
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 20 September 2023
Sec. Viral Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1227905
Background: Nirmatrelvir has been authorized for the treatment of both hospitalized and non-hospitalized COVID-19 patients. However, the association between T lymphocyte subsets and the outcome of hospitalized COVID-19 patients treated with oral Nirmatrelvir has not been investigated. The objective of this study was to examine whether lymphocyte subsets could serve as biomarkers to assess the risk of mortality in COVID-19 patients undergoing Nirmatrelvir treatment, with the aim of enhancing medication management for COVID-19 patients.
Methods: We conducted a retrospective cohort study at the Xiangya Hospital of Central South University in China between December 5, 2022 and January 31, 2023. The study reported demographic, clinical, T lymphocyte subsets, and inflammatory cytokine data of COVID-19 patients. We evaluated the associations of T lymphocyte subsets on admission with the composite outcome or death of patients using univariate and multivariable Cox regression analyses with hazards ratios (HRs) and 95% confidence intervals (CIs).
Results: We identified 2118 hospitalized COVID-19 patients during the study period, and conducted a follow-up of up to 38 days. Of these, 131 patients received Nirmatrelvir, with 56 (42.7%) in the composite outcome group, and 75 (57.3%) in the non-composite outcome group. Additionally, 101 (77.1%) patients were discharged, while 30 (22.9%) died. Our results showed a significant decrease in the CD3+, CD4+, and CD8+ T cell counts of patients in the composite outcome group and mortality group compared to the non-composite outcome group and discharged group, respectively. Multivariate Cox regression analysis showed that the significant decrease in CD8+ T cell count in peripheral blood was independently associated with the composite outcome in COVID-19 patients treated with Nirmatrelvir, with an HR of 1.96 (95%CI: 1.01-3.80). The significant decrease in CD4+ and CD8+ T cell counts in peripheral blood increased the hazard of developing mortality, with HRs of 6.48 (95%CI: 1.47-28.63) and 3.75 (95%CI: 1.27-11.11), respectively.
Conclusion: Our study revealed a significant positive correlation between a decrease in CD8+ T cell counts and progression and mortality of hospitalized COVID-19 patients treated with Nirmatrelvir. Lower counts (/μL) of CD8+ T cell (<201) were associated with a higher risk of in-hospital severity and death. Our findings may provide valuable references for physicians in optimizing the use of Nirmatrelvir.
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) is regarded as one of the most critical threats to human health and the global economy. Since its first report in 2019, its impact will continue as the Omicron strain mutates and spreads (1). Although COVID-19 initially invades the respiratory system, neurological manifestations have been reported in most patients, including anosmia, ageusia, headache, fatigue, myalgia, dizziness, and psychiatric disorders (2, 3). However, it is still necessary to understand whether the clinical manifestation of COVID-19 patients will change as Omicron mutates. Current evidence shows that COVID-19 infection induces a rapidly coordinated immune response and subsequent inflammatory cytokine storm, which is associated with disease progression (3–5).
Several studies have confirmed the impaired immune system caused by COVID-19 and a significant reduction of lymphocytes, especially a decrease of B cells, natural killer (NK) cells, CD3+ T cells, CD4+ T cells and CD8+ T cells in peripheral blood examination (3, 6–8). At the beginning of the COVID-19 outbreak, some early studies suggested that T lymphocyte cells and their subsets could predict disease severity and mortality (4, 8, 9). Cantenys‐Molina et al. (10) suggested that decreased CD4+ and CD8+T cell counts and expansion of NK lymphocytes proportion at admission were prognostic factors for COVID-19 patients. Mirsharif et al. (3) included 100 COVID-19 patients compared to 70 healthy controls to identify biomarkers for assigning the risk of mortality, and demonstrated that CD8+ HLA‐DR+ T cells count significantly decreased in severe patients and may be the best biomarker for mortality outcome. However, it is unclear whether T lymphocyte subsets can predict the outcome of COVID-19 patients who are treated with antiviral drugs.
Nirmatrelvir is an oral antiviral drug that can effectively inhibit the SARS-CoV-2 3-chymotrypsin–like cysteine protease enzyme (11). Nirmatrelvir has been authorized for COVID-19 patients in many countries worldwide (12, 13). Current clinical evidence suggests that Nirmatrelvir can reduce the risk of 28-days hospitalization or death of outpatients, while showing a well-curative effect in hospitalized patients (12–14). However, the supply of Nirmatrelvir cannot meet the global COVID-19 patients’ demand, and it needs to be used for the most suitable patients. Therefore, in this retrospective study, we focus on the clinical manifestations and immunophenotyping characteristics of COVID-19 patients in the context of the prevalence of Omicron variants. Additionally, we aimed to investigate the potential use of lymphocyte subsets as biomarkers to evaluate the risk of mortality in COVID-19 patients who were undergoing Nirmatrelvir treatment.
