- 1Department of Hematology, The First Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Hematopathology, Duke Cancer Institute, Duke University Medical Center, Durham, NC, United States
Advanced studies have shown a biological correlation between hepatitis B virus (HBV) and B-cell lymphoma, especially diffuse large B-cell lymphoma (DLBCL). Patients with DLBCL infected with HBV (HBV-associated DLBCL) are clinically characterized by an advanced clinical stage, poor response to front-line immunochemotherapy regimens, and worse clinical prognosis. HBV-associated DLBCL often exhibits abnormal activation of the nuclear factor kappa B pathway as well as mutations in oncogenes, including Myc and BCL-6. Currently, there is no consensus on any specific and effective treatment for HBV-associated DLBCL. Therefore, in this review, we comprehensively and mechanistically analyzed the natural history of HBV infection and immunity, including HBV-mediated oncogenes, immune escape, epigenetic alterations, dysregulated signaling pathways, and potential therapeutic approaches for HBV-associated DLBCL. We hope that an improved understanding of the biology of HBV-associated DLBCL would lead to the development of novel therapeutic approaches, enhance the number of effective clinical trials, and improve the prognosis of this disease.
1 Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of B cell lymphoma, comprising 30%–40% of non-Hodgkin lymphomas (NHL). Most patients with DLBCL can be cured using current front-line immunochemotherapy regimens based on a combination of anthracyclines and anti-CD20 antibodies, such as R-CHOP; however, 30%–40% of patients have disease relapse or are refractory to first-line treatment, meaning that DLBCL is a possibly heterogeneous disease (1). Over the past few decades, considerable efforts have been made to decipher the molecular basis of this heterogeneity. First, according to the gene-expression profiles of the cell of origin, DLBCL has been divided into three subtypes, namely activated-B-cell-like (ABC), germinal-center B-cell-like (GCB), and an unclassified group; patients with GCB DLBCL have been reported to have a significantly better overall survival (OS) than those with ABC DLBCL (2). Moreover, DLBCLs harboring Myc, BLC-2, and/or BCL-6 translocations are called “double-hit” lymphoma (DHL) or “triple-hit” lymphoma (THL), which have a poor prognosis. In the recent 5th edition of the World Health Organization classification of lymphoid neoplasms, the DHL/THL category is recognized as “high-grade B-cell” lymphoma owing to the rearrangements of Myc and BCL-2 (3). However, there are certain disadvantages to first-line chemotherapy of DLBCL, including hepatitis B virus (HBV) infection (HBV-associated DLBCL). In this review, we focus on whether HBV infection worsens the prognosis of patients with HBV-associated DLBCL through the aforementioned related molecules and/or cell signaling.
2 HBV-associated DLBCL with poor prognosis
2.1 Clinical features
Many studies have validated that HBV can lead to other kinds of cancers besides hepatocellular carcinoma (HCC), including gastrointestinal tumors and B-cell non-Hodgkin’s lymphoma (B-NHL), in Asia, Africa and Western countries (4–7). Moreover, among patients with B-NHL, DLBCL has been reported to have a strong and more significant association with HBV infection (8–11) compared to that with indolent lymphoma (12–14); for instance, a study from west Africa showed that the prevalence of HBV-associated DLBCL is 14.3% (5). Moreover, a previous study of ours showed that the incidence of HBV-associated DLBCL is 13.2% (15), meaning that HBV infection is closely related to DLBCL.
HBV-associated DLBCL is characterized by a high prevalence among younger individuals, a high incidence at advanced clinical stages, mainly involving the peritoneal lymph nodes and spleen, and a relatively poor prognosis (16).Our previous study showed that patients with DLBCL and HBV infection had poor prognosis (three-year OS: HBsAg-negative patients, 90.6% vs. patients with HBV-associated DLBCL 54.5%) (17). Cheng et al. (18) also found that patients with HBV-associated DLBCL had a lower overall response rate (ORR: 76.5% vs. 85.5%, p=0.043), poorer 5-year OS rate (57.2% vs. 73.5%, p <0.001), and a shorter 5-year progression-free survival (PFS: 47.2% vs. 60.7%, p=0.013) than HBsAg-negative patients. HBsAg-positivity is an independent adverse prognostic factor for HBV-associated DLBCL (18, 19). It is also the main factor leading to the poor efficacy of immunochemotherapy, including R-CHOP regimens. Accordingly, HBV infection is closely associated with DLBCL and is a crucial factor leading to poor prognosis (17, 20, 21). Huang et al. indicated that antiviral agents, entecavir, combined with chemotherapy could improve the prognosis of patients with HBV-associated DLBCL (median OS not reached vs. 35.61 months) (22). In this review, we focus on the dysregulation in molecules related to the poor prognosis of HBV-associated DLBCL, including oncogenes, immune escape, epigenetic modification, and dysregulated signaling pathways, as well as chemo-resistance and potential therapeutic approaches.
2.2 Genomic structure of HBV, immunity, and dysregulation
The HBV genome encompasses four open overlapping reading frames (ORFs)-pre-C/C (pre-core/core), pre-S/S (surface proteins), X (transcriptional co-activator), and P (DNA polymerase) (23) (Figure 1A). The preS1, preS2, and S genes mainly code HBsAg; pre-C/C code HBeAg; and the HBV X gene codes the HBx protein (Figure 1B). After HBV infection, the human body initially carries the virus for a period of time and subsequently expresses the relevant antigens, including HBsAg and HBeAg, thereby eliciting immune response by stimulating the B-cell receptor (BCR) and subsequently activating the abnormal BCR signaling pathway. B-cells produce antibodies HBsAb, HBeAb, and HBcAb to block further infection and support effective viral clearance (24). The natural history of HBV infection has four phases, including immune tolerance, immune clearance, immune-control, and reactive phase (25–27). (1) The immune tolerant phase is characterized by HBeAg seropositivity with high viral loads (>106-7 IU/mL) but near-normal liver histology. (2) The immune clearance phase is characterized by HBeAg positivity with a declining serum HBV-DNA level and active inflammation in the liver; this phase may eventually lead to HBV-DNA seroclearance and the seroconversion of HBeAg to its antibody (HBeAb) in most patients. (3) In the immune-control phase, following HBeAg seroconversion, most patients enter the immune-control phase with low serum HBV-DNA (<2000 IU/mL). (4) The reactive phase is characterized by HBeAg negativity with HBeAb positivity, detectable serum HBV-DNA levels, and active inflammation in the liver; this phase is associated with immune escape (Figure 1C).
