
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 04 October 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1219785
Nanotechnology-enhanced photodynamic therapy (PDT) and immunotherapy are emerging as exciting cancer therapeutic methods with significant potential for improving patient outcomes. By combining these approaches, synergistic effects have been observed in preclinical studies, resulting in enhanced immune responses to cancer and the capacity to conquer the immunosuppressive tumor microenvironment (TME). Despite challenges such as addressing treatment limitations and developing personalized cancer treatment strategies, the integration of nanotechnology-enabled PDT and immunotherapy, along with advanced photosensitizers (PSs), represents an exciting new avenue in cancer treatment. Continued research, development, and collaboration among researchers, clinicians, and regulatory agencies are crucial for further advancements and the successful implementation of these promising therapies, ultimately benefiting cancer patients worldwide.
In 2020, there will be approximately 19.3 million new cases of cancer and 10 million cancer-related deaths globally, making cancer the primary cause of death (1). Surgical, chemotherapeutic, and radiotherapeutic interventions are the traditional cancer treatment approaches. Although significant advancements have been made in cancer treatment, these conventional methods frequently result in serious side effects, limited therapeutic efficacy, and the development of drug resistance (2). Consequently, there is an ongoing need for innovative and effective cancer treatment approaches.
PDT is a minimally invasive cancer treatment that uses a PS, a particular wavelength of light, and oxygen to generate reactive oxygen compounds that can cause tumor cell death (3). Upon absorbing a photon, a PS transitions from its ground state (S0) to an excited singlet state (S1 or S2). The S2 state quickly decays to S1 via internal conversion, and the unstable S1 either emits light (fluorescence) or generates heat, or may even undergo intersystem crossing to a more stable triplet state (T1). T1, with a longer lifetime, can lead to phosphorescent emission and energy transfer to O2 to generate singlet oxygen (1O2) – a process referred to as type II PDT. Alternatively, T1 can react with intracellular substrates to form radicals that further react with H2O or O2 to produce other reactive oxygen species (ROS) like hydroxyl and superoxide radicals - known as the type I PDT process. In most cases, the predominant photosensitizing mechanism involves the formation of 1O2, causing PDT destruction to biological tissues and cells (4).
PDT has demonstrated promising results in the treatment of different malignancies, including superficial bladder cancer, early and obstructive lung cancer, Barrett’s esophagus, head and neck cancers, and skin cancer (4, 5). Immunotherapy, on the other hand, aims to harness the immune system of patients to combat cancer by activating the immune system’s cells to recognize and eliminate cancer cells (6). Several immunotherapy approaches, including immune checkpoint inhibitors, cancer vaccines, and adoptive T cell therapy, have demonstrated remarkable effectiveness in the treatment of malignancies like melanoma and non-small cell lung cancer (7, 8). Although immune checkpoint therapy offers numerous advantages, a considerable proportion of patients with different cancer types do not respond effectively to immune checkpoint inhibitors (9). This lack of sensitivity can be attributed to their tumors’ low immunogenicity, which limits the therapeutic potential of this approach for these particular patients (10, 11).
Recent studies have highlighted the potential synergistic effects of combining PDT and immunotherapy (12). PDT can induce immunogenic cell death (ICD), which promotes the release of tumor antigens and enhances the activation of antigen-presenting cells (13, 14). This process can stimulate a robust immune response and improve the efficacy of immunotherapy (15, 16). Furthermore, combining PDT and immunotherapy can overcome the limitations of each individual treatment modality, such as the hypoxic TME in PDT and the low response rates of some patients to immunotherapy (17).
Nanotechnology is now recognized as a promising instrument for improving the delivery, efficacy, and safety of cancer therapeutics, including PSs and immunotherapeutic agents (18). The integration of PDT and immunotherapy has resulted in promising advances in cancer treatment (19–21). The use of nanotechnology to improve the safety and effectiveness of PSs and immunotherapeutic agents has opened new avenues for further exploration. This article provides a thorough analysis of the applications of nanomedicine in PDT and immunotherapy. In addition, this article also discusses the advantages of nanomedicines as PSs. Finally, the challenges and prospective future developments in the field of nanomedicine-driven photodynamic tumor immunotherapy are highlighted.
The pioneering PSs, known as first-generation PSs, largely comprise hematoporphyrin derivative (HpD) and Photofrin. Hematoporphyrin was one of the first agents discovered to have photosensitizing properties and was later developed into HpD and Photofrin to enhance its effectiveness in PDT (3). HpD and Photofrin are complexes of various porphyrin derivatives. The primary characteristic of these PSs is the presence of porphyrin structures, which absorb light, particularly in the red region of the electromagnetic spectrum, and interact with molecular oxygen to generate ROS that induce cell death (22). Despite their effectiveness in causing photo-damage to cells, these first-generation PSs have limitations. They have a relatively weak absorption at therapeutic wavelengths (>600 nm), limiting the depth of tissue penetration. Moreover, these agents also have low tumor selectivity, leading to damage to healthy tissues. Prolonged skin phototoxicity is another drawback, with patients becoming sensitive to light for several weeks after treatment (23).
Due to the drawbacks of the first-generation PSs, more research was conducted on the second-generation PSs with strong 1O2 production and near-infrared (NIR) activation. Second-generation PSs include both porphyrinoid and non-porphyrinoid compounds (Figure 1) (24). The former consists of macrocyclic structures like porphyrin, chlorins, bacteriochlorins, phthalocyanines, pheophorbides, bacteriopheophorbides, Porphycene, and texaphyrins, whereas the latter comprises anthraquinones, phenothiazines, xanthenes, cyanines, and curcuminoids (24). Metalated derivatives such as aluminum phthalocyanine tetrasulfonate (AlPcS4), Si (IV)-naphthalocyanine (SiNC), zinc phthalocyanine (ZnPC), and tin ethyl etiopurpurin (SnET2) are also included, although metalation doesn’t consistently enhance photodynamic activity (25). Second-generation PSs are superior to HpD in terms of 1O2 quantum yields, tumor-to-normal tissue concentrations, and antitumor effects (26). They also offer practical advantages like shorter tissue accumulation time, enabling same-day treatment and making PDT more convenient for out-patient procedures. Furthermore, these PSs exhibit a reduced window of cutaneous photosensitivity (27). Several factors, including lipophilicity, the type and number of charged groups, charge-to-mass ratio, and the type and number of ring and core substituents, influence the properties of the PSs (28). Most of the second-generation PSs, particularly those with porphyrin ring structures, are hydrophobic, affecting their administration, biodistribution, and pharmacokinetic profile (11). Although hydrophobicity enables cellular penetration, extreme hydrophobicity could cause aggregation in aqueous solution, compromising its pharmacokinetics and photophysical properties. Moreover, it could restrict solubility in physiological solvents and body fluids, thus limiting clinical applications. Hence, a balance between hydrophilicity and lipophilicity is critical for a clinically successful PS. This balance can be achieved by introducing hydrophilic polar substituents in the lead PS structure to synthesize amphiphilic derivatives. Other modifications, such as oxidation, extension, or modification of the porphyrin ring system to carry a central ion, can also enhance photophysical and pharmacological properties (29).
Present research primarily focuses on the development of third-generation PSs that are expected to enhance tumor specificity, minimize generalized photosensitivity, and be activated with longer-wavelength light (30). These improvements could be accomplished by modifying existing PSs with biological conjugates like peptides, antibodies, or antisense molecules for tumor-specific targeting, or by chemically conjugating or encapsulating PSs in efficient delivery vehicles or carriers (31–33). In essence, these third-generation PSs represent advancements over their second-generation counterparts in terms of improved delivery and targeting abilities. Specifically, they are designed to bind selectively to tumor cells or elements of the TME, consequently boosting tumor localization while reducing phototoxicity to healthy tissue.
Nanotechnology has emerged as a promising instrument for enhancing the delivery, efficacy, and safety of PSs in PDT (4). The application of nanoparticles as carriers of drugs offers several advantages, including improved solubility and stability, controlled drug release, and tumor-specific delivery of PSs (34). Additionally, targeted delivery of PSs and immunotherapeutic agents can be achieved using nanoparticles functionalized with specific ligands or antibodies, which can reduce off-target toxicity and enhance therapeutic efficacy (35). Nanoparticle-based systems have also been used to co-deliver PSs and immunotherapeutic agents, leading to a more potent and coordinated antitumor immune response (36, 37).
Organic nanoparticles like micelles, liposomes, and dendrimers have been extensively investigated for PS delivery in PDT. Liposomes are spherical vesicles composed of phospholipid bilayers, which can encapsulate both hydrophilic and hydrophobic PSs (38). Micelles are self-assembled aggregates of amphiphilic molecules that can solubilize hydrophobic PSs within their hydrophobic core (39). Dendrimers are highly branched, tree-like polymers with a high degree of molecular uniformity and tunable surface functional groups, which can be used to conjugate PSs (39). Chen et al. developed a hybrid protein oxygen nanocarrier with chlorine e6 (Ce6) for oxygen self-sufficient PDT, which substantially decreased tumor hypoxia and improved the efficacy of PDT and the infiltration of CD8+ T cells at the cancer location (40). The increased PDT caused strong ICD and effectively inhibited primary tumors and suppressed pulmonary metastasis by stimulating more dendritic cells (DCs), NK cells, and cytotoxic T lymphocytes (CTLs) by increasing the release of damage-associated molecular patterns (DAMPs) (Figure 2A). A recent study improved the efficacy of PDT by using oxygen-laden hemoglobin (Hb) loaded auxiliary liposomes in conjunction with indocyanine green (ICG) modified gold nanospheres (41). Upon reaching the hypoxic tumor environment, the oxygen is rapidly released from the Hb, aiding the ICG in generating robust ROS and enhancing the immune response’s intensity (Figure 2B).
