- 1Department of Dermatology, Shanghai Skin Disease Hospital, Tongji University School of Medicine, Shanghai, China
- 2Institute of Psoriasis, Tongji University School of Medicine, Shanghai, China
Autoimmune bullous disease (AIBD) is a severe skin disorder caused by autoantibodies that target intercellular or cell-matrix adhesion proteins. Currently, the preferred treatment for AIBD involves the use of glucocorticoids or traditional immunosuppressants. Additionally, the utilization of biological agents such as rituximab, omalizumab, and dupilumab is on the rise. However, effectively managing AIBD remains a challenge. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway has been implicated in various inflammatory diseases. In recent years, a range of drugs known as JAK inhibitors, which target this pathway, have been developed. Several studies have explored the efficacy and safety of JAK inhibitors for treating AIBD. Consequently, this review begins by examining the role of the JAK/STAT pathway in AIBD, summarizing the application of different JAK inhibitors in AIBD treatment, and emphasizing the importance of disease management in treating AIBD with JAK inhibitors. Furthermore, it highlights the need for a better understanding of the JAK/STAT pathway’s role in AIBD, as well as the effectiveness and safety of JAK inhibitors for treating this disease.
1 Introduction
Autoimmune bullous diseases (AIBDs) are rare conditions characterized by erosion, blistering, and bullous lesions on the skin and mucous membranes. These diseases arise from the production of autoantibodies that target proteins responsible for maintaining cell-to-cell and cell-to-matrix adhesion (1, 2). AIBDs can be categorized into two groups: pemphigus disease (intraepidermal immunobullous disease) and pemphigoid disease (subepidermal immunobullous disease). Pemphigus diseases encompass pemphigus vulgaris (PV), pemphigus foliaceus (PF), paraneoplastic pemphigus (PNP), immunoglobulin A (IgA) pemphigus, and others. Pemphigoid diseases include bullous pemphigoid (BP), epidermolysis bullosa acquisita (EBA), anti-lamininγ1 pemphigoid, mucous membrane pemphigoid (MMP), dermatitis herpetiformis (DH), linear IgA bullous dermatosis, pemphigoid gestationis, lichen planus pemphigoid (LPP), and bullous systemic lupus erythematosus (BSLE).
Since the availability of glucocorticoids and, later, non-steroidal immunosuppressive drugs, from the late 1960s, the mortality rate of AIBD patients has significantly decreased. However, as critical dermatological diseases, AIBDs are still associated with a high mortality rate (3, 4). In recent years, treatment modalities have gradually shifted toward more precise and targeted immune suppression and/or immune regulation. In particular, immunotherapy with biologics, such as rituximab, dupilumab, and omalizumab, is being clinically applied to treat various types of AIBDs with relatively good efficacy (5–9). Nevertheless, despite the availability of various treatment options, including glucocorticoids, traditional immunosuppressive drugs, and biological reagents, some patients still exhibit poor treatment responses or experience serious drug-related complications. Hence, there is a need for new therapeutic approaches that offer improved efficacy and fewer adverse effects (AEs).
The Janus kinase/signal transducers and activators of the transcription (JAK/STAT) pathway is vital for immune regulation, cell differentiation, apoptosis, and proliferation, affecting various cytokines and growth factors (10). This signaling pathway is associated with the pathophysiology of several autoimmune and autoinflammatory diseases. Therefore, blocking the JAK/STAT pathway has become an attractive approach for treating these diseases. In recent years, a variety of JAK pathway inhibitors have been used for the treatment of skin diseases such as psoriasis (11), alopecia areata (12), and atopic dermatitis (13). Since the JAK/STAT pathway is thought to play a role in the development of AIBDs, inhibiting this pathway could be a promising therapeutic strategy for these diseases.
This review aims to provide a concise overview of the potential involvement of the JAK/STAT pathway in the development of AIBDs and list the reported JAK inhibitors that have been used to treat AIBD. This information will serve as a foundation for further research on the pathogenesis, clinical diagnosis, and treatment of AIBDs.
2 The JAK/STAT pathway
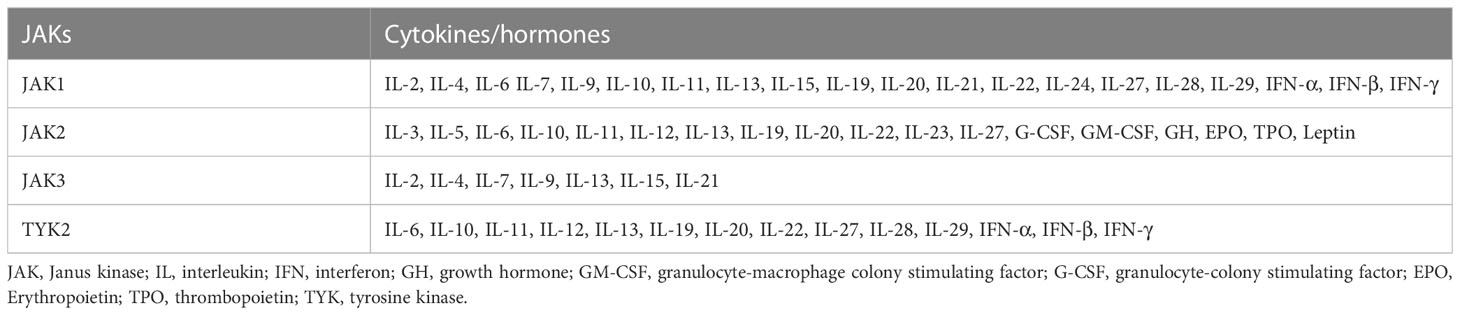
To the best of our knowledge, only JAK inhibitors have been reported for the treatment of AIBD, while STAT inhibitors have not been similarly reported. Therefore, we will just summarize the function of JAK proteins. The JAK family comprises four proteins called TYK2, JAK1, JAK2, and JAK3 (14). JAK-dependent cytokine receptors transmit signals through various JAKs (Table 1), with each receptor having multiple subunits associated with JAK. A primary function of protein kinases is to transfer phosphate groups from guanosine triphosphate or adenosine triphosphate to amino acid hydroxyl groups (15). This fundamental mechanism is essential for receptors that lack intrinsic enzyme activity to facilitate biological processes. As a non-receptor tyrosine protein kinase, JAK can also conduct signal transduction through this mechanism. Typically, cytokine binding to their receptors triggers inflammatory signals. Receptors of type I and type II cytokines, including interferon (IFN), interleukin (IL)-2, IL-6, IL-12, IL-23, etc., are intrinsically deficient in enzyme activity and highly dependent on JAK for signal transduction (16). The JAKs initiate signaling pathways in the cell membrane that ultimately reach the nucleus after cytokines bind to their receptors (17). As part of this reaction, type I and II cytokine receptors undergo oligomerization to recruit JAKs and phosphorylate tyrosine residues, including tyrosine residues within the receptor chain. STAT proteins are then recruited, regulating the expression of related genes, resulting in antibody production, lymphocyte differentiation, increased inflammation, blister formation, and other pathophysiological processes.
