- 1Department of Immunology, Beaumont Hospital, Royal College of Surgeons in Ireland (RCSI) Hospital Group, Dublin, Ireland
- 2Department of Acute Medical Assessment Unit (AMAU)/ General Internal Medicine (GIM), St. James’s Hospital, Trinity College Dublin, Dublin, Ireland
Background: Data on non-infectious cryoglobulinemic vasculitis (NICV) is scarce, especially concerning the management of relapses, which are troublesome. We aimed to investigate risk factors for relapse in NICV.
Methods: A systematic literature search of CINAHL, Embase, MEDLINE, Scopus, and the Web of Science databases was implemented until April 2023. Eligible studies included randomized control trials, observational studies, and case series with ≥4 patients. Two reviewers independently extracted data and assessed the quality of the eligible studies.
Results: A total of 3,724 articles were retrieved from a database search, with 27 studies meeting the inclusion criteria for review. Most studies (n = 23) detailed relapses, with the time to relapse varying between 1 and 80 months. The relapse rate was reported at 28% in Type I NICV and ranged from 22% to 60% in mixed NICV. Risk factors for relapse in NICV were identified based on the cryoglobulin subtype and correlated with clinical and immunological responses to varying treatment regimens. Type I NICV with an associated lymphoproliferative disorder exhibited a response-relapse pattern. Cutaneous and articular involvement and incomplete clinical and immunological responses to treatment, particularly corticosteroid monotherapy and occasionally rituximab, influence the risk of relapse in Type II and Type III NICV.
Conclusion: Our findings underscore the significance of attaining both clinical and immunological responses and identifying risk factors for relapse in NICV. Appropriate risk stratification for NICV patients is essential for the successful implementation of effective treatment strategies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023408140.
Background
Cryoglobulins are immunoglobulins (Ig) that undergo precipitation at temperatures below 37°C and re-dissolve upon rewarming (1). Cryoglobulinemia is a heterogeneous condition defined by the presence of circulating cryoglobulins in the sera and their deposition into small to medium blood vessels, causing chronic inflammation and end-organ damage (2). According to Brouet et al., cryoglobulinemia can be classified into three subtypes: Type I cryoglobulins comprising isolated monoclonal Ig, usually IgM, and this can be associated with plasma cell dyscrasias; Type II cryoglobulins comprising polyclonal IgG and monoclonal IgM with rheumatoid factor (RF) activity; and Type III cryoglobulins comprising polyclonal IgG and polyclonal IgM with RF activity, commonly referred to as mixed cryoglobulinemia (MC) (2, 3). Cryocrit, defined as the relative volume of the precipitate as a percentage of the total serum volume, is elevated in Type 1 and lowest in Type III cryoglobulins (4). Rocatello et al. recently introduced a novel concept of idiopathic hypocryoglobulinemia in patients with clinically suspicious vasculitis who have negative cryoglobulins by standard techniques. Through a modified precipitation technique in a hypoionic medium, a subset of patients are found to have trace levels of circulating polyclonal Type III cryoglobulins (hypocryoglobulins), redefining the diagnostic pathways for cryoglobulinemia (5).
Cryoglobulinemic vasculitis (CV) is an immune complex-mediated small- to medium-vessel vasculitis. The prevalence of CV is rare, estimated at approximately 1 per 100,000 worldwide. It is commonly observed in patients between 45 and 65 years of age, with a predominance of a female-to-male ratio of 3:1 (3). CV can be classified into infectious CV (ICV), where Hepatitis C virus (HCV) infection accounts for 90% of cases, and non-infectious CV (NICV), which is associated with autoimmune diseases and hematological malignancies, mainly B-cell lymphoproliferative disorders (6).
A triad of clinical presentations, including fatigue, arthralgia, and palpable purpura, are typically found in type II and type III cryoglobulinemia. In comparison, type I cryoglobulinemia is usually associated with conditions of the skin such as Raynaud’s phenomenon, ulcers, and gangrene (3). The literature describing the clinical characteristics and effective treatment of NICV is limited. The pathogenesis of NICV is less understood compared to ICV, but it is well acknowledged that chronic inflammation resulting from autoimmune conditions stimulates B-cell proliferation through complement and macrophage activation, subsequently inducing cryoglobulinemia (2, 3).
Various treatment approaches have been used in CV, which involve the use of corticosteroids (CS), rituximab (RTX), azathioprine (AZA), and plasmapheresis (PLEX) either individually or in combination. However, the treatment response in CV is variable, and treatment failures have been reported. In type I cryoglobulinemia, targeted therapies addressing the underlying lymphoproliferative disorder did not affect relapses (7). In HCV-related mixed cryoglobulinemia (MC), successful eradication is achieved using direct-acting antivirals (8). The combined administration of pegylated interferon α or ribavirin and/or novel antiviral drugs has also been effective in inducing remission in most cases of MC (9). However, despite successful treatment, there are instances of treatment failures, which can result in persistent disease manifestations or relapses (8). These treatment failures emphasize the importance of understanding the underlying disease mechanisms in different subtypes of cryoglobulinemia and developing more effective therapeutic strategies.
Clinical relapse is defined as the reappearance or recurrence of vasculitic clinical manifestations following the achievement of a complete treatment response. A complete immunological response refers to the normalization of the C4 complement level and the reduction/disappearance of cryoglobulins in sera. Only one study examined possible predictors for early relapse, where the absence of achieving a complete immunological response influenced relapse (10). Pulmonary involvement, gastrointestinal involvement, renal insufficiency, and advancing age >65 years were notable factors independently associated with death (11). To date, there is limited data on risk factors for relapse in NICV. Therefore, this systematic review aims to explore possible risk factors for relapse to guide effective patient management.
Materials and methods
This systematic review was conducted according to ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (12). We registered our protocol with the International prospective register of systematic reviews (PROSPERO) on 14 March 2023 (PROSPERO registration CRD42023408140).
Search strategy
A literature search was implemented between March and April 2023 across key electronic databases Embase and MEDLINE by a trained librarian and one reviewer through OVID access (https://ovidsp.ovid.com) for thoroughness—MEDLINE (EBSCO Interface), MEDLINE (Ovid Interface), CINAHL (Complete Interface), CINAHL (EBSCOHost Interface), Embase (Ovid Interface), Web of Science, and Scopus using agreed key search terms. Articles published from the inception of databases (Embase 1974; Medline 1946) until 2023 were included. Only articles published in English were reviewed. Key search terms used in the literature search were: cryoglobulinemic vasculitis, cryoglobulinemia vasculitis, essential mixed cryoglobulinemia, non-infectious cryoglobulinemia vasculitis, and relapse.
Study selection
Eligibility criteria/inclusion criteria
1. Articles on clinical features, investigations, treatment, and outcomes, including relapse, prognosis, and mortality in patients with NICV
2. Studies including randomized control trials (RCT), observational studies, and case series with ≥4 patients
Exclusion criteria
1. Articles not published in English
2. Duplicates
3. Letters to the editor, opinions, commentaries, communication, reviews and meta-analyes, conference abstracts, and case series with a sample size of fewer than four patients
Two reviewers (NR and PR) thoroughly examined the electronic databases for relevant titles and abstracts. Articles suitable for further review were independently explored for full text or abstract. Eligible studies matching inclusion criteria were analyzed, and references within eligible studies were assessed retrospectively as potential additions to our review. Any disagreement on article selection was achieved through consensus-building face-to-face meetings with a third reviewer.