We performed a retrospective, single-center cohort study at Xiangya hospital from December 5, 2022 to January 31, 2023. The study included hospitalized patients with a positive RT-PCR for SARS-CoV-2 infection who received Nirmatrelvir treatment and underwent peripheral blood T lymphocyte subset testing via flow cytometry. Participants under the age of 18, those who received antiviral agents other than Nirmatrelvir, or those who received non-invasive or invasive respiratory support upon admission were excluded. The Xiangya Hospital Institutional Review Committee (202002024) approved our research, and all participants in the retrospective cohort study remained anonymous with no need for individual informed consent.
We retrieved electronic health records of COVID-19 patients from the inpatient system of Xiangya Hospital, which included demographic characteristics, admission date, time from symptom onset to admission, time from symptom onset to treatment exposure (within or beyond 5 days), pre-existing conditions, prescription and drug dispensing records, laboratory tests, ICU admission, and date of discharge or death. Collected data were recorded consecutively until the planned time point.
The primary outcome was a composite outcome of disease progression including non-invasive respiratory support, initiation of endotracheal intubation, ICU admission and all-cause death. The secondary outcome was each of these individual disease progression outcomes. Patient outcomes were recorded from the date of admission to the occurrence of outcome events, the discharge date, or the date of death, whichever came first.
Continuous variables were presented as median and interquartile range (IQR) and analyzed using the Mann-Whitney test, as most laboratory data had a skewed distribution. Categorical variables were presented as counts and proportions and analyzed using the Chi-square test or Fisher’s exact test. The univariate Cox regression model was used to estimate a HR with a 95% confidence interval (CI) for each result between the groups. The multivariable Cox regression model was then used to control for the impact of confounding variables, including gender, age, comorbidities, and severity at admission. The reference range for normal T lymphocyte subsets was based on the standard of Xiangya Hospital (15). All statistical analyses were performed with SPSS (version 26.0, IBM), and R (version 4.2.1). The level of significance was two-tailed 0.05 for statistical tests.
Data of 2118 hospitalized patients with confirmed diagnosis of SARS-CoV-2 infection were consecutively collected, and followed up for 38 days. Following the inclusion and exclusion criteria, a total of 131 Nirmatrelvir recipients were enrolled in our cohort (Figure 1). Table 1 showed the demographic and clinical characteristics of patients on admission. Of the enrolled patients, 94 (71.8%) were male and 37 (28.2%) were female with 85 (64.9%) patients aged 65 years or older. Based on disease progression, we divided the COVID-19 patients into two groups - composite outcome (n = 56, 42.7%) and non-composite outcome (n = 75, 57.3%). We found significant differences in gender and age between the two groups (P = 0.031 and P<0.001, respectively). The common clinical symptoms of COVID-19 patients were fever (61.8%), dry cough (86.3%), expectoration (75.6%), poor appetite (48.1%), polypnea (45.8%), fatigue (33.6%), stuffiness (18.3%), myalgia (19.8%), dyspnea (11.5%), and headache (7.6%). On admission, the dry cough, expectoration, and myalgia rates were significantly higher in the non-composite outcome group than in the composite outcome group (P = 0.027, P = 0.003, and P = 0.024, respectively), while the dyspnea rate was lower in the composite outcome group (P = 0.047). Patients with preexisting conditions mainly included hypertension (50.4%), diabetes mellitus (30.5%), coronary disease (26.0%), cancer (13.7%), and chronic obstructive pulmonary disease (9.9%). The rate of patients with hypertension on admission was higher in the composite outcome group than in the no composite outcome group (P = 0.002). However, there was no significant difference in admission severity between the two groups (P = 0.085). Based on medical records, 38 (29.0%) patients received systemic steroid treatment, including 22 (39.3%) in the composite outcome group and 16 (21.3%) in the non-composite outcome group. In addition, 61 (46.6%) patients received antibiotic therapy, and no significant differences were found between the two groups.