3 The JAK/STAT pathway in AIBD
Multiple immune mechanisms, including cellular and humoral immunity, contribute to the development of pemphigus diseases. Researches have demonstrated that serum levels of tumor necrosis factor-α (TNF-α) and several type I and II cytokines, such as IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, and IL-15, are increased in patients with PV, while levels of IFN-γ are decreased (18–20). Furthermore, desmoglein (Dsg)3-reactive helper T cell (Th)1 and Th2 cells are found in patients with pemphigus disease. The presence of immunoglobulin (Ig)G4 and IgG1 antibodies against Dsg3 is directly related to the ratio of Dsg3 reactive Th1/Th2 cells (21). Thus, both Th1 and Th2 cells play an integral role in the pathogenesis of PV. Th1 cells overexpress IFN-γ to further mediate the immune response, while IL4, as a Th2-derived cytokine, regulates immunoglobulin conversion and antibody production by stimulating B cell proliferation (22). Cytokines such as IFN and IL-4 have been shown to play a biological role through JAK proteins. JAK1, JAK3, STAT6, and IL-4 signaling pathways are also important for the differentiation and proliferation of helper T cells (18). These discoveries suggest that the JAK/STAT signaling pathway plays a significant role in the pathogenesis of pemphigus diseases.
BP, a subepidermal form of AIBDs, is associated with autoantibodies targeting hemidesmosome components BP180 and BP230 (1). Studies using immunohistochemistry and western blotting analysis have shown that the expression of JAK/STAT proteins is significantly higher in BP-associated skin lesions compared to surrounding skin and healthy individuals (23). Furthermore, an increasing number of studies have demonstrated that Th2 cells play an important role in the production of antibodies in BP. It is worth noting that the chemotactic attraction of the Th2-like cytokine IL-4 towards eosinophils is very strong. IL-4 also takes part in the maturation and functional activity of eosinophils, as well as antibody production and the autoimmune response to BP (24, 25). The role of IL-17 in BP progression is evidenced by its upregulation of proteases involved in blister formation such as matrix metalloproteinase 9 and neutrophil elastase (26). The JAK signaling pathway has been proven to be closely related to the function of IL-4 and IL-17 (27, 28), which warrants further exploration of the role of the JAK signaling pathway in BP. Additionally, many JAK-dependent cytokines such as IL-6, IL-8, and IL-23 have been implicated in BP (29).
The role of JAK in other pemphigoid diseases, such as DH, MMP, and EBA, is similar to that observed for BP. For example, in DH and MMP, the expression of JAK/STAT-related proteins in the skin lesions of patients was significantly elevated (23). In the case of DH, eosinophilin and Th2 cytokines, such as IL-13, IL-4, and IL-5, also play an important role in the disease development (30). Additionally, a decrease in Treg cells and IL-10 has been observed in DH lesions (31), and the IL-31 concentration differs between healthy individuals and those with DH (32). This change in IL-31 concentration may be related to the intense itching typically associated with DH. In MMP, IL-4 and IL-13 not only affect disease onset but also influence the function of conjunctival fibroblasts, regulate scar formation, and thus, affect the prognosis of ocular MMP (33, 34). MMP is also associated with an increased localization of Th17 lymphocytes in lesions, especially in the conjunctiva, as well as local overexpression of IL-6, IL-12, and IL-17 (35). In EBA, high levels of IL-1β, IL-2, IL-6, IL-10, IL-21, TNF-β, and IFN-γ have been detected in serum samples (36). Similar to BP, in EBA, the Th2 pathway plays a key role in pathogenesis and itching. Consequently, the Th2 signaling pathway has received increasing attention in studies on pemphigoid diseases. Regarding treatment, an increasing number of reports have shown that the IL-4R monoclonal antibody dupilumab exhibits good efficacy in treating pemphigoid diseases (37). In addition to IL-4, the JAK/STAT pathway plays an important role in the function of other Th2 cytokines. In other words, inhibiting the JAK/STAT pathway can affect multiple Th2 cytokines. Therefore, evaluating the role of JAK/STAT in AIBDs is important for further research on pathogenesis and the development of therapeutic targets.
Animal models play a crucial role in understanding the mechanism of JAK inhibitors in treating AIBDs. However, there is a lack of research specifically using JAK inhibitors to treat AIBDs in mouse models, despite the existence of numerous reports on AIBDs in mouse models induced by antibody transfer, lymphocyte transfer, and immunization (38, 39). Interestingly, in veterinary medicine, oclacitinib, a selective JAK1 inhibitor for dogs, has shown promise in treating AIBDs like PF (40, 41). These observations suggest that JAK inhibitors may be beneficial for animals with AIBDs. It is still necessary to conduct animal model experiments focusing on AIBDs to gain further insights into the role of JAK inhibitors, representing an important avenue for future research.
4 Application of JAK inhibitors in AIBDs
4.1 Baricitinib
Baricitinib, which belongs to the class of JAK inhibitors, functions by blocking the JAK1 and JAK2-STAT signaling pathways. Additionally, it also inhibits the activity of IL-6, IL-12, and IL-23, thereby suppressing the differentiation of pathogenic Th17 cells. Baricitinib is used for treating rheumatoid arthritis, alopecia areata, and COVID-19, and has also been recommended for the treatment of psoriasis and other inflammatory-mediated diseases due to its considerable efficacy in controlling exaggerated inflammatory responses (42). Furthermore, some researchers have explored the potential of baricitinib as an off-label treatment for AIBDs (Table 2).
Baricitinib was initially utilized for the treatment of MMP in individual cases reported by Sarny et al. (43) and Burningham et al. (44). According to their reports, recalcitrant MMP was successfully managed with systemic glucocorticoids, methotrexate, and baricitinib. The favorable side effects of baricitinib and its oral administration make it a promising alternative to current interventions for MMP. Nevertheless, prospective studies are necessary to assess baricitinib’s effectiveness and establish its position within the treatment approach for MMP.