Data extraction
Two independent reviewers (NR and PR) performed an in-depth analysis of eligible articles meeting inclusion criteria using a standardized data extraction form [S1]. The data extraction form included: 1) article title, author information, publication date, and country of publication; 2) study design; 3) sample size and demographics; 4) clinical characteristics, investigations, and management; 5) outcome of interest—clinical manifestations for relapse, risk factors of relapse, and treatment adverse event(s).
Quality assessment
The quality of included studies was assessed independently by two reviewers (NR and PR) using the Newcastle-Ottawa Scale (NOS) for cohort studies (13) (S2) and Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) for RCTs (14) (S3). A STROBE checklist was used to assess bias risk in cohort studies (15) (S4).
Results
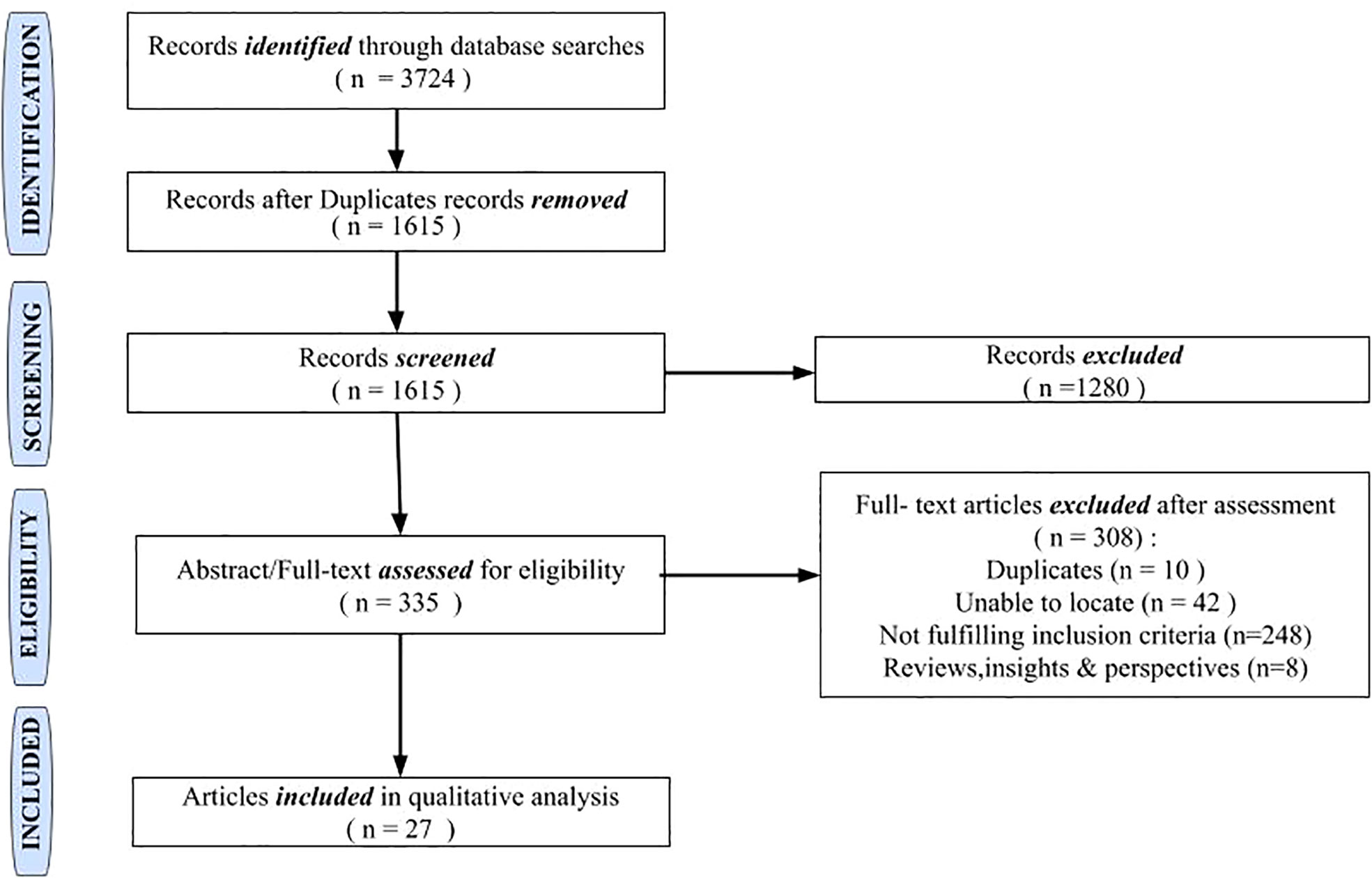
A total of 3,724 records were retrieved from the database search (Figure 1). Following deduplication, 1,615 records were screened through a review of titles. A total of 335 records were retained for full-text assessment following the exclusion of 1,280 records that did not fulfill our inclusion criteria. A total of 27 studies comprising randomized controlled trials (n = 1), non-randomized clinical trials (n = 1), observational studies (n = 23), and case series ≥4 (n = 2) were included in the final review. Due to the scarcity of clinical trials and large prospective cohort studies on NICV, we were unable to perform a meta-analysis.
