorphisms can lead to decreased transport activity, including influx and efflux (12, 15, 23, 24), which decrease MTX entering cells. This may explain why patients with the SLCO1B1 variant genotype showed a higher incidence of Peri-ES than those with the wild-type genotype. Another explanation for our conclusion is that although several studies had shown that SLCO1B1 (1865+4846T>C) variant leads to increasing plasma concentration and thus results in MTX-related toxicity, these are all focused on the use of HD-MTX (13, 25, 26). Currently, there is no research on the influence of SLC family gene polymorphism on MTX after HSCT. The pharmacokinetics of the SLC family in the allo-HSCT population remains unclear. Therefore, we plan to utilize high-performance liquid chromatography (HPLC) to detect the plasma concentration of MTX after allo-HSCT to verify our results in the future.
Another interesting finding in our research was that ABCB1 (1236C>T) was associated with platelet recovery day. MTX is pumped out from the cells by ABC subfamily transporters, including ABCB1 (27). ABCB1 (1236C>T) was the most common and extensively studied ABCB1 SNP, which involves a C to T transition at position 1236 within exon 12 (28). Bo Jiang discovered that the T allele of ABCB1 (1236C>T) gene variant increases the efflux of MTX (29). This implies that in wild-type patients, there is a decrease in the efflux of MTX in hematopoietic cells, leading to the inhibition of cell proliferation by MTX. Consequently, this may explain why wild-type patients with the ABCB1 (1236C>T) variant are more prone to experiencing slowed recovery of hematopoietic cells.
Our study has several limitations, including the absence of information regarding the donor’s gene polymorphism status and the small sample size to analyze the association between transplantation outcome and MTX-related transporter and metabolizing enzyme gene polymorphisms. Consequently, we need future studies to validate our findings in a larger subsequent cohort, especially in a prospective study design.
In summary, our study suggests that SLCO1B1 genotype is correlated with Peri-ES in pediatric patients undergoing haplo-HSCT with MTX as GvHD prophylaxis. Specifically, the SLCO1B1 (1865+4846T>C) TT genotype was an independent protective factor against the development of Peri-ES, and the dose of reinfused MNC was significantly associated with II–IV aGvHD. Our findings contribute to a better understanding of the inter-individual difference in efficacy and facilitate the development of individualized approaches to GvHD prophylaxis, ultimately leading to improved HSCT outcomes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb) CNP0004415 (https://db.cngb.org).
The studies involving humans were approved by Children’s Hospital of Soochow University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
SH and JQ designed the study and approved the final manuscript. QJ, YZ, YH, LG, BL, YT, LK, SW, JLing, PX, JLu, JLi, and YY collected the clinical sample and data. LL and CS performed the NGS platform. QJ, YZ, YH, LL, and JQ did the statistical analysis. QJ, JQ, and SH wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key R&D Program of China (2022YFC2502700), National Natural Science Foundation of China (NSFC) (81970163, 82170218, 82100229, and 82200177), the Jiangsu Project (BE2021654 and BK20210097), the Suzhou project (SZS201615, GSWS2020039, SKY2022183, and SYS2020153), and the National Clinical Research Center for Hematological Disorders (2020ZKPB02).
We thank all the patients who participated in this study.
