- 1Infectious Diseases Divison, icddr, b (International Centre for Diarrhoeal Disease Research, Bangladesh), Dhaka, Bangladesh
- 2Department of Microbiology and Immunology, Institute of Biomedicine, University of Gothenburg, Gothenburg, Sweden
- 3Department of Clinical Immunology and Transfusion Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden
Background: Infection with enterotoxigenic Escherichia coli (ETEC) gives rise to IgA antibodies against both the heat labile toxin (LT) and colonization factors (CFs), which are considered to synergistically protect against ETEC diarrhea. Since the development of ETEC-specific long lived plasma cells and memory B cells is likely to be dependent on T helper (Th) cells, we investigated if natural ETEC diarrhea elicits ETEC-specific Th cells and their relation to IgA responses.
Methods: Th cell subsets were analyzed in adult Bangladeshi patients hospitalized due to ETEC diarrhea by flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) isolated from blood collected day 2, 7, 30 and 90 after hospitalization as well as in healthy controls. The LT- and CF-specific Th responses were determined by analysis of IL-17A and IFN-γ in antigen stimulated PBMC cultures using ELISA. ETEC-specific IgA secreted by circulating antibody secreting cells (plasmablasts) were analyzed by using the antibodies in lymphocyte supernatants (ALS) ELISA-based method and plasma IgA was also measured by ELISA.
Results: ETEC patients mounted significant ALS and plasma IgA responses against LTB and CFs on day 7 after hospitalization. ETEC patients had significantly elevated proportions of memory Th cells with a Th17 phenotype (CCR6+CXCR3-) in blood compared to controls, while frequencies of Th1 (CCR6-CXCR3+) or Th2 (CCR6-CXCR3-) cells were not increased. Antigen stimulation of PBMCs revealed IL-17A responses to LT, most clearly observed after stimulation with double mutant heat labile toxin (dmLT), but also with LT B subunit (LTB), and to CS6 in samples from patients with LT+ or CS6+ ETEC bacteria. Some individuals also mounted IFN-γ responses to dmLT and LTB. Levels of LTB specific IgA antibodies in ALS, but not plasma samples correlated with both IL-17A (r=0.5, p=0.02) and IFN-γ (r=0.6, p=0.01) responses to dmLT.
Conclusions: Our results show that ETEC diarrhea induces T cell responses, which are predominantly of the Th17 type. The correlations between IL-17A and IFN-g and intestine-derived plasmablast responses support that Th responses may contribute to the development of protective IgA responses against ETEC infection. These observations provide important insights into T cell responses that need to be considered in the evaluation of advanced ETEC vaccine candidates.
1 Introduction
Enterotoxigenic Escherichia coli (ETEC) is one of the most common causative agents of acute watery diarrhea in children and adults in low- and middle-income countries and is a key public health problem that results in considerable child mortality and morbidity (1). ETEC enters the gut through contaminated foods or water and subsequently attaches and colonizes the epithelial cells using different appendages present on the bacterial surfaces known as colonization factors (CFs) (2). So far over 25 different CFs have been identified and among them, CFA/I, CS5, CS6 and CS3 are often the most common (3, 4). ETEC produces heat-labile enterotoxin (LT) and/or heat-stable enterotoxin (ST), which modulate ion channels and increase the loss of salt and water into the intestinal lumen and ultimately cause watery diarrhea (2, 5).
ETEC is a non-invasive bacterium and protective immunity towards ETEC is considered to be mainly mediated by mucosal secretory IgA (SIgA) antibodies (2, 6). At present, there is no licensed vaccine available against ETEC. However, different preclinical and clinical trials with candidate ETEC vaccines are ongoing, including the oral vaccine ETVAX developed and evaluated by our study group (6, 7). The ETVAX vaccine contains inactivated E. coli bacteria expressing common CFs plus a LT-toxoid and is currently the most advanced vaccine candidate, currently undergoing Phase II testing, and Phase III studies are planned to be started soon (6, 8). Vaccination against ETEC mainly targets antibody mediated immunity. ETEC infection and vaccination both induce increased levels of mucosal and plasma antibodies against the B subunit of LT (LTB) and CFs (2, 6). Responses include transient increases in ETEC specific antibody secreting cells (ASCs; i.e. plasmablasts) in peripheral blood after antigenic exposure and development of memory B cell responses (6, 9, 10). Similar to in many other enteric infections, antigen specific ASCs detected in blood during early ETEC infection are considered good proxy measurement of a mucosal B cell response (9, 11–13). ETEC specific ASCs in blood correlate with the number of ASCs detected in intestinal biopsy specimens one week later (11) and ASCs specific for ETEC CFs and toxoid can also be efficiently isolated from peripheral blood of ETEC infected patients 7 days after onset of symptoms using magnetic beads coated with antibodies directed against the mucosal homing receptor α4β7 integrin (9). Natural ETEC infection provides protection against subsequent infection with homologous ETEC strains and memory B cells are likely to contribute to this protection (10, 14, 15).
The CD4+ T helper (Th) cells are well known for their role in the development, affinity maturation and isotype switching of antibody responses as well as in the generation of memory B cells following infection. However, T cell-mediated immune responses to natural ETEC infection remains to be described. In this study, we analyzed Th cells with a focus on Th17 responses, because of their important role in induction of mucosal immunity and protection against other gastrointestinal infections, including Vibrio cholerae, Shigella, Salmonella, Klebsiella pneumoniae and Helicobacter pylori (16–19). The Th17 cells are proinflammatory cells, characterized by the expression of the CCR6 chemokine receptor, which play a critical role in host defense and clearance of both extracellular and intracellular bacterial infections and in maintaining the mucosal epithelial barrier (20). Th17 cells typically produce the cytokine IL-17A, which is critical for the regulation of both protective and pathogenic immune responses, and may also promote the upregulation of polymeric immunoglobulin receptor on the epithelial cells to facilitate IgA secretion in the gut lumen (21). IL-17A is also required for IgA and IgG1 antibody production, stimulates fibroblasts and epithelial cells to secrete proinflammatory mediators, including G-CSF, GM-CSF, and IL-8, which have roles in neutrophil recruitment, granulopoiesis, and inflammatory responses (20, 22).
Our previous studies of Th responses to ETVAX suggest that the vaccine induces ETEC specific Th17 and Th1 cells as well as activated T follicular helper (Tfh)-like cells with predominantly a Th17 phenotype in the circulation of healthy Swedish adults (23) and Lundgren A. et al., unpublished). The Tfh-like responses were found to correlate with ETEC specific antibody responses in the vaccinees (23). The ETVAX vaccine has been administered together with the mucosal adjuvant double mutant LT (dmLT) (7, 24–27), which is also known to predominantly enhance Th17 responses to several types of vaccines and vaccine antigens, both in vitro and in vivo (28–31). dmLT is homologous to ETEC LT, except for two mutations in the enzymatically active A subunit, which inhibit the toxicity but not the adjuvanticity of the molecule (24). However, to what extent the T cell responses induced by the ETVAX vaccine plus dmLT adjuvant resemble T cell responses induced by natural ETEC infection is unclear at present. Considering the ability of ETEC infection to induce protective immunity and the importance of Th cells for induction of long-lived protective IgA responses in general, Th cell responses to natural ETEC infection is thus an important target for investigation.
The overall aim of this study was to investigate Th responses to natural ETEC infection and their relation to IgA antibody responses in hospitalized adult diarrheal patients, both during acute infection and the convalescence period. This in-depth analysis of Th responses after natural infection will provide important T cell benchmarks for evaluating leading ETEC vaccine candidates, including ETVAX, in future clinical studies and field trials.
2 Methods and materials
2.1 Study design
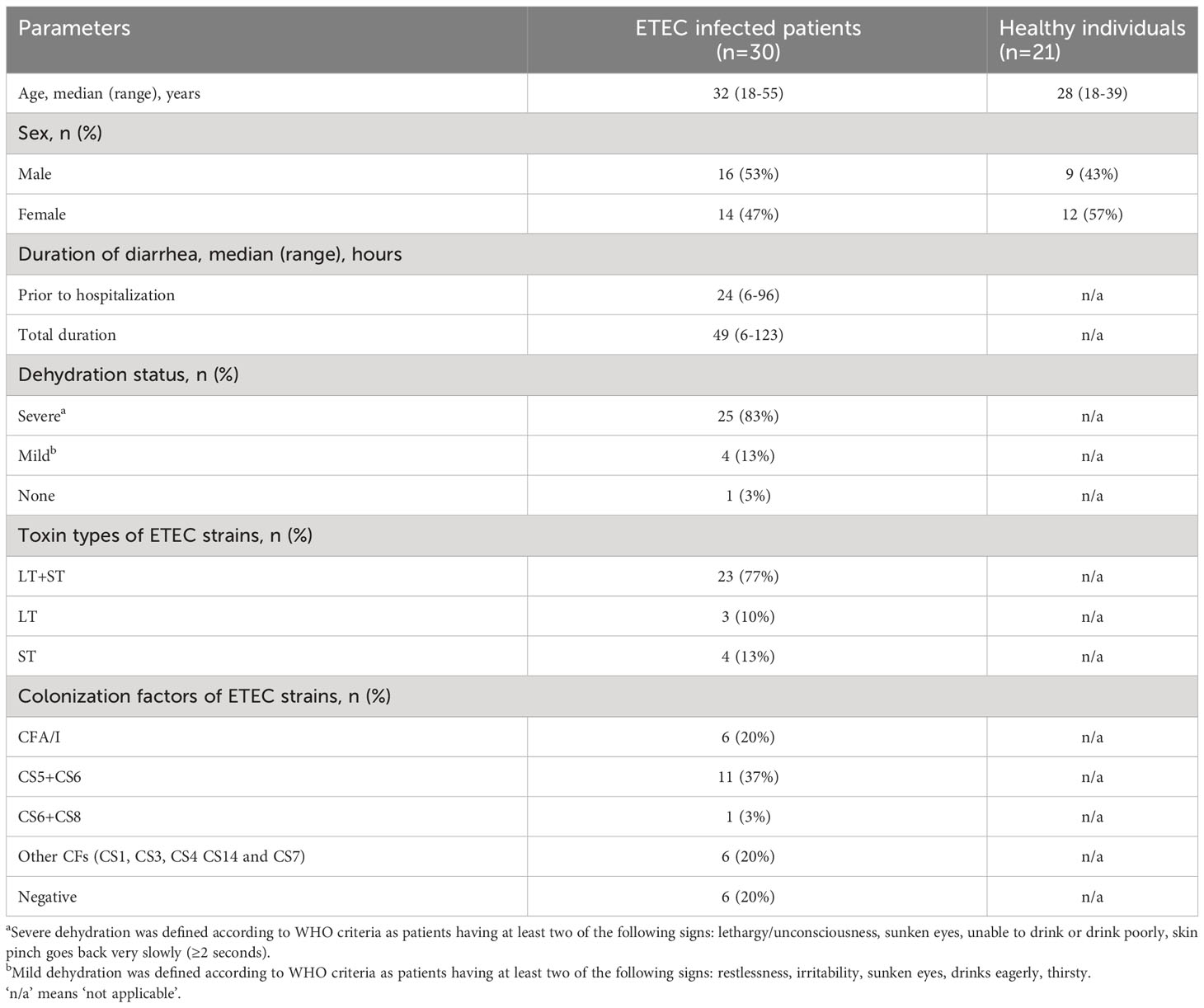
Diarrheal patients hospitalized at the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) in Dhaka, Bangladesh were screened for ETEC, Vibrio cholerae O1, Shigella, Salmonella and enteric parasites on the first day of hospitalization. Adult male and female patients (n=30) with stool specimens positive for ETEC, as determined by the presence of LT and/or ST enterotoxin genes using polymerase chain reaction (PCR) (32), but negative for other enteric pathogens, were enrolled (Table 1). Patients had experienced diarrhea for 6-96 hours (median 24 hours) prior to hospitalization and most had severe dehydration (83%). Expression of ETEC-specific CFs by the isolated ETEC strains was determined by a dot blot assay (33). Among the enrolled patients, 77% were infected with ETEC strains expressing both LT and ST, 10% were LT only and 13% were ST only (Table 1). A majority of isolated ETEC strains expressed known CFs; CS5+CS6+ and CFA/I+ strains were most common (37% and 20%, respectively), whereas strains expressing other CFs were less frequent (20%). In 20% of the strains, no CF was identified. In addition, healthy adult participants (n=21) who had no history of gastrointestinal disorders, diarrhea, febrile illness or antibiotic use during two weeks before enrollment, based on the clinical assessment by study physicians, were recruited from a similar urban area in Dhaka (Table 1). Informed written consent was obtained from each participant. The study was approved by the research review and the ethical review committees of icddr,b.