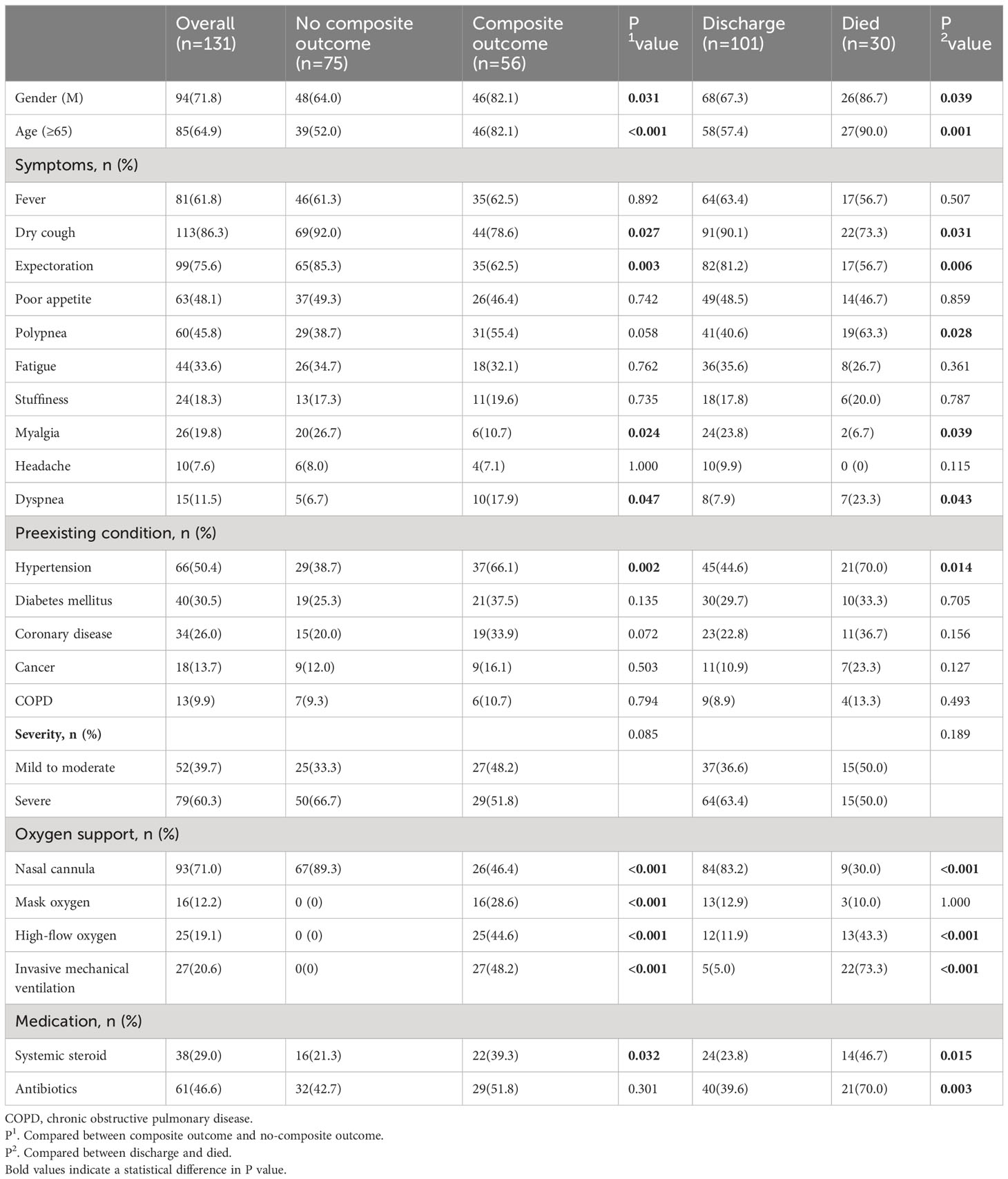
Table 1 Characteristics of included patients (overall and categorized by disease progression and final outcome).
Patients were categorized into two groups based on their final outcome - discharged (n = 101, 77.1%) or mortality (n = 30, 22.9%). The rate of male and age in the mortality group were significantly higher than in the discharge group (P = 0.039 and P = 0.001, respectively). Furthermore, the common symptom of polypnea and dyspnea was more frequent in the mortality group compared to the discharged group. Of the patients who received oxygen therapy, 93 (71.0%) patients received nasal cannula, 16 (12.2%) patients received mask oxygen, 12 (11.9%) patients received high-flow oxygen, and 5 (5.0%) patients received invasive mechanical ventilation. The rate of receiving high-flow oxygen (43.3%) and invasive mechanical ventilation (73.3%) was significantly higher in the mortality group than in the discharged group. Meanwhile, systemic steroid and antibiotic treatment were more frequent in the mortality group.
Table 2 presents detailed characteristics of immune cells. We found that the counts of white blood cells (WBC) and neutrophils were higher in the composite outcome group than in the non-composite outcome group, but the lymphocyte count was lower in the composite outcome group (P < 0.001). Compared with the mortality group, the counts of WBC and neutrophils were lower in the discharged group (P < 0.001), but the lymphocyte count was higher (P = 0.002). Additionally, we found that the levels of T lymphocyte subsets varied according to disease progression and final outcome (Table 2). There was a significant decrease in CD3+ T cell, CD4+ T cell, and CD8+ T cell counts in the composite outcome group (Figure 2A) and mortality group (Figure 2B) compared to the non-composite outcome group and discharged group, respectively. Moreover, we detected the concentration of C-reactive protein (CRP), interleukin-6 (IL-6), and interleukin-10 (IL-10) cytokines produced by T cells in plasma. We found that the concentration of CRP and IL-10 was significantly higher in the composite outcome group (P < 0.01). Notably, the median concentration of CRP, IL-6, and IL-10 in the mortality group was over 2-fold higher than in the discharged group.