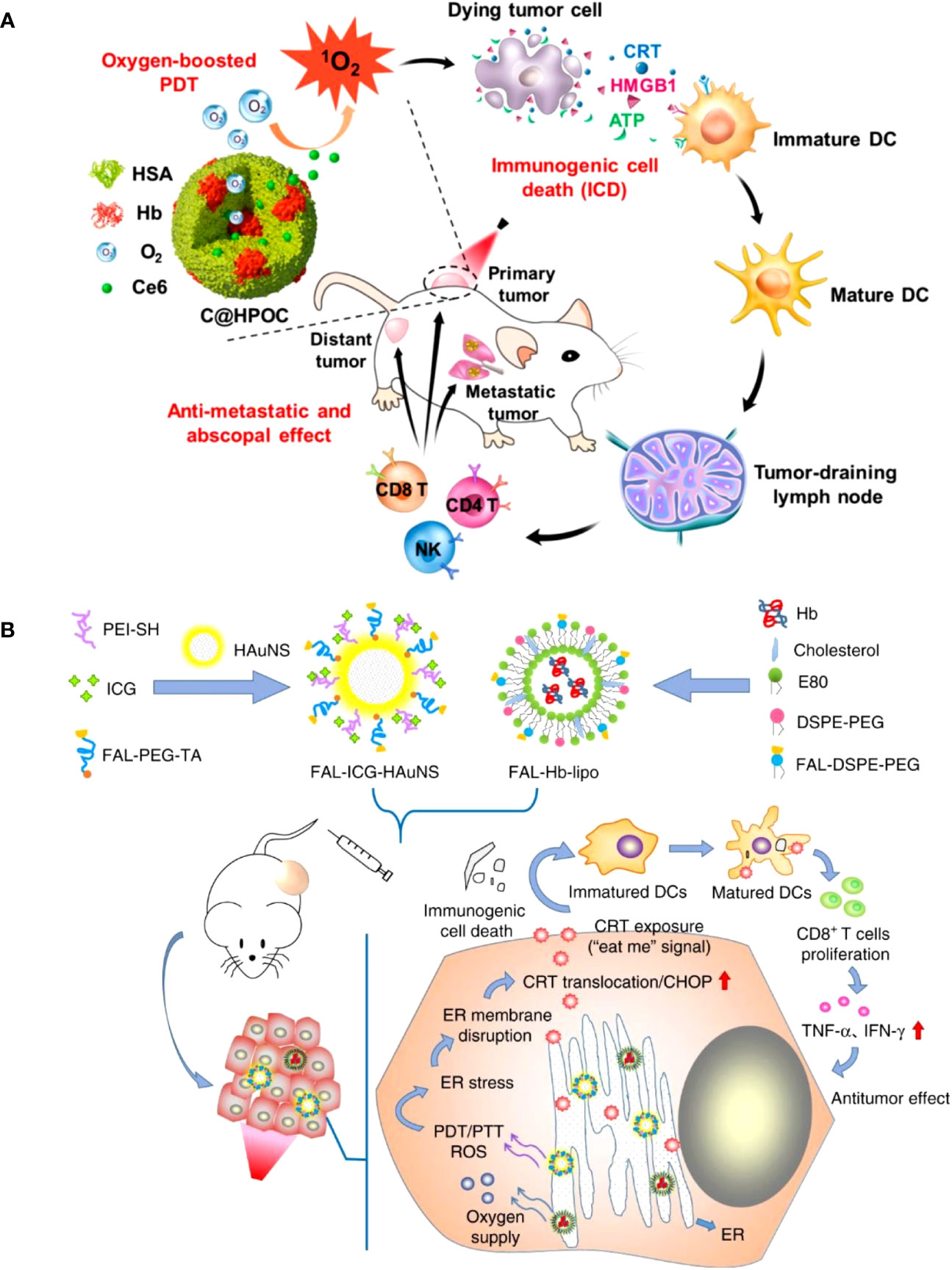
Figure 2 (A) Schematic depiction of oxygen-augmented immunogenic PDT with C@HPOC for eliciting the anti-metastatic and abscopal effect (40). Copyright 2018, American Chemical Society. (B) The antitumor mechanism of FAL-ICG-HAuNS plus FAL-Hb-lipo. Schematic illustration of enhanced immunogenic cancer cell death and anticancer effect induced by endoplasmic reticulum-targeting photothermal/photodynamic therapy (41). Copyright 2013, Nature.
Inorganic nanoparticles, such as gold nanoparticles, silica nanoparticles, and quantum dots (Figure 3), have also been investigated for PS delivery (45, 46). Gold nanoparticles (AuNCs) can be easily functionalized with various ligands and PSs, allowing for targeted delivery and controlled release (47). Silica nanoparticles are highly biocompatible and can be modified with various functional groups to improve PS loading and release (43). Quantum dots, semiconductor nanoparticles with size-tunable optical properties, can act as both PSs and imaging agents in PDT (44). Manganese dioxide (MnO2) can stimulate the overproduction of hydrogen peroxide in tumor cells, resulting in the production of oxygen (48). Additionally, AuNCs act as intrinsic inorganic PSs that can induce ROS production, and the use of gold-based nanomedicines can enhance the effectiveness of other PSs in PDT due to the presence of a localized electric field (42, 49).
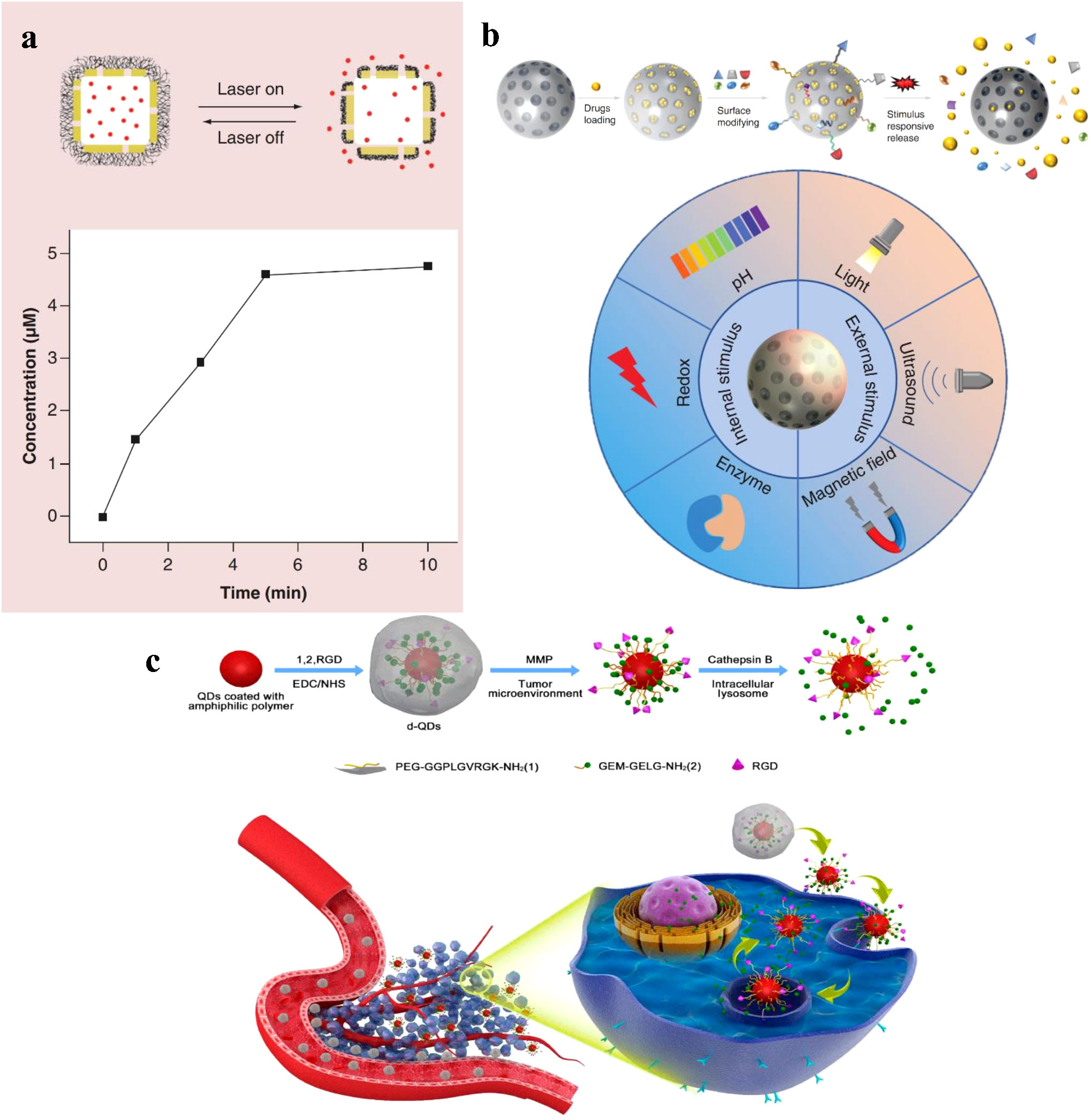
Figure 3 (A) A schematic illustration of controlled release enabled by a smart polymer, poly(N-isopropylacrylamide), coated on the surface of Au nanocages, and release profile of Dox from the system shown in above, as triggered by a NIR laser (42). Copyright 2016, Future Medicine Ltd. (B) Characteristics of controlled-release of mesoporous silica nanoparticles, and Characteristics of controlled-release of mesoporous silica nanoparticles (43). Copyright 2022, Future Medicine Ltd. (C) Schematic illustration of the nanovectors preparation protocol and their enzyme sensitive behavior, and Schematic illustration of the nanovectors delivering GeM to pancreatic cancer cells (44). Copyright 2017, American Chemical Society.