2.2 Blood collection and processing
Heparinized venous blood was collected from ETEC infected patients at the acute stage of illness (the second day after hospitalization, day 2), at early convalescence (day 7, range day 6-10), and late convalescence (30 ± 7 days and 90 ± 7 days after hospitalization). From healthy individuals, blood specimens were collected once after enrollment. Plasma and peripheral blood mononuclear cells (PBMCs) were separated by density-gradient centrifugation using Ficoll-Isopaque (Pharmacia, Sweden). Plasma specimens were stored at -20°C. From subsets of patients, CD4+ cells were depleted from PBMCs using magnetic beads (Dynabeads, Invitrogen, USA), according to the manufacturer´s instructions. The frequency of CD4+ T cells among lymphocytes after depletion was <3% (mean 1%), as determined by flow cytometric analysis.
2.3 Cell culture and stimulation
For analysis of T cell responses, PBMCs were cultured in DMEM F12 medium (Thermo Fisher Scientific, USA) supplemented with 50 mg/ml gentamicin (Sigma, USA) and 5% human AB+ serum (Sigma-Aldrich) at 37°C in a 5% CO2 incubator. Whole PBMCs and PBMCs depleted of CD4+ cells (105 cells/well) were cultured in duplicate wells in U-bottomed 96-well plates. Cells were left untreated or stimulated with phytohaemagglutinin (PHA,1 µg/ml, Remel, USA), dmLT (10 µg/ml), LTB (10 µg/ml), LTE112K (10 µg/ml), LT (10 µg/ml), CFA/I (1 µg/ml), CS5 (5 µg/ml) or CS6 (1 µg/ml). After 5 days culture, supernatants were collected and stored at -80°C for cytokine analysis. The indicated stimuli concentrations were found to induce maximal cytokine responses in initial set-up experiments testing a range of different concentrations. Details regarding production of different CF antigens, LTB and dmLT have been previously described (27). LTE112K, LT and LTB used in control experiments were kindly provided by J. Clements, Tulane University, USA.
For analysis of ETEC-specific ASCs, the antibodies in lymphocytes secretions (ALS) assay was used. Several studies have shown that results from the ALS assay, in which the levels of antigen-specific antibodies spontaneously secreted by peripheral blood ASCs in vitro are measured by ELISA, closely correlate with results from the more traditional ELISPOT assay where the numbers of antigen-specific ASCs are determined by counting spots developed on a membrane (13, 34–36). In the ALS assay, PBMCs were cultured at 107 cells/ml in RPMI complete medium (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), Na-pyruvate (1mM, Invitrogen), L-Glutamine (2mM, Invitrogen), 100 IU/ml Penicillin (Invitrogen) and 0.1mg/ml Streptomycin (Invitrogen). Cells were incubated in 96-well flat-bottomed tissue culture plates (2 x 106 cells/well) for 48 hours at 37°C with 5% CO2. Supernatants were collected and stored at -80°C.
2.4 ELISA analysis of cytokines and antibodies
The concentrations of IL-17A and IFN-γ were determined using sandwich ELISA (eBioscience, USA) following the manufacturer’s instructions. IgA antibodies specific for LTB, CFA/I, CS5 and CS6 in ALS and plasma specimens were analyzed by ELISA. Briefly, as described previously (26), to measure LTB-specific IgA antibodies in ALS/plasma specimens, 96-well ELISA plates (Nunc, USA) were coated with GM1 ganglioside (0.3 nmol/mL) overnight at room temperature followed by recombinant LTB subunit (1 μg/mL). To determine CF specific IgA antibody responses, high binding 96-well ELISA plates (Greiner, Austria) were coated with CFA/I (0.5 µg/mL), CS3 (0.3 µg/mL), CS5 (0.5 µg/mL) or CS6 (0.5 µg/mL). Plasma/ALS specimen was added to each well; initially diluted 1:10 (plasma LTB and CFs), 1:4 (ALS LTB) or 1:2 (ALS CFs) in PBS with 0.1% bovide serum albumin (BSA) and 0.05% Tween and a serially 3-fold dilution (plasma LTB and CFs and ALS LTB) or 2-fold (ALS CFs) was performed. The presence of antigen-specific antibodies was detected using horseradish peroxidase (HRP)-conjugated anti-human IgA (Jackson ImmunoResearch; 1:1500 dilution in 0.1% BSA-PBS-Tween) and ortho phenylenediamine (Sigma-Aldrich) in 0.1 M sodium citrate buffer (pH 4.5) and 30% hydrogen peroxide (Merck, USA). For plasma, the reactions were stopped after 20 minutes and endpoint titers were determined as the reciprocal interpolated dilutions of the samples at 450 nm that were 0.4 above background. For ALS, the reactions were stopped after 20 minutes by adding 1 M H2SO4 and endpoint titers were determined as the reciprocal interpolated dilutions of the samples at 492 nm that were 0.2 above background. All plasma, ALS and T cell supernatant samples from one individual collected at different time points were analyzed on the same plate in each type of assay and reference samples were included in all analyses to verify consistency between different runs.
2.5 Flow cytometric analysis
Fresh PBMCs (106) were stained with Aqua fluorescent live/dead (Fixable Dead Cells Stain Kit, Invitrogen) to exclude dead cells. To analyze the frequency of different Th cell subsets, PBMCs were stained with anti-CD3-PETR (clone S4.1, Invitrogen), anti-CD4-PerCP (SK3, Becton Dickinson, USA), anti-CD45RO-PECy7 (UCHL1, BD), anti-CXCR3-PE (1C6/CXCR3, BD) and anti-CCR6-BV421 (11A9, BD). To analyze the purity of CD4-depleted PBMCs, cells were stained with anti-CD3-PETR (clone S4.1, Invitrogen) and anti-CD4-PerCP (SK3, BD). After staining, cells were acquired (100,000 total events) using FACSAria™ Fusion (BD, USA). Results were analyzed using FlowJo software (version 10, FlowJo LLC, BD, USA). Among the lymphocytes, 99% cells were found to be alive as determined by live/dead staining. Different subsets of live T cells were analyzed following the gating strategy shown in Supplementary Figure 1.
2.6 Statistical analyses
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., USA). The Kruskal Wallis test with Dunn’s multiple comparison post-test was used to compare immunological responses in healthy controls and individual sampling time points of ETEC patients. The Mann-Whitney test was used to compare maximum responses at any time point between ETEC patients and healthy individuals. The increases (fold-rises) in magnitudes of immune responses in patients were calculated either as the antibody or cytokine levels found in patient samples on day 2, 7, 30 or 90 after hospitalization divided by the corresponding mean+2SD values in samples from healthy controls or as the antibody or cytokine levels found in patients on day 7, 30 or 90 after hospitalization divided by the corresponding levels on day 2, as indicated for each analysis. A responder was defined as having ≥2-fold increase in the magnitude of response. The Fisher’s Exact test was used to compare the responder frequencies at different time points. Correlation analyses were performed using the Spearman test. P values <0.05 were considered significant.
3 Results
3.1 Intestine derived and plasma IgA responses
We first investigated the mucosal and plasma IgA responses to ETEC infection in adult hospitalized ETEC infected diarrheal patients. Mucosal responses were analyzed by measuring LTB- and CF-specific IgA antibodies secreted from intestine-derived ASCs migrating in peripheral circulation using the ALS method. Analysis of ASCs using ALS or ELISPOT has been shown to be a good correlate of mucosal B-cell responses in many studies of enteric infections and vaccines (11–13, 37). IgA antibodies specific for LTB and CFs most commonly expressed by the infecting strains (ETEC positive for CS6, CS5 and CFA/I) were analyzed in samples from both diarrheal patients infected with ETEC strains expressing the corresponding antigens and healthy controls. Two days after hospitalization, ALS IgA responses were low and infrequent in most patients, but 8-33% of the patients responded to LTB, CS6 and CFA/I with antibody levels at least 2-fold higher than those observed in healthy controls already at this early time point (Figure 1 and Table 2). On day 7 after hospitalization, significant increases in levels of ALS IgA responses were observed against both LTB and all CFs compared to healthy individuals (Figure 1 and Table 2). CF-specific ALS responses were more frequent (82-100%) than LTB responses (56%) at this time point (Table 2). Responses were low in most participants on day 30 except for IgA against CFA/I, which remained elevated in about half of the analyzed participants (P>0.05). The ALS antibody levels on day 7 and day 30 were also compared to levels measured in samples collected two days after hospitalization (corresponding to 6 hours to 4 days after onset of symptoms) (Supplementary Table 1). This control analysis gave similar responder frequencies as comparisons to the healthy controls for most antigens, but slightly lower responder frequencies to CFA/I.

Figure 1 ALS IgA antibody responses to LTB and CFs in ETEC infected patients and healthy adults. (A) LTB-, (B) CS6-, (C) CS5- and (D) CFA/I-specific IgA titers were analyzed in ALS samples in healthy adults (n=10) and diarrheal patients on day 2, 7 and 30 after hospitalization (A); n=20-26, (B); n=10-12, (C); 9-11 and (D); n=5-6. Each symbol represents one individual, and bars indicate median values. Statistical analysis was performed using the Kruskal Wallis test with Dunn’s multiple comparison post test. ***P <0.001, ns, not significant; P >0.05.