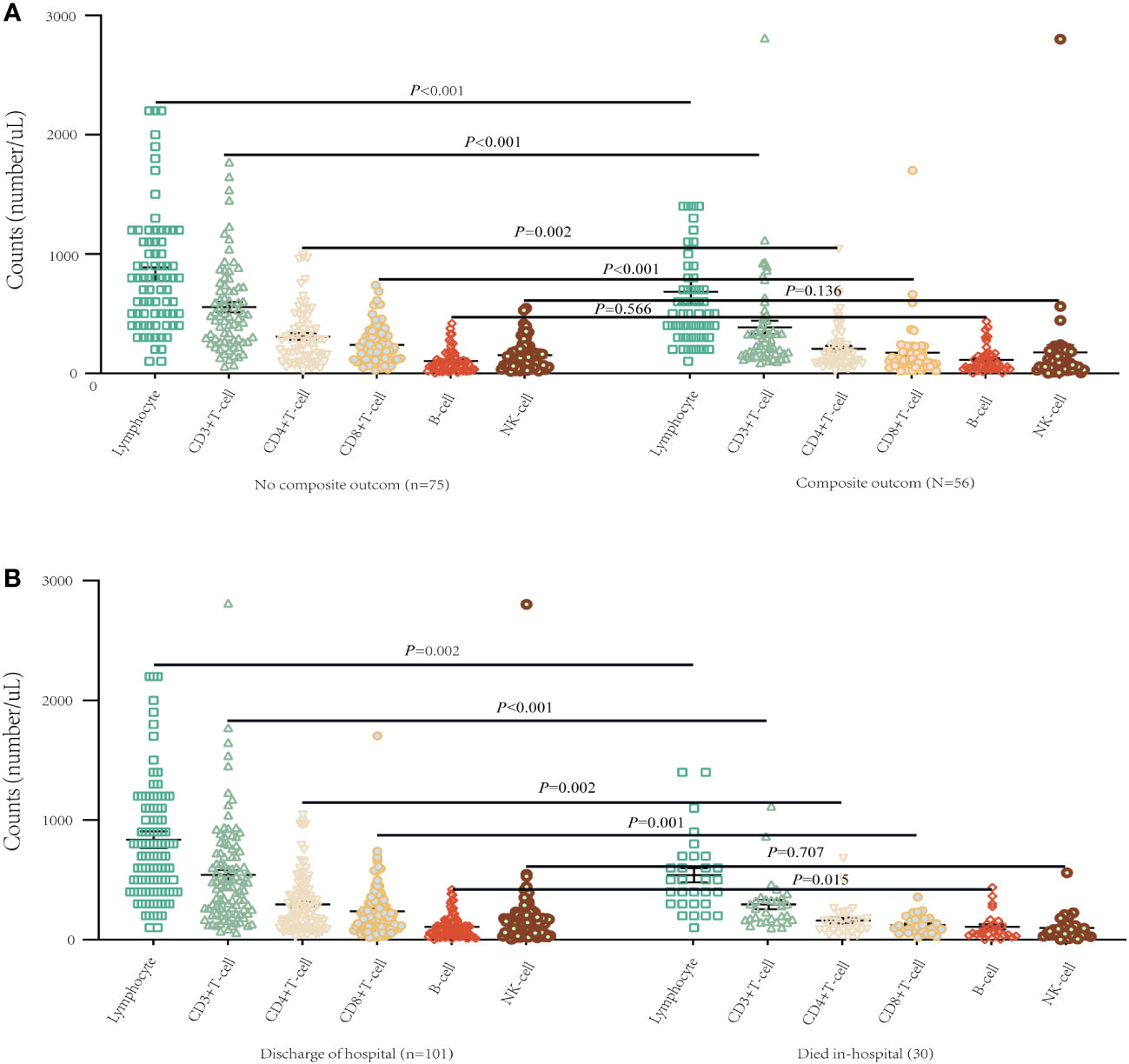
Figure 2 T lymphocyte subsets of different progression of illness and survival conditions in patients on admission with COVID-19. (A) Differences of T lymphocyte subsets among composite outcome and no composite outcome-ill patients (mean with SD). (B) Differences of T lymphocyte subsets between survivor and non-survivor (mean with SD).
We further explored the association of different lymphocyte subsets with disease progression and final outcome in hospitalized COVID-19 patients treated with Nirmatrelvir. The results of univariate Cox regression were shown in Table 3. We found that the risk of in-hospital composite outcome was statistically higher in those who had lower counts of CD8+ T cells (HR = 2.54, 95%CI: 1.34-4.84). Moreover, the lower counts of CD4+ T cells and CD8+ T cells were significantly associated with in-hospital death (HR: 4.61; 95%CI: 1.10-19.38, HR: 3.90; 95%CI: 1.36-11.19, respectively). Furthermore, multivariate Cox regression analysis revealed that a significant decrease in CD8+ T cell count in peripheral blood was independently associated with composite outcome in COVID-19 patients treated with Nirmatrelvir, with an HR of 2.01 (95%CI: 1.03-3.90), adjusted for age, gender, comorbidities, severity at admission and the Nirmatrelvir treatment within or beyond 5 days of symptom onset (Table 4). We also found that a significant decrease in CD4+ T cell and CD8+ T cell count in peripheral blood increased the hazard of developing mortality (HR: 6.05; 95%CI: 1.39-26.29, HR: 3.61; 95%CI: 1.22-10.63, respectively), adjusted for age, gender, comorbidities, severity at admission and Nirmatrelvir treatment within or beyond 5 days of symptom onset (Table 4). Figure 3 represents the cumulative risk of developing composite outcome and mortality in COVID-19 patients treated with Nirmatrelvir, whose peripheral blood CD4+ T cell and CD8+ T cell counts decreased significantly at admission.
