- 1Department of Orthopaedic Surgery, Duke Molecular Physiology Institute, Duke University, Durham, NC, United States
- 2Department of Physiology and Pharmacology, Schulich School of Medicine & Dentistry, University of Western Ontario, London, ON, Canada
Editorial on the Research Topic
The immune system and inflammation in musculoskeletal health, aging, and disease
The musculoskeletal and immune systems are intricately linked in anatomical space and function, with crosstalk between immune cells and musculoskeletal tissues, including bone, cartilage, muscle, and tendons, being essential for normal development and homeostasis (1–4). Such a relationship is also critical during injury and repair, with inflammation and immune cells being necessary for initiating and resolving injury-induced tissue responses and altered extracellular matrix composition and turnover, likewise regulating immune cell engagement (5–9). Over the last decade, growing evidence has demonstrated that alterations to immune cell populations caused by aging and metabolic dysfunction underly the impaired tissue repair responses seen in chronic musculoskeletal diseases and acute injuries, including those affecting synovial joints (e.g., osteoarthritis), bones (e.g., osteoporosis, fracture healing), muscles (e.g., sarcopenia), and tendons/ligaments (e.g., tendinopathy, rupture).
The burden from musculoskeletal conditions continues to rise globally, impacting patients’ quality of life, independence, and health, social and economic systems due to increasing care costs and work loss. Over 1.7 billion people globally live with musculoskeletal conditions, according to the World Health Organization (10) and findings from the Lancet’s Global Burden of Disease Study 2019 (11–13). Low back pain is the main contributor to this overall burden, while osteoarthritis (OA) shows the most rapid increase of these conditions. While advances have been made in treating osteoporosis in the past decade, intervertebral disc degeneration (IVDD), OA, and many others where disease pathogenesis is less understood lack disease-modifying therapeutics. Progress in understanding how diseases such as IVDD and OA progress has revealed an essential role for inflammatory dysregulation in these conditions; however, several important questions must be resolved before this can be mobilized as a therapeutic strategy. In this Frontiers of Immunology – Inflammation Section Research Topic; The Immune System and Inflammation in Musculoskeletal Health, Aging, and Disease; we present a collection of articles focused on the aging skeleton and aim to shed light on age-associated dysregulation of inflammatory processes that lead to musculoskeletal morbidity with the hope of identifying targets for future disease-modifying therapeutics (Figure 1).
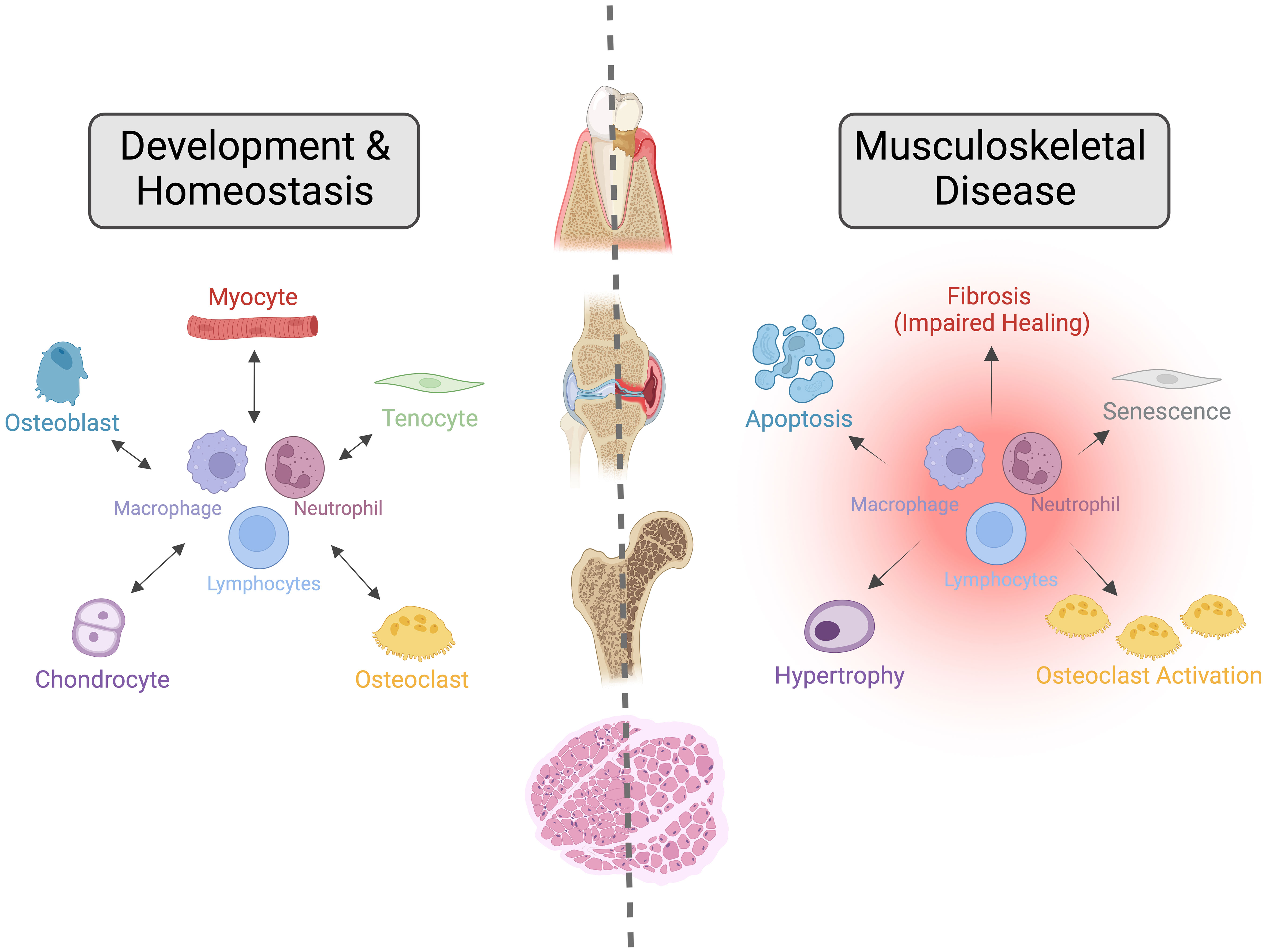
Figure 1 The Importance of Crosstalk between the Immune and Musculoskeletal Systems during Development, Homeostasis, and Disease. Interactions between the immune and musculoskeletal systems are critical for their development and homeostasis. Alterations to these interactions caused by aging, metabolic dysfunction, or genetic mutations lead to impaired repair responses and dysregulated tissue homeostasis, resulting in various musculoskeletal diseases such as osteoarthritis, osteoporosis, sarcopenia, periodontal disease and dental implant loosening, and intervertebral disc degeneration. In this Frontiers of Immunology – Inflammation Section research topic, we present a collection of articles that aim to shed light on age-associated dysregulation of inflammatory processes that lead to musculoskeletal morbidity. Image created with BioRender.com under Academic License.
