- 1Department of Thoracic Surgical Oncology, Shanghai Lung Cancer Center, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Thoracic Surgery, Shanghai Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Oncology, Shanghai Lung Cancer Center, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Thoracic Surgery, Putuo District People’s Hospital, Zhejiang, China
Background: Neoadjuvant immunochemotherapy has been increasingly applied to treat non-small cell lung cancer (NSCLC). However, the comparison between robotic-assisted thoracoscopic surgery (RATS) and video-assisted thoracoscopic surgery (VATS) in the feasibility and oncological efficacy following neoadjuvant immunochemotherapy is scarce. This study aims to assess the superiorities of RATS over (VATS) concerning short-term outcomes in treating NSCLC patients with neoadjuvant immunochemotherapy.
Methods: NSCLC patients receiving RATS or VATS lobectomy following neoadjuvant immunochemotherapy at Shanghai Chest Hospital from 2019 to 2022 were retrospectively identified. Baseline clinical characteristics, perioperative outcomes, and survival profiles were analyzed.
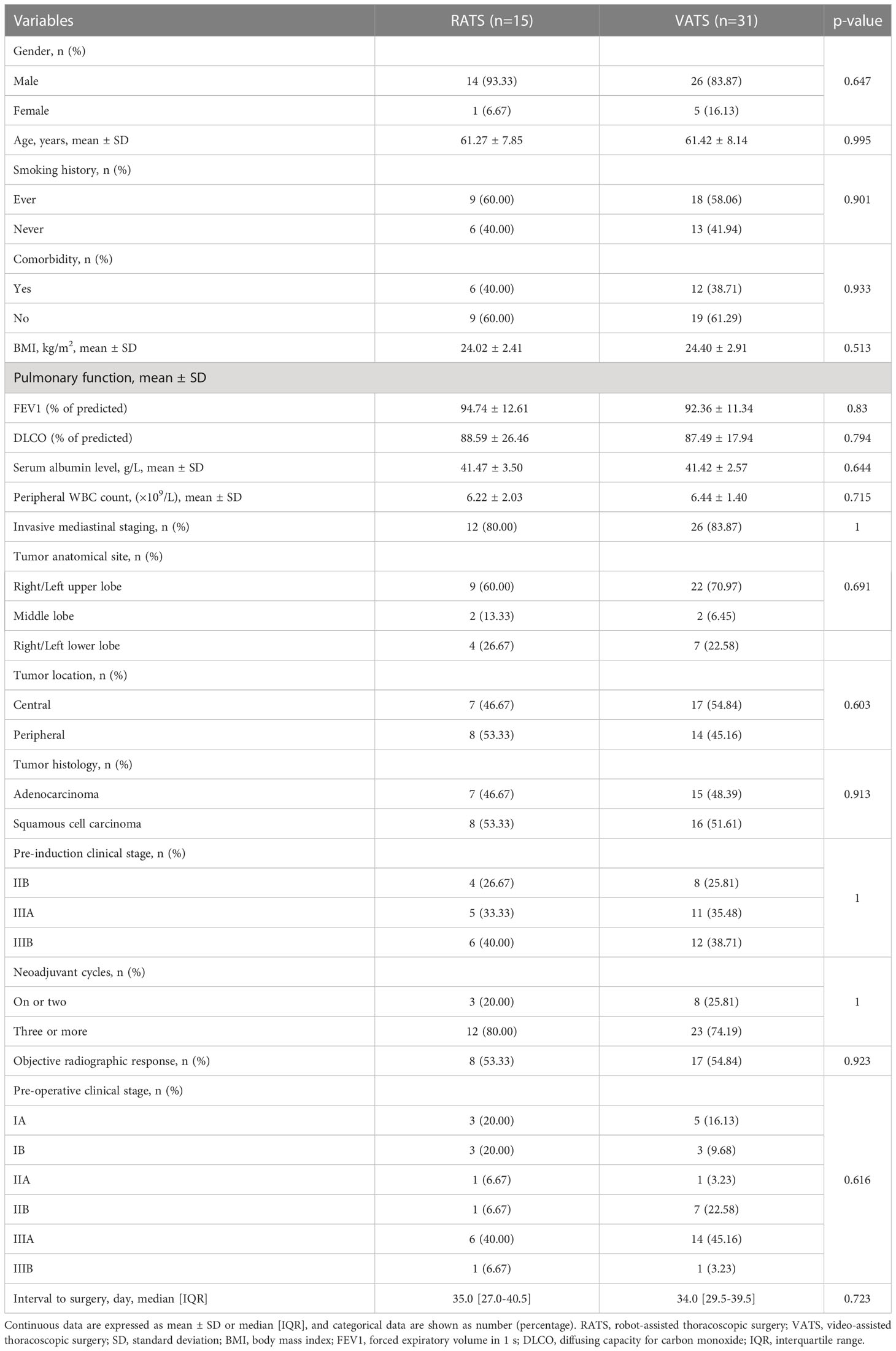
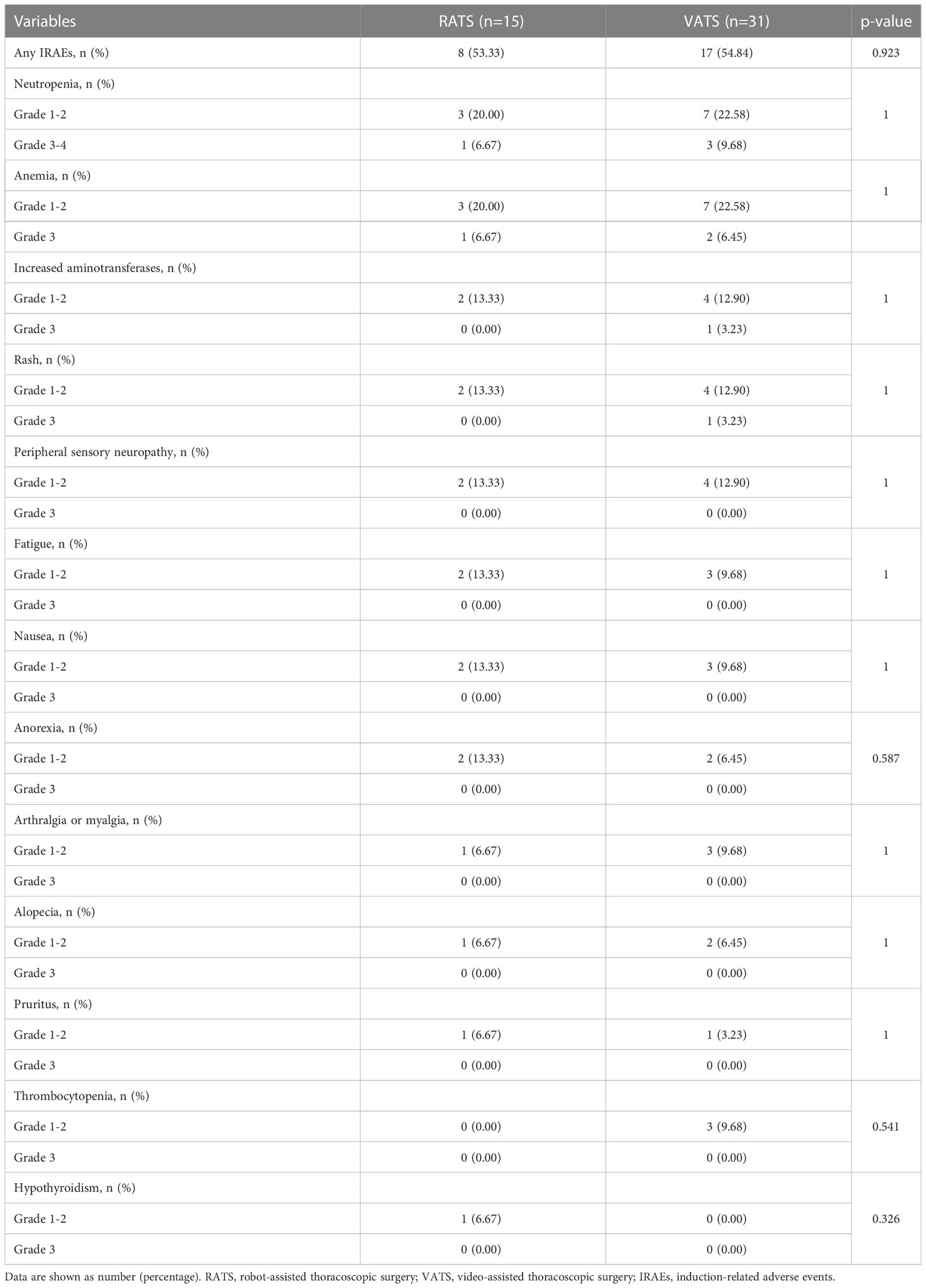
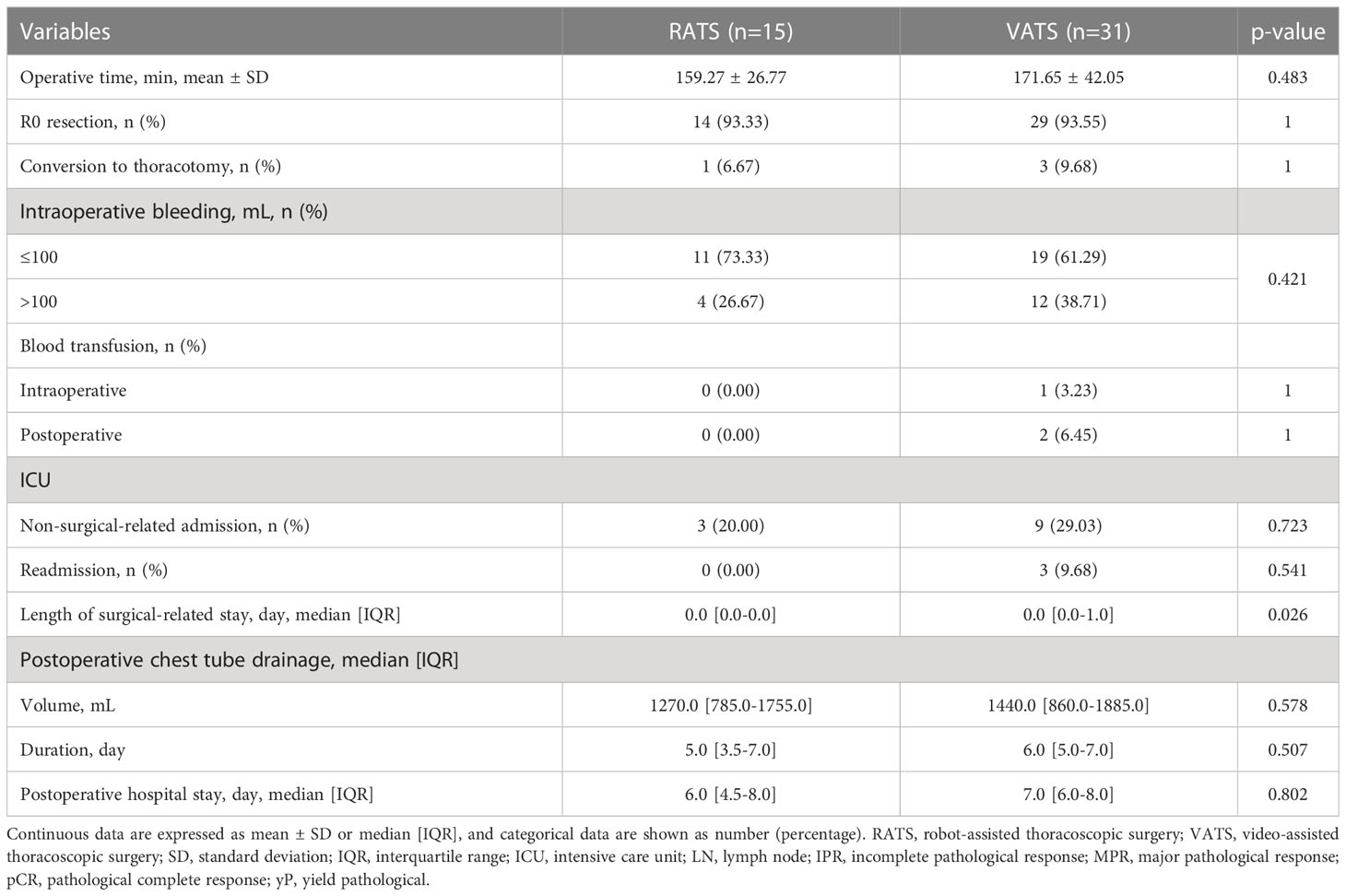
Results: Forty-six NSCLC patients with neoadjuvant immunochemotherapy were included and divided into the RATS (n=15) and VATS (n=31) groups. The baseline clinical characteristics and induction-related adverse events were comparable between the two groups (all p>0.050). The 30-day mortality in the RATS and VATS groups were 0% and 3.23%, respectively (p=1.000). Patients undergoing RATS were associated with reduced surgical-related intensive unit care (ICU) stay than those receiving VATS (0.0 [0.0-0.0] vs. 0.0 [0.0-1.0] days, p=0.026). Moreover, RATS assessed more N1 LNs (6.27 ± 1.94 vs 4.90 ± 1.92, p=0.042) and LN stations (3.07 ± 1.03 vs 2.52 ± 0.57, p=0.038) compared with VATS. By comparison, no difference was found in surgical outcomes, pathological results, and postoperative complications between the RATS and VATS groups (all p>0.050). Finally, RATS and VATS achieved comparable one-year recurrence-free survival (82.96% vs. 85.23%, p=0.821) and the timing of central nervous system, LN, and bone recurrences (all p>0.050).
Conclusion: RATS is safe and feasible for NSCLC patients with neoadjuvant immunochemotherapy, reducing surgical-related ICU stay, assessing increased N1 LNs and stations, and achieving similar survival profiles to VATS.
1 Introduction
Nowadays, non-small cell lung cancer (NSCLC) remains one of the most frequently diagnosed malignancies and the primary contributors to cancer-related death worldwide (1). However, despite undergoing a curative-intent surgical resection, most patients with locally advanced disease (stage IIIA-IIIB) subsequently experience tumor recurrence, mainly at distant locations, resulting in a poor prognosis with 5-year overall survival (OS) rate of less than 30% (2, 3). Unfortunately, the additional application of neoadjuvant or adjuvant chemotherapy merely leads to an improvement of about 5% in the 5-year OS rate (4). Over recent years, immunotherapy has revolutionized the treatment of NSCLC, and the oncological efficacy of immune checkpoint inhibitors (ICIs) has been well-established in patients with stage IV disease. Given this, numerous studies have further investigated the value of neoadjuvant ICIs treatment alone or with chemotherapy for resectable (stage IB-IIIA) and potentially resectable (stage IIIB) NSCLC, indicating the favorable safety and oncological efficacy of this promising therapy strategy (5–11).
Surgical resection remains the ultimate curative treatment for NSCLC if complete resection is feasible, with lobectomy remaining the gold standard. Video-assisted thoracoscopic surgery (VATS) has been widely applied, and its safety, feasibility, and oncological efficacy are well-established for early-stage and advanced NSCLC with or without neoadjuvant chemotherapy. Compared with traditional thoracotomy, VATS is associated with less surgical trauma, reduced surgical-related pain, and fewer postoperative complications (12–15). However, patients receiving neoadjuvant ICIs treatments are frequently associated with dense adhesions and fibrosis in the chest cavity, especially those with notable therapy responses, making lung resection more technically demanding (16, 17). Given this, traditional thoracotomy is still the most common surgical approach for this group of patients, with growing interest in the application of minimally invasive surgery (MIS). Several recent publications have assessed the safety and feasibility of VATS following neoadjuvant immunotherapy, suggesting that VATS is associated with low surgical-related mortality and morbidity and an acceptable conversion rate (17–20). In 2022, Zhang et al. compared VATS and thoracotomy, indicating that VATS achieved comparable surgical-related outcomes, postoperative recovery, and comorbidities to thoracotomy, with the benefit of fewer postoperative intensive care unit (ICU) stays (21). However, VATS exhibited inferiority in lymph node (LN) assessment than thoracotomy. Therefore, debate persists on the optional surgical modality for patients following ICIs treatment.