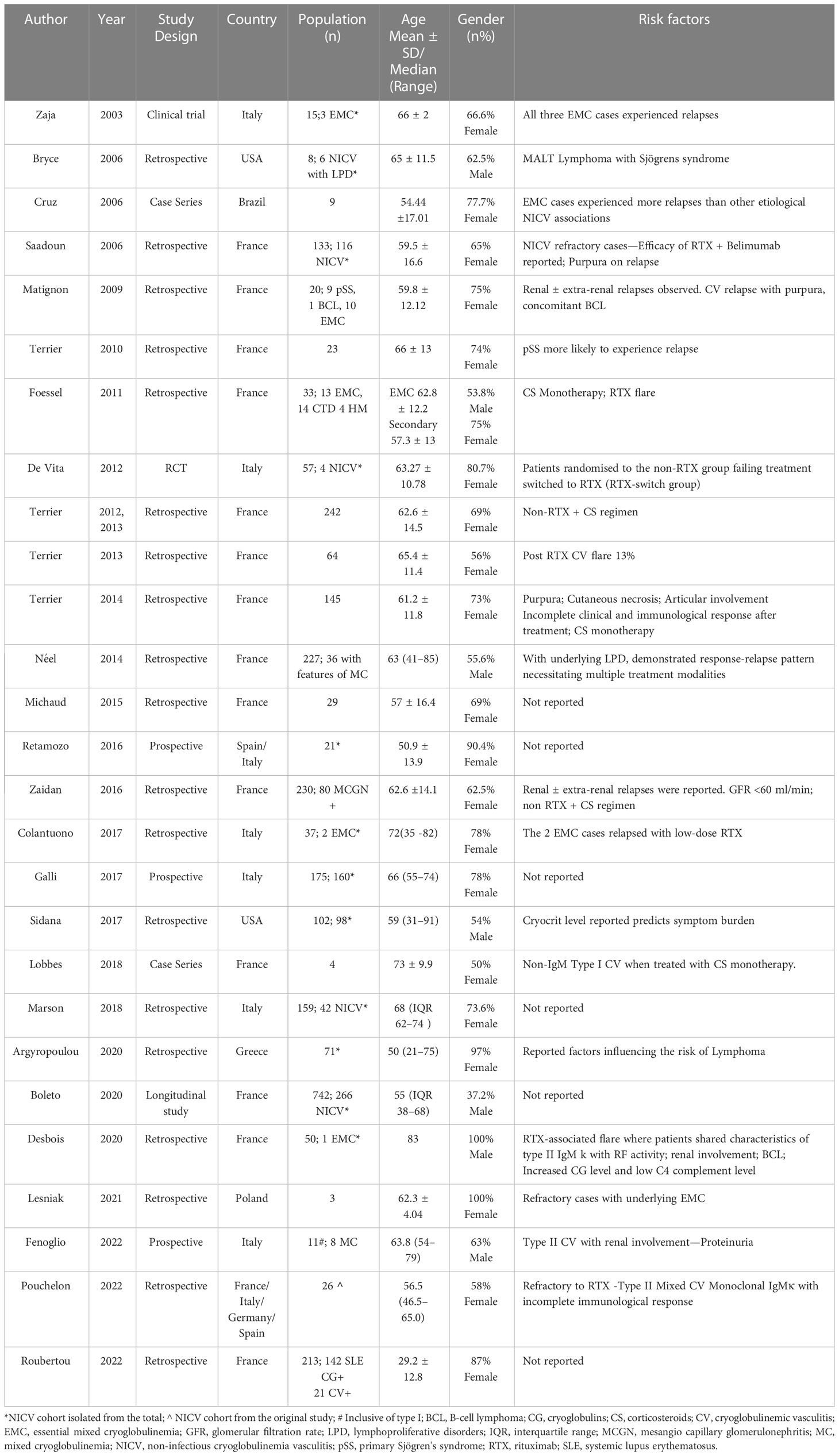
For this review, the sample size has been adjusted with the NICV cohort segregated from the total population in 10 studies (16–25). The total population from the included studies was 1,519, with a mean of 60.76; the study population ranged between 1 and 266. This review encompassed several studies that included a broad spectrum of patient characteristics in the context of relapses in CV, shedding light on its clinical characteristics, therapeutic approaches, and prognosis. The patient population included age groups ranging from 21 to 91 years, with a female gender preponderance. Studies included a range of countries: France (fourteen studies), Italy (six studies), the USA (two studies), and one study each from Brazil, Greece, and Poland. Multiple countries were involved in two studies (Table 1).
The most frequently reported non-infectious etiological associations were connective tissue diseases accounting for 41.8% (n = 636), predominantly primary Sjögren’s Syndrome (pSS) and systemic lupus erythematosus (SLE), and haematological diseases comprising 27.6% (n = 416) encompassing MGUS and haematological malignancies (HM). Approximately 26.1% consisted of EMC (n = 397), whose underlying etiology was unidentified.
The included studies consistently found prevalent cutaneous manifestations in the patient population at baseline, while articular involvement, neuropathy, and nephropathy were observed to varying degrees. Immunological findings such as elevated cryoglobulin levels, low complement C4 levels, and positive RF activity in MC were commonly detected. Treatment strategies varied and included CS, RTX, cyclophosphamide (CYC), AZA, PLEX, and others. An in-depth summary of the study and patient characteristics are outlined in Tables 2, 3.

Table 2 Summary of study characteristics on risk factors for relapse in type 1 cryoglobulinemic vasculitis.
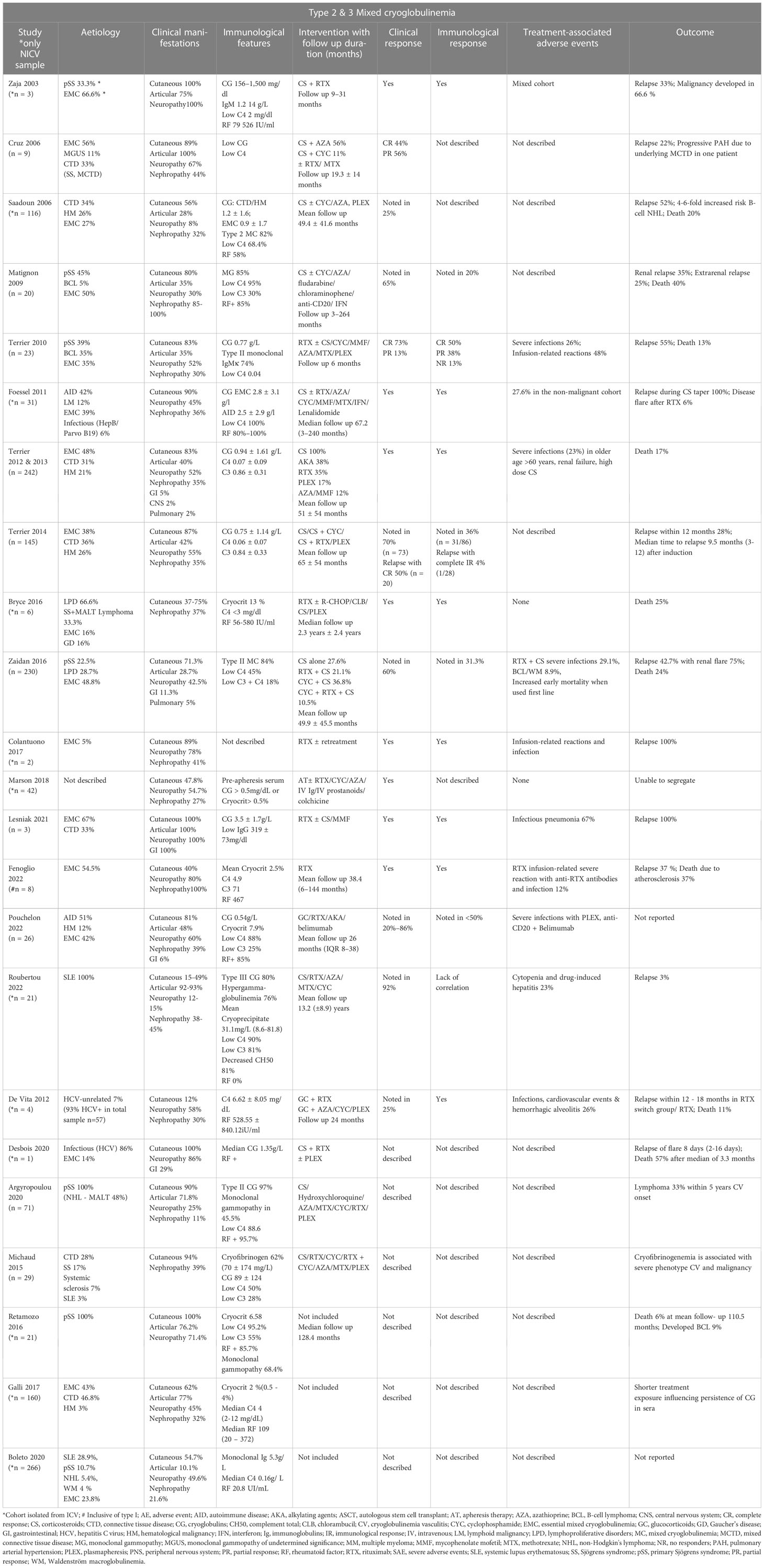
Table 3 Summary of study characteristics on risk factors for relapse in type 2 & 3 mixed cryoglobulinemia.