OA is a multifactorial disease affecting synovial joints and one of the most common musculoskeletal diseases worldwide (14, 15). The disease is characterized by progressive loss of articular cartilage, subchondral bone and peri-articular bone remodeling, and intra-articular inflammation with synovitis, culminating in chronic pain and reduced mobility. The role of immune cells in OA progression was long recognized but remains poorly understood. In this Research Topic, Haubruck et al. outline the role of monocytic cells in post-traumatic OA (PTOA) and discuss therapeutic interventions to potentially improve disease outcomes. In addition to modifying disease outcomes, improved metrics for classifying OA severity are required to better manage the disease and for the development of disease-modifying drugs. In this regard, the search for biomarkers that can identify the stage of OA progression and distinguish between OA at different anatomical sites has become increasingly important. In this Research Topic, Zhang et al. identify extracellular vesicle sub-species that are more prevalent in patients with knee OA and carry a pro-inflammatory cargo, including tumor necrosis factor-alpha (TNF-α). In a second study in this Research Topic, Ratneswaran et al. investigated patients with hand OA and identified that circulating cytokines could distinguish OA severity of the trapeziometacarpal joint. Specifically, interleukin-7 (IL-7) was identified as a marker capable of differentiating disease severity with higher levels associated with a decreased likelihood of trapeziometacarpel joint OA needing surgical intervention. In terms of targeting inflammation to treat OA, Vachhani et al. investigated whether CD200R1 agonists could delay the progression of PTOA; however, neither the protein therapeutic CD200Fc nor the synthetic DNA aptamer CCS13 were able to attenuate cartilage degeneration or synovitis, despite their ability to blunt inflammatory response in the knee. This study points to the complexities of targeting inflammation in complex diseases such as OA.
In recent years, synoviocytes and resident immune cells within the synovium have emerged as key players in the progression of OA and other joint diseases (16, 17). In this Research Topic, Jones et al. used ChIP-seq to assay fibroblast-like synoviocytes and identified that SOX4 and RELA physically interact on chromatin, with TNF-responsive genes being the primary targets of this transcriptional complex. Sodhi et al. identified sex-dependent differences between complement and synovial microvascular pathology in patients, with higher synovial fluid C5 levels being associated with increased complement activation and decreased synovial vascularization in males but not in females with OA. Farina et al. identified that the binding of pro-nerve growth factor to p75NTR on synoviocytes elicits an inflammatory response, resulting in the release of IL-1β, IL-6 and TNF-α. Inhibition of this binding prevented this inflammatory response and represents a novel therapeutic approach in chronic arthritis. Finally, the role of Hippo pathway targets, the mechanoresponsive Yap/Taz transcription factors, was investigated in the context of rheumatoid arthritis (RA) by Caire et al., where the authors demonstrated that RA activates Yap/Taz within synoviocytes. Treatment with a Yap/Taz inhibitor reversed the RA phenotype indicating their transcriptional inhibition could be relevant to treat inflammatory-related diseases.