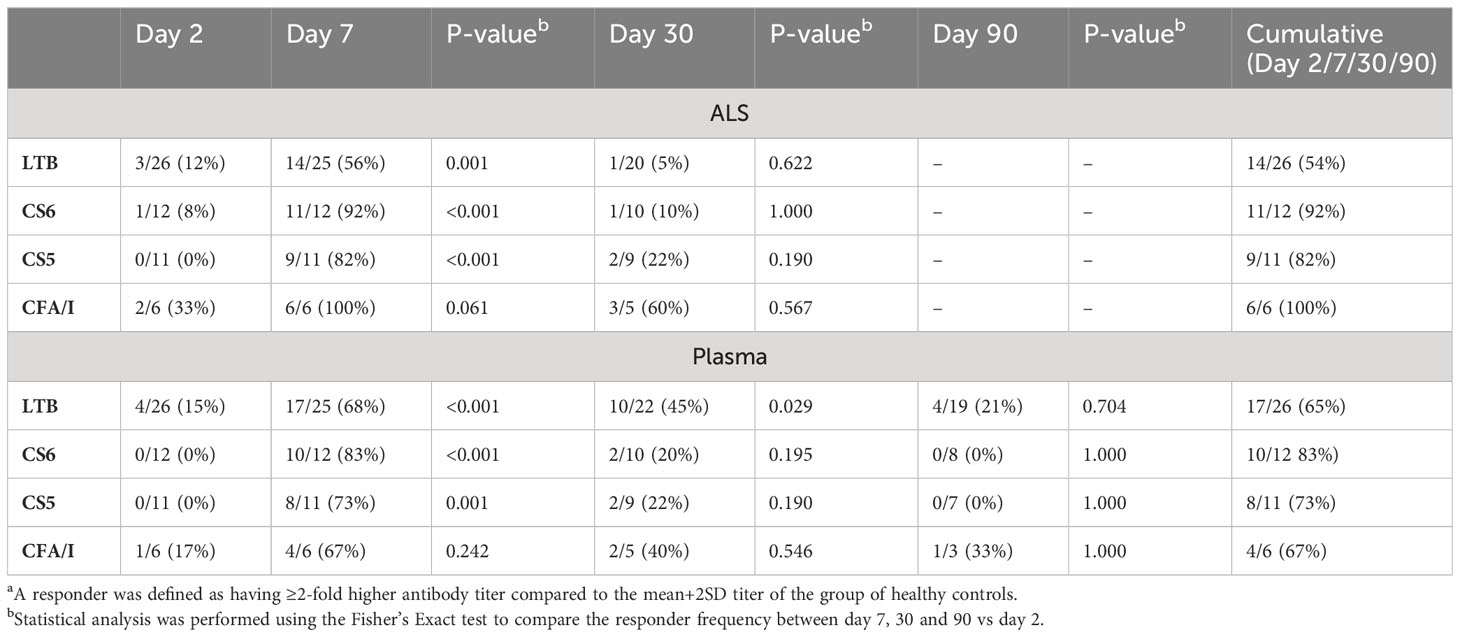
Table 2 Frequencies of IgA antibody respondersa against LTB and CF antigens in ALS and plasma specimens from ETEC patients.
ETEC patients also mounted significant IgA responses in plasma. Magnitudes of IgA responses to LTB were increased already on study day 2 in 15% of the participants compared to healthy controls, whereas few patients responded to CFs in plasma at this early time point (Figure 2 and Table 2). On day 7, magnitudes of responses to both LTB and all CFs were significantly higher in patients compared to healthy controls and reached the highest levels detected during the study period (Figure 2). Among the patients, 68% had at least 2-fold increased levels of LTB-specific IgA and 67-83% CF-specific IgA in plasma on day 7 compared to healthy controls (Table 2). The responder frequencies were slightly higher for most antigens when comparisons were made to levels measured on day 2 instead of to healthy controls (Supplementary Table 1). Plasma responses against all antigens declined until day 30, but LTB- (both magnitudes and responder frequencies) and CFA/I-specific (only magnitudes) responses still remained significantly elevated compared to levels in healthy controls at this time point (Figure 2 and Table 2). All plasma responses declined to levels comparable to those observed in controls by day 90.
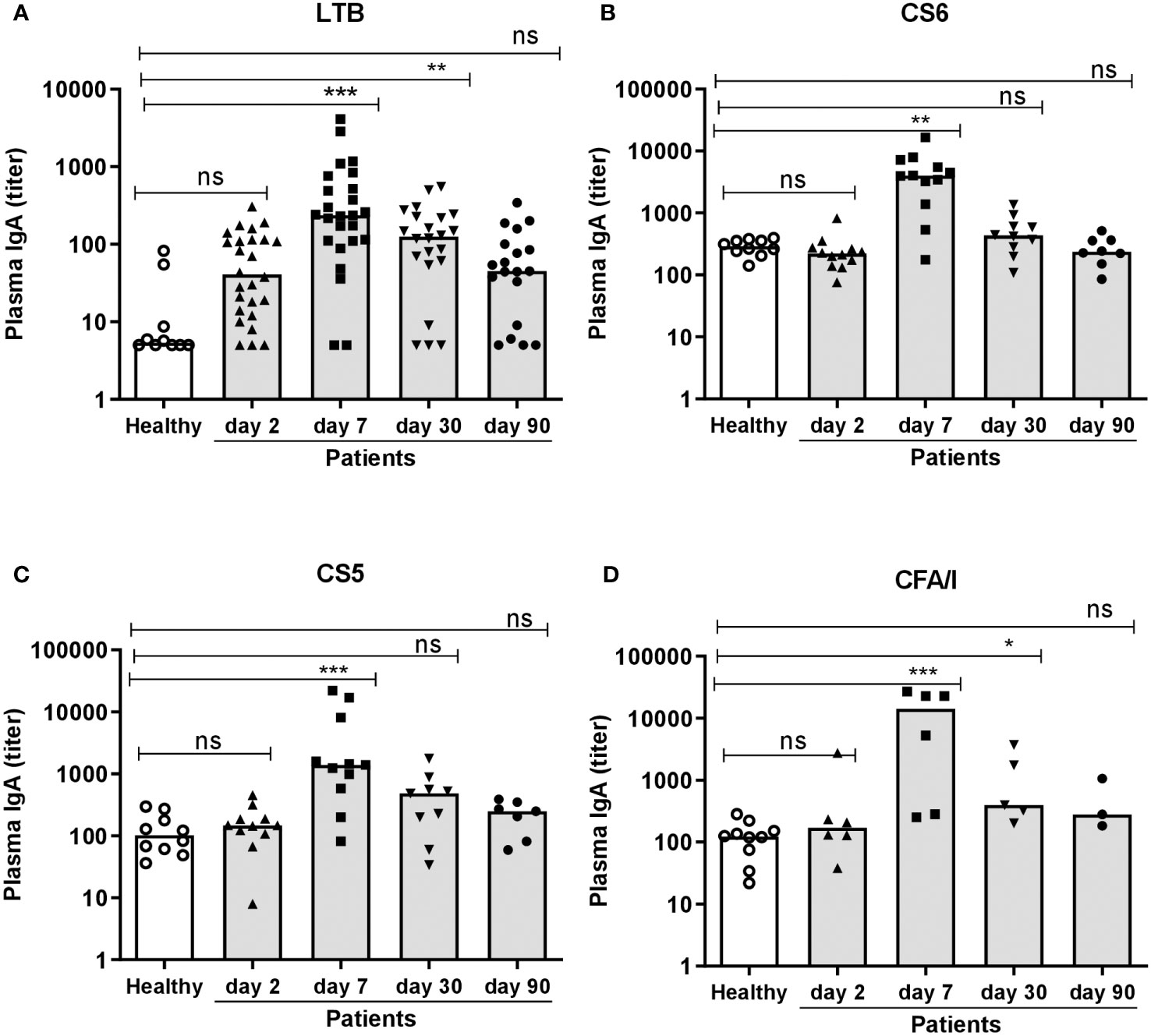
Figure 2 Plasma IgA antibody responses to LTB and CFs in ETEC infected patients and healthy adults. (A) LTB-, (B) CS6-, (C) CS5- and (D) CFA/I-specific IgA titers were analyzed in plasma samples in healthy adults (n=10) and diarrheal patients on day 2, 7, 30 and 90 after hospitalization (A); n=19-26, (B); n=8-12, (C); 7-11 and (D); n=3-6. Each symbol represents one individual, and bars indicate median values. Statistical analysis was performed using the Kruskal Wallis test with Dunn’s multiple comparison post test. *P <0.05, **P <0.01, ***P <0.001, ns, not significant; P >0.05.
Collectively, these results show that a majority of the ETEC infected patients enrolled in the study mounted strong mucosal and plasma IgA responses to the LT and CFs expressed by the infecting ETEC strains, with maximal antibody levels detected on day 7 after hospitalization.
3.2 CD4+ memory Th cell responses
To investigate the Th response to ETEC, we first analyzed the total frequencies of memory Th (CD4+CD45RO+) cells in fresh PBMCs collected from ETEC patients infected with ETEC strains expressing LT, CFA/I, CS5 or CS6 and healthy controls. Frequencies of memory Th cells among lymphocytes were comparable in ETEC patients and controls both in the acute and convalescence phase of infection (Figure 3A). However, when maximum frequencies of memory Th cells at any time point were compared with proportions in the healthy controls, a small but significant increase was observed in ETEC patients (Figure 3B), which was not seen when analyzing levels at each time point separately (Figure 3A).
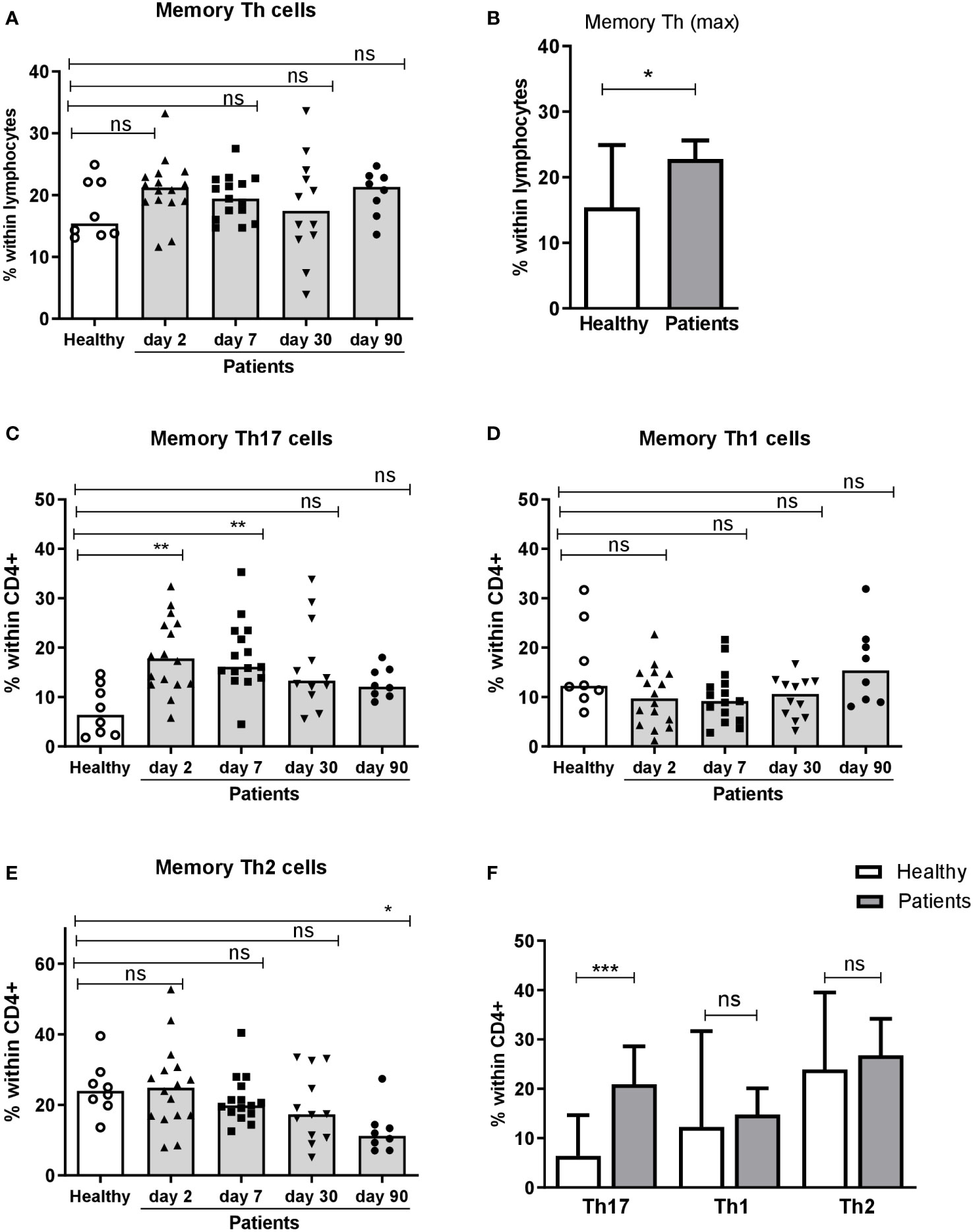
Figure 3 Frequencies of CD4+ memory Th cells and Th17, Th1 and Th2 subsets in peripheral blood of ETEC infected patients and healthy adults. Frequencies of (A, B) memory Th cells (CD4+CD45RO+) within lymphocytes, and (C) Th17 (CCR6+CXCR3-), (D) Th1 (CCR6-CXCR3+) and (E) Th2 (CCR6-CXCR3-) populations within CD4+ cells in PBMCs isolated from healthy adults (n=8) and ETEC infected patients on day 2, 7, 30 and 90 after hospitalization (n=8-16). (A, C E) Frequencies at each individual time point. Each symbol represents the percentage of cells for one individual, and bars indicate median values. (B, F) Maximum frequencies at any time point. Bars represent median + 95% confidence interval. Statistical analysis was performed by the Kruskal Wallis test with Dunn’s multiple comparison post test (A, C, E) or the Mann-Whitney test (B, F). *P <0.05, **P <0.01, ***P <0.001, ns, not significant; P >0.05.