Of the 27 studies included in this review, 20 provided insights into the risk factors associated with CV relapse. Four studies investigated relapse in type 1 CV-associated MGUS and HM (25–28), while the remaining twenty studies focused on relapse in mixed CV (10, 29–40). Interestingly, seven studies (14, 16, 20–22, 41) failed to identify any identifiable risk factors for relapse. Below is a comprehensive summary of risk factors for relapse in NICV, including type I cryoglobulinemia.
Risk factors for relapse in type I CV
Patient characteristics
In our review, four studies involving a total population of 206 patients (25–28) were explored. The included studies encompassed patients with a wide age range at the time of diagnosis, spanning from 31 to 91 years (25–28). The restricted availability of data made it difficult to accurately capture the age at relapse. However, it is important to highlight that based on the limited data, females (50%) had a greater probability of CV relapses, especially among those with underlying haematological malignancies, when compared to those with MGUS (28) (Table 2).
Clinical manifestations
Among the studies that were considered, there were differences in the clinical baseline traits of patients who had relapses (Table 2). Skin manifestations were observed in a range of 63% to 100% of cases, where purpura was the most common skin symptom reported in 67% to 69% of cases, followed by necrotic ulcers/gangrene in 27% to 55% of cases, and livedo reticularis in 28% of the population. Peripheral neuropathy was observed in 25% to 47% of patients, while vasomotor symptoms, primarily Raynaud’s phenomenon, were present in 25% to 31% of patients. Articular features, predominantly arthralgia and arthritis, were deduced in 19% to 75% of patients, while renal involvement was observed in 14% and 31%, respectively.
Immunological parameters
The median cryoglobulin level was 1.55 g/L in a study by Terrier et al. (26). Furthermore, the mean cryoglobulin level was 2.5 g/L in a study by Néel et al. (27). In a study by Sidana et al. (25), the median cryocrit levels were 10% in HM and 5% in MGUS. Specific cryoglobulin patterns are summarized in Table 2. Low C3 and C4 were observed in the study by Terrier et al. (26).
Treatment, relapse rate, and time to relapse
All the studies that were included used different treatment regimens (Table 2). CS and AKA, RTX and CS/AKA, Rituximab and CS, and PLEX have all been associated with relapses. Due to the variety of the included studies, the follow-up period in each study ranged from 46 to 63 months, and the time to relapse varied. In the included studies, 29% (n = 32) of patients did not respond to first-line therapy, and about 28% (n = 30) of patients relapsed following partial improvement (25–28).
After receiving first-line therapy, Terrier et al. observed a median duration to relapse of 12 months (range: 3–144) for MGUS and 13 months (range: 4–59) for hematological malignancies, whereas Lobbes et al. reported relapses taking place between 4 and 18 months after receiving Rituximab (26, 28). The observed relapse rate varied from 13% to 68%, and this variation was explained using several treatment protocols (26). Terrier et al. found that 68% of patients relapsed after responding to AKA-based regimens, and 40% relapsed after receiving RTX-based therapy. Notably, 13% of patients with underlying hematological malignancies and significant levels of cryoglobulin experienced further CV flares after receiving RTX (26). Additionally, one patient experienced a relapse upon re-treatment with RTX (26). Three studies identified adverse reactions to treatment, such as severe infections (13%–27%) (26, 27) and worsened neuropathy brought on by Rituximab (25%) (28).
Factors influencing relapse
Type I CV, occurring in conjunction with an underlying lymphoproliferative disorder, often exhibits a pattern of response followed by relapse, necessitating the use of multiple treatment modalities (27). Frequent relapses were observed in patients with non-IgM type I CV treated with CS, necessitating intense therapy with AKA or RTX (28). RTX-associated CV flare was observed in type I CV (26).
Prognosis
Compared to IgM cryoglobulin, type I IgG cryoglobulin was linked with more severe CV features (p = 0.006), with notable cutaneous symptoms and eventual renal involvement (27). Cryoglobulin level and renal involvement, two separate predictor variables, were investigated in relation to symptom burden (25, 27). While Néel et al. agreed that extended follow-up durations permitted early discovery and treatment, favorably affecting prognosis (27), Terrier et al. indicated that underlying hematological malignancies were positively related to lower survival (26). Two studies have consistently shown that advanced age significantly affects prognosis. However, the extent of organ damage varied, with neurological involvement (25) and kidney disease (27) being recognized as significant determinants. According to Lobbes et al., Rituximab can regulate monoclonal proteins, accelerating clinical recovery; however, its efficacy in averting relapse was found to be limited (28).
Risk factors for relapse in type 2 and type 3 MC
Sixteen studies investigated relapses in NICV (sample size n = 927) (10, 18, 19, 23, 24, 29–40) whereas the remaining seven studies (sample size n = 552) were ambiguous on risk factors for relapse (16, 17, 20–22, 41, 42) consisting of a total sample size of 1,479.
Patient characteristics
The mean age at diagnosis ranged between 29 and 66 years, with a female predominance of 70.78%. The mean age for renal disease diagnosis in CV was 60 years (30). There were difficulties separating mean age and gender from the total population in three studies (18, 19, 24), and two studies examined all three CV subtypes (35, 37). The individual study characteristics are summarized in Table 3. The etiological associations observed were EMC in 40% (n = 529 cases; total 1,320) (10, 18, 21–24, 29–32, 34–39, 41, 42), autoimmune disease/connective tissue disease in 44% (n = 637; total 1,433) (10, 17, 20, 21, 24, 29–42), hematological malignancies/solid tumors in 19.3% (n = 255; total 1,320), with one case of Gaucher’s disease with hypersplenism (10, 18, 21–24, 29–32, 34–39, 41, 42).
Clinical manifestations
The baseline characteristics of the included studies indicated that most studies reported cutaneous features, primarily purpura (10, 16, 17, 20, 21, 24, 29–34, 36–39, 41, 42). A subgroup of studies (18.7%, n = 3) exclusively described nephropathy (24, 30, 35) and neuropathy (19, 23, 39) as cardinal features, while a minority of studies (12%, n = 2) focused on articular features (37, 39). In SLE-associated CV, arthralgia was observed as the leading manifestation, followed by skin features (40) (Table 3).
Immunological parameters
Common immunological features such as high cryoglobulin levels consisting of monoclonal/polyclonal IgM with RF activity preponderant Kappa light chain or polyclonal IgG (38), cryocrit (>0.5% to 13%) (17, 35, 42), along with low serum C4 levels (7, 19–21, 23, 24, 29–34, 36, 37, 40, 41) were noted.
