- 1Division of Nephrology, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand
- 2Excellence Center for Organ Transplantation (ECOT), King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand
- 3Renal Immunology and Transplantation Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 4HIV-NAT, Thai Red Cross AIDS Research Centre, Bangkok, Thailand
- 5Center of Excellence on Translational Research in Inflammation and Immunology (CETRII), Department of Microbiology, Chulalongkorn University, Bangkok, Thailand
- 6Immunology Unit, Department of Microbiology, Chulalongkorn University, Bangkok, Thailand
- 7Biostatistics Excellence Centre, Research Affairs, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 8The Kirby Institute, University of New South Wales, Sydney, NSW, Australia
Background: Evidence has demonstrated inferior humoral immune responses after SARS-CoV-2 vaccination in kidney transplant recipients compared to the general population. However, data on cellular immune responses in this population have not been established.
Methods: We searched the MEDLINE, Scopus, and Cochrane databases and included studies reporting cellular immune response rates in kidney transplant recipients after receiving SARS-CoV-2 vaccines. Studies that reported factors associated with cellular immune responders or non-responders were also included (PROSPERO: CRD42022375544).
Results: From a total of 1,494 articles searched, 53 articles were included in the meta-analysis. In all, 21 studies assessed cellular immune response by interferon-γ enzyme-linked immunosorbent spot (IFN-γ ELISPOT), 22 studies used interferon-γ release assay (IGRA), and 10 studies used flow cytometric analysis. The pooled response rate after two doses (standard regimen) and three doses of vaccination was 47.5% (95%CI 38.4-56.7%) and 69.1% (95%CI 56.3-80.6%) from IFN-γ ELISPOT, 25.8% (95%CI 19.7-32.4%) and 14.7% (95%CI 8.5-22.2%) from IGRA, and 73.7% (95%CI 55.2-88.8%) and 86.5% (95%CI 75.3-94.9%) from flow cytometry, respectively. Recipients with seroconversion were associated with a higher chance of having cellular immune response (OR 2.58; 95%CI 1.89-3.54). Cellular immune response in kidney transplant recipients was lower than in dialysis patients (OR 0.24; 95%CI 0.16-0.34) and the general population (OR 0.10; 95%CI 0.07-0.14). Age and immunosuppressants containing tacrolimus or corticosteroid were associated with inferior cellular immune response.
Conclusion: Cellular immune response after SARS-CoV-2 vaccination in kidney transplant recipients was lower than in dialysis patients and the general population. Age, tacrolimus, and corticosteroid were associated with poor response. Cellular immune response should also be prioritized in vaccination studies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022375544.
Introduction
Mortality after infection with coronavirus disease (COVID-19) in kidney transplant recipients is higher compared to the general population (1). Immunosuppressive medications used in transplant recipients blunt the immune response against the SARS-CoV-2 vaccine, thereby increasing the risk of severe COVID-19. Despite global inequities in vaccine availability, COVID-19 vaccines are available in most settings, but transplant recipients are still at risk of severe disease and death because of the insufficient immune response after vaccination (2, 3).
To date, many studies and meta-analyses have demonstrated that humoral immune responses against SARS-CoV-2 vaccines, defined by the presence of anti-spike protein antibodies or neutralizing antibodies, are poor in kidney transplant recipients (4, 5). Factors associated with decreased antibody responses include older age, deceased donor transplantation, antimetabolite use, and recent rituximab or anti-thymocyte globulin use (5). However, protective immunity against SARS-CoV-2 does not depend on humoral immune responses alone but also requires a robust cell-mediated immune response to clear the virus and enhance humoral immune system function (6–8). The current evidence demonstrates that while the humoral immune response is particularly important for blocking SARS-CoV-2 infection, cellular immunity is of relatively greater importance for the prevention of severe disease, hospitalization, and death (9). Both neutralizing antibody and S2-specific interferon-γ T-cell responses protect against breakthrough SARS-CoV-2 infection after vaccination in kidney transplant recipients (10).
Although both humoral and cellular immune response are crucial for viral clearance and protection against COVID-19, only a limited number of studies have reported data on cellular immune responses. Most COVID-19 vaccination studies in kidney transplant recipients have described only seroconversion rates or neutralizing antibody concentrations without providing details regarding the cellular immune response. This systematic review and meta-analysis was conducted to summarize the current evidence on the cellular immune response after SARS-CoV-2 vaccination in kidney transplant recipients. Cellular immune response rates after COVID-19 vaccines were compared with humoral responses, and the factors associated with cellular immune response were explored.
Methods
Data source and searches
This systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) Statement (11). Electronic databases, including MEDLINE, Scopus, and Cochrane Central Register of Controlled Trials, were searched for eligible studies published in English up to 7 May 2023. The search strategy for MEDLINE using Medical Subject Headings (MeSH) was as followed: (“COVID-19 Vaccines” [MeSH]) AND (“Kidney Transplantation” [MeSH]). The search term in Cochrane Central Register of Controlled Trials included COVID and vaccine and kidney transplantation, exploding all trees of MeSH descriptors. For Scopus, the search strategy was TITLE-ABS-KEY (COVID AND vaccine AND kidney AND transplant). We also reviewed the reference lists in the qualified articles and manually included relevant articles. The protocol for systematic review and meta-analysis was registered in PROSPERO (CRD42022375544).
Study selection
This primary aim of this systematic review and meta-analysis was to explore cellular immune responses against SARS-CoV-2 vaccination and to determine factors associated with cell-mediated immunity responses in kidney transplant recipients. The included studies were required to report cellular immune response rates, determined by the proportion of patients with cellular immune responses above the specific cutoff used in individual studies, after stimulating with entire spike protein or the S1 subunit. Since cellular immune responses can be evaluated using different assays, including the interferon-γ enzyme-linked immunosorbent spot (IFN-γ ELISPOT), interferon-γ release assay (IGRA), and flow cytometric analysis, studies that presented only the absolute assay results without referencing the positive or negative cellular response rates were excluded from the final analyses. Studies that reported only antibody response were excluded. Evidence regarding factors associated with cellular response was derived from studies assessing associations between potential risk factors in cellular responders and non-responders. We only included studies that reported cellular immune responses in kidney transplant recipients, whether a comparator group was included or not. Two authors (S.U. and S.K.) independently screened the titles and abstracts of the articles and extracted data from full-text articles using a custom-designed spreadsheet. Disagreements were resolved through consensus by all the coauthors.
Data extraction and quality assessment
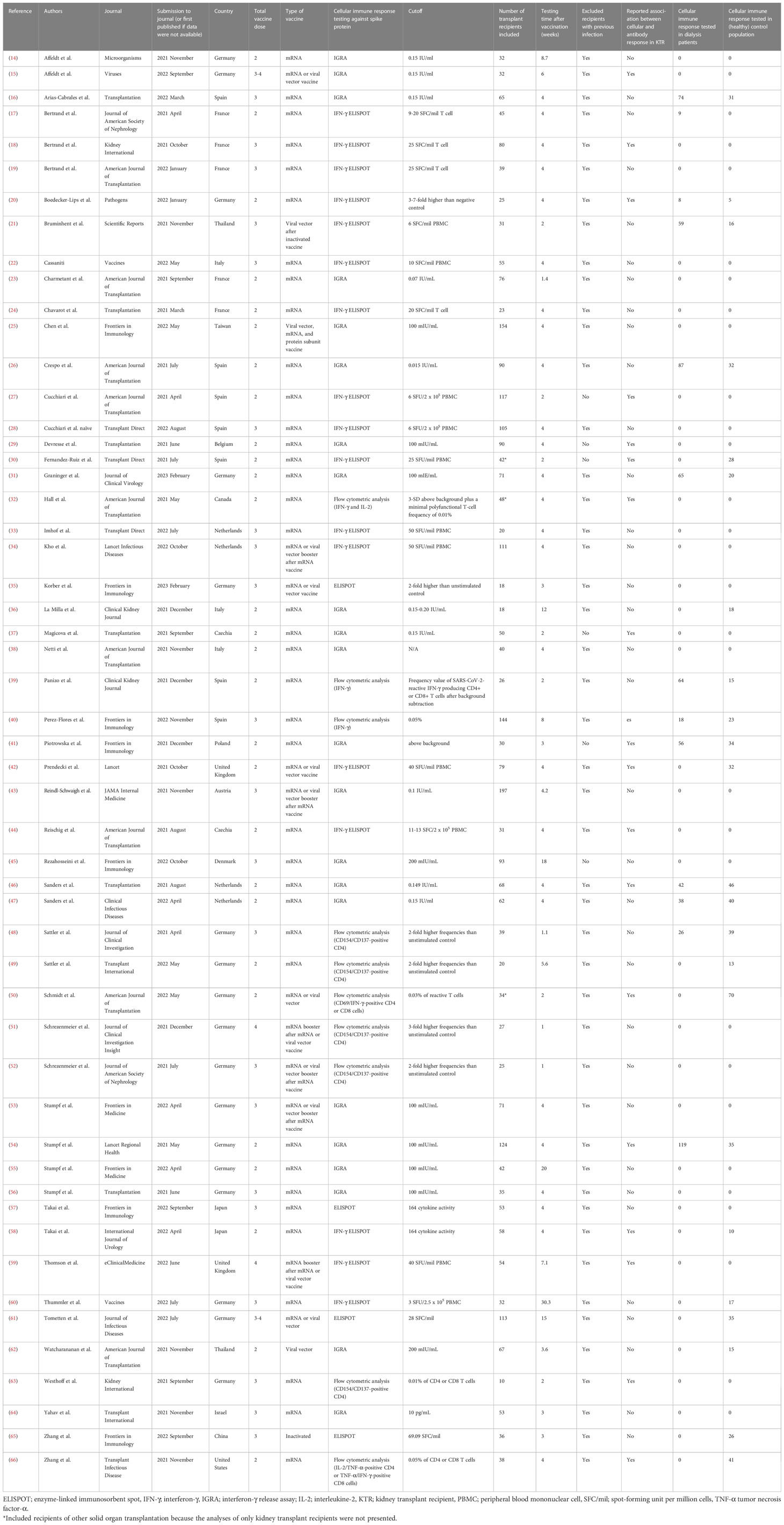
The following information was extracted from each study: author names, journal, month and year of article submission (or publication), country of origin, type of COVID-19 vaccine, dose of vaccination, cellular immune response assays and their positive cutoff values, number of kidney transplant recipients included for the evaluation of cellular immune responses, results of cellular immune responses, time of assessment post-vaccination, antibody responses (if presented), cellular immune responses in dialysis patients or (healthy) control population (if presented), and the characteristics of kidney transplant recipients between responders or non-responders. Quality assessment was conducted by using the Newcastle–Ottawa scale (12), which is categorized into three domains: selection, comparability, and outcome. Total scores of 0-3 were considered poor quality, 4-6 fair quality, and 7-9 were considered good quality studies.