Many PSs exhibit poor solubility and stability in aqueous environments, which can limit their clinical application. Nanoparticles can improve the solubility and stability of PSs by encapsulating them within their hydrophobic core or conjugating them to the nanoparticle surface (50, 51). For example, liposomal formulations of the PS verteporfin have shown improved solubility and stability, resulting in enhanced PDT efficacy in preclinical studies (52). Huang et al. designed a dual-functional drug conjugate comprised of protoporphyrin IX and NLG919, a strong indoleamine-2,3-dioxygenase (IDO) inhibitor, to enhance the biocompatibility and tumor accumulation of the drug conjugate (PpIX-NLG@Lipo) (Figure 4A). In vitro and in vivo experiments demonstrated the strong ROS generation ability of PpIX-NLG@Lipo, which directly damages cancer cells through PDT. Concurrently, PpIX-NLG@Lipo induces ICD to stimulate the host immune system. Moreover, by interfering with IDO activity, PpIX-NLG@Lipo amplifies PDT-induced immune responses, resulting in an increased infiltration of CD8+ T lymphocytes at the tumor site and ultimately inhibiting both primary and distant tumors (53).
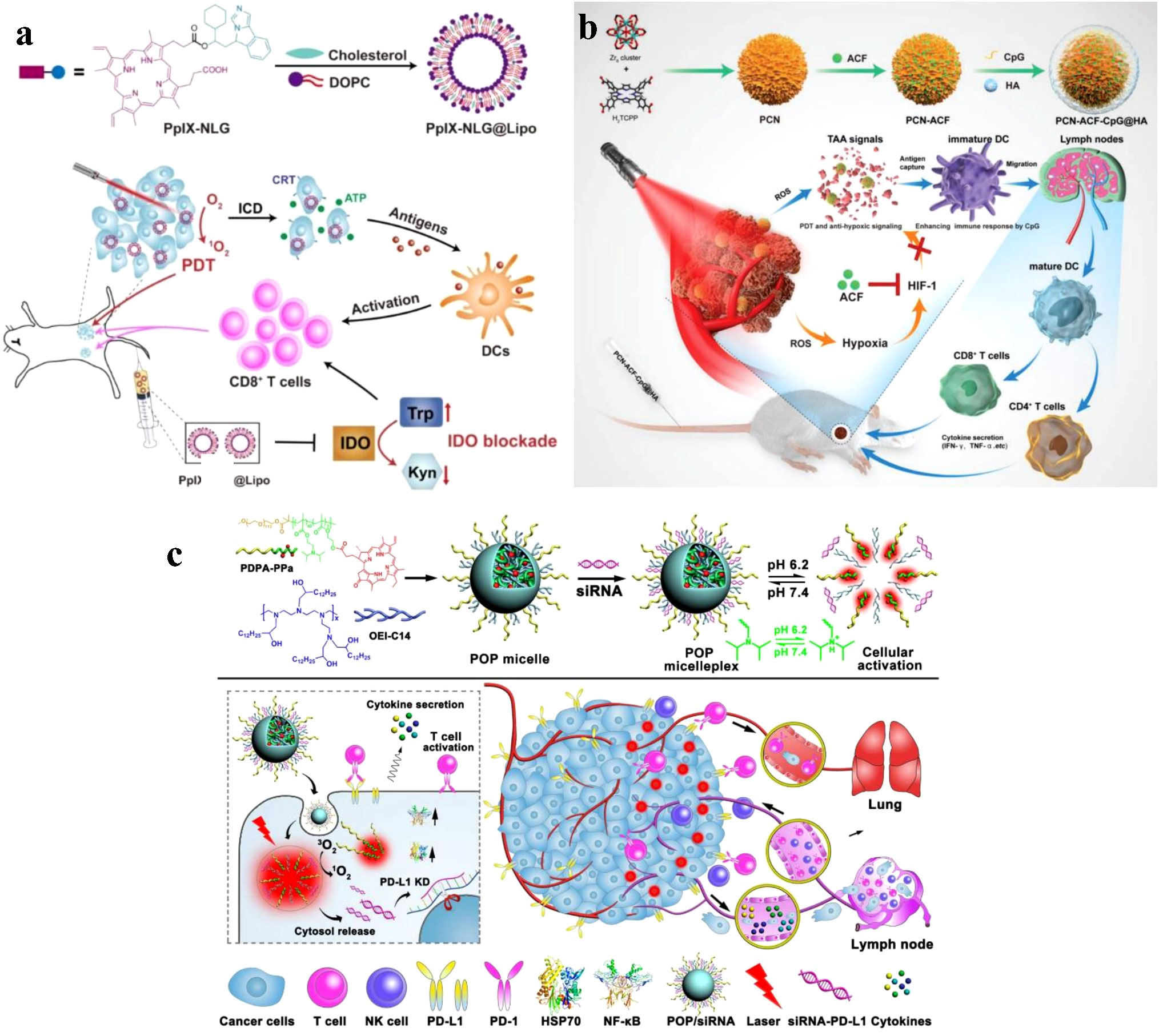
Figure 4 (A) Schematic illustration of PpIX-NLG@Lipo for combined PDT and IDO blockade (53). Copyright 2019, Ivyspring International. (B) Schematic illustration of the preparation procedure and the working principle of PCN-ACF-CpG@HA to integrate PDT, antihypoxic signaling, and CpG adjuvant as in situ tumor vaccine (54). Copyright 2019, John Wiley and Sons. (C) Schematic illustration of the acid-activatable micelleplexes for PD-L1 blockade-enhanced photodynamic cancer immunotherapy (55). Copyright 2016, American Chemical Society.
Nanoparticles can be designed for controlled payload release, which improves the therapeutic index of PDT by maintaining optimal PS concentrations at the tumor site (56). By incorporating stimuli-responsive nanoparticles that release their payload in response to specific triggers, such as pH, temperature, or light, the spatiotemporal control of PS release can be further enhanced (57). This approach can potentially overcome the aggregation-caused quenching (ACQ) effect often seen in PDT, as well as increase the efficacy of the treatment by facilitating ROS diffusion (58, 59). Nanosized metal-organic Frameworks (nMOFs) incorporate PSs as structural units, producing adjustable and permeable structures that surmount the ACQ effect and permit significant PS loads (60, 61). These permeable structures not only efficiently disperse PSs but also promote ROS diffusion, thus improving the efficacy of PDT (58, 62). Cai et al. developed in situ tumor vaccines composed of MOF-based nanoparticles (PCNs), immunologic adjuvants (CpG), and hypoxia-inducible factor inhibitors (acriflavine) (54). To accomplish superior tumor targeting, the PCN-CpG-acriflavine surface was coated with hyaluronic acid (HA) to identify the increased CD44 receptor on the surfaces of tumor cells. In the research they conducted, self-assembled PCNs were made up of zirconium ions and H2TCPP, and the well-dispersed H2TCPP inside the framework counteracted the ACQ effect, resulting in equivalent PDT efficacy. In addition, the complementary impact of PDT-generated tumor-associated antigens (TAAs) and CpG resulted in robust immune responses (Figure 4B). Zhang et al. developed a hybrid cytomembrane (FM)-coated nMOF for photoactivatable immunotherapy of cancer to further enhance immune responses. FMs originate from DCs and tumor cells (63). The FMs not only exhibited exceptional tumor-targeting capacity because of their self-targeting properties toward homologous tumors but also facilitated tumor-specific immune responses related to the presence of extremely expressed tumor antigens. In addition, the DC-derived immunomodulatory molecules in FMs improved the presentation of antigen and encouraged antigen-specific T cell reactions.
Compared to normal tissues, solid tumors exhibit unique TME characteristics, including low pH, acute hypoxia, and increased glutathione (GSH) amounts (64). Designing intelligent, stimuli-responsive nanomedicines with TME-sensitive chemical linkages or components can therefore efficiently regulate cargo discharge. In addition to advanced nMOFs, TME-responsive nanoparticles can effectively surmount the ACQ by cleaving the TME-sensitive linkers, allowing for the quick dissolution of PSs at the tumor location and significantly boosting the generation of ROS (65). To facilitate PDT-based cancer immunotherapy, Wang et al. designed a pH-responsive, multipurpose nanoplatform (Figure 4C). This nanoplatform is composed of a pH-sensitive diblock copolymer (PDPA), pheophorbide A (PPa) attached to PDPA, and an inhibitor of the anti-programmed death-1 (PD-1)- anti-programmed death-ligand 1 (PD-L1) interaction (55). Due to the ACQ effect, the nanoplatform’s hydrophobic core encases PPa, reducing phototoxicity during blood circulation. The rapid micelle dissociation induced by PDPA’s “proton sponge effect” upon delivery to the mildly acidic TME restores PPa’s photoactivity. Consequently, irradiation-activated PPa generates ROS via PDT, inducing an immune response by stimulating the expression of NF-κB and HSP70.
PSs can be conjugated with targeting ligands, such as antibodies, peptides, or small molecules, to enhance their tumor specificity and uptake (66, 67). Targeted delivery of PSs can be achieved using nanoparticles functionalized with specific ligands or antibodies that recognize receptors overexpressed on tumor cells or within the TME (68, 69). This strategy can improve the selectivity of PDT, reducing off-target toxicity and enhancing therapeutic efficacy. For example, folic acid-functionalized gold nanoparticles loaded with the PS chlorin e6 have demonstrated improved targeting and PDT efficacy in folate receptor-overexpressing cancer cells (70) A PS conjugated with an epidermal growth factor receptor (EGFR)-targeting peptide demonstrated improved PDT efficacy in EGFR-overexpressing cancer cells (71).