Next, we analyzed the proportions of Th1, Th2 and Th17 subsets within memory Th cells based on the expression of the chemokine receptors CXCR3 and CCR6 in both patients and controls. The frequencies of Th17 (CCR6+CXCR3-) cells were significantly increased in patients already on day 2 compared to the healthy controls and Th17 responses remained significantly elevated until day 7 of infection (Figure 3C). The proportions of Th17 cells decreased on day 30-90. In contrast, ETEC diarrhea did not induce any increases in proportions of Th1 (CCR6-CXCR3+) or Th2 (CCR6-CXCR3-) cells at any time point (Figures 3D, E). Analysis of maximum frequencies of cells at any time point during infection gave the same result; strongly increased proportions of Th17 cells, whereas frequencies of the Th1 and Th2 subsets were comparable to the controls (Figure 3F). These flow cytometric results suggest that ETEC infection induces a Th response that is predominantly of the Th17 type, but did not reveal the antigen specificity of the responding cells.
3.3 LT-specific Th responses
To determine if the increased proportions of Th17 cells in the circulation of ETEC infected patients were due to LT- and/or CF-specific T cell responses, PBMCs from both patients and controls were stimulated with LTB, dmLT or CFs matching the infecting strain and the secretion of IL-17A and IFN-γ was analyzed in the culture medium.
T cell responses to LT were seen more clearly after stimulation with dmLT than LTB. A few patients with LT+ ETEC infection had IL-17A responses against dmLT or LTB on day 2 (9-13%), but significant responses to dmLT were observed first on day 7 after hospitalization, when 41% of the participants showed elevated IL-17A levels compared to controls (Figure 4A and Table 3). Responses peaked on day 7, but remained elevated until day 30 in 22% of patients (P>0.05 compared to day 2). In most patients, IL-17A responses returned to the levels observed in healthy controls on day 90. A trend for increased IL-17A responses after stimulation with LTB was also observed on day 7 (Figure 4C) when 33% patients responded in comparison to healthy controls (Table 3). When the maximum levels of IL-17A responses at any time point of infection were analyzed, increased IL-17A responses were found against both dmLT (56%) and LTB (45%) (Figure 4E and Table 3). Depletion of CD4+ Th cells from PBMCs isolated on day 7 after infection resulted in almost complete loss of IL-17A production in response to stimulation with dmLT and LTB (Supplementary Figures 2A, B), supporting that the IL-17A responses to LT were derived from CD4+ Th cells.
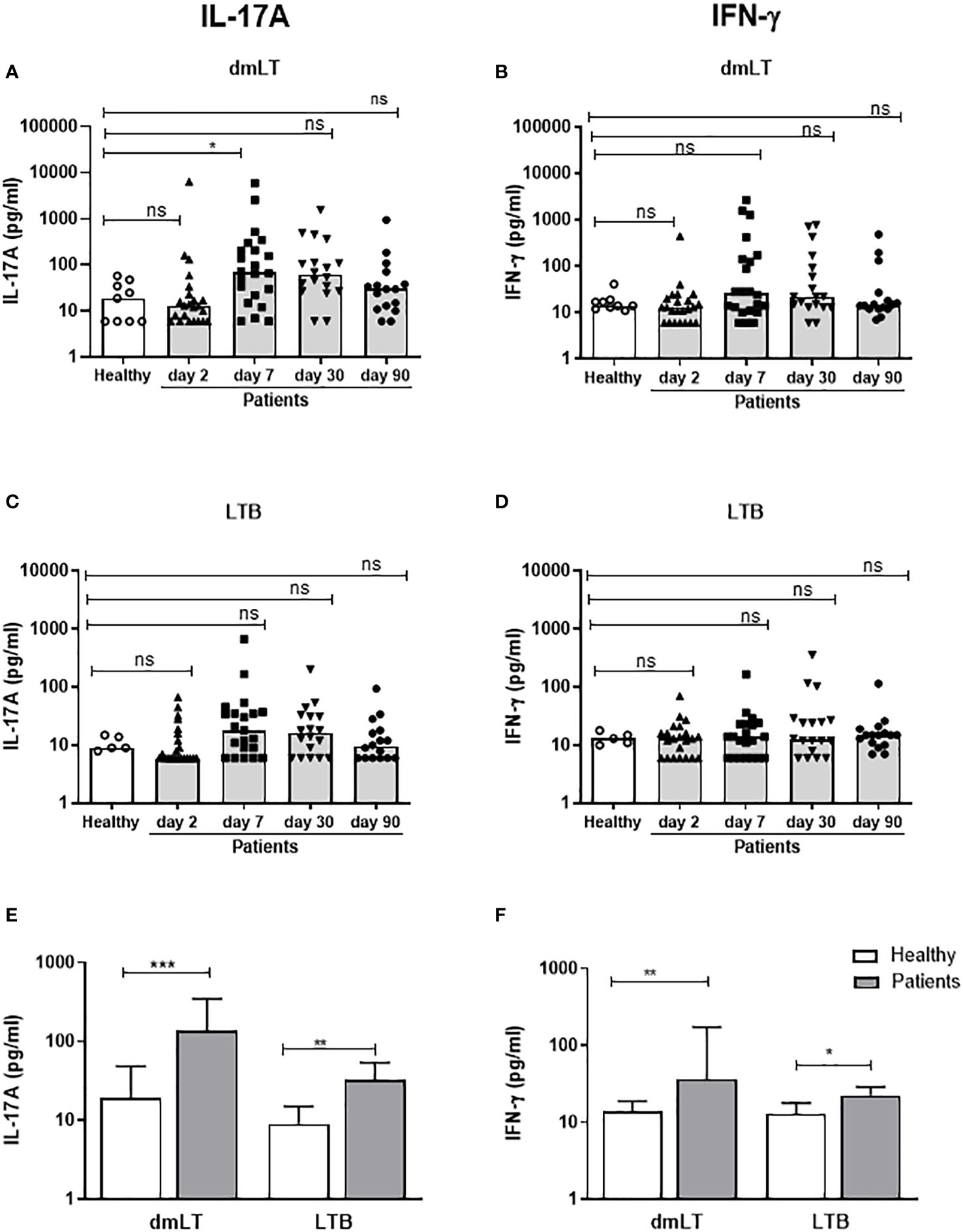
Figure 4 IL-17A and IFN-γ responses to dmLT and LTB in PBMCs from ETEC infected patients and healthy adults. IL-17A (A, C, E) and IFN-γ (B, D, F) concentrations in cultures with PBMCs stimulated with dmLT (A, B) and LTB (C, D) in healthy controls (n=5-9) and diarrheal patients on day 2, 7, 30 and 90 after hospitalization (A, B); n=16-23 and (C, D); n=16-22. (A-D) Responses at each individual time point. Each symbol represents one individual, and bars indicate median values. (E, F) Maximum responses at any time point. Bars represent median + 95% confidence interval. Statistical analysis was performed using the Kruskal Wallis test with Dunn’s multiple comparison post test (A-D) or Mann-Whitney test (E, F). *P <0.05, **P <0.01, ***P <0.001, ns; not significant; P >0.05.
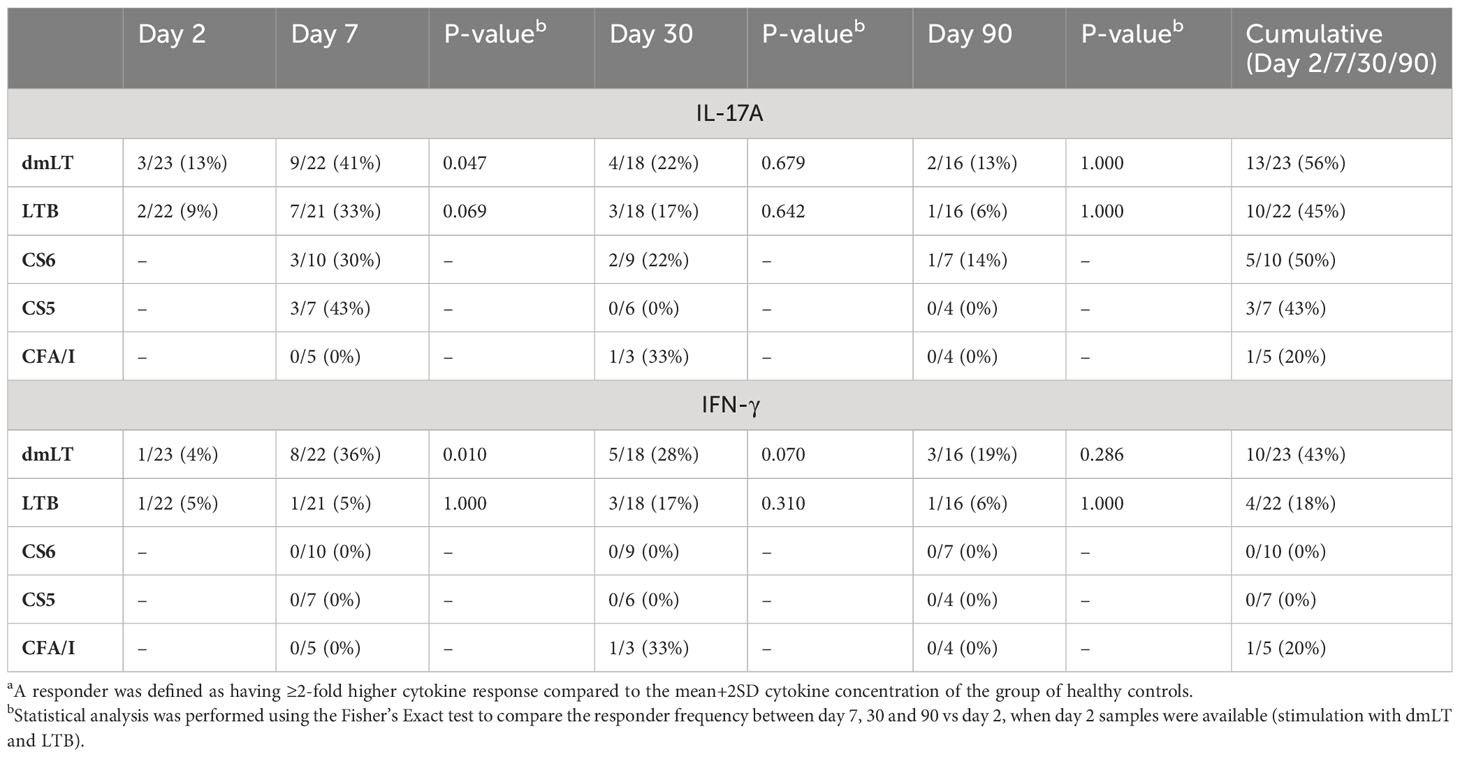
Table 3 Frequencies of IL-17A and IFN-γ respondersa against dmLT, LTB and CF antigens in ETEC infected patients.