Data synthesis and analysis
Random-effects model meta-analysis was used to 1) calculate the pooled cellular immune response rates following different numbers of vaccine doses and assess cellular immune responses by seroconversion status, 2) to compare cellular immune responses between kidney transplant recipients and other populations and the factors associated with being the responders. The timing of evaluation was considered post-vaccination if the evaluation was performed within 1-8 weeks after receiving the vaccines. Studies that reported the immune response more than 12 weeks after the previous dose (and before the next dose) were reported separately from those reported in the immediate post-vaccination category. If available, antibody and cellular response data were compared and categorized into E+A+ (both cellular and antibody response), E+A- (cellular response without antibody response), E-A+ (antibody response without cellular response), and E-A- (lack of both cellular and antibody response). Pooled odds ratios (ORs) were calculated using the logarithm of the effect size and standard error from each study. If study provided both ORs and adjusted ORs for the cellular immune response, the adjusted ORs were to be used for this analysis. All pooled estimates were provided with a 95% confidence interval (95%CI). Heterogeneity of the pooled effect sizes was evaluated by the I2 index and Q-test p-value. An I2 index higher than 75% implies medium to high heterogeneity. Small-study effect was assessed by Egger’s test. Funnel plots were used to graphically assess the possibility of publication bias of the included studies. Skewed or asymmetric scatter plots of the pooled effect estimates relative to the standard error as a measure of study size indicated possible publication bias or other biases (13). The analyses were performed using Stata 17.0 (StataCorp LLC, College Station, TX) and GraphPad Prism 9.4.0 (GraphPad Software, San Diego, CA).
Role of the funding sources
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Characteristics of included studies
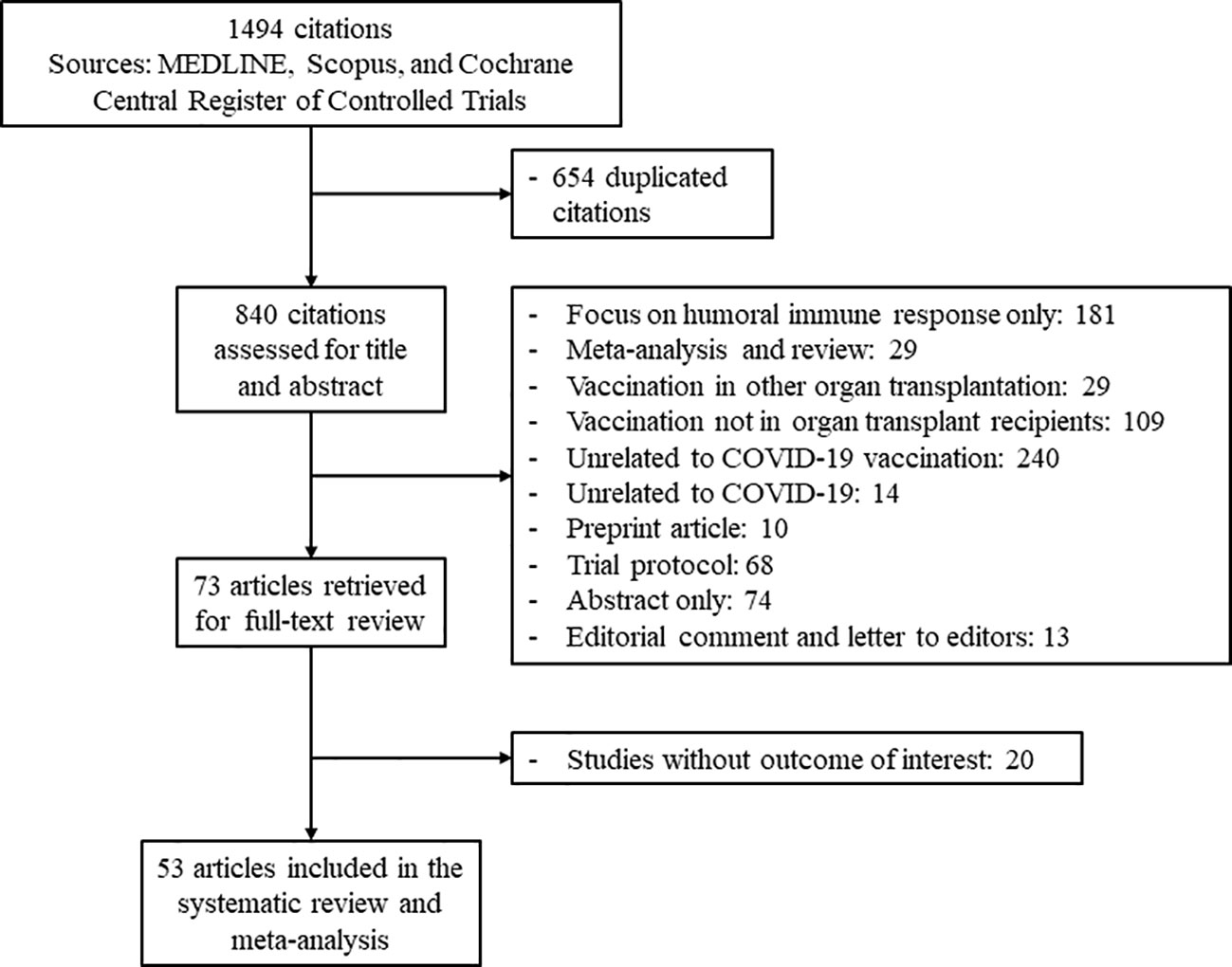
The study flow diagram is shown in Figure 1. From a total of 1,494 citations retrieved based on our criteria, 53 articles reporting outcomes on 3,138 transplant recipients were included in the analyses (14–66). In all, 28 studies examined cellular immune responses after two doses of SARS-CoV-2 vaccine, and 25 studies evaluated the response of booster (third or fourth) doses. The characteristics of each study are presented in Table 1. Immune responses after mRNA vaccination were evaluated in 50 studies, and 12 studies investigated the response after viral vector vaccination. The inactivated vaccine and protein subunit vaccine were utilized in one study each. Spike protein antigen from SARS-CoV-2 was used to stimulate cellular immune responses after vaccination. A total of 21 studies assessed this cellular response by the IFN-γ ELISPOT assay, 22 studies used IGRA to identify responders and non-responders, and 10 studies explored the cellular immune response using flow cytometric analysis with intracellular cytokine and/or surface antigen staining. Supplementary Table S1 shows the quality assessment of the included studies.