Due to the enhanced penetration and retention (EPR) effect that results from tumor vessel permeability, nanoparticles accumulate in tumor cells (72–74). Song et al. reported the development of PS-conjugated immune checkpoint inhibitor nanoparticles. As shown in Figure 5A, because of the EPR effect, these nanoparticles collect within tumors, Targeted delivery of PS and checkpoint inhibitor slowed tumor regrowth, prevented lung metastasis, and increased CD8+ T cells systemically (75). The hybrid nanoparticles that release PS and glucocorticoid-induced tumor necrosis factor receptor family-related protein, or poly (lactic-co-glycolic acid) (GITR-PLGA), utilize the immune activating role of PDT and GITR-PLGA-mediated suppression of immunosuppression to increase the amount of anti-tumor CD8+ T cells in the tumor (16).
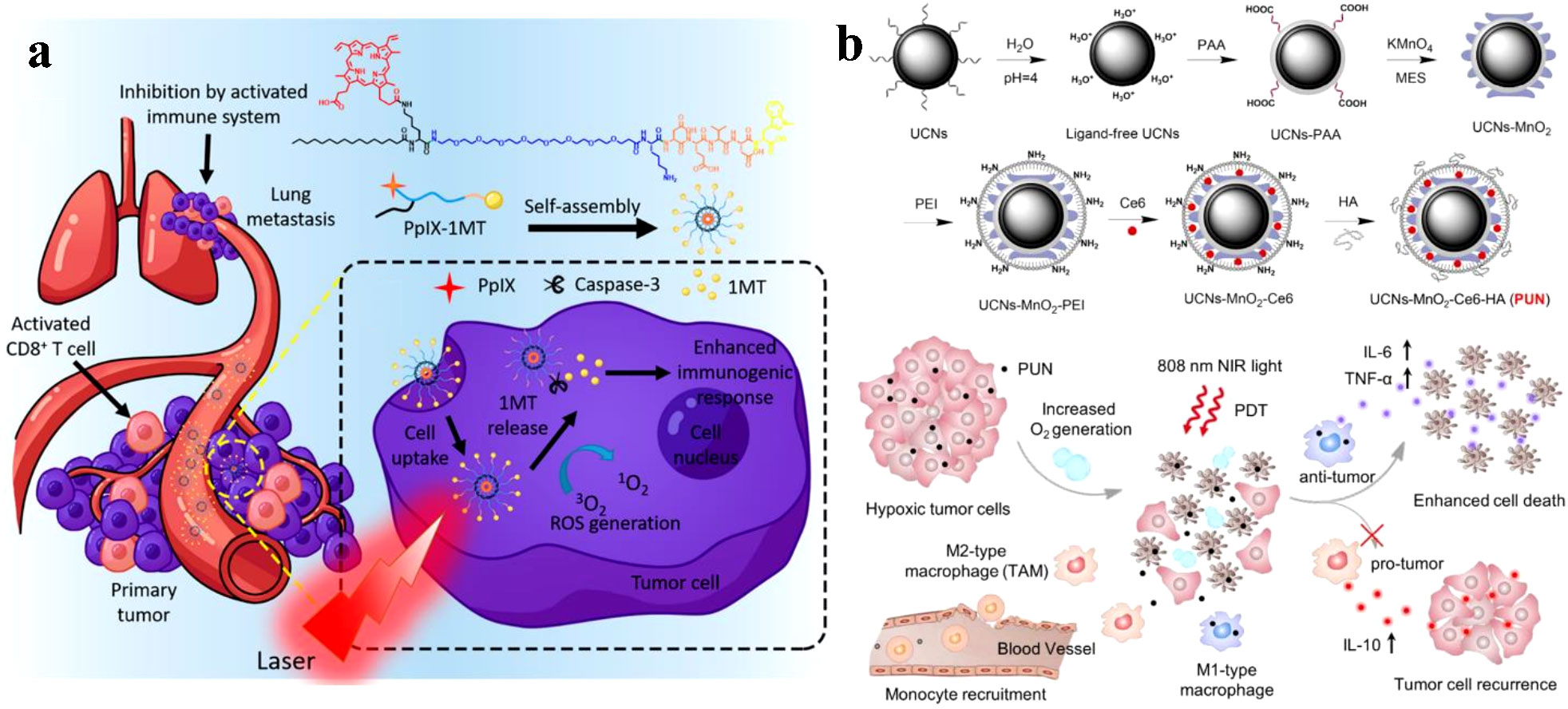
Figure 5 (A) PpIX-1MT chimeric peptide nanoparticles target tumor areas via the EPR effect, activate CD8+ T cells through cascade activations, and effectively inhibit primary tumors and lung metastasis while enabling in situ PDT to trigger apoptosis and caspase-3 production (75). Copyright 2018, American Chemical Society. (B) Illustration of NIR light-mediated PDT strategy for the enhanced cellular ablation in tumor microenvironment (76). Copyright 2018, American Chemical Society.
Dual-function PSs have attracted considerable interest because of their multifunctional characteristics, that make them appropriate for imaging and treatment using PDT. This integrated method enables continuous tracking of the therapy process, enhancing the efficiency and precision of PDT (77). ICG is a well-known example of a dual-function PS. It is a water-soluble tricarbocyanine dye with widespread use in medical diagnostics for its fluorescence imaging capabilities (78). In recent years, researchers have also discovered its potential for PDT applications. ICG has shown efficacy in both in vitro and in vivo experiments, showing its potential for imaging and treating malignant tumors simultaneously (79). Porphysomes, another example of dual-function PSs, are self-assembled porphyrin-lipid nanoparticles. These unique structures exhibit excellent photoacoustic imaging and PDT properties. Porphysomes have been shown to generate a strong photoacoustic signal in response to laser irradiation, allowing for high-resolution imaging of targeted tissues. Moreover, porphysomes can generate cytotoxic ROS upon light activation, leading to targeted destruction of cancer cells (80).
The restricted absorption of light into tissues limits treatment to surface tumors or requires invasive procedures for deeper tumors (27, 81). To overcome this limitation, researchers have explored strategies such as the use of upconversion nanoparticles (UCNPs), which convert NIR light to higher-energy light that is visible, allowing deeper tissue penetration without damaging surrounding tissues (82, 83).
UCNPs are nanoscale materials that convert low-energy light to high-energy light by sequentially exciting multiple photons via an anti-Stokes emission process. Compared with downconverted nanoparticles, UCNs can absorb NIR light and have a relatively high depth of tissue penetration, while the light can be converted into strong ultraviolet or visible light, enabling the activation of PSs in deep tissues (84). When coupled with PSs, UCNPs can extend the tissue penetration depth of PDT, improving its efficacy in the treatment of deep-seated tumors (85–89).
UCN-based PDTs have been extensively studied for tumor therapy due to their ability to improve tissue penetration depth. A study by Ai et al. utilized this feature by constructing Ce6-loaded UCNs combined with HA and MnO2 nanosheets to enhance NIR light-mediated PDT (Figure 5B). By converting 808 nm light excitation into 655 nm emissions, the Ce6-loaded UCNs efficiently generated sufficient 1O2 to induce deep-tissue cellular ablation. Additionally, the surface-anchored HA inhibited tumor recurrence post-PDT treatment by producing M1-type macrophages instead of M2-type macrophages (76).
PDT has been demonstrated to elicit ICD, increasing immune responses, which stimulates the immune system against dying cancer cells (90, 91). When combined with Adoptive T cell therapy, Cancer vaccine, and Immune checkpoint inhibitor, the ideal mechanism is currently speculated as Figure 6 (92–94). ICD promotes the release of DAMPs and TAAs, which act as danger signals to activate antigen-presenting cells (APCs) and initiate an immune response (95). PDT can activate APCs, such as DCs, through the release of DAMPs and the upregulation of costimulatory molecules. When immature DCs migrate from peripheral organs to neighboring draining lymph nodes, they gather antigens from the surrounding fluid, convert them into peptides, and then present the peptide major histocompatibility complex (MHC) at T cell receptors (TCR) triggering T cell activation, thereby priming and activating tumor-specific CTLs (96, 97). PDT has been reported to enhance the infiltration of T cells into the TME by modulating the expression of chemokines and adhesion molecules, thereby promoting the attraction of CTLs along with additional immune cells to the tumor location (98).