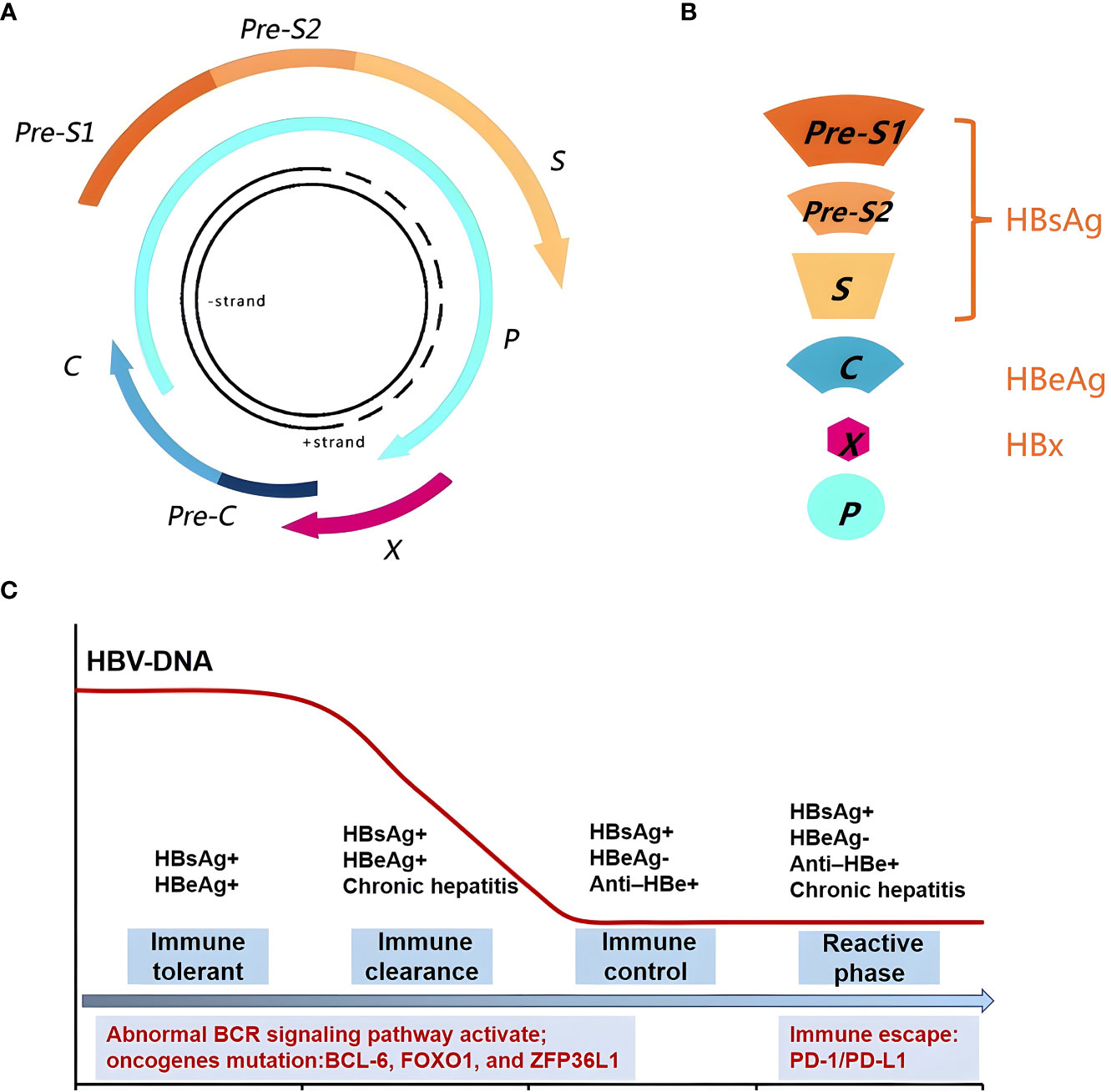
Figure 1 Genomic structure of HBV and relationship with the body immune function. (A) The HBV Genomic structure. (B) HBV gene code associated protein. (C) The natural history of HBV infection has four phases, including immune tolerant, immune clearance, immune-control and reactive phase, could activate the abnormal BCR signaling pathway and oncogenes mutation. The reactive phase is associated with immune escape.
As for genes, HBx is the most frequently integrated viral gene following HBV infection, which plays a critical role in tumor pathogenesis (28). Studies have reported that HBx was detected in the tissues of patients with HBV-associated DLBCL (29, 30). Our previous study has also shown that HBx was detected in tissues from patients with HBV-associated DLBCL and that HBx expression was correlated with c-Myc expression (31) (Figure 2).
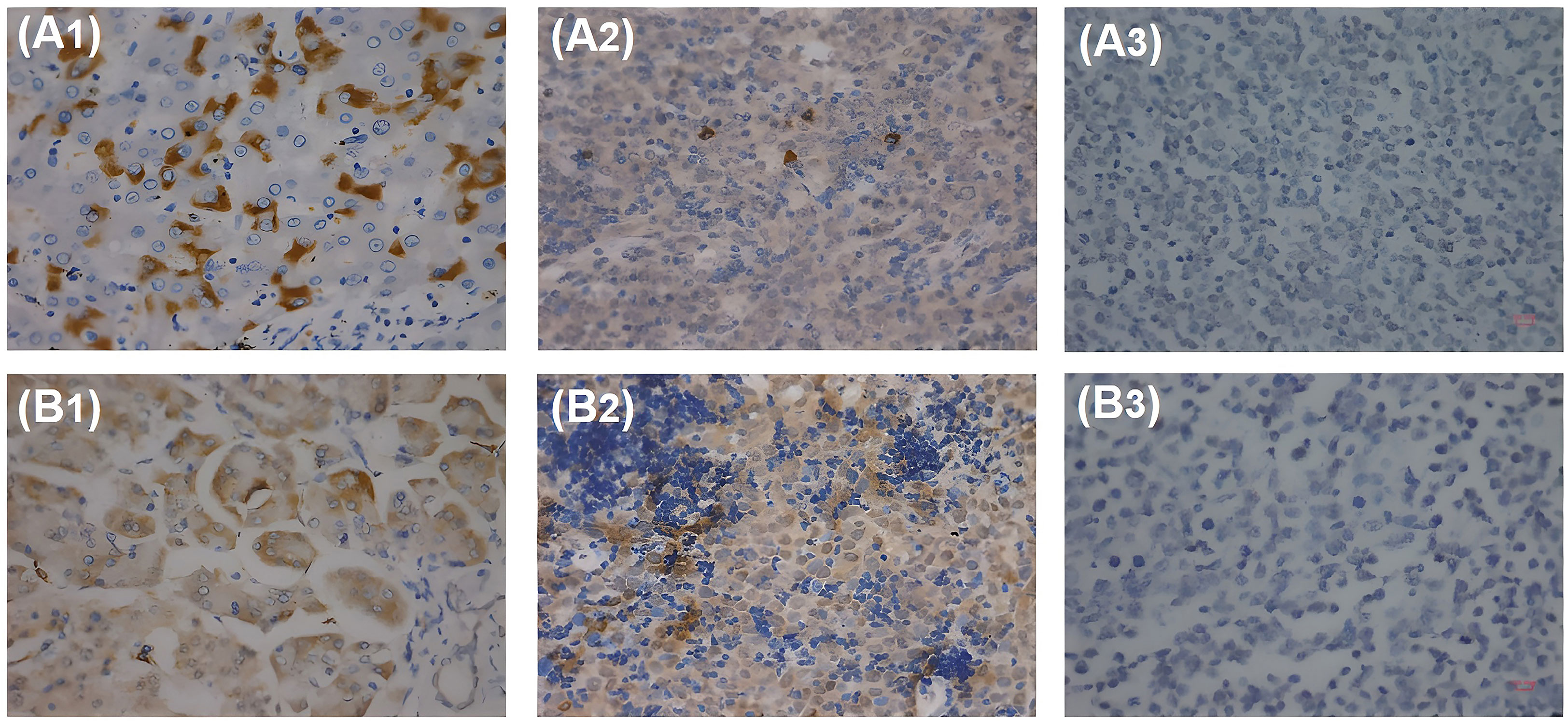
Figure 2 HBx and pre-S2 detected in patients with HBV-associated DLBCL with IHC. (A1) HBx antigen expressed in HBV-associated hepatocytes. (A2) HBx antigen expressed in HBV-associated DLBCL lymphoma cells. (A3) HBx antigen cannot be detected in HBV-negative DLBCL lymphoma cells. (B1) Pre-S2 antigen expressed in HBV-associated hepatocytes. (B2) Pre-S2 antigen expressed in HBV-associated DLBCL lymphoma cells. (B3) Pre-S2 antigen cannot be detected in HBV-negative DLBCL lymphoma cells.