In the case of BP, Xiao et al. first reported a case of aggressive BP alongside plaque psoriasis that was effectively managed using baricitinib (45). The co-existence of psoriasis and the poor health conditions of the patient made the use of systematic glucocorticoids difficult, so baricitinib was chosen as an alternative. This report underscores the potential of baricitinib as a promising alternative treatment for co-existing plaque psoriasis and BP, or either condition individually.
Moussa et al. successfully treated a case of refractory LPP with baricitinib, highlighting its potential as an effective therapy for persistent cases (46). The limited availability of treatment options for LPP makes baricitinib a valuable addition. Concerning the underlying mechanism, Shao et al. discovered that in LPP, cytotoxic T-cell-mediated injury in keratinocytes was dependent on JAK2 and STAT1 signaling, and was inhibited by the JAK1/2 inhibitor baricitinib (47). These findings suggest that baricitinib may be able to alleviate the lichenoid tissue reaction in LPP.
One case report described a patient with epidermolysis bullosa pruriginosa (EBP) who had severe skin lesions and intense itching. Treatment with baricitinib resulted in a marked improvement in the patient’s condition (48).
4.2 Tofacitinib
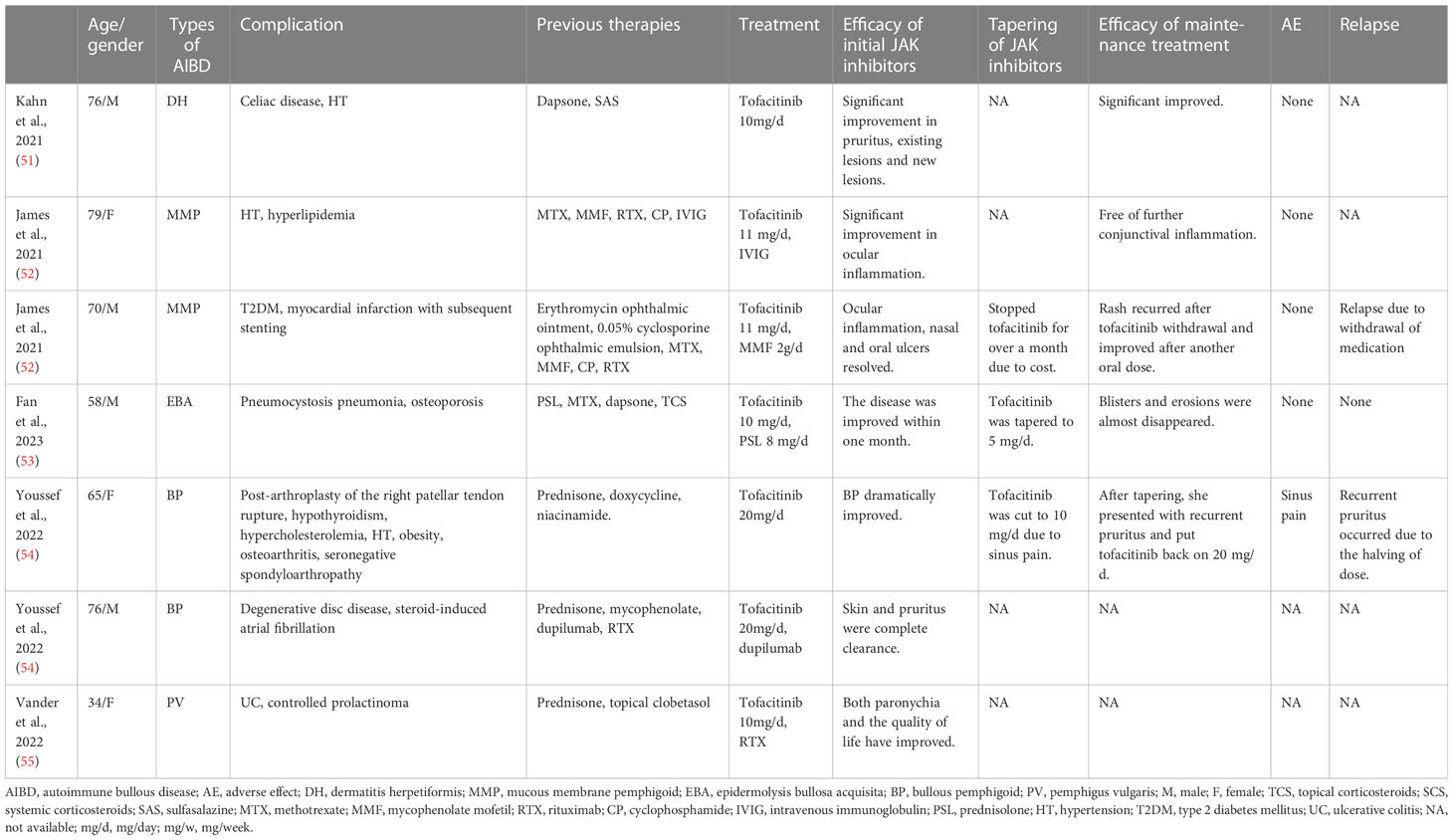
Tofacitinib, an oral inhibitor targeting JAK1/3, has received U.S. Food and Drug Administration (FDA) approval for treating moderate-to-severe rheumatoid arthritis, active psoriatic arthritis, and ulcerative colitis in adult patients (49), as well as polyarticular juvenile idiopathic arthritis in children. In vitro studies have indicated that tofacitinib is capable of reducing TNF, IL-1β, and type I IFN production in dendritic cells derived from monocytes stimulated with antigenic lipopolysaccharide (50). Further, the use of tofacitinib for treating DH, MMP, EBA, BP, and PV has been reported in the literature, as described below (Table 3).
Tofacitinib was initially used for DH that did not respond to conventional treatment. Kahn et al. presented a DH patient treated with tofacitinib, observing notable clinical improvement and inhibition of new lesion development (51). These findings suggest that tofacitinib could be a potentially effective alternative for managing DH in patients who are unable to adhere to a gluten-free diet or have contraindications to dapsone, or in cases where these approaches prove unsuccessful.
Tofacitinib has also shown promising results as a treatment for MMP, as reported in some cases (52). Two patients suffering from ocular MMP had failed to respond to several therapies and were treated with tofacitinib, resulting in long-term control of conjunctival inflammation and no observed progression of sub-conjunctival fibrosis.
Fan et al. described a recurrent EBA patient that responded well to tofacitinib treatment (53). In this patient, they observed a decrease in circulating neutrophil counts, although their association with the clinical response is debated, as well as the anti-COL-7 IgG titer. These observations indicate the potential therapeutic value of JAK inhibitors for EBA.