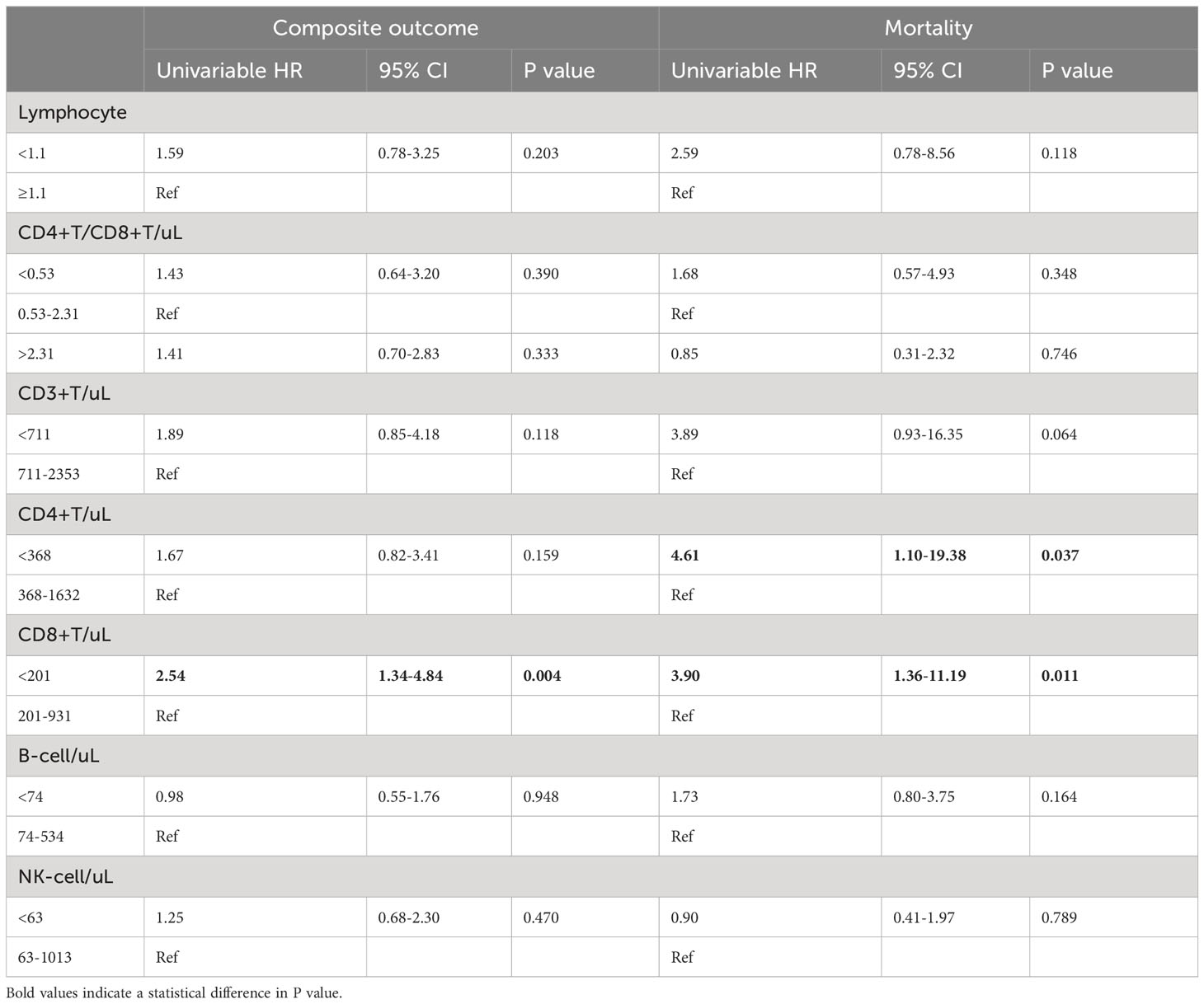
Table 3 Comparison of the distribution of lymphocyte subsets as risk factors of clinical outcome for patients with COVID‐19.