We also analyzed IFN-γ responses to dmLT and LTB in patients and controls. Neither dmLT- nor LTB-stimulation induced significant magnitudes of IFN-γ responses at any individual time point after infection (Figures 4B, D), but the responder frequencies to dmLT were significantly higher on day 7 compared to day 2 (36 vs 4%, Table 3). When maximum magnitudes of responses were evaluated at any time point, patients had significantly higher IFN-γ responses against both dmLT and LTB compared to healthy individuals (Figure 4F), although the median magnitudes of responses were lower than for IL-17A. Maximum increases in magnitudes (fold-rises) of IL-17A responses to dmLT or LTB did not correlate significantly with increases in magnitudes of IFN-γ responses to the same antigens (Supplementary Figure 3).
We have previously shown that dmLT enhances IL-17A responses to both antigens and mitogens/superantigens in vitro (29–31). To determine if the strong IL-17A response observed after stimulation with dmLT compared to LTB was related to the adjuvant effect of dmLT, cells from patients infected by LT+ ETEC were also stimulated with native LT and the LT-derivative LTE112K, which has a single mutation in the A-subunit that inhibits the ability of the molecule to induce cAMP and act as an oral adjuvant (38, 39). First, the adjuvant activity of dmLT and LTE112K was evaluated by stimulating PBMCs with the mitogen PHA with or without addition of dmLT or LTE112K. Only dmLT enhanced IL-17A responses to PHA (Supplementary Figure 4A), confirming that dmLT but not LTE112E has adjuvant function. We did not measure IFN-γ responses, since our previous studies have shown that dmLT cannot enhance IFN-γ responses in vitro (29–31). Next, the responses to dmLT on day 2 and day 7 were compared to responses induced by native LT or the other homologues LTE112K and LTB. dmLT, LT and LTE112K all induced significantly increased levels of IL-17A on day 7 compared to day 2 (Supplementary Figure 4B, P=0.02, P=0.01, P=0.02, respectively). IFN-γ responses were weaker and more variable between patients and only significant for dmLT and LT (P=0.01, P=0.03, respectively; Supplementary Figure 4C). Magnitudes of IL-17A and IFN-γ responses to LT and LTE112K were comparable to the responses induced by dmLT, whereas responses to LTB were significantly lower (Supplementary Figures 4B, C). Responses to LTB produced in the same laboratory (denoted by LTB2) as LT and LTE112K were also comparable to responses induced by stimulation with the LTB used in all other T cell and antibody analyses in the study (denoted by LTB1). The similar cytokine responses to dmLT and LTE112K, but lower responses to LTB, indicate that the strong response to dmLT is not directly related to the enzymatic effect of the A subunit included to the dmLT molecule.
Taken together, these results suggest that ETEC infected patients mount Th responses to LT that are predominantly of the Th17 type and can be detected at maximal levels 7 days after hospitalization.
3.4 CF-specific Th responses
We also analyzed Th responses to CFs expressed by the infecting strains on day 7, 30 and 90 after hospitalization. We could not measure CF-specific T cell responses on day 2, since the CF expression of the infecting ETEC strain was not known at this time point and the cell numbers did not allow stimulation with several different CFs. Patients infected with CS6+ ETEC had significantly increased magnitudes of IL-17A responses against CS6 on day 7 compared to healthy controls (30% responder frequency) but responses remained elevated in only a few patients on day 30-90 (Figure 5A and Table 3). Some patients responded with increased levels IL-17A production to stimulation with CS5 and CFA/I on day 7, however, the number of patients tested for these antigens was small (n=3-7) and responses were not significant (Figures 5C, E). Considering the maximum IL-17A responses at any time point, increased responses were again only observed against CS6 (Figure 5G). Depletion of CD4+ Th cells from PBMCs isolated on day 7 after infection substantially reduced IL-17A production in response to stimulation with CS6 and CS5 (Supplementary Figure 2C), supporting that the observed IL-17A responses to CFs were derived from CD4+ Th cells. Patients had almost no detectable CF-specific IFN-γ responses at any time point after infection (Figures 5B, D, F). However, a very small but significant increase in IFN-γ was found for CS5 when maximum responses at any time point were considered (Figure 5H).
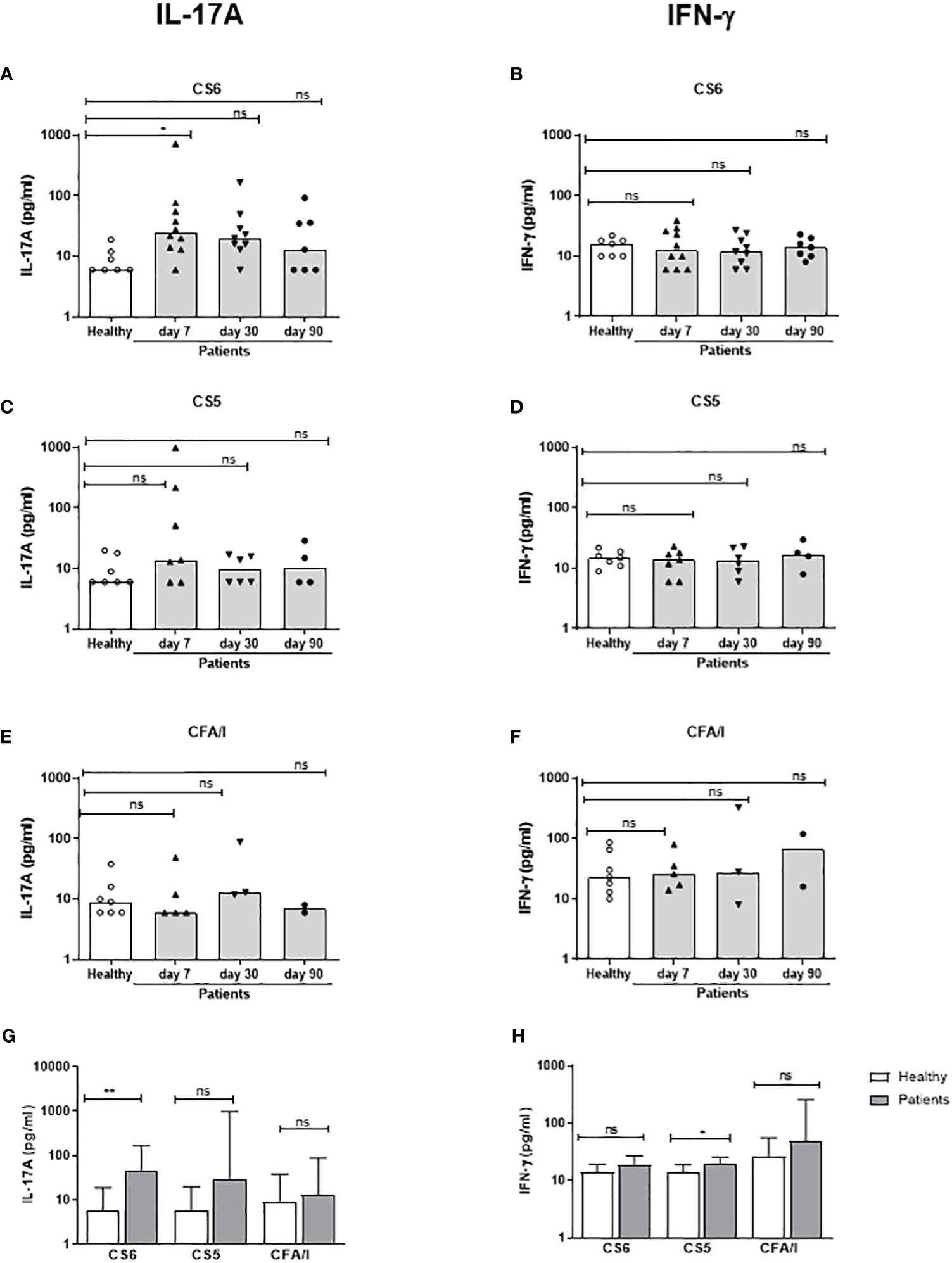
Figure 5 IL-17A and IFN-γ responses to CFs in PBMCs from ETEC infected patients and healthy adults. IL-17A (A, C, E, G) and IFN-γ (B, D, F, H) concentrations in cultures with PBMCs stimulated with CS6 (A, B), CS5 (C, D) and CFA/I (E, F) in healthy controls (n=7) and diarrheal patients (A, B); n=7-10, (C, D); n=4-7, (E, F); n=2-5) on day 7, 30 and 90 after hospitalization. (A-F) Responses at each individual time point. Each symbol represents one individual, and bars indicate median values. (G, H) Maximum responses at any time point. Bars represent median + 95% confidence interval. Statistical analysis was performed using the Kruskal Wallis test with Dunn’s multiple comparison post test (A-F) or the Mann-Whitney test (G, H). *P <0.05, **P <0.01, ns, not significant; P >0.05.
3.5 Correlations between IgA and cytokine responses
We next evaluated the relationship between the IgA and T cell cytokine responses induced by ETEC infection. We first investigated the correlation between the maximum increases in magnitudes (fold-rises between maximum responses at any time point of infection versus day 2) of LTB-specific ALS and plasma IgA responses versus the maximum increases in magnitudes of IL-17A and IFN-γ responses induced by stimulation with dmLT, resulting in several significant correlations, as described below. Correlation analyses were also performed using the maximal response level minus the level measured on day 2, but these analyses did not reveal any significant correlations (data not shown). The magnitudes (fold-rises) of ALS IgA responses to LTB correlated significantly with the IL-17A responses (r=0.51, P=0.02) (Figure 6A) as well as IFN-γ responses (r=0.56, p=0.01) (Figure 6C) to dmLT (Figure 6C). In contrast, plasma IgA LTB antibody responses did not correlate with either the IL-17A or IFN-γ responses to dmLT (Figures 6B, D). We also analyzed the correlation between the weaker IL-17A and IFN-γ responses to LTB versus ALS and plasma IgA antibody responses to the same antigen. Significant correlations were observed between ALS IgA and IFN-γ responses to LTB (r=0.50, p=0.02) (Supplementary Figure 5). These results support a relation between both Th17 and Th1 responses and ASC-IgA responses, most likely reflecting mucosal IgA responses to ETEC.