Cellular immune response after SARS-CoV-2 vaccination in kidney transplant recipients
Figure 2 shows the cellular response rates by the different testing methods. For studies that used IFN-γ ELISPOT assays, the pooled response rate after the first vaccine dose was 18.3% (95%CI 7.2-32.5%; I2 68.3%), after the second dose it was 47.5% (95%CI 38.4-56.7%; I2 86.5%), and after a third booster dose it was 69.1% (95%CI 56.3-80.6%; I2 88.5%) (Figure 2A). Pooled cellular immune response rates assessed by using IGRA were 18.2% (95%CI 7.7-31.6%, I2 86.8%) after the first dose, 25.8% (95%CI 19.7-32.4%; I2 82.1%) after the second dose, and 14.7% (95%CI 8.5-22.2%; I2 69.6%) after a third booster dose (Figure 2B). Studies presenting results from flow cytometric analysis demonstrated pooled response rates of 16.1% (95%CI 8.5-25.4%) after the first dose, 73.7% (95%CI 55.2-88.8%; I2 85.6%) after the second dose, and 86.5% (95%CI 75.3-94.9%; I2 70.1%) after a third booster dose (Figure 2C). Only six studies included recipients with previous SARS-CoV-2 infection (27, 29, 30, 37, 41, 45). Supplementary Figure S1 illustrates the cellular immune response rate after excluding the results from these studies, and the results were not different from the primary analyses that included recipients with previous SARS-CoV-2 infection. Some studies that evaluated the response after a third booster dose included only kidney transplant recipients who did not achieve seroconversion after the second dose; these included 3/12 estimates (33, 34) using the IFN-γ ELISPOT assay, 5/8 estimates (43, 53, 56) using the IGRA test, and 3/5 estimates (51, 52, 63) using flow cytometric analysis. Supplementary Figure S2 shows the cellular immune response after excluding these studies, in which the response rates were comparable to the primary analyses that included all studies. The pooled seroconversion rates based on anti-spike IgG production are shown in Supplementary Figure S3. Supplementary Figure S4 demonstrates funnel plots of the overall cellular and antibody responses of all the included studies. The plots are predominantly symmetrical except the studies using flow cytometric analysis, suggesting possible publication bias or heterogeneity among different flow cytometry techniques.
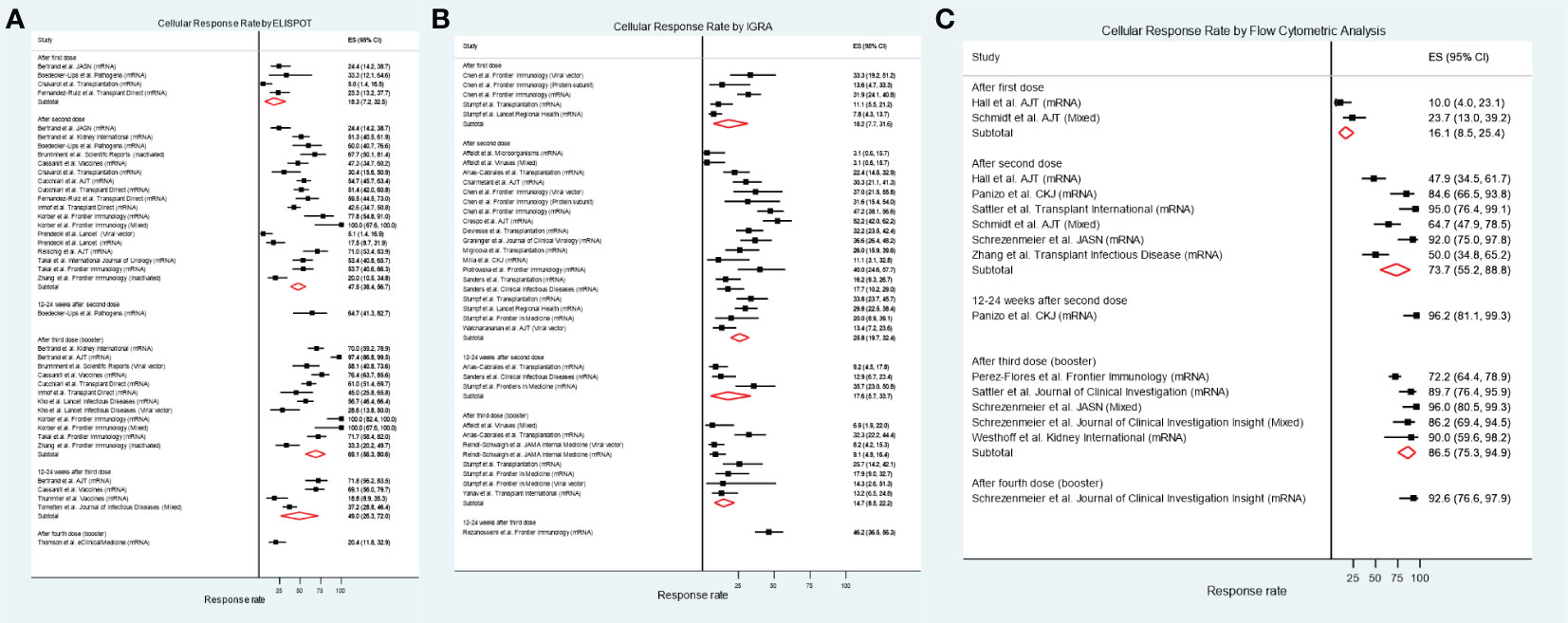
Figure 2 Cellular immune response rate in kidney transplant recipients after receiving different numbers of SARS-CoV-2 vaccine doses. (A) IFN-γ ELSIPOT assay. (B) IGRA. (C) Flow cytometric analysis.
Figure 3 illustrates a forest plot of the cellular immune response in kidney transplant recipients who seroconverted compared to those who did not seroconvert. Recipients who seroconverted were more likely to achieve cellular immune responses (pooled OR 2.58; 95%CI 1.89-3.54; 95% prediction interval 1.26-5.28; p-value<0.001; I2 18.0%; Q-test 0.569; Egger’s test 0.161) than recipients who failed to seroconvert. This also means that recipients with cellular immune responses had 2.58-fold higher odds of having seroconverted compared with recipients without cellular immune response. A funnel plot of this analysis is shown in Supplementary Figure S5, showing some evidence of asymmetry, which may be due to publication bias.
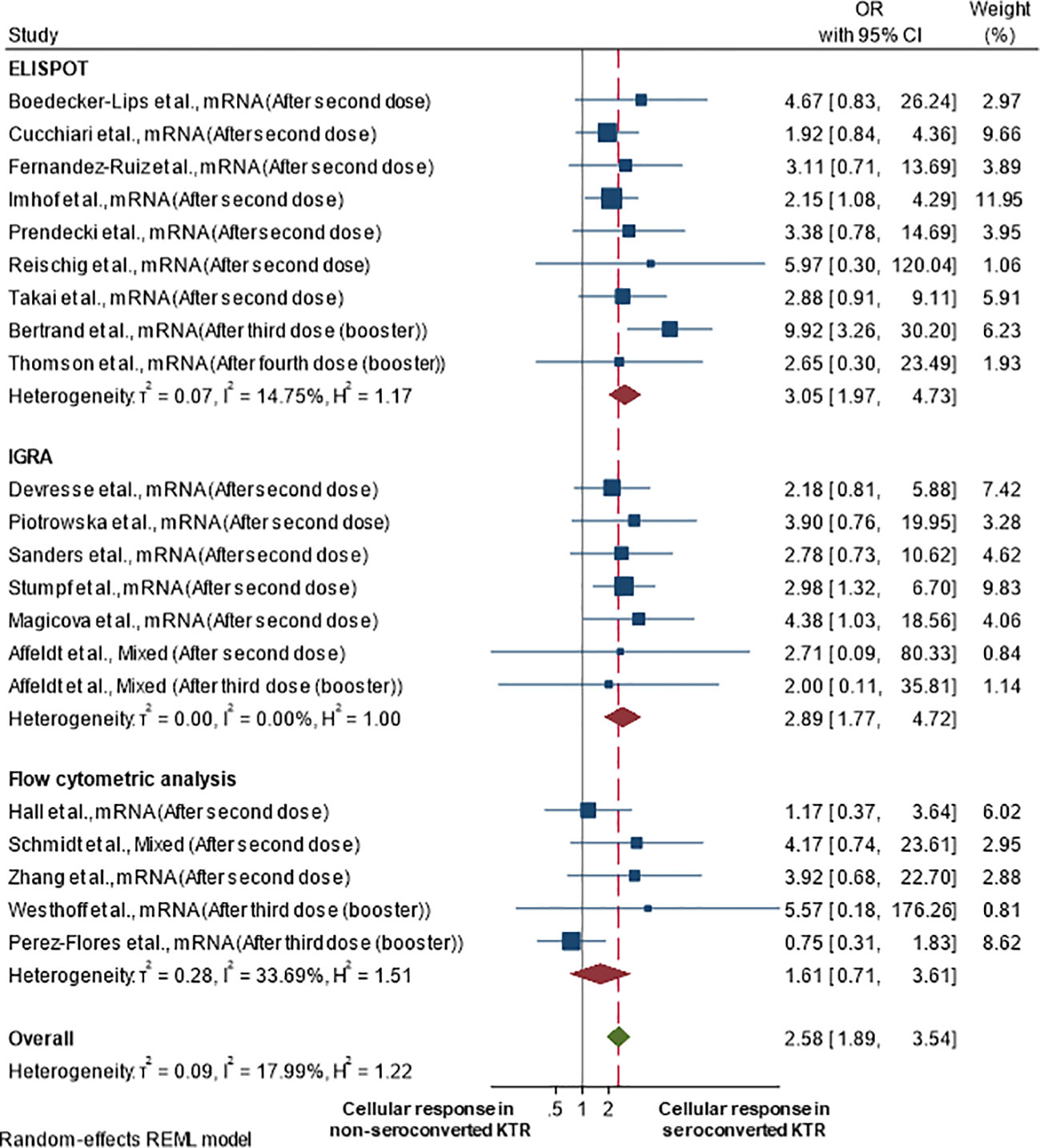
Figure 3 Cellular immune response rate in kidney transplant recipients by seroconversion status after at least two vaccine doses.