With regard to BP, Youssef et al. found that tofacitinib was effective in treating two cases of BP, achieving treatment goals while avoiding the side effects of standard therapies, and improving itch control (54). This study represents the initial report highlighting the benefits of oral tofacitinib in the management of BP. Fan et al. subsequently published a case series that supported these findings (56), reporting that all patients were relieved of itching after one week of tofacitinib treatment, and levels of serum autoantibodies, eosinophils, IL-6, IL-17, and TNF-α were lower after tofacitinib administration compared to before. These findings indicate that tofacitinib has good therapeutic prospects in BP.
In 2018, a review indicated that tofacitinib could potentially serve as an alternative treatment for pemphigus diseases (57). It was suggested that both systemic and topical tofacitinib could have positive therapeutic effects on pemphigus diseases. However, determining the ideal dosage requires further exploration through clinical trials. Compared to rituximab, tofacitinib offers the advantage of being available in both oral and topical forms, whereas rituximab can only be administered intravenously. Furthermore, tofacitinib may be more efficacious than rituximab because it targets both T cells and B cells, while rituximab mainly affects B cells. In 2022, Vander et al. reported a case of a female patient presenting with mild to moderate PV with nail involvement (55). The combination of oral tofacitinib and rituximab infusions resulted in a strikingly rapid improvement in her nail symptoms. Although rituximab has been shown to achieve long-lasting remission in pemphigus patients, its onset of action is slow. Therefore, tofacitinib may have contributed to the swift symptom improvement in the Vander et al. case report. The case findings suggest that combining tofacitinib with rituximab could potentially lead to rapid disease improvement and long-term remission.
The treatment of EBP with tofacitinib has also been reported (58), and it has been found to inhibit the inflammatory response, relieve pruritus, and decrease the recurrent onset.
4.3 Ruxolitinib
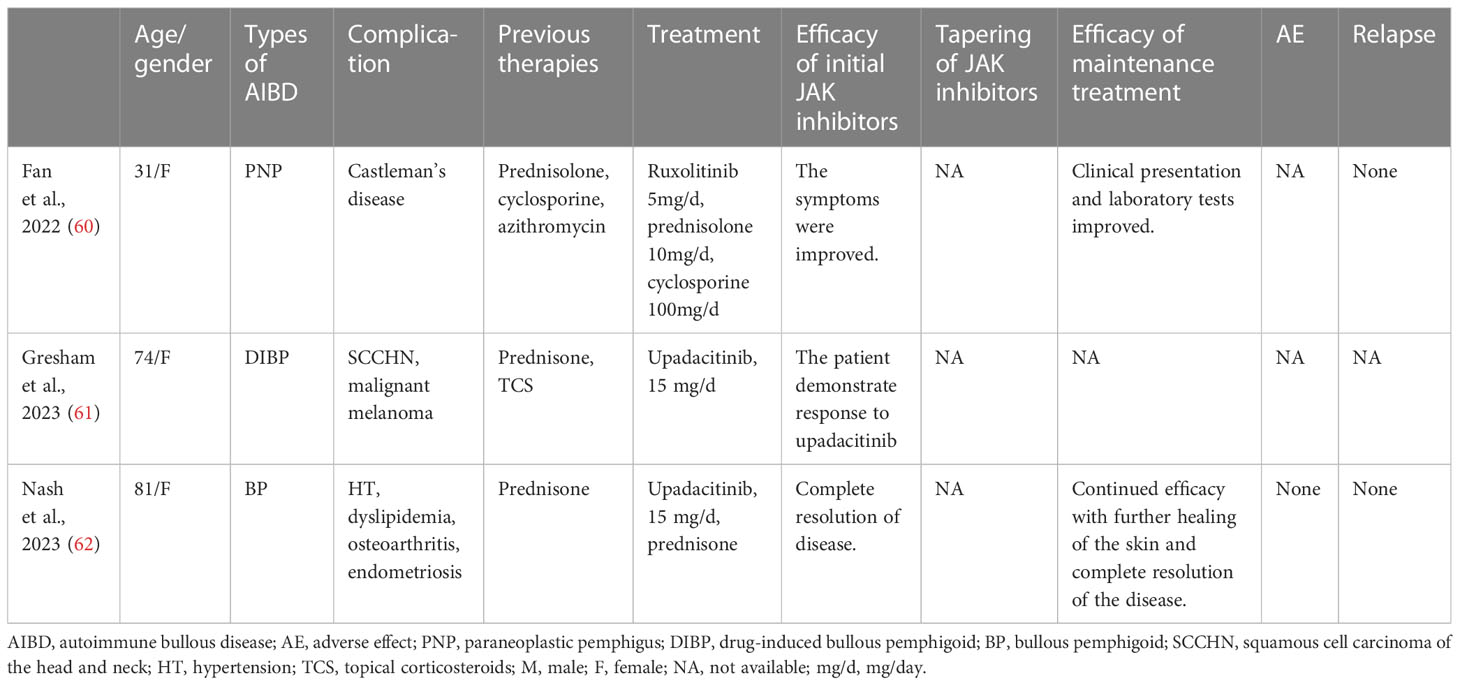
Ruxolitinib is an oral JAK1/2 inhibitor approved for the treatment of polycythemia vera, myelofibrosis, vitiligo, and steroid-refractory graft-versus-host disease (SR-GVHD). It has shown efficacy in treating dermatologic diseases like psoriasis and alopecia areata as an oral or topical agent. In SR-GVHD, ruxolitinib is used as a salvage therapy, particularly for cases with oral involvement and bronchiolitis obliterans (BO). This is because ruxolitinib plays a critical role in inflammation and T-cell activation (59). Some studies report high efficacy and survival rates with ruxolitinib for treating SR-GVHD. Based on the similarities in pathogenesis and clinical features of PNP and GVHD, ruxolitinib was considered a potential option for managing persistent stomatitis and BO in a female PNP patient (60) (Table 4). Despite the patient experiencing consistent healing of the skin with prednisolone, azithromycin, and cyclosporine, there was no noticeable improvement in oral lesions and respiratory function until ruxolitinib was added. The potent immunosuppressive and anti-inflammatory activities of JAK inhibitors were considered valuable for managing BO in this refractory PNP case.
4.4 Upadacitinib
Upadacitinib is a selective small molecule that functions as a JAK1 inhibitor. It has been approved for treating several medical conditions, including Crohn’s disease, psoriatic arthritis, atopic dermatitis, ulcerative colitis, and rheumatoid arthritis. Regarding AIBDs, researchers have used upadacitinib to treat BP (Table 4).