Treatment, relapse rate, and time to relapse
Different treatment regimens were used across the studies, including CS and AKA, RTX with CS/AKA, Rituximab with CS, and PLEX. The follow-up duration in these studies ranged from 3 months to 13.2 years. Relapse rates varied from 22% to 60%, and the time to relapse ranged between 1 and 80 months across eligible studies (10, 18, 24, 29–35, 37–40). Combination regimens such as CS + RTX or CS + CYC showed efficacy in preventing early relapse. RTX + CS was associated with a reduced relapse rate (31–34). Three studies (31, 32, 34) investigated the impact of different treatment regimens on response rates. One study found that the combination of RTX and CS had better efficacy compared to AKA + CS as first- or second-line therapy (32). Another study reported that a high percentage (91%) of relapses occurred within three months in patients treated with CS, while seven patients (42%) showed a partial response to RTX (31). In a different study, relapse rates of approximately 42.7% were observed, with renal relapses being particularly prevalent (75%), in patients treated with CYC + CS (n = 11/20) or CYC + RTX + CS (n = 13/21) (34). A few studies demonstrated that the combination of RTX and CS as a treatment regimen resulted in a decreased relapse rate compared to other treatment approaches (31–34). However, PLEX was found to be ineffective in patients with peripheral neuropathy (16).
De Vita et al. discussed the superiority of RTX to conventional treatment (consisting of CS, AZA, CYC, or PLEX) in refractory cases where subsequent responses were achieved on RTX retreatment. Patients who were switched to the RTX group after showing resistance to non-RTX regimens had an increased risk of clinical relapse. This group, referred to as the “RTX switch group,” consisted of randomly assigned patients who had not responded adequately to previous non-RTX treatments (16). Moreover, Desbois et al. observed that 3.4% of disease flares were specifically linked to Type II mixed cryoglobulinemia. Patients who encountered vasculitis flares following RTX treatment demonstrated a lower 1-year survival rate compared to those who did not experience such flares (20). In three studies, the determination of relapse rates was limited due to the inclusion of cohorts with mixed characteristics, making it difficult to ascertain relapse rates, or due to a combination of the literature review and the original study (19, 23, 36).
Factors influencing relapse
Terrier et al. demonstrated that certain factors observed at baseline, including cutaneous necrosis, purpura, and joint involvement, were associated with an elevated risk of experiencing a relapse within a 12-month period. Furthermore, the lack of achieving a complete clinical response (p = 0.034) and immunological response after treatment (p = 0.0005) was also associated with an increased risk of relapse, highlighting the importance of achieving both clinical and immunological responses to reduce the risk of relapse (10). Debois et al. investigated potential factors contributing to RTX-associated vasculitis, where patients who experienced relapses had a higher likelihood of renal involvement (p = 0.0008), B-cell lymphoproliferation (p = 0.015), elevated cryoglobulin levels (2.1 vs 0.4 g/l, p = 0.0004), and decreased levels of IgG (2.9 vs 10.1 g/l, p = 0.005) and C4 prior to receiving RTX (0.02 vs 0.05, p = 0.023), compared to those who did not experience a flare after RTX treatment (22). Foessel et al. observed that there was an increased occurrence of relapses in patients who were treated solely with CS, on tapering, or in those who received a combination of CS and CYC. Moreover, RTX-associated CV flare was also observed (31). This finding is consistent with the study conducted by Terrier et al., indicating that the use of corticosteroids alone (p = 0.026) may be associated with a higher risk of relapse (10).
Several studies have discussed the occurrence of renal (membranous glomerulonephritis) ± CV relapses, particularly in cases where the glomerular filtration rate (GFR) is below 60 ml/min (30, 31, 34). Foessel et al. reported a relapse involving cutaneous manifestations in a patient with underlying EMC (31). Additionally, there have been observations of refractory cases of EMC, especially when treated with non-RTX + CS regimens. Terrier et al. reported that patients with pSS experienced more relapse than patients with EMC (38). pSS-CV patients had a higher frequency of extra glandular manifestations and B-cell lymphoma, which were attributed to underlying Type II IgMκ with RF (20).
Prognosis
The mortality rate was higher in pSS (31), and an increased risk of B-cell lymphoproliferative disorder was observed (29). Furthermore, older age as well as renal, pulmonary, and gastrointestinal involvement were found to be poor prognostic factors due to their increased association with severe infections (33). A patient who had MALT and pSS had a relapse of lymphoma, which resulted in subsequent death (24). Another study demonstrated a 4- to 6-fold increased risk of developing B-cell NHL with NICV and low IgG levels (29). Fenoglio et al. reported an increased rate of relapses in type I CV and delineated the development of anti-RTX antibodies in a patient with type III cryoglobulinemia (35).
Inarguably, most relapses are concordant with an incomplete response or refractoriness to treatment, attributed to persistently low C4 levels and high levels of circulating cryoglobulin. Multiple relapses were deduced in patients who were treated with different second-line treatment regimens (33, 34, 39).
Studies comparing ICV and NICV
The remaining studies (n = 4) provided comparisons of both ICV and NICV cohorts (21), with minimal emphasis on risk factors for relapse (17, 21, 41, 42). Boleto et al. described the spectrum of both HCV and non-infectious MC in the era of direct antiviral agents (21). Two studies focused on the classification criteria and clinical spectrum of pSS with concomitant CV, respectively (17, 20). Michaud et al. provided unique insights on cryofibrinogenemia as a marker of the severe CV phenotype (41). Galli et al. critically analyzed the influence of clinical and immunological parameters on overall outcomes in NICV, emphasizing poor prognosis in the male gender, the presence of purpura, and/or type II mixed cryoglobulins irrespective of the underlying disease (42). Nonetheless, a large proportion of studies recognized type II MC as a significant factor influencing outcome (17, 20, 22, 42). Retamozo et al. demonstrated a higher mean cryocrit level, a higher frequency of monoclonal gammopathy (68% vs. 29%, P = 0.009), and lymphopenia (47.6% vs. 20.9%, p = 0.042) among patients with CV (17).
Discussion
NICV is commonly associated with non-infectious underlying causes, such as autoimmune diseases and hematological malignancies, in contrast to HCV-related CV. Treatments aiming at eliminating HCV infection have reduced relapse risk. In our systematic review, by synthesizing and analyzing existing evidence, we categorized risk factors for relapse in NICV based on Ig isotype. Due to the heterogeneity of the included studies, we delineated risk factors for relapse by identifying common patterns through the correlation of their clinical and immunological responses to various therapeutic approaches across the literature. Notably, each underlying condition impacted the clinical severity and cryoprecipitation in NICV (42).
Our analysis showed that the mean age at diagnosis of NICV was ≥60 years, and the male gender had poor survival outcomes, particularly if there was renal involvement (33, 39, 42). Terrier et al. (2014) described earlier relapses within 12 months in patients with a GFR <60 ml/min/1.73 m2 (10).