2.2.1 Crucial oncogene mutation and corresponding cell signaling pathways
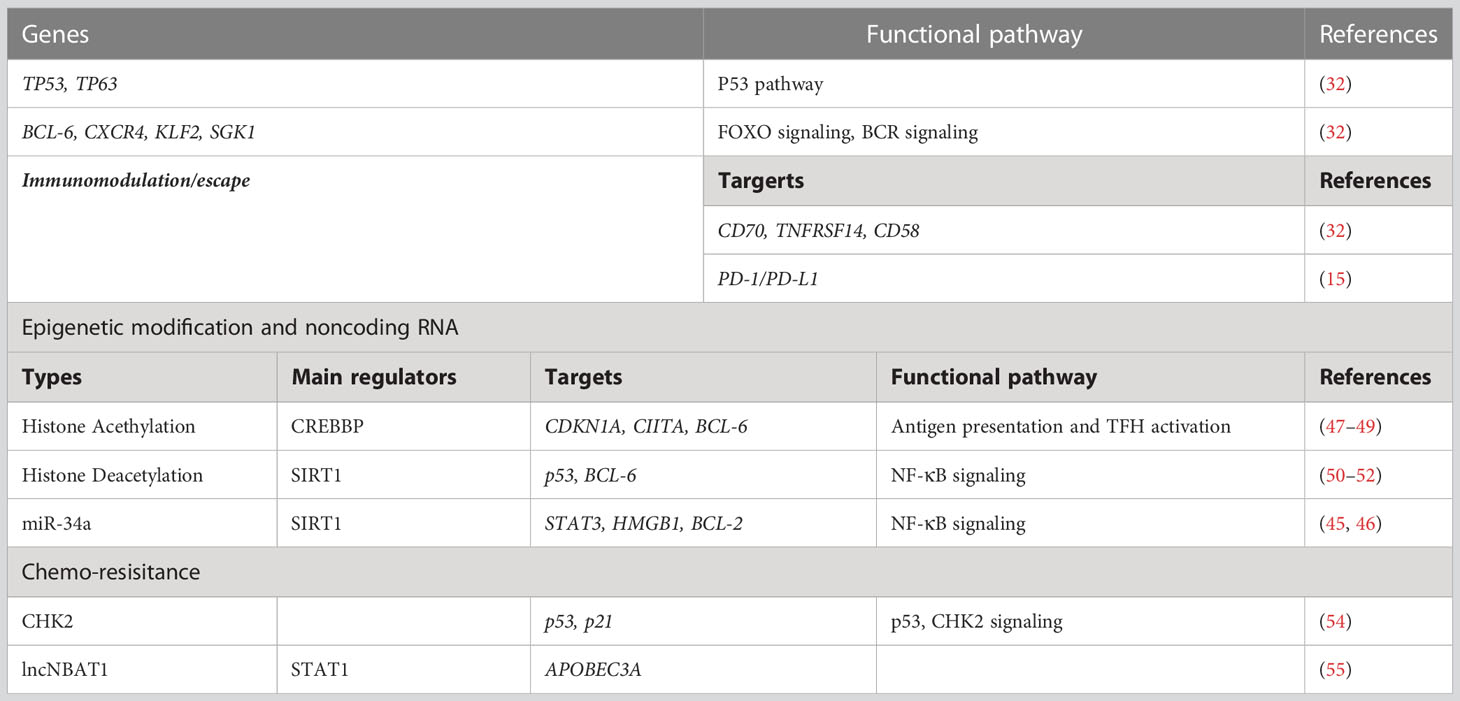
Whole exome sequencing/whole genome sequencing (WES/WGS) showed that HBV infection was associated with DLBCL. Studies indicated several mutations in genes related to the AID/APOBEC enzymes in patients with HBV-associated DLBCL (32, 33). Ren et al. (32) detected the genes mutation in 275 patients of DLBCL, including 20% patients with HBsAg positive (56/275). And the tumor biopsy specimens of 229 patients were taken at diagnosis, and 46 were taken at relapse. The quantitation of viral DNA was performed in 44 HBsAg positive patients. Fourteen genes were confirmed to be preferentially mutated in the HBV infection group, including KLF2, TMSB4X, CD70, BCL-6, FAS, TNFRSF14, UBE2A, CD58, SGK1, ZFP36L1, CXCR4, FOXO1, CSK, and MSL2. In these genes, the enrichment of genes regulated by BCL-6, FOXO1, and ZFP36L1 and involved in signaling pathways, including BCR, janus kinase/signal transducer and activator of transcription (JAK/STAT), and nuclear factor kappa B (NF-κB), contributed to the gene expression signature (Figure 3).
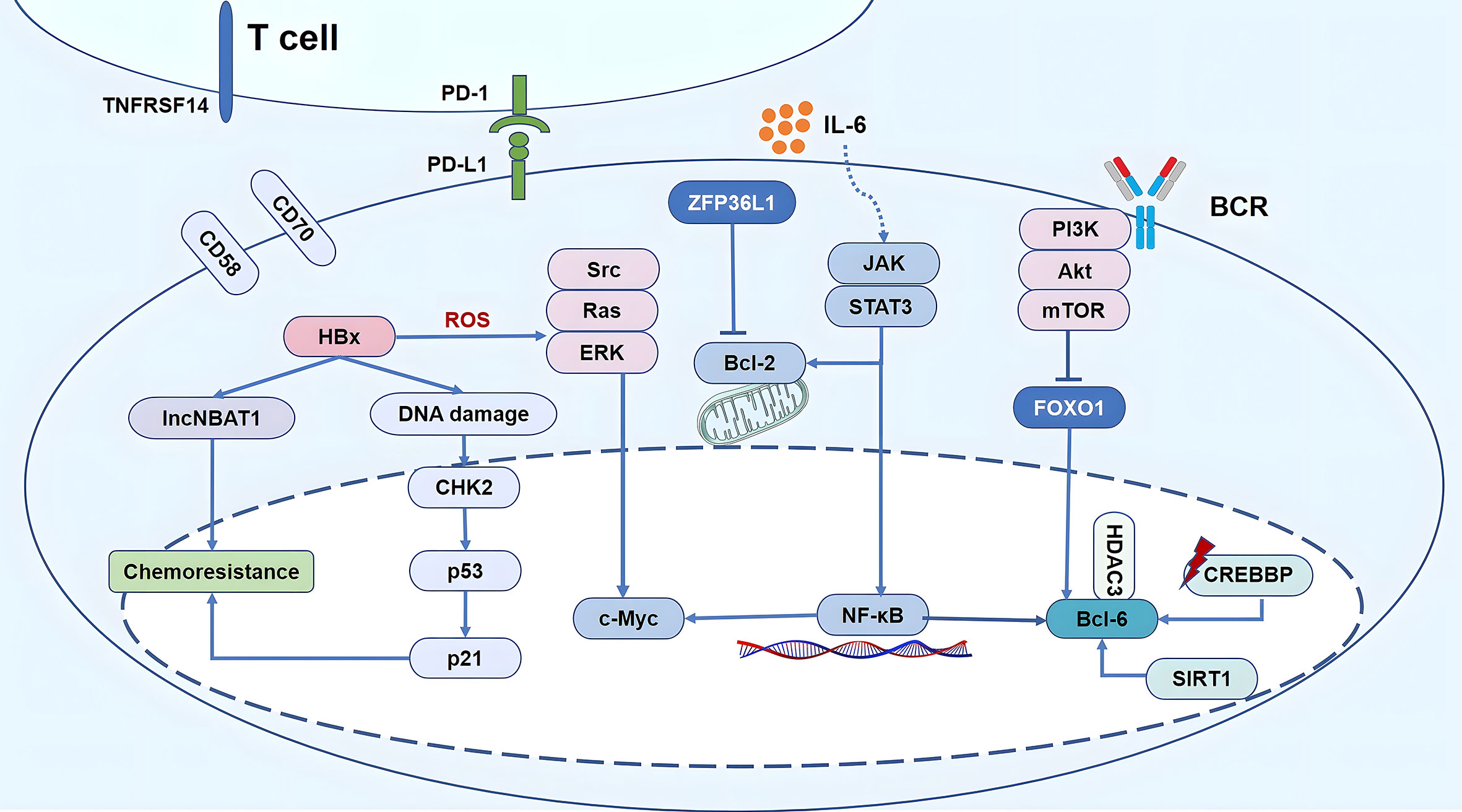
Figure 3 Schematic diagram of HBV-mediated oncogenic signaling pathway activation, epigenetic modification, immune escape and chemo-resistance in HBV-associated DLBCL. 1) Replication of the virus leads to abnormal host epigenetic regulation, accompanied by CREBBP mutations up-regulation of SIRT1, inducing the up-regulation of deacetylatation levels, resulting in imbalance of CREBBP-BCL-6/HDAC3 axis, finally increasing the activity of BCL-6 as proto–oncogenes. Gene expression signature of HBV-associated DLBCL was contributed by the enrichment of genes regulated by BCL-6, FOXO1, and ZFP36L1 and involved in signaling pathways, including BCR, PI3K, JAK/STAT, and NF-κB. In addition, HBx simultaneously activates the Src/Ras/ERK pathway, promoting proliferation and anti-apoptosis owing to the accumulation of ROS and endoplasmic reticulum stress. 2) Tumor-infiltrating immune cells CD8+ T cells are in a state of functional depletion, highly expressed PD-1. The activation of the PD-1/PD-L1 cell signaling pathway can suppress the function of the immune system. CD70, TNFRSF14, and CD58 were mainly mutated in HBV-associated DLBCL, led to a decrease in T cell infusion in tumor microenvironment. 3) HBx over expression significantly reduces the activation of CHK2 signals induced by S-period blockers and p53 in HBV-associated DLBCL, thereby reducing the sensitivity of chemotherapy drug; HBx directly up-regulated the expression of lncNBAT1, reduced the sensitivity of DLBCL cells to chemotherapeutic agents that induced S phase arrest.