Aging also affects other tissues of the skeleton. The effect of oxidative stress on IVDD was studied by Cao et al. Specifically, this group found that Ccnb1 and Pkd1 help to regulate oxidative stress during intervertebral disc degeneration and lead to CD8+ T cell infiltration. This work presents Ccnb1 & Pkd1 as potential targets for treatment in IVDD. A lesser-considered modality of the aging skeleton is dental health. Schluessel et al. investigated the loosening of dental implants and related gene expression profiles with the loosening of orthopedic implants. Using co-culture systems, disparate and overlapping gene profiles were established, identifying potential therapeutic targets for improving and maintaining the integration of dental and orthopedic implants. Ma et al. investigated the ratio of pro-inflammatory and anti-inflammatory macrophages within the infrapatellar fat pad and subcutaneous fat tissue of patients receiving total knee arthroplasties. The macrophage ratio differed between infrapatellar and subcutaneous fat, with the infrapatellar environment presenting a more inflammatory niche that could be targeted for therapeutic intervention in joint disease.
The works within this collection shed light on the importance of immune cell populations and signaling mechanisms in musculoskeletal disease progression; however, more work is certainly needed before therapeutics targeting these mechanisms can be developed to modify complex diseases such as OA and IVDD.
In conclusion, we thank the contributing authors for sharing their findings and insights in these critical areas. Musculoskeletal diseases have long presented a challenge to the medical and scientific communities. However, with an improved understanding of how the immune and musculoskeletal systems interact, better and more selective therapies could be on the horizon.
Author contributions
All authors listed have made equal substantial, direct, and intellectual contributions to the work and approved it for publication.
Funding
G.S.B. was supported by a Borden Scholars award, Duke Claude D. Pepper Older Americans Independence Center Pilot Award (P30AG028716), and by the NIH/NIA (R21AG067245). M.W.G. was supported by funding from the Canada Research Chairs Program and the Canadian Institutes of Health Research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guder C, Gravius S, Burger C, Wirtz DC, Schildberg FA. Osteoimmunology: a current update of the interplay between bone and the immune system. Front Immunol (2020) 11:58. doi: 10.3389/fimmu.2020.00058
2. Longoni A, Knezevic L, Schepers K, Weinans H, Rosenberg A, Gawlitta D. The impact of immune response on endochondral bone regeneration. NPJ Regener Med (2018) 3:22. doi: 10.1038/s41536-018-0060-5
3. Sun Z, Liu B, Luo ZJ. The immune privilege of the intervertebral disc: implications for intervertebral disc degeneration treatment. Int J Med Sci (2020) 17:685–92. doi: 10.7150/ijms.42238
4. Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol (2017) 17:165–78. doi: 10.1038/nri.2016.150
5. Baht GS, Vi L, Alman BA. The role of the immune cells in fracture healing. Curr Osteoporos Rep (2018) 16:138–45. doi: 10.1007/s11914-018-0423-2
6. Crosio G, Huang AH. Innate and adaptive immune system cells implicated in tendon healing and disease. Eur Cell Mater (2022) 43:39–52. doi: 10.22203/eCM.v043a05
7. Woodell-May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J Orthop Res (2020) 38:253–7. doi: 10.1002/jor.24457
8. Xiong Y, Mi BB, Lin Z, Hu YQ, Yu L, Zha KK, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res (2022) 9:65. doi: 10.1186/s40779-022-00426-8
9. Ye F, Lyu FJ, Wang H, Zheng Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine (2022) 5:e1196. doi: 10.1002/jsp2.1196
11. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
12. Liu S, Wang B, Fan S, Wang Y, Zhan Y, Ye D. Global burden of musculoskeletal disorders and attributable factors in 204 countries and territories: a secondary analysis of the global burden of disease 2019 study. BMJ Open (2022) 12:e062183. doi: 10.1136/bmjopen-2022-062183
13. Sebbag E, Felten R, Sagez F, Sibilia J, Devilliers H, Arnaud L. The world-wide burden of musculoskeletal diseases: a systematic analysis of the world health organization burden of diseases database. Ann Rheum Dis (2019) 78:844–8. doi: 10.1136/annrheumdis-2019-215142
14. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum (2012) 64:1697–707. doi: 10.1002/art.34453
15. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers (2016) 2:16072. doi: 10.1038/nrdp.2016.72
16. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther (2017) 19:18. doi: 10.1186/s13075-017-1229-9
Keywords: inflammation, immune system, musculoskeletal disorders, musculoskeletal system, osteoarthritis, rheumatoid arthritis, intervertebral disc degeneration, dental implants
Citation: Baht GS and Grol MW (2023) Editorial: The immune system and inflammation in musculoskeletal health, aging, and disease. Front. Immunol. 14:1218118. doi: 10.3389/fimmu.2023.1218118
Received: 06 May 2023; Accepted: 09 May 2023;
Published: 18 May 2023.
Edited and Reviewed by:
Pietro Ghezzi, University of Urbino Carlo Bo, ItalyCopyright © 2023 Baht and Grol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gurpreet S. Baht, Z3VycHJlZXQuYmFodEBkdWtlLmVkdQ==; Matthew W. Grol, bWdyb2wyQHV3by5jYQ==
 Gurpreet S. Baht
Gurpreet S. Baht Matthew W. Grol
Matthew W. Grol