Gresham et al. presented a 74-year-old woman with recurrent squamous cell carcinoma of the head and neck who developed drug-induced BP while undergoing immunotherapy with a novel immunoglobulin-like transcript 4 inhibitor (MK-4830) and pembrolizumab (61). As her condition worsened, the patient decided to transition to end-of-life care. Upadacitinib was used to manage the BP symptoms, and after four weeks of treatment, the patient responded well, suggesting the potential therapeutic efficacy of upadacitinib in BP. It is essential to emphasize that this case involved the use of upadacitinib under exceptional circumstances to provide palliative care for skin symptoms in a patient receiving end-of-life care for metastatic cancer.
In a case study presented by Nash et al., an 81-year-old woman with BP showed an incomplete response to prednisone but achieved complete resolution with upadacitinib (62). BP is commonly observed in elderly patients who have multiple systemic comorbidities; therefore, it is essential to investigate the use of safer drugs for such individuals. Conducting further research on JAK inhibitors like upadacitinib would be useful in expanding the treatment options for this condition.
Kim et al. reported a case study of a patient suffering from EBP. The patient experienced intense itching and had widespread lesions. Treatment with upadacitinib led to a substantial reduction in itching and lesions without any adverse effects (63).
5 Systematic management of AIBDs
AIBDs are chronic conditions that can persist for several years or a lifetime with a high likelihood of relapse. The primary aim of treatment is to facilitate the healing of bullous and erosive cutaneous and/or mucous lesions, with the additional objectives of reducing pruritus, preventing or minimizing the recurrence of blistering eruptions, enhancing the quality of life of patients, and promptly identifying serious adverse effects associated with long-term therapy, especially in the elderly. Glucocorticoids and immunosuppressants are still the main treatment agents for AIBDs. Furthermore, the emergence of monoclonal antibody drugs has provided more options for the treatment of AIBDs. However, in clinical practice, doctors may still encounter patients for whom none of these treatments work. Therefore, discovering newer, safer, and more effective drugs has become a future trend in AIBDs therapy. JAK inhibitors interfere with the function of various inflammatory cytokines by targeting the JAK/STAT pathway, and thus, they have become new therapeutic agents for AIBDs. However, until now, only individual cases treated with JAK inhibitors have been reported, and no large-scale prospective clinical studies have been conducted to prove their efficacy. Based on the case reports available, JAK inhibitors are highly effective in patients who have failed to respond to treatment with glucocorticoids, immunosuppressive agents, and biologics. Therefore, it appears that, in general, JAK inhibitors are an attractive option for the treatment of AIBDs.
In addition to effectiveness, safety is also a significant concern regarding the current treatment options for AIBDs. The adverse effects of glucocorticoids include skin atrophy, fat redistribution, acne, weight gain, impaired glucose tolerance, insomnia, secondary infections, and femoral head necrosis (64). Conventional immunosuppressants may have side effects on liver and kidney function, and the potential consequences of systemic immunosuppression cannot be overlooked either. In recent years, advancements in immunological research have led to the development of new immunosuppressants, with rituximab being a representative example for treating AIBDs. However, it could lead to serious infections resulting from immunosuppression, and the invasiveness of its intravenous route of administration may also carry additional risks (65). Although JAK inhibitors have shown promising efficacy in clinical reports, their safety issues should not be ignored. For instance, one of the serious adverse effects of most JAK inhibitors is the activation of infections, including tuberculosis, herpes zoster, and hepatitis B (66). In addition, JAK inhibitors are associated with the risk of malignant tumors, such as lymphoma, which may be induced with the use of baricitinib, especially in patients with cancer (67).
Furthermore, attention should be paid to vascular events and cardiovascular risks, as several studies have shown that JAK inhibitors may cause thrombosis and platelet loss (68). Since the FDA added a black box warning to tofacitinib in 2019 and warned about the safety of JAK inhibitors, there has been ongoing controversy over cardiovascular events and thrombosis caused by JAK inhibitors. Some post-hoc analyses have shown that certain cardiovascular events and thrombosis are attributable to the use of baricitinib and tofacitinib (69, 70), but multiple meta-analyses have failed to confirm that the use of JAK inhibitors increases the risk of cardiovascular events (71, 72). Large, multi-center clinical studies of two highly selective JAK1 inhibitors (abrocitinib and upadacitinib) have also shown a very high safety profile, with no reported increase in cardiovascular events or higher thromboembolic risk (73, 74). This seems to imply that highly selective JAK inhibitors may have a lower cardiovascular risk. However, studies of abrocitinib and upadacitinib have mostly been conducted in patients with AD, who have a lower median age and have not been followed for a long duration.
In general, the safety of JAK inhibitors is a matter of debate. Considering that BP patients are predominantly elderly and prone to cardiovascular and metabolic diseases (1), the pros and cons of JAK inhibitors need to be carefully weighed before treatment. However, it is essential to note that the mechanisms by which JAK inhibitors cause cardiovascular events and thromboembolism risk are still unknown, so all patients should be educated and monitored. Despite this, based on current case reports, there have been no instances of severe adverse reactions to JAK inhibitor treatment for AIBDs. However, it cannot be ruled out that patients with severe adverse reactions have not been reported. Due to the potential adverse effects of JAK inhibitors, it is crucial to screen patients before administering them and to closely monitor them during treatment. Additionally, timely management of any adverse effects that may occur is vital when using JAK inhibitors.
6 Conclusions
We have reviewed the literature on the effectiveness of several JAK inhibitors in the treatment of AIBDs, including PV, PNP, BP, MMP, EBA, LPP, and DH. Based on the reported findings, we recommend careful monitoring, screening, and management of adverse effects on patients during the treatment of AIBDs with these agents. Regarding the gaps in the literature, the specific mechanisms of JAK/STAT in the pathogenesis and progression of AIBDs remain unclear, and more experimental studies are needed to further explore the role of this pathway in this group of diseases. Moreover, no clinical trials have been conducted to test the efficacy and safety of JAK inhibitors in the treatment of AIBDs. Larger and higher-quality long-term follow-up studies are essential to determine the efficacy and safety of JAK inhibitors in treating AIBDs. However, it must be mentioned that this review is a narrative review rather than a systematic review, and due to the scarcity of studies and lack of reports in the relevant fields, the conclusions are largely dependent on theoretical hypotheses and case reports. Therefore, the clinical use of JAK inhibitors in AIBDs should be concluded after joint discussions with patients.
Author contributions
DH: writing – original draft. YZ: writing – original draft. LK: writing – original draft. JL: resources, methodology, writing – review & editing. YS: writing – review & editing. All authors contributed to the article and approved to the published version of the manuscript.