BCR signaling activates downstream oncogenic pathways, including NF-κB or PI3K (34). The PI3K-Akt-mTOR signaling cascade is known to be dysregulated and represents a major regulator of cell survival-proliferation (35). NF-κB regulates the proliferation of B cells and promotes the expression of proto-oncogenes (Myc and BCL-6) and cellular anti-apoptotic protein (BCL-2), as well as the secretion of cytokines, including tumour Necrosis Factor alpha (TNFα), lymphotoxin-α, and interleukin-6; it further positively regulates the JAK/STAT3 pathway and cell proliferation and promotes tumorigenesis. As a key transcription factor, BCL-6 plays a crucial role in the clearance of HBV (36). Furthermore, BCL-6 is a crucial gene of HBV-associated DLBCL. There was no significant difference between the HBsAg positive and HBsAg negative groups in the percentage of GCB vs. non-GCB patients. The incidence of GCB DLBCL was 37.5% vs. 38.1%, respectively; non-GCB was 62.5% vs. 61.9%, respectively (32). Zhang et al. showed that HBV was important in DHL/THL DLBCL and that the incidence of Myc/BCL-6 DHL was higher than that of Myc/BCL-2 DHL with HBV infection (37). A previous study of ours showed that the rate of Myc rearrangements in patients with HBV-associated DLBCL was significantly higher than that in HBV-free individuals (38). Moreover, HBx simultaneously activated the Src/Ras/ERK pathway, promoting proliferation and anti-apoptosis owing to the accumulation of ROS and endoplasmic reticulum stress (39). Therefore, HBV-associated DLBCL is involved in the signaling pathways, including BCR, NF-κB, and JAK/STAT3, and further dysregulates oncogenes, including BCL-6, Myc, which are associated with poor prognosis. Moreover, following HBV infection, the human body activates the abnormal BCR signaling pathway, which could be more directly related to HBV-associated DLBCL.
2.2.2 Immune escape
In the reactive phase, HBV could not be completely eliminated and induce immune escape, which is associated with the poor survival of patients with HBV-associated DLBCL. During chronic HBV infections, virus-specific T-cells appear deeply exhausted. Both CD8 and CD4 T-cells up-regulate co-inhibitory receptors, which can inhibit T-cell function following the cross-linking of their ligands, entailing T-cell exhaustion and tumor escape. For instance, the programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) cell signaling pathway is closely related to the functioning of the immune system, which is currently a research hotspot in the field of tumor immunotherapy. The activation of the PD-1/PD-L1 cell signaling pathway can suppress the function of the immune system and contribute to the immune escape of cancer cells (40). Our previous study showed that the incidence of PD-1 expression among patients with HBV-associated DLBCL was 4.3-fold higher than that among HBV-free individuals (40.0% vs. 9.4%; p=0.010). Moreover, the median OS and PFS were the worst in PD-1-positive patients with HBV-associated DLBCL. These results indicate that the dismal prognosis of patients with HBV-associated DLBCL may be related to the high rate of PD1 expression (15) (Figure 3).
Moreover, the number of CD4-positive lymphocytes has been reported to decrease significantly after chemotherapy in patients with HBV-associated DLBCL, and the CD4:CD8 ratio decreased for a longer time in the aforementioned population than in HBV-free individuals, elucidating the reason behind the poor prognosis of patients with HBV-associated: The host immune system weakened the tumor surveillance effect, leading to rapid disease recurrence and poor prognosis (41). Moreover, Ren et al. (32) showed that CD70, TNFRSF14, and CD58 were the main mutated genes among patients with HBV-associated DLBCL, leading to a decrease in T-cell infusion in the tumor microenvironment and thereby weakening tumor immune surveillance and accelerating their escape.
In summary, the continuous high expression of multiple co-inhibitory molecules is the key cause of the depletion of HBV-specific T-cell function. Blocking these molecules can significantly restore HBV-specific T cell function and subsequently remove the virus in vivo. Therefore, the blocking of co-stimulatory molecules might represent a novel strategy for the clinical treatment of HBV infection.
2.2.3 Noncoding RNAs and epigenetic modification
HBx does not bind to DNA, and HBx-activated RNA polymerase (pol) I-, II-, and III-dependent promoters directly interact with some transcription factors and stimulate signal-transduction pathways. The HBx protein can initiate epigenetic modifications to dysregulate noncoding RNAs expression, which, consequently, can regulate downstream epigenetic changes throughout the pathogensis of HBV-associated DLBCL. Bruni et al. (42) found that patients with HBV-associated indolent B-NHL have dysregulated miRNA. Chen et al. (43) also showed that this dysregulation of miRNAs was closely related to the proliferation and differentiation of B-cells. Of note, miRNAs regulate through histone deacetylase (HDAC) (44) or the NF-κB pathway (45). While HDAC inhibitors can break this negative regulation by promoting the expression of miR-34a (46). Several studies have shown that epigenetic modification play a significant role in patients with HBV-associated DLBCL, which have a higher frequency of the CREB-binding protein (CREBBP) mutations (27.2%) compared to the general population (47). CREBBP mainly regulates the activity of histone acetyl transferase (HAT), maintains the activity of HAT domain, regulates transcription factors, and affects immune regulation. However, CREBBP mutation (loss-of-function) inactivates the HAT domain and impairs acetylation-deacetylation balance, thereby increasing BCL-6 expression (Figure 3). Nevertheless, the selective inhibition of HDAC3 reverts the molecular phenotype of CREBBP mutations (48, 49). Moreover, SIRT1 plays an important role in the acetylation of B-cell germinal center and could lead to an imbalance of acetylation/deacetylation. SIRT1 could cause the deacetylation of BCL-6, p53, and other transcription factors (50) and up-regulate the expression of BCL-6 (51). BO et al. showed that chronic stimulation with HBsAg promoted the viability of the human B lymphoblastoid cell line through regulation of the SIRT1-NF-κB pathway (52) (Figure 3). In conclusion, noncoding RNAs and epigenetic regulation may play roles in the pathogenesis of HBV associated DLBCL.