Type I cryoglobulinemia with an underlying lymphoproliferative disorder tended to be more frequently associated with severe skin manifestations ranging from purpuric lesions to skin necrosis to ulcers in comparison to MC (25–28). Type I cryoglobulinemia lacked RF activity by conventionally abstaining from complement activation (26). In contrast, Kolopp-Sarda et al. suggested possible complement engagement and complement-inducing vasculopathy in type I cryoglobulinemia (43). There is an increased prevalence of hyperviscosity in type I, although some studies have reported leukocytoclastic vasculitis on histopathology, which is typical for mixed CV (44).
A huge proportion of the study population had an underlying autoimmune disease in the spectrum of NICV, where pSS was the leading etiology, followed by SLE. These often present with cutaneous manifestations (17, 20, 21, 32, 40). Additionally, few studies have associated symptoms such as low-grade fever, purpuric lesions, skin ulcers, low C4 complement level, and higher frequency of CV and severity of disease activity in pSS with concomitant cryoglobulin in sera with an increased risk of lymphoma and/or death (17, 20, 45).
Interestingly, Type II MC with monoclonal IgM is more susceptible to precipitation upon cold exposure. This is probably due to the pentameric conformation of IgM exhibiting increased avidity and the presence of multiple Fc sites on the same IgM molecule, making it accessible for rapid and/or multiple C1q binding, subsequently activating the complement pathway. Furthermore, binding affinity properties, glycosylation, amino acid formation, and temperature-induced molecular changes favor cryoprecipitation of IgM (46).
The persistence of cryoglobulinemia despite treatment constitutes a significant risk factor for relapse in NICV. Potential mechanisms for inadequate clearance include defective complement C1q receptor (47), failure of crosstalk between antibodies and complement non-interference with immune precipitation via the classical pathway, and impaired solubilization of immune precipitates impeding clearance by the alternative complement pathway in SLE (48). Circulating cryoglobulins activate complements, forming immune complexes with subsequent complement C4 consumption. Another plausible mechanism postulated for low C4 levels is competent cryoglobulin interaction with the C1q protein (47).
More than 40% of cases were EMC with type III cryoglobulinemia in the GISC study (42), compared to the CryoVas French series (10, 32, 33). Furthermore, most patients with type III EMC progressed to type II during their follow-up, highlighting that ineffective treatment of EMC could impact the clearance of cryoglobulin (42). In our review, frequent relapses were encountered in patients with underlying EMC (23, 31, 37, 39).
A study by Fayed et al. identified predictors of relapses following sustained viral response in HCV-related CV. The main predictors determined the presence of severe clinical manifestations at the end of direct-acting antiviral therapy such as skin ulcers, renal disease, peripheral neuropathy, and male gender (49). These findings align with our review of NICV, which also demonstrated that severe skin manifestations were associated with relapse, while male gender and renal disease were linked to a poorer prognosis. Moreover, the study conducted by Fayed et al. did not find a significant association between relapse and B-cell lymphoma or immunological parameters due to the limited availability of data (49).
The reactivation of RF-expressing B cells occurs when B-cell receptor (BCR) binds to immune complexes and Toll-like receptor-7 (TLR-7) and Toll-like receptor-9 (TLR-9), in response to microbial nucleic acid (50). Unlike TLR7, which had a limited impact on relapses, TLR9 had a significant role in disrupting immune tolerance and promoting relapse, even in patients who had successfully eliminated the virus (51). HCV-related CV patients who initially responded to B-cell targeting agents such as RTX experienced relapses due to ongoing viral mutations, leading to the emergence of different B-cell clones (52). Elevated levels of TNF ligand superfamily member 13B (also known as the B-cell-activating factor of the TNF family [BAFF]) and TNF ligand superfamily member 13 (also known as a proliferation-inducing ligand [APRIL]), which are implicated in B-cell survival and autoimmunity, have been observed in both HCV infection and autoimmune diseases (53). Considering the underlying pathogenic mechanisms, B-cell proliferation is a potential risk factor for relapse. Furthermore, BAFF and APRIL may serve as potential biomarkers to predict disease progression and/or relapse (49).
Several studies explored various therapeutic regimes; conspicuously, the RTX and CS regimen on induction, followed by maintenance RTX, demonstrated better efficacy and safety than CS or AKA, particularly in relapsing and/or refractory NICV patients (10, 23–26, 30–34, 37–40). RTX-associated CV flares have been observed in some patients and may be considered a risk factor for relapse, highlighting the importance of individualized treatment strategies (22, 26, 31). Strikingly, patients who are refractory to RTX share similar characteristics such as female predominance, age >50 years, type II cryoglobulinemia with monoclonal IgM kappa component, pSS, and EMC (32, 36). Saadoun et al. demonstrated significant improvement with the belimumab and RTX combination regimen in refractory cases (54). Further studies are needed to investigate the safety and efficacy of plausible treatment regimens for RTX-refractory cases.
A study by Lobbes et al. observed that patients with type I cryoglobulinemia who received treatment with Rituximab and experienced a reduction in circulating cryoglobulin levels showed rapid clinical improvement (28). Factors including advancing age with renal impairment were associated with poor prognosis (19, 27), and monitoring of cryocrit levels in type I predicted symptom improvement, serving as a prognostic tool in guiding management (25).
Our study has limitations due to the rarity of NICV, cohort size, and heterogeneity of the included studies. Most studies were retrospective observational studies discussing relapses of NICV and the efficacy of multiple treatment regimens. RCTs and/or prospective studies on relapse with a larger sample size are necessary to achieve explicit results. Some studies had mixed cohorts inclusive of ICV, alluding to the possibility of segregation bias. Case reports and case series of less than four were excluded; articles in languages other than English were excluded, indicative of selection and language bias.
To our knowledge, this is the first systematic review exploring identifiable risk factors for relapse with an underlying immunopathological etiology in NICV in relation to Ig isotype. Given the disease’s rarity and lack of RCTs and prospective studies, we could not perform a meta-analysis. Nonetheless, the achievement of remission in HCV-related CV with direct-acting antiviral agents paves the way for NICV as the next leading cause of CV (21). Abating the likelihood of relapse at the time of diagnosis is cumbersome; however, identification of those at risk might be critical for efficiently treating patients with NICV.
Conclusion
In conclusion, type I NICV accompanied by lymphoproliferative disorder often shows a response-relapse pattern, particularly when treated with CS alone. In MC, baseline factors such as cutaneous necrosis, purpura, and joint involvement, along with incomplete clinical and immunological responses to treatment, increase the risk of relapse. CS monotherapy is associated with a higher relapse risk. RTX-associated CV flare is more common in patients with renal involvement, B-cell lymphoproliferation, elevated cryoglobulin levels, decreased IgG levels, and low C4 prior to RTX treatment. These findings emphasize the importance of achieving both clinical and immunological responses and identifying relapse risk factors in NICV. Further research is needed to optimize treatment strategies and improve long-term outcomes for patients with NICV and associated lymphoproliferative disorders.