Funding
This work was sponsored by grants from National Natural Science Foundation of China (No. 82073429, 82273510, 82003335), Innovation Program of Shanghai Municipal Education Commission (No.2019-01-07-00-07-E00046), Clinical Research Plan of SHDC (No. SHDC2020CR1014B) and Program of Shanghai Academic Research Leader (No. 20XD1403300).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet (2013) 381:320–32. doi: 10.1016/S0140-6736(12)61140-4
2. Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol (2016) 11:175–97. doi: 10.1146/annurev-pathol-012615-044313
3. Cortés B, Marazza G, Naldi L, Combescure C, Borradori L. Mortality of bullous pemphigoid in switzerland: a prospective study. Br J Dermatol (2011) 165:368–74. doi: 10.1111/j.1365-2133.2011
4. Kridin K. Pemphigus group: overview, epidemiology, mortality, and comorbidities. Immunol Res (2018) 66:255–70. doi: 10.1007/s12026-018-8986-7
5. Heymann WR. Bullous pemphigoid: rituximab to the rescue? J Am Acad Dermatol (2020) 82:1089–90. doi: 10.1016/j.jaad.2020.02.058
6. Kianfar N, Dasdar S, Mahmoudi H, Tavakolpour S, Balighi K, Daneshpazhooh M. Rituximab in childhood and juvenile autoimmune bullous diseases as first-line and second-line treatment: a case series of 13 patients. J Dermatol Treat (2022) 33:869–74. doi: 10.1080/09546634.2020.1788702
7. Chen DM, Odueyungbo A, Csinady E, Gearhart L, Lehane P, Cheu M, et al. Rituximab is an effective treatment in patients with pemphigus vulgaris and demonstrates a steroid-sparing effect. Br J Dermatol (2020) 182:1111–9. doi: 10.1111/bjd.18482
8. Tavakolpour S. Dupilumab: a revolutionary emerging drug in atopic dermatitis and its possible role in pemphigus. Dermatol Ther (2016) 29:299. doi: 10.1111/dth.12327
9. Amber KT, Maglie R, Solimani F, Eming R, Hertl M. Targeted therapies for autoimmune bullous diseases: current status. Drugs (2018) 78:1527–48. doi: 10.1007/s40265-018-0976-5
10. Owen KL, Brockwell NK, Parker BS. Jak-stat signaling: a double-edged sword of immune regulation and cancer progression. Cancers (Basel) (2019) 11:2002. doi: 10.3390/cancers11122002
11. Zhang L, Guo L, Wang L, Jiang X. The efficacy and safety of tofacitinib, peficitinib, solcitinib, baricitinib, abrocitinib and deucravacitinib in plaque psoriasis - a network meta-analysis. J Eur Acad Dermatol Venereol (2022) 36:1937–46. doi: 10.1111/jdv.18263
12. Phan K, Sebaratnam DF. Jak inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol (2019) 33:850–6. doi: 10.1111/jdv.15489
13. Silverberg JI, Thyssen JP, Fahrbach K, Mickle K, Cappelleri JC, Romero W, et al. Comparative efficacy and safety of systemic therapies used in moderate-to-severe atopic dermatitis: a systematic literature review and network meta-analysis. J Eur Acad Dermatol Venereol (2021) 35:1797–810. doi: 10.1111/jdv.17351
14. Clark JD, Flanagan ME, Telliez JB. Discovery and development of janus kinase (jak) inhibitors for inflammatory diseases. J Med Chem (2014) 57:5023–38. doi: 10.1021/jm401490p
15. Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev (2009) 228:273–87. doi: 10.1111/j.1600-065X.2008.00754.x
16. Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be? Nat Immunol (2009) 10:356–60. doi: 10.1038/ni.1701
17. O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The jak-stat pathway: impact on human disease and therapeutic intervention. Annu Rev Med (2015) 66:311–28. doi: 10.1146/annurev-med-051113-024537
18. Juczynska K, Wozniacka A, Waszczykowska E, Danilewicz M, Wągrowska-Danilewicz M, Zebrowska A. Expression of jak3, stat2, stat4, and stat6 in pemphigus vulgaris. Immunol Res (2020) 68:97–103. doi: 10.1007/s12026-020-09122-y
19. Balighi K, Hatami P, Sheikh AM, Daneshpazhooh M, Ghiasi M, Mahmoudi HR, et al. Multiple cycles of rituximab therapy for pemphigus: a group of patients with difficult- to-treat disease or a consequence of late rituximab initiation? Dermatol Ther (2022) 35:e15249. doi: 10.1111/dth.15249
20. Lee SH, Hong WJ, Kim SC. Analysis of serum cytokine profile in pemphigus. Ann Dermatol (2017) 29:438–45. doi: 10.5021/ad.2017.29.4.438
21. Veldman C, Stauber A, Wassmuth R, Uter W, Schuler G, Hertl M. Dichotomy of autoreactive th1 and th2 cell responses to desmoglein 3 in patients with pemphigus vulgaris (pv) and healthy carriers of pv-associated hla class ii alleles. J Immunol (2003) 170:635–42. doi: 10.4049/jimmunol.170.1.635
22. Paul WE, Zhu J. How are t(h)2-type immune responses initiated and amplified? Nat Rev Immunol (2010) 10:225–35. doi: 10.1038/nri2735
23. Juczynska K, Wozniacka A, Waszczykowska E, Danilewicz M, Wagrowska-Danilewicz M, Wieczfinska J, et al. Expression of the jak/stat signaling pathway in bullous pemphigoid and dermatitis herpetiformis. Mediators Inflamm (2017) 2017:6716419. doi: 10.1155/2017/6716419
24. Zhang J, Fang H, Shen S, Dang E, Li Q, Qiao P, et al. Identification of immunodominant th2-cell epitopes in chinese patients with bullous pemphigoid. J Invest Dermatol (2018) 138:1917–24. doi: 10.1016/j.jid.2018.03.1515
25. Feliciani C, Toto P, Mohammad PS, Coscione G, Amerio P, Amerio P. A th2-like cytokine response is involved in bullous pemphigoid. the role of il-4 and il-5 in the pathogenesis of the disease. Int J Immunopathol Pharmacol (1999) 12:55–61. doi: 10.1177/205873929901200202
26. Le Jan S, Plée J, Vallerand D, Dupont A, Delanez E, Durlach A, et al. Innate immune cell-produced il-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol (2014) 134:2908–17. doi: 10.1038/jid.2014.263