2.2.4 Chemo-resistance
HBx elicits a DNA damage response. It binds to p53 in the nucleus, inhibits the expression of p53-responsive genes, promotes the phosphorylation (inactivation) of Rb, lowers the activities of CDK inhibitors, promotes vascular regeneration, and inhibits p53 function through Rb, thereby inhibiting cell apoptosis and cell cycle (53). HBx itself can inhibit p53 from functioning as a cell cycle blocker, thereby promoting cell survival. Moreover, the overexpression of HBx significantly reduces the activation of CHK2 signals induced by S-period blockers and p53 and p21 in patients with HBV-associated DLBCL, consequently reducing the sensitivity of chemotherapy drugs and suggesting that CHK2 may be a potential factor in HBx-induced chemotherapy resistance (54). Li et al. (55) found that HBx directly up-regulated the expression of lncNBAT1, a long non-coding RNA (lncRNA) that is closely associated with the chemotherapy outcomes of patients with HBV-associated DLBCL. The up-regulation of lncNBAT1 reduced the sensitivity of DLBCL cells to the chemotherapeutic agents that induced S-phase arrest, whereas the knockdown of lncNBAT1 significantly relieved the chemo-resistance of HBx-expressing DLBCLs (Figure 3).
Therefore, patients with HBV-associated DLBCL have a poor prognosis owing to genomic instability, immune escape, epigenetic regulation, and chemo-resistance, leading to an uncontrolled cell cycle, obstruction of apoptosis, and increased frequency of gene mutations. Moreover, many signal pathways, including BCR, JAK/STAT3, and the NF-κB pathway, are involved in the pathogenesis of HBV-associated DLBCL (Table 1).
3 Future prospects of targeted treatment to improve prognosis
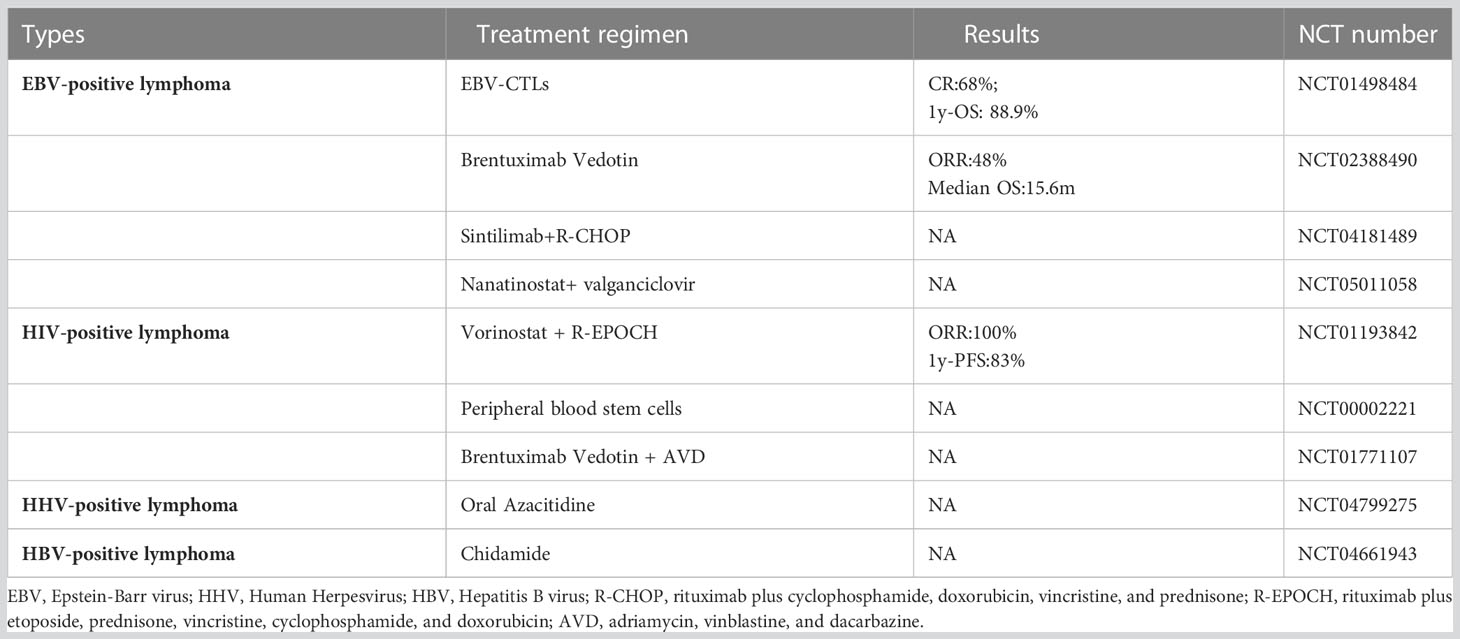
Patients with HBV-associated DLBCL respond poorly to current first-line immunochemotherapy. Therefore, novel therapeutic approaches for patients with HBV-associated DLBCL, including other virus-related lymphomas, should be considered (Table 2). Possible therapeutic approaches include: (1) anti-virus therapy; (2) small molecule kinase inhibitors, including epigenetic regulators, PI3K inhibitors, BCL-6 inhibitors, and BCL-2 inhibitors; and (3) immunotherapy, including CAR-T and immune-checkpoint inhibitors (Figure 4).

Figure 4 Therapeutic modulation of HBV infection and associated signaling pathways. Many therapeutic targets have been identified. (1) NAs suppress viral replication by inhibiting reverse transcription of pre-genomic RNA to HBV DNA in the cytoplasm. (2) small molecule kinase inhibitors, including epigenetic regulators, PI3K inhibitors, BCL-6 inhibitors and BCL-2 inhibitors. (3) immunotherapy, CAR-T, including CD19 and CD70 CAR-T; PD-1/PD-L1 mAb can activate T cells by regulating the PD-1/PD-L1 axis.
3.1 Anti-virus therapy
Nucleos(t)ide analogs (NAs) and interferons are two classes of antiviral agents currently licensed for the treatment of HBV infection. NAs, including entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide, suppress viral replication by inhibiting the reverse transcription of pre-genomic RNA to HBV DNA in the cytoplasm. Study indicated that entecavir combined with chemotherapy could improve the prognosis of patients with HBV-associated DLBCL (22). Moreover, Yamauchi et al. also showed that the prophylactic use of entecavir could reduce the occurrence of HBV reactivation-related hepatitis and mortality in patients with HBV-associated DLBCL receiving rituximab-containing chemotherapy (56).
3.2 Small molecule kinase inhibitors
3.2.1 Epigenetic regulators
With advances in epigenetic research in recent years, the use of epigenetic regulators has gradually increased in clinical practice. Several clinical trials have confirmed the effectiveness and safety of HDAC inhibitors, including chidamide, in first-line consolidation and rescue therapy for the recurrence of lymphoma. Chidamide is a selective inhibitor of histone deacetylases (HDACs), which is mainly targeted at class I HDACs, and has a regulatory effect on the epigenetic function of tumor abnormalities. At our center, the First Hospital of Jilin University, Changchun, China, a clinical trial (Table 2; registered in www.clinicaltrials.gov under number NCT04661943) of chidamide maintenance treatment was conducted for patients with HBV-associated DLBCL. The inclusion criteria were achievement of complete response and complete response lasting for 1 year following systemic treatment before enrollment. The trial aimed at exploring the efficacy of epigenetic regulators in patients with HBV-associated lymphoma.