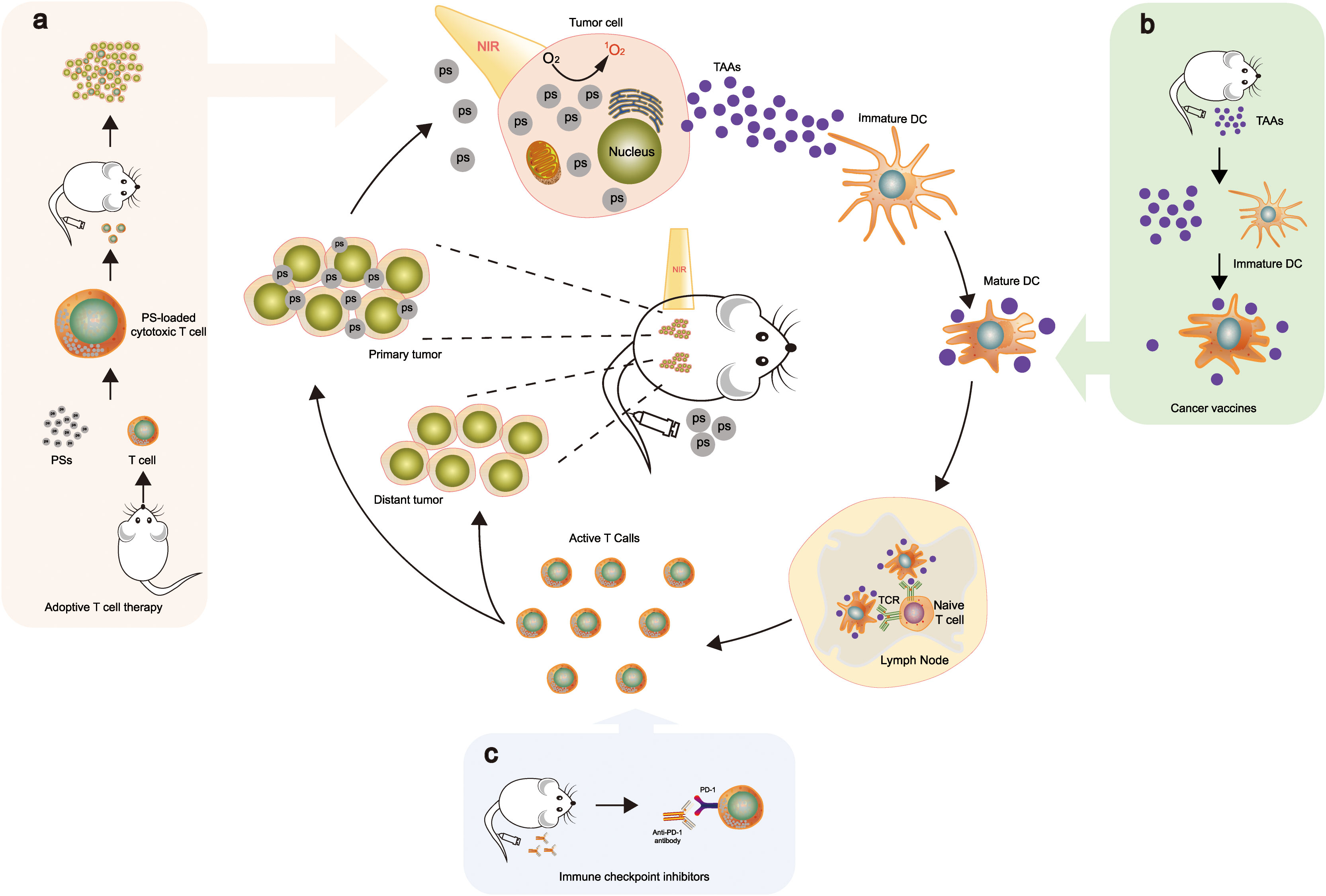
Figure 6 PDT triggers systemic antitumor immunity. This combination stimulates ICD and fosters an inflammatory environment at the primary tumor location, thereby releasing TAAs. These TAAs are then processed and introduced by dendritic cells to naive T cells, facilitating the growth and multiplication of tumor-specific effector T cells in lymphoid organs. The tumor-specific effector T cells infiltrate not only in primary but also in distant tumors. When combined with Adoptive T cell therapy, Cancer vaccine, and Immune checkpoint inhibitor, the mechanisms are as follows: (A) Ps is loaded into cytotoxic T cells; upon intravenous injection, they accumulate in the tumor tissues (92). (B) TAAs are utilized as a vaccine to enhance tumor-associated immune responses by dendritic cells presenting antigens to T cells (93). (C) Integration with PD-1 checkpoint blockade boosts the formation and infiltration of tumor-specific effector T cells in primary and distant tumors (94). The mechanisms (A–C) illustrate part of the drug mechanism of this therapy. Reproduced with permission from (92–94).
In preclinical investigations, the combination of PDT with immune checkpoint inhibitors such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4), anti-PD-1, anti-PD-L1 antibodies and IDO inhibitor demonstrated synergistic effects (94, 99). This review synthesizes recent investigations into the utilization of nanomaterials in the combination of PDT with immune checkpoint inhibitors (Table 1). The combination boosts the immune system’s response to tumors and can surmount the immunosuppressive TME (105, 106). Liu et al. developed an effective approach to eliminate metastatic malignancies by combining oxygen-boosted PDT with checkpoint blockade therapy (107). Using a light-activated hydrogel with Ce6-modified CAT, biodegradable polymer, and an immune adjuvant, which induced strong ICD and regulated macrophage polarization, leading to a significantly enhanced immune response. This was achieved by raising the intratumoral CD8+ T cell accumulation. Wang et al. created a sequential delivery strategy to enhance therapeutic efficacy. They initially assembled a biocompatible polymer nanoparticle system by combining dextran-modified HAase (DEX-HAase) with pH-responsive linkers. Due to the second infusion of Ce6@liposome and irradiation, the remodeled TME enhanced the efficacy of PDT after the initial infusion of DEX-HAase nanoparticles. Notably, the intensified PDT induced a robust immune response, which increased by combining it with PD-L1 blockade therapy after the third anti-PD-L1 administration. This sequential infusion of therapeutic agents facilitated synergistic PDT-immunotherapy, effectively suppressing the growth of both primary and distant tumors (102).
Furthermore, nMOFs can efficiently load immune checkpoint inhibitors into their open channels due to their high co-loading capacity. For example, Lin et al. constructed H4TBC-based nMOFs to encapsulate IDO inhibitors within their highly porous structure, inducing systemic antitumor immunity (100). Their results showed that H4TBC-based nMOFs provided both PDT and ICD, and when combined with checkpoint blockade therapy using an IDO inhibitor, enhanced T cell infiltration in the TME. In another study, Zhang et al. developed benzoporphyrin-based nMOFs composed of a Zr6 cluster and PS (TBP) in combination with αPD-1 to inhibit tumor metastasis (108). The TBP effectively avoided the ACQ effect due to its good dispersion in the nMOF structure, thereby facilitating PDT. Based on these advantages, TBP-mediated PDT induced a strong ICD to recruit tumor-infiltrating CTLs. Importantly, the combined application of αPD-1 restored the activity of CTLs suppressed in the immunosuppressive TME. In another study, Lin et al. constructed a cationic nMOF (W-TBP) highly loaded with anionic CpG through electrostatic interaction to enhance cancer immunotherapy (109). The combination of αPD-1 with antigen availability significantly improved the penetration and stimulation of CTLs in bilateral tumors, resulting in potent cancer immunotherapy.
Combining PDT with cancer vaccines, which aim to motivate the immune system to identify and target tumor antigens, is possible. In preclinical models, the combination of PDT and cancer vaccines has demonstrated improved antitumor efficacy and immune memory (12, 110–113). Table 2 summarizes the representative results of the current combination of PDT with cancer vaccines. In a mouse model, one study examined the combination of PDT and TLR5 agonist flagellin-adjuvanted tumor-specific peptide vaccination (FlaB-Vax) for increased PD-1 blockade-mediated melanoma inhibition. Results show the combination therapy significantly increased tumor-infiltrating effector memory CD8+ T cells and systemic IFNγ secretion, improving the therapeutic benefits of PD-1-targeting checkpoint inhibitor therapy for malignant melanoma (93).
Adoptive T cell therapy (ACT) involves the diversion of ex vivo expanded or genetically engineered T cells into a patient to target cancer cells. Combining PDT with ACT has shown potential for increasing T cell infiltration and activation in the TME, leading to improved antitumor responses (114). Blaudszun et al. introduced a combined photodynamic and cancer immunotherapy strategy involving the adoptive transfer of PS-loaded cytotoxic T cells (PS-OT-1 cells). They loaded OT-1 cells with temoporfin, a clinically useful porphyrin derivative, to create PS-OT-1 cells. Remarkably, under visible light irradiation in culture, PS-OT-1 cells produced a substantial quantity of ROS in an efficient manner. In addition, the combination of PDT and ACT with PS-OT-1 cells resulted in substantially increased cytotoxicity in comparison to ACT alone with unloaded OT-1 cells (92).
Several preclinical studies have demonstrated the potential of combining PDT with immunotherapy, resulting in improved antitumor effects and long-lasting immune responses (36, 101). In a mouse model of melanoma, the combination of PDT and anti-PD-1 therapy led to enhanced tumor regression and improved survival rates (99, 115, 116). Another study showed that combining PDT with a cancer vaccine increased antitumor immunity and prevented tumor recurrence in a mouse model of colon cancer (117). Bao et al. targeted IRDye700 to subcutaneous murine 4T1 tumors using a Fab portion of an antibody that attaches to CD276, an antigen specifically expressed on tumor cells. While targeted NIR therapy reduced tumor regeneration, PD-L1 expression on tumor cells increased significantly. By combining CD276-targeted NIR-PIT and anti-PD-L1 therapy, they were able to prevent subcutaneous tumor regrowth and lung metastasis (69). Hao et al. developed a combined therapy approach using PDT and immune checkpoint blockade to optimize tumor control. They incorporated a 25% thermosensitive polymer 407 hydrogel as a co-delivery platform for this treatment strategy. NIR PDT at 808 nm irradiation, along with CTLA4 and PD-L1 checkpoint blockade, suppressed solid tumor growth and extended the survival rate of colorectal tumor-bearing mice. This was achieved by eliciting a range of immune responses, including macrophage and DCs phagocytosis of tumor debris, acute inflammation induction, leukocyte infiltration, as well as maturation and activation of DCs (20). In the study by Santos and colleagues, the use of PDT with redaporfin successfully eradicated all observable tumor tissue. Subsequently, the application of an immune checkpoint inhibitor contributed to a sustained complete response in the clinical setting. This particular case exemplifies the potential effectiveness of such a combined therapeutic approach, laying the foundation for the development of a novel treatment strategy (118).