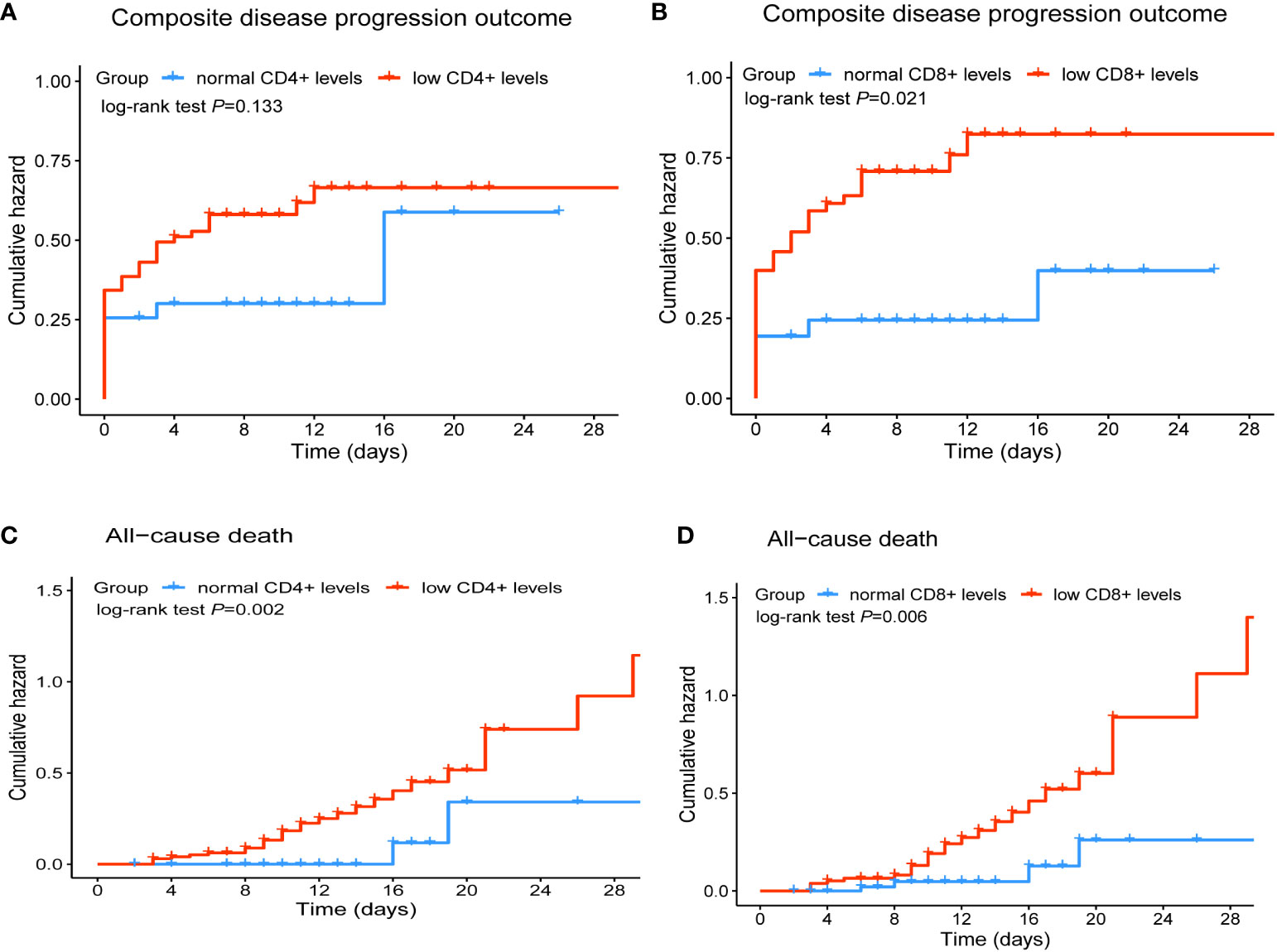
Figure 3 Cumulative incidence of composite disease progression outcome (A, B) and all-cause death (C, D) of COVID-19 patients for lower CD4+ and CD8+ T cell counts versus normal CD4+and CD8+ T cell counts.
This retrospective study investigated hospitalized patients during the Omicron variant epidemic. We analyzed the demographic, immunophenotype, and clinical characteristics of 131 COVID-19 patients treated with Nirmatrelvir. Neurological manifestations such as anosmia, ageusia, and psychiatric disorders were not common in enrolled patients. It may also be the widespread of full vaccination, which protects the nervous system from Omicron damage. A recent study shows that children who are not fully vaccinated may develop omicron-related neurological complications (16). In composite-outcome patients, the median counts of lymphocytes decreased, and CD3+ T cell, CD4+ T cell, and CD8+T cell counts were almost decreased to half of median counts of non-composite-outcome patients. Similar results were observed in patients who died in the hospital compared to discharged patients. Meanwhile, inflammatory cytokines were significantly higher in patients with composite outcome and death than in patients with non-composite outcome and discharge, respectively. The median concentrations of CRP, IL-6, and IL-10 in died patients were more than two-fold of that of discharged patients. Previous evidence has shown that lymphocyte subsets significantly reduced, including CD3+T cells, CD4+T cells, and CD8+T cells, B cells, NK cells, and cytokine storms such as CRP are common in COVID-19 patients (8, 10, 17). In this study, we explored the association of peripheral blood T-lymphocyte subsets with the prognosis of COVID-19 patients treated with Nirmatrelvir. We found that lower counts (/μL) of T lymphocyte subsets CD8+T cells (<201) were associated with a higher risk of composite outcome, and lower counts (/μL) of CD4+T cells (<368) and CD8+T cells (<201) were significantly associated with the death outcome of COVID‐19 patients with Nirmatrelvir therapy. Identifying biomarkers that predict the curative effect of Nirmatrelvir may help physicians conduct evidence-based treatments for COVID-19.