Robot-assisted thoracoscopic surgery (RATS), an innovative minimally invasive surgical (MIS) technic, was introduced into the thoracic surgery field in 2002 and performed firstly by our team in mainland China in 2009 (22). Nowadays, RATS is gaining increasing interest among thoracic surgeons and has emerged as a viable option for treating NSCLC, serving as a potential alternative to both thoracotomy and VATS (23). RATS offers a high-definitional, magnified, 3-dimensional (3D) visualization, allowing operators to perform complicated surgery precisely, and has a highly flexible mechanical wrist that can maneuver even more efficiently than human hands, providing great convenience in radical lymphadenectomy (24). Compared with VATS, RATS has shown the advantages of increased LN assessment, shorter surgical durations, faster postoperative recoveries, and higher cost-effectiveness for NSCLC patients (24–26). Additionally, RATS may even provide benefits over VATS with reduced conversion risk and blood loss for patients following neoadjuvant therapy (27, 28). However, the research on the safety and feasibility of RATS versus VATS in treating NSCLC patients with neoadjuvant immunochemotherapy is scarce, and the comparison of oncological efficacy has never been reported.
Herein, we compared the perioperative and survival outcomes of NSCLC patients receiving RATS or VATS following neoadjuvant immunochemotherapy, aiming to assess the advantage of RATS over VATS for these patients.
2 Materials and methods
2.1 Patients
In the present study, we retrospectively reviewed NSCLC patients receiving MIS lobectomy following immunochemotherapy induction from June 2019 to December 2022 at Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine. Echocardiography, pulmonary function testing, and electrocardiogram were performed to assess the surgical tolerance of patients. Brain-enhanced magnetic resonance imaging (MRI), positron emission tomography/CT (PET/CT), bone scintigraphy, and abdominal ultrasound were utilized to evaluate distant metastasis. To determine mediastinal LN status, PET/CT was conducted for all patients, and invasive mediastinal assessment, including endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) and mediastinoscopy, were further performed if necessary. All patients were staged by the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Before immunochemotherapy induction, the pathological biopsy was performed for all patients, and EGFR (epidermal growth factor receptor) mutation and ALK (anaplastic lymphoma kinase) translocation status were determined. Other oncogene events were tested, if applicable, by adopting next-generation sequencing, fluorescence in situ hybridization, or polymerase chain reaction. The preoperative assessment of patients between the two groups was identical.
Eligible patients had a stage IIB to IIIB NSCLC and underwent the simple single lobectomy in a curative intent following neoadjuvant ICIs immunotherapy plus platinum-based doublet chemotherapy. All cases were associated with radiographically measurable lesions following the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. The following were the exclusion criteria (1): cases with missing information (2); clinical N3 stage of the disease (3); intrapulmonary or distant metastasis assessed by preoperative tests.
2.2 Therapeutic regimens and treatment
The neoadjuvant regimens and indications for surgery were discussed and determined by a multidisciplinary team (MDT). All patients received PD-1 monoclonal antibodies (Nivolumab, Pembrolizumab, Sintilimab, Tislelizumab, Toripalimab, or Camrelizumab) combined with guideline-recommended platinum-based doublets (pemetrexed, docetaxel, or gemcitabine plus cisplatin or carboplatin), and routinely underwent surgery four to six weeks after the last cycle of therapy. For the selected stage IIIB (T3N2M0) NSCLC patients with single-level N2 involvement, the operation was also performed if an MDT assessment considered they could benefit from surgical resection (29, 30). Nevertheless, cases with initial stage-IV NSCLC who downgraded to the operative clinical stage were excluded due to the controversial oncological efficacy of neoadjuvant immunochemotherapy and various therapeutic approaches to metastatic lesions preoperatively.