27. Traves PG, Murray B, Campigotto F, Galien R, Meng A, Di Paolo JA. Jak selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis (2021) 80:865–75. doi: 10.1136/annrheumdis-2020-219012
28. van de Kerkhof PC. From empirical to pathogenesis-based treatments for psoriasis. J Invest Dermatol (2022) 142:1778–85. doi: 10.1016/j.jid.2022.01.014
29. Maglie R, Solimani F, Didona D, Pipitò C, Antiga E, Di Zenzo G. The cytokine milieu of bullous pemphigoid: current and novel therapeutic targets. Front Med (Lausanne) (2023) 10:1128154. doi: 10.3389/fmed.2023.1128154
30. Marzano AV, Genovese G. Eosinophilic dermatoses: recognition and management. Am J Clin Dermatol (2020) 21:525–39. doi: 10.1007/s40257-020-00520-4
31. Antiga E, Quaglino P, Pierini I, Volpi W, Lami G, Bianchi B, et al. Regulatory t cells as well as il-10 are reduced in the skin of patients with dermatitis herpetiformis. J Dermatol Sci (2015) 77:54–62. doi: 10.1016/j.jdermsci.2014.11.003
32. Kulczycka-Siennicka L, Cynkier A, Waszczykowska E, Woźniacka A, Żebrowska A. The role of intereukin-31 in pathogenesis of itch and its intensity in a course of bullous pemphigoid and dermatitis herpetiformis. BioMed Res Int (2017) 2017:5965492. doi: 10.1155/2017/5965492
33. Razzaque MS, Ahmed BS, Foster CS, Ahmed AR. Effects of il-4 on conjunctival fibroblasts: possible role in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci (2003) 44:3417–23. doi: 10.1167/iovs.02-1084
34. Saw VP, Offiah I, Dart RJ, Galatowicz G, Dart JK, Daniels JT, et al. Conjunctival interleukin-13 expression in mucous membrane pemphigoid and functional effects of interleukin-13 on conjunctival fibroblasts in vitro. Am J Pathol (2009) 175:2406–15. doi: 10.2353/ajpath.2009.090579
35. Suelves AM, Zhao TZ, Siddique SS, Foster CS. Profile of local interleukin expression in a cohort of ocular cicatricial pemphigoid patients. Invest Ophthalmol Vis Sci (2012) 53:8112–7. doi: 10.1167/iovs.11-9322
36. Park SH, Park HS, Kim JH, Kim SC. Elevation of serum interleukin-21 in patients with epidermolysis bullosa acquisita. J Dermatol (2019) 46:279–80. doi: 10.1111/1346-8138.14789
37. Zhang Y, Xu Q, Chen L, Chen J, Zhang J, Zou Y, et al. Efficacy and safety of dupilumab in moderate-to-severe bullous pemphigoid. Front Immunol (2021) 12:738907. doi: 10.3389/fimmu.2021.738907
38. Emtenani S, Hertl M, Schmidt E, Hudemann C. Mouse models of pemphigus: valuable tools to investigate pathomechanisms and novel therapeutic interventions. Front Immunol (2023) 14:1169947. doi: 10.3389/fimmu.2023.1169947
39. Ghorbanalipoor S, Emtenani S, Parker M, Kamaguchi M, Osterloh C, Pigors M, et al. Cutaneous kinase activity correlates with treatment outcomes following pi3k delta inhibition in mice with experimental pemphigoid diseases. Front Immunol (2022) 13:865241. doi: 10.3389/fimmu.2022.865241
40. Carrasco I, Martínez M, Albinyana G. Beneficial effect of oclacitinib in a case of feline pemphigus foliaceus. Vet Dermatol (2021) 32:299–301. doi: 10.1111/vde.12949
41. Aymeric E, Bensignor E. A case of presumed autoimmune subepidermal blistering dermatosis treated with oclacitinib. Vet Dermatol (2017) 28:123–512. doi: 10.1111/vde.12458
42. Nash P, Kerschbaumer A, Dörner T, Dougados M, Fleischmann RM, Geissler K, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with janus kinase inhibitors: a consensus statement. Ann Rheum Dis (2021) 80:71–87. doi: 10.1136/annrheumdis-2020-218398
43. Sarny S, Hucke M, El-Shabrawi Y. Treatment of mucous membrane pemphigoid with janus kinase inhibitor baricitinib. JAMA Ophthalmol (2018) 136:1420–2. doi: 10.1001/jamaophthalmol.2018.3789
44. Burningham KM, Cao J, Dominguez AR. Successful treatment of recalcitrant mucous membrane pemphigoid with multisystem involvement with baricitinib and methotrexate. JAAD Case Rep (2022) 27:67–9. doi: 10.1016/j.jdcr.2022.07.013
45. Xiao Y, Xiang H, Li W. Concurrent bullous pemphigoid and plaque psoriasis successfully treated with janus kinase inhibitor baricitinib. Dermatol Ther (2022) 35:e15754. doi: 10.1111/dth.15754
46. Moussa A, Colla TG, Asfour L, Bhoyrul B, Sinclair RD. Effective treatment of refractory lichen planus pemphigoides with a janus kinase-1/2 inhibitor. Clin Exp Dermatol (2022) 47:2040–1. doi: 10.1111/ced.15344
47. Shao S, Tsoi LC, Sarkar MK, Xing X, Xue K, Uppala R, et al. Ifn-γ enhances cell-mediated cytotoxicity against keratinocytes via jak2/stat1 in lichen planus. Sci Transl Med (2019) 11:eaav7561. doi: 10.1126/scitranslmed.aav7561
48. Jiang X, Wang H, Lee M, Lin Z. Epidermolysis Bullosa Pruriginosa Treated with Baricitinib. JAMA Dermatol (2021) 157:1243–44. doi: 10.1001/jamadermatol.2021.3174
49. Varyani F, Argyriou K, Phillips F, Tsakiridou E, Moran GW. Profile of tofacitinib in the treatment of ulcerative colitis: an evidence-based review of recent data. Drug Des Devel Ther (2019) 13:4091–105. doi: 10.2147/DDDT.S182891
50. Kubo S, Yamaoka K, Kondo M, Yamagata K, Zhao J, Iwata S, et al. The jak inhibitor, tofacitinib, reduces the t cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis (2014) 73:2192–8. doi: 10.1136/annrheumdis-2013-203756
51. Kahn JS, Moody K, Rosmarin D. Significant improvement of dermatitis herpetiformis with tofacitinib. Dermatol Online J (2021) 27:6. doi: 10.5070/D327754365
52. James H, Paley GL, Brasington R, Custer PL, Margolis TP, Paley MA. Tofacitinib for refractory ocular mucous membrane pemphigoid. Am J Ophthalmol Case Rep (2021) 22:101104. doi: 10.1016/j.ajoc.2021.101104