Author contributions
Conceptualization: NR. Data curation: NR and PR. Formal analysis: NR, PR, and HA. Methodology: NR and PR. Validation: NR and PR. Visualization: NR and PR. Writing: original draft: NR and PMR. Writing: review and editing: NR, PR, and HA. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Ms. Breffni Smith, RCSI Librarian, for her contributions to the literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1215345/full#supplementary-material
Abbreviations
AKA, alkylating agent; AZA, azathioprine; CS, corticosteroids; CV, cryoglobulinemic vasculitis; CYC, cyclophosphamide; EMC, essential mixed cryoglobulinemia; HCV, Hepatitis C virus; ICV, infectious cryoglobulinemic vasculitis; Ig, immunoglobulins; RF, rheumatoid factor; MC, mixed cryoglobulinemia; NICV, non-infectious cryoglobulinemic vasculitis; pSS, primary Sjögren’s syndrome; RCT, randomized control trial; RTX, rituximab; SLE, systemic lupus erythematosus.
References
1. Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet (2012) 379(9813):348–60. doi: 10.1016/S0140-6736(11)60242-0
2. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. a report of 86 cases. Am J Med (1974) 57(5):775–88. doi: 10.1016/0002-9343(74)90852-3
3. Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol (2002) 55(1):4–13. doi: 10.1136/jcp.55.1.4
4. Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, et al. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian group for the study of cryoglobulinaemias. QJM (1995) 88(2):115–26. doi: 10.1093/oxfordjournals.qjmed.a069032
5. Roccatello D, Sciascia S, Naretto C, Barreca A, Solfietti L, Battaglia L, et al. Recognizing the new disorder “idiopathic hypocryoglobulinaemia” in patients with previously unidentified clinical conditions. Sci Rep (2022) 12:14904. doi: 10.1038/s41598-022-18427-x
6. Ferri C, Ramos-Casals M, Zignego AL, Arcaini L, Roccatello D, Antonelli A, et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A Multidiscip Expert statement. Autoimmun Rev (2016) 15(12):1145–60. doi: 10.1016/j.autrev.2016.09.006
7. Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood (2017) 129(3):289–98. doi: 10.1182/blood-2016-09-719773
8. Comarmond C, Cacoub P, Saadoun D. Treatment of chronic hepatitis c-associated cryoglobulinemia vasculitis at the era of direct-acting antivirals. Therap Adv Gastroenterol (2020) 13:1756284820942617. doi: 10.1177/1756284820942617
9. Giuggioli D, Sebastiani M, Colaci M, Fallahi P, Gragnani L, Zignego AL, et al. Treatment of HCV-related mixed cryoglobulinemia. Curr Drug Targets (2017) 18(7):794–802. doi: 10.2174/1389450116666150825112105
10. Terrier B, Marie I, Launay D, Lacraz A, Belenotti P, de Saint-Martin L, et al. Predictors of early relapse in patients with non-infectious mixed cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey. Autoimmun Rev (2014) 13(6):630–4. doi: 10.1016/j.autrev.2013.11.006
11. Cacoub P, Comarmond C, Domont F, Savey L, Saadoun D. Cryoglobulinemia vasculitis. Am J Med (2015) 128(9):950–5. doi: 10.1016/j.amjmed.2015.02.017
12. PRISMA (2019). Available at: http://www.prisma-statement.org/documents/PRISMA-ScR-Fillable-Checklist_11Sept2019.pdf.
13. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2013). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
14. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
15. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol (2008) 61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008
16. De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheumatol (2012) 64(3):843–53. doi: 10.1002/art.34331
17. Retamozo S, Gheitasi H, Quartuccio L, Kostov B, Corazza L, Bové A, et al. Cryoglobulinaemic vasculitis at diagnosis predicts mortality in primary sjögren syndrome: analysis of 515 patients. Rheumatol (Oxford) (2016) 55(8):1443–51. doi: 10.1093/rheumatology/kew194
18. Colantuono S, Mitrevski M, Yang B, Tola J, Carlesimo M, De Sanctis GM. Efficacy and safety of long-term treatment with low-dose rituximab for relapsing mixed cryoglobulinemia vasculitis. Clin Rheumatol (2017) 36(3):617–23. doi: 10.1007/s10067-017-3552-6
19. Marson P, Monti G, Montani F, Riva A, Mascia MT, Castelnovo L, et al. Apheresis treatment of cryoglobulinemic vasculitis: a multicentre cohort study of 159 patients. Transfus Apher Sci (2018) 57(5):639–45. doi: 10.1016/j.transci.2018.06.005
20. Argyropoulou OD, Pezoulas V, Chatzis L, Critselis E, Gandolfo S, Ferro F, et al. Cryoglobulinemic vasculitis in primary sjögren's syndrome: clinical presentation, association with lymphoma and comparison with hepatitis c-related disease. Semin Arthritis Rheumatol (2020) 50(5):846–53. doi: 10.1016/j.semarthrit.2020.07.013
21. Boleto G, Ghillani-Dalbin P, Musset L, Biard L, Mulier G, Cacoub P, et al. Cryoglobulinemia after the era of chronic hepatitis c infection. Semin Arthritis Rheumatol (2020) 50(4):695–700. doi: 10.1016/j.semarthrit.2020.05.004
22. Desbois AC, Biard L, Sène D, Brocheriou I, Rouvier P, Lioger B, et al. Rituximab-associated vasculitis flare: incidence, predictors, and outcome. J Rheumatol (2020) 47(6):896–902. doi: 10.3899/jrheum.190076
23. Zaja F, De Vita S, Mazzaro C, Sacco S, Damiani D, De Marchi G, et al. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood (2003) 101(10):3827–34. doi: 10.1182/blood-2002-09-2856
24. Bryce AH, Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Inwards DJ, et al. Response to rituximab in patients with type II cryoglobulinemia. Clin Lymphoma Myeloma (2006) 7(2):140–4. doi: 10.3816/CLM.2006.n.052
25. Sidana S, Rajkumar SV, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol (2017) 92(7):668–73. doi: 10.1002/ajh.24745
26. Terrier B, Karras A, Kahn JE, Le Guenno G, Marie I, Benarous L, et al. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Med (Baltimore) (2013) 92(2):61–8. doi: 10.1097/MD.0b013e318288925c
27. Néel A, Perrin F, Decaux O, Dejoie T, Tessoulin B, Halliez M, et al. Long-term outcome of monoclonal (type 1) cryoglobulinemia. Am J Hematol (2014) 89(2):156–61. doi: 10.1002/ajh.23608
28. Lobbes H, Grobost V, Lemal R, Rieu V, Le Guenno G, Ruivard M. Efficacy of Rituximab in non-IgM type I cryoglobulinaemic vasculitis: a single-centre retrospective case series. Eur J Haematol (2018) 101(5):635–42. doi: 10.1111/ejh.13154
29. Saadoun D, Sellam J, Ghillani-Dalbin P, Crecel R, Piette JC, Cacoub P. Increased risks of lymphoma and death among patients with non-hepatitis c virus-related mixed cryoglobulinemia. Arch Intern Med (2006) 166(19):2101–8. doi: 10.1001/archinte.166.19.2101