2.3 Surgical technics
RATS and VATS were performed according to the procedure reported by our surgical team previously (23–25). All patients received general intravenous (i.v.) anesthesia with double-lumen intubation and single-lung ventilation managed by dedicated thoracic anesthesiologists. RATS was carried out by adopting the da Vinci Surgical System (Intuitive Surgical, CA, USA) via four minimal incisions of the non-rib spreading technic. The camera port was located on the 7th or 8th intercostal space along the posterior axillary line. Then, two incisions were symmetrically made at the 7th and 9th intercostal spaces along the mid-axillary and infrascapular lines, respectively. A utility port was created at the 3rd or 4th intercostal space on the anterior axillary line for the bedside assistant to expose the operating field, tract lung, and retrieve specimens. Conventionally, VATS was performed via three or four minimal incisions without spreading the ribs. The camera port was created at the 7th intercostal space along the anterior axillary line. Then, two incisions were made at the 3rd or 4th, and 8th intercostal spaces on the anterior and posterior axillary lines, respectively. If deemed necessary, a fourth port was created at the 9th intercostal space along the posterior axillary line for assistance. A radical lobectomy with systematic mediastinal LN dissection was carried out for all patients, with the resection margin being evaluated by the intraoperative frozen section. After confirming no air leak and active bleeding, the chest wall was closed with one or two 24F chest tubes placed in the pleural cavity. The conversion was defined as the operation starting with RATS or VATS dissection and finishing as the rib spreading thoracotomy.
2.4 Postoperative management and follow up
After operations, patients in the two groups were managed following identical protocols, which included early postoperative activities, breathing training, and specific postoperative analgesia. Dedicated rehabilitation therapists participated throughout the postoperative recovery process for every patient. The decision of ICU administration was made by the surgical team. Generally, the following patients would be treated by ICU after surgery: 1) with life-threaten surgical-related complications; 2) experiencing severe events during operation, such as conversion and blood transfusion; and 3) with the preoperative-assessed baseline potential for serious postoperative comorbidities, for example, elderly, with impaired cardiac or pulmonary function, and with cardiocerebrovascular diseases. The chest tube was removed when the absence of apparent air leak and subcutaneous emphysema was confirmed, the drainage volume of <200 mL/day, no densely bloody, cloudy, or purulent pleural effusion, and the chest X-ray images indicated excellent resorption of the lung. After surgery, patients were routinely evaluated by an MTD and received adjuvant therapy.
The lifelong follow-up assessment was planned one month after the operation, followed by every three months for the first two years, every half year from years three to five, and annually afterward. Thoracic CT scans, abdominal ultrasounds, routine blood tests, and serum tumor marker tests were routinely performed. PET-CT, brain MRI, bone scintigraphy, or TBNA were further applied if deemed necessary. Telephone follow-up was performed every six months until death or May 2023 for patients who did not regularly visit the outpatient clinic. The latest electronic medical profiles were recorded if patients lost to follow-up.
2.5 Clinical assessment and outcome measurement
Induction-related adverse events (IRAEs) were assessed in all patients using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (https://ctep.cancer.gov/), a widely applied grade system for induction-related morbidities (31–33). The thoracic CT scan or PET-CT radiographic assessment was conducted after induction therapy. The radiographic response was determined using RECIST version 1.1 by at least one dedicated thoracic radiologist. Interval to surgery was measured from the end of neoadjuvant treatment to the surgery date, and operation time was measured from incision to wound closure. R0 resection was defined as no microscopic residual tumor confirmed by paraffin pathologic reports. The 30-day postoperative complications were classified per the Clavien-Dindo classification system as follows: grade I, any deviation from the ordinary postoperative course without the need for pharmacological or operational intervention, or merely needing drugs such as analgesics, antipyretics, antiemetics, diuretics, or electrolytes; grade II, complication requiring pharmacological treatment, including blood transfusion and total parenteral nutrition; grade III, comorbidities requiring surgical or endoscopic intervention; grade IV, severe complication requiring ICU treatment; and grade V, death of the patient (34). Specifically, pulmonary comorbidities included pneumonia, acute respiratory distress syndrome (ARDS), respiratory failure requiring reintubation, empyema, and pulmonary embolism. Cardiac comorbidities included arrhythmia and myocardial ischemia or infarction. Anastomotic complications included prolonged air leaks and bronchopleural fistula. Other comorbidities included chylothorax, recurrent laryngeal nerve injury, and wound infection. Pathological response to therapy was evaluated regarding the volume of residual viable tumor cells in relation to the tumor bed following the principle described previously (35). Major pathological response (MPR) was defined as the presence of 10% viable residual tumor cells in the resected specimen, among which pathological complete response (pCR) was indicated when no viable residual tumor cell was found. The PD-L1 status of the tumor cells was assessed, and the positivity was indicated by a tumor proportion score of 1% or more. Recurrence-free survival (RFS) was calculated from the surgy to the date of any local or distant tumor recurrence, while central nervous system (CNS)-free survival was calculated from the surgery to the date of CNS tumor recurrence.
2.6 Statistical analysis
Mean ± standard deviation (SD) or median and interquartile range (IQR) were used to express the continuous variables, and frequencies and percentages were applied to define the categorical variables. If the Kolmogorov-Smirnov test indicated a normal distribution and homogeneous variance of the variable, the Student’s t-test was conducted to compare continuous variables. Otherwise, the Mann-Whitney U test was carried out. Pearson’s χ2 or Fisher’s exact test was applied to compare categorical variables. Kaplan-Meier curves log-rank (Mantel-Cox) test was adopted to analyze survival profiles. A prespecified two-sided p-value <0.05 was considered statistically significant. IBM SPSS Statistics v.26.0 (IBM Corporation, Armonk, NY, USA) was applied to perform the statistical analysis, while GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA) was adopted to analyze survival profiles.
3 Results
3.1 Baseline clinical characteristics of patients
A total of 46 NSCLC patients receiving MISs with neoadjuvant immunochemotherapy were retrospectively identified according to the inclusion and exclusion criteria and then split into RATS (n=15) and VATS (n=31) groups (Figure 1). The baseline clinical characteristics of the identified patients are summarized in Table 1. The male patients occupied the most dominant in the RATS (93.33%) and VATS (83.37%) groups. The average age of patients receiving RATS and VATS was 61.27 and 61.42 years, respectively. Moreover, 80.00% and 83.87% of cases underwent invasive mediastinal staging in the RATS and VATS groups, respectively. Most participants received three or more neoadjuvant cycles before undergoing RATS (80.00%) or VATS (74.19%). Additionally, patients in the RATS and VATS groups were associated with 53.33% and 54.84% objective radiographic response before the surgery. Finally, the median interval to operation in the RATS and VATS groups were 35.0 and 34.0 days, respectively. By comparison, no significant difference was found between the two groups concerning gender (p=0.647), age (p=0.995), smoking history (p=0.901), presence of comorbidities (p=0.933), BMI (p=0.513), FEV1% (p=0.830), DLCO% (p=0.794), serum albumin level (p=0.644), peripheral white blood cell (WBC) count (p=0.715), tumor anatomic site (p=0.691), tumor location (p=0.603), invasive mediastinal staging rate (p=1.000), tumor histology type (p=0.913), pre-induction TNM stage (p=1.000), neoadjuvant cycle (p=1.000), objective radiographic response rate (p=0.923), and pre-operative TNM stage (p=0.616), and interval to surgery (p=0.723). Given the small sample size and balanced baseline features of included cases, propensity score matching was not further applied.
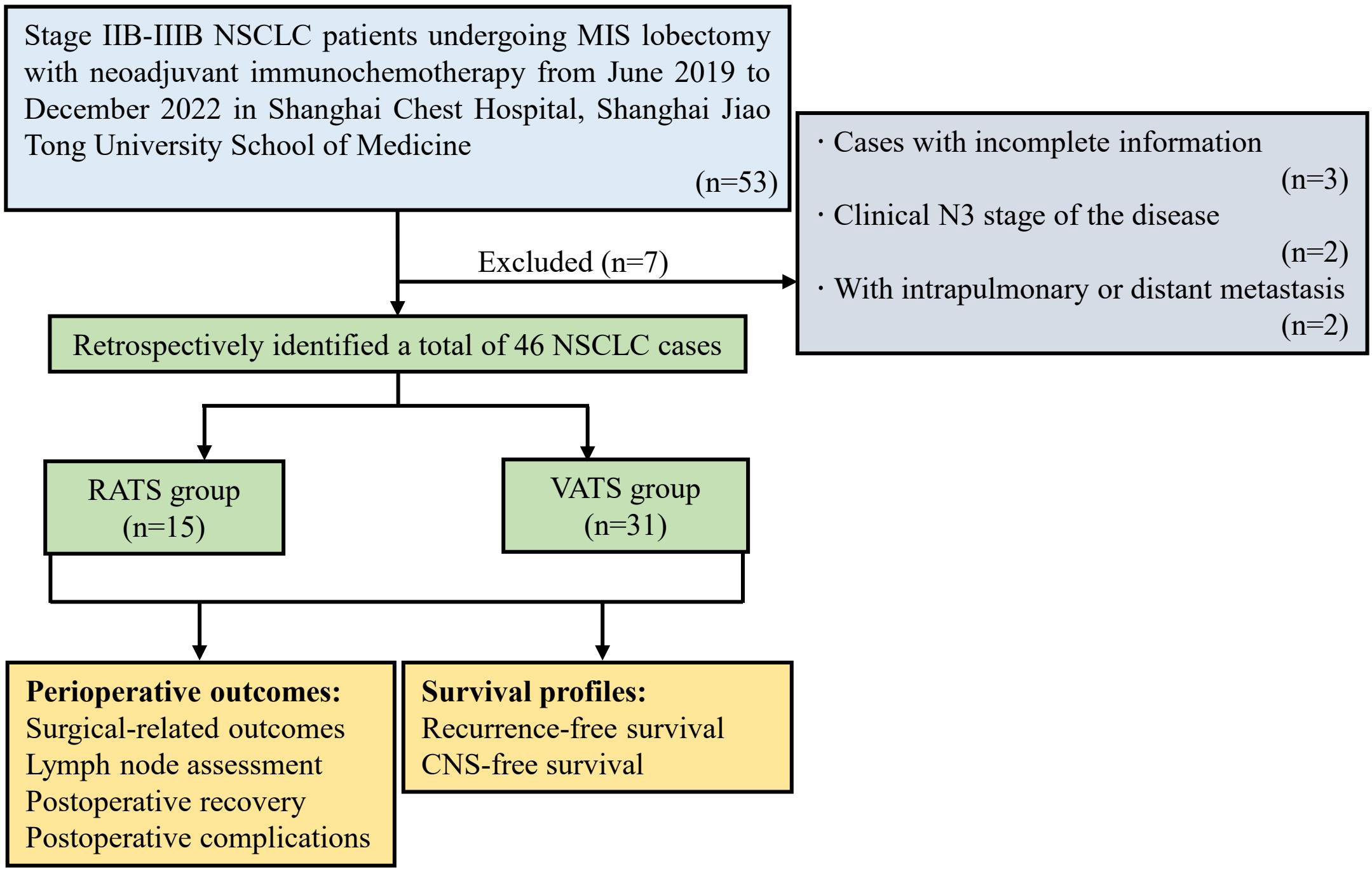
Figure 1 Flow chart of the study population. NSCLC, non-small cell lung cancer; MIS, minimally invasive surgery; RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; CNS, central nervous system.