Authors LL, SC, and JQ are employed by the company Acornmed Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1229266/full#supplementary-material
1. Maqbool S, Nadeem M, Shahroz A, Naimat K, Khan I, Tahir H, et al. Engraftment syndrome following Hematopoietic stem cell transplantation: a systematic approach toward diagnosis and management. Med Oncol (2022) 40(1):36. doi: 10.1007/s12032-022-01894-7
2. Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol (2010) 11(4):331–8. doi: 10.1016/S1470-2045(09)70352-3
3. Matsui H, Arai Y, Imoto H, Mitsuyoshi T, Tamura N, Kondo T, et al. Risk factors and appropriate therapeutic strategies for thrombotic microangiopathy after allogeneic HSCT. Blood Adv (2020) 4(13):3169–79. doi: 10.1182/bloodadvances.2020002007
4. Iguchi A, Terashita Y, Sugiyama M, Ohshima J, Sato TZ, Cho Y, et al. Graft-versus-host disease (GVHD) prophylaxis by using methotrexate decreases pre-engraftment syndrome and severe acute GVHD, and accelerates engraftment after cord blood transplantation. Pediatr Transplant (2016) 20(1):114–9. doi: 10.1111/petr.12621
5. AlJohani NI. Role of folinic acid in methotrexate-based prophylaxis of graft-versus-host disease following hematopoietic stem cell transplantation. Hematology (2021) 26(1):620–7. doi: 10.1080/16078454.2021.1966222
6. Hong KT, Kang HJ, Kim NH, Kim MS, Lee JW, Kim H, et al. Peri-engraftment syndrome in allogeneic hematopoietic SCT. Bone Marrow Transplant (2013) 48(4):523–8. doi: 10.1038/bmt.2012.171
7. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transplant (2016) 22(1):4–10. doi: 10.1016/j.bbmt.2015.09.001
8. Jin L, Sun Z, Liu H, Zhu X, Zhou Y, Fu B, et al. Inflammatory monocytes promote pre-engraftment syndrome and tocilizumab can therapeutically limit pathology in patients. Nat Commun (2021) 12(1):4137. doi: 10.1038/s41467-021-24412-1
9. Park M, Lee SH, Lee YH, Yoo KH, Sung KW, Koo HH, et al. Pre-engraftment syndrome after unrelated cord blood transplantation: a predictor of engraftment and acute graft-versus-host disease. Biol Blood Marrow Transplant (2013) 19(4):640–6. doi: 10.1016/j.bbmt.2013.01.014
10. Schulte RR, Choi L, Utreja N, Van Driest SL, Stein CM, Ho RH. Effect of SLCO1B1 polymorphisms on high-dose methotrexate clearance in children and young adults with leukemia and lymphoblastic lymphoma. Clin Transl Sci (2021) 14(1):343–53. doi: 10.1111/cts.12879
11. Lima A, Sousa H, Monteiro J, Azevedo R, Medeiros R, Seabra V. Genetic polymorphisms in low-dose methotrexate transporters: current relevance as methotrexate therapeutic outcome biomarkers. Pharmacogenomics (2014) 15(12):1611–35. doi: 10.2217/pgs.14.116
12. Trevino LR, Shimasaki N, Yang W, Panetta JC, Cheng C, Pei D, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol (2009) 27(35):5972–8. doi: 10.1200/JCO.2008.20.4156
13. Yang FF, Xue TL, Gao C, Wu Y, Lin W, Li J, et al. Effects of SLCO1B1 on elimination and toxicities of high-dose methotrexate in pediatric acute lymphoblastic leukemia. Pharmacogenomics (2022) 23(15):821–34. doi: 10.2217/pgs-2022-0098
14. Li J, Wang XR, Zhai XW, Wang HS, Qian XW, Miao H, et al. Association of SLCO1B1 gene polymorphisms with toxicity response of high dose methotrexate chemotherapy in childhood acute lymphoblastic leukemia. Int J Clin Exp Med (2015) 8(4):6109–13.