53. Fan B, Wang M. Treatment of recurrent epidermolysis bullosa acquisita with tofacitinib. JAMA Dermatol (2023) 159:342–4. doi: 10.1001/jamadermatol.2022.6250
54. Youssef S, Gallitano S, Bordone LA. Two cases of bullous pemphigoid effectively treated with oral tofacitinib. JAAD Case Rep (2023) 32:77–80. doi: 10.1016/j.jdcr.2022.10.028
55. Vander DA, Gamret AC, Yosipovitch G. Nail loss in mild to moderate pemphigus vulgaris. Skin Appendage Disord (2022) 8:504–7. doi: 10.1159/000525462
56. Fan B, Wang M. Tofacitinib in recalcitrant bullous pemphigoid: a report of seven cases. Br J Dermatol (2023) 188:432–4. doi: 10.1093/bjd/ljac078
57. Tavakolpour S. Tofacitinib as the potent treatment for refractory pemphigus: a possible alternative treatment for pemphigus. Dermatol Ther (2018) 31:e12696. doi: 10.1111/dth.12696
58. Chen KJ, Fang S, Ye Q, Jia M. Successful use of tofacitinib in epidermolysis bullosa pruriginosa. Clin Exp Dermatol (2022) 47:598–600. doi: 10.1111/ced.14998
59. Zhao Y, OuYang G, Shi J, Luo Y, Tan Y, Yu J, et al. Salvage therapy with low-dose ruxolitinib leads to a significant improvement in bronchiolitis obliterans syndrome in patients with cgvhd after allogeneic hematopoietic stem cell transplantation. Front Pharmacol (2021) 12:668825. doi: 10.3389/fphar.2021.668825
60. Fan B, Wang M. Ruxolitinib, a jak1/2 inhibitor as treatment for paraneoplastic pemphigus: a case report. Acta Derm Venereol (2022) 102:v732. doi: 10.2340/actadv.v102.1378
61. Gresham LM, Kirchhof MG. A case of drug-induced bullous pemphigoid secondary to immunotherapy treated with upadacitinib: a case report. SAGE Open Med Case Rep (2023) 11:2050313X–231160926X. doi: 10.1177/2050313X231160926
62. Nash D, Kirchhof MG. Bullous pemphigoid treated with janus kinase inhibitor upadacitinib. JAAD Case Rep (2023) 32:81–3. doi: 10.1016/j.jdcr.2022.12.006
63. Kim N, Jue MS, Huh YJ, Sung KJ, Kim H, Kim JE, et al. Epidermolysis bullosa pruriginosa with extensive truncal involvement treated with upadacitinib. J Eur Acad Dermatol Venereol (2023) 37:e215–7. doi: 10.1111/jdv.18487
64. Oray M, Abu SK, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf (2016) 15:457–65. doi: 10.1517/14740338.2016.1140743
65. Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol (2022) 13:928621. doi: 10.3389/fimmu.2022.928621
66. Winthrop KL, Cohen SB. Oral surveillance and jak inhibitor safety: the theory of relativity. Nat Rev Rheumatol (2022) 18:301–4. doi: 10.1038/s41584-022-00767-7
67. Roskoski RJ. Properties of fda-approved small molecule protein kinase inhibitors. Pharmacol Res (2019) 144:19–50. doi: 10.1016/j.phrs.2019.03.006
68. Atzeni F, Popa CD, Nucera V, Nurmohamed MT. Safety of jak inhibitors: focus on cardiovascular and thromboembolic events. Expert Rev Clin Immunol (2022) 18:233–44. doi: 10.1080/1744666X.2022.2039630
69. King B, Maari C, Lain E, Silverberg JI, Issa M, Holzwarth K, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol (2021) 22:395–405. doi: 10.1007/s40257-021-00602-x
70. Charles-Schoeman C, Buch MH, Dougados M, Bhatt DL, Giles JT, Ytterberg SR, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from oral surveillance. Ann Rheum Dis (2023) 82:119–29. doi: 10.1136/ard-2022-222259
71. Shehab M, Alrashed F, Alkazemi A, Lakatos PL, Bessissow T. Impact of biologic therapies and small molecules on the risk of major adverse cardiovascular events in patients with inflammatory bowel diseases: systematic review and meta-analysis of randomized controlled trials. Expert Rev Gastroenterol Hepatol (2023) 17:469–77. doi: 10.1080/17474124.2023.2194631
72. Campanaro F, Zaffaroni A, Cacioppo E, Cappelli A, Bertù L, Donadini MP, et al. Venous and arterial thromboembolic risk of jak inhibitors: a systematic review with meta-analysis. Rheumatol (Oxford) (2023) kead211. doi: 10.1093/rheumatology/kead211
73. Fleischmann R, Curtis JR, Charles-Schoeman C, Mysler E, Yamaoka K, Richez C, et al. Safety profile of upadacitinib in patients at risk of cardiovascular disease: integrated post hoc analysis of the select phase iii rheumatoid arthritis clinical programme. Ann Rheum Dis (2023) ard-2023-223916. doi: 10.1136/ard-2023-223916
74. Flohr C, Cork MJ, Ardern-Jones MR, Eichenfield LF, Barbarot S, Feeney C, et al. Efficacy and safety of abrocitinib monotherapy in adolescents and adults: a post hoc analysis of the phase 3 jak1 atopic dermatitis efficacy and safety (jade) regimen clinical trial. J Dermatol Treat (2023) 34:2200866. doi: 10.1080/09546634.2023.2200866
Keywords: autoimmune bullous disease, Janus kinase inhibitor, pemphigus, pemphigoid, JAK-STAT signaling pathway
Citation: Huang D, Zhang Y, Kong L, Lu J and Shi Y (2023) Janus kinase inhibitors in autoimmune bullous diseases. Front. Immunol. 14:1220887. doi: 10.3389/fimmu.2023.1220887
Received: 11 May 2023; Accepted: 27 June 2023;
Published: 10 July 2023.
Edited by:
Christian David Sadik, University of Lübeck, GermanyReviewed by:
Andreas Recke, University of Lübeck, GermanyWilliam D. Shipman, Yale University, United States
Copyright © 2023 Huang, Zhang, Kong, Lu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajing Lu, Ym9ubmllMTY2MTY2QDEyNi5jb20=; Yuling Shi, c2hpeXVsaW5nMTk3M0B0b25namkuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Dawei Huang1,2†
Dawei Huang1,2† Yuexin Zhang
Yuexin Zhang Jiajing Lu
Jiajing Lu Yuling Shi
Yuling Shi