The immune response is closely related to the pathogenesis, progression, and prognosis of COVID-19 patients, especially the activation of adaptive immune function (18). CD4+ and CD8+ T cells are the most basic components of adaptive immunity, with various helper and effector functionalities and the ability to kill infected cells respectively (7). Previous studies on CD4+ and CD8+ T cells in COVID-19 patients have mostly focused on the progression and prognosis of the disease from mild to severe. However, there are no reports on CD4+ and CD8+T cells regarding progression and mortality for COVID-19 patients who treated with Nirmatrelvir. One study on 701 COVID-19 patients reported that the counts of CD4+ T cells (≤500) and CD8+ T cells (100) were significantly associated with mortality (10). The earliest study by Du et al. showed a significant decrease in 21 deceased patients compared with 158 survivors, and when CD8+T cells reduced (≤75), the risk of death increased more than five-fold (9). Xu et al. in the evaluation of 187 hospitalized patients with COVID‐19, reported that the counts of total lymphocytes, CD3+ T cells, CD4+ T cells, CD8+ T cells, B cells, and NK cells decreased significantly. And the sensitivity analysis indicated that the count of lymphocytes (<500), CD3+ T cells (<100), CD4+ T cells (<100), CD8+ T cells (<100), and B cells (<50), were risk factors for COVID-19 patients’ death (8). We note that several studies reported the significant decrease in CD4+ T cells, CD8+ T cells of COVID-19 patients with increasing disease severity (17, 19, 20).
Our study also has some limitations. Firstly, we conducted a single-center retrospective study in Hunan province, limiting the generalizability of our findings. Future studies on the association of T lymphocyte subsets with the prognosis of COVID-19 patients in different regions and ethnicities should be carried out. Secondly, the supply of other antiviral drugs was insufficient, and hence we only evaluated the association between T lymphocyte subsets and prognosis in COVID-19 patients treated with Nirmatrelvir. Thirdly, our population of COVID-19 patients was mainly composed of females (71.8%), which may not be sufficient to predict the prognosis of Nirmatrelvir in male patients. Fourth, the absence of data before and after the administration of Nirmatrelvir may limit the ability to fully assess its impact on lymphocyte subsets as biomarkers. Finally, due to the small sample size, we did not perform ROC curve analysis to confirm the warning values of T lymphocytes. To our best knowledge, we are the first to explore the association of T lymphocyte subsets with the prognosis of COVID-19 patients treated with Nirmatrelvir. Flow cytometry is a quick and convenient method to detect peripheral blood lymphocyte subsets for hospitalized COVID-19 patients, which could help to identify the most suitable patients treated with Nirmatrelvir.
In summary, our study reveals a significant correlation between decreased CD8+ T cell counts and in-hospital progression and mortality of COVID-19 patients treated with Nirmatrelvir. Specifically, patients with lower CD8+ T cell counts (/μL < 201) exhibited a higher risk of in-hospital severity and death. These findings may provide valuable references for physicians in optimizing the use of Nirmatrelvir.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The Xiangya Hospital Institutional Review Committee (202002024) approved this research, and all participants in the retrospective cohort study remained anonymous with no need for individual informed consent.
Conception and design: GD and QG. Acquisition of data: YS and YD. Interpretation of data, statistical analysis and manuscript writing: YS and GD. Revision of manuscript and administrative, technical, or material support: GD, QG, YS, and YD. All authors contributed to the article and approved the submitted version.