Comparison of cellular immune response between types of SARS-CoV-2 vaccination
Four studies directly compared cellular immune responses in kidney transplant recipients vaccinated with mRNA versus the viral vector vaccine (34, 42, 43, 53). Although comparison was not significant at <0.05, the pooled effect sizes and 95%CI for both two- and three-dose regimens demonstrated higher cellular immune response rates in kidney transplant recipients who received mRNA versus viral vector vaccines (Supplementary Figure S6). Three studies performed head-to-head comparisons of BNT162b2 and mRNA-1273, two after a single dose and a complete primary vaccination series (25, 31) and one after a booster dose (53), which did not show a significant difference of cellular immune responses between these mRNA vaccines.
Comparison of cellular immune response rates between kidney transplant recipients and other populations
Figures 4A, B demonstrate the OR of achieving a cellular immune response in kidney transplant recipients compared to dialysis patients or control (non-kidney disease) populations, respectively. Being a kidney transplant recipient was associated with lower odds of being a cellular immune responder compared to both dialysis patients (pooled OR 0.24; 95%CI 0.16-0.34; 95% prediction interval 0.06-1.01; p-value<0.001; I2 65.7%; Q-test<0.001; Egger’s test 0.869) or controls without kidney disease (pooled OR 0.10; 95%CI 0.07-0.14; 95% prediction interval 0.02-0.51; I2 58.6%; p-value<0.001; Q-test<0.001; Egger’s test 0.629). The funnel plots presented in Supplementary Figure S7 appear to be symmetrical, suggesting no publication bias.
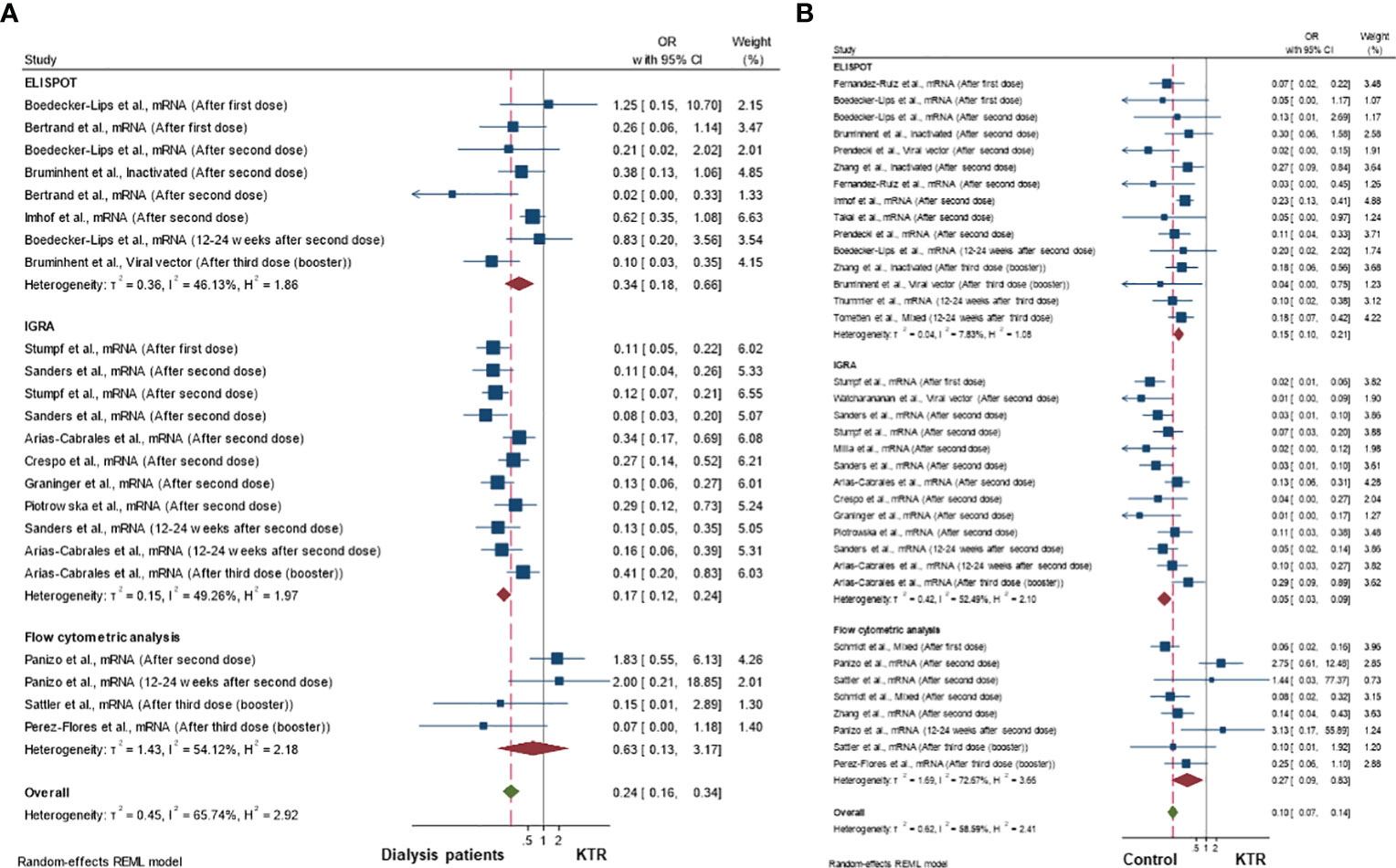
Figure 4 Cellular immune response rates in kidney transplant recipients compared to (A) dialysis patients and (B) healthy controls population.
In studies that followed kidney transplant recipients from primary vaccination to boosting doses and categorized them according to their cellular and antibody responses, 19.6 (95%CI 17.2-22.2) % of recipients had both cellular and antibody responses after two doses of vaccination, and this increased to 43.5 (38.0 – 49.2) % after receiving a third booster dose (Figures 5A, B). Compared to kidney transplant recipients, dialysis patients and controls were more likely to have both cellular and antibody immune responses after two doses of vaccination (69.9% and 90.9%, respectively) (Figures 5C, D).
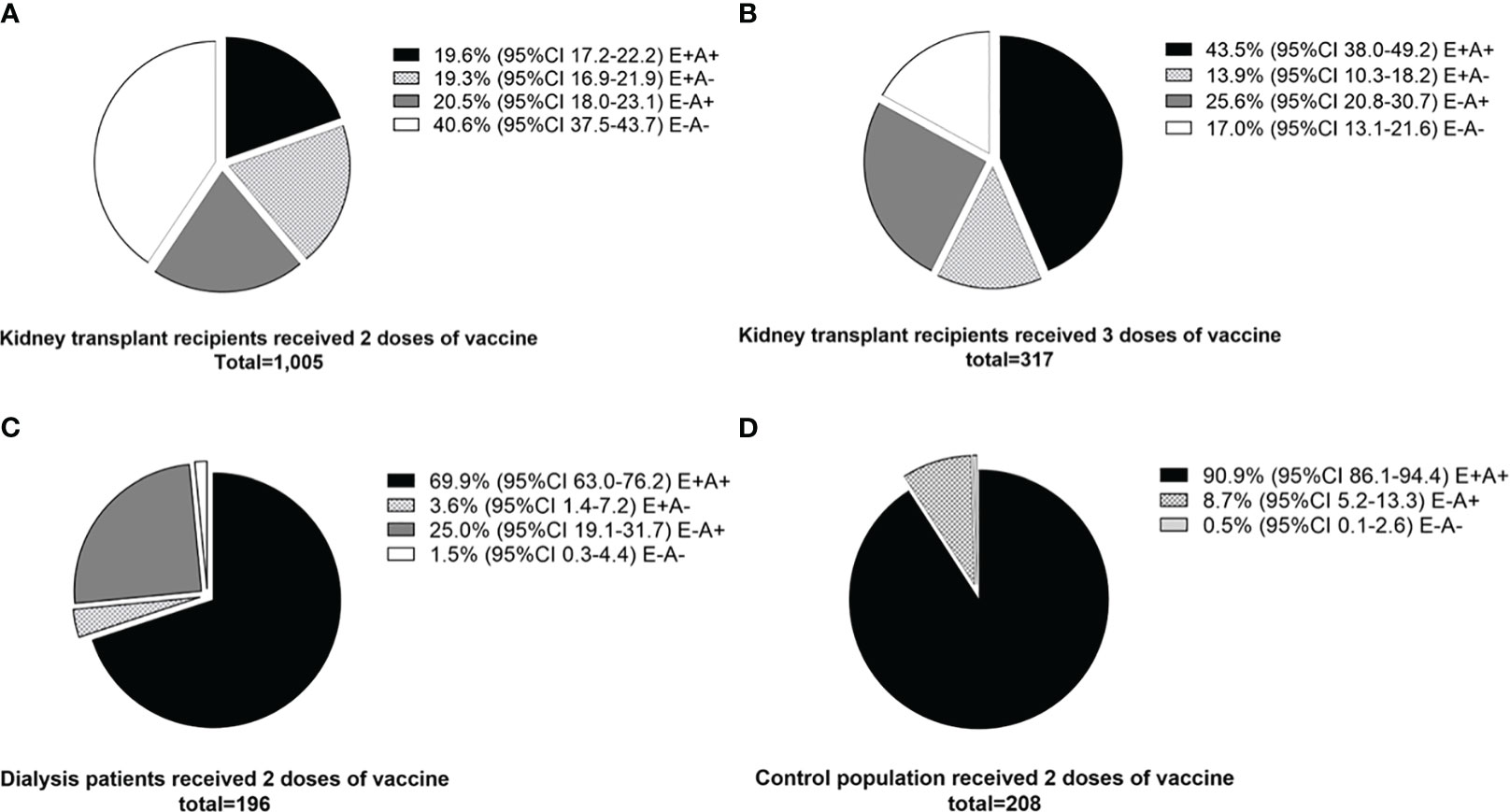
Figure 5 Comparison of cellular and antibody immune responses between different populations. (A) Kidney transplant recipients who received two doses of vaccine (standard regimen). (B) Kidney transplant recipients who received three doses of vaccine (after booster dose). (C) Dialysis patients who received two doses of vaccine. (D) Control population who received two doses of vaccine. E+A+; both cellular and antibody response, E+A-; cellular response but no antibody response, E-A+; antibody response but no cellular response, E-A-; both negative cellular and antibody response.