3.2 Neoadjuvant treatment-related adverse events
The induction toxicity in the preoperative setting is expressed in Table 2. Overall, the proportion of patients with IRAEs in the RATS and VATS groups was 53.33% (8 of 15 patients) and 54.84% (17 of 31 patients), respectively. Altogether, the most frequent IRAEs in the two groups were neutropenia (30.43%), anemia (28.26%), increased aminotransferases (15.22%), rash (15.22%), and peripheral sensory neuropathy (13.04%), with all the grade-4 IRAEs being myelosuppression (6.52%). By comparison, the induction-related AEs were similar between the RATS and VATS groups (all p>0.050). All IRAEs were manageable with symptomatic treatment or observation only.
3.3 Surgical outcomes, pathological results, and LN assessment
As expressed in Table 3, RATS and VATS led to comparable operative time (159.27 ± 26.77 vs. 171.65 ± 42.05 min, p=0.483), R0 resection rate (93.33% vs. 93.55%, p=1.000), and incidence of conversion to thoracotomy (6.67% vs. 9.68%, p=1.000). Additionally, blood transfusion was not required in patients undergoing RATS, while was performed for one and two patients receiving VATS intraoperatively and postoperatively, respectively. By comparison, patients in the RATS and VATS groups were associated with similar intraoperative bleeding (p=0.421) and incidence of intraoperative (p=1.000) and postoperative blood transfusion (p=1.000).
Additionally, the regression in tumor area with viable tumor cells in resection specimens of the individual patient undergoing RATS or VATS was shown in Figures 2A, B. Overall, in the RATS and VATS groups, the MPR rates of patients were 53.33% (8 of 15 patients) and 51.61% (16 of 31 patients), respectively, and the pCR rates were 46.67% (7 of 15 cases) and 35.48% (11 of 31 cases), respectively (Figure 2C). By comparison, there was no difference in the two groups concerning the pathological response (p=0.913) and pCR rate (p=0.466). Finally, RATS and VATS achieved the comparable ypN stage (p=0.818, Figure 2D).
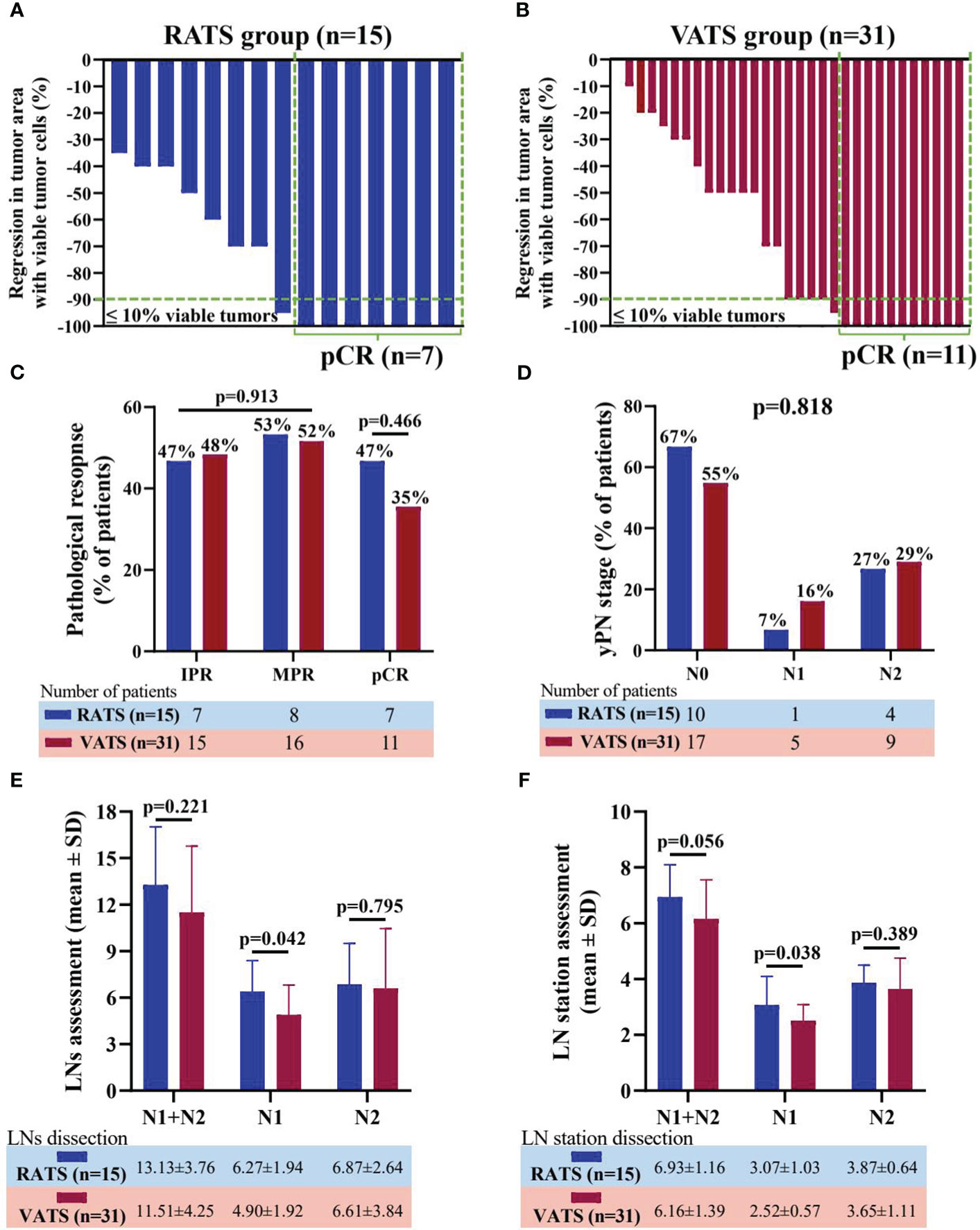
Figure 2 Waterfall plots of regression in tumor area with viable tumor cells in resection specimen of patients receiving RATS (A) or VATS (B) following neoadjuvant immunochemotherapy. Each bar represents one patient. Comparison of pathological response and pCR rate (C) and yPN stage (D) between the RATS and VATS groups. Comparison of dissected LNs (E) and LN stations (F) between the RATS and VATS groups. NSCLC, non-small cell lung cancer; RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; IPR, incomplete pathological response; MPR, major pathological response; pCR, pathological complete response; yP, yield pathological; LNs, lymph nodes.
In terms of LN dissection, RATS assessed significantly increased N1 LNs (6.27 ± 1.94 vs. 4.90 ± 1.92, p=0.042) and stations (3.07 ± 1.03 vs. 2.52 ± 0.57, p=0.038) than VATS (Figure 2E, F). Nevertheless, RATS and VATS were comparable in harvesting N2 (6.87 ± 2.64 vs 6.61 ± 3.84, p=0.795) and total LNs (13.13 ± 3.76 vs 11.51 ± 4.25, p=0.221), and N2 (3.87 ± 0.64 vs 3.65 ± 1.11, p=0.389) and total LN stations (6.93 ± 1.16 vs 6.16 ± 1.39, p=0.056).
3.4 Postoperative recovery and surgical-related complications
As expressed in Table 3, RATS reduced the duration of surgical-related ICU stay compared with VATS (0.0 [0.0-0.0] vs. 0.0 [0.0-1.0] days, p=0.026). Meanwhile, the two groups had a comparable incidence of ICU readmission (0.00% vs. 9.68%, p=0.541) and non-surgical-related admission (20.00% vs. 29.03%, p=0.723). Additionally, patients in the RATS and VATS groups were associated with similar postoperative chest tube drainage volume (1270.0 [785.0-1755.0] vs. 1440.0 [860.0-1885.0] mL, p=0.578) and duration (5.0 [3.5-7.0] vs. 6.0 [5.0-7.0] days, p=0.507), and postoperative hospital stay (6.0 [4.5-8.0] vs. 7.0 [6.0-8.0] days, p=0.802).