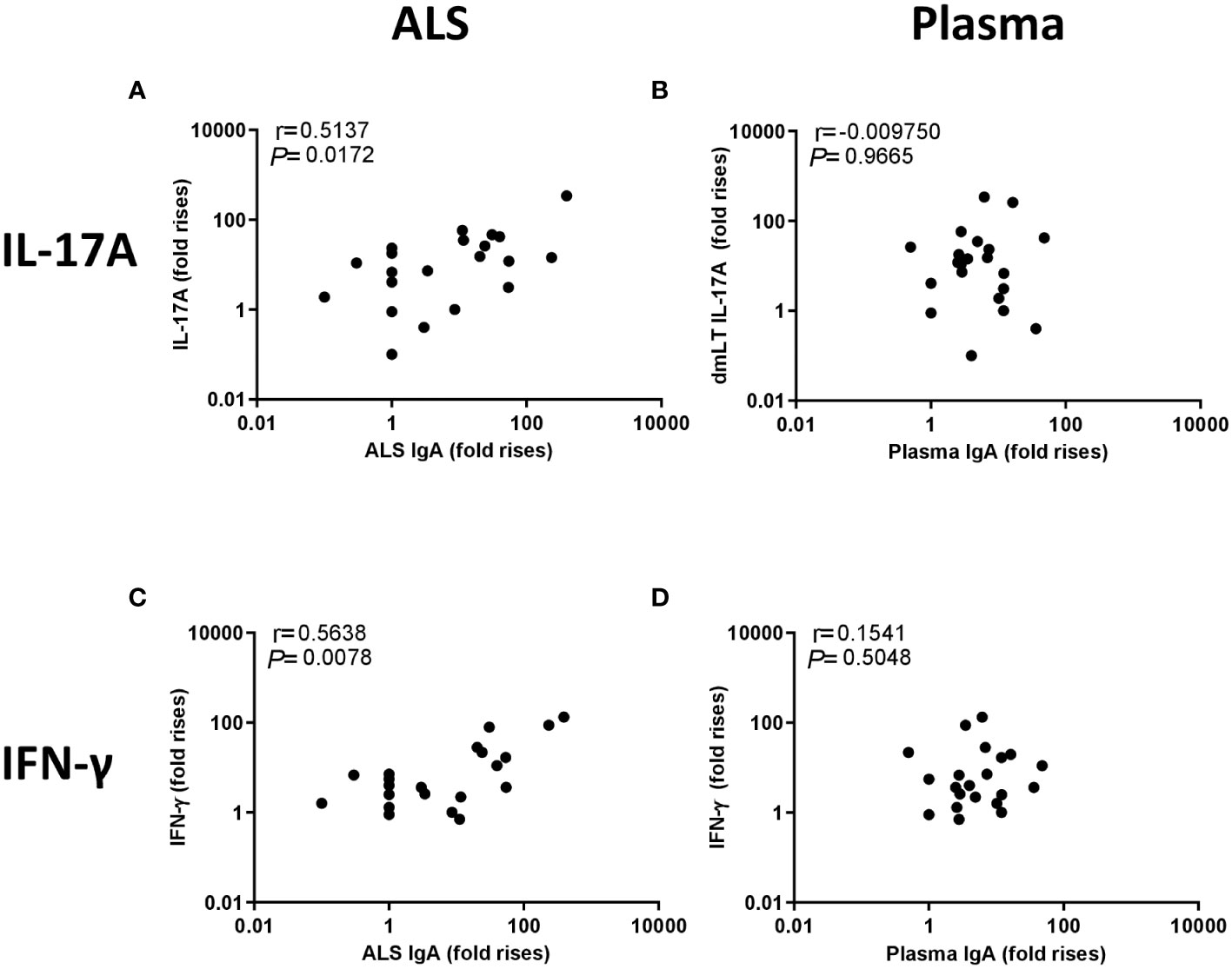
Figure 6 Correlation between IL-17A and IFN-γ responses to dmLT and IgA responses to LTB in ALS and plasma. Correlation between the fold-increases in cytokines and IgA levels (maximum responses at any time point divided by the response on day 2). (A) IL-17A response to dmLT vs. ALS IgA to LTB, (B) IL-17A response to dmLT vs plasma IgA to LTB, (C) IFN-γ response to dmLT vs. ALS IgA to LTB and (D) IFN-γ response to dmLT vs. plasma IgA to LTB. Statistical analysis was performed using the Spearman test.
4 Discussion
Although T cells are likely to play an active role in promoting the development of SIgA-mediated protection against ETEC, T cell responses against natural ETEC infection have, to our knowledge, not previously been investigated. In this study, we analyzed Th responses in hospitalized Bangladeshi patients with ETEC diarrhea during acute infection and convalescence. Consistent with previous studies, the patients showed significant IgA ASC as well as plasma IgA responses to LTB and CFs expressed by the infecting strains (9–11). The IgA responses were concomitant with significantly higher frequencies of circulating Th cells expressing chemokine receptors associated with a Th17 cytokine profile (CCR6+CXCR3-) in blood during the early phase of ETEC infection and convalescence (day 2 and 7), whereas the proportions of cells with Th1 or Th2 phenotypes did not change. The induction of ETEC-specific Th17 responses was further supported by the increases in IL-17A secretion from PBMCs in response to stimulation with dmLT, LT, LTB and CS6 in ETEC patients expressing the corresponding antigens. We also observed IL-17A responses to CS5 and CFA/I in some patients, but due to lower prevalence of ETEC strains expressing these CFs in the study group, smaller numbers of participants were tested for responses to these antigens and larger studies are needed to fully characterize T cell responses to CS5 and CFA/I.
Th17 cells represent the most abundant cellular source of IL-17A in human gut tissue (20), but IL-17A can also be produced from innate cells (40). Results from depletion experiments verified that the IL-17A detected in our experiments was mainly produced by CD4+ T cells. IFN-γ responses to ETEC antigen stimulation were lower in magnitude and less frequent compared to IL-17A responses. IFN-γ is mainly produced by activated T cells, particularly Th1 cells as well as innate cells like natural killer cells, macrophages and dendritic cells (41). Due to low and infrequent IFN-γ responses observed in ETEC infected patients, we were not able to determine the cellular source of IFN-γ in our study.
Our results are consistent with a few previous reports of T cell responses in experimental ETEC human challenge models. After challenge of Norwegian volunteers with the ETEC strain TW10722, which expresses ST, CS5 and CS6, but not LT, Th responses to CS5 and CS6 were detected in peripheral blood, with particularly strong and long-lived responses to CS5 (42). Challenge with the TW11681 strain, that expresses ST, CFA/I and CS21, also induced responses to CFA/I in some individuals (43). In both studies, T cell responses to the E. coli mucinase YghJ expressed by both strains were also noted (42, 43). However, in these studies the Th responses were analyzed using a flow cytometric assay based on expression of Th activation markers after antigen stimulation, and the cytokine profiles of the ETEC specific cells were not determined. A very small flow cytometric study of CD4+ T cell responses to the LT+ ST+CFAI/+ H10407 ETEC challenge strain in American volunteers supported the presence of Th cells specific for CFA/I in peripheral blood of ETEC infected individuals with mild diarrhea, whereas such responses were not observed in patients with more severe symptoms (44). The ETEC-specific cells predominantly produced TNF-α and IL-2, but IFN-γ and IL-17A responses were also detected in some individuals. However, since only five participants were included in the T cell analysis, it is difficult to draw any firm conclusions of the general characteristics or the potentially protective effect of T cells from these results. Experimental infection with the same ETEC H10407 strain has also been shown to induce significantly increased levels of IL-17A and IFN-γ in serum of volunteers who developed symptomatic infection (45). The cellular source of the serum cytokines was not defined and since the serum cytokine levels increased very early after challenge, innate cells may be involved. However, early IL-17A production has also been described in mucosal CD3+CD4+ T cells after infection of naïve piglets with ETEC expressing F4 fimbriae (46). The animals also mounted ETEC specific IL-17A and IFN-γ responses in peripheral blood, as revealed by analysis of cytokine mRNA in unstimulated PBMCs from infected animals as well as by analysis of cytokines in culture supernatants from antigen stimulated PBMCs. ETEC is an important diarrheal pathogen in pigs, and although the ETEC strains which infect pigs do not infect humans, several studies confirm similar pathogenic mechanisms of ETEC in pigs and humans and also support the importance of SIgA responses in protection against the infection (47, 48). Collectively, our studies of T cell responses to natural ETEC infection, and the results from different human and animal ETEC challenge models, support that ETEC induce Th responses to both LT and CFs. Furthermore, Th17 responses seem to be an important component of the response both in naïve people and individuals living in an ETEC endemic area, who are most likely are primed by previous ETEC infections.
It was noteworthy that the IL-17A responses in ETEC patients infected by LT positive strains in our study were stronger after stimulation with dmLT and LT compared to LTB. LT and dmLT can act both as antigens and adjuvants and can boost both T and B cell responses to various types of vaccines, including ETVAX, in animals as well as humans (7, 25, 28). We have also previously shown that LT, dmLT and cholera toxin (CT) primarily promote Th17 but not Th1 type responses to both vaccine antigens and polyclonal stimuli in vitro, whereas LTB does not have any adjuvant effect (29–31). Since we found that stimulation with the LT mutant LTE112K, which contains an A subunit which lacks ability to induce cAMP due to a single amino acid substitution and has no adjuvant activity when administered orally (38, 39), gave rise to similar strong IL-17A responses as dmLT or LT, our results indicate that the strong T cell response to dmLT and LT compared to LTB may not be directly related to the adjuvant effect. The stronger T cell responses to dmLT may instead be a result of responses to T cell epitopes in the A subunit, and/or be related to increased binding, uptake and presentation of dmLT by antigen presenting cells. Antibodies to the A subunit have been demonstrated in ETEC infected individuals (49), and the A subunit has also been shown to have adjuvant function independently of the B subunits (50). These observations also indicate that the inclusion of dmLT in the ETVAX vaccine formulation may be an advantage not only from the perspective of its adjuvant potential, but also given it ability to induce responses to the A subunit of the LT toxin, which may be an important contributor to vaccine induced protection. To gain further mechanistic insights into how protective immunity to ETEC is induced, T- and B cell responses to both the A- and B-subunits of LT should be monitored in future studies.