Factors associated with cellular immune response in kidney transplant recipients
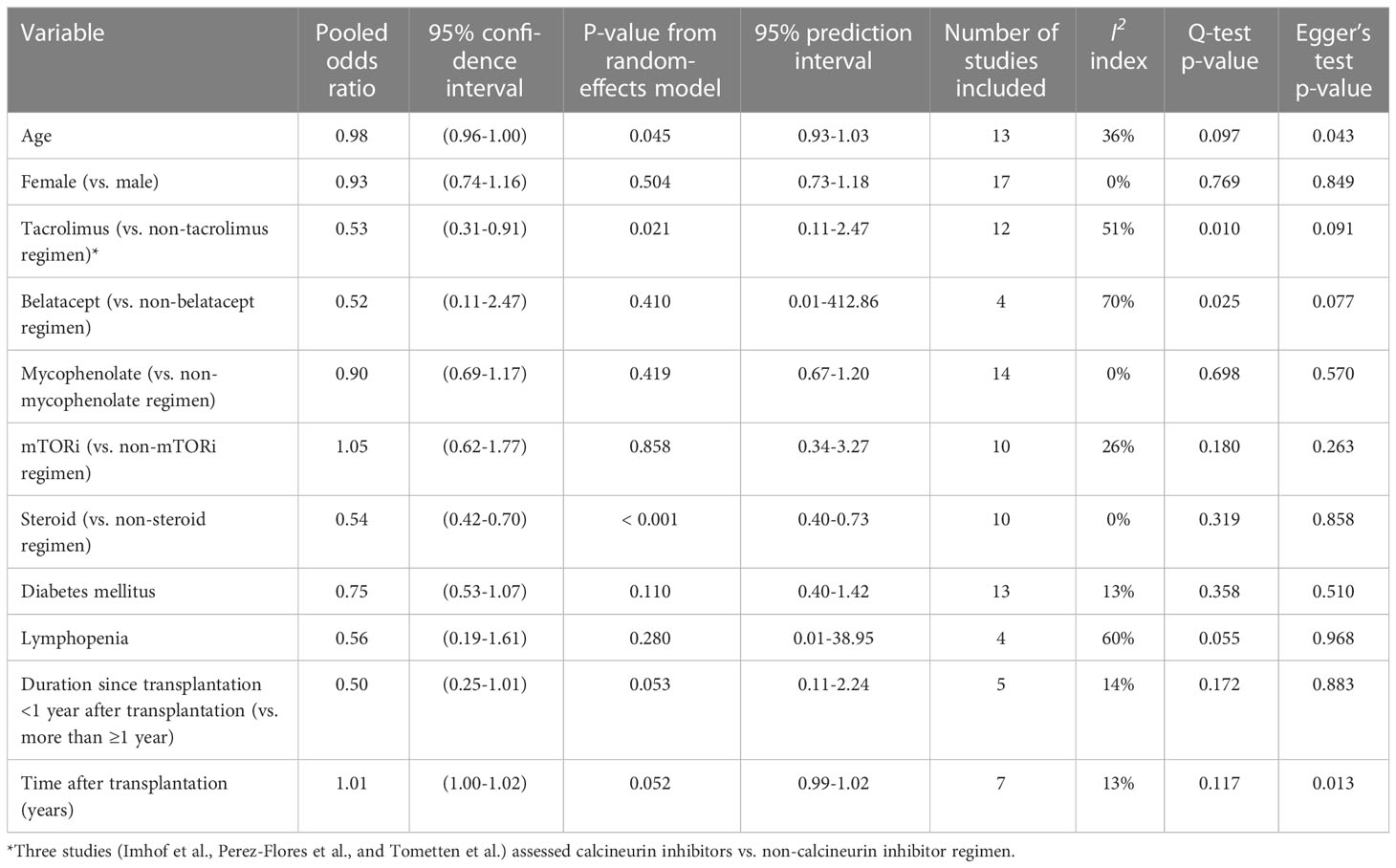
Table 2 demonstrates the pooled OR for factors that were evaluated for their association with cellular immune responder in the studies (16–18, 22, 26, 27, 30, 32, 33, 35, 37, 38, 40–42, 45, 50, 55, 57–59, 61, 66). Increasing age was associated with being a cellular immune non-responder (pooled OR 0.98; 95%CI 0.96-1.00; p-value=0.045; I2 36%, Q-test 0.097; Egger’s test 0.043). Tacrolimus (vs. non-tacrolimus regimens) was associated with a reduced odds of being a cellular immune responder (pooled OR 0.53; 95%CI 0.31-0.91; p-value=0.021; I2 51%, Q-test 0.010; Egger’s test 0.091). Corticosteroid-containing regimens were also associated with being non-responders (pooled OR 0.54; 95%CI 0.42-0.70; p-value<0.001; I2 0%, Q-test 0.319; Egger’s test 0.858). Although not reaching statistical significance, the time from transplant showed an association with cellular response rates, with patients less likely to achieve a response if they were <1 year from transplant and more likely to achieve a response as the duration since transplant increased. The forest plots and funnel plots for the factors evaluated in two or more studies for their association with cellular immune responses are illustrated in Supplementary Figures S8, S9. Many funnel plots show evidence of asymmetry, which might be due to publication bias or the heterogeneity of the included studies, although the interpretation of funnel plots with <10 studies is challenging (67).
Discussion
To our knowledge, this study is the first systematic review and meta-analysis to examine the cellular immune response in kidney transplant recipients after SARS-CoV-2 vaccination. Although our primary focus was on kidney transplant recipients, we made comparisons of cellular and humoral immune response rates in dialysis patients and controls after vaccination when these groups were included in individual studies. Positive cellular immune responses were more frequently found in the control population or dialysis patients than in kidney transplant recipients. Kidney transplant recipients who experienced seroconversion after vaccination were more likely to have a cellular immune response. Based on the available data, mRNA vaccines were not more likely to elicit a cellular immune response compared to viral vector vaccines. Age and post-transplant immunosuppressive regimens containing tacrolimus or corticosteroids were associated with a decreased chance of being a cellular immune responder.
The evaluation of cell-mediated immunity in kidney transplant recipients has been shown to provide valuable information on identifying recipients at risk of post-transplant complications. For example, the donor-specific IFN-γ ELSIPOT assay can determine recipients with a high risk of acute rejection (68). Kidney transplant recipients with positive CMV and BK virus-specific IFN-γ ELSIPOT assay or IGRA have a lower risk of CMV or BK virus infection (69, 70). For SARS-CoV-2, T-cell responses are needed to generate and maintain levels of high-affinity antibodies (6). T-cell immunity also plays an important role in preventing initial infection and limiting the extent of disease after infection, thus reducing the severity of disease. Cellular sensitization without seroconversion has been described in individuals with mild or asymptomatic COVID-19, which potentially indicates a role for the cellular immune system in clearing early infection and limiting the spread of the virus (71). A generally accepted concept is that high levels of neutralizing antibodies mediate protection from SARS-CoV-2 infection, and T cells and memory B cells help prevent severe disease and hospitalization (7). Importantly, T-cell immunity provides durable protection and can recognize a broad range of SARS-CoV-2 antigens, including those from variants of concern, where specific neutralizing antibody responses are greatly reduced or absent. T-cell responses are less sensitive to the single amino acid mutations seen in these variants, so loss of cross-protective immunity is unlikely (6–8).