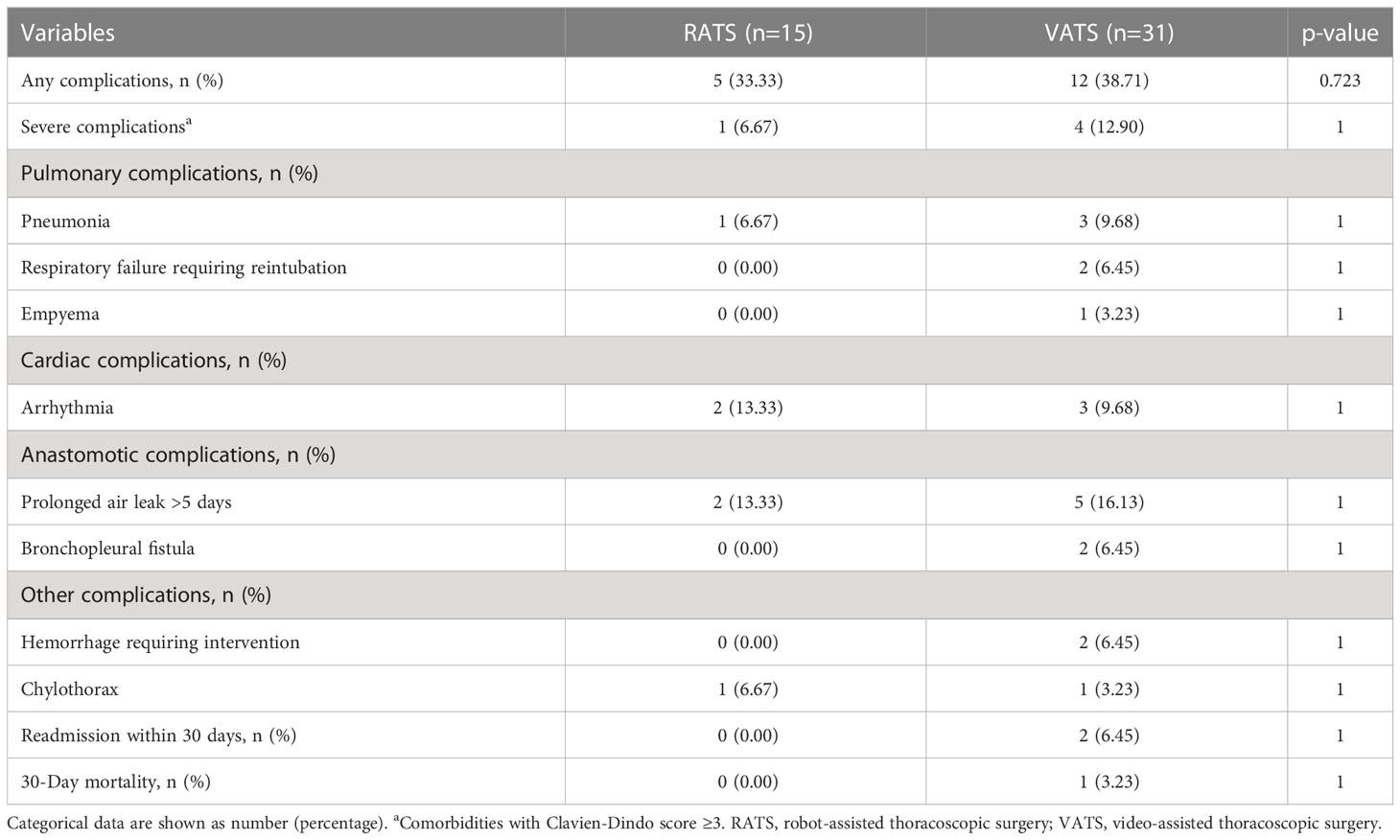
The postoperative complications of patients are shown in Table 4. Five and twelve patients had postoperative complications in the RATS and VATS groups, respectively, and one and three had severe comorbidities (Clavien-Dindo score ≥3). Surgical-related mortality was not occurred in the RATS groups, while one patient died within 30 days after VATS. Overall, prolonged air leak, arrhythmia, and pneumonia were the most common complications in patients receiving MISs following neoadjuvant immunochemotherapy. By comparison, RATS and VATS led to comparable incidences of overall (33.33% vs. 38.71%, p=0.723) and severe complications (6.47% vs. 12.90%, p=1.000) and any individual comorbidities (all p>0.050). Additionally, the distribution of complications was similar in the two groups (all p>0.050, Figure 3A). Finally, patients in the RATS and VATS groups were associated with an equal distribution of 30-day Clavien-Dindo postoperative complication scores (p=1.000, Figure 3B).
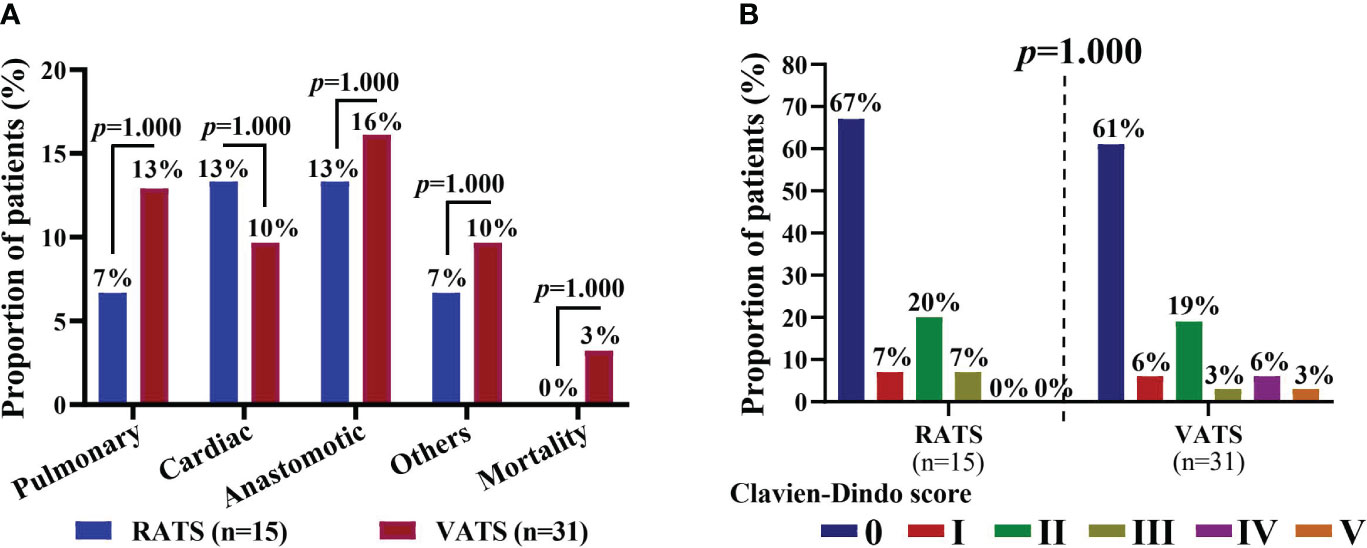
Figure 3 (A) Distribution of 30-day postoperative complications of NSCLC patients in the RATS and VATS groups. Patients may be associate with multiple (≥2) complications. (B) Comparison of Clavien-Dindo scores of NSCLC patients in the RATS and VATS groups. The highest grade was indicated if a patient was associated multiple comorbidities. NSCLC, non-small cell lung cancer; RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
3.5 Survival outcomes
RFS is deemed an indicator of oncological efficacy, and therefore one patient who experienced surgical-related mortality in the VATS group was excluded when evaluating RFS. As shown in Figure 4, in a median follow-up time of 26.5 months [IQR, 16.0-37.0 months], patients in the RATS and VATS groups were associated with comparable one-year RFS profiles (82.96% vs. 85.23% months, p=0.821, Figure 4A). The swimming plot of survival profiles is presented in Figure S1. By the additional comparison, no significant difference was found between RATS and VATS considering the one-year CNS-free (93.33% vs. 92.72%, p=0.506, Figure 4B), LN-free (88.89% vs. 89.01%, p=0.661, Figure S2A), and bone-free (87.50% vs. 92.98%, p=0.937, Figure S2B) survivals.
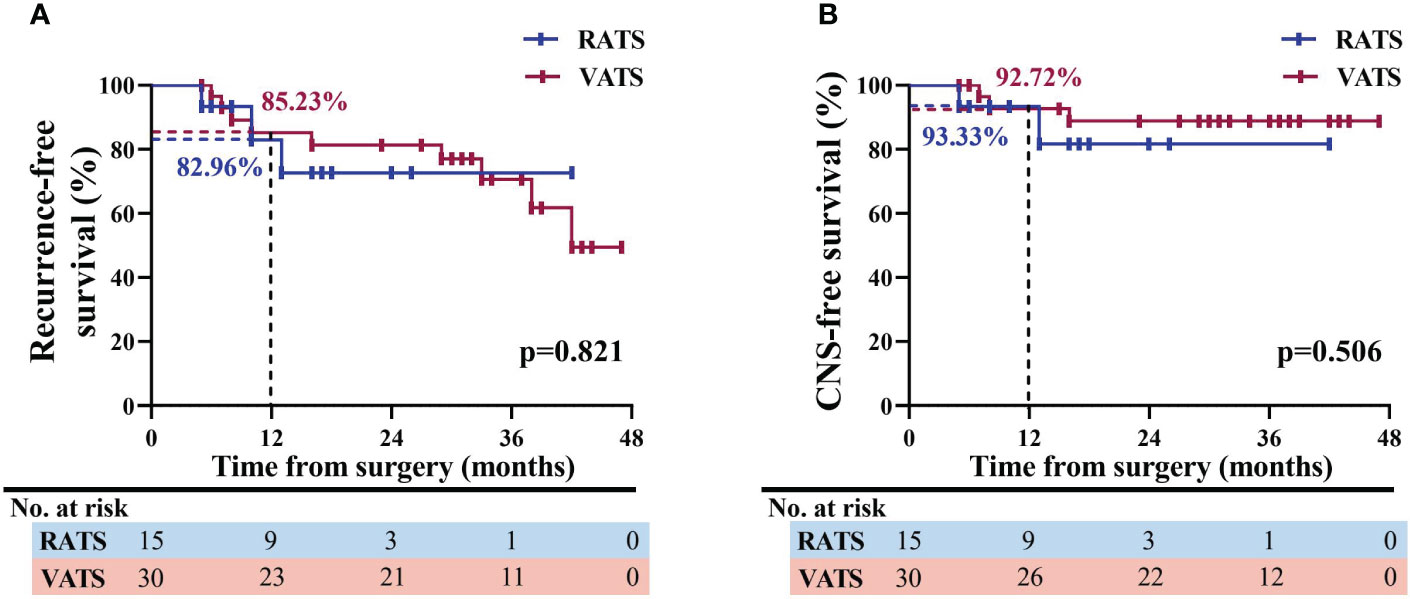
Figure 4 Analysis of survival profiles of NSCLC patients receiving RATS or VATS following neoadjuvant immunochemotherapy. Kaplan-Meier comparison of RFS (A) and CNS-free survival (B) profiles between the RATS and VATS groups. NSCLC, non-small cell lung cancer; RATS, robotic-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; RFS, recurrence-free survival; CNS, central nervous system.
4 Discussion
Nowadays, neoadjuvant immunochemotherapy has revolutionized the treatment of NSCLC, providing dramatic survival benefits compared with traditional chemotherapy. Although induction therapy usually adds surgical difficulty and perioperative challenges, VATS is becoming increasingly prevalent and has been considered safe and feasible in treating NSCLC patients following neoadjuvant immunochemotherapy (18, 21). Nevertheless, the application of RATS, an innovative MIS technic offering perioperative and even survival improvement than VATS for early-stage NSCLC, in treating patients with neoadjuvant immunochemotherapy has been rarely reported (25, 36–38). Consequently, research on the safety, feasibility, and oncological efficacy of RATS versus VATS for these patients is scarce. In the present study, we compared short-term outcomes of RATS versus VATS for NSCLC patients following immunochemotherapy, indicating that RATS exhibited superiorities in reducing postoperative ICU stay and dissecting more N1 LNs and stations, though achieving similar survival profiles to VATS.