We thank all the fundings supported by the National Natural Science Foundation of China (Grant Nos. 82102803, 82272849 to GD), National Natural Science Foundation of Hunan Province (Grant Nos. 2021JJ40976 to GD). We also thank all the hospital staff members for their efforts in collecting the information that used in this study; thank the patients who participated in this study, their families, and the medical, nursing, and research staff at Xiangya hospital of Central South University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li L, Taeihagh A, Tan SY. A scoping review of the impacts of COVID-19 physical distancing measures on vulnerable population groups. Nat Commun (2023) 14(1):599. doi: 10.1038/s41467-023-36267-9
2. Hensley MK, Markantone D, Prescott HC. Neurologic manifestations and complications of COVID-19. Annu Rev Med (2022) 73:113–27. doi: 10.1146/annurev-med-042320-010427
3. Mirsharif ES, Chenary MR, Bozorgmehr M, Mohammadi S, Hashemi SM, Ardestani SK, et al. Immunophenotyping characteristics of COVID-19 patients: Peripheral blood CD8+ HLA-DR+ T cells as a biomarker for mortality outcome. J Med Virol (2023) 95(1):e28192. doi: 10.1002/jmv.28192
4. He R, Lu Z, Zhang L, Fan T, Xiong R, Shen X, et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol (2020) 127:104361. doi: 10.1016/j.jcv.2020.104361
5. Wei LL, Wang WJ, Chen DX, Xu B. Dysregulation of the immune response affects the outcome of critical COVID-19 patients. J Med Virol (2020) 92(11):2768–76. doi: 10.1002/jmv.26181
6. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest (2020) 130(5):2620–9. doi: 10.1172/JCI137244
7. Sette A, Crotty S. Adaptive immunity to SARS-coV-2 and COVID-19. Cell (2021) 184(4):861–80. doi: 10.1016/j.cell.2021.01.007
8. Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect (2020) 81(1):e51–60. doi: 10.1016/j.jinf.2020.04.012
9. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J (2020) 55(5):2000524. doi: 10.1183/13993003.00524-2020
10. Cantenys-Molina S, Fernandez-Cruz E, Francos P, Lopez Bernaldo de Quiros JC, Munoz P, Gil-Herrera J. Lymphocyte subsets early predict mortality in a large series of hospitalized COVID-19 patients in Spain. Clin Exp Immunol (2021) 203(3):424–32. doi: 10.1111/cei.13547
11. Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science (2021) 374(6575):1586–93. doi: 10.1126/science.abl4784
12. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med (2022) 386(15):1397–408. doi: 10.1056/NEJMoa2118542
13. Wan EYF, Yan VKC, Mok AHY, Wang B, Xu W, Cheng FWT, et al. Effectiveness of molnupiravir and nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A target trial emulation study. Ann Intern Med (2023) 176(4):505–14. doi: 10.7326/M22-3057
14. Cao Z, Gao W, Bao H, Feng H, Mei S, Chen P, et al. VV116 versus nirmatrelvir-ritonavir for oral treatment of Covid-19. N Engl J Med (2023) 388(5):406–17. doi: 10.1056/NEJMoa2208822
15. Jiao Y, Qiu Z, Xie J, Li D, Li T. Reference ranges and age-related changes of peripheral blood lymphocyte subsets in Chinese healthy adults. Sci China C Life Sci (2009) 52(7):643–50. doi: 10.1007/s11427-009-0086-4
16. Tso WW, Kwan MY, Kwok JS, Tsang JO, Yip CC, Leung LK, et al. Clinical characteristics of unvaccinated or incompletely vaccinated children with neurological manifestations due to SARS-CoV-2 Omicron infection. J Med Virol (2023) 95(7):e28895. doi: 10.1002/jmv.28895
17. Bobcakova A, Petriskova J, Vysehradsky R, Kocan I, Kapustova L, Barnova M, et al. Immune profile in patients with COVID-19: lymphocytes exhaustion markers in relationship to clinical outcome. Front Cell Infect Microbiol (2021) 11:646688. doi: 10.3389/fcimb.2021.646688
18. Yan J, Wang J, Ding L, Liu S, Zhan Y, Lu J, et al. Adaptive immune dysfunction in patients with COVID-19 and impaired kidney function during the omicron surge. Clin Immunol (2023) 248:109271. doi: 10.1016/j.clim.2023.109271
19. Allardet-Servent J, Ait Belkacem I, Miloud T, Benarous L, Galland F, Halfon P, et al. The association of low CD4 expression on monocytes and low CD8+ T-cell count at hospital admission predicts the need for mechanical ventilation in patients with COVID-19 pneumonia: A prospective monocentric cohort study. Crit Care Explor (2022) 4(12):e0810. doi: 10.1097/CCE.0000000000000810
Keywords: COVID-19, lymphocyte, T cell, biomarker, CD8+ T cell
Citation: Sun Y, Dian Y, Gao Q and Deng G (2023) Immunophenotyping characteristics and outcome of COVID‐19 patients: peripheral blood CD8+T cell as a prognostic biomarker for patients with Nirmatrelvir. Front. Immunol. 14:1227905. doi: 10.3389/fimmu.2023.1227905
Received: 24 May 2023; Accepted: 01 September 2023;
Published: 20 September 2023.
Edited by:
Pei-Hui Wang, Shandong University, ChinaReviewed by:
Furong Qi, Shenzhen Third People’s Hospital, ChinaCopyright © 2023 Sun, Dian, Gao and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangtong Deng, ZGVuZ2d1YW5ndG9uZ0BvdXRsb29rLmNvbQ==; Qian Gao, Z2FvcWlhbkBjc3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.