Our study shows that different methods of evaluation resulted in different cellular immune response rates. It is important to recognize that there is currently no single best test for assessing cellular immunity. The negative IFN-γ ELISPOT assay or IGRA results do not imply the complete absence of cellular immune responses in the patients, since several cytokines are involved in the T-cells response, including tumor necrosis-α and interleukin-2 (72). A robust panel of tests would ideally be needed to accurately classify patients as immune responders or non-responders. Currently, flow cytometric analysis of the surface and intracellular staining offer simultaneous assessments of multiple aspects of cellular immunity by determining individual cytokine responses and whether naïve, memory, regulatory, or effector cells are involved. However, flow cytometric analysis requires well-trained personnel and still lacks methodological standardization between centers. On the contrary, commercial ELISPOT and IGRA kits are available but cannot capture every aspect of cellular immune responses (8). Future studies should explore the performance of advanced assays such as multiplex polymerase chain reaction or combinations of multiple cellular response assays to provide a more comprehensive assessment of cellular immune response signatures. This multifaceted assay has the potential to facilitate a more complete picture of the cellular immune response against SARS-CoV-2, consequently aiding in the identification of a precise diagnostic test capable of establishing an optimal threshold for immune protection. Such a study comparing different assays with a large number of included participants would also be useful to standardize evaluations.
The clinical outcomes of a COVID-19 infection, such as infectivity rate, severity and duration of symptoms, and mortality, would be the best indicators to determine the true effect of cellular immune responses versus humoral immune responses. Since some kidney transplant recipients in the studies in this meta-analysis uniquely had isolated cellular immune responses (Figure 5), this population could be a candidate to determine the optimal protection thresholds of pure cellular immunity. Additionally, assessing the effect of SARS-CoV-2 vaccines on allograft and patient survival would be interesting to explore in preexisting kidney transplant prediction models (73).
Humoral immune responses after SARS-CoV-2 vaccines in kidney transplant recipients were inferior to those observed in dialysis patients or control populations (74). In addition, the vaccination response rates in dialysis patients were lower compared to the general population, similarly to other vaccines that are routinely prescribed in clinical practice (75, 76). Tacrolimus- and corticosteroid-containing regimens were associated with lower cellular immune response rates. Since tacrolimus inhibits calcineurin activity in T cells (77), one could reasonably expect that tacrolimus significantly affects cell-mediated immunity after vaccination. Glucocorticoids have a wide spectrum of immunosuppressive effects, including the induction of apoptosis of T cells and B cells (78), which also influences the post-vaccination immune response. Although the estimates did not reach a significant level, the longer period between kidney transplantation and vaccination showed a trend of being associated with cellular immune responses. The lower net immunosuppression used in the later period after transplantation compared to the earlier period may explain this association. It should be noted that although age and tacrolimus-containing regimens were significant factors for cellular non-responders based on the 95%CI from the random-effect model meta-analysis, their 95% prediction intervals were wider and included the no-effect OR of 1.0. Relatively few studies provided sufficient information to make a comparison according to age and tacrolimus use. Therefore, this uncertainty might be explained by confounding from other individual factors shown to impact cellular immune response, and further studies may help with more precise estimates.
A strength of this systematic review and meta-analysis is the comprehensive analysis of multiple aspects regarding the cellular immune response against SARS-CoV-2 vaccines in kidney transplant recipients. The results from this study could serve as a reference of cellular response to two doses and booster doses of SARS-CoV-2 vaccines. Future studies can compare these results with those of newer generations of the SARS-CoV-2 vaccine.
Limitations of the study
There are also some limitations in this study. First, the clinical outcomes after vaccination were not reported in most studies, which precludes the comparison of COVID-19 infection rates and their clinical outcomes between cellular immune responders and non-responders. Second, the information on variant-specific cellular immune responses was not reported in most of the studies. Third, although we presented funnel plots for each outcome, these plots and the corresponding Egger tests results are difficult to interpret when the number of studies is low. Although it seems plausible that authors who conducted cellular immune response studies in specialized disease cohorts would publish their results, the possibility of publication bias cannot be completely discounted. Furthermore, there is evidence of asymmetry in multiple funnel plots, indicating possible publication bias or the heterogeneity of the included studies, although in funnel plots of less than 10 studies, it is difficult to differentiate the chance of asymmetry from real asymmetry, and these plots require careful interpretation (67). Some cohorts might not be able to fully exclude individuals with prior SARS-CoV-2 infection before receiving vaccinations, potentially impacting the rate of cellular immune response. Nonetheless, we mitigated this confounding by conducting a sensitivity analysis of the response rate after excluding studies that included recipients with known SARS-CoV-2 infection. Fourth, most of the included studies assessed the cellular immune response in only a proportion of their entire kidney transplant recipient cohorts without describing whether the selected patients were representative of the entire cohort. For this reason, we did not attempt any meta-regression of the response rates against individual cohort characteristics as the results would be confounded by potential selection bias. Finally, there is a potential for bias in assessing the odds of achieving cellular immune responses in kidney transplant recipients who have seroconverted compared to the non-seroconverters. This bias arises from the inclusion of studies that provided cellular immune response rates while excluding studies that solely reported humoral immune responses without reporting cellular response rates.
Conclusions
In conclusion, the cellular immune response following SARS-CoV-2 vaccine administration in kidney transplant recipients was lower than in dialysis patients and the control population. Recipients who seroconverted had a higher odds of developing cellular immune responses. Increasing age and taking immunosuppressive regimens that included tacrolimus and corticosteroid were associated with being a non-responder. More studies are needed to standardize the assays used for the evaluation of cellular immune responses and correlate the values with clinical outcomes. The new generation of vaccines should not solely aim for the improvement of humoral immunity but also for achieving an adequate cellular immune response.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Idea and study design: SU and SK. Study selection: SU and SK. Data collection and analysis: SU and SK. Writing of the article: SU. Manuscript review and edit: SG, AL, and SK. Funding acquisition: SU. All authors have read the manuscript and approved this submission.
Funding
This study is supported by the Ratchadapisek Sompoch Endowment Fund of Chulalongkorn University (CU_GR_63_157_30_60).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1220148/full#supplementary-material
References
1. Caillard S, Chavarot N, Francois H, Matignon M, Greze C, Kamar N, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant (2021) 21(3):1295–303. doi: 10.1111/ajt.16424
2. Udomkarnjananun S, Kerr SJ, Townamchai N, Susantitaphong P, Tulvatana W, Praditpornsilpa K, et al. Mortality risk factors of COVID-19 infection in kidney transplantation recipients: a systematic review and meta-analysis of cohorts and clinical registries. Sci Rep (2021) 11(1):20073. doi: 10.1038/s41598-021-99713-y
3. Chen JJ, Lee TH, Tian YC, Lee CC, Fan PC, Chang CH. Immunogenicity rates after SARS-CoV-2 vaccination in people with end-stage kidney disease: A systematic review and meta-analysis. JAMA Netw Open (2021) 4(10):e2131749. doi: 10.1001/jamanetworkopen.2021.31749
4. Li J, Ayada I, Wang Y, den Hoed CM, Kamar N, Peppelenbosch MP, et al. Factors associated with COVID-19 vaccine response in transplant recipients: A systematic review and meta-analysis. Transplantation (2022) 106(10):2068–75. doi: 10.1097/TP.0000000000004256
5. Manothummetha K, Chuleerarux N, Sanguankeo A, Kates OS, Hirankarn N, Thongkam A, et al. Immunogenicity and risk factors associated with poor humoral immune response of SARS-CoV-2 vaccines in recipients of solid organ transplant: A systematic review and meta-analysis. JAMA Netw Open (2022) 5(4):e226822. doi: 10.1001/jamanetworkopen.2022.6822
6. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol (2022) 23(2):186–93. doi: 10.1038/s41590-021-01122-w
7. Vardhana S, Baldo L, Morice WG 2nd, Wherry EJ. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci Immunol (2022) 7(71):eabo1303. doi: 10.1126/sciimmunol.abo1303
8. Paramithiotis E, Sugden S, Papp E, Bonhomme M, Chermak T, Crawford SY, et al. Cellular immunity is critical for assessing COVID-19 vaccine effectiveness in immunocompromised individuals. Front Immunol (2022) 13:880784. doi: 10.3389/fimmu.2022.880784
9. Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med (2022) 387(11):1011–20. doi: 10.1056/NEJMra2206573
10. Kemlin D, Gemander N, Depickère S, Olislagers V, Georges D, Waegemans A, et al. Humoral and cellular immune correlates of protection against COVID-19 in kidney transplant recipients. Am J Transplant (2023) 23(5):649–58. doi: 10.1016/j.ajt.2023.02.015
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71.
12. Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2014).