With regard to surgical-related outcomes, our results showed that RATS led to a 6.67% of conversion rate, which was comparable to VATS (9.68%), and thus both surgical methods appear to be feasible with acceptable conversion rates. This conclusion is aligned with many previous publications enrolling early-stage or locally advanced NSCLC cases (23, 24, 39). However, at least four research revealed that fewer patients who received RATS converted to thoracotomy when compared with those undergoing VATS (27, 40–42). Importantly, RATS was also found to reduce conversion incidence for NSCLC patients with neoadjuvant immunochemotherapy than VATS (43). We notice that in our study, the conversion rate in NSCLC patients undergoing RATS after neoadjuvant immunochemotherapy was consistent with the previous ones, which ranged from 4.5% to 7% (43, 44). Nevertheless, it is much lower in patients receiving VATS in our study (9.68%) than that reported by previous publications ranging from 19% to over 50% (16, 20, 21, 45). Therefore, the controversial conclusion comparing the conversion incidence of RATS and VATS between our study and previous ones may largely be attributed to the decreased conversion rate of VATS. Given this, a high-volume medical center may reduce the risk of conversion of VATS for NSCLC patients following neoadjuvant immunochemotherapy. Finally, RATS and VATS achieved excellent bleeding management, with most of the patients having blood loss of less than 100 mL, and only one patient in the VATS group required intraoperative blood transfusion. For these reasons, both approaches seem to be safe concerning bleeding control.
In our study, RATS significantly reduced the length of surgical-related ICU stay than VATS. This superiority of RATS is potentially due to the great flexibility of the robot arms and high-quality surgical view that enable thoracic surgeons to perform resection and LN retrieval more precisely and thus minimize unnecessary damage to normal tissues, especially for patients with adhesive, fibrotic, and brittle tissues and fibro-calcified LNs caused by induction therapy, which accelerated the patient recovery (39, 43). Moreover, the fewer surgical-related complications requiring ICU administration in the RATS group than VATS (0.00% vs 9.68%), despite no statistical significance being found, could also contribute to the reduction in ICU stay. Nevertheless, the faster recovery from ICU in the RATS group did not dramatically lead to a more immediate discharge, although RATS appeared to be associated with a shorter chest tube duration and postoperative hospitalization to VATS. This might be attributed to the small sample size of cases in the present study. Given this, further study based on a larger cohort of patients is necessary further to compare the postoperative recoveries between the two approaches.
LN assessment is pivotal for the surgical treatment of NSCLC and a critical standard measuring the operative quality, and concerns about LN dissection have commonly been a drawback for VATS in treating NSCLC patients, especially those with the involved LNs or neoadjuvant therapy (21, 24, 46). Many previous publications have reported the comparison of LNs and LN station assessment between RATS and VAST but have drawn conflicting conclusions. At least two large-scale retrospective studies have revealed that RATS could dissect more LNs than VATS, which was further verified by two clinical trials reported recently (39, 47–49). However, several other research did not observe this superiority of RATS over VATS (50–52). In the present study, RATS dissected more N1 LNs and stations than VATS, which was in line with a previous publication, suggesting that RATS may possess the superiority over VATS in LN assessment for NSCLC patients with neoadjuvant immunochemotherapy (43). This is primarily attributed to the surgical view with 3D, high-definition, and ten-fold magnification and robotic arms with excellent maneuverability and improved dexterity provided by the robotic-assisted surgical system that offers operators great convenience to harvest LNs around vessels and bronchi (24, 39). Additionally, our results showed that RATS did assess an increased number of N2 and total LNs and LN stations than VATS. However, no statistical difference was found, which might be attributed to the small sample size included, and thus a larger cohort is necessary to validate our conclusion. Moreover, our study indicated that the increased LN assessment by RATS over VATS was not correlated with higher ypN upstaging in our study, which might be attributed to the good LN response to neoadjuvant immunochemotherapy and more than half of patients being associated with the stage ypN0 disease, as well as the comparable N2 LNs and stations assessed by the two approaches.
Although the increased examined LNs might lead to a more thorough elimination of remnants, its correlation to potential survival benefits remains controversial. At least four research found that an increased LN dissection contributed to prolonged survival, and at least 10 LNs should be harvested, while extra assessment of more than 16 LNs did not lead to better oncological outcomes (53–56). However, three others did not find this association (21, 57, 58). Moreover, many studies have found increased capability of RATS in LN assessment, but none of them observed its superiority in survival profiles over VTAS, which is consistent with our results (24, 25, 59, 60). Until now, the correlation between LN dissection and survival for NSCLC patients with neoadjuvant immunochemotherapy remains unrevealed, and further research is needed. Previous studies have found that lymphocytes in tumor-drainage LNs exhibit robust anti-tumor efficacies, and their activation and cytotoxicity effects upon immunotherapy dramatically improve survival in NSCLC patients (61, 62). Given this, the remaining LNs may also enhance the therapeutic efficacy of adjuvant immunotherapy ± chemotherapy to eliminate for patients receiving surgery following neoadjuvant ICI treatment. Paradoxically, the deficient LN dissection could lead to undiscovered metastatic LNs, disrupting the therapy and ultimately resulting in recurrence and distant dissemination (63). Therefore, the adequate LN examination and the preservation of the regional immune microenvironment should be balanced, and the optimal number of examined LNs and LN stations for NSCLC patients with neoadjuvant immunotherapy requires further investigation.
In terms of survival outcomes, our results showed that RATS and VATS achieved comparable 1-year RFS and CNS-free survival, and thus the two approaches appear to have similar oncological efficacies. In previous studies, RATS and VATS usually achieved comparable long-term outcomes in treating NSCLC (24, 41, 64). These conclusions, together with ours, indicated that the approach of MISs may not impact the long-term survival of NSCLC patients. Nevertheless, RATS may be associated with less long-term postoperative pain, improved life quality, and better mental health than VATS for NSCLC patients (64–66). Since neoadjuvant immunochemotherapy could dramatically prolong the survival period of patients with locally advanced NSCLC, evaluating their postoperative life qualities is necessary. We are now performing further follow-ups to compare the oncological and life-quality effectiveness of RATS versus VATS for NSCLC patients with neoadjuvant immunochemotherapy.
Previous studies have revealed that the robot-assisted surgical system could be adapted to perform highly difficult thoracic surgeries, including sleeve or double-sleeve resection for centrally located NSCLC (67–69). Nowadays, sleeve lobectomy has become a preferred surgical approach for centrally located NSCLC due to its reduced postoperative morbidity, lower mortality, better long-term survival and quality of life, and comparable oncological efficacy compared to pneumonectomy (70–72). More importantly, sleeve lobectomy has also proven feasible and oncologically effective for NSCLC patients following chemotherapy or immunochemotherapy induction (32, 73). Therefore, further evaluation of RATS for sleeve resection following neoadjuvant immunochemotherapy is necessary to expand its application for centrally located NSCLC patients.
We have acknowledged some limitations of our research. First, the sample size of our study was small, resulting in many negative results, and PSM was not further applied. These could have influenced the data validity and prejudiced the representative of the results. Secondly, the retrospective nature of the present study may result in undiscovered patient selection bias, despite the comparable baseline clinical features between the RATS and VATS groups. Finally, the long-term outcomes were unavailable due to the relatively short period of MIS’s application for NSCLC patients following neoadjuvant immunochemotherapy. Therefore, the multi-center, prospective study enrolling a more significant number of cases is necessary to confirm the conclusion of our research, and further follow-up is needed to analyze the oncological efficacy of RATS versus VATS. Nevertheless, MIS is likely to be increasingly applied with neoadjuvant immunochemotherapy due to its potential advantages over thoracotomy for NSCLC patients after induction therapy, though it may require a high surgical technic of thoracic surgeons (14, 21, 46, 74, 75). Thus, we believe that it is necessary to compare RATS and VATS, two pivotal MIS technics, for these patients based on the current practice, which might provide a reference for thoracic surgeons in further research and clinical practice.