30. Matignon M, Cacoub P, Colombat M, Saadoun D, Brocheriou I, Mougenot B, et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis c virus infection. Med (Baltimore) (2009) 88(6):341–8. doi: 10.1097/MD.0b013e3181c1750f
31. Foessel L, Besancenot JF, Blaison G, Magy-Bertrand N, Jaussaud R.L, Etienne Y, et al. Clinical spectrum, treatment, and outcome of patients with type II mixed cryoglobulinemia without evidence of hepatitis c infection. J Rheumatol (2011) 38(4):716–22. doi: 10.3899/jrheum.100898
32. Terrier B, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, et al. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood (2012) 119(25):5996–6004. doi: 10.1182/blood-2011-12-396028
33. Terrier B, Carrat F, Krastinova E, Marie I, Launay D, Lacraz A, et al. Prognostic factors of survival in patients with non-infectious mixed cryoglobulinaemia vasculitis: data from 242 cases included in the CryoVas survey. Ann Rheum Dis (2013) 72(3):374–80. doi: 10.1136/annrheumdis-2012-201405
34. Zaidan M, Terrier B, Pozdzik A, Frouget T, Rioux-Leclercq N, Combe C, et al. Spectrum and prognosis of noninfectious renal mixed cryoglobulinemic GN. J Am Soc Nephrol (2016) 27(4):1213–24. doi: 10.1681/ASN.2015020114
35. Fenoglio R, Sciascia S, Rossi D, Naretto C, Alpa M, Roccatello D. Non HCV-related mixed cryoglobulinemic vasculitis with biopsy-proven renal involvement: the effects of rituximab. Front Med (Lausanne) (2022) 9:819320. doi: 10.3389/fmed.2022.819320
36. Pouchelon C, Visentini M, Emmi G, le Guern V, Quartuccio L, Samson M, et al. Management of nonviral mixed cryoglobulinemia vasculitis refractory to rituximab: data from a European collaborative study and review of the literature. Autoimmun Rev (2022) 21(4):103034. doi: 10.1016/j.autrev.2022.103034
37. Cruz B, de Melo AL, de Almeida Cruz Filho A, Gouvea PS, Soares CG, Battle VS, et al. Non-hepatitis c virus cryoglobulinemic vasculitis: a case series of nine patients. Rev Bras Reumatol (2006) 46(5):310–4. doi: 10.1590/S0482-50042006000500002
38. Terrier B, Launay D, Kaplanski G, Hot A, Larroche C, Cathébras P, et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French autoimmunity and rituximab registry. Arthritis Care Res (Hoboken) (2010) 62(12):1787–95. doi: 10.1002/acr.20318
39. Leśniak K, Rymarz A, Lubas A, Niemczyk S. Noninfectious, severe cryoglobulinemic vasculitis with renal involvement - safety and efficacy of long-term treatment with rituximab. Int J Nephrol Renovasc Dis (2021) 14:267–77. doi: 10.2147/IJNRD.S315388
40. Roubertou Y, Mainbourg S, Hot A, Fouque D, Confavreux C, Chapurlat R, et al. Cryoglobulinemia in systemic lupus erythematosus: a retrospective study of 213 patients. Arthritis Res Ther (2022) 24(1):167. doi: 10.1186/s13075-022-02857-z
41. Michaud M, Moulis G, Puissant B, Balardy L, Huart A, Gaches F, et al. Cryofibrinogenemia: a marker of severity of cryoglobulinemic vasculitis. Am J Med (2015) 128(8):916–21. doi: 10.1016/j.amjmed.2015.03.009
42. Galli M, Oreni L, Saccardo F, Castelnovo L, Filippini D, Marson P, et al. HCV-unrelated cryoglobulinaemic vasculitis: the results of a prospective observational study by the Italian group for the study of cryoglobulinaemias (GISC). Clin Exp Rheumatol (2017) 35 Suppl 103(1):67–76.
43. Kolopp-Sarda MN, Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol (2021) 33(1):1–7. doi: 10.1097/BOR.0000000000000757
44. Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol (1991) 25(1 Pt 1):21–7. doi: 10.1016/0190-9622(91)70168-2
45. Quartuccio L, Isola M, Baldini C, Priori R, Bartoloni Bocci E, Carubbi F, et al. Biomarkers of lymphoma in sjögren's syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J Autoimmun (2014) 51:75–80. doi: 10.1016/j.jaut.2013.10.002
46. Andersen BR, Tesar JT, Schmid FR, Haisty WK, Hartz WH Jr. Biological and physical properties of a human m-cryoglobulin and its monomer subunit. Clin Exp Immunol (1971) 9(6):795–807.
47. Gorevic PD. Rheumatoid factor, complement, and mixed cryoglobulinemia. Clin Dev Immunol (2012) 2012:439018. doi: 10.1155/2012/439018
48. Adu D, Williams DG. Complement activating cryoglobulins in the nephritis of systemic lupus erythematosus. Clin Exp Immunol (1984) 55(3):495–501.
49. Fayed A, Hegazy MT, Biard L, Vieira M, El Shabony T, Saadoun D, et al. Relapse of hepatitis c virus cryoglobulinemic vasculitis after sustained viral response after interferon-free direct-acting antivirals. Am J Gastroenterol (2022) 117(4):627–36. doi: 10.14309/ajg.0000000000001667
50. Suthers AN, Sarantopoulos S. TLR7/TLR9- and b cell receptor-signaling crosstalk: promotion of potentially dangerous b cells. Front Immunol (2017) 8:775. doi: 10.3389/fimmu.2017.00775
51. Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol (2014) 10(6):365–73. doi: 10.1038/nrrheum.2014.33
52. Saadoun D, Delluc A, Piette JC, Cacoub P. Treatment of hepatitis c-associated mixed cryoglobulinemia vasculitis. Curr Opin Rheumatol (2008) 20(1):23–8. doi: 10.1097/BOR.0b013e3282f1330c
53. Comarmond C, Lorin V, Marques C, Maciejewski-Duval A, Joher N, Planchais C, et al. TLR9 signalling in HCV-associated atypical memory b cells triggers Th1 and rheumatoid factor autoantibody responses. J Hepatol (2019) 71(5):908–19. doi: 10.1016/j.jhep.2019.06.029
Keywords: small-vessel vasculitis, cryoglobulinemia vasculitis, cryoglobulinemia, non infectious cryoglobulinemia vasculitis, non-HCV related cryoglobulinemia, essential cryoglobulinemia, systematic review, relapse
Citation: Rajendran N, Rameli PM and Awad H (2023) Risk factors for relapse in non-infectious cryoglobulinemic vasculitis, including type I cryoglobulinemia: a systematic review. Front. Immunol. 14:1215345. doi: 10.3389/fimmu.2023.1215345
Received: 01 May 2023; Accepted: 12 June 2023;
Published: 07 July 2023.
Edited by:
Luca Quartuccio, University of Udine, ItalyReviewed by:
Mohamed Tharwat Hegazy, Cairo University, EgyptMarcella Visentini, Sapienza University of Rome, Italy
Paolo Fraticelli, Ospedali Riuniti Umberto I, Italy
Copyright © 2023 Rajendran, Rameli and Awad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Puteri Maisarah Rameli, cmFtZWxpcEB0Y2QuaWU=
†These authors have contributed equally to this work and share first authorship
 Nithya Rajendran
Nithya Rajendran Puteri Maisarah Rameli
Puteri Maisarah Rameli Hanaa Awad
Hanaa Awad