13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
14. Affeldt P, Koehler FC, Brensing KA, Adam V, Burian J, Butt L, et al. Immune responses to SARS-CoV-2 infection and vaccination in dialysis patients and kidney transplant recipients. Microorganisms (2021) 10(1). doi: 10.3390/microorganisms10010004
15. Affeldt P, Koehler FC, Brensing KA, Gies M, Platen E, Adam V, et al. Immune response to third and fourth COVID-19 vaccination in hemodialysis patients and kidney transplant recipients. Viruses (2022) 14(12). doi: 10.3390/v14122646
16. Arias-Cabrales C, Folgueiras M, Faura A, Eguia J, Padilla E, Hurtado S, et al. Dynamics of humoral and cellular responses in renal transplant recipients receiving 3 doses of SARS-CoV-2 mRNA vaccine. Transplantation (2023) 107(2):457–65. doi: 10.1097/TP.0000000000004433
17. Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Hanoy M, Laurent C, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. (2021) 32(9):2147–52. doi: 10.1681/ASN.2021040480
18. Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Laurent C, Etienne I, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int (2021) 100(6):1337–40. doi: 10.1016/j.kint.2021.09.014
19. Bertrand D, Lemée V, Laurent C, Lemoine M, Hanoy M, Le Roy F, et al. Waning antibody response and cellular immunity 6 months after third dose SARS-Cov-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Am J Transplant (2022) 22(5):1498–500. doi: 10.1111/ajt.16954
20. Boedecker-Lips SC, Lautem A, Runkel S, Klimpke P, Kraus D, Keil P, et al. Six-month follow-up after vaccination with BNT162b2: SARS-CoV-2 antigen-specific cellular and humoral immune responses in hemodialysis patients and kidney transplant recipients. Pathogens (2022) 11(1). doi: 10.3390/pathogens11010067
21. Bruminhent J, Setthaudom C, Kitpermkiat R, Kiertiburanakul S, Malathum K, Assanatham M, et al. Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients. Sci Rep (2022) 12(1):3587. doi: 10.1038/s41598-022-07574-w
22. Cassaniti I, Gregorini M, Bergami F, Arena F, Sammartino JC, Percivalle E, et al. Effect of a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine on humoral and cellular responses and serum anti-HLA antibodies in kidney transplant recipients. Vaccines (Basel) (2022) 10(6). doi: 10.3390/vaccines10060921
23. Charmetant X, Espi M, Barba T, Ovize A, Morelon E, Mathieu C, et al. Predictive factors of a viral neutralizing humoral response after a third dose of COVID-19 mRNA vaccine. Am J Transplant (2022) 22(5):1442–50. doi: 10.1111/ajt.16990
24. Chavarot N, Ouedrani A, Marion O, Leruez-Ville M, Vilain E, Baaziz M, et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation (2021) 105(9):e94–e5. doi: 10.1097/TP.0000000000003784
25. Chen CC, Huang YJ, Lai MJ, Lin MH, Lin WC, Lin HY, et al. Immunogenicity and safety of two-dose SARS-CoV-2 vaccination via different platforms in kidney transplantation recipients. Front Immunol (2022) 13:951576. doi: 10.3389/fimmu.2022.951576
26. Crespo M, Barrilado-Jackson A, Padilla E, Eguía J, Echeverria-Esnal D, Cao H, et al. Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am J Transplant (2022) 22(3):786–800. doi: 10.1111/ajt.16854
27. Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant (2021) 21(8):2727–39. doi: 10.1111/ajt.16701
28. Cucchiari D, Egri N, Rodriguez-Espinosa D, Montagud-Marrahi E, Casals-Urquiza J, Del Risco-Zevallos J, et al. Humoral and cellular immune responses after a 3-dose course of mRNA-1273 COVID-19 vaccine in kidney transplant recipients: A prospective cohort study. Transplant Direct (2022) 8(11):e1389.
29. Devresse A, Saad Albichr I, Georgery H, Yombi JC, De Greef J, Belkhir L, et al. T-cell and antibody response after 2 doses of the BNT162b2 vaccine in a belgian cohort of kidney transplant recipients. Transplantation (2021) 105(10):e142–e3. doi: 10.1097/TP.0000000000003892
30. Fernández-Ruiz M, Almendro-Vázquez P, Carretero O, Ruiz-Merlo T, Laguna-Goya R, San Juan R, et al. Discordance between SARS-CoV-2-specific cell-mediated and antibody responses elicited by mRNA-1273 vaccine in kidney and liver transplant recipients. Transplant Direct (2021) 7(12):e794.
31. Graninger M, Stumpf J, Bond G, Görzer I, Springer DN, Kessel F, et al. Prediction of humoral and cellular immune response to COVID-19 mRNA vaccination by TTV load in kidney transplant recipients and hemodialysis patients. J Clin Virol (2023) 162:105428. doi: 10.1016/j.jcv.2023.105428
32. Hall VG, Ferreira VH, Ierullo M, Ku T, Marinelli T, Majchrzak-Kita B, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J transplantation (2021) 21(12):3980–9. doi: 10.1111/ajt.16766
33. Imhof C, Messchendorp AL, van der Heiden M, Baan CC, van der Molen RG, Remmerswaal EBM, et al. SARS-CoV-2 spike-specific IFN-γ T-cell response after COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplant Direct (2022) 8(11):e1387. doi: 10.1097/TXD.0000000000001387
34. Kho MML, Messchendorp AL, Frölke SC, Imhof C, Koomen VJ, Malahe SRK, et al. Alternative strategies to increase the immunogenicity of COVID-19 vaccines in kidney transplant recipients not responding to two or three doses of an mRNA vaccine (RECOVAC): a randomised clinical trial. Lancet Infect Dis (2022). doi: 10.2139/ssrn.4176376
35. Körber N, Holzmann-Littig C, Wilkens G, Liao BH, Werz ML, Platen L, et al. Comparable cellular and humoral immunity upon homologous and heterologous COVID-19 vaccination regimens in kidney transplant recipients. Front Immunol (2023) 14:1172477. doi: 10.3389/fimmu.2023.1172477
36. La Milia V, Tonolo S, Luzzaro F, Bonato C, Casartelli D, Limardo M, et al. Humoral and T-cell response to SARS-CoV-2 mRNA BNT162b2 vaccination in a cohort of kidney transplant recipients and their cohabitant living kidney donor partners. Clin Kidney J (2022) 15(4):820–1. doi: 10.1093/ckj/sfac010
37. Magicova M, Zahradka I, Fialova M, Neskudla T, Gurka J, Modos I, et al. Determinants of immune response to anti-SARS-CoV-2 mRNA vaccines in kidney transplant recipients: A prospective cohort study. Transplantation (2022) 106(4):842–52. doi: 10.1097/TP.0000000000004044
38. Netti GS, Infante B, Troise D, Mercuri S, Panico M, Spadaccino F, et al. mTOR inhibitors improve both humoral and cellular response to SARS-CoV-2 messenger RNA BNT16b2 vaccine in kidney transplant recipients. Am J Transplant. (2022) 22(5):1475–82. doi: 10.1111/ajt.16958
39. Panizo N, Albert E, Giménez-Civera E, Puchades MJ, D'Marco L, Gandía-Salmerón L, et al. Dynamics of SARS-CoV-2-Spike-reactive antibody and T-cell responses in chronic kidney disease patients within 3 months after COVID-19 full vaccination. Clin Kidney J (2022) 15(8):1562–73. doi: 10.1093/ckj/sfac093
40. Pérez-Flores I, Juarez I, Aiffil Meneses AS, Lopez-Gomez A, Romero NC, Rodriguez-Cubillo B, et al. Role of mTOR inhibitor in the cellular and humoral immune response to a booster dose of SARS-CoV-2 mRNA-1273 vaccine in kidney transplant recipients. Front Immunol (2023) 14:1111569. doi: 10.3389/fimmu.2023.1111569
41. Piotrowska M, Zieliński M, Tylicki L, Biedunkiewicz B, Kubanek A, Ślizień Z, et al. Local and systemic immunity are impaired in end-stage-renal-disease patients treated with hemodialysis, peritoneal dialysis and kidney transplant recipients immunized with BNT162b2 Pfizer-BioNTech SARS-CoV-2 vaccine. Front Immunol (2022) 13:832924. doi: 10.3389/fimmu.2022.832924
42. Prendecki M, Thomson T, Clarke CL, Martin P, Gleeson S, De Aguiar RC, et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet (2021) 398(10310):1482–4. doi: 10.1016/S0140-6736(21)02096-1
43. Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, Jabbour R, Hofbauer TM, Merrelaar A, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: A randomized clinical trial. JAMA Intern Med (2022) 182(2):165–71. doi: 10.1001/jamainternmed.2021.7372
44. Reischig T, Kacer M, Vlas T, Drenko P, Kielberger L, Machova J, et al. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am J Transplant (2022) 22(3):801–12. doi: 10.1111/ajt.16902
45. Rezahosseini O, Hamm SR, Heftdal LD, Pérez-Alós L, Møller DL, Perch M, et al. Humoral and T-cell response 12 months after the first BNT162b2 vaccination in solid organ transplant recipients and controls: Kinetics, associated factors, and role of SARS-CoV-2 infection. Front Immunol (2022) 13:1075423. doi: 10.3389/fimmu.2022.1075423
46. Sanders JF, Bemelman FJ, Messchendorp AL, Baan CC, van Baarle D, van Binnendijk R, et al. The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation (2022) 106(4):821–34. doi: 10.1097/TP.0000000000003983