5 Conclusion
In conclusion, RATS is a safe and feasible approach in treating NSCLC patients following neoadjuvant immunochemotherapy, exhibiting superiorities over VATS in shortening postoperative ICU stay and assessing increased N1 LNs and stations, though the two surgical approaches achieved similar survival profiles.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine (approval number: IS23017). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conception and design: LJ, JH, and QL. Resources: NZ, YT, ZG, HZ, ZL, and LJ. Methodology: HP, NZ, YT, JN, and WJ. Software: YT and WJ. Formal analysis: HP, NZ, JZ. Investigation: HP, NZ, YT, ZG, JZ, WK. Validation: HP, NZ. Visualization: HP, NZ, and YT. Writing-original draft: HP, NZ, and YT. Writing-review & editing: ZL, LJ, JH, and QL. Supervision: JH, and QL. Data curation: JH and QL. Project administration: QL. Funding acquisition: QL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Nature Science Foundation (Grant No. 81772482), the Joint Clinical Research Centre of Institute of Medical Robotics@SJTU-Shanghai Chest Hospital (IMR-XKH202104), and the Shanghai Hospital Development Center (Grant No. SHDC12016113).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RJ declared a shared affiliation with the authors to the handling editor at time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1228451/full#supplementary-material
References
1. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet (2021) 398(10299):535–54. doi: 10.1016/S0140-6736(21)00312-3
2. Ulas EB, Dickhoff C, Schneiders FL, Senan S, Bahce I. Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: a systematic review. ESMO Open (2021) 6(5):100244. doi: 10.1016/j.esmoop.2021.100244
3. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11(1):39–51. doi: 10.1016/j.jtho.2015.09.009
4. Burdett S, Rydzewska LHM, Tierney JF, Auperin A, Le Pechoux C, Le Chevalier T, et al. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet (2014) 383(9928):1561–71. doi: 10.1016/S0140-6736(13)62159-5
5. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
6. Cao C, Guo A, Chen C, Chakos A, Bott M, Yang CJ, et al. Systematic review of neoadjuvant immunotherapy for patients with non-small cell lung cancer. Semin Thorac Cardiovasc Surg (2021) 33(3):850–7. doi: 10.1053/j.semtcvs.2020.12.012
7. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(6):786–95. doi: 10.1016/S1470-2045(20)30140-6
8. Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol (2020) 15(5):816–26. doi: 10.1016/j.jtho.2020.01.017
9. Zhang F, Guo W, Zhou B, Wang S, Li N, Qiu B, et al. Three-year follow-up of neoadjuvant programmed cell death protein-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol (2022) 17(7):909–20. doi: 10.1016/j.jtho.2022.04.012
10. Rosner S, Reuss JE, Zahurak M, Zhang J, Zeng Z, Taube J, et al. Five-year clinical outcomes after neoadjuvant nivolumab in resectable non-small cell lung cancer. Clin Cancer Res (2023) 29(4):705–10. doi: 10.1158/1078-0432.CCR-22-2994
11. Cascone T, Leung CH, Weissferdt A, Pataer A, Carter BW, Godoy MCB, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med (2023) 29(3):593–604. doi: 10.1038/s41591-022-02189-0
12. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol (2016) 17(6):836–44. doi: 10.1016/S1470-2045(16)00173-X
13. Lee PC, Kamel M, Nasar A, Ghaly G, Port JL, Paul S, et al. Lobectomy for non-small cell lung cancer by video-assisted thoracic surgery: effects of cumulative institutional experience on adequacy of lymphadenectomy. Ann Thorac Surg (2016) 101(3):1116–22. doi: 10.1016/j.athoracsur.2015.09.073
14. Yang CJ, Nwosu A, Mayne NR, Wang YY, Raman V, Meyerhoff RR, et al. A minimally invasive approach to lobectomy after induction therapy does not compromise survival. Ann Thorac Surg (2020) 109(5):1503–11. doi: 10.1016/j.athoracsur.2019.09.065
15. Boffa D, Fernandez FG, Kim S, Kosinski A, Onaitis MW, Cowper P, et al. Surgically managed clinical stage IIIA-clinical N2 lung cancer in the society of thoracic surgeons database. Ann Thorac Surg (2017) 104(2):395–403. doi: 10.1016/j.athoracsur.2017.02.031
16. Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg (2019) 158(1):269–76. doi: 10.1016/j.jtcvs.2018.11.124
17. Deng H, Liu J, Cai X, Chen J, Rocco G, Petersen RH, et al. Radical minimally invasive surgery after immuno-chemotherapy in initially-unresectable stage IIIB non-small cell lung cancer. Ann Surg (2022) 275(3):e600–e2. doi: 10.1097/SLA.0000000000005233
18. Bott MJ, Cools-Lartigue J, Tan KS, Dycoco J, Bains MS, Downey RJ, et al. Safety and feasibility of lung resection after immunotherapy for metastatic or unresectable tumors. Ann Thorac Surg (2018) 106(1):178–83. doi: 10.1016/j.athoracsur.2018.02.030
19. Yao Y, Tang D, Gao W, Zhang H. Neoadjuvant immuno-chemotherapy: a new perspective for stage III NSCLC? Front Surg (2022) 9:843987. doi: 10.3389/fsurg.2022.843987
20. Romero Román A, Campo-Cañaveral de la Cruz JL, Macía I, Escobar Campuzano I, Figueroa Almánzar S, Delgado Roel M, et al. Outcomes of surgical resection after neoadjuvant chemoimmunotherapy in locally advanced stage IIIA non-small-cell lung cancer. Eur J Cardiothorac Surg (2021) 60(1):81–8. doi: 10.1093/ejcts/ezab007
21. Zhang B, Xiao Q, Xiao H, Wu J, Yang D, Tang J, et al. Perioperative outcomes of video-assisted thoracoscopic surgery versus open thoracotomy after neoadjuvant chemoimmunotherapy in resectable NSCLC. Front Oncol (2022) 12:858189. doi: 10.3389/fonc.2022.858189
22. Huang J, Luo Q, Tan Q, Lin H, Qian L, Lin X. Initial experience of robot-assisted thoracoscopic surgery in China. Int J Med Robot. (2014) 10(4):404–9. doi: 10.1002/rcs.1589
23. Huang J, Tian Y, Zhou QJ, Ning JW, Gu ZN, Lu PJ, et al. Comparison of perioperative outcomes of robotic-assisted versus video-assisted thoracoscopic right upper lobectomy in non-small cell lung cancer. Transl Lung Cancer Res (2021) 10(12):4549–57. doi: 10.21037/tlcr-21-960
24. Pan H, Tian Y, Wang H, Jiang L, Gu Z, Zhu H, et al. Perioperative and oncological outcomes of robotic-assisted, video-assisted thoracoscopic and open lobectomy for patients with N1-metastatic non-small cell lung cancer: a propensity score-matched study. Cancers (Basel) (2022) 14(21):5249–63. doi: 10.3390/cancers14215249
25. Pan H, Gu Z, Tian Y, Jiang L, Zhu H, Ning J, et al. Propensity score-matched comparison of robotic- and video-assisted thoracoscopic surgery, and open lobectomy for non-small cell lung cancer patients aged 75 years or older. Front Oncol (2022) 12:1009298. doi: 10.3389/fonc.2022.1009298
26. Jiang Y, Su Z, Liang H, Liu J, Liang W, He J. Video-assisted thoracoscopy for lung cancer: who is the future of thoracic surgery? J Thorac Dis (2020) 12(8):4427–33. doi: 10.21037/jtd-20-1116
27. Baig MZ, Razi SS, Agyabeng-Dadzie K, Stroever S, Muslim Z, Weber J, et al. Robotic-assisted thoracoscopic surgery demonstrates a lower rate of conversion to thoracotomy than video-assisted thoracoscopic surgery for complex lobectomies. Eur J Cardiothorac Surg (2022) 62(3):281–9. doi: 10.1093/ejcts/ezac281
28. Nelson DB, Mehran RJ, Mitchell KG, Rajaram R, Correa AM, Bassett RL Jr., et al. Robotic-assisted lobectomy for non-small cell lung cancer: a comprehensive institutional experience. Ann Thorac Surg (2019) 108(2):370–6. doi: 10.1016/j.athoracsur.2019.03.051
29. Wu J, Hou L, E H, Zhao Y, Yu X, Xu L, et al. Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer (2022) 165:115–23. doi: 10.1016/j.lungcan.2022.01.019
30. Gu L, Wang X, Sun Y, Xu Y, Niu X, Zhao R, et al. An open, observational, three-arm clinical study of 2-3 cycles of treatment as neoadjuvant therapy in operable locally advanced non-small cell lung cancer: an interim analysis. Front Immunol (2022) 13:938269. doi: 10.3389/fimmu.2022.938269
31. Shi Y, Li B, Wu L, Pan Y, Pan Z, Liu Y, et al. Efficacy and safety of limertinib (ASK120067) in patients with locally advanced or metastatic EGFR Thr790Met-mutated NSCLC: a multicenter, single-arm, phase 2b study. J Thorac Oncol (2022) 17(10):1205–15. doi: 10.1016/j.jtho.2022.05.011
32. Chen T, Ning J, Shen J, Pan H, Fu L, Xu E, et al. Sleeve lobectomy after neoadjuvant chemoimmunotherapy versus chemotherapy for squamous cell lung cancer: a multicenter, retrospective study. JTO Clin Res Rep (2023) 4(4):100472. doi: 10.1016/j.jtocrr.2023.100472
33. Zhao ZR, Lin ZC, Shen JF, Xie ZH, Jiang L. Neoadjuvant immunotherapy in oncogene-positive non-small cell lung cancer: a multicenter study. Ann Thorac Surg (2022) 4975(22):1541–7. doi: 10.1016/j.athoracsur.2022.11.035
34. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
35. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol (2018) 29(8):1853–60. doi: 10.1093/annonc/mdy218
36. Casiraghi M, Mariolo AV, Mohamed S, Sedda G, Maisonneuve P, Mazzella A, et al. Long-term outcomes of robotic-assisted, video-assisted and open surgery in non-small cell lung cancer: a matched analysis. J Clin Med (2022) 11(12):3363–74. doi: 10.3390/jcm11123363
37. Wei S, Chen M, Chen N, Liu L. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol (2017) 15(1):98. doi: 10.1186/s12957-017-1168-6
38. Reddy RM, Gorrepati ML, Oh DS, Mehendale S, Reed MF. Robotic-assisted versus thoracoscopic lobectomy outcomes from high-volume thoracic surgeons. Ann Thorac Surg (2018) 106(3):902–8. doi: 10.1016/j.athoracsur.2018.03.048
39. Jin R, Zheng Y, Yuan Y, Han D, Cao Y, Zhang Y, et al. Robotic-assisted versus video-assisted thoracoscopic lobectomy: short-term results of a randomized clinical trial (RVlob trial). Ann Surg (2022) 275(2):295–302. doi: 10.1097/SLA.0000000000004922