ETEC LT has 80% structural similarity to CT produced by Vibrio cholerae O1 and causes diarrhea by similar mechanisms. The SIgA antibodies against both CT and somatic cholera antigens such as lipopolysaccharide (LPS) and toxin-coregulated pilus (TCP) are thought to contribute to protection against cholera (51). Interestingly, natural cholera infection has been shown to induce IL-17A responses in duodenal tissue from adult Bangladeshis early after hospitalization (day 2) and the responses remained until day 30 (52). A marked increase in IL-17A and IFN-γ production was also noted in whole blood cultures from cholera patients after stimulation with a membrane protein preparation (MP) of cholera antigens (52). In Bangladeshi children, MP as well as CT, CT B subunit (CTB), dmLT and a single mutant CT molecule (mCT) with retained adjuvanticity, but less binding to GM1, have been shown to induce significant Th cell proliferation, and mCT-stimulation of cells in whole blood cultures was also demonstrated to result in significant IL-17A, IFN-γ and IL-13 responses (16). These results suggest that ETEC and cholera both induce T cell responses of the Th17 type, but also indicate that Th1 responses may be stronger after cholera compared to ETEC infection.
Th17 cells have been implicated in mediating protective immunity against a variety of additional bacterial infections in the intestinal tract, including Salmonella enterica serovar Typhimurium, Helicobacter pylori and Citrobacter rodentium (19, 53–55). Th17 cells may contribute to protection by different mechanisms, including induction of inflammation via recruitment of neutrophils, enhancement of production of antimicrobial peptides or by promotion of the development of IgA responses or secretion of IgA over the mucosal epithelium, and the importance of these mechanisms are likely to vary considerably between different infections (20, 56). We demonstrate here that the magnitudes of both IL-17A and IFN-γ responses to dmLT and IFN-γ responses to LTB correlate with the magnitude of ASC responses to LTB measured by the ALS method. Although the correlations were moderate r=(0.51-0.56), these results indicate that both types of Th responses may be involved in promoting development of B cell responses. In contrast, no correlation was seen between T cell and plasma IgA responses. The proportions of patients responding to dmLT with IL-17A responses and IgA LTB were comparable (about 50%), whereas ALS IgA responses to CS6 and CS5 were more frequent than T cell responses to the same antigens (80-90% compared to 40-50%). However, it is possible that differences in kinetics between T and B cell responses may have influenced these results. Although maximal cytokine as well as ALS responses were detected on day 7 in our study, the enrolled patients had experienced ETEC symptoms 6-96 h (median 24 hours) before being hospitalized and sampled, and we may have missed transient responses or analyzed submaximal responses in some individuals.
Vaccines against ETEC currently under development are aiming to induce mucosal SIgA responses against both CFs and toxins. ETEC bacteria are non-invasive and localized in the gut, therefore mucosal SIgA antibodies directed against CFs and LTB are considered to confer immune protection after natural infection or vaccination (2, 6). In a number of different studies we have shown that the oral ETEC vaccine ETVAX induces both blood ASC responses, characterized by prominent expression of integrin α4β7, and fecal SIgA against LT, CFs, and LPS and that such responses are promoted by addition of dmLT in both mice and humans (6, 7, 23, 25, 57). We have also shown that the vaccine induces both Th1 and Th17 responses to LTB and CFs, and that the strongest CS6 specific T cell as well as ASC responses were detected in those participants receiving vaccine with dmLT (25) and Lundgren A, unpublished). The results from the current study support the similarity between both T and B cell responses induced by ETVAX and natural ETEC infection, indicating that the vaccine is able to induce protective responses. In addition, we have previously shown in Swedish vaccinees that activated Tfh-like cells are released in peripheral blood after ETVAX vaccination and that the magnitude of the response correlated with development of plasmablast responses and long-lived B cell memory (23). Tfh cells are located in the germinal centers, where they promote isotype switching, affinity maturation and development of long lived plasma cells and memory B cells. Circulating Tfh-like (cTfh) cells clonally related to Tfh cells in lymph nodes have been demonstrated and have been shown to be associated with development of highly functional antibodies to both vaccination and infection (58–60). cTfh cells were not evaluated specifically in the present study, but since cTfh have been shown to be able to produce both IL-17A and IFN-γ (61), some of the cytokines detected here may be produced by cTfh cells. Increased proportions of ETEC-specific cTfh cells have been observed in participants challenged with ST+LT+ ETEC (44). cTfh responses to natural ETEC infection will be specifically investigated in our continued studies of immunity to ETEC infection.
In conclusion, we have shown that natural ETEC infection in adults living in an ETEC endemic area gives rise to Th responses to both LT and CFs. Responses appear to be dominated by Th17 cells, but magnitudes of both IL-17A and IFN-γ responses correlate with ASC IgA responses, most likely derived from and homing to intestinal mucosa, supporting that several Th subtypes may be important in the development of intestinal IgA responses against ETEC. Our study has several strengths, particularly the longitudinal analysis of antigen specific Th cells in the same individuals at several time points during both acute infection and convalescence, the comprehensive comparison of Th responses with both circulating ASC and plasma IgA responses, and the ETEC endemic study setting, which enabled analysis of immune responses to natural ETEC infection in previously ETEC primed subjects. Study limitations include the low number of patients infected with CFA/I and CS5 expressing ETEC strains enrolled in the study and that most patients had severe dehydration, which limits the possibility to investigate the relation of disease severity and immune responses. Methodological limitations include that mucosal IgA responses were estimated by measurement of antigen specific ASCs migrating in peripheral blood rather than by direct evaluation of SIgA responses in fecal samples or mucosal biopsies, and that T cell responses were measured by analysis of cytokines in culture supernatants and not by flow cytometric analysis of intracellular cytokines, which may have provided additional information about the frequency and phenotype of antigen specific T cells. Further detailed characterization of T and B cell responses to ETEC infection in larger studies, including different age groups and populations with different levels of priming by natural ETEC infection, is likely to provide increased mechanistic insights into how protective immunity against ETEC develops and may also result in the identification of biomarkers useful for identifying the most promising vaccine candidates.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research review committee and Ethical review committee, icddr,b. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AL, MA, TB and FQ designed and planned the studies. MA, SB, NN and LH performed the immunological analyses. MA and AL wrote the manuscript. All authors contributed to the interpretation of results and critical review and revision of the manuscript and have approved the final version.
Funding
This work was supported by SIDA (grant number GR-01455), Adlerbertska forskningsstiftelsen, the Bill & Melinda Gates Foundation (OPP1112376), and the UK’s Foreign and Commonwealth Development Office (204139–101). Under the grant conditions of the Bill & Melinda Gates Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript. icddr,b is thankful to the donors for their support to its research efforts. icddr,b also gratefully acknowledges the following donors who provided unrestricted support: Governments of Bangladesh, Canada, Sweden and the UK.
Acknowledgments
We acknowledge the support of the study participants as well as the dedicated hospital, field and laboratory workers in this study at icddr,b. We thank Scandinavian Biopharma, Solna, Sweden, and John Clements, Tulane University, USA for providing antigens, adjuvants and toxins used in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1220130/full#supplementary-material
Supplementary Table 1 | Frequencies of IgA antibody responders against LTB and CF antigens in ALS and plasma specimens from ETEC patients (comparison with day 2 responsesa). aA responder was defined as having ≥2-fold higher antibody titer on day 2/30/90 compared to day 2.
Supplementary Table 2 | Frequencies of IL-17A and IFN-γ responders against LTB and dmLT in ETEC infected patients (comparison with day 2 responsesa). aA responder was defined as having ≥2-fold higher cytokine response on day 2/30/90 compared to day 2.
Abbreviations
ALS, antibody in lymphocyte supernatant; ASC, antibody secreting cell; CFs, colonization factors; CT, cholera toxin; CTB, cholera toxin B subunit; dmLT, double mutant heat labile toxin; ETEC, enterotoxigenic Escherichia coli; LPS, lipopolysaccharide; LT, heat labile toxin; LTB, heat labile toxin B subunit; mCT, single mutant cholera toxin; MP, membrane protein preparation; PBMC, peripheral blood mononuclear cell; PCR, polymerase chain reaction; PHA, phytohaemagglutinin; SIgA, secretory IgA; ST, heat stable toxin; TCP, toxin-coregulated pilus; Tfh, T follicular helper; Th, T helper.
References
1. Lanata CF, Black RE. Estimating the true burden of an enteric pathogen: enterotoxigenic Escherichia coli and Shigella spp. Lancet Infect Dis (2018) 18(11):1165–6. doi: 10.1016/s1473-3099(18)30546-2
2. Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev (2005) 18(3):465–83. doi: 10.1128/CMR.18.3.465-483.2005
3. Begum YA, Baby NI, Faruque AS, Jahan N, Cravioto A, Svennerholm AM, et al. Shift in phenotypic characteristics of enterotoxigenic Escherichia coli (ETEC) isolated from diarrheal patients in Bangladesh. PloS Negl Trop Dis (2014) 8(7):e3031. doi: 10.1371/journal.pntd.0003031
4. Vidal RM, Muhsen K, Tennant SM, Svennerholm AM, Sow SO, Sur D, et al. Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS). PloS Negl Trop Dis (2019) 13(1):e0007037. doi: 10.1371/journal.pntd.0007037
5. Evans DG, Evans DJ Jr., Tjoa WS, DuPont HL. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun (1978) 19(2):727–36. doi: 10.1128/iai.19.2.727-736.1978
6. Svennerholm AM, Lundgren A, Leach S, Akhtar M, Qadri F. Mucosal immune responses against an oral ETEC vaccine evaluated in clinical trials. J Infect Dis (2021) 224(12 Suppl 2):S821–8. doi: 10.1093/infdis/jiab475
7. Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, et al. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine (2013) 31(20):2457–64. doi: 10.1016/j.vaccine.2013.03.027
8. Walker R, Kaminski RW, Porter C, Choy RKM, White JA, Fleckenstein JM, et al. Vaccines for protecting infants from bacterial causes of diarrheal disease. Microorganisms (2021) 9(7):1382. doi: 10.3390/microorganisms9071382