47. Sanders JSF, Lianne Messchendorp A, de Vries RD, Baan CC, van Baarle D, van Binnendijk R, et al. Antibody and T-cell responses 6 months after COVID-19 mRNA-1273 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Clin Infect Dis (2022). doi: 10.2139/ssrn.4070419
48. Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest (2021) 131(14). doi: 10.1172/JCI150175
49. Sattler A, Thumfart J, Tóth L, Schrezenmeier E, Proß V, Stahl C, et al. SARS-CoV2 mRNA vaccine-specific B-, T- and humoral responses in adolescents after kidney transplantation. Transpl Int (2022) 35:10677. doi: 10.3389/ti.2022.10677
50. Schmidt T, Klemis V, Schub D, Schneitler S, Reichert MC, Wilkens H, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant (2021) 21(12):3990–4002. doi: 10.1111/ajt.16818
51. Schrezenmeier E, Rincon-Arevalo H, Jens A, Stefanski AL, Hammett C, Osmanodja B, et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight (2022) 7(9). doi: 10.1172/jci.insight.157836
52. Schrezenmeier E, Rincon-Arevalo H, Stefanski AL, Potekhin A, Straub-Hohenbleicher H, Choi M, et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol. (2021) 32(12):3027–33. doi: 10.1681/ASN.2021070966
53. Stumpf J, Schwöbel J, Karger C, Schirutschke H, Mauer R, Klimova A, et al. Anti-SARS-CoV-2 revaccination success in kidney transplant recipients with no initial humoral response is linked to primary vaccine type. Front Med (Lausanne) (2022) 9:910987. doi: 10.3389/fmed.2022.910987
54. Stumpf J, Siepmann T, Lindner T, Karger C, Schwöbel J, Anders L, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur (2021) 9:100178. doi: 10.1016/j.lanepe.2021.100178
55. Stumpf J, Siepmann T, Schwöbel J, Glombig G, Paliege A, Steglich A, et al. MMF/MPA is the main mediator of a delayed humoral response with reduced antibody decline in kidney transplant recipients after SARS-CoV-2 mRNA vaccination. Front Med (Lausanne) (2022) 9:928542. doi: 10.3389/fmed.2022.928542
56. Stumpf J, Tonnus W, Paliege A, Rettig R, Steglich A, Gembardt F, et al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation (2021) 105(11):e267–e9. doi: 10.1097/TP.0000000000003903
57. Takai S, Nishida H, Ito H, Fukuhara H, Nawano T, Narisawa T, et al. Humoral and cellular immune response and the safety of third SARS-CoV-2 mRNA vaccine with longer interval after the second vaccination in kidney transplant recipients. Front Immunol (2022) 13:1050211. doi: 10.3389/fimmu.2022.1050211
58. Takai S, Nishida H, Ito H, Fukuhara H, Nawano T, Narisawa T, et al. Immunogenicity and safety of two doses of SARS-CoV-2 mRNA vaccine in kidney transplant recipients with low-dose rituximab. Int J Urol (2022) 29(11):1279–86. doi: 10.1111/iju.14978
59. Thomson T, Prendecki M, Gleeson S, Martin P, Spensley K, De Aguiar RC, et al. Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients. EClinicalMedicine (2022) 53:101642. doi: 10.1016/j.eclinm.2022.101642
60. Thümmler L, Gäckler A, Bormann M, Ciesek S, Widera M, Rohn H, et al. Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients. Vaccines (Basel) (2022) 10(8). doi: 10.3390/vaccines10081348
61. Tometten I, Landmann S, Kantauskaite M, Lamberti J, Hillebrandt J, Müller L, et al. Factors associated with vaccine-induced T-cell immune responses against severe acute respiratory syndrome Coronavirus 2 in kidney transplant recipients. J Infect Dis (2023) 227(5):641–50. doi: 10.1093/infdis/jiac449
62. Watcharananan SP, Jaru-Ampornpan P, Sahawongcharoen S, Naitook N, Himananto O, Jongkaewwattana A, et al. Comparison of the immunogenicity of ChAdOx1 nCoV-19 vaccine against the wild-type and delta variants in kidney transplant recipients and healthy volunteers. Am J Transplant (2022) 22(5):1459–66. doi: 10.1111/ajt.16966
63. Westhoff TH, Seibert FS, Anft M, Blazquez-Navarro A, Skrzypczyk S, Zgoura P, et al. A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int (2021) 100(5):1135–6. doi: 10.1016/j.kint.2021.09.001
64. Yahav D, Rahamimov R, Mashraki T, Ben-Dor N, Steinmetz T, Agur T, et al. Immune response to third dose BNT162b2 COVID-19 vaccine among kidney transplant recipients-A prospective study. Transpl Int (2022) 35:10204. doi: 10.3389/ti.2022.10204
65. Zhang L, Yang J, Lai C, Wan L, Xiong S, Kong W, et al. Immunity against Delta and Omicron variants elicited by homologous inactivated vaccine booster in kidney transplant recipients. Front Immunol (2022) 13:1042784. doi: 10.3389/fimmu.2022.1042784
66. Zhang R, Shin BH, Gadsden TM, Petrosyan A, Vo A, Ammerman N, et al. Assessment of humoral and cellular immune responses to SARS CoV-2 vaccination (BNT162b2) in immunocompromised renal allograft recipients. Transpl Infect Dis (2022) 24(2):e13813. doi: 10.1111/tid.13813
67. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj (2006) 333(7568):597–600. doi: 10.1136/bmj.333.7568.597
68. Udomkarnjananun S, Kerr SJ, Townamchai N, van Besouw NM, Hesselink DA, Baan CC. Donor-specific ELISPOT assay for predicting acute rejection and allograft function after kidney transplantation: A systematic review and meta-analysis. Clin Biochem (2021) 94:1–11. doi: 10.1016/j.clinbiochem.2021.04.011
69. Udomkarnjananun S, Kerr SJ, Francke MI, Avihingsanon Y, van Besouw NM, Baan CC, et al. A systematic review and meta-analysis of enzyme-linked immunosorbent spot (ELISPOT) assay for BK polyomavirus immune response monitoring after kidney transplantation. J Clin Virol (2021) 140:104848. doi: 10.1016/j.jcv.2021.104848
70. Ruan Y, Guo W, Liang S, Xu Z, Niu T. Diagnostic performance of cytomegalovirus (CMV) immune monitoring with ELISPOT and QuantiFERON-CMV assay in kidney transplantation: A PRISMA-compliant article. Med (Baltimore) (2019) 98(16):e15228. doi: 10.1097/MD.0000000000015228
71. Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell (2020) 183(1):158–68.e14. doi: 10.1016/j.cell.2020.08.017
72. Guo Y, Hu K, Li Y, Lu C, Ling K, Cai C, et al. Targeting TNF-α for COVID-19: recent advanced and controversies. Front Public Health (2022) 10:833967. doi: 10.3389/fpubh.2022.833967
73. Udomkarnjananun S, Townamchai N, Kerr SJ, Tasanarong A, Noppakun K, Lumpaopong A, et al. The first asian kidney transplantation prediction models for long-term patient and allograft survival. Transplantation (2020) 104(5):1048–57. doi: 10.1097/TP.0000000000002918
74. Kolb T, Fischer S, Müller L, Lübke N, Hillebrandt J, Andrée M, et al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360 (2021) 2(9):1491–8. doi: 10.34067/KID.0003512021
75. Udomkarnjananun S, Takkavatakarn K, Praditpornsilpa K, Nader C, Eiam-Ong S, Jaber BL, et al. Hepatitis B virus vaccine immune response and mortality in dialysis patients: a meta-analysis. J Nephrol (2020) 33(2):343–54. doi: 10.1007/s40620-019-00668-1
76. Udomkarnjananun S, Townamchai N, Wattanatorn S, On-Yim L, Vanichanan J, Jutivorakool K, et al. Maximizing anti-HBs seroresponsiveness in kidney transplant waitlist patients: A tertiary-center perspective. Clin Nephrol (2019) 92(4):190–200. doi: 10.5414/CN109767
77. Udomkarnjananun S, Francke MI, De Winter BCM, Mulder MB, Baan CC, Metselaar HJ, et al. Therapeutic drug monitoring of immunosuppressive drugs in hepatology and gastroenterology. Best Pract Res Clin Gastroenterol (2021) 54-55:101756. doi: 10.1016/j.bpg.2021.101756
Keywords: cellular immune response, COVID-19, Elispot, flow cytometry, interferon-γ, kidney transplantation, SARS-CoV-2 vaccine, T cells
Citation: Udomkarnjananun S, Gatechompol S, Leelahavanichkul A and Kerr SJ (2023) Cellular immune response of SARS-CoV-2 vaccination in kidney transplant recipients: a systematic review and meta-analysis. Front. Immunol. 14:1220148. doi: 10.3389/fimmu.2023.1220148
Received: 10 May 2023; Accepted: 05 July 2023;
Published: 26 July 2023.
Edited by:
Alain Le Moine, Université libre de Bruxelles, BelgiumReviewed by:
Naoki Tanimine, National Hospital Organization Kure Medical Center, JapanCaroline La, Université libre de Bruxelles, Belgium
Nicolas Gemander, Université libre de Bruxelles, Belgium, in collaboration with reviewer CL
Copyright © 2023 Udomkarnjananun, Gatechompol, Leelahavanichkul and Kerr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suwasin Udomkarnjananun, c3V3YXNpbi51QGdtYWlsLmNvbQ==
 Suwasin Udomkarnjananun
Suwasin Udomkarnjananun Sivaporn Gatechompol4
Sivaporn Gatechompol4 Asada Leelahavanichkul
Asada Leelahavanichkul Stephen J. Kerr
Stephen J. Kerr