3.2.2 Small molecule kinase inhibitors of cell signaling pathways
Such as PI3K, BCL-6, and BCL-2 inhibitors, and the immunomodulator lenalidomide have shown efficacy in treating recurrent and refractory DLBCL (57–60); however, clinical trials on the efficacy of these treatments for HBV-associated DLBCL are warranted.
3.3 Immunotherapy
3.3.1 Chimeric antigen receptor-T cell therapy
CAR-T for the failure of multiline therapy of DLBCL has been demonstrated to have significant efficacy. Patients with HBV-associated DLBCL undergoing CAR-T cell therapy did not increase the risk of severe cytokine release syndrome (61). However, Yang et al. showed that patients with HBV-associated DLBCL receiving CD19-CAR-T cell therapy are at risk of HBV reactivation, especially in HBeAg-positive patients; the close monitoring of HBV-DNA levels and adequate antiviral prophylaxis are essential for the prevention of HBV reactivation (62). HBV-associated lymphoma presents immune escape-related gene mutations such as CD70, and targeted CD70 CAR-T may have potential efficacy.
3.3.2 Immune-checkpoint inhibitors
PD-1 expression was higher in patients with HBV-associated DLBCL than in HBV-free individuals. Thus, immune-checkpoint inhibitors may have a satisfactory effect on patients with HBV-associated DLBCL. However, there is no standard first-line therapy, and clinical trials exploring specific treatment regimens for HBV-associated DLBCL are rare compared with those for other virus-related lymphomas. Further studies to explore more effective therapy regimens are warranted.
4 Conclusion
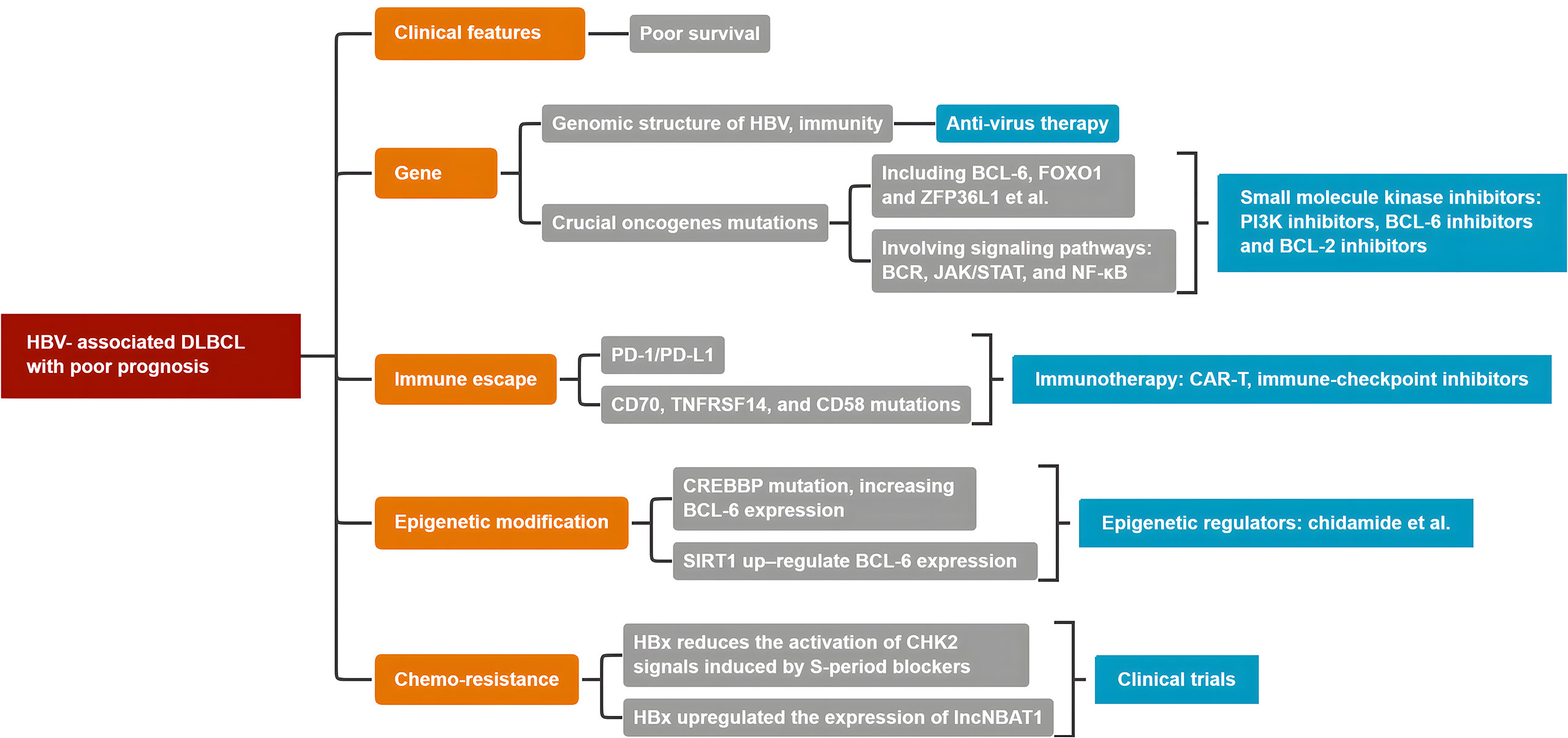
Patients with HBV-associated DLBCL experience worse clinical prognosis and chemo-resistance. HBV infection and integration triggers inflammation favoring the accumulation of genetic and epigenetic lesions, involving many oncogenes, including crucial BCL-6, Myc, and CREBBP. Moreover, many signal pathways, including crucial BCR, JAK/STAT3, and the NF-κB pathway, are involved. Therefore, HBV, as a major risk factor, contributes to the development of HBV-associated DLBCL. There is no standard first-line immunochemotherapy in clinical settings. Figure 5 presents a conclusive algorithm of crucial factors for HBV-associated DLBCL. Therefore, more clinical trials to implement personalized treatment approaches for HBV-associated DLBCL are warranted.