Potential side effects of PDT, including skin photosensitivity, pain, and edema, stem from prolonged presence and systemic distribution of PSs (119). Addressing these concerns, nanotechnology provides targeted PS delivery, reducing off-target phototoxicity. Biodegradable CaCO3/MnO2-based nanocarriers have been successfully used to transport PSs, demonstrating significant advancements over potentially toxic gold-based nanomedicines (120). These nanoparticles, engineered to accumulate in tumors through the enhanced EPR effect, limit PS exposure in healthy tissues (103). Surface modifications with tumor-specific ligands further enhance nanoparticle specificity, minimizing non-target skin photosensitivity (4, 104). Some nanoparticles also provide controlled PS release in response to stimuli such as pH, temperature, or enzymes, localizing PS activation to the treatment area (121).
An emerging trend in cancer treatment is the development of personalized medicine, which tailors treatments to the distinctive features of a patient’s tumor (122, 123). The combination of PDT and immunotherapy allows for a more tailored approach by considering factors such as TME, immune profile, and response to treatment (94, 124). Identifying predictive biomarkers and developing treatment algorithms based on individual patient profiles will be crucial for improving patient outcomes (124).
Translating preclinical research findings into clinical practice remains a challenge for the field. Issues such as regulatory approval, manufacturing, and standardization of treatment protocols need to be addressed to ensure the successful clinical translation of nanotechnology-enabled PDT and immunotherapy (18, 125). Collaborative efforts between researchers, clinicians, and regulatory agencies are necessary to accelerate the development and approval of these innovative therapies (126, 127).
In conclusion, the exciting interplay of nanotechnology-enabled PDT, advanced PSs, and immunotherapy heralds a promising new era in cancer treatment. The potential to overcome limitations such as tissue penetration and side effects, coupled with the opportunity to develop personalized cancer treatment strategies, paves the way for successful clinical translation. At the heart of this revolutionary approach lies the convergence of several disciplines. Material science, molecular biology, and biomedical engineering come together to optimize nanomaterials for improved delivery and activation of PSs, enhancing biocompatibility and maximizing therapeutic payloads. Moreover, an in-depth understanding of the TME is vital to design nanoparticles capable of navigating and penetrating these complex terrains effectively. Simultaneously, insights from the intersection of immunology and oncology spotlight the potential of leveraging PDT to stimulate potent immune responses against tumors. This accentuates the exciting prospects of integrating PDT and immunotherapy for comprehensive cancer treatment. Furthermore, the introduction of machine learning and computational modeling can advance nanotherapeutic design by predicting biological interactions, leading to more effective and targeted therapies. The translation of these advancements from lab to clinic necessitates meticulous testing and a thorough understanding of regulatory pathways. This underlines the significance of collaborative efforts between researchers, clinicians, and regulatory agencies. Ultimately, the future of PDT and nanotechnology, through this multi-disciplinary collaboration, holds immense potential in revolutionizing cancer therapy, improving patient outcomes, and providing benefits to cancer patients worldwide.
JJ and XW made equal contributions to this manuscript. JJ and XW prepared and wrote the manuscript. JY and GL revised the manuscript for clarity, grammar, and style. HZ and WH commented on previous versions of the manuscript. ZY and DW provided critical feedback on the manuscript. JT designed and revised the manuscript. All authors discussed the relevant literature and composed the sections of the review article. All authors contributed to the article and approved the submitted version.
This work is supported by the Sanming Project of Medicine in Shenzhen (Grant Number: SZSM202111003), Shenzhen High-level Hospital Construction Fund, the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital Shenzhen Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College (SZ2020MS003). The funding sources had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst (1998) 90(12):889–905. doi: 10.1093/jnci/90.12.889
4. Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev (2015) 115(4):1990–2042. doi: 10.1021/cr5004198
5. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin (2011) 61(4):250–81. doi: 10.3322/caac.20114
6. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
7. Sharma P, Allison JP. The future of immune checkpoint therapy. Science (2015) 348(6230):56–61. doi: 10.1126/science.aaa8172
8. Luo J-Q, Liu R, Chen F-M, Zhang J-Y, Zheng S-J, Shao D, et al. Nanoparticle-mediated CD47-SIRPα Blockade and calreticulin exposure for improved cancer chemo-immunotherapy. ACS Nano (2023) 17(10):8966–79. doi: 10.1021/acsnano.2c08240
9. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
10. Xu L, Zou C, Zhang S, Chu TSM, Zhang Y, Chen W, et al. Reshaping the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J Hematol Oncol (2022) 15(1):87. doi: 10.1186/s13045-022-01307-2
11. Chen J, Fan T, Xie Z, Zeng Q, Xue P, Zheng T, et al. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials (2020) 237:119827. doi: 10.1016/j.biomaterials.2020.119827
12. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer (2006) 6(7):535–45. doi: 10.1038/nrc1894
13. Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta (2010) 1805(1):53–71. doi: 10.1016/j.bbcan.2009.08.003
14. Gellén E, Fidrus E, Péter M, Szegedi A, Emri G, Remenyik É. Immunological effects of photodynamic therapy in the treatment of actinic keratosis and squamous cell carcinoma. Photodiagnosis Photodyn Ther (2018) 24:342–8. doi: 10.1016/j.pdpdt.2018.10.018
15. Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol (2019) 25:101084. doi: 10.1016/j.redox.2018.101084
16. O’Shaughnessy MJ, Murray KS, La Rosa SP, Budhu S, Merghoub T, Somma A, et al. Systemic antitumor immunity by PD-1/PD-L1 inhibition is potentiated by vascular-targeted photodynamic therapy of primary tumors. Clin Cancer Res (2018) 24(3):592–9. doi: 10.1158/1078-0432.CCR-17-0186
17. Chen X-J, Zhang X-Q, Liu Q, Zhang J, Zhou G. Nanotechnology: a promising method for oral cancer detection and diagnosis. J Nanobiotechnol (2018) 16(1):52. doi: 10.1186/s12951-018-0378-6
18. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer (2017) 17(1):20–37. doi: 10.1038/nrc.2016.108
19. Xu T, Liu Z, Huang L, Jing J, Liu X. Modulating the tumor immune microenvironment with nanoparticles: A sword for improving the efficiency of ovarian cancer immunotherapy. Front Immunol (2022) 13:1057850. doi: 10.3389/fimmu.2022.1057850
20. Hao Y, Chung CK, Gu Z, Schomann T, Dong X, Veld R, et al. Combinatorial therapeutic approaches of photodynamic therapy and immune checkpoint blockade for colon cancer treatment. Mol Biomed (2022) 3(1):26. doi: 10.1186/s43556-022-00086-z
21. Xiong W, Qi L, Jiang N, Zhao Q, Chen L, Jiang X, et al. Metformin liposome-mediated PD-L1 downregulation for amplifying the photodynamic immunotherapy efficacy. ACS Appl Mater Interfaces (2021) 13(7):8026–41. doi: 10.1021/acsami.0c21743
22. Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol (2004) 5(8):497–508. doi: 10.1016/S1470-2045(04)01529-3
23. Allison RR, Downie GH, Cuenca R, Hu X-H, Childs CJ, Sibata CH. Photosensitizers in clinical PDT. Photodiagnosis Photodyn Ther (2004) 1(1):27–42. doi: 10.1016/S1572-1000(04)00007-9
24. Ormond AB, Freeman HS. Dye sensitizers for photodynamic therapy. Mater (Basel) (2013) 6(3):817–40. doi: 10.3390/ma6030817
25. Josefsen LB, Boyle RW. Photodynamic therapy and the development of metal-based photosensitisers. Met Based Drugs (2008) 2008:276109. doi: 10.1155/2008/276109
26. van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ. Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers (Basel) (2017) 9(2):19. doi: 10.3390/cancers9020019
27. Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer (2003) 3(5):380–7. doi: 10.1038/nrc1071
28. Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: Part three-Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn Ther (2005) 2(2):91–106. doi: 10.1016/S1572-1000(05)00060-8
29. Patito IA, Rothmann C, Malik Z. Nuclear transport of photosensitizers during photosensitization and oxidative stress. Biol Cell (2001) 93(5):285–91. doi: 10.1016/S0248-4900(01)01118-2
30. Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat (2005) 4(3):283–93. doi: 10.1177/153303460500400308
31. Hudson R, Carcenac M, Smith K, Madden L, Clarke OJ, Pèlegrin A, et al. The development and characterisation of porphyrin isothiocyanate-monoclonal antibody conjugates for photoimmunotherapy. Br J Cancer (2005) 92(8):1442–9. doi: 10.1038/sj.bjc.6602517
32. Malatesti N, Smith K, Savoie H, Greenman J, Boyle RW. Synthesis and in vitro investigation of cationic 5,15-diphenyl porphyrin-monoclonal antibody conjugates as targeted photodynamic sensitisers. Int J Oncol (2006) 28(6):1561–9. doi: 10.3892/ijo.28.6.1561
33. Staneloudi C, Smith KA, Hudson R, Malatesti N, Savoie H, Boyle RW, et al. Development and characterization of novel photosensitizer: scFv conjugates for use in photodynamic therapy of cancer. Immunology (2007) 120(4):512–7. doi: 10.1111/j.1365-2567.2006.02522.x
34. Huang P, Wang D, Su Y, Huang W, Zhou Y, Cui D, et al. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J Am Chem Soc (2014) 136(33):11748–56. doi: 10.1021/ja505212y
35. Miao L, Guo S, Zhang J, Kim WY, Huang L. Nanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancer. Adv Funct Mater (2014) 24(42):6601–11. doi: 10.1002/adfm.201401076
36. He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun (2016) 7:12499. doi: 10.1038/ncomms12499
37. Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl (2019) 58(3):670–80. doi: 10.1002/anie.201804882
38. Bovis MJ, Woodhams JH, Loizidou M, Scheglmann D, Bown SG, Macrobert AJ. Improved in vivo delivery of m-THPC via pegylated liposomes for use in photodynamic therapy. J Control Release (2012) 157(2):196–205. doi: 10.1016/j.jconrel.2011.09.085
39. Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res (2007) 24(1):1–16. doi: 10.1007/s11095-006-9132-0