9. Bhuiyan TR, Hoq MR, Nishat NS, Al Mahbuba D, Rashu R, Islam K, et al. Enumeration of Gut-Homing β7-Positive, Pathogen-Specific Antibody-Secreting Cells in Whole Blood from Enterotoxigenic Escherichia coli- and Vibrio cholerae-Infected Patients, Determined Using an Enzyme-Linked Immunosorbent Spot Assay Technique. Clin Vaccine Immunol CVI (2016) 23(1):27–36. doi: 10.1128/cvi.00526-15
10. Alam MM, Aktar A, Afrin S, Rahman MA, Aktar S, Uddin T, et al. Antigen-specific memory B-cell responses to enterotoxigenic Escherichia coli infection in Bangladeshi adults. PloS Negl Trop Dis (2014) 8(4):e2822. doi: 10.1371/journal.pntd.0002822
11. Wenneras C, Qadri F, Bardhan PK, Sack RB, Svennerholm AM. Intestinal immune responses in patients infected with enterotoxigenic Escherichia coli and in vaccinees. Infect Immun (1999) 67(12):6234–41. doi: 10.1128/IAI.67.12.6234-6241.1999
12. Svennerholm AM, Lundgren A. Recent progress toward an enterotoxigenic Escherichia coli vaccine. Expert Rev Vaccines (2012) 11(4):495–507. doi: 10.1586/erv.12.12
13. Saletti G, Cuburu N, Yang JS, Dey A, Czerkinsky C. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat Protoc (2013) 8(6):1073–87. doi: 10.1038/nprot.2013.058
14. Qadri F, Ahmed F, Ahmed T, Svennerholm AM. Homologous and cross-reactive immune responses to enterotoxigenic Escherichia coli colonization factors in Bangladeshi children. Infect Immun (2006) 74(8):4512–8. doi: 10.1128/iai.00474-06
15. Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun (2007) 75(8):3961–8. doi: 10.1128/IAI.00459-07
16. Arifuzzaman M, Rashu R, Leung DT, Hosen MI, Bhuiyan TR, Bhuiyan MS, et al. Antigen-specific memory T cell responses after vaccination with an oral killed cholera vaccine in Bangladeshi children and comparison to responses in patients with naturally acquired cholera. Clin Vaccine Immunol CVI (2012) 19(8):1304–11. doi: 10.1128/cvi.00196-12
17. Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev (2008) 226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x
18. Schulz SM, Köhler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol (2008) 20(9):1129–38. doi: 10.1093/intimm/dxn069
19. Bhuiyan TR, Islam MM, Uddin T, Chowdhury MI, Janzon A, Adamsson J, et al. Th1 and Th17 responses to Helicobacter pylori in Bangladeshi infants, children and adults. PloS One (2014) 9(4):e93943. doi: 10.1371/journal.pone.0093943
20. Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol (2009) 2(5):403–11. doi: 10.1038/mi.2009.100
21. Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol (Baltimore Md 1950) (2012) 189(9):4666–73. doi: 10.4049/jimmunol.1200955
22. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and th17 cells. Annu Rev Immunol (2009) 27:485–517. doi: 10.1146/annurev.immunol.021908.132710
23. Cardeno A, Magnusson MK, Quiding-Jarbrink M, Lundgren A. Activated T follicular helper-like cells are released into blood after oral vaccination and correlate with vaccine specific mucosal B-cell memory. Sci Rep (2018) 8(1):2729. doi: 10.1038/s41598-018-20740-3
24. Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol CVI (2011) 18(4):546–51. doi: 10.1128/CVI.00538-10
25. Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, et al. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine (2014) 32(52):7077–84. doi: 10.1016/j.vaccine.2014.10.069
26. Akhtar M, Chowdhury MI, Bhuiyan TR, Kaim J, Ahmed T, Rafique TA, et al. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine (2018) 37(37):5645–56. doi: 10.1016/j.vaccine.2018.11.040
27. Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis (2019) 20(2):208–19. doi: 10.1016/S1473-3099(19)30571-7
28. Clements JD, Norton EB. The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. mSphere (2018) 3(4):e00215-18. doi: 10.1128/mSphere.00215-18
29. Leach S, Clements JD, Kaim J, Lundgren A. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PloS One (2012) 7(12):e51718. doi: 10.1371/journal.pone.0051718
30. Larena M, Holmgren J, Lebens M, Terrinoni M, Lundgren A. Cholera toxin, and the related nontoxic adjuvants mmCT and dmLT, promote human Th17 responses via cyclic AMP-protein kinase A and inflammasome-dependent IL-1 signaling. J Immunol (Baltimore Md 1950) (2015) 194(8):3829–39. doi: 10.4049/jimmunol.1401633
31. Akhtar M, Nizam NN, Basher SR, Hossain L, Akter S, Bhuiyan TR, et al. dmLT adjuvant enhances cytokine responses to T cell stimuli, whole cell vaccine antigens and lipopolysaccharide in both adults and infants. Front Immunol (2021) 12:654872. doi: 10.3389/fimmu.2021.654872
32. Rodas C, Iniguez V, Qadri F, Wiklund G, Svennerholm AM, Sjoling A. Development of multiplex PCR assays for detection of enterotoxigenic Escherichia coli colonization factors and toxins. J Clin Microbiol (2009) 47(4):1218–20. doi: 10.1128/jcm.00316-09
33. Rudin A, Svennerholm AM. Colonization factor antigens (CFAs) of enterotoxigenic Escherichia coli can prime and boost immune responses against heterologous CFAs. Microbial pathogenesis (1994) 16(2):131–9. doi: 10.1006/mpat.1994.1014
34. Carpenter CM, Hall ER, Randall R, McKenzie R, Cassels F, Diaz N, et al. Comparison of the antibody in lymphocyte supernatant (ALS) and ELISPOT assays for detection of mucosal immune responses to antigens of enterotoxigenic Escherichia coli in challenged and vaccinated volunteers. Vaccine (2006) 24(18):3709–18. doi: 10.1016/j.vaccine.2005.07.022
35. Chang HS, Sack DA. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin Diagn Lab Immunol (2001) 8(3):482–8. doi: 10.1128/cdli.8.3.482-488.2001
36. Lundgren A, Leach S, Tobias J, Carlin N, Gustafsson B, Jertborn M, et al. Clinical trial to evaluate safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli prototype vaccine containing CFA/I overexpressing bacteria and recombinantly produced LTB/CTB hybrid protein. Vaccine (2013) 31(8):1163–70. doi: 10.1016/j.vaccine.2012.12.063
37. Ahren C, Jertborn M, Svennerholm AM. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect Immun (1998) 66(7):3311–6. doi: 10.1128/IAI.66.7.3311-3316.1998
38. Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol (1992) 22(9):2277–81. doi: 10.1002/eji.1830220915
39. Cheng E, Cardenas-Freytag L, Clements JD. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine (1999) 18(1-2):38–49. doi: 10.1016/S0264-410X(99)00168-1
40. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol (2010) 10(7):479–89. doi: 10.1038/nri2800
41. Ivashkiv LB. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol (2018) 18(9):545–58. doi: 10.1038/s41577-018-0029-z
42. Sakkestad ST, Steinsland H, Skrede S, Lillebo K, Skutlaberg DH, Guttormsen AB, et al. A new human challenge model for testing heat-stable toxin-based vaccine candidates for enterotoxigenic Escherichia coli diarrhea - dose optimization, clinical outcomes, and CD4+ T cell responses. PloS Negl Trop Dis (2019) 13(10):e0007823. doi: 10.1371/journal.pntd.0007823
43. Todnem Sakkestad S, Steinsland H, Skrede S, Kleppa E, Lillebo K, Saevik M, et al. Experimental infection of human volunteers with the heat-stable enterotoxin-producing enterotoxigenic escherichia coli strain TW11681. Pathog (Basel Switzerland) (2019) 8(2):84. doi: 10.3390/pathogens8020084
44. McArthur MA, Chen WH, Magder L, Levine MM, Sztein MB. Impact of CD4+ T Cell Responses on Clinical Outcome following Oral Administration of Wild-Type Enterotoxigenic Escherichia coli in Humans. PloS Negl Trop Dis (2017) 11(1):e0005291. doi: 10.1371/journal.pntd.0005291
45. Brubaker J, Zhang X, Bourgeois AL, Harro C, Sack DA, Chakraborty S. Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. coli infection and impact on intestinal colonization and ETEC specific immune responses in an experimental human challenge model. Gut Microbes (2021) 13(1):1–13. doi: 10.1080/19490976.2021.1891852
46. Luo Y, Van Nguyen U, de la Fe Rodriguez PY, Devriendt B, Cox E. F4+ ETEC infection and oral immunization with F4 fimbriae elicits an IL-17-dominated immune response. Veterinary Res (2015) 46:121. doi: 10.1186/s13567-015-0264-2
47. Melkebeek V, Goddeeris BM, Cox E. ETEC vaccination in pigs. Vet Immunol Immunopathol (2013) 152(1-2):37–42. doi: 10.1016/j.vetimm.2012.09.024
48. Van den Broeck W, Cox E, Goddeeris BM. Induction of immune responses in pigs following oral administration of purified F4 fimbriae. Vaccine (1999) 17(15-16):2020–9. doi: 10.1016/s0264-410x(98)00406-x
49. Norton EB, Branco LM, Clements JD. Evaluating the A-subunit of the heat-labile toxin (LT) as an immunogen and a protective antigen against enterotoxigenic escherichia coli (ETEC). PloS One (2015) 10(8):e0136302. doi: 10.1371/journal.pone.0136302
50. Norton EB, Lawson LB, Mahdi Z, Freytag LC, Clements JD. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect Immun (2012) 80(7):2426–35. doi: 10.1128/iai.00181-12
51. Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. Lancet (2017) 390(10101):1539–49. doi: 10.1016/S0140-6736(17)30559-7
52. Kuchta A, Rahman T, Sennott EL, Bhuyian TR, Uddin T, Rashu R, et al. Vibrio cholerae O1 infection induces proinflammatory CD4+ T-cell responses in blood and intestinal mucosa of infected humans. Clin Vaccine Immunol CVI (2011) 18(8):1371–7. doi: 10.1128/cvi.05088-11
53. Mayuzumi H, Inagaki-Ohara K, Uyttenhove C, Okamoto Y, Matsuzaki G. Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology (2010) 131(3):377–85. doi: 10.1111/j.1365-2567.2010.03310.x
54. Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med (2008) 14(4):421–8. doi: 10.1038/nm1743
55. Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity (2009) 30(1):108–19. doi: 10.1016/j.immuni.2008.11.009
56. Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev (2008) 226:160–71. doi: 10.1111/j.1600-065X.2008.00703.x
57. Svennerholm AM, Qadri F, Lundgren A, Kaim J, Rahman Bhuiyan T, Akhtar M, et al. Induction of mucosal and systemic immune responses against the common O78 antigen of an oral inactivated ETEC vaccine in Bangladeshi children and infants. Vaccine (2022) 40(2):380–9. doi: 10.1016/j.vaccine.2021.10.056
58. Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity (2013) 39(4):758–69. doi: 10.1016/j.immuni.2013.08.031
59. Vella LA, Buggert M, Manne S, Herati RS, Sayin I, Kuri-Cervantes L, et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest (2019) 129(8):3185–200. doi: 10.1172/JCI125628
60. Law H, Venturi V, Kelleher A, Munier CML. Tfh cells in health and immunity: potential targets for systems biology approaches to vaccination. Int J Mol Sci (2020) 21(22):8524. doi: 10.3390/ijms21228524
61. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity (2011) 34(1):108–21. doi: 10.1016/j.immuni.2010.12.012
Keywords: ETEC, T helper cell, Th17, Th1, IL-17A, IFN-γ
Citation: Akhtar M, Basher SR, Nizam NN, Hossain L, Bhuiyan TR, Qadri F and Lundgren A (2023) T helper cell responses in adult diarrheal patients following natural infection with enterotoxigenic Escherichia coli are primarily of the Th17 type. Front. Immunol. 14:1220130. doi: 10.3389/fimmu.2023.1220130
Received: 10 May 2023; Accepted: 31 August 2023;
Published: 20 September 2023.
Edited by:
Felix Ngosa Toka, Ross University School of Veterinary Medicine, Saint Kitts and NevisReviewed by:
Rezwanul Wahid, University of Maryland, United StatesLi Liang, University of California, Irvine, United States
Copyright © 2023 Akhtar, Basher, Nizam, Hossain, Bhuiyan, Qadri and Lundgren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Lundgren, YW5uYS5sdW5kZ3JlbkBtaWNyb2Jpby5ndS5zZQ==
 Marjahan Akhtar
Marjahan Akhtar Salima Raiyan Basher
Salima Raiyan Basher Nuder Nower Nizam
Nuder Nower Nizam Lazina Hossain
Lazina Hossain Taufiqur Rahman Bhuiyan
Taufiqur Rahman Bhuiyan Firdausi Qadri
Firdausi Qadri Anna Lundgren
Anna Lundgren