Author contributions
XW, KY, and OB designed the study, provided vital data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Agency of Jilin province (20220402064GH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the groupe d’Etudes des lymphomes de l’Adulte. Blood (2010) 116(12):2040–5. doi: 10.1182/blood-2010-03-276246
2. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large b-cell lymphoma identified by gene expression profiling. Nature (2000) 403(6769):503–11. doi: 10.1038/35000501
3. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia (2022) 36(7):1720–48. doi: 10.1038/s41375-022-01620-2
4. Spradling PR, Xing J, Zhong Y, Rupp LB, Moorman AC, Lu M, et al. Incidence of malignancies among patients with chronic hepatitis b in US health care organizations, 2006-2018. J Infect Dis (2022) 226(5):896–900. doi: 10.1093/infdis/jiac011
5. Jaquet A, Boni SP, Boidy K, Tine J, Tchounga B, Touré SA, et al. Chronic viral hepatitis, HIV infection and non-Hodgkin lymphomas in West Africa, a case-control study. Int J Cancer (2021) 149(8):1536–43. doi: 10.1002/ijc.33709
6. Tian T, Song C, Jiang L, Dai J, Lin Y, Xu X, et al. Hepatitis b virus infection and the risk of cancer among the Chinese population. Int J Cancer (2020) 147(11):3075–84. doi: 10.1002/ijc.33130
7. Mahale P, Engels EA, Koshiol J. Hepatitis b virus infection and the risk of cancer in the elderly US population. Int J Cancer (2019) 144(3):431–9. doi: 10.1002/ijc.31643
8. Su TH, Liu CJ, Tseng TC, Chou SW, Liu CH, Yang HC, et al. Chronic hepatitis b is associated with an increased risk of b-cell non-hodgkin’s lymphoma and multiple myeloma. Aliment Pharmacol Ther (2019) 49(5):589–98. doi: 10.1111/apt.15132
9. Taborelli M, Polesel J, Montella M, Libra M, Tedeschi R, Battiston M, et al. Hepatitis b and c viruses and risk of non-Hodgkin lymphoma: a case-control study in Italy. Infect Agent Cancer (2016) 11:27. doi: 10.1186/s13027-016-0073-x
10. Kang X, Bai L, Han C, Qi X. Clinical analysis and prognostic significance of hepatitis b virus infections with diffuse Large b-cell lymphoma. Cancer Manag Res (2020) 12:2839–51. doi: 10.2147/CMAR.S244381
11. Zhou X, Pan H, Yang P, Ye P, Cao H, Zhou H. Both chronic HBV infection and naturally acquired HBV immunity confer increased risks of b-cell non-Hodgkin lymphoma. BMC Cancer (2019) 19(1):477. doi: 10.1186/s12885-019-5718-x
12. Wang C, Xia B, Ning Q, Zhao H, Yang H, Zhao Z, et al. High prevalence of hepatitis b virus infection in patients with aggressive b cell non-hodgkin’s lymphoma in China. Ann Hematol (2018) 97(3):453–7. doi: 10.1007/s00277-017-3188-2
13. Chen MH, Hsiao LT, Chiou TJ, Liu JH, Gau JP, Teng HW, et al. High prevalence of occult hepatitis b virus infection in patients with b cell non-hodgkin’s lymphoma. Ann Hematol (2008) 87(6):475–80. doi: 10.1007/s00277-008-0469-9
14. Wang F, Xu RH, Luo HY, Zhang DS, Jiang WQ, Huang HQ, et al. Clinical and prognostic analysis of hepatitis b virus infection in diffuse large b-cell lymphoma. BMC Cancer (2008) 8:115. doi: 10.1186/1471-2407-8-115
15. Liu Z, Li S, Liu Y, Guo W, Bai O. PD1 is highly expressed in diffuse large b-cell lymphoma with hepatitis b virus infection. PloS One (2017) 12(6):1–12. doi: 10.1371/journal.pone.0180390
16. Rong X, Wang H, Ma J, Pan S, Wang H, Jing S, et al. Chronic hepatitis b virus infection is associated with a poorer prognosis in diffuse large b-cell lymphoma: a meta-analysis and systemic review. J Cancer (2019) 10(15):3450–8. doi: 10.7150/jca.31033
17. Guo W, Zhang W, Liu C, Song Y, Bai O. Clinical analysis of the HBV infection status of 135 patients with diffuse Large b cell lymphoma treated with r-CHOP or CHOP/CHOP-like chemotherapy. PloS One (2015) 10(6):e0129064. doi: 10.1371/journal.pone.0129064
18. Cheng CL, Huang SC, Chen JH, Wei CH, Fang WQ, Su TH, et al. Hepatitis b surface antigen positivity is an independent unfavorable prognostic factor in diffuse Large b-cell lymphoma in the rituximab era. Oncologist (2020) 25(9):793–802. doi: 10.1634/theoncologist.2019-0756
19. Wei Z, Zou S, Li F, Cheng Z, Li J, Wang J, et al. HBsAg is an independent prognostic factor in diffuse large b cell lymphoma patients in rituximab era: result from a multicenter retrospective analysis in China. Med Oncol (2014) 31(3):845. doi: 10.1007/s12032-014-0845-3
20. Qi X, Gui X, Zhuang K. Establishment and characterization of HBV-associated b lymphocytes with an immortalization potential. PloS One (2019) 14(5):1–16. doi: 10.1371/journal.pone.0217161
21. Deng L, Song Y, Young KH, Hu S, Ding N, Song W, et al. Hepatitis b virus-associated diffuse large b-cell lymphoma: unique clinical features, poor outcome, and hepatitis b surface antigen-driven origin. Oncotarget (2015) 6(28):25061–73. doi: 10.18632/oncotarget.4677
22. Huang HH, Hsiao FY, Chen HM, Wang CY, Ko BS. Antiviral prophylaxis for hepatitis b carriers improves the prognosis of diffuse large b-cell lymphoma in Taiwan - a population-based study. Br J Haematol (2021) 192(1):110–8. doi: 10.1111/bjh.17142
23. Tong S, Revill P. Overview of hepatitis b viral replication and genetic variability. J Hepatol (2016) 64:S4–S16. doi: 10.1016/j.jhep.2016.01.027
24. Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, et al. Hepatitis b virus infection. Nat Rev Dis Primers (2018) 4:18035. doi: 10.1038/nrdp.2018.35
25. Liaw YF. Natural history of chronic hepatitis b virus infection and long-term outcome under treatment. Liver Int (2009) 29 Suppl 1:100–7. doi: 10.1111/j.1478-3231.2008.01941.x
26. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis b: special emphasis on disease progression and prognostic factors. J Hepatol (2008) 48(2):335–52. doi: 10.1016/j.jhep.2007.11.011
27. Kwon H, Lok AS. Hepatitis b therapy. Nat Rev Gastroenterol Hepatol (2011) 8(5):275–84. doi: 10.1038/nrgastro.2011.33
28. Lucifora J, Arzberger S, Durantel D, Belloni L, Strubin M, Levrero M, et al. Hepatitis b virus X protein is essential to initiate and maintain virus replication after infection. J Hepatol (2011) 55(5):996–1003. doi: 10.1016/j.jhep.2011.02.015
29. Yoffe B, Noonan CA, Melnick JL, Hollinger FB. Hepatitis b virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis (1986) 153(3):471–7. doi: 10.1093/infdis/153.3.471
30. Wang Y, Wang H, Pan S, Hu T, Shen J, Zheng H, et al. Capable infection of hepatitis b virus in diffuse Large b-cell lymphoma. J Cancer (2018) 9(9):1575–81. doi: 10.7150/jca.24384
31. Huang X, Young KH, Guo W, Wang Y, Wang X, Xi Y, et al. Identification of hepatitis b virus aetiologic antigens, HBx and pre-S2, in diffuse large b-cell lymphoma. J Viral Hepatitis (2020) 27(9):948–50. doi: 10.1111/jvh.13301
32. Ren W, Ye X, Su H, Li W, Liu D, Pirmoradian M, et al. Genetic landscape of hepatitis b virus-associated diffuse large b-cell lymphoma. Blood (2018) 131(24):2670–81. doi: 10.1182/blood-2017-11-817601
33. Gu X, Booth CJ, Liu Z, Strout MP. AID-associated DNA repair pathways regulate malignant transformation in a murine model of BCL6-driven diffuse large b-cell lymphoma. Blood (2016) 127(1):102–12. doi: 10.1182/blood-2015-02-628164
34. Burger JA, Wiestner A. Targeting b cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer (2018) 18(3):148–67. doi: 10.1038/nrc.2017.121
35. Xu W, Berning P, Lenz G. Targeting b-cell receptor and PI3K signaling in diffuse large b-cell lymphoma. Blood (2021) 138(13):1110–9. doi: 10.1182/blood.2020006784
36. Lin CT, Hsieh YT, Yang YJ, Chen SH, Wu CH, Hwang LH. B-cell lymphoma 6 (BCL6) is a host restriction factor that can suppress HBV gene expression and modulate immune responses. Front Microbiol (2018) 9:3253. doi: 10.3389/fmicb.2018.03253
37. Zhang J, Weng Z, Huang Y, Li M, Wang F, Wang Y, et al. High-grade b-cell lymphoma with MYC, BCL2, and/or BCL6 Translocations/Rearrangements: clinicopathologic features of 51 cases in a single institution of south China. Am J Surg Pathol (2020) 44(12):1602–11. doi: 10.1097/PAS.0000000000001577
38. Liu Z, Li S, Guo W, Wang Y, Wang M, Bai O. MYC gene rearrangements are closely associated with poor survival of diffuse Large b cell lymphoma with hepatitis b virus infection. BioMed Res Int (2017) 2017:1967648. doi: 10.1155/2017/1967648
39. Lin HD, Tseng YK, Yuh CH, Chen SC. Low concentrations of 4-ABP promote liver carcinogenesis in human liver cells and a zebrafish model. J Hazard Mater (2022) 423(Pt A):126954. doi: 10.1016/j.jhazmat.2021.126954
40. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res (2020) 10(3):727–42.