15. Ramsey LB, Panetta JC, Smith C, Yang W, Fan Y, Winick NJ, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood (2013) 121(6):898–904. doi: 10.1182/blood-2012-08-452839
16. Goricar K, Kovac V, Jazbec J, Zakotnik B, Lamovec J, Dolzan V. Influence of the folate pathway and transporter polymorphisms on methotrexate treatment outcome in osteosarcoma. Pharmacogenet Genomics (2014) 24(10):514–21. doi: 10.1097/FPC.0000000000000083
17. Avivi I, Zuckerman T, Krivoy N, Efrati E. Genetic polymorphisms predicting methotrexate blood levels and toxicity in adult non-Hodgkin lymphoma. Leuk Lymphoma (2014) 55(3):565–70. doi: 10.3109/10428194.2013.789506
18. Eldem I, Yavuz D, Cumaogullari O, Ileri T, Unal Ince E, Ertem M, et al. SLCO1B1 polymorphisms are associated with drug intolerance in childhood leukemia maintenance therapy. J Pediatr Hematol Oncol (2018) 40(5):e289–e94. doi: 10.1097/MPH.0000000000001153
19. Türková A, Zdrazil B. Current advances in studying clinically relevant transporters of the solute carrier (SLC) family by connecting computational modeling and data science. Comput Struct Biotechnol J (2019) 17:390–405. doi: 10.1016/j.csbj.2019.03.002
20. Ackland SP, Schilsky RL. High-dose methotrexate: a critical reappraisal. J Clin Oncol (1987) 5(12):2017–31. doi: 10.1200/JCO.1987.5.12.2017
21. Jolivet J, Chabner BA. Intracellular pharmacokinetics of methotrexate polyglutamates in human breast cancer cells. Selective retention and less dissociable binding of 4-NH2-10-CH3-pteroylglutamate4 and 4-NH2-10-CH3-pteroylglutamate5 to dihydrofolate reductase. J Clin Invest (1983) 72(3):773–8. doi: 10.1172/JCI111048
22. Narimatsu H, Terakura S, Matsuo K, Oba T, Uchida T, Iida H, et al. Short-term methotrexate could reduce early immune reactions and improve outcomes in umbilical cord blood transplantation for adults. Bone Marrow Transplant (2007) 39(1):31–9. doi: 10.1038/sj.bmt.1705539
23. Radtke S, Zolk O, Renner B, Paulides M, Zimmermann M, Möricke A, et al. Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood (2013) 121(26):5145–53. doi: 10.1182/blood-2013-01-480335
24. Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Piñan MA, Garcia-Miguel P, Navajas A, et al. Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia. Pediatr Blood Canc (2011) 57(4):612–9. doi: 10.1002/pbc.23074
25. Xu M, Wu S, Wang Y, Zhao Y, Wang X, Wei C, et al. Association between high-dose methotrexate-induced toxicity and polymorphisms within methotrexate pathway genes in acute lymphoblastic leukemia. Front Pharmacol (2022) 13:1003812. doi: 10.3389/fphar.2022.1003812
26. Taylor ZL, Vang J, Lopez-Lopez E, Oosterom N, Mikkelsen T, Ramsey LB. Systematic review of pharmacogenetic factors that influence high-dose methotrexate pharmacokinetics in pediatric Malignancies. Cancers (Basel) (2021) 13(11):2837. doi: 10.3390/cancers13112837
27. Han J, Liu L, Meng L, Guo H, Zhang J, Han ZQ, et al. Effect of polymorphisms of ABCB1 and MTHFR on methotrexate-related toxicities in adults with hematological Malignancies. Front Oncol (2021) 11:759805. doi: 10.3389/fonc.2021.759805
28. Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics (2003) 13(8):481–94. doi: 10.1097/00008571-200308000-00006
Keywords: MTX, Peri-ES, GvHD, SLCO1B1, gene polymorphism
Citation: Ji Q, Zhang Y, Hu Y, Liu L, Cao S, Gao L, Li B, Tian Y, Kong L, Wu S, Ling J, Xiao P, Lu J, Li J, Yao Y, Qin J and Hu S (2023) The influence of methotrexate-related transporter and metabolizing enzyme gene polymorphisms on peri-engraftment syndrome and graft-versus-host disease after haplo-hematopoietic stem cell transplantation in pediatric patients with malignant hematological diseases. Front. Immunol. 14:1229266. doi: 10.3389/fimmu.2023.1229266
Received: 26 May 2023; Accepted: 14 August 2023;
Published: 05 September 2023.
Edited by:
Ying-Jun Chang, Peking University People’s Hospital, ChinaReviewed by:
Ting Yang, Fujian Medical University, ChinaCopyright © 2023 Ji, Zhang, Hu, Liu, Cao, Gao, Li, Tian, Kong, Wu, Ling, Xiao, Lu, Li, Yao, Qin and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoyan Hu, aHVzaGFveWFuQHN1ZGEuZWR1LmNu; Jiayue Qin, anlxaW5AbGl2ZS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.