40. Chen Z, Liu L, Liang R, Luo Z, He H, Wu Z, et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano (2018) 12(8):8633–45. doi: 10.1021/acsnano.8b04371
41. Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun (2019) 10(1):3349. doi: 10.1038/s41467-019-11269-8
42. Pang B, Yang X, Xia Y. Putting gold nanocages to work for optical imaging, controlled release and cancer theranostics. Nanomed (Lond) (2016) 11(13):1715–28. doi: 10.2217/nnm-2016-0109
43. Li Y, Deng G, Hu X, Li C, Wang X, Zhu Q, et al. Recent advances in mesoporous silica nanoparticle-based targeted drug-delivery systems for cancer therapy. Nanomed (Lond) (2022) 17(18):1253–79. doi: 10.2217/nnm-2022-0023
44. Han H, Valdepérez D, Jin Q, Yang B, Li Z, Wu Y, et al. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano (2017) 11(2):1281–91. doi: 10.1021/acsnano.6b05541
45. Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic therapy mediated by nontoxic core-shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc (2016) 138(51):16686–95. doi: 10.1021/jacs.6b09538
46. Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun (2017) 8(1):902. doi: 10.1038/s41467-017-01050-0
47. Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev (2012) 41(7):2740–79. doi: 10.1039/c1cs15237h
48. Chung C-H, Lu K-Y, Lee W-C, Hsu W-J, Lee W-F, Dai J-Z, et al. Fucoidan-based, tumor-activated nanoplatform for overcoming hypoxia and enhancing photodynamic therapy and antitumor immunity. Biomaterials (2020) 257:120227. doi: 10.1016/j.biomaterials.2020.120227
49. Li Y, Wen T, Zhao R, Liu X, Ji T, Wang H, et al. Localized electric field of plasmonic nanoplatform enhanced photodynamic tumor therapy. ACS Nano (2014) 8(11):11529–42. doi: 10.1021/nn5047647
50. Zhao X, Yang C-X, Chen L-G, Yan X-P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat Commun (2017) 8:14998. doi: 10.1038/ncomms14998
51. Wang Z, Zhang F, Shao D, Chang Z, Wang L, Hu H, et al. Janus nanobullets combine photodynamic therapy and magnetic hyperthermia to potentiate synergetic anti-metastatic immunotherapy. Adv Sci (Weinheim Baden-Wurttemberg Germany) (2019) 6(22):1901690. doi: 10.1002/advs.201901690
52. Ghosh S, Carter KA, Lovell JF. Liposomal formulations of photosensitizers. Biomaterials (2019) 218:119341. doi: 10.1016/j.biomaterials.2019.119341
53. Huang Z, Wei G, Zeng Z, Huang Y, Huang L, Shen Y, et al. Enhanced cancer therapy through synergetic photodynamic/immune checkpoint blockade mediated by a liposomal conjugate comprised of porphyrin and IDO inhibitor. Theranostics (2019) 9(19):5542–57. doi: 10.7150/thno.35343
54. Cai Z, Xin F, Wei Z, Wu M, Lin X, Du X, et al. Photodynamic therapy combined with antihypoxic signaling and cpG adjuvant as an in situ tumor vaccine based on metal-organic framework nanoparticles to boost cancer immunotherapy. Adv Healthc Mater (2020) 9(1):e1900996. doi: 10.1002/adhm.201900996
55. Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B, et al. Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Letters (2016) 16(9):5503–13. doi: 10.1021/acs.nanolett.6b01994
56. Chen H, Tian J, He W, Guo Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J Am Chem Soc (2015) 137(4):1539–47. doi: 10.1021/ja511420n
57. Yu Z, Sun Q, Pan W, Li N, Tang B. A near-infrared triggered nanophotosensitizer inducing domino effect on mitochondrial reactive oxygen species burst for cancer therapy. ACS Nano (2015) 9(11):11064–74. doi: 10.1021/acsnano.5b04501
58. Lan G, Ni K, Lin W. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord Chem Rev (2019) 379:65–81. doi: 10.1016/j.ccr.2017.09.007
59. Ni K, Lan G, Lin W. Nanoscale metal-organic frameworks generate reactive oxygen species for cancer therapy. ACS Cent Sci (2020) 6(6):861–8. doi: 10.1021/acscentsci.0c00397
60. Anderson SL, Boyd PG, Gładysiak A, Nguyen TN, Palgrave RG, Kubicki D, et al. Nucleobase pairing and photodimerization in a biologically derived metal-organic framework nanoreactor. Nat Commun (2019) 10(1):1612. doi: 10.1038/s41467-019-09486-2
61. Fernandes PD, Magalhães FD, Pereira RF, Pinto AM. Metal-organic frameworks applications in synergistic cancer photo-immunotherapy. Polymers (Basel) (2023) 15(6):1490. doi: 10.3390/polym15061490
62. Lan G, Ni K, Xu Z, Veroneau SS, Song Y, Lin W. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J Am Chem Soc (2018) 140(17):5670–3. doi: 10.1021/jacs.8b01072
63. Liu W-L, Zou M-Z, Liu T, Zeng J-Y, Li X, Yu W-Y, et al. Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells. Adv Mater (2019) 31(18):e1900499. doi: 10.1002/adma.201900499
64. Volk T, Jähde E, Fortmeyer HP, Glüsenkamp KH, Rajewsky MF. pH in human tumour xenografts: effect of intravenous administration of glucose. Br J Cancer (1993) 68(3):492–500. doi: 10.1038/bjc.1993.375
65. Yang G, Phua SZF, Lim WQ, Zhang R, Feng L, Liu G, et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv Mater (2019) 31(25):e1901513. doi: 10.1002/adma.201901513
66. Klausen M, Ucuncu M, Bradley M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules (2020) 25(22):5239. doi: 10.3390/molecules25225239
67. Luo Y, Zeng Z, Shan T, Xu X, Chen J, He Y, et al. Fibroblast activation protein α activatable theranostic pro-photosensitizer for accurate tumor imaging and highly-specific photodynamic therapy. Theranostics (2022) 12(8):3610–27. doi: 10.7150/thno.70308
68. Kim H, Beack S, Han S, Shin M, Lee T, Park Y, et al. Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv Mater (2018) 30:1701460. doi: 10.1002/adma.201701460
69. Bao R, Wang Y, Lai J, Zhu H, Zhao Y, Li S, et al. Enhancing anti-PD-1/PD-L1 immune checkpoint inhibitory cancer therapy by CD276-targeted photodynamic ablation of tumor cells and tumor vasculature. Mol Pharm (2019) 16(1):339–48. doi: 10.1021/acs.molpharmaceut.8b00997
70. Li Z, Huang H, Tang S, Li Y, Yu X-F, Wang H, et al. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials (2016) 74:144–54. doi: 10.1016/j.biomaterials.2015.09.038
71. Savellano MD, Hasan T. Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem Photobiol (2003) 77(4):431–9. doi: 10.1562/0031-8655(2003)077<0431:TCTOTE>2.0.CO;2
72. Grimland JL, Wu C, Ramoutar RR, Brumaghim JL, McNeill J. Photosensitizer-doped conjugated polymer nanoparticles with high cross-sections for one- and two-photon excitation. Nanoscale (2011) 3(4):1451–5. doi: 10.1039/c0nr00834f
73. Kim H, Mun S, Choi Y. Photosensitizer-conjugated polymeric nanoparticles for redox-responsive fluorescence imaging and photodynamic therapy. J Mater Chem B (2013) 1(4):429–31. doi: 10.1039/c2tb00287f
74. Yang Y, Hu Y, Wang H. Targeting antitumor immune response for enhancing the efficacy of photodynamic therapy of cancer: recent advances and future perspectives. Oxid Med Cell Longev (2016) 2016:5274084. doi: 10.1155/2016/5274084
75. Song W, Kuang J, Li C-X, Zhang M, Zheng D, Zeng X, et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano (2018) 12(2):1978–89. doi: 10.1021/acsnano.7b09112
76. Ai X, Hu M, Wang Z, Lyu L, Zhang W, Li J, et al. Enhanced cellular ablation by attenuating hypoxia status and reprogramming tumor-associated macrophages via NIR light-responsive upconversion nanocrystals. Bioconjug Chem (2018) 29(4):928–38. doi: 10.1021/acs.bioconjchem.8b00068
77. Lovell JF, Liu TWB, Chen J, Zheng G. Activatable photosensitizers for imaging and therapy. Chem Rev (2010) 110(5):2839–57. doi: 10.1021/cr900236h
78. Ma R, Alifu N, Du Z, Chen S, Heng Y, Wang J, et al. Indocyanine green-based theranostic nanoplatform for NIR fluorescence image-guided chemo/photothermal therapy of cervical cancer. Int J Nanomed (2021) 16:4847–61. doi: 10.2147/IJN.S318678
79. Kaibori M, Matsui K, Hayashi M. Theranostics using indocyanine green lactosomes. Cancers (Basel) (2022) 14(15):3840. doi: 10.3390/cancers14153840
80. Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater (2011) 10(4):324–32. doi: 10.1038/nmat2986
81. Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev (2010) 110(5):2795–838. doi: 10.1021/cr900300p
82. Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials (2011) 32(26):6145–54. doi: 10.1016/j.biomaterials.2011.05.007
83. Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano (2017) 11(5):4463–74. doi: 10.1021/acsnano.7b00715
84. Wang C, Cheng L, Liu Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics (2013) 3(5):317–30. doi: 10.7150/thno.5284
85. Jiang Y, Pu K. Multimodal biophotonics of semiconducting polymer nanoparticles. Acc Chem Res (2018) 51(8):1840–9. doi: 10.1021/acs.accounts.8b00242
86. Ju Y, Liao H, Richardson JJ, Guo J, Caruso F. Nanostructured particles assembled from natural building blocks for advanced therapies. Chem Soc Rev (2022) 51(11):4287–336. doi: 10.1039/d1cs00343g
87. Sun B, Bte Rahmat JN, Zhang Y. Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials (2022) 291:121875. doi: 10.1016/j.biomaterials.2022.121875
88. Bonferoni MC, Rassu G, Gavini E, Sorrenti M, Catenacci L, Torre ML, et al. Electrochemotherapy of deep-seated tumors: state of art and perspectives as possible “EPR effect enhancer” to improve cancer nanomedicine efficacy. Cancers (Basel) (2021) 13(17):4437. doi: 10.3390/cancers13174437
89. Li W-P, Yen C-J, Wu B-S, Wong T-W. Recent advances in photodynamic therapy for deep-seated tumors with the aid of nanomedicine. Biomedicines (2021) 9(1):69 doi: 10.3390/biomedicines9010069
90. Garg AD, Agostinis P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol Rev (2017) 280(1):126–48. doi: 10.1111/imr.12574
91. Kleinovink JW, Fransen MF, Lowik CW, Ossendorp F. Photodynamic-immune checkpoint therapy eradicates local and distant tumors by CD8(+) T cells. Cancer Immunol Res (2017) 5(10):832–8. doi: 10.1158/2326-6066.CIR-17-0055
92. Blaudszun A-R, Kim WJ, Um W, Yoon HY, Shim MK, Kim K. Adoptive transfer of photosensitizer-loaded cytotoxic T cells for combinational photodynamic therapy and cancer immuno-therapy. Pharmaceutics (2023) 15(4):1295. doi: 10.3390/pharmaceutics15041295
93. Hwang HS, Cherukula K, Bang YJ, Vijayan V, Moon MJ, Thiruppathi J, et al. Combination of photodynamic therapy and a flagellin-adjuvanted cancer vaccine potentiated the anti-PD-1-mediated melanoma suppression. Cells (2020) 9(11):2432. doi: 10.3390/cells9112432
94. Gao L, Zhang C, Gao D, Liu H, Yu X, Lai J, et al. Enhanced anti-tumor efficacy through a combination of integrin αvβ6-targeted photodynamic therapy and immune checkpoint inhibition. Theranostics (2016) 6(5):627–37. doi: 10.7150/thno.14792
95. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol (2017) 17(2):97–111. doi: 10.1038/nri.2016.107
96. Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res (2002) 62(6):1604–8.