41. Wei Zheng CY-F, Wang Z-M, Zou S-H. The impact of hepatitis b virus concurrent infection on peripheral T cells in diffuse large b cell lymphoma patients. China Oncol (2014) 24(10):765–9.
42. Bruni R, Marcantonio C, Pulsoni A, Tataseo P, De Angelis F, Spada E, et al. microRNA levels in paraffin-embedded indolent b-cell non-Hodgkin lymphoma tissues from patients chronically infected with hepatitis b or c virus. BMC Infect Dis (2014) 14 Suppl 5(Suppl 5):S6. doi: 10.1186/1471-2334-14-S5-S6
43. Chen Z, Diaz G, Pollicino T, Zhao H, Engle RE, Schuck P, et al. Role of humoral immunity against hepatitis b virus core antigen in the pathogenesis of acute liver failure. Proc Natl Acad Sci U States A (2018) 115(48):E11369–E78. doi: 10.1073/pnas.1809028115
44. Han R, Chen X, Li Y, Zhang S, Li R, Lu L. MicroRNA-34a suppresses aggressiveness of hepatocellular carcinoma by modulating E2FI, E2F3, and caspase-3. Cancer Manage Res (2019) 11:2963–76. doi: 10.2147/CMAR.S202664
45. Zhang Y, Ren H, Li J, Xue R, Liu H, Zhu Z, et al. Elevated HMGB1 expression induced by hepatitis b virus X protein promotes epithelial-mesenchymal transition and angiogenesis through STAT3/miR-34a/NF-kappaB in primary liver cancer. Am J Cancer Res (2021) 11(2):479–94.
46. Peng J, Li SJ, Fu X, Liu Y, Zhao XL. Chidamide acts on the histone deacetylase-mediated miR-34a/Bcl-2 axis to regulate NB4 cell line proliferation and apoptosis. Kaohsiung J Med Sci (2020) 36(12):1004–13. doi: 10.1002/kjm2.12283
47. Li WY, Jiang XM, Guo HG, Wei XJ, Chen FL, Liu SC, et al. Next generation sequencing highlights the role of cell cycle and apoptosis dysregulation and hepatitis b infection in refractory diffuse Large b cell lymphoma. Blood (2017) 130:5131. doi: 10.1182/blood.V130.Suppl_1.5131.5131
48. Mondello P, Tadros S, Teater M, Fontan L, Chang AY, Jain N, et al. Selective inhibition of HDAC3 targets synthetic vulnerabilities and activates immune surveillance in lymphoma. Cancer Discovery (2020) 10(3):440–59. doi: 10.1158/2159-8290.CD-19-0116
49. Jiang Y, Ortega-Molina A, Geng H, Ying H-Y, Hatzi K, Parsa S, et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov (2017) 7(1):38–53. doi: 10.1158/2159-8290.CD-16-0975
50. Bereshchenko OR, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet (2002) 32(4):606–13. doi: 10.1038/ng1018
51. Frazzi R, Tigano M, Valli R, Tamagnini I, Fragliasso V, Merli F. SIRT1 expression is higher in human follicular lymphoma and correlates with Ki67 and bcl-6 overexpression. Blood (2014) 124(21):1649. doi: 10.1182/blood.V124.21.1649.1649
52. Bo J, Wang X, Li J, Wang W, Zhang J. Consecutive stimulation of HBsAg promotes the viability of the human b lymphoblastoid cell line IM-9 through regulating the SIRT1-NF-κB pathway. Oncol Lett (2017) 14(1):433–40. doi: 10.3892/ol.2017.6114
53. Kim S, Lee HS, Ji JH, Cho MY, Yoo YS, Park YY, et al. Hepatitis b virus X protein activates the ATM-Chk2 pathway and delays cell cycle progression. J Gen Virol (2015) 96(8):2242–51. doi: 10.1099/vir.0.000150
54. Zhao X, Guo X, Xing L, Yue W, Yin H, He M, et al. HBV infection potentiates resistance to s-phase arrest-inducing chemotherapeutics by inhibiting CHK2 pathway in diffuse large b-cell lymphoma. Cell Death Dis (2018) 9(2):61. doi: 10.1038/s41419-017-0097-1
55. Li J, Chen Y, Guo X, Bai X, Xu X, Han T, et al. lncNBAT1/APOBEC3A is a mediator of HBX-induced chemoresistance in diffuse large b cell lymphoma cells. Mol Ther Nucleic Acids (2022) 27:1064–77. doi: 10.1016/j.omtn.2022.01.015
56. Yamauchi N, Maruyama D, Choi I, Atsuta Y, Sakai R, Miyashita K, et al. Prophylactic antiviral therapy for hepatitis b virus surface antigen-positive patients with diffuse large b-cell lymphoma treated with rituximab-containing chemotherapy. Cancer Sci (2021) 112(5):1943–54. doi: 10.1111/cas.14846
57. Zinzani PL, Rodgers T, Marino D, Frezzato M, Barbui AM, Castellino C, et al. RE-MIND: comparing tafasitamab + lenalidomide (L-MIND) with a real-world lenalidomide monotherapy cohort in relapsed or refractory diffuse Large b-cell lymphoma. Clin Cancer Res (2021) 27(22):6124–34. doi: 10.1158/1078-0432.CCR-21-1471
58. Major A, Kline J, Karrison TG, Fishkin PAS, Kimball AS, Petrich AM, et al. Phase I/II clinical trial of temsirolimus and lenalidomide in patients with relapsed and refractory lymphomas. Haematologica (2022) 107(7):1608–1618. doi: 10.3324/haematol.2021.278853
59. Wilson WH, Wright GW, Huang DW, Hodkinson B, Balasubramanian S, Fan Y, et al. Effect of ibrutinib with r-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell (2021) 39(12):1643–1653. doi: 10.1016/j.ccell.2021.10.006
60. Bertram K, Leary PJ, Boudesco C, Fullin J, Stirm K, Dalal V, et al. Inhibitors of bcl-2 and bruton’s tyrosine kinase synergize to abrogate diffuse large b-cell lymphoma growth in vitro and in orthotopic xenotransplantation models. Leukemia 36(4):1035–1047. doi: 10.1038/s41375-021-01470-4
61. Cui R, Lyu C, Li Q, Jiang Y, Mou N, Yang Z, et al. Humanized anti-CD19 chimeric antigen receptor-T cell therapy is safe and effective in lymphoma and leukemia patients with chronic and resolved hepatitis b virus infection. Hematol Oncol (2021) 39(1):75–86. doi: 10.1002/hon.2807
Keywords: hepatitis B virus, DLBCL, epigenetics, prognosis, chemo-resistance
Citation: Wan X, Young KH and Bai O (2023) HBV-associated DLBCL of poor prognosis: advance in pathogenesis, immunity and therapy. Front. Immunol. 14:1216610. doi: 10.3389/fimmu.2023.1216610
Received: 04 May 2023; Accepted: 14 June 2023;
Published: 07 July 2023.
Edited by:
Maria Teresa Sabrina Bertilaccio, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2023 Wan, Young and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ken H. Young, a2VuLnlvdW5nQGR1a2UuZWR1; Ou Bai, YmFpb3VAamx1LmVkdS5jbg==
 Xin Wan
Xin Wan Ken H. Young
Ken H. Young Ou Bai
Ou Bai