40. Muslim Z, Stroever S, Poulikidis K, Weber JF, Connery CP, Herrera LJ, et al. Conversion to thoracotomy in non-small cell lung cancer: risk factors and perioperative outcomes. Innov (Phila) (2022) 17(2):148–55. doi: 10.1177/15569845221091979
41. Shagabayeva L, Fu B, Panda N, Potter AL, Auchincloss HG, Mansur A, et al. Open, video- and robot-assisted thoracoscopic lobectomy for stage II-IIIA non-small cell lung cancer. Ann Thorac Surg (2023) 115(1):184–90. doi: 10.1016/j.athoracsur.2022.01.026
42. Kent MS, Hartwig MG, Vallières E, Abbas AE, Cerfolio RJ, Dylewski MR, et al. Pulmonary open, robotic, and thoracoscopic lobectomy (PORTaL) study: an analysis of 5721 cases. Ann Surg (2023) 277(3):528–33. doi: 10.1097/SLA.0000000000005115
43. Zeng J, Yi B, Chang R, Chen Y, Yu Z, Gao Y. Safety and feasibility of robotic-assisted thoracic surgery after neoadjuvant chemoimmunotherapy in non-small cell lung cancer. Front Oncol (2023) 13:1134713. doi: 10.3389/fonc.2023.1134713
44. Gao Y, Jiang J, Xiao D, Zhou Y, Chen Y, Yang H, et al. Robotic-assisted thoracic surgery following neoadjuvant chemoimmunotherapy in patients with stage III non-small cell lung cancer: a real-world prospective cohort study. Front Oncol (2022) 12:969545. doi: 10.3389/fonc.2022.969545
45. Yang CJ, McSherry F, Mayne NR, Wang X, Berry MF, Tong B, et al. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann Thorac Surg (2018) 105(3):924–9. doi: 10.1016/j.athoracsur.2017.09.030
46. Wang YF, Deng HY, Huang W, Zhou Q. Is video-assisted thoracoscopic surgery comparable with thoracotomy in perioperative and long-term survival outcomes for non-small-cell lung cancer after neoadjuvant treatment? Interact Cardiovasc Thorac Surg (2022) 35(6):271–7. doi: 10.1093/icvts/ivac271
47. Zhang Y, Chen C, Hu J, Han Y, Huang M, Xiang J, et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: a multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg (2020) 160(5):1363–72. doi: 10.1016/j.jtcvs.2019.12.112
48. Kodia K, Razi SS, Alnajar A, Nguyen DM, Villamizar N. Comparative analysis of robotic segmentectomy for non-small cell lung cancer: a national cancer database study. Innov (Phila) (2021) 16(3):280–7. doi: 10.1177/1556984521997805
49. Veronesi G, Abbas AE, Muriana P, Lembo R, Bottoni E, Perroni G, et al. Perioperative outcome of robotic approach versus manual videothoracoscopic major resection in patients affected by early lung cancer: results of a randomized multicentric study (ROMAN study). Front Oncol (2021) 11:726408. doi: 10.3389/fonc.2021.726408
50. Liang H, Liang W, Zhao L, Chen D, Zhang J, Zhang Y, et al. Robotic versus video-assisted Lobectomy/Segmentectomy for lung cancer: a meta-analysis. Ann Surg (2018) 268(2):254–9. doi: 10.1097/SLA.0000000000002346
51. Ma J, Li X, Zhao S, Wang J, Zhang W, Sun G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer (2021) 21(1):498. doi: 10.1186/s12885-021-08241-5
52. Merritt RE, Abdel-Rasoul M, D'Souza DM, Kneuertz PJ. Lymph node upstaging for robotic, thoracoscopic, and open lobectomy for stage T2-3N0 lung cancer. Ann Thorac Surg (2023) 115(1):175–82. doi: 10.1016/j.athoracsur.2022.05.041
53. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-Small-Cell lung cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol (2017) 35(11):1162–70. doi: 10.1200/JCO.2016.67.5140
54. Chen D, Mao Y, Wen J, Shu J, Ye F, She Y, et al. Numbers and stations: impact of examined lymph node on precise staging and survival of radiologically pure-solid NSCLC: a multi-institutional study. JTO Clin Res Rep (2020) 1(3):100035. doi: 10.1016/j.jtocrr.2020.100035
55. Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest (2005) 128(3):1545–50. doi: 10.1378/chest.128.3.1545
56. Samayoa AX, Pezzi TA, Pezzi CM, Greer Gay E, Asai M, Kulkarni N, et al. Rationale for a minimum number of lymph nodes removed with non-small cell lung cancer resection: correlating the number of nodes removed with survival in 98,970 patients. Ann Surg Oncol (2016) 23(Suppl 5):1005–11. doi: 10.1245/s10434-016-5509-4
57. Hennon MW, DeGraaff LH, Groman A, Demmy TL, Yendamuri S. The association of nodal upstaging with surgical approach and its impact on long-term survival after resection of non-small-cell lung cancer. Eur J Cardiothorac Surg (2020) 57(5):888–95. doi: 10.1093/ejcts/ezz320
58. Fang L, Wang L, Wang Y, Lv W, Hu J. Video assisted thoracic surgery vs. thoracotomy for locally advanced lung squamous cell carcinoma after neoadjuvant chemotherapy. J Cardiothorac Surg (2018) 13(1):128. doi: 10.1186/s13019-018-0813-7
59. Li C, Hu Y, Huang J, Li J, Jiang L, Lin H, et al. Comparison of robotic-assisted lobectomy with video-assisted thoracic surgery for stage IIB-IIIA non-small cell lung cancer. Transl Lung Cancer Res (2019) 8(6):820–8. doi: 10.21037/tlcr.2019.10.15
60. Merritt RE, Abdel-Rasoul M, D'Souza DM, Kneuertz PJ. Comparison of the long-term oncologic outcomes of robotic-assisted and video-assisted thoracoscopic lobectomy for resectable non-small cell lung carcinoma. J Robot Surg (2022) 16(6):1281–8. doi: 10.1007/s11701-022-01368-y
61. Kimura H, Matsui Y, Ishikawa A, Nakajima T, Iizasa T. Randomized controlled phase III trial of adjuvant chemoimmunotherapy with activated cytotoxic T cells and dendritic cells from regional lymph nodes of patients with lung cancer. Cancer Immunol Immunother (2018) 67(8):1231–8. doi: 10.1007/s00262-018-2180-6
62. Zhang M, Yang W, Wang P, Deng Y, Dong YT, Liu FF, et al. CCL7 recruits cDC1 to promote antitumor immunity and facilitate checkpoint immunotherapy to non-small cell lung cancer. Nat Commun (2020) 11(1):6119. doi: 10.1038/s41467-020-19973-6
63. Rahim MK, Okholm TLH, Jones KB, McCarthy EE, Liu CC, Yee JL, et al. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell (2023) 186(6):1127–43.e18. doi: 10.1016/j.cell.2023.02.021
64. Zheng L, Song P, Jiang Y, Fan X, Yang C, Zhang L, et al. Outcomes and quality of life after robot-assisted lobectomy/segmentectomy for lung cancer compared to video-assisted thoracoscopic surgery: both three-port procedures performed by a single surgeon. J Thorac Dis (2022) 14(3):689–98. doi: 10.21037/jtd-22-238
65. Heiden BT, Mitchell JD, Rome E, Puri V, Meyers BF, Chang SH, et al. Cost-effectiveness analysis of robotic-assisted lobectomy for non-small cell lung cancer. Ann Thorac Surg (2022) 114(1):265–72. doi: 10.1016/j.athoracsur.2021.06.090
66. Chen D, Kang P, Tao S, Li Q, Wang R, Tan Q. Cost-effectiveness evaluation of robotic-assisted thoracoscopic surgery versus open thoracotomy and video-assisted thoracoscopic surgery for operable non-small cell lung cancer. Lung Cancer (2021) 153:99–107. doi: 10.1016/j.lungcan.2020.12.033
67. Pan X, Gu C, Yang J, Shi J. Robotic double-sleeve resection of lung cancer: technical aspects. Eur J Cardiothorac Surg (2018) 54(1):183–4. doi: 10.1093/ejcts/ezy070
68. Gu C, Pan X, Chen Y, Yang J, Zhao H, Shi J. Short-term and mid-term survival in bronchial sleeve resection by robotic system versus thoracotomy for centrally located lung cancer. Eur J Cardiothorac Surg (2018) 53(3):648–55. doi: 10.1093/ejcts/ezx355
69. Pan X, Gu C, Wang R, Zhao H, Shi J, Chen H. Initial experience of robotic sleeve resection for lung cancer patients. Ann Thorac Surg (2016) 102(6):1892–7. doi: 10.1016/j.athoracsur.2016.06.054
70. Abdelsattar ZM, Shen KR, Yendamuri S, Cassivi S, Nichols FC 3rd, Wigle DA, et al. Outcomes after sleeve lung resections versus pneumonectomy in the united states. Ann Thorac Surg (2017) 104(5):1656–64. doi: 10.1016/j.athoracsur.2017.05.086
71. Chen J, Soultanis KM, Sun F, Gonzalez-Rivas D, Duan L, Wu L, et al. Outcomes of sleeve lobectomy versus pneumonectomy: a propensity score-matched study. J Thorac Cardiovasc Surg (2021) 162(6):1619–28.e4. doi: 10.1016/j.jtcvs.2020.08.027
72. Yang M, Zhong Y, Deng J, She Y, Zhang L, Wang Y, et al. Comparison of bronchial sleeve lobectomy with pulmonary arterioplasty versus pneumonectomy. Ann Thorac Surg (2022) 113(3):934–41. doi: 10.1016/j.athoracsur.2021.04.007
73. Maurizi G, D'Andrilli A, Anile M, Ciccone AM, Ibrahim M, Venuta F, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol (2013) 8(5):637–43. doi: 10.1097/JTO.0b013e318286d145
74. Jeon YJ, Choi YS, Cho JH, Kim HK, Kim J, Zo JI, et al. Thoracoscopic vs open surgery following neoadjuvant chemoradiation for clinical N2 lung cancer. Semin Thorac Cardiovasc Surg (2022) 34(1):300–8. doi: 10.1053/j.semtcvs.2021.01.002
Keywords: non-small cell lung cancer, robot-assisted thoracoscopic surgery, video-assisted thoracoscopic surgery, neoadjuvant immunochemotherapy, perioperative outcomes, recurrence-free survival
Citation: Pan H, Zou N, Tian Y, Zhu H, Zhang J, Jin W, Gu Z, Ning J, Li Z, Kong W, Jiang L, Huang J and Luo Q (2023) Short-term outcomes of robot-assisted versus video-assisted thoracoscopic surgery for non-small cell lung cancer patients with neoadjuvant immunochemotherapy: a single-center retrospective study. Front. Immunol. 14:1228451. doi: 10.3389/fimmu.2023.1228451
Received: 24 May 2023; Accepted: 27 June 2023;
Published: 11 July 2023.
Edited by:
Fabrizio Minervini, University of Lucerne, SwitzerlandReviewed by:
Wenxiong Zhang, Second Affiliated Hospital of Nanchang University, ChinaYunlang She, Tongji University, China
Runsen Jin, Shanghai Jiao Tong University, China
Qingyuan Huang, Fudan University, China
Copyright © 2023 Pan, Zou, Tian, Zhu, Zhang, Jin, Gu, Ning, Li, Kong, Jiang, Huang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingquan Luo, bHVvcWluZ3F1YW5AaG90bWFpbC5jb20=; Jia Huang, aHVhbmdqaWFkcmFnb25AMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Hanbo Pan
Hanbo Pan Ningyuan Zou1†
Ningyuan Zou1† Yu Tian
Yu Tian Junwei Ning
Junwei Ning Long Jiang
Long Jiang Jia Huang
Jia Huang Qingquan Luo
Qingquan Luo