97. Liu J, He S, Luo Y, Zhang Y, Du X, Xu C, et al. Tumor-microenvironment-activatable polymer nano-immunomodulator for precision cancer photoimmunotherapy. Adv Mater (2022) 34(8):e210665. doi: 10.1002/adma.202106654
98. Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res (2005) 65(3):1018–26. doi: 10.1158/0008-5472.1018.65.3
99. Zhang R, Zhu Z, Lv H, Li F, Sun S, Li J, et al. Immune checkpoint blockade mediated by a small-molecule nanoinhibitor targeting the PD-1/PD-L1 pathway synergizes with photodynamic therapy to elicit antitumor immunity and antimetastatic effects on breast cancer. Small (Weinheim an Der Bergstrasse Germany) (2019) 15(49):e1903881. doi: 10.1002/smll.201903881
100. Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR, et al. Chlorin-based nanoscale metal-organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J Am Chem Soc (2016) 138(38):12502–10. doi: 10.1021/jacs.6b06663
101. Mroz P, Hashmi JT, Huang Y-Y, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol (2011) 7(1):75–91. doi: 10.1586/eci.10.81
102. Wang H, Han X, Dong Z, Xu J, Wang J, Liu Z. Hyaluronidase with pH-responsive dextran modification as an adjuvant nanomedicine for enhanced photodynamic-immunotherapy of cancer. Adv Funct Mater (2019) 29:1902440. doi: 10.1002/adfm.201902440
103. Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev (2017) 46(12):3830–52. doi: 10.1039/c6cs00592f
104. Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, et al. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat Commun (2015) 6:8785. doi: 10.1038/ncomms9785
105. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell (2008) 133(5):775–87. doi: 10.1016/j.cell.2008.05.009
106. Cramer GM, Moon EK, Cengel KA, Busch TM. Photodynamic therapy and immune checkpoint blockade(dagger). Photochem Photobiol (2020) 96(5):954–61. doi: 10.1111/php.13300
107. Meng Z, Zhou X, Xu J, Han X, Dong Z, Wang H, et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater (2019) 31(24):e1900927. doi: 10.1002/adma.201900927
108. Zeng J-Y, Zou M-Z, Zhang M, Wang X-S, Zeng X, Cong H, et al. π-extended benzoporphyrin-based metal-organic framework for inhibition of tumor metastasis. ACS Nano (2018) 12(5):4630–40. doi: 10.1021/acsnano.8b01186
109. Ni K, Luo T, Lan G, Culbert A, Song Y, Wu T, et al. A nanoscale metal-organic framework to mediate photodynamic therapy and deliver cpG oligodeoxynucleotides to enhance antigen presentation and cancer immunotherapy. Angew Chem Int Ed Engl (2020) 59(3):1108–12. doi: 10.1002/anie.201911429
110. Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer (2019) 1872(2):188308. doi: 10.1016/j.bbcan.2019.07.003
111. Fang L, Zhao Z, Wang J, Zhang P, Ding Y, Jiang Y, et al. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci Adv (2020) 6(25):eaba4024. doi: 10.1126/sciadv.aba4024
112. Ji J, Fan Z, Zhou F, Wang X, Shi L, Zhang H, et al. Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncotarget (2015) 6(19):17135–46. doi: 10.18632/oncotarget.3529
113. Trempolec N, Doix B, Degavre C, Brusa D, Bouzin C, Riant O, et al. Photodynamic therapy-based dendritic cell vaccination suited to treat peritoneal mesothelioma. Cancers (Basel) (2020) 12(3):545. doi: 10.3390/cancers12030545
114. Srivastava S, Riddell SR. Engineering CAR-T cells: Design concepts. Trends In Immunol (2015) 36(8):494–502. doi: 10.1016/j.it.2015.06.004
115. Kleinovink JW, van Driel PB, Snoeks TJ, Prokopi N, Fransen MF, Cruz LJ, et al. Combination of photodynamic therapy and specific immunotherapy efficiently eradicates established tumors. Clin Cancer Res (2016) 22(6):1459–68. doi: 10.1158/1078-0432.CCR-15-0515
116. Xu J, Yu S, Wang X, Qian Y, Wu W, Zhang S, et al. High affinity of chlorin e6 to immunoglobulin G for intraoperative fluorescence image-guided cancer photodynamic and checkpoint blockade therapy. ACS Nano (2019) 13(9):10242–60. doi: 10.1021/acsnano.9b03466
117. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun (2016) 7:13193. doi: 10.1038/ncomms13193
118. Santos LL, Oliveira J, Monteiro E, Santos J, Sarmento C. Treatment of head and neck cancer with photodynamic therapy with redaporfin: A clinical case report. Case Rep Oncol (2018) 11(3):769–76. doi: 10.1159/000493423
119. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol (2020) 17(11):657–74. doi: 10.1038/s41571-020-0410-2
120. Liu Y, Pan Y, Cao W, Xia F, Liu B, Niu J, et al. A tumor microenvironment responsive biodegradable CaCO3/MnO2- based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics (2019) 9(23):6867–84. doi: 10.7150/thno.37586
121. Chen Q, Chen J, Yang Z, Xu J, Xu L, Liang C, et al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv Mater (2019) 31(10):e1802228. doi: 10.1002/adma.201802228
122. Kim S, Kim SA, Nam G-H, Hong Y, Kim GB, Choi Y, et al. In situ immunogenic clearance induced by a combination of photodynamic therapy and rho-kinase inhibition sensitizes immune checkpoint blockade response to elicit systemic antitumor immunity against intraocular melanoma and its metastasis. J Immunother Cancer (2021) 9(1):e001481. doi: 10.1136/jitc-2020-001481
123. Kaneko K, Acharya CR, Nagata H, Yang X, Hartman ZC, Hobeika A, et al. Combination of a novel heat shock protein 90-targeted photodynamic therapy with PD-1/PD-L1 blockade induces potent systemic antitumor efficacy and abscopal effect against breast cancers. J Immunother Cancer (2022) 10(9):e004793. doi: 10.1136/jitc-2022-004793
124. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer (2016) 16(5):275–87. doi: 10.1038/nrc.2016.36
125. Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer (2005) 5(3):161–71. doi: 10.1038/nrc1566
126. Jain V, Kumar H, Anod HV, Chand P, Gupta NV, Dey S, et al. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J Control Release (2020) 326:628–47. doi: 10.1016/j.jconrel.2020.07.003
Keywords: nanotechnology, photodynamic therapy, immunotherapy, photosensitizers, cancer treatment, photosensitizer
Citation: Jia J, Wu X, Long G, Yu J, He W, Zhang H, Wang D, Ye Z and Tian J (2023) Revolutionizing cancer treatment: nanotechnology-enabled photodynamic therapy and immunotherapy with advanced photosensitizers. Front. Immunol. 14:1219785. doi: 10.3389/fimmu.2023.1219785
Received: 09 May 2023; Accepted: 20 September 2023;
Published: 04 October 2023.
Edited by:
Giulio Fracasso, University of Padova, ItalyCopyright © 2023 Jia, Wu, Long, Yu, He, Zhang, Wang, Ye and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Tian, dGlhbmp1bjE